Abstract
Rationale:
Primary malignant melanoma of the lung (PMML) is an extremely rare neoplasm with a dismal prognosis. The diagnosis of PMML is very difficult and is based on several clinical, radiological, and histopathological criteria.
Patient concerns:
A 61-year-old women was admitted with a 2-month history of a productive cough and chest pain provoked by breathing and coughing. Computed tomography (CT) scans of the chest showed a large, solid tumor in the right middle lobe of the lung. Puncture biopsy of the right lung lesion was performed using B-ultrasound guidance, and immunohistochemical tests were performed.
Diagnoses:
The diagnosis of PMML was histopathologically confirmed by puncture biopsy with B-ultrasound guidance of the right lung lesion.
Interventions:
The patient refused to receive surgery, adjuvant chemotherapy, or radiation therapy.
Outcomes:
The patient died 6 months after the diagnosis.
Lessons:
The clinical manifestation and imaging features of PMML are not specific, and it does not differ from the more common primary bronchogenic carcinoma. In addition, it cannot be discriminated from other forms of primary melanoma according to its histology and immunohistochemistry. The treatment of choice is an aggressive surgical approach, combined with radiation therapy, chemotherapy, and immunotherapy.
Keywords: diagnosis, primary malignant melanoma of the lung, treatment
1. Introduction
Malignant melanoma is a refractory disease that generally presents as a primary neoplasm of the skin.[1,2] Primary malignant melanoma of the lung (PMML) is rare, accounting for only 0.01% of all primary lung tumors.[3] Prior to the diagnosis of primary melanoma of the lung, we must prove that there is no primary lesion at cutaneous or nonpulmonary extracutaneous sites.[4] In this report, we present a case of an elderly woman who was diagnosed with PMML at our hospital, and the related literature was reviewed. The family of the patient provided written informed consent.
2. Case report
A 61-year-old female presented to Xinhua Hospital (Shanghai, China) on April 11, 2015, reporting a 2-month history of a productive cough with mucous sputum and chest pain provoked by breathing and coughing. She denied hemoptysis, fever, night sweats, dyspnea, exercise limitation, skin rash, or joint pains. She did not have pets, recent travel, or exposure to tuberculosis. She had no smoking history. The only medical history was significant for hypertension. She was exhibited a weight loss of 2.5 kg over 2 months. At the outpatient clinic, she was found to have an abnormal shadow in the right middle lung on chest X-ray (Fig. 1A).
Figure 1.

Imaging features of the case. (A) Chest X-ray showed a high-density shadow in the right middle lung. (B) Chest CT showed a peripheral mass in the right middle lobe with enlarged lymph nodes at nodal station 4R. (C) PET-CT revealed no other tumors that could have been the primary lesion. CT = computed tomography, PET = positron emission tomography.
On physical examination, auscultation of the lungs revealed diminished breath sounds over the right middle and lower lung fields. The patient was apyrexic and hemodynamically stable, with an oxygen saturation of 94% while inspiring room air. Laboratory examination results showed her white blood cell count was increased to 10.6 × 109/L and her erythrocyte sedimentation rate was 54 mm/hour. Neuron-specific enolase levels were slightly increased to 20.47 (μg/L) in tumor marker detection. The serum chemistry finding was an elevated level of lactic dehydrogenase (747 U/L [upper normal value of 211 U/L]). Sputum culture results showed the dominant growth of Haemophilus influenzae. The remaining results from the laboratory examination, including analyses of hemoglobin, platelet count, serum chemistry, fungal serologies, tuberculosis antibody, rheumatoid factor, cyclic citrullinated peptide, antinuclear antibodies, antineutrophil cytoplasmic antibodies, and urine, were normal or negative.
Chest X-ray (Fig. 1A) showed a high-density shadow in the right middle lung. Subsequent computed tomography (CT) scan of the chest (Fig. 1B) showed a large, solid tumor in the right middle lobe of the lung, with a clear boundary adjacent to the pleura, enlarged lymph nodes at the mediastinal, right hilus pulmonis and left neck root, and a small amount of right pleural effusion. The findings from positron emission tomography-CT (PET-CT) were consistent with a malignant, space-occupying lesion of the right lung, with multiple lymph node metastases and bone metastases, and it revealed no other lesions that could have represented a primary site (Fig. 1C).
To determine the exact histopathological nature of the lesion, bronchoscopy was performed. It showed tracheobronchial stenosis and bronchial mucosal invasion in the right middle lobe (Fig. 2). However, the results from biopsy and brush biopsy were all negative, and the pathologic diagnosis remained unclear. Consequently, B-ultrasound-guided percutaneous transthoracic biopsy of the lung lesions was performed. The histology showed the tumor cells were epithelioid, rich in cytoplasm, eosinophilic staining, and discohesive, with a sheet distribution and frequent mitoses. Immunohistochemical analysis showed positive cytoplasmic staining of the tumor cells for HMB-45, MelanA, and S100 and negative staining for TTF1, cytokeratin (CK), SYN, and CD3, findings compatible with malignant melanoma (Fig. 3). To distinguish between primary and metastatic pulmonary melanoma, we performed a thorough examination of the skin, mucosae, scalp, anogenital region, and eyes, but no melanocytic lesion was identified. Furthermore, PET-CT revealed no other tumors that could be a primary site. Finally, we diagnosed the lung tumor as a PMML. She refused to receive surgery, adjuvant chemotherapy, or radiation therapy and died 6 months after the diagnosis.
Figure 2.
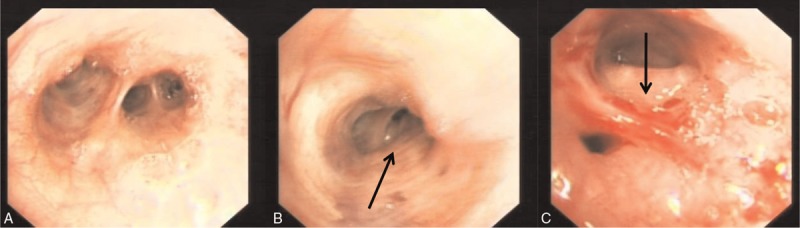
Bronchoscopic features of the right middle lobe. (A) Right middle lobe. (B) Medial segment of right middle lobe tracheobronchial stenosis. (C) Lateral segment of right middle lobe bronchial mucosal invasion.
Figure 3.

Immunohistochemical features of lung tissue biopsy (×400). Positive cytoplasmic staining of the tumor cells for HMB-45, MelanA, and S100 and negative for cytokeratin (CK).
3. Discussion
PMML is an extremely rare neoplasm, accounting for 0.01% of all primary lung malignancies. Only a few cases have been reported in the world literature.[5–8] The clinical manifestation and imaging features of PMML are not specific, and it does not differ from the more usual primary bronchogenic carcinoma. In addition, it cannot be discriminated from other forms of primary melanoma according to its histology and immunohistochemistry. Therefore, the diagnosis is very difficult and is based on several clinical, radiological, and histopathological criteria.
Patients with PMML have a wide age distribution, and the mean age at diagnosis is 51 years (reported range, 45–71 years). There is no difference in the incidence of PMML between men and women.[6] The symptoms exhibited at presentation were cough, expectoration, hemoptysis, chest tightness, or chest pain. Some patients may be symptomless, with an incidental finding on routine chest X-ray. The most common abnormal chest radiological findings are a solitary, central endobronchial neoplasm, although in this case, there was a peripheral distribution.[3,9]
The histogenesis of PMML remains controversial.[3] Several theories have been proposed to explain the occurrence of PMML. The most plausible theory is that during embryogenesis some melanocytes can migrate into the lower respiratory anlage.[10,11] The histological characteristics of PMML are large epithelioid cells with large, round hyperchromatic nuclei and prominent eosinophilic nucleoli. A large amount of melanin granules is observed in the cytoplasm of the neoplastic cells.[5,6] HMB-45 is a specific marker of primary melanoma, and the positive ratio is greater than 96.8%. MelanA and Vimentin have high sensitivity in the diagnosis of malignant melanoma, but their specificity is worse than that of HMB-45.[12] In the present case, HMB-45 and MelanA stains appear to have a diffuse strong positivity, which is helpful for the diagnosis of diseases. However, immunohistochemical staining offers little help in discriminating between PMML and other primary melanomas because these results can also be found in primary melanomas at other sites. Hence, we must prove that there are no primary lesions at cutaneous or nonpulmonary extracutaneous sites. The findings from PET-CT scan showed no evidence of malignancy, and a thorough examination of the skin showed no melanocytic lesion was identified. Thus, we diagnosed the lung tumor as PMML.
There are 4 clinical criteria that should be satisfied for the diagnosis of PMML: invasion or destruction of the bronchial epithelium and intact bronchial mucosa; the above changes were proven to be a malignant melanoma by histology and immunohistochemistry; radiological manifestation of a solitary lung tumor; and no history or present clinical or laboratory findings of a cutaneous, mucosal, or ocular melanoma.[3,5] The present case fulfills the above-mentioned criteria.
The treatment of choice for PMML is an aggressive surgical approach, combined with radiation therapy, chemotherapy, and immunotherapy.[5,6] The incision range of surgery should be large, which is at least above 5 cm from the margin to the lesion, to avoid recurrence. However, many patients show postoperative recurrence, rapid progression, and a short survival period. Therefore, postoperative adjuvant chemotherapy is proposed with agents such as dacarbazine, IL-2, and interferon.[13] Radiation therapy can be used for postoperative patients with locally advanced melanoma, and it can be used as palliation treatment for patients with distant metastases (such as brain or bones).[14] Novel immunotherapy, with effective immune checkpoint blockers, such as ipilimumab (anticytotoxic T-lymphocyte antigen-4 antibody) or nivolumab and pembrolizumab (antiprogrammed cell death monoclonalantibodies), has been used for treatment in patients with advanced melanoma in several clinic trials, which have been reported to have impressive antitumor effects.[15,16] In the present case, the patient had multiple lymph node metastasis and bone metastasis and refused to receive surgery, adjuvant chemotherapy, or radiation therapy; she died 6 months after the diagnosis.
In conclusion, PMML is an extremely rare neoplasm and has a high degree of malignancy, a powerful capacity for invasiveness, a tendency toward recurrence, and a poor prognosis. The treatment of choice is an aggressive surgical approach, combined with radiation therapy and chemotherapy. Novel immunotherapy has shown promising results and further studies are needed.
Footnotes
Abbreviations: CT = computed tomography, PET = positron emission tomography, PMML = primary malignant melanoma of the lung.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Allen AC, Spitz S. Malignant melanoma: a clinicopathologic analysis of the criteria for diagnosis and prognosis. Cancer 1953;6:1–45. [DOI] [PubMed] [Google Scholar]
- [2].Ruiter DJ. Clinical and pathologic diagnosis, staging and prognostic factors of melanoma and management of primary disease. Curr Opin Oncol 1992;4:357–67. [DOI] [PubMed] [Google Scholar]
- [3].Wilson RW, Moran CA. Primary melanoma of the lung: a clinicopathologic and immunohistochemical study of eight cases. Am J Surg Pathol 1997;21:1196–202. [DOI] [PubMed] [Google Scholar]
- [4].Maeda R, Isowa N, Onuma H, et al. Primary malignant melanoma of the lung with rapid progression. Gen Thorac Cardiovasc Surg 2009;57:671–4. [DOI] [PubMed] [Google Scholar]
- [5].Dountsis A, Zisis C, Karagianni E, et al. Primary malignant melanoma of the lung: a case report. World J Surg Oncol 2003;1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pan XD, Zhang B, Guo LC, et al. Primary malignant melanoma of the lung in the elderly: case report and literature review. Chin Med J (Engl) 2010;123:1815–7. [PubMed] [Google Scholar]
- [7].Kundranda MN, Clark CT, Chaudhry AA, et al. Primary malignant melanoma of the lung: a case report and review of the literature. Clin Lung Cancer 2006;7:279–81. [DOI] [PubMed] [Google Scholar]
- [8].Reddy VS, Mykytenko J, Giltman LI, et al. Primary malignant melanoma of the lung: review of literature and report of a case. Am Surg 2007;73:287–9. [PubMed] [Google Scholar]
- [9].Ost D, Joseph C, Sogoloff H, et al. Primary malignant melanoma: case report and literature review. Mayo Clin Proc 1999;74:62–6. [DOI] [PubMed] [Google Scholar]
- [10].Busuttil A. Dendritic pigmented cells within human laryngeal mucosa. Arch Otolaryngol 1976;102:43–4. [DOI] [PubMed] [Google Scholar]
- [11].Wenig BM. Laryngeal mucosal malignant melanoma: a clinicopathologic, immunohistochemical, and ultrastructural study of four patients and a review of the literature. Cancer 1995;75:1568–77. [DOI] [PubMed] [Google Scholar]
- [12].Achilles E, Schröder S. Postive cytokeratin results in malignant melanoma. Pitfall in differential immunohistologic diagnosis of occult neoplasms. Pathologe 1994;15:235–41. [DOI] [PubMed] [Google Scholar]
- [13].Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7–17. [DOI] [PubMed] [Google Scholar]
- [14].Bastiaannet E, Beukema JC, Hoekstra HJ. Radiation therapy following lymph node dissection in melanoma patients: treatment, outcome and complications. Cancer Treat Rev 2005;31:18–26. [DOI] [PubMed] [Google Scholar]
- [15].Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hao C, Tian J, Liu H, et al. Efficacy and safety of anti-PD-1 and anti-PD-1 combined with anti-CTLA-4 immunotherapy to advanced melanoma: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e7325. [DOI] [PMC free article] [PubMed] [Google Scholar]


