Abstract
Rapé, a diverse group of smokeless tobacco products indigenous to South America, is generally used as a nasal snuff and contains substantial amount of plant material with or without tobacco. Previously uncharacterized, rapé contains addictive and harmful chemicals that may have public health implications for users. Here we report % moisture, pH and the levels of total nicotine, un-ionized nicotine, flavor-related compounds, tobacco-specific N-nitrosamines (TSNAs) and polycyclic aromatic hydrocarbons (PAHs) for manufactured and hand-made rapé. Most rapé products were mildly acidic (pH 5.17 – 6.23) with total nicotine ranging from 6.32 to 47.6 milligram per gram of sample (mg/g). Calculated un-ionized nicotine ranged from 0.03 to 18.5 mg/g with the highest values associated with hand-made rapés (pH 9.75 – 10.2), which contain alkaline ashes. In tobacco-containing rapés, minor alkaloid levels and Fourier transform infrared spectra were used to confirm the presence of Nicotiana rustica, a high nicotine tobacco species. There was a wide concentration range of TSNAs and PAHs among the rapés analyzed. Several TSNAs and PAHs identified in the products are known or probable carcinogens according to the International Agency for Research in Cancer. Milligram quantities of some non-tobacco constituents, such as camphor, coumarin, and eugenol, warrant additional evaluation.
Keywords: Smokeless Tobacco, Nasal snuff, Rapé, GC/MS, Nicotine, Tobacco-specific Nitrosamines, Flavor chemicals
1. Introduction
The use of tobacco in South and Central America dates back several thousand years and is thoroughly integrated into the culture of northwest Amazonian tribes (Groark, 2010; Zagorevski and Loughmiller-Newman, 2012). Among South American Indians, tobacco preparations are most commonly sniffed nasally although they may also be held under the tongue for sublingual absorption. Recreational use of tobacco among South American Indians is generally in the form of a variety of nasal snuff known as rapé. While typically used for recreational purposes, some rapé varieties are used in the medicinal and spiritual practices of some tribes (e.g., Kaxinawá, Nu-nu, Yawanawá, Katukina (Wilbert, 1987; Duke and Vasquez, 1994; Lima, 1994; Perez-Gil, 2001; Lagrou, 1996).
The non-commercial preparation of rapé is similar across tribes. A high nicotine tobacco species, Nicotiana rustica, is reportedly used in rapé preparations. Tobacco leaves are air cured or heated over a low fire. The dry leaves are pulverized, finely sifted, and, depending on the intended use (medicinal, ritualistic, or recreational), mixed with finely ground plant materials (tonka bean, cinnamon, clove buds, etc.) or alkaline ashes (Cardoso and Nascimento, 2008). The ashes can made be from pharmacologically active plants (Macambo, Theobroma bicolor; Tsunu, Platycyamus regnellii; Copaíba, Copaiba sp.) (McKenna, 1993). Some custom-blended rapés reportedly have hallucinogenic properties (Duke and Vasquez 1994).
Although rapé has existed for thousands of years, presently only sociological and anthropological data are available on its composition and use. There is no known chemical data for levels of nicotine, flavor compounds, and toxicants in rapés. The present study provides data on the major chemical components and flavor compounds of this diverse and understudied smokeless tobacco product. Global travel and immigration can introduce previously unknown products to urban populations. Clinicians, regulators, and public health decision makers benefit from characterization of previously unknown tobacco products when high levels of pharmacologically active or toxic chemical components raise questions of their potential to cause harm.
2. Materials and methods
2.1. Sample collection
Samples were collected by the National Agency for Sanitary Surveillance (ANVISA) in Brazil and provided to the Centers for Disease Control and Prevention (CDC) for chemical analysis and characterization. Manufactured rapé samples, packaged in round metal tins (Figure 1), were from southern Brazil (Maringá, Paraná State); whereas, two custom-made rapés, including Rapé Kashinawá (from the Kashinawá tribe) and Rapé Nu-nu (from the Apurinã tribe) were purchased in municipal markets in northern Brazil (Rio Branco, Acre State). Ingredient labeling in Portuguese was interpreted by ANVISA staff. Additionally, samples of Nicotiana glauca, tonka bean, cinnamon, clove buds, and cassava were provided by AVISA. An exudate from the camphor laurel tree (Cinnamomum camphora), a common source of the chemical camphor (Duke Phytochemical Database, 2014), was purchased from a medicinal shop in Atlanta, GA. Upon receipt, smokeless samples were logged into a custom database and assigned barcodes with a unique ID.
Figure 1. Photograph of manufactured rapé product packaging.

Manufactered Products: 1) Rapé Caratinga Imburana, 2) Rapé Caratinga Mentolado, 3) Rapé Araçá Cravo e Canela, 4) Rapé Guarany Especial (Canela), 5) Rapé Moeda Girassol, 6) Rapé Moeda Especial, 7) Rapé Cacitral, 8) Rapé Guarany Cravo, 9) Rapé Guarany Cristal, 10) Rapé Real (flavored), and 11) Rapé Real (unflavored). Products 7) and 9) did not contain tobacco. Products 12) Rapé Guarany Especial is not shown.
Custom-made Products (not shown): 13) Rapé Kaxinawa and 14) Rapé Nu-nu did not have manufactured packaging.
2.2. Characterization using Fourier transform infrared (FT/IR) spectroscopy
Fourier transform infrared (FT/IR) spectroscopy is a valuable tool for identifying Nicotiana species (e.g., N. tabacum, N. rustica, and N. glauca), nontobacco plant components (e.g., tonka bean, cinnamon, clove buds, camphor laurel exudate, cassava, Peruvian cocoa), individual chemicals (e.g., coumarin, camphor, menthol, eugenol), and alkaline ashes (calcium carbonate). Infrared analysis parameters used in this study are described elsewhere (Stanfill et al., 2011).
2.3. Measurement of moisture content
Total moisture content was determined by the weight difference between fresh and oven dried samples as described elsewhere (Lawler et al., 2013).
2.4. Quantification of total nicotine by GC-MS
Nicotine concentrations were measured by gas chromatography-mass spectrometry (GC/MS) in selected ion monitoring (SIM) mode as described elsewhere (Stanfill et al., 2009). Measurements of product pH, required for calculating percent and total un-ionized nicotine, were performed in this study as described previously (Lawler et al., 2013).
2.5. Quantification of tobacco minor alkaloids by GC-MS/MS
Concentrations of minor tobacco alkaloids (nornicotine, myosmine, anatabine, anabasine, and isonicoteine) were determined (expressed as microgram per gram of sample (μg/g)) using GC-Triple Quadruple Mass Spectrometry (MS/MS) in multiple reaction monitoring (MRM) mode. Parameters are published elsewhere (Lisko et al., 2013).
2.6. Quantification of tobacco-specific N-nitrosamines by LC-MS/MS
Concentrations of five TSNA analytes, included N-nitrosonornicotine (NNN), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), N-nitrosoanatabine (NAT), N-nitrosoanabasine (NAB), and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) were determined and expressed as nanogram per gram of sample (ng/g). All sample preparation and liquid chromatography tandem mass spectrometry (LC-MS/MS) parameters in this study were the same as reported previously (Lawler et al., 2013).
2.7. Quantification of Polycyclic Aromatic Hydrocarbons by GC/MS
Fourteen PAH compounds, which included acenaphthene (ACL), acenaphthylene (ACT), anthracene (ANT), benz[a]anthracene (BAA), benzo[a]pyrene (BAP), benzo[b]fluoranthene (BBF), benzo[e]pyrene (BEP), benzo[k]fluoranthene (BKF), chrysene (CHR), fluoranthene (FLR), fluorene (FLU), naphthalene (NAP), phenanthrene (PHE), and pyrene (PYR), were quantified using GC/MS in selected ion monitoring (SIM) mode and expressed in ng/g. Sample preparation and analysis parameters were performed as described elsewhere (Hearn et al., 2013).
2.8. Quantification of ten flavor compounds
Concentrations of eucalyptol, camphor, menthol, pulegone, ethyl salicylate, methyl salicylate, cinnamaldehyde, eugenol, diphenyl ether and coumarin were measured using GC/MS in selected ion monitoring (SIM) and expressed in μg/g. The analytical methodology has been previously described (Lisko et al., 2014).
3. Results and Discussion
Most manufactured rapés were packaged in colorful labeled tins with product name and descriptive ingredient labeling in Portuguese (Figure 1). Three products (Rapé Real, Rapé Kaxinawá, Rapé Nu-nu) lacked descriptive ingredient labeling and required additional chemical characterization. There was a limited amount of one sample available (Rapé Real, unflavored) so all analyses could not be performed for this sample.
The rapé products in this study can be divided into three groups: 1) manufactured non-tobacco rapé containing a mixture of plant materials, such as tonka bean, clove, camphor, cinnamon and cassava (2 products); 2) manufactured tobacco-containing rapé with tobacco alone or tobacco mixed with varying amounts of plant materials (tonka bean, clove, camphor, etc.) and flavorings such as menthol (10 products); or 3) custom-made rapés from indigenous Brazilian tribes that contained tobacco mixed with non-tobacco plant material and alkaline ashes (2 products). The rapés analyzed in this study had a variety of colors (dark brown, reddish-brown, tan, light pink, gray, bright green) based on tobacco and nontobacco ingredients (Figure 2). Rapés, which are normally sniffed, are finely milled, dry powders with a moisture content ranging from 3.68 to 9.46%. The pH values of non-tobacco manufactured rapé products ranged from pH 5.71 – 5.93; whereas, pH values of tobacco-containing manufactured rapé products ranged from pH 5.17 – 6.42. Two custom-made rapés had higher pH values (pH 9.75 and 10.2, respectively) than manufactured rapés. Individual moisture and pH values are presented (Table 1).
Figure 2.

Photographs of various rapés showing product texture and product color, which is influenced by the presence of tobacco and non-tobacco plant materials.
Table 1.
Levels of pH, un-ionized nicotine (%), moisture (%), total nicotine and un-ionized nicotine (mg/g) in rapé samples.
| Product | pH1 | % Un-ionized Nicotine | Moisture (%)1 |
Total Nicotine1 (wet, mg/g) |
Un-ionized Nicotine2 (wet, mg/g) |
Total Nicotine (dry, mg/g) |
Total Nicotine (dry, %) |
|---|---|---|---|---|---|---|---|
| Rapé (manufactured, non-tobacco) | |||||||
| Rapé Cacitral | 5.71 | n.a. | 7.65 | <LOD | n.a. | n.a. | n.a. |
| Rapé Guarany Cristal | 5.93 | n.a. | 5.77 | <LOD | n.a. | n.a. | n.a. |
|
| |||||||
| Rapé (manufactured, tobacco-containing) | |||||||
| Rapé Guarany Especial | 5.63 | 0.40 | 5.85 | 6.32 | 0.03 | 6.72 | 0.67 |
| Rapé Guarany Especial (Canela) | 5.73 | 0.52 | 6.66 | 6.72 | 0.03 | 7.20 | 0.72 |
| Rapé Guarany Cravo | 5.34 | 0.21 | 9.46 | 15.0 | 0.03 | 16.6 | 1.66 |
| Rapé Moeda Especial | 5.17 | 0.14 | 7.21 | 20.7 | 0.03 | 22.4 | 2.24 |
| Rapé Caratinga Mentolado | 6.42 | 2.46 | 5.56 | 21.6 | 0.53 | 22.9 | 2.29 |
| Rapé Caratinga Imburana | 6.23 | 1.59 | 6.15 | 24.2 | 0.39 | 25.8 | 2.58 |
| Rapé Araçá Cravo e Canela | 5.83 | 0.64 | 5.61 | 31.1 | 0.20 | 33.0 | 3.30 |
| Rapé Moeda Girassol | 5.78 | 0.57 | 6.21 | 32.7 | 0.19 | 34.8 | 3.48 |
| Rapé Real (flavored) | 5.91 | 0.77 | 5.80 | 44.7 | 0.34 | 47.5 | 4.75 |
| Rapé Real (unflavored)3 | 5.98 | 0.91 | 6.51 | 47.6 | 0.43 | 50.9 | 5.09 |
|
| |||||||
| Rapé (custom-made, tobacco-containing) | |||||||
| Rapé Kaxinawá | 9.75 | 98.2 | 4.91 | 18.9 | 18.5 | 18.5 | 1.85 |
| Rapé Nu-nu | 10.2 | 99.3 | 3.68 | 15.5 | 15.4 | 15.4 | 1.54 |
|
| |||||||
| Minimum Detected (tobacco-containing) | 5.17 | 0.14 | 3.68 | 6.32 | 0.03 | 6.72 | 0.67 |
| Maximum Detected (tobacco-containing) | 10.2 | 99.3 | 9.46 | 47.6 | 18.5 | 50.9 | 5.09 |
Measurements were made in triplicate except for pH and % moisture (n=2).
Total and un-ionzied nicotine was calculated using measured pH and total nicotine values. The % un-ionized is calculated using the Henderson-Hasselbalch equation.
Based on nicotine values, FT/IR and GC/MS full scan analyses, Rapé Real (unflavored) was determined to be essentially pure Nicotiana rustica tobacco with no other discernable flavorings or plant material added.
All reported analytical measurements are on a wet weight basis to allow comparisons of constituents on an “as used” basis. Two manufactured non-tobacco rapé products, Rapé Cacitral and Rapé Guarany Cristal, were confirmed to contain no detectable nicotine. Total nicotine concentrations in the manufactured tobacco-containing rapés ranged from 6.3 – 47.6 mg/g, wet. The custom-made rapés contained total nicotine concentrations ranging from 15.5 – 18.9 mg/g. Calculated total un-ionized nicotine concentrations in the custom-made preparations, Rapé Kashinawá (18.5 mg/g) and Rapé Nu-nu (15.4 mg/g), were much higher than the manufactured rapés (0.03 – 0.53 mg/g) (Table 1). Infrared spectroscopy was used to confirm the presence of Nicotiana rustica, a high nicotine tobacco species. In the tobacco-containing products, anatabine (131 – 2,090 μg/g) and nornicotine (181 – 1,130 μg/g) had the highest concentrations, followed by anabasine (37.6 – 366 μg/g). Two other minor alkaloids, myosmine (3.19 – 63.3 μg/g) and isonicoteine (31.5 – 193 μg/g), had lower levels.
Certain flavor-related compounds detectable by GC/MS can be used as markers for the presence of plant materials (Table 2). Fourier transform infrared spectroscopy (FT/IR) adds additional confirmation of the identity of tobacco and non-tobacco plant material (Figure 4). We found indicators of non-tobacco flavor compounds, including clove, cinnamon, tonka bean, menthol flavoring, and camphor laurel in some of the products. In contrast with the non-tobacco containing rapes, most of the chemical indicators, except coumarin, were below detectable levels in tobacco-containing manufactured and custom-made rapés. Several locally obtained raw materials were also analyzed with GC/MS and FT/IR for the indicator compounds (Table 2).
Table 2.
Concentrations (μg/g, wet) of seven flavor-related compounds (n=3) detected in various rapé samples and raw materials used to make rapé.
| Ingredients | Coumarin | Camphor | Eugenol | Methyl Salicylate | Cinnamaldehyde | Menthol | Pulegone | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Listed on packaging1 | Not listed on packaging2 | Average (μg/g, wet weight; n=3) | |||||||
|
|
|||||||||
| Rapé (non-tobacco, manufactured) | |||||||||
| Rapé Cacitral | TB, Cm, M | 17400 | 6640 | <LOD | <LOD | <LOD | 18600 | 40.1 | |
| Rapé Guarany Cristal | TB, Cs, M | Cl, Cm, Cn | 5830 | 1360 | 73.3 | 5.52 | 7.22 | 2780 | <LOD |
|
| |||||||||
| Rapé (tobacco-containing, manufactured) | |||||||||
| Rapé Guarany Especial | T, Cn | TB, Cl, Cm, M | 596 | 6.02 | 1330 | <LOD | 532 | 42.1 | <LOD |
| Rapé Guarany Especial (Canela) | T, Cn | TB, Cl, Cm, M | 623 | 29.3 | 1340 | <LOD | 545 | 40.0 | <LOD |
| Rapé Guarany Cravo | T, Cl | TB, Cm, M | 2760 | 1010 | 34000 | 14.9 | <LOD | 1580 | 17.1 |
| Rapé Moeda Especial | T, Cl, Cn | TB | 35.4 | <LOD | 975 | <LOD | <LOD | <LOD | <LOD |
| Rapé Caratinga Mentolado | T, M | TB | 10.9 | <LOD | <LOD | <LOD | <LOD | 212 | <LOD |
| Rapé Caratinga Imburana | T,TB | Cm | 1560 | 21.6 | <LOD | <LOD | <LOD | <LOD | <LOD |
| Rapé Araçá Cravo e Canela3 | Cl, Cn | T, TB | 13.3 | <LOD | 3670 | <LOD | <LOD | <LOD | <LOD |
| Rapé Moeda Girassol | T, S | TB, M, Cm | 22.3 | 17.2 | <LOD | <LOD | <LOD | 887 | <LOD |
| Rapé Real (flavored) | – | T, Cl, M | <LOD | <LOD | 5.13 | <LOD | <LOD | 1100 | <LOD |
|
| |||||||||
| Rapé (custom-made, tobacco-containing) | |||||||||
| Rapé Kashinawá3 | – | T, AA | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Rapé Nu-nu3 | – | T, PC, AA, TB | 8.23 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
|
| |||||||||
| Raw Materials | |||||||||
| Tonka Bean (pod and seeds) | 1428 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | ||
| Tonka Bean (seeds only) | 46900 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | ||
| Clove Buds | <LOD | <LOD | 84600 | 83.4 | <LOD | <LOD | <LOD | ||
| Cinnamon Bark | 44.5 | <LOD | 108 | <LOD | 195 | <LOD | <LOD | ||
| Cassava | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | ||
| Camphor Laurel tree (exudate) | <LOD | 670000 | <LOD | <LOD | <LOD | <LOD | 1960 | ||
Note: Eucalyptol, ethyl salicylate and diphenyl ether were not detected in any product analyzed. Nicotiana rustica was analyzed and did not contain any of the flavor additives above method LOD.
Ingredient designations are based on those stated on labeling.
Ingredients suspected to contain the product based on FT-IR and GC/MS analysis. Key: T=Tobacco; TB=Tonka Bean; M=Menthol; Cm=Camphor; Cl=Clove; Cn=Cinnamon; PC=Peruvian Cocoa; AA=Alkaline Ashes; S=Sunflower; Cs=Cassava Flour.
Packaging did not include ingredient list; although Cravo e Canela means ‘clove and cinnamon’.
Figure 4.
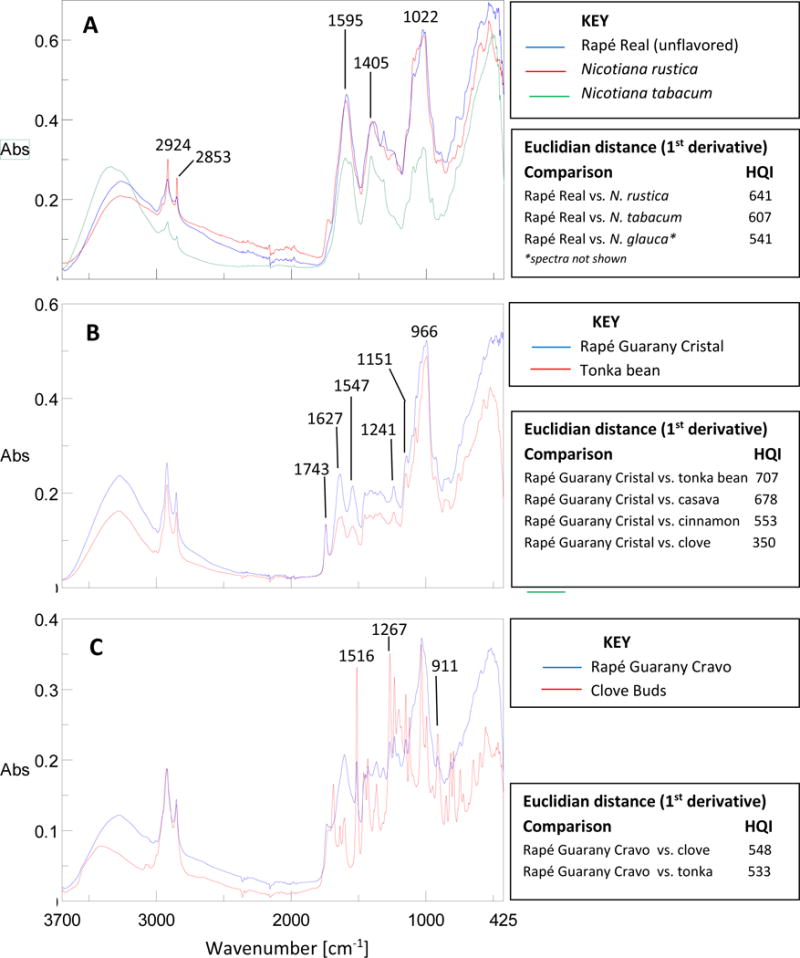
Fourier transform infrared (FT/IR) spectra in the range from 425 – 3700 cm−1. Comparison of: [A] Rape Real with Nicotiana tabacum and N. rustica; the IR spectra for N. glauca is not shown [B] Rapé Guarany Cristal (non-tobacco product) overlaid with pure tonka bean (seed and pods combined), [C] Rapé Guarany Cravo overlaid with ground clove buds. The hit quality index (HQI) for the Euclidian distance (1st derivative) is shown. The HQI scale is from 1 to 1000 for least similar to most similar to the reference spectra.
Eleven of 13 rapé products contained detectable levels of coumarin ranging from 8.23 – 17,400 μg/g. Tonka beans (Imburana in Portuguese) are a common source of coumarin; however, tonka bean was listed in the product name (Rape Caratinga Imburana) or as an ingredient in two more products (Rapé Cacitral and Rapé Guarany Cristal). Among the products tested, Rapé Cacitral had the highest coumarin levels (17,400 μg/g, wet) but its FT/IR spectral pattern did not match the samples of tonka seeds or intact pods (data not shown). Other plants known to contain high coumarin levels include woodruff (Galium odoratum), Korean date (Ziziphus jujuba) and Peru Balsam (Myroxylon balsamum) (Duke’s Phytochemical and Ethnobotanical Databases, 2014). Hence, it is possible that the coumarin in Rapé Cacitral is derived from a different type of tonka bean or another plant with high coumarin levels.
A cinnamon bark (Cinnamomum sp.) sample purchased in Brazil was analyzed and found to contain coumarin (44.5 μg/g) and cinnamaldehyde (195 μg/g). Coumarin is attributable to tonka bean when cinnamaldehyde is not detectable in most samples. In this study, only two products contained cinnamon (Rapé Guarany Especial (Canela) and Rapé Guarany Especial) as indicated by the presence of both cinnamaldehyde and coumarin. Rapé Moeda Especial and Rapé Araca Cravo e Canela listed cinnamon as an ingredient; however, neither GC/MS nor FT/IR analysis of these products indicated the presence of cinnamon. Seven rapé products had detectable levels of camphor, a chemical found at high levels in an exudate of the camphor laurel tree (Cinnamomum camphora). Three products had camphor concentrations exceeding 1 mg/g, with one product (Rapé Cacitral) exceeding 6 mg/g.
Eugenol, a major constituent of clove (Syzygium aromaticum), was detected in seven products. One product had a eugenol concentration of 34,000 μg/g suggesting high clove bud content while other rapés had detectable levels ranging from 5.13 – 3,670 μg/g. Methyl salicylate, a constituent of clove buds (Duke Phytochemical Database, 2014), was only detected in two products which also contained eugenol. Eight rapé products contained menthol across a wide concentration range (40.0 – 18,600 μg/g) of these, four brands, Rapé Cacitral, Rapé Guarany Cristal, Rapé Guarany Cravo, and Rapé Real (flavored) had menthol levels exceeding 1 mg/g. Pulegone, a terpene ketone often found with menthol (Duke’s Phytochemical and Ethnobotanical Databases, 2014) was present in two brands with high menthol levels. The concentrations of these flavor-related chemicals were determined quantitatively (Table 2).
Among all rapé products, carcinogenic NNN was the TSNA present at the highest concentration in most samples (Table 3). For carcinogenic TSNAs, the concentration range for NNN (13.2 – 14,500 ng/g) exceeded that of NNK (12 – 3,300 ng/g). For the tobacco containing manufactured products, the concentration range for total TSNAs (NAB, NAT, NNAL, NNK, and NNN) ranged from 88 – 24,200 ng/g. Interestingly, only NNN were detected at low levels (13.2 ng/g) in one non-tobacco manufactured brands, Rapé Guarany Cristal; whereas, Rapé Cacitral did not contain detectable levels of TSNAs (Table 3). Seven manufactured rapés had total TSNAs ranging from 1710 – 5990 ng/g; whereas, three products, Rapé Caratinga Mentolado, Rapé Guarany Especial, Rapé Guarany Especial (Canela), had higher total TSNA levels (19,300 – 24,200 ng/g) (Table 3).
Table 3.
Levels of five tobacco-specific N-Nitrosamines (n=3) present in various rapé samples.
| Product | NAB | NAT | NNAL | NNK | NNN | NNN+NNK | Total TSNAs* |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Average (ng/g, wet weight; n=3) | |||||||
| Rapé (manufactured, non-tobacco) | |||||||
| Rapé Cacitral | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Rapé Guarany Cristal | <LOD | <LOD | <LOD | <LOD | 13.2 | n.a. | 13.2 |
|
| |||||||
| Rapé (manufactured, tobacco-containing) | |||||||
| Rapé Guarany Especial | 376 | 2570 | 554 | 3300 | 13000 | 16300 | 19800 |
| Rapé Guarany Especial (Canela) | 364 | 2510 | 530 | 3250 | 12600 | 15900 | 19300 |
| Rapé Guarany Cravo | 58.1 | 735 | 49.4 | 133 | 739 | 872 | 1710 |
| Rapé Moeda Especial | 135 | 2080 | 118 | 1160 | 2390 | 3540 | 5870 |
| Rapé Caratinga Mentolado | 742 | 7290 | 277 | 1360 | 14500 | 15800 | 24200 |
| Rapé Caratinga Imburana | 268 | 2440 | 166 | 670 | 2450 | 3120 | 5990 |
| Rapé Araçá Cravo e Canela | 237 | 2610 | 132 | 425 | 2150 | 2576 | 5556 |
| Rapé Moeda Girassol | 158 | 1740 | 38.2 | 251 | 1790 | 2040 | 3980 |
| Rapé Real (flavored) | 86.1 | 1180 | 42.4 | 131 | 1020 | 1150 | 2460 |
| Rapé Real(unflavored) | 157 | 1700 | 50.3 | 232 | 1440 | 1680 | 3580 |
|
| |||||||
| Rapé (custom-made, tobacco-containing) | |||||||
| Rapé Kashinawá | 45.2 | 437 | 12.9 | 470 | 152 | 621 | 1120 |
| Rapé Nu-nu | 22.9 | 21.3 | 2.35 | 12 | 29 | 42 | 88 |
|
| |||||||
| Minimum detected | 9.16 | 6.85 | 2.35 | 3.99 | 13.2 | 17.2 | 36.7 |
| Maximum detected | 742 | 7290 | 554 | 3300 | 14500 | 16300 | 24200 |
Note: Total TSNAs is the sum of NAB, NAT, NNAL, NNK and NNN concentrations; n.a. = not applicable
TSNA analytes in this study, included N-nitrosonornicotine (NNN), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), N-nitrosoanatabine (NAT), N-nitrosoanabasine (NAB), and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL).
Polycyclic aromatic hydrocarbons (PAHs), which are generated during burning of organic material, are present in tobacco smoke and fire-cured tobacco (Hearn et al., 2013). Among the rapés, detectable levels for individual PAHs compounds ranged from 1.18 – 211 ng/g; albeit, at much lower levels than found in fire-cured leaf, fire-cured twist, and moist snuff (Hearn et al., 2013). Rapé likely contains some PAHs because the tobacco in rapé products is often dried over a low fire (Cardoso and Nascimento, 2008). Detectable levels of three IARC carcinogens, including a group 1 carcinogen, benzo[a]pyrene, and two group 2B carcinogens (benzo[b] fluoranthene (BBF) and benzo[k] fluoranthene) (BKF), were found among the tobacco-containing rapés. When detectable PAH levels were summed, it was found that rapés contained between 16.1 – 596 ng/g PAH (Table 4).
Table 4.
Concentrations of twelve polycyclic aromatic hydrocarbons (n=3), including one IARC group 1 carcinogen and two IARC group 2B carcinogens, detected in the various rapé samples.
| ACL | ACT | FLU | PHE | ANT | FLR | PYR | BAA | CHR | BBFa | BKFa | BAPa | PAH Sum b ng/g, wet | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ----------Average, ng/g, wet weight, n=3---------- | |||||||||||||||
| LOD (ng/g) | 10.3 | 2.16 | 13.3 | 6.71 | 3.56 | 7.35 | 5.65 | 1.56 | 1.42 | 0.79 | 0.53 | 1.01 | |||
|
| |||||||||||||||
| Rapé (manufactured, non-tobacco) | |||||||||||||||
| Rapé Cacitral | <LOD | <LOD | <LOD | 11.4 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 1.18 | 3.51 | 16.1 | ||
| Rapé Guarany Cristal | <LOD | <LOD | <LOD | 10.4 | <LOD | <LOD | <LOD | <LOD | <LOD | 1.41 | <LOD | 4.6 | 16.4 | ||
|
| |||||||||||||||
| Rapé (manufactured, tobacco-containing) | |||||||||||||||
| Rapé Guarany Especial | <LOD | <LOD | <LOD | 36.8 | 19 | 11.2 | 37.5 | <LOD | 3.18 | 2.69 | 1.27 | 11.1 | 123 | ||
| Rapé Guarany Especial (Canela) | 12.3 | 8.04 | <LOD | 35.3 | 21.2 | 13.9 | 25.6 | <LOD | 3.15 | 2.91 | 1.24 | 7.29 | 131 | ||
| Rapé Guarany Cravo | <LOD | 2.86 | 35.5 | 106 | 12.5 | 40.7 | 60.8 | 50.0 | 7.59 | 4.28 | 2.64 | 10.4 | 333 | ||
| Rapé Moeda Especial | 11.0 | 4.83 | <LOD | 132 | 14.2 | 81.7 | 68.5 | 17.8 | 28.6 | 23.2 | 9.83 | 16.3 | 408 | ||
| Rapé Caratinga Mentolado | LOD | 2.26 | <LOD | 15.6 | <LOD | <LOD | 9.4 | <LOD | 4.21 | 2.44 | <LOD | 3.59 | 37.5 | ||
| Rapé Caratinga Imburana | <LOD | 4.7 | <LOD | 41.0 | 3.68 | 11.4 | 22.7 | <LOD | 7.64 | 3.72 | 2.35 | 5.86 | 103 | ||
| Rapé Araçá Cravo e Canela | 16.3 | 3.27 | <LOD | 51.4 | 6.59 | 9.43 | 15.2 | <LOD | 5.85 | 3.3 | 2.43 | 5.54 | 119 | ||
| Rapé Moeda Girassol | 46.4 | 9.9 | 29.4 | 127 | 34.2 | 42.9 | 102 | 12.8 | 23.0 | 14.4 | 4.04 | 12.7 | 459 | ||
| Rapé Real (flavored) | <LOD | 2.23 | 20.8 | 68.6 | 10.4 | 13.8 | 46.5 | 3.72 | 8.28 | 6.3 | 4.23 | 15.1 | 200 | ||
|
| |||||||||||||||
| Rapé (custom-made, tobacco-containing) | |||||||||||||||
| Rapé Kashinawá | <LOD | <LOD | 20.4 | 99 | 15.1 | 7.9 | 60.2 | 1.84 | 24.41 | 5.4 | 2.69 | 5.22 | 242 | ||
| Rapé Nu-nu | 14.9 | 13.6 | 16.2 | 159 | 10.4 | 83.1 | 53.5 | <LOD | 211 | 3.16 | 6.38 | 24.3 | 596 | ||
|
| |||||||||||||||
| Minimum detected | 11.0 | 2.23 | 16.2 | 10.4 | 1.87 | 7.9 | 9.4 | 1.84 | 3.00 | 1.41 | 1.18 | 3.51 | 16.1 | ||
| Maximum detected | 62.9 | 16.5 | 35.5 | 159 | 34.2 | 83.1 | 102 | 50.0 | 211 | 23.2 | 9.83 | 24.3 | 596 | ||
Abbreviations: acenaphthene (ACL), acenaphthylene (ACT), anthracene (ANT), benzo[e]pyrene (BEP), chrysene (CHR), fluoranthene (FLR), fluorine (FLU), naphthalene (NAP), phenanthrene (PHE), pyrene (PYR), and benzo[a]pyrene (BAP). Note: Naphthalene (NAP) was not detected above the LOD in any of the products analyzed.
Three IARC carcinogens, including a group 1 carcinogen, benzo[a]pyrene, and two group 2B carcinogens (benzo[b] fluoranthene (BBF) and benzo[k] fluoranthene) (BKF), were analyzed in these samples. IARC=International Agency for Research on Cancer.
PAH Sum is the sum of all PAHconcentrations above the analyte LOD.
Without the presence of label information, further characterization was needed for Rapé Nu-nu; visual inspection suggested the presence of plant ashes. Calcium carbonate is a major constituent of wood ash (Misra et al., 1993). It was suspected that Rapé Nu-nu contained calcium carbonate based on high pH (pH 10.2) and IR absorbances (875 and 1410 cm−1) characteristic of calcium carbonate (not shown). Our results suggest that this Rapé Nu-Nu sample was a mixture of ashes from the inner bark of the Macambo tree (Theobroma bicolor; Peruvian cocoa) (Cultivodetabaco website, 2013) mixed with N. rustica.
Table 5 summarized the analyses performed and the confirmation of tobacco species, presence or absence of fire-curing, and the use of various plant materials, flavor additives, and alkaline ashes. Based on our full scan flavor characterization, Rapé Real (flavored) was the least complex chemically of the flavored products as it contained N. rustica with menthol flavoring and minute amounts of clove. Characterization revealed that Rapé Guarany Cravo was the most complex product analyzed, as containing fire-cured tobacco (N. rustica) combined with high concentrations of clove, tonka bean, camphor, and menthol flavoring.
Table 5.
Summary of tobacco and non-tobacco constituents confirmed in various rapé samples using gas chromatography/mass spectrometry (MS), Fourier transform infrared (IR) and pH analyses.
| Tobacco (Species/Curing) Chemical markers |
Non-tobacco plant material1 Chemical markers, if available |
Other additives chemical markers or other indicators |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Nicotiana Rustica | Fire-cured | Tonka Bean |
Camphor Laurel |
Clove | Cinnamon | Cassava | Peruvian Cocoa |
Menthol Flavoring 1 | Alkaline Ashes 2 | |||
|
|
||||||||||||
| Products | Alkaloids, TSNAs3 | PAHs | Coumarin | Camphor | Eugenol | Methyl Salicylate |
Cinnam- aldehyde |
n.a.4 | n.a.4 | Menthol | Pulegone | Calcium Carbonate IR spectra; pH |
| Rapé Cacitral | No Tobacco | MS only5 | MS | MS | MS, IR | MS | ||||||
| Rapé Guarany Cristal | No Tobacco | MS, IR6 | MS, IR | MS MS | MS, IR | IR only | MS | |||||
|
| ||||||||||||
| Rapé Guarany Especial | MS, IR | MS | MS, IR6 | MS | MS, IR | MS, IR | MS, IR | |||||
| Rapé Guarany Especial (Canela) | MS, IR | MS | MS, IR6 | MS | MS, IR | MS, IR | MS, IR | |||||
| Rapé Guarany Cravo | MS, IR | MS | MS, IR | MS | MS, IR | MS | MS | MS | ||||
| Rapé Moeda Especial | MS, IR | MS | MS, IR | MS, IR | Not found7 | |||||||
| Rapé Caratinga Mentolado | MS, IR | MS8 | MS, IR | |||||||||
| Rapé Caratinga Imburana | MS, IR | MS, IR | MS | |||||||||
| Rapé Araçá Cravo e Canela9 | MS, IR | MS8 | MS, IR | Not found6 | ||||||||
| Rapé Moeda Girassol | MS, IR | MS | MS, IR | MS | MS | |||||||
| Rapé Real (flavored)10 | MS, IR | MS | MS | |||||||||
|
| ||||||||||||
| Rapé Kaxinawá | MS, IR | MS | IR; high pH11 | |||||||||
| Rapé Nu-nu | MS, IR | MS | MS, IR | IR only | IR; high pH11 | |||||||
Scientific names: Tonka bean (Dipteryx odorata); Camphor laurel (Cinnamomum camphora); Clove (Syzygium aromaticum), Cinnamon (Cinnamomum sp.); Cassava (Manihot esculenta); Peruvian cocoa (Theobroma bicolor)
Menthol can be extracted from various mint plants, including, cornmint (Mentha arvensis) and peppermint (Mentha piperita).
The alkaline ashes in some rapé products can be taken from Peruvian cocoa, which is also known as the Macambo tree (Theobroma bicolor).
Alkaloids include nicotine and minor alkaloids (nornicotine, anatabine, anabasine, myosmine, isonicoteine).
Suitable chemical markers for cassava and Peruvian Cocoa were not available.
Although this product contains coumarin, the identification of it sources being tonka bean was inconclusive based on FT/IR spectra.
Although tonka bean is likely the primary source of coumarin, a small amount of coumarin could be attributable to cinnamon in these products.
Although cinnamon was listed on the packaging; its presence was not confirmed by FT/IR or chemical markers (e.g., cinnamaldehyde).
Products with coumarin concentrations less than 20µg/g did not have a positive identification using FT/IR spectra.
In the product name Rapé Araçá Cravo e Canela, ‘Cravo e Canela’ means cloves and cinnamon. GC/MS and FT/IR analysis confirmed that cinnamon is not present in this product despite its name.
Although not shown Rapé Real (unflavored) was essentially pure Nicotiana rustica based on FT/IR and GC/MS analysis.
Although the presence of calcium carbonate, a major component of ashes, is confirmed by FT/IR, other product constituents including additives could contribute to the higher pH observed.
4. Conclusion
Although rapé and similar preparations have been used for thousands of years, this study is the first comprehensive chemical characterization of this tobacco product type. Using FT/IR analysis, we confirmed the presence of non-tobacco materials (e.g. tonka bean, camphor laurel, cloves, cinnamon, and alkaline plant ashes). The majority of rapé products were tobacco mixed with non-tobacco materials. Two products lacked tobacco but contained various amounts of tonka bean, camphor, clove, menthol flavoring, cinnamon, and cassava. All of the tobacco-containing rapés analyzed in this study contained N. rustica, which is native to South America and is associated with higher total nicotine and TSNA levels than seen in products made with N. tabacum (Idris et al., 1991; Stanfill et al., 2010).
Manufactured products were mildly acidic (pH 5.17 – 6.42) and contained low total un-ionized nicotine levels (0.03 – 0.43 mg/g), suggesting that they do not contain alkaline ashes. Calcium carbonate is a major constituent of wood ashes (Demeyer et al., 2001) and acts as an alkalinizing agent when ashes are added to the tobacco mixture. In both Rapé Kaxinawá and Rapé Nu-nu, elevated pH values (pH 9.75 – 10.2) were observed and the presence of calcium carbonate was confirmed by FT/IR spectral analysis. Alkaline ashes are used in the preparation of other custom-made oral tobacco products with high pH such as chimó and iq’mik (Hearn et al., 2013, Stanfill et al., 2010). Use of an alkalinizing agent is one way to increase the amount of un-ionized nicotine which is absorbed more rapidly in the mouth than ionized nicotine (Richter and Spierto 2003).
For Rapé Kaxinawá and Rapé Nu-nu, the high pH levels resulted in almost all (>98%) of the total nicotine being converted to un-ionized nicotine (15.4 – 18.5 mg/g) (see Table 1). For comparison, commercial moist snuff products sold in the United States contain total un-ionized nicotine concentrations ranging from 0.01 – 7.81 mg/g (Richter et al., 2008). Tobacco-specific nitrosamine levels suggest that the rapés did not contain fermented tobacco. Products containing fermented tobacco, such as commercial moist snuff, commercial dry snuff, and Sudanese toombak (Richter et al., 2008; Lawler et al., 2013; Idris et al., 1991; Stanfill et al., 2010) have higher TSNA levels than products typically manufactured without fermented tobacco, such as snus, plug, loose leaf and twist tobaccos (Lawler et al., 2013; Stepanov et al., 2012). The presence of PAHs in seven products was a clear indication of use of fire-cured tobacco in these rapés. All of the tobacco-containing products contained PAHs classified as group 1 or 2B carcinogens by International Agency on Research in Cancer (IARC).
While undetectable in some rapes, a few products had milligram levels of coumarin, camphor, and eugenol. Because of the exceptionally high levels of some of these potentially toxic chemicals (Martin et al., 2004; Ehlers et al., 1995; Uc et al., 2000) and the absence of information on their toxicity following nasal administration further evaluation of the toxicity of these products is warranted. A number of products clearly contained ingredients not suggested by the product name or included on the ingredient list. Therefore, consumers and public health officials may be unaware that tonka bean or other ingredients, such as menthol flavoring, camphor laurel exudate, clove, or cinnamon are present in rapé products even when not listed as an ingredient. Coumarin, a major constituent of tonka bean, was present in the majority of rapé products; although only three rapé product packages listed tonka bean as an ingredient in the product name or on the package labelling. This study reveals that rapé product packaging does not accurately present product ingredients.
As of 2011, more than 2.7 million South American immigrants reside in the U.S.; this is roughly 7% of all immigrants (U.S. Census Bureau, 2011). As people visit or immigrate to other countries they may introduce tobacco products used in their native countries (Stanfill and Stepanov, 2014). Physicians, regulators, and public health decision makers may encounter these unfamiliar products and require information on the physical and chemical properties that contribute to their potential for addiction and toxicity.
This publication presents for the first time a thorough characterization of pH, moisture, and concentrations of nicotine, minor alkaloids, TSNAs, PAHs, and flavor compounds in rapé products. The use of fire cured tobacco with higher nicotine (N. rustica), alkaline ashes that boost product pH and un-ionized nicotine content, and use of certain non-tobacco plant material (tonka bean, camphor, clove, cassava, etc.) may influence the addictiveness, toxicity or carcinogenicity of certain rapés products. Information gained through this thorough characterization of rapés provides the basis for a better understanding of these products and suggests topics of further research to characterize the health impact associated with the use of rapé and similar products.
Figure 3.
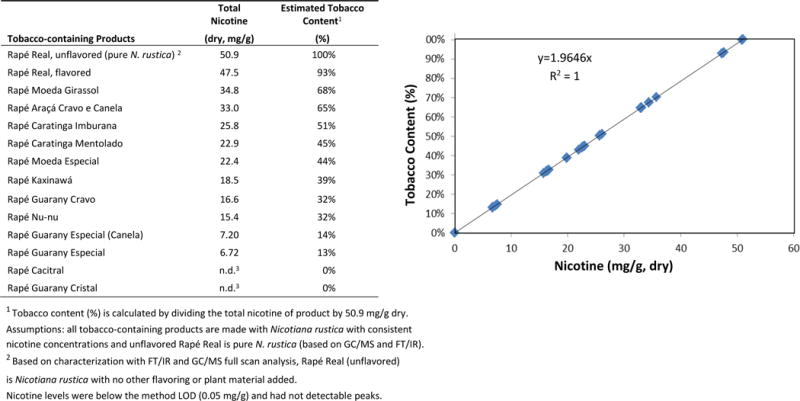
Tobacco Content (%) is based on a dose-dependent correlation between total nicotine (dry wt.) and tobacco content for rapé samples. Rapé Real (unflavored) was determined to be pure N. rustica (set as 100% tobacco, 50.9 mg/g) based on GC/MS and FT/IR analyses. Non-tobacco containing products with no detectable tobacco (i.e., 0 mg nicotine), including Rapé Cacitral and Rapé Guarany Cristal, and were set as 0% tobacco content in this regression.
Footnotes
Disclaimer: This information is distributed solely for the purpose of pre dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by the Centers for Disease Control and Prevention. It does not represent and should not be construed to represent any agency determination or policy.
References
- Cardoso CM, Nascimento S. Etnobotany and Umbanda temples. College of Theology Umbanda. São Paulo, Brazil: 2008. p. 133. [translation] [Google Scholar]
- Cultivo de tabaco (translation: Tobacco Cultivation) Accessed on December 12, 2014 at http://cultivodetabaco.com/comunidad/temas/rape-nunu.392/
- Demeyer A, Voundi Nkana JC, Verloo MG. Characteristics of wood ash and influence on soil properties and nutrient uptake: an overview. Bioresource Technology. 2001;77(3):287–95. doi: 10.1016/S0960-8524(00)00043-2. [DOI] [PubMed] [Google Scholar]
- Duke’s Phytochemical and Ethnobotanical Databases. Online Database access on December 12, 2014 at http://www.ars-grin.gov/duke/
- Duke JA, Vasquez R. Amazonian Ethnobotanical Dictionary. CRC Press; 1994. p. 224. [Google Scholar]
- Ehlers D, Pfister M, Bork WR, Toffel-Nadolny P. HPLC analysis of tonka bean extracts. Z Lebensm Unters Forsch. 1995 Sep;201(3):278–82. doi: 10.1007/BF01193004. [DOI] [PubMed] [Google Scholar]
- Hearn BA, Ding YS, England L, Kim S, Vaughan C, Stanfill SB, Zhang L, Polzin GP, Ashley DL, Watson CH, Renner CC, Enoch C, Nevak C. Chemical Analysis of Alaskan Iq’mik Smokeless Tobacco. Nicotine & Tobacco Research. 2013 doi: 10.1093/ntr/nts270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris AM, Nair J, Ohshima H, Friesen M, Brouet I, Faustman EM, Bartsch H. Unusually high levels of carcinogenic tobacco-specific nitrosamines in Sudan snuff (toombak) Carcinogenesis. 1991;12:1115–1118. doi: 10.1093/carcin/12.6.1115. [DOI] [PubMed] [Google Scholar]
- Lagrou E. Shamanism and representation among the Kaxinawa. In: Langdon EJ, editor. Shamanism in Brazil: New Perspectives. Florianopolis: Federal University of Santa Catarina Publishing House; 1996. [Google Scholar]
- Lawler TS, Stanfill SB, Zhang L, Ashley DL, Watson CH. Chemical characterization of domestic oral tobacco products: total nicotine, pH, unprotonated nicotine and tobacco-specific N-nitrosamines. Food and Chemical Toxicology. 2013;57:380–386. doi: 10.1016/j.fct.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima EC. Dissertation - Graduate Program in Social Anthropology. University of São Paulo; 1994. Katukina: History and organization of a Pano group from the high Juruá; p. 193. [Google Scholar]
- Lisko JG, Stanfill SB, Watson CH. Quantitation of Ten Flavor Compounds in Unburned Tobacco Products. Analytical Methods. 2014 doi: 10.1039/C4AY00271G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisko JG, Stanfill SB, Duncan BW, Watson CH. Application of GC-MS/MS for the Analysis of Tobacco Alkaloids in Cigarette Filler and Various Tobacco Species. Analytical Chemistry. 2013 doi: 10.1021/ac400077e. dx.doi.org/10.1021/ac400077e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Valdez J, Boren J, Mayersohn M. Dermal absorption of camphor, menthol, and methyl salicylate in humans. J Clin Pharmacol. 2004 Oct;44(10):1151–7. doi: 10.1177/0091270004268409. [DOI] [PubMed] [Google Scholar]
- McKenna T. Food of the Gods: The Search for the Original Tree of Knowledge - A Radical History of Plants, Drugs, and Human Evolution. Bantam Books; New York: 1993. p. 336. [Google Scholar]
- U.S. Census Bureau. American Community Survey (ACS) 2011 Table B05006, “Place of Birth for the Foreign-Born Population.”. [Google Scholar]
- Misra MK, Ragland KW, Baker AJ. Wood Ash Composition as a Function of Furnace Temperature. Biomass and Bioenergy. 1993;4(2):103–116. [Google Scholar]
- Perez-Gil L. O sistema médico Yawanáwa e seus especialistas: cura, poder e iniciação xamânica. [translation: The Yawanáwa Medical system and its specialists: cure, power and shamanic initiation] Cad. Saúde Pública [online] 2001;17(2):333–344. doi: 10.1590/s0102-311x2001000200008. Accessed at: http://www.readcube.com/articles/10.1590/S0102-311X2001000200008 on December 12, 2014. [DOI] [PubMed] [Google Scholar]
- Richter P, Spierto FW. Surveillance of smokeless tobacco nicotine, pH, moisture, and unprotonated nicotine content. Nicotine and Tobacco Research. 2003;5:885–889. doi: 10.1080/14622200310001614647. [DOI] [PubMed] [Google Scholar]
- Richter P, Hodge K, Stanfill S, Zhang L, Watson C. Surveillance of moist snuff: total nicotine, moisture, pH, un-ionized nicotine, and tobacco-specific nitrosamines. Nicotine and Tobacco Research. 2008;10:1645–1652. doi: 10.1080/14622200802412937. [DOI] [PubMed] [Google Scholar]
- Stanfill SB, Jia LT, Watson CH, Ashley DL. Rapid and chemically-selective quantification of nicotine in smokeless tobacco products using gas chromatography/mass spectrometry. Journal of Chromatographic Science. 2009;47(10):902–909. doi: 10.1093/chromsci/47.10.902. [DOI] [PubMed] [Google Scholar]
- Stanfill SB, Connolly GN, Zhang L, Jia TL, Henningfield J, Richter P, Lawler T, Ayo-Yusuf L, Ashley DL, Watson CH. Surveillance of international oral tobacco products: Total nicotine, un-ionized nicotine and tobacco-specific nitrosamines. Tobacco Control. 2010;20:e2. doi: 10.1136/tc.2010.037465. [DOI] [PubMed] [Google Scholar]
- Stepanov I, Jensen J, Hatsukami D, Hecht SS. New and traditional smokeless tobacco: comparison of toxicant and carcinogen levels. Nic Tob Res. 2008;10(12):1773–82. doi: 10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Villalta PW, Knezevich A, Jensen J, Hatsukami DK, Hecht SS. Analysis of 23 polycyclic aromatic hydrocarbons in smokeless tobacco by gas chromatography–mass spectrometry. Chem Res Toxicol. 2010 Jan 18;23(1):66–73. doi: 10.1021/tx900281u. Erratum in: Chem Res Toxicol. 2010 Apr 19;23(4):845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Biener L, Knezevich A, Nyman AL, Bliss R, Jensen J, et al. Monitoring tobacco-specific N-nitrosamines and nicotine in novel Marlboro and Camel smokeless tobacco products: findings from round 1 of the New Product Watch. Nicotine Tob Res. 2012 Mar;14(3):274–81. doi: 10.1093/ntr/ntr209. Epub 2011 Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uc A, Bishop WP, Sanders KD. Camphor hepatotoxicity. South Med J. 2000;93(6):596–8. [PubMed] [Google Scholar]
- Wilbert J. Tobacco and Shamanism in South America. Yale University Press; New Haven, CT: 1987. [Google Scholar]
- Zagorevski DV, Loughmiller-Newman JA. The detection of nicotine in a Late Mayan period flask by gas chromatography and liquid chromatography mass spectrometry methods. Rapid Communications in Mass Spectrometry. 2012;26(4):403–411. doi: 10.1002/rcm.5339. 2014. [DOI] [PubMed] [Google Scholar]


