Abstract
A female can develop a diabetes-like disease due to a high fat content in her father's diet before she was conceived. Epigenetic modifications of the father's sperm DNA might underlie this peculiar observation. See Letter p.963
On page 963 of this issue, Ng et al.1 report one of the first observations that a father's diet can affect his daughters' health. When the authors fed male rats a highfat diet, the outcome was not surprising: the animals' body weight and body fat increased, and they exhibited glucose intolerance and resistance to the hormone insulin. Unexpectedly, however, although these males' daughters did not show altered body weight or body fat, in adulthood they developed a diabeteslike condition of impaired glucose tolerance and insulin secretion. Ng and colleagues also found that the gene-expression profile of the insulin-secreting pancreatic islet cells obtained from the daughters was abnormal, affecting several gene networks and cellular pathways. This indicates that the fathers' high-fat diets altered the development of their sperm, which then promoted an adult-onset disease in the daughters.
Although diet undoubtedly influences many somatic (non-germ) cells and disease states (obesity and diabetes), none of the somatic-cell effects can be transmitted to the next generation2. For environmental factors such as diet to exert the type of generational effects that Ng et al. describe, the process of sperm formation in the testis and molecular programming of the germ line must be affected. Previous studies3–6 have shown that genetic abnormalities in sperm caused by chemotherapeutic drugs and environmental factors can be transmitted to the next (F1) generation. But such genetic effects are random and occur at extremely low frequency — and thus cannot explain the high frequency and reproducibility of Ng and co-workers' observations.
An alternative explanation could be that sperm and its precursors undergo alterations in epigenetic programming (this is mediated by molecular factors around DNA that alter gene expression independently of the DNA sequence). This could lead to reproducible traits (phenotypes) at high frequency. Among epigenetic modifications, DNA methylation patterns are predominantly altered in — and transmitted through — the germ line2. Transgenerational transmission of adult-onset disease affecting the prostate, kidney, testis and mammary gland through alterations in the sperm's DNA-methylation patterns has been documented2,7. Alterations in the small percentage of DNA-associated histone proteins that sperm retain could also play a part, but the functional role of sperm histones remains unclear8. Ng and colleagues' finding that a large number of genes have altered expression in the pancreatic islet cells also supports a role for epigenetics in mediating the generational effects that these authors describe.
Following fertilization, the early embryo's pattern of DNA methylation is reset genome-wide by a transient demethylation event, which erases the majority of the parental ‘epigenome’ effects on the germ line, and creates a stem-cell population capable of differentiating into almost any cell type2. During gonadal sex determination in the fetus, however, environmental exposures can promote a permanent epigenetic reprogramming of the primordial germ cell that alters the phenotypes of subsequent generations2,7. As part of this event, the epigenome of the embryo's germ line becomes permanently programmed, making the associated phenotypes transgenerational, irrespective of subsequent direct environmental exposures2.
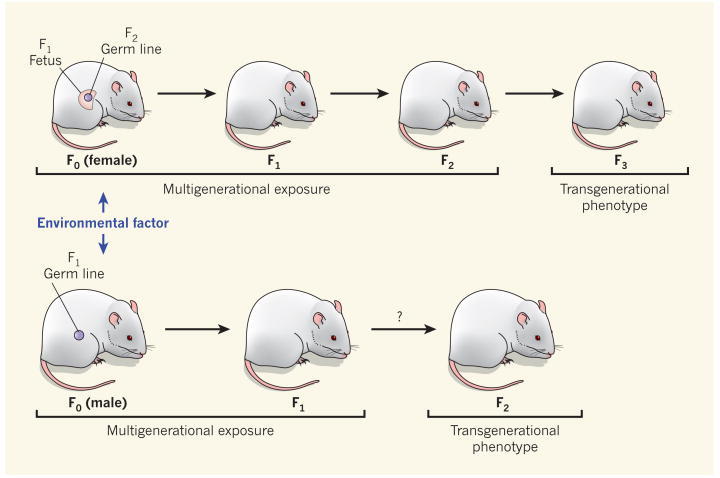
Epigenetic inheritance can involve multigenerational exposures, allowing its persistence across generations2 (Fig. 1). Ng and colleagues' results are an example of multigenerational exposure. The father — the F0 generation — is directly exposed, as is his sperm, which will generate the F1 generation. Similarly, exposure of a pregnant female to an environmental factor can affect not only herself, but also the F1-generation fetus that she carries and the fetus's germ line, which will eventually generate the F2 generation2. The multigenerational exposures — involving a somatic-cell-mediated effect on the F0 generation and a potentially epigenetic effect on the germ line of the F1 generation — could indirectly promote a ‘generational phenotype’, such as an adult-onset disease in subsequent generations.
Figure 1. Environmental effects across generations.
Whereas most environmental factors cannot alter an animal's DNA sequence, many promote epigenetic alterations that influence somatic cells and so the disease status of the individual exposed (F0 generation). In pregnant females, environmental exposure could also cause epigenetic modifications in the next two generations (F1 and F2) through the fetus and its germ line. The effect of such multigenerational exposure in subsequent generations (F3 and beyond) would be considered a transgenerational phenotype. By contrast, multigenerational exposure in males is limited to the F0 and F1 generations. Ng and colleagues' observations1 fit well into a multigenerational exposure. However, they did not explore whether the high-fat diet of their male rats also causes a transgenerational phenotype in the F2 generation.
Epidemiological evidence has long suggested that the environment has a significant effect on human health and disease. Examples include regional differences in disease frequency, discordant diseases in identical twins, drastic increases in disease frequency in a population, and chemical exposures directly affecting adult-onset disease2. Numerous studies have also reported a maternal impact on disease in offspring — often due to fetal exposure. For instance, in the female Agouti mouse, diet can cause epigenetic alterations in a genomic region called the Agouti locus to promote, in the offspring, changes in coat colour and in the risk of adult-onset diseases, including obesity and diabetes9. However, the influence of environmental factors on the next generation through the father is not well documented.
In light of Ng and colleagues' observations1 additional studies are required. For instance, it remains to be seen whether the generational phenotype these authors describe — the effect of environmental exposure of the F0-generation fathers on the F1-generation daughters — is transgenerational, being further transmitted to yet the next (F2) generation (Fig. 1). Moreover, whether adult exposure can promote an epigenetic transgenerational inheritance — beyond the effects of multigenerational exposure — should be more thoroughly investigated.
Indeed, in this study1, the high frequency of disease in the F1 generation, the reproducibility of the diabetes-like condition and the extent to which the gene-expression profile is modified in the pancreatic islet cells all suggest the involvement of an epigenetic molecular mechanism. Nonetheless, the direct role of epigenetics in the sperm-mediated process must be demonstrated experimentally.
The dramatic increase in human metabolic disorders such as obesity and diabetes warrants considering the influence of environmental factors on the germ line. Ng and colleagues' observations1 certainly support a role for environmental factors and generational effects in contributing to metabolic disease. Epi genetics provides a molecular mechanism for environmental factors such as diet to affect health and to influence subsequent generations through the germ line2. It is likely, therefore, that epigenetic biomarkers would be useful to aid in the diagnosis and potential treatment of such cases.
References
- 1.Ng SF, et al. Nature. 2010;467:963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 2.Skinner MK, Manikkam M, Guerrero-Bosagna C. Trends Endocrinol Metab. 2010;21:214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton TS, Robaire B, Hales BF. Toxicol Sci. 2007;100:495–503. doi: 10.1093/toxsci/kfm242. [DOI] [PubMed] [Google Scholar]
- 4.Aitken RJ, Koopman P, Lewis SEM. Nature. 2004;432:48–52. doi: 10.1038/432048a. [DOI] [PubMed] [Google Scholar]
- 5.Sharpe RM. Phil Trans R Soc B. 2010;365:1697–1712. doi: 10.1098/rstb.2009.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du Plessis SS, Cabler S, McAlister DA, Sabanegh E, Agarwal A. Nature Rev Urol. 2010;7:153–161. doi: 10.1038/nrurol.2010.6. [DOI] [PubMed] [Google Scholar]
- 7.Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. PLoS ONE. 2010;5:e13100. doi: 10.1371/journal.pone.0013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puri D, Dhawan J, Mishra RK. Epigenetics. 2010 doi: 10.4161/epi.5.5.12005. [DOI] [PubMed] [Google Scholar]
- 9.Jirtle RL, Skinner MK. Nature Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]