Abstract
The location of nucleosomes in SV40 virions and minichromosomes isolated during infection were determined by next generation sequencing (NGS). The patterns of reads within the regulatory region of chromatin from wild-type virions indicated that micrococcal nuclease-resistant nucleosomes were specifically positioned at nt 5223 and nt 363, while in minichromosomes isolated 48 h post-infection we observed nuclease-resistant nucleosomes at nt 5119 and nt 212. The nucleosomes at nt 5223 and nt 363 in virion chromatin would be expected to repress early and late transcription, respectively. In virions from the mutant cs1085, which does not repress early transcription, we found that these two nucleosomes were significantly reduced compared to wild-type virions confirming a repressive role for them. In chromatin from cells infected for only 30 min with wild-type virus, we observed a significant reduction in the nucleosomes at nt 5223 and nt 363 indicating that the potential repression by these nucleosomes appeared to be relieved very early in infection.
Keywords: SV40, Epigenome, Nucleosome Positioning, Transcription, Next Generation Sequencing
1. Introduction
It has been known for many years that the promoter-regulatory region of genes licensed for transcription contains specific chromatin architecture. This structure is different from the bulk of the cellular chromatin based upon differences in its sensitivity to treatment with a number of enzymes and chemical agents. The difference in sensitivity of the chromatin to various treatments generally manifests itself either as an extended region encompassing much of the regulatory region or as a small region corresponding to a specific transcription factor binding site which is highly sensitive to the treatment (Gross and Garrard, 1988; Workman and Kingston, 1998; Yaniv and Cereghini, 1986). The extended region of differential sensitivity encompassing the whole regulatory region is thought to represent a nucleosome-free region based upon an analysis of chromatin structure by electron microscopy primarily in the Simian Virus 40 (SV40) model system described below (Jakobovits et al., 1980; Saragosti et al., 1980). Similar differences in structure have also been noted for other regulatory regions including transcription termination sites and intron boundaries (Spies et al., 2009).
Simian Virus 40 (SV40), a small circular double-stranded DNA virus, is found associated with host-cellular histones and auxiliary proteins and is organized into typical chromatin structure when present as an intracellular minichromosome and within the virion (Tooze and Acheson, 1981). Like its cellular counterpart, SV40 chromatin also possesses typical regulatory hallmarks such as a nucleosome-free regulatory region (NFR) and nuclease hypersensitivity at certain transcription factor binding sites (Kube and Milavetz, 1989, 1996; Saragosti et al., 1980; Scott et al., 1984; Scott and Wigmore, 1978; Varshavsky et al., 1978; Waldeck et al., 1978). The organization of the SV40 genome along with an expansion of the regulatory region indicating the location of important sites in regulation is shown in Fig. 1.
Fig. 1.
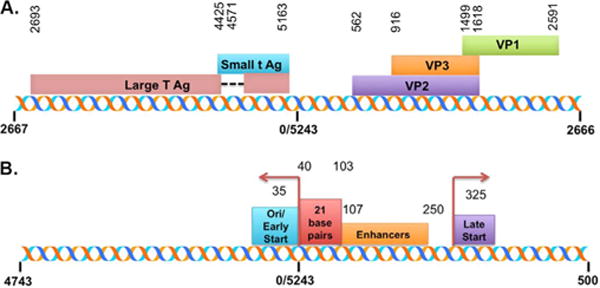
Schematic representation of the SV40 genome including the coding and regulatory regions. The genomic locations of the major SV40 proteins are indicated in A with the start site and termination for each protein. The genome has been linearized between nt 2666 and nt 2667 to be consistent with the data presented. An expansion of the regulatory region is shown in B. The positions of the major regulatory elements and the early and late transcription start sites are also indicated.
While the presence of these structural features of SV40 chromatin have been unequivocally established, their fine structure has not been determined due to the low resolution of the available technology when these features were being characterized. With the advent of next generation sequencing (NGS), it is now possible to probe chromatin structure at potentially much higher resolution.
Although NGS is most commonly used in conjunction with chromatin immunoprecipitation (ChIP) to characterize the location of proteins of interest on a genome, NGS can also be used to determine the location of nucleosomes [reviewed in (Mensaert et al., 2014; Meyer and Liu, 2014)]. Chromatin can be sheared into mononucleosome sized DNA fragments by either sonication for low resolution analysis or micrococcal nuclease digestion for high resolution analysis and sequencing libraries prepared from the DNA. The latter has the potential to yield single base resolution as long as one takes into consideration certain limitations inherent to the procedure (Allan et al., 2012; Chung et al., 2010). For example, micrococcal nuclease prefers to cleave within AT rich regions and is capable of digesting DNA associated with the nucleosome core. However, by using multiple similar biological samples, purifying libraries so that they include only nucleosome-sized DNA, using paired-end sequencing, normalizing analyses across the biological samples, and limiting the analyses to reads of nucleosome-sized DNA, the location of nucleosomes on a genome can be determined (Allan et al., 2012; Mensaert et al., 2014; Meyer and Liu, 2014). By selecting only nucleosome-sized DNA fragments during library preparation and from the generated paired-end sequencing reads, transcription factor binding sites are excluded from the analysis since they are smaller in size (Kasinathan et al., 2014).
We report here the analysis of SV40 chromatin from minichromosomes isolated at 30 min, and 48 h post-infection and from disrupted wild-type and mutant cs1085 virions using next generation sequencing (NGS) with libraries prepared by either sonication or micrococcal nuclease digestion. Our results indicate that the chromatin structure within the SV40 regulatory regions obtained from different sources of SV40 contains some features in common and others that vary depending upon the source of the chromatin.
2. Results
2.1. Analysis of sonicated SV40 chromatin by NGS
In order to determine whether NGS could be used with various sources of sonicated SV40 chromatin to identify differences in structural features like nucleosome-free regions, we prepared libraries from SV40 chromatin obtained from disrupted virions, minichromosomes, and purified SV40 DNA for sequencing. The results of this analysis are displayed as the number of reads relative to the underlying SV40 genome. Since the genome is circular, it was arbitrarily linearized at nt 2666 in order to optimally display the regulatory region where we anticipated differences to occur. Reads over the complete genome for disrupted virions, minichromosomes, and DNA are shown in Fig. 2, A, C, and E respectively. The regulatory region between nt 5000 and nt 500 in the SV40 genome was expanded in B, D, and F for each of the samples respectively for higher resolution.
Fig. 2.
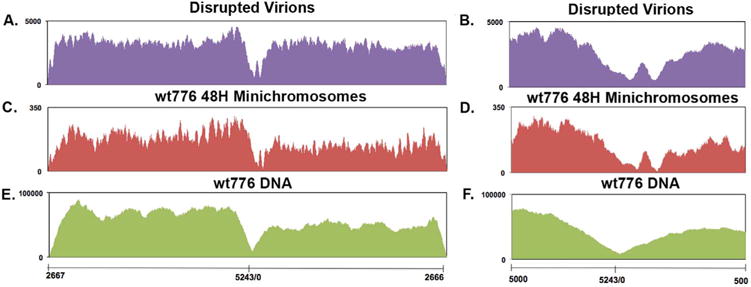
NGS analysis of sonicated SV40 chromatin prepared from wild-type virions and minichromosomes. Libraries were prepared from sonicated wild-type SV40 chromatin obtained from disrupted virions (A and B), minichromosomes isolated at 48 h post-infection (C and D) or naked DNA (E and F) and paired-end sequenced by NGS using an Illumina MiSeq. The sequence reads were plotted against the wild-type SV40 genome, which was linearized between nts 2666 and 2667 for display and analysis. Reads over the complete genome for each sample is shown in A–C and an expansion of the regulatory region for each sample is shown in D–F. On the X–axis is shown the nt numbers of the SV40 genome. On the Y–axis is shown the number of reads obtained from each site.
All three samples yielded the same general pattern of reads over the SV40 genome with similar relatively high numbers of reads throughout the genome with the exception of the regulatory region from approximately nt 0 to nt 250. Despite the general similarity between the patterns of reads for the three samples, there were major differences observed between the two chromatin samples and the sample of sonicated DNA. First, the two chromatin samples contained two sites which were hypersensitive to sonication located at nt 136 and nt 208, while the sonicated DNA contained only one site located at nt 98. Moreover, the sensitivity to sonication in the chromatin and DNA was very different. In the chromatin the number of reads at the site of greatest sensitivity was approximately 0.05% of the maximal number of reads in the sample, while in the DNA, the site of maximal sensitivity was 10% of the maximum. In addition to the differences in the sites of maximal sensitivity to sonication, there was also a difference in the region, which surrounded this site. In the chromatin samples, the region was somewhat asymmetric with more of the sensitivity on the early side of the hypersensitive sites than on the late side. In the DNA, the region appeared to be more or less symmetrical around the single hypersensitive site and extended from −117 to +362, which was much larger than what was observed in the chromatin. The results indicate that a nucleosome-free region was present in SV40 chromatin over the 21 bp repeat region and at least part of the enhancer regions.
2.2. Analysis of micrococcal nuclease digested SV40 chromatin by NGS
In an alternative approach, libraries of chromatin fragments obtained from micrococcal nuclease digestion of chromatin from disrupted SV40 virions and SV40 minichromosomes were sequenced by NGS. We did not prepare libraries from purified DNA because purified DNA was completely digested by the conditions used to prepare the digested chromatin.
In order to minimize the variation inherent in individual samples, the data presented are composites of a minimum of three sequenced libraries each of which was prepared from distinct biological preparations of SV40 chromatin. The actual number of biological repeats for each type of chromatin is indicated in the figure legend. For this analysis only SV40 DNA inserts between 120 and 150 bp in length from the paired-end sequencing data were used. This was done so that only DNA fragments corresponding in size to the core nucleosome would be included in the subsequent analysis. In other analyses inserts from 60 to 100 bp in length were used to search for possible binding of transcription factors (Kasinathan et al., 2014) (data not shown).
For the bedgraph data shown, the SV40 genome was linearized at nt 2666 to display the promoter-regulatory region in the center of the graph and the number of reads at each nucleotide was displayed relative to the SV40 genome. To generate the composite data shown for each form of chromatin, the sequencing reads obtained for a sample at each base in the genome was first normalized by dividing the number of reads at a particular base by the total number of reads for the sample. The number of normalized reads at each base in each of the samples was then added together and divided by the total number of samples used. Graphs were then generated from this combined data and used for subsequent peak-calling analysis and the generation of heatmaps.
The sequencing results for chromatin fragments from wild-type mini chromosomes isolated at 48 h post-infection are shown in Fig. 3A and for disrupted wild-type virionsh in Fig. 3B. A heatmap comparing the frequency of reads throughout the genome in each form of chromatin is shown in 3C and an expansion of the regulatory region is shown in 3D.
Fig. 3.
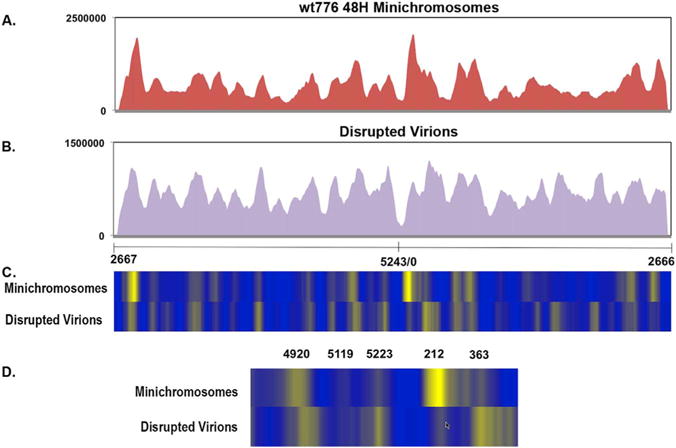
NGS analysis of micrococcal nuclease digested SV40 chromatin prepared from wild-type virions and minichromosomes. Libraries were prepared from micrococcal nuclease digested wild-type SV40 chromatin obtained from minichromosomes isolated at 48 h post-infection (A) or disrupted virions (B) and paired-end sequenced by NGS using an Illumina MiSeq. The sequence reads were plotted against the wild-type SV40 genome which was linearized between nts 2666 and 2667 for display and analysis. On the X–axis is shown the nt numbers of the SV40 genome. On the Y–axis is shown the number of reads obtained from each site. The data was derived from 5 distinct samples of disrupted virions and 4 different samples from minichromosomes isolated 48 h post-infection. In (C) is a heatmap comparison of the data from disrupted virions and minichromosomes over the entire genome and in (D) is shown the same data with an expansion of the regulatory region.
Unlike the results seen with sonicated chromatin, the results with micrococcal nuclease digested chromatin showed a clear pattern of peaks containing high numbers of reads and valleys with relatively lower numbers of reads. The former corresponds to regions of chromatin relatively resistant to micrococcal nuclease digestion as expected for most nucleosomes, while the latter are regions which are susceptible. The location of these nucleosomes in the two forms of chromatin can be compared most easily in the heatmaps (Fig. 3C and D).
From the number of individual lines seen in the heatmaps we estimated that there were from 20 to 25 nucleosomes in each form of chromatin, a number similar to what has been previously reported (22+2) based upon electron microscopy and the measurement of supercoiling resulting from nucleosome formation (Ambrose et al., 1986; Coca-Prados and Hsu, 1979; Jakobovits et al., 1980, 1982; Moyne et al., 1982). An analysis of the distance between most of the peaks in this chromatin indicated a spacing of approximately 200 base pairs again in good agreement with previous reports for chromatin (Eissenberg et al., 1985). However, the 200 base pairs is only an average since some of the peaks were closer than the 200 base pairs while others were significantly greater than this number.
Also from the heatmaps, it is apparent that there are a number of differences in the relative location of nucleosomes throughout the SV40 genome in virion chromatin compared to minichromosomes. There were at least five nucleosomes represented by bright lines in the heatmap of virion chromatin which were not present or significantly reduced in minichromosomes, and at least four new nucleosome positions in the minichromosomes which did not appear to be present in the virion chromatin suggesting that there was a substantial change in nucleosome location within the two types of SV40 chromatin.
Since nucleosome positioning in the promoter is thought to play an important regulatory role for many genes and because we observed differences specific to this region in the SV40 heatmaps, we focused our attention on this region. In the chromatin of disrupted virions, prominent bands were present in the heatmap centered on nt 5223 and nt 363 and weaker bands centered on nt 5119 and 212. In marked contrast in the minichromosomes isolated at 48 h post-infection, the band at nt5223 appeared to be substantially reduced or shifted to nt 5119, while the band at 363 appeared to be shifted to nt 212. The band centered on nt 5223 would correspond to a nucleosome overlapping the early transcription start site and origin of replication, while the band at nt 363 would correspond to a nucleosome overlapping the late transcription start site (see Fig. 1). Together, these two nucleosomes would be expected to effectively repress transcription and replication in virion chromatin. These two regions would be open and potentially available for transcription or replication in many of the minichromosomes isolated at 48 h post-infection.
In addition to differences in the location of nucleosomes within the regulatory regions between the two forms of chromatin, there also appears to be a difference in the size of the region between the nucleosomes bounding the region in the majority of each form of chromatin. In the minichromosomes this was approximately 440 base pairs, while in the chromatin from disrupted virions only 100 base pairs, a difference of slightly more than 340 base pairs.
2.3. Analysis of cs1085 chromatin from disrupted virions by NGS
In order to test whether the nucleosomes located at nt 5223 and nt 363 in the chromatin of disrupted wild-type virions were associated with the repression of transcription, we analyzed chromatin from virions of the mutant cs1085. This mutant is characterized by a deletion of T-antigen binding Site I and as a consequence does not repress early transcription like the wild-type virus (DiMaio and Nathans, 1980, 1982). We have used this mutant virus extensively to characterize various aspects of chromatin structure with and without repression of early transcription (Balakrishnan and Milavetz, 2006; Kallestad et al., 2014, 2013; Kube and Milavetz, 1989, 1996; Milavetz et al., 2012).
The bedgraph results obtained from an analysis of sonicated chromatin from disrupted virions is shown in Fig. 4A and a corresponding analysis with chromatin digested with micrococcal nuclease is shown in Fig. 4B. Heatmap comparisons between cs1085 and wild-type chromatin from virions prepared by micrococcal nuclease digestion are shown in Fig. 4C (entire genome) and Fig. 4D (expanded regulatory region). In both Fig. 4A and B, there is a new region with relatively few reads centered around nt 5202 which is approximately the middle of T-antigen binding Site I which has been deleted in this virus. The relative absence of reads in this region would be expected from the absence of this region in the mutant virus. With the exception of the deletion present in the mutant the sequencing results from the two viruses prepared by sonication looked substantially the same, compare Figs. 2A and 4A respectively. In both forms of chromatin we observed the same size and location of a region of increased sensitivity. In the heatmap, the deletion was taken into account during the bioinformatics analysis.
Fig. 4.
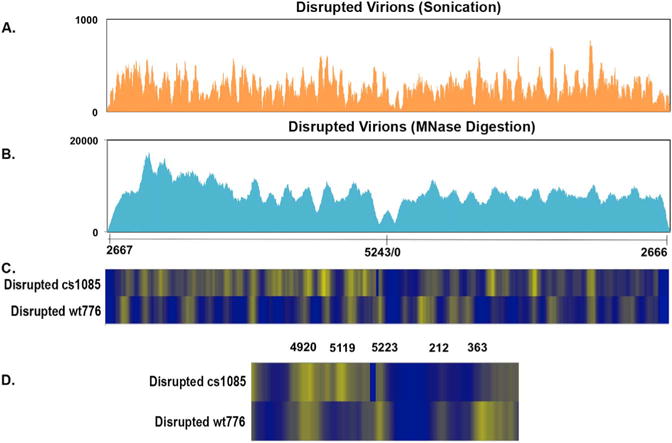
NGS analysis of sonicated and micrococcal nuclease digested SV40 chromatin prepared from virions of the mutant cs1085. Libraries were prepared from sonicated (A) or micrococcal nuclease digested (B) mutant cs1085 SV40 chromatin obtained from disrupted virions and paired-end sequenced by NGS using an Illumina MiSeq. The sequence reads were plotted against the wild-type SV40 genome which was linearized between nts 2666 and 2667 for display and analysis. On the X–axis is shown the nt numbers of the SV40 genome. On the Y–axis is shown the number of reads obtained from each site. The data for cs1085 was derived from 3 separate biological samples. A heatmap comparison between wild-type disrupted virions as in Fig. 2 and the cs1085 disrupted virion chromatin is shown in (C) for the entire genome and (D) for the expanded regulatory region.
As shown in the heatmap for the entire genome (Fig. 4C), a comparison of results from chromatin prepared by micrococcal nuclease digestion from wild-type and mutant virions indicated a number of major differences. Notably, the nucleosome-free region in cs1085 appears to be much larger than in the wild-type chromatin and to extend from approximately nt 5150 to nt 450. The prominent band at nt 5223 in the wild-type disrupted virions appears to have shifted to nt 5119 where a new prominent band is found while the band centered at nt 363 in wild-type chromatin appears to be shifted to the right in the genome into a series of bands. The minor band at nt 212 appears to have substantially disappeared in the mutant compared to the wild-type chromatin. In addition, there were other differences in the location of nucleosomes throughout the genome. Since cs1085 does not repress early transcription, the apparent loss or shifting of the nucleosomes at nt 5223 and nt 363 in this virus would be consistent with these nucleosomes being involved in repression of transcription in wild-type virions. The differences are more pronounced in the heatmap comparison of the expanded regulatory region, Fig. 4D.
2.4. Analysis of wild-type chromatin isolated 30 min post-infection by NGS
If the nucleosome centered on nt 5223 in wild-type virions blocked early transcription as suggested by the results with the cs1085 chromatin, the repression by this nucleosome would have to be relieved early in an infection so that transcription could be initiated. Since early transcription can occur as early as 30 min post-infection in our system (Balakrishnan and Milavetz, 2006; Kallestad et al., 2014), we analyzed minichromosomes isolated at this time by NGS. A bedgraph of the results of an analysis of chromatin obtained at 30 min post-infection digested with micrococcal nuclease is shown in Fig. 5A. A heatmap comparison of the sequencing results from disrupted wild-type virions and minichromosomes isolated at 30 min post-infection is shown in Fig. 5B (entire genome) and 5 C (expanded regulatory region). While there were changes in intensity of many of the bands in the heatmap, the location of most of the bands were identical in the figure with a few major exceptions. The prominent band at nt 5223 over the early transcription site in the virion chromatin appears to be significantly reduced in the 30 min minichromosomes along with the band located at nt 363. The specific loss of the nucleosome centered on nt 5223 within the promoter would be consistent with a role in blocking transcription in virion chromatin which is relieved during the early stages of infection. However, it appears that the nucleosome located at nt 363 is also affected to a certain extent. Interestingly, unlike the large increase in intensity of the nucleosome located at nt 212 which we observed in chromatin isolated at 48 h post-infection which we assume was associated with late transcription, this nucleosome appeared to be reduced in intensity early in infection.
Fig. 5.
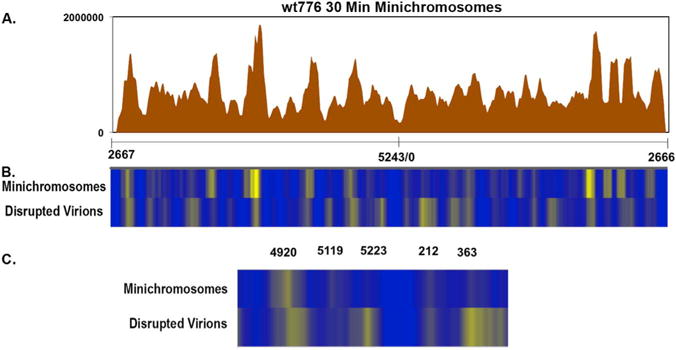
NGS analysis of micrococcal nuclease digested chromatin prepared from wild-type minichromosomes isolated 30 min post-infection Libraries were prepared from micrococcal nuclease digested SV40 wild-type minichromosomes isolated 30 min post-infection and paired-end sequenced by NGS using an Illumina MiSeq (A). The sequence reads were plotted against the wild-type SV40 genome, which was linearized between nts 2666 and 2667 for display and analysis. On the X–axis is shown the nt numbers of the SV40 genome. On the Y–axis is shown the number of reads obtained from each site. The data for minichromosomes was derived from 4 separate biological samples. A heatmap comparison between wild-type disrupted virions as in Fig. 2 and the minichromosomes isolated 30 min post-infection is shown in (C) for the entire genome and (D) for the expanded regulatory region.
3. Discussion
The results described here substantially extend our understanding of the nature of the nucleosome-free region present in SV40 chromatin in two important ways. First, the organization of nucleosomes observed in the regulatory region explains many of the differences noted in earlier studies using nuclease sensitivity assays, and second, the differences in the location of nucleosomes in minichromosomes and virions suggests a simple mechanism to regulate the appropriate initiation of transcription during the establishment of an infection.
A schematic representation of the results from these analyses of chromatin from disrupted virions (A) and minichromosomes isolated at 48 h post-infection (B) along with the location of critical regulatory elements (C) is shown in Fig. 6. The location of nucleosomes along with the principal regulatory sequences including the start sites for early transcription (Wasylyk et al., 1983) and late transcription (Ghosh et al., 1982; Piatak et al., 1983) are all indicated.
Fig. 6.
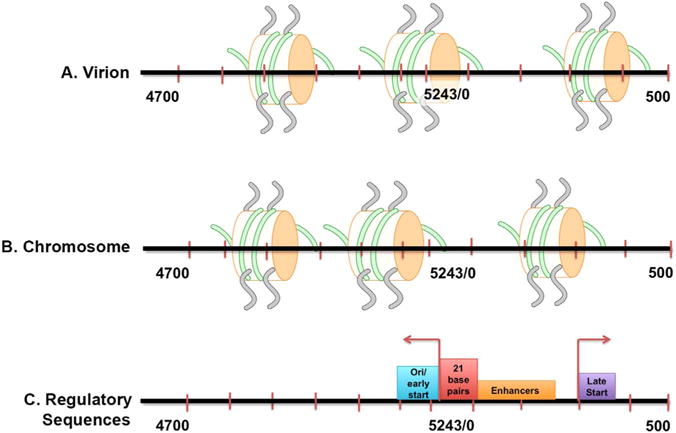
Schematic representation of the position of nucleosomes in the major forms of SV40 chromatin present in virions and minichromosomes isolated 48 h post-infection. The location of nucleosomes in SV40 chromatin from disrupted virions (A) and minichromosomes isolated at 48 h post-infection (B) based upon peak data from the respective NGS data are indicated. By comparison the location of critical regulatory sequences including the early and late transcription start sites, enhancers, 21 base pair repeats (SP1 binding sites) and the origin of replication (ori) are indicated in (C). The location of the peaks was determined from the NGS data by peak-calling using the program R/bioconductor package nucleR v2.20. The results are shown to scale with the center of each nucleosome from the peak-calling data used to determine its location on the genome.
The indicated nucleosome positions most likely represent the organization of nucleosomes in the majority of SV40 chromatin in minichromosomes and disrupted virions, since we have previously shown that SV40 chromatin is heterogeneous and exists as multiple epigenomes with presumably different biological functions in both minichromosomes and virions (Milavetz et al., 2012).
In SV40 minichromosomes isolated at 48 h post-infection the organization of nucleosomes in the majority of the chromatin shows the presence of a NFR. Nucleosomes were centered on nt 5119 and nt 212 which allows for most regulatory sequences including the enhancers, 21 bp repeats, Site I, early start sites, late transcription elements, late start site, and the origin of replication to be available for binding by their cognizant regulatory factors (Ghosh et al., 1982; Piatak et al., 1983). This organization probably explains why the regulatory region around the origin of replication is both nucleosome-free by electron microscopy and hypersensitive to various probes as previously described (Kube and Milavetz, 1989, 1996; Saragosti et al., 1980; Scott et al., 1984; Scott and Wigmore, 1978; Varshavsky et al., 1978; Waldeck et al., 1978). Interestingly, the nucleosome-free region is not continuous from the early promoter through the late promoter but appears to contain a single nucleosome between the two regulatory regions at nt 212. The presence of this nucleosome most likely explains why restriction endonuclease sensitivity was significantly greater at the BglI site in the origin of replication compared to the KpnI and MspI sites, which might be partially covered by this nucleosome (Kube and Milavetz, 1989, 1996; Milavetz, 1986; Varshavsky et al., 1978). This nucleosome may be the reason for the small peak observed at approximately the same position in the NFR in the analyses of sonicated chromatin in Figs. 2 and 3.
The organization of nucleosomes in the chromatin from disrupted virions is significantly different from minichromosomes, with a nucleosome centered at nt 5223 which would cover the early transcription start sites (nt 5235 and nt 35) (Wasylyk et al., 1983) and the origin of replication and a second nucleosome centered at nt 363 over the late start site (nt 325) (Ghosh et al., 1982). The nucleosome at nt 5223 appears to be part of a rather broad peak that potentially could consist of two very close nucleosomes. The presence of nucleosomes at these two sites in the regulatory region would be expected to inhibit the activity of restriction endonucleases targeting this region such as BglI, KpnI, and MspI and likely explains the reduction in restriction endonuclease digestion with these enzymes which we have previously described in encapsidation intermediates (Milavetz, 1986).
Our relatively low resolution results with sonicated chromatin are quite consistent with these results and previous studies using DNAase I hypersensitivity in which hypersensitive sites were observed at the Oct-1/TEF-1 transcription factor binding site found in each of the tandemly duplicated enhancers while regions of lower sensitivity were observed throughout the rest of the early promoter (Cereghini and Yaniv, 1984; Chu et al., 1990).
The organization of nucleosomes in disrupted virions, which we observed likely explains why a nucleosome-free region was reported in virions by psoralen crosslinking analysis (Kondoleon et al., 1989) in contrast to the results from most other researchers using nuclease sensitivity. Most likely, the psoralen crosslinks occurred within the enhancer region, which we find to be nucleosome-free even in chromatin from disrupted virions.
For technical reasons, there have been only a few attempts in the past to determine the location of the ends of the nucleosome-free region in SV40 minichromosomes Bal31 was used in conjunction with restriction endonuclease digestion to show that on the early side a barrier to digestion occurs in the region between nucleotides (nts) 5100 and 5200 and on the late side between nts 390 and 450 (Scott et al., 1984). Our results with micrococcal nuclease digestion and NGS are within 100 bases of these results. In a second approach, clones were prepared from micrococcal nuclease digested chromatin, the clones amplified, and subsequently sequenced by the Sanger procedure (Ambrose et al., 1990, 1989). In this analysis a number of clones were identified where nucleosomes were centered between nts 5050–5150 and nts 275–350. Our results are also in reasonable agreement with these published results in particular with respect to the late side of the regulatory region where we find nucleosomes centered on nt 212. The differences between what we observed and what was reported previously most likely is a consequence of the different techniques used to analyze the chromatin, although there may be other differences including the strain of virus used and how the SV40 chromatin was prepared. For example, in the prior studies with micrococcal nuclease digestion (Ambrose et al., 1990, 1989), minichromosomes were isolated from cells grown and infected at 40 °C instead of 37 °C as described here.
The presence of nucleosomes in different locations within the regulatory region of disrupted virions and minichromosomes suggests a simple mechanism for controlling the appropriate initiation of early transcription upon SV40 infection. Since the incoming chromatin contains nucleosomes positioned over the critical binding sites for early and late transcription, transcription could not occur immediately at these sites upon infection. However, because the enhancers and upstream 21 base pair repeat regions are available for interaction with transcription factors, appropriate early transcription driven by transcription factor binding in these regions would be permitted. Sliding the nucleosome centered on nt 5223 in the chromatin from disrupted virions to the left approximately 60 bases would be sufficient to open up the early-early transcription start site. Based upon the results with minichromosomes isolated 30 min post-infection it would appear that the nucleosome at nt 5223 is simply lost since there is no evidence for a new nucleosome position. However, other nucleosomes including the one at nt 363 within the core regulatory region also appear to be lost early in infection suggesting that an extended region is opened.
A role for the nucleosomes at nt 5223 and nt 363 in repressing transcription was confirmed by the analysis of the mutant cs1085, a mutant which does not repress early transcription. In the mutant chromatin the nucleosomes at nt 5223 and nt 363 in wild-type chromatin were either lost or shifted away from the regulatory region as expected if they were involved in repression.
While the analysis of nucleosome positioning by the sequencing of DNA libraries obtained from micrococcal nuclease digestion of chromatin is a well-established technique, it is important to consider the potential variability inherent to the technique. This variability is due to the fact that micrococcal nuclease digestion to mononucleosome sized DNA is very difficult to control and that chromatin itself can vary. To address this issue we show bedgraphs of all of the sequencing analyses (total reads not size selected) used in this study (supplementary data). We observed very good reproducibility for nucleosomes located at some sites regardless of the source of SV40 chromatin such as the nucleosomes located at nt 3700, nt 4885, and nt 2850. However, within the regulatory region we observed some variability. The most likely reason for this variability is the observation made many years ago that SV40 virus particles can be easily disrupted during the extraction of SV40 minichromosomes at 30 min and 48 h post-infection (Seidman et al., 1979). The disruption of virus particles to varying extents in the SV40 chromatin prepared at these times would account for the differences in nucleosome location within the regulatory region observed in the duplicate samples. Because of these differences we chose to normalize and merge the results of multiple analyses to minimize the differences.
These studies also begin to address the role of chromatin structure in the encapsidation of SV40 chromatin into virions. First, the difference in nucleosome location in the disrupted virions compared to minichromosomes indicates that changes in nucleosome location are an important part of the encapsidation process. Second, the results of the analysis of cs1085 chromatin from virions which differ from chromatin from wild-type virions suggest that there is not a specific organization of nucleosomes that is required for encapsidation to occur. Clearly, more work needs to be focused in this area in order to conclusively prove the above observations.
4. Materials and methods
4.1. Cells and viruses
SV40 virus and minichromosomes were prepared in African Green Monkey kidney cells, BS-C-1 line, obtained from ATCC (CCL-26). Both the wild-type 776 and mutant cs1085 SV40 viruses were gifts from Dr. Daniel Nathans. The conditions utilized for cell culture have been described previously (Kallestad et al., 2014, 2013; Milavetz et al., 2012).
4.2. Infections and Purification of Minichromosomes
The procedures used for the preparation of minichromosomes have been described previously (Kallestad et al., 2014, 2013; Milavetz et al., 2012). Briefly, sub-confluent monolayers of cells were infected with 50 PFU per cell of SV40 virus prepared by low multiplicity of infection for 48 h at which time the nuclei were prepared. Minichromosomes were extracted in low-ionic strength buffer from the nuclei and subsequently purified by glycerol gradient centrifugation. Aliquots of 200 μl from the top of the centrifuge tube were taken and fractions 3–5 were pooled since the fractions have previously been shown to contain the minichromosomes and used for further analysis.
4.3. Purification of virions and chromatin from virions
Crude wild-type 776 or mutant cs1085 virus was prepared by infecting sub-confluent monolayers of monkey cells in 75 cm3 T-flasks with 1 μl of stock virus (equivalent to 0.05 PFU per cell) in 10 ml of medium per flask. Low multiplicity of infection was used for virus preparation in order to lessen the likelihood that defective SV40 virus would be produced. When the infected cells displayed almost complete cell death from infection (approximately two weeks), the T-flasks were frozen and thawed at least twice to completely disrupt the cells. 1 ml of crude virus was placed in each of four 1.4 ml Eppendorf tubes and centrifuged at 50,000 Xg for 35 min to pellet the virus present in the crude preparation. The pellet was suspended in 170 μl Tris buffer (10 mM Tris) 1 mM EDTA (pH 7.4), adjusted to digestion conditions with 20 μl of the 10X buffer supplied with the DNAase I (New England Biolabs), and digested with 10 μl of DNAase I for 30 min at 37 °C. An aliquot (20 μl) was removed and analyzed by submerged agarose gel electrophoresis to ensure that all DNA other than the expected size of SV40 was removed. The digested resuspension was purified by glycerol gradient centrifugation at 50,000×g for 35 min to pellet the DNAase I resistant virus. The pelleted virus was suspended in 189 μl of Tris buffer as above and the virus disrupted by addition of 5 μl 0.1 M EGTA and 6 μl of 1 M dithiothreitol (DTT) for 30 min at room temperature. The mixture was then frozen and subjected to two more rounds of disruption. In each subsequent round of disruption, an additional 6 μl of DTT was added. Following three rounds of disruption, the released minichromosomes were purified by glycerol gradient centrifugation as described above for the preparation of minichromosomes from infected cells.
4.4. Preparation of sequencing libraries from SV40 chromatin fragmented by either sonication or micrococcal nuclease digestion
Sonicated chromatin for library preparation was obtained by sonication for 6 min in a Branson Digital Sonifier at 50% power. The sample (200 μl) in an Eppendorf tube was placed in a cup containing cold flowing water for cooling. Following sonication the sample was purified using Zymo Research ChIP DNA Clean and Concentrator columns according to their protocol and eluted in 51 μl H2O. Micrococcal nuclease digested chromatin was obtained by treating 200 μl of SV40 chromatin with micrococcal nuclease (New England Biolabs, M0247S) at 4 °C. The amount of enzyme and time of digestion was empirically determined for each sample so that approximately 90% of the chromatin was digested. Following digestion, the DNA was purified as described for sonicated DNA.
Libraries were prepared using Illumina TruSeq reagents and protocols with only one change. For samples containing small amounts of DNA, the amount of adapter DNA ligated was reduced from 2.5 μl to 1 μl and the difference was made up with H2O. Following library preparation the presence of SV40 inserts was confirmed using PCR with amplification of a region of the SV40 genome near the termination of early and late transcription. Confirmation of library preparation was determined by PCR using the amplification primers supplied in the TruSeq kit. Appropriately sized libraries were obtained by agarose gel electrophoresis and purification using BioRad certified molecular biology agarose. Bands of DNA corresponding in size to 200–250 base pairs were separated, cut out from the agarose gel, purified on Zymo Research Gel DNA Recovery columns using the protocol and reagents in the kit and eluted from the columns in 21 μl water.
4.5. Next generation sequencing (NGS)
The libraries were sequenced on an Illumina MiSeq in the epigenetics core laboratory at the University of North Dakota using protocols and reagents from Illumina. For each form of SV40 chromatin a minimum of three libraries each prepared from distinct biological samples were sequenced. Prior to sequencing the libraries were analyzed for quality and quantity using an Agilent Bioanalyzer.
Preliminary quality control analysis of fastq files was performed using FastQC v.0.11.2 (Andrews, 2010).. If necessary, adapter trimming was performed using scythe v0.981 (Buffalo, 2011), and quality trimming was carried out using sickle v1.33 (Joshi and Fass, 2011) with a phred score of 33 as the quality threshold; reads with a length less than 45 bp were discarded. To remove contaminating cellular sequences, reads were aligned to the host genome Chorocebus_sabeus1.1, and hg19 using Bowtie2 (Langmead et al., 2009). The remaining unmapped reads were aligned to the appropriate viral genome. Wild-type (776) reads were aligned to the SV40 genome (RefSeqAcc: NC_001669.1). The cs1085 genome had site 1 deleted from nt 5186 to 5207 of the SV40 genome. Viral reads were aligned to the appropriate genome using Bowtie2 v2.2.4 twice; first with the viral genome linearized at 0 nt, and secondly with the viral genome linearized at nt 2666. Alignments were filtered to contain only fragments between 120 bp and 150 bp. DANPOS-2.2.2 was used to determine nucleosome occupancy (Chen et al., 2013). Briefly, clonal reads were removed, then reads were extended toward the 3′prime end by 80 bps. Counts were scaled by the mean of the total number of hits. Occupancy was determined by the number of reads at each nucleotide. Heatmaps were generated using the Z-scores of the occupancy values. In order to compare the heatmaps of wild-type occupancy to cs1085, occupancy values of zero were used to represent the site I deletion.
Supplementary Material
Acknowledgments
The authors would like to thank Emily Blake, Epigenetic Core Laboratory at the University of North Dakota for sequencing some of the samples and part of the bioinformatics analysis. This work was funded by grants from the National Institutes of Health, AI094441 (to B.M.) and GM098328 (to L.B).
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.virol.2016.12.023.
References
- Allan J, Fraser RM, Owen-Hughes T, Keszenman-Pereyra D. Micrococcal nuclease does not substantially bias nucleosome mapping. J Mol Biol. 2012;417:152–164. doi: 10.1016/j.jmb.2012.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose C, Blasquez V, Bina M. A block in initiation of simian virus 40 assembly results in the accumulation of minichromosomes containing an exposed regulatory region. Proc Natl Acad Sci. 1986;83:3287–3291. doi: 10.1073/pnas.83.10.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose C, Rajadhyaksha A, Lowman H, Bina M. Locations of nucleosomes on the regulatory region of simian virus 40 chromatin. J Mol Biol. 1989;210:255–263. doi: 10.1016/0022-2836(89)90328-8. [DOI] [PubMed] [Google Scholar]
- Ambrose C, Lowman H, Rajadhyaksha A, Blasquez V, Bina M. Location of nucleosomes in simian virus 40 chromatin. J Mol Biol. 1990;214:875–884. doi: 10.1016/0022-2836(90)90342-J. [DOI] [PubMed] [Google Scholar]
- Andrews S. FastQC: a quality control for high throughput sequence data 2010 [Google Scholar]
- Balakrishnan L, Milavetz B. Reorganization of RNA polymerase II on the SV40 genome occurs coordinately with the early to late transcriptional switch. Virology. 2006;345:31–43. doi: 10.1016/j.virol.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Buffalo V. Scythe: a bayesian adapter trimmer 2011 [Google Scholar]
- Cereghini S, Yaniv M. Assembly of transfected DNA into chromatin: structural changes in the origin-promoter-enhancer region upon replication. EMBO J. 1984;3:1243–1253. doi: 10.1002/j.1460-2075.1984.tb01959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Xi Y, Pan X, Li Z, Kaestner K, Tyler J, Dent S, He X, Li W. DANPOS: dynamic analysis of nucleosome position and occupancy by sequencing. Genome Res. 2013;23:341–351. doi: 10.1101/gr.142067.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Huang TS, Hsu MT. P1 nuclease defines a subpopulation of active SV40 chromatin–a new nuclease hypersensitivity assay. Nucleic Acids Res. 1990;18:3705–3711. doi: 10.1093/nar/18.13.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HR, Dunkel I, Heise F, Linke C, Krobitsch S, Ehrenhofer-Murray AE, Sperling SR, Vingron M. The effect of micrococcal nuclease digestion on nucleosome positioning data. PloS One. 2010;5:e15754. doi: 10.1371/journal.pone.0015754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coca-Prados M, Hsu MT. Intracellular forms of simian virus 40 nucleoprotein complexes. II Biochemical and electron microscopic analysis of simian virus 40 virion assembly. J Virol. 1979;31:199–208. doi: 10.1128/jvi.31.1.199-208.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D, Nathans D. Cold-sensitive regulatory mutants of simian virus 40. J Mol Biol. 1980;140:129–142. doi: 10.1016/0022-2836(80)90359-9. [DOI] [PubMed] [Google Scholar]
- DiMaio D, Nathans D. Regulatory mutants of simian virus 40. Effect of mutations at a T antigen binding site on DNA replication and expression of viral genes. J Mol Biol. 1982;156:531–548. doi: 10.1016/0022-2836(82)90265-0. [DOI] [PubMed] [Google Scholar]
- Eissenberg JC, Cartwright IL, Thomas GH, Elgin SC. Selected topics in chromatin structure. Annu Rev Genet. 1985;19:485–536. doi: 10.1146/annurev.ge.19.120185.002413. [DOI] [PubMed] [Google Scholar]
- Ghosh PK, Piatak M, Mertz JE, Weissman SM, Lebowitz P. Altered utilization of splice sites and 5′ termini in late RNAs produced by leader region mutants of simian virus 40. J Virol. 1982;44:610–624. doi: 10.1128/jvi.44.2.610-624.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross DS, Garrard WT. Nuclease hypersensitive sites in chromatin. Annu Rev Biochem. 1988;57:159–197. doi: 10.1146/annurev.bi.57.070188.001111. [DOI] [PubMed] [Google Scholar]
- Jakobovits EB, Bratosin S, Aloni Y. A nucleosome-free region in SV40 minichromosomes. Nature. 1980;285:263–265. doi: 10.1038/285263a0. [DOI] [PubMed] [Google Scholar]
- Jakobovits EB, Bratosin S, Aloni Y. Formation of a nucleosome-free region in SV40 minichromosomes is dependent upon a restricted segment of DNA. Virology. 1982;120:340–348. doi: 10.1016/0042-6822(82)90035-6. [DOI] [PubMed] [Google Scholar]
- Joshi NA, Fass JN. Sickle - A windowed adaptive trimming tool for FASTQ files using quality 2011 [Google Scholar]
- Kallestad L, Christensen K, Woods E, Milavetz B. Transcriptional repression is epigenetically marked by H3K9 methylation during SV40 replication. Clin Epigenetics. 2014;6:21. doi: 10.1186/1868-7083-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallestad L, Woods E, Christensen K, Gefroh A, Balakrishnan L, Milavetz B. Transcription and replication result in distinct epigenetic marks following repression of early gene expression. Front Genet. 2013;4:140. doi: 10.3389/fgene.2013.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasinathan S, Orsi GA, Zentner GE, Ahmad K, Henikoff S. High-resolution mapping of transcription factor binding sites on native chromatin. Nat Methods. 2014;11:203–209. doi: 10.1038/nmeth.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoleon SK, Kurkinen NA, Hallick LM. The SV40 nucleosome-free region is detected throughout the virus life cycle. Virology. 1989;173:129–135. doi: 10.1016/0042-6822(89)90228-6. [DOI] [PubMed] [Google Scholar]
- Kube D, Milavetz B. Generation of a nucleosome-free promoter region in SV40 does not require T-antigen binding to site I. Virology. 1989;172:100–105. doi: 10.1016/0042-6822(89)90111-6. [DOI] [PubMed] [Google Scholar]
- Kube D, Milavetz B. Differential regulation by SV40 T-antigen binding at site I defines two distinct classes of nucleosome-free promoter. Anat Rec. 1996;244:28–32. doi: 10.1002/(SICI)1097-0185(199601)244:1<28::AID-AR3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensaert K, Denil S, Trooskens G, Van Criekinge W, Thas O, De Meyer T. Next-generation technologies and data analytical approaches for epigenomics. Environ Mol Mutagen. 2014;55:155–170. doi: 10.1002/em.21841. [DOI] [PubMed] [Google Scholar]
- Meyer CA, Liu XS. Identifying and mitigating bias in next-generation sequencing methods for chromatin biology. Nat Rev Genet. 2014;15:709–721. doi: 10.1038/nrg3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milavetz B. Analysis of the origin-specific nucleosome-free region in SV40 encapsidation intermediates. Virology. 1986;153:310–313. doi: 10.1016/0042-6822(86)90034-6. [DOI] [PubMed] [Google Scholar]
- Milavetz B, Kallestad L, Gefroh A, Adams N, Woods E, Balakrishnan L. Virion-mediated transfer of SV40 epigenetic information. Epigenetics: Off J DNA Methylation Soc. 2012;7:528–534. doi: 10.4161/epi.20057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyne G, Harper F, Saragosti S, Yaniv M. Absence of nucleosomes in a histone-containing nucleoprotein complex obtained by dissociation of purified SV40 virions. Cell. 1982;30:123–130. doi: 10.1016/0092-8674(82)90018-6. [DOI] [PubMed] [Google Scholar]
- Piatak M, Ghosh PK, Norkin LC, Weissman SM. Sequences locating the 5′ ends of the major simian virus 40 late mrna forms. J Virol. 1983;48:503–520. doi: 10.1128/jvi.48.2.503-520.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saragosti S, Moyne G, Yaniv M. Absence of nucleosomes in a fraction of SV40 chromatin between the origin of replication and the region coding for the late leader RNA. Cell. 1980;20:65–73. doi: 10.1016/0092-8674(80)90235-4. [DOI] [PubMed] [Google Scholar]
- Scott WA, Wigmore DJ. Sites in simian virus 40 chromatin which are preferentially cleaved by endonucleases. Cell. 1978;15:1511–1518. doi: 10.1016/0092-8674(78)90073-9. [DOI] [PubMed] [Google Scholar]
- Scott WA, Walter CF, Cryer BL. Barriers to nuclease Bal31 digestion across specific sites in simian virus 40 chromatin. Mol Cell Biol. 1984;4:604–610. doi: 10.1128/mcb.4.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman M, Garber E, Levine AJ. Parameters affecting the stability of SV40 virions during the extraction of nucleoprotein complexes. Virology. 1979;95:256–259. doi: 10.1016/0042-6822(79)90427-6. [DOI] [PubMed] [Google Scholar]
- Spies N, Nielsen CB, Padgett RA, Burge CB. Biased chromatin signatures around polyadenylation sites and exons. Mol Cell. 2009;36:245–254. doi: 10.1016/j.molcel.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J, Acheson NH. DNA Tumor Viruses. 2nd. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y.: 1981. [Google Scholar]
- Varshavsky AJ, Sundin OH, Bohn MJ. SV40 viral minichromosome: preferential exposure of the origin of replication as probed by restriction endonucleases. Nucleic Acids Res. 1978;5:3469–3477. doi: 10.1093/nar/5.10.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck W, Fohring B, Chowdhury K, Gruss P, Sauer G. Origin of DNA replication in papovavirus chromatin is recognized by endogenous endonuclease. Proc Natl Acad Sci USA. 1978;75:5964–5968. doi: 10.1073/pnas.75.12.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B, Wasylyk C, Matthes H, Wintzerith M, Chambon P. Transcription from the SV40 early-early and late-early overlapping promoters in the absence of DNA replication. EMBO J. 1983;2:1605–1611. doi: 10.1002/j.1460-2075.1983.tb01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman JL, Kingston RE. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu Rev Biochem. 1998;67:545–579. doi: 10.1146/annurev.biochem.67.1.545. [DOI] [PubMed] [Google Scholar]
- Yaniv M, Cereghini S. Structure of transcriptionally active chromatin. CRC Crit Rev Biochem. 1986;21:1–26. doi: 10.3109/10409238609113607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


