Abstract
Objectives
Gaps in vaccination coverage leave populations vulnerable to illnesses. Since the 1990s, there has been a growing movement to improve vaccination access by giving pharmacists the authority to administer vaccines according to state laws. Understanding the variation of pharmacist vaccination laws over time is critical to understanding the effect of improving access to vaccination services.
Methods
We identified relevant statutes and regulations with the use of Westlaw legal databases. A 4-stage coding process identified 220 legal variables of pharmacist vaccination authority. Each jurisdiction’s laws were coded against these 220 legal variables. The resulting legal dataset was then evaluated to determine whether jurisdictions expanded or restricted pharmacist vaccination authorities over time.
Results
From 1971 to 2016, jurisdictions made 627 changes to statutes and regulations relating to pharmacist vaccination authority. There were 85 expansions, 3 restrictions, and 22 regulatory clarifications. Eight changes were deemed to be unclear, and 479 changes did not substantively alter the scope of pharmacist vaccination authority.
Conclusion
Collectively, the laws in 50 states and DC paint a clear picture: the scope of pharmacists’ vaccination authority is expanding. Jurisdictions are allowing pharmacists to administer more vaccines to younger patients with less direct prescriber oversight. This clear expansion of pharmacist vaccination authority stands in contrast to the reservations expressed by some physician groups for pharmacists as vaccination providers. However, laws in some states still do not permit pharmacists to vaccinate according to the Advisory Committee on Immunization Practices recommendations.
Vaccination is one of the greatest public health achievements of the twentieth century.1 Systematic vaccination programs have resulted in decreases in illnesses, fewer deaths, longer lifespans, and even the eradication of some diseases. Nevertheless, gaps in vaccination coverage leave significant proportions of the population vulnerable to illnesses, with outbreaks of vaccine-preventable diseases occurring regularly. One perceived barrier to vaccination is a lack of adequate access to vaccination services.2
Since the 1990s, there has been a growing movement to improve access to immunization services by giving pharmacists the authority to administer vaccines.2–5 Expanding pharmacists’ scope of practice to include vaccination significantly increases the number of health care providers who can offer vaccination services and significantly improves the convenience of those services by authorizing them at community pharmacies frequented by prospective patients.2,6 For example, pharmacies frequently have longer operation hours than traditional health care providers, permitting busy individuals to get vaccinated on the way home from work or while on errands.2,7,8 Moreover, vaccinating retail pharmacies are 1 possible access point for individuals not currently in the health care system or in rural areas with fewer traditional providers.7,9 Increasing pharmacists’ authority also leads to increased public awareness of vaccination’s importance and availability through routine interaction with pharmacists and advertisements.8,10–13 Finally, pharmacists can supplement the vaccinating workforce of health facilities.14 If pharmacists are able to increase vaccination rates (as opposed to displacing vaccines normally administered in physician offices), then pharmacists can potentially play a significant role in the prevention of future outbreaks and even the eradication of some diseases.9
Despite the possible upside, one-third of physicians express reservations about pharmacists as vaccination providers.3,15,16 For example, early pharmacist vaccination programs raised pediatricians’ concerns that vaccinations of young children outside of the clinical setting could disrupt regular office visits.3 One study suggests that some physicians are concerned that documentation and communication are inadequate between “alternate vaccinators” and primary care providers, and some physicians are concerned that some pharmacists do not have adequate training to administer vaccines.15 These reservations, however, have not prevented states from granting pharmacists the authority to administer vaccines.
Currently, legal authorities in all states permit pharmacists to administer vaccines to varying extents.2,3,17 These authorities are rooted in state statutes and regulations prescribing the scope of pharmaceutical practice. These laws vary across jurisdictions in many ways.2,18
Authorities defining pharmacists’ vaccination practice
Constitutions
Constitutions provide the legal framework for government entities to act. Constitutions often provide protections against certain government actions, such as due process restrictions on state licensure actions against pharmacists. However, constitutions rarely describe specific legal authorizations or restrictions for licensed health care professionals (e.g., whether pharmacists can administer vaccines).
Statutes
Statutes are laws passed by state legislative bodies that authorize or prohibit certain activities. Statutes can also authorize executive agencies, such as a state health department or licensing authority, to promulgate regulations. Statutes are powerful legal authorities that can significantly change pharmacists’ vaccination authority. However, passing or amending statutes requires opportunity and political will that are not always present.
Regulations
Regulations are laws promulgated by executive agencies to provide procedural or substantive specifics as to what the law authorizes or prohibits. Regulations are powerful tools to refine pharmacists’ vaccination authority, but new regulations must be consistent with their statutory authorization. For example, a regulation cannot authorize pharmacists to administer the human papillomavirus (HPV) vaccine to minors if the statute restricts pharmacist vaccinations to adult patients. State statutes require executive agencies to undergo a rule-making process before implementing new regulations which can last several months to several years.
Executive orders
Governors can issue executive orders that direct executive agencies’ implementation of their statutory authority. In some cases, statutes will provide governors authority to modify statutory or regulatory requirements in specified circumstances and may allow governors to expand pharmacists’ vaccination authority for outbreak response after declaring a state of emergency. Unlike regulations, these orders have no formal process to implement, revise, or revoke. Consequently, new administrations will frequently summarily revoke old executive orders from previous administrations to promote a new agenda. Executive orders cannot contradict a statute or regulation.
Agency guidance
State agencies can provide guidance to aid the public with understanding the requirements of statutes and regulations. Agency guidance must be consistent with existing statutes and regulations. Moreover, courts view agency guidance as persuasive but not binding. In many cases, courts are free to ignore agency guidance when interpreting statutes or regulations.
External guidance
States can incorporate guidance from external entities, such as nongovernmental organizations or the federal government, into their legal authorities. Some states incorporate the Advisory Committee on Immunization Practices (ACIP)–recommended vaccinations by reference in their statutes and regulations. States that adopt this approach allow statutes and regulations to adapt to advances in practice, industry, and evidence. However, incorporation by reference might be prohibited in some states, and external guidance and recommendations cannot be contrary to the state’s statutes or regulations.
Understanding these authorities is critical to understanding the extent to which pharmacists have the legal authority to improve access to vaccination services.19 State laws place significant limitations on pharmacists’ scope of practice, including restrictions on the types of vaccines that pharmacists can administer and the patient groups that pharmacists can serve, among other practice requirements.
Statutes and regulations have the most impact and are the most lasting legal authorities on pharmacists’ scope of practice. Examining the changes to these authorities over time would provide important insights on the states’ experience with pharmacists’ vaccination authorities and provides hints of future national trends. We examined statutes and regulations specifically because they are the foundation of pharmacists’ scope of practice authority.
Many states passed laws authorizing pharmacists to administer vaccines to improve vaccination access with the assumption that increased access will improve vaccination rates.2 The study of the effect of law on health outcomes, called legal epidemiology, represents a new era in the field of public health law. Legal epidemiology differs from traditional legal research methods, which suffer from transparency and reliability issues, by using scientific research principles to enhance transparency, reliability, and validity of the research results.
With the use of legal epidemiology methods, we created a robust legal dataset that can be used in research to identify the specific attributes of laws that have the greatest effect in achieving the desired outcome. We announced the existence this legal dataset previously (The research protocol for the dataset of pharmacist vaccination laws is available as a Supplemental Appendix online).20 This dataset of pharmacist vaccination laws is publicly available and contains information on more than 200 legal attributes. These data enable ongoing and future research on the specific types of laws that best promote population vaccination and other desired outcomes. Pharmacists, professional associates, and policymakers can use this data and derivative research to better understand how specific types of pharmacist vaccination laws affect health outcomes.18
Methods
The Public Health Law Program (PHLP) at the Centers for Disease Control and Prevention (CDC) assessed the statutes and regulations relating to pharmacists’ authority to administer vaccines in the 50 states and DC (jurisdictions). PHLP collected and analyzed statutes and regulations in effect from 1971 to January 1, 2016.20
We followed generally accepted public health law research guidelines21,22 for policy surveillance, including the use of redundant “blind” and “naive” coders, with modifications. We identified relevant statutes and regulations with the use of Westlaw legal databases first during July 19, 2013, to July 2, 2014, and then updating them on March 29, 2016.
Search terms
Initial searches used specific terms identified in trade organization data, for example, “influenza,” “zoster,” “protocol,” “prescription!,” and “standing order.” These initial searches were deemed to be too narrow. We designed subsequent searches to be comprehensive, repetitive, and redundant.
Laws containing language regulating pharmacists’ vaccination authority must contain a word indicating the regulated profession (i.e., pharmacy, pharmacist) and a word indicating the regulated practice (i.e., vaccination, immunization). Consequently, we used repeated searches containing “pharmac!,” and “vaccin!” or “immuniz!.” We also used searches using “pharmac!,” and “administ!” to capture laws without express vaccination language (e.g., administration of drugs). After we found 1 state regulation that used the colloquialism “flu shot” instead of formal terminology, we searched all jurisdictions for “pharmac!” and “flu” or “shot.”
Identifying historical laws
We identified historical versions of laws with the use of Westlaw’s annotations (e.g., to identify renumbered laws), Westlaw’s historical archives (e.g., to obtain prior text), and, in rare cases, publicly available state regulatory and legislative resources (e.g., to identify or reconstruct earlier text when the Westlaw resources were exhausted). The earliest law we identified was from 1971, but most jurisdictions did not have relevant laws before 1990. We ended our search for historical laws when a jurisdiction’s laws no longer contained relevant text. For example, New York defined the practice of pharmacy in 2008 as including the administration of “drugs, medicines, and therapeutic devices,” but the earlier version of the law, in effect since 1971, did not include a provision relating to the administration of substances. We retained this 1971 law to capture the status of law before the introduction of administration authority and to indicate that no earlier versions of this New York law were relevant to pharmacists’ vaccination authority.
Coding jurisdictional statutes and regulations
We used a 4-stage coding process to develop the coding questions to analyze the laws. The 4 stages were: 1) development of a question set; 2) testing questions on a batch of legal provisions; 3) analysis of question adequacy; and 4) revision of questions. This iterative process resulted in 10 revisions to the coding questions and identified 220 legal variables of pharmacist vaccination authority.
Each jurisdiction’s laws were coded against these 220 legal variables by trained legal researchers using Public Health Law Research’s policy surveillance tool Workbench. All laws in effect on January 1, 2014, and later were redundantly coded by 2 blinded legal researchers. For the laws in effect before 2014, 2 blinded legal researchers coded 10 jurisdictions and a single legal researcher coded the remaining 41 jurisdictions’ laws. Coding meetings determined consensus codes when redundant coding was used.
The 220 distinct attributes fall within 4 broad categories of pharmacist vaccination law variables. The first category covers the fundamental questions of whether pharmacists can administer substances generally and administer vaccines specifically. The second category addresses additional practice requirements for pharmacists who administer vaccines, including: 1) training and education; 2) state certification or notification; 3) record-keeping; 4) reporting; 5) liability insurance; 6) patient notification; 7) facility requirements for administration; and 8) authorizations for pharmacists to opt out of vaccination services. The third category relates to requirements for a third-party prescriber authorization for vaccine administration, including provisions that grant pharmacists prescriptive authority, require a patient-specific third-party prescriber authorization, and permit use of general authorizations to vaccinate a specified group of patients (e.g., standing orders). The fourth category covers restrictions (i.e., patient age, vaccines, modes of vaccine administration) that are specific to a type of authorization.
Independent data validation and quality control
Public Health Law Research conducted independent evaluation and quality control for the final dataset values with the use of comparison data from 2 different secondary sources.23,24 Independent evaluators compared 2 random samples of our findings (25 states each) with data from these 2 secondary sources. The independent evaluators found 1 coding difference for each of the secondary sources, but determined that these differences were due to differences in coding scheme (as opposed to coding error).20
Classifying changes in jurisdictional laws
We evaluated a subset of this dataset’s attributes to determine whether jurisdictions expanded or restricted pharmacist vaccination authorities over time (i.e., those attributes that relate to the substantive scope of practice rather than practice requirements such as reporting or recordkeeping). To accomplish this, we used Microsoft Excel to assess whether each change in the law was an expansion, restriction, regulatory clarification, or no change to the scope of pharmacist vaccination authority using the following groups of legal variables:
Vaccine authorization type (3 variables)
Vaccine restrictions (60 variables)
Modes of vaccine administration (18 variables)
Minimum patient age restrictions (66 variables)
Emergency exceptions (5 variables)
Other select restrictions on authorization types (6 variables)
Changes to the laws were coded as regulatory clarifications if the change was a regulatory change occurring subsequent to a statutory change that was ambiguous or silent, or that otherwise deferred to executive agencies for clarification. The first change in a jurisdiction’s law expressly authorizing vaccination was omitted from this analysis. Changes were deemed to be unclear if they included at least 1 criterion indicating an expansion and 1 indicating a restriction of vaccination authority. The full dataset, codebook, protocol describing the validation process, and full text of all included laws are publicly available (the protocol is included as a Supplemental Appendix to this article online).20
Results
We found a clear national trend to expand pharmacists’ vaccination authority. From 1971 to 2016, jurisdictions made 627 changes to statutes and regulations relating to pharmacist vaccination authority. Of these, 479 changes did not substantively alter the scope of pharmacist vaccination authority. There were 85 expansions, 3 restrictions, and 22 clarifications. Eight changes were deemed to be unclear. California, New Hampshire, and North Carolina were the only 3 jurisdictions that changed their laws to restrict pharmacist vaccination authority, but all 3 subsequently expanded the authority beyond the initial restriction.
Vaccination law restrictions
California
On January 1, 2000, California added a new condition to Cal.Bus. & Prof.Code § 4052 that limited immunizations under a standing orderx to those “provided by a health care facility, a licensed clinic in which there is physician oversight, or a provider who contracts with a licensed health care plan.” All other immunizations required patient-specific authorizations. California subsequently amended its laws to expand pharmacists’ vaccination authority in 2006, 2007, and 2014. In particular, the 2014 enactment of Cal.Bus. & Prof.Code § 4052.8 was significant because it authorized pharmacists to “independently initiate and administer vaccines” listed on the ACIP immunization schedules to patients 3 years of age and older.
New Hampshire
On September 23, 2009, New Hampshire enacted a new regulation that restricted pharmacists’ vaccination authority.25 Before the regulation’s enactment, a state statute authorized pharmacists to “administer influenza vaccines to the general public provided all of the criteria in this section have been met….”26 The statute did not contain any requirement for a third-party prescriber authorization, such as a prescription or a standing order. This law was coded as authorizing prescriptive vaccination authority. The 2009 regulation added the following requirement: a pharmacist who administers influenza vaccines “shall comply with the following procedures: (a) Administer pursuant to a standing order from a practitioner practicing within their scope of practice….” This new requirement restricted pharmacists’ vaccination authority by adding express language requiring a standing order. In 2012, New Hampshire removed this requirement for a standing order and granted pharmacists prescriptive authority.27
North Carolina
On December 29, 2009, North Carolina granted pharmacists a temporary authority to administer influenza vaccines to patients 14 years of age and older “as a result of the H1N1 influenza pandemic of 2009.”28 This new law contained an automatic expiration provision. When the automatic expiration was triggered, the expanded authorization for influenza vaccination was removed from law. This change was classified as a restriction. In 2013, North Carolina expanded its laws to permit influenza vaccine administration (and others) beyond the temporary H1N1 expansion.29
Jurisdictions with express vaccination laws
We identified 47 jurisdictions with express language relating to pharmacists’ authority to administer vaccines. The remaining 4 jurisdictions (AL, MS, TN, and WA) had laws authorizing pharmacists to administer drugs. The legal definition of “drugs” in all of these states include substances that are intended for use in the “prevention of disease” or “intended to affect the structure or any function of the body.”30–33 We did not find legal language prohibiting pharmacists from vaccinating in these states.
Third-party vaccination authorizations
The longitudinal analysis revealed widespread adoption of laws authorizing administration pursuant to general authorizations and a recent trend to grant pharmacists prescriptive authority to administer vaccines without a third-party prescriber authorization. Despite an increasing number of jurisdictions with express vaccination laws, the number of jurisdictions requiring pharmacists to have a patient-specific order has remained relatively stagnant (Figure 1).
Figure 1.
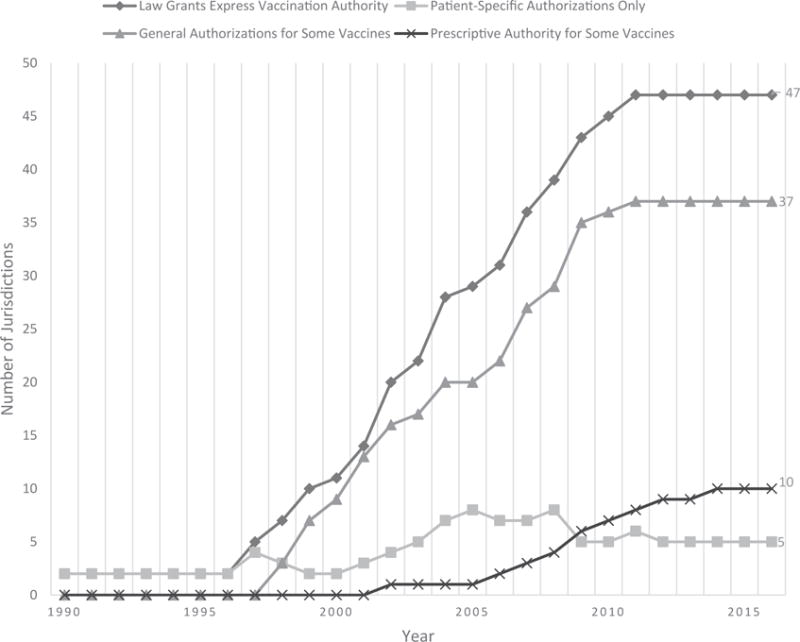
Numbers of jurisdictions requiring specific authorization types for pharmacist vaccine administrations, 1990–2016.
As of 2016, 10 jurisdictions permitted pharmacists to administer a vaccine without a third-party prescriber authorization in some circumstances (AZ, CA, ID, LA, ME, MT, NH, SD, WV, WY). Thirty-five jurisdictions had laws that permit use of general authorizations, such as standing orders, to vaccinate patients (AK, AR, AZ, CA, DC, DE, FL, GA, HI, IA, IL, IN, KS, KY, MD, ME, MN, MO, MT, NC, ND, NJ, NM, NV, NY, OH, OK, OR, PA, RI, SC, TX, UT, VA, VT, WI, WV). Five jurisdictions had statutes or regulations that do not specifically authorize the use of general authorizations or grant prescriptive authority (CO, CT, MA, MI, NE); however, the laws in these states did not expressly prohibit general authorizations, either.
Patient age restrictions
Our analysis also revealed a dramatic decrease in minimum patient age restrictions. In 2005, 19 jurisdictions had express patient age restrictions, with an average minimum age of 16.8 years (median 18, mode 18; 10 states did not have express patient age restrictions). By 2016, the average minimum age dropped to 8.76 years (median 7, mode <1) and the number of jurisdictions with express age restrictions increased to 39 (8 states did not have express patient-age restrictions). This drop in average age restrictions indicates that more states are adopting or amending laws that permit pharmacists to vaccinate younger patients.
As of 2016, 7 jurisdictions limited pharmacist vaccinations to adult patients (CT, DE, FL, MA, NY, VT, WV). The remaining states had minimum patient age restrictions of 14 years (HI, NC), 12 years (ID, MT, SC), 10 years (IL), 9 years (KY, MD, PA, RI), 7 years (AR, LA, ME, NJ, OH, OR, WY), 6 years (AZ, IA, KS, MN, WI), 5 years (ND), and 3 years (CA). Eight jurisdictions had laws with language indicating no minimum patient age restriction (AK, DC, GA, IN, MO, NH, NV, VA), and 8 states (CO, MI, NE, NM, OK, SD, TX, UT) did not mention patient age restrictions (AL, MS, TN, and WA do not have express vaccination laws).
Vaccine type restrictions
We also identified a clear trend for jurisdictions to authorize pharmacists to administer more vaccines (Figure 2). Three types of laws contributed to this trend: laws that expressly list vaccines pharmacists are authorized to administer; laws that authorize administration of any ACIP-recommended vaccine; and laws that do not have express language restricting the authority to administer specific vaccines. Changes to vaccine restrictions were common among pharmacist vaccination laws.
Figure 2.
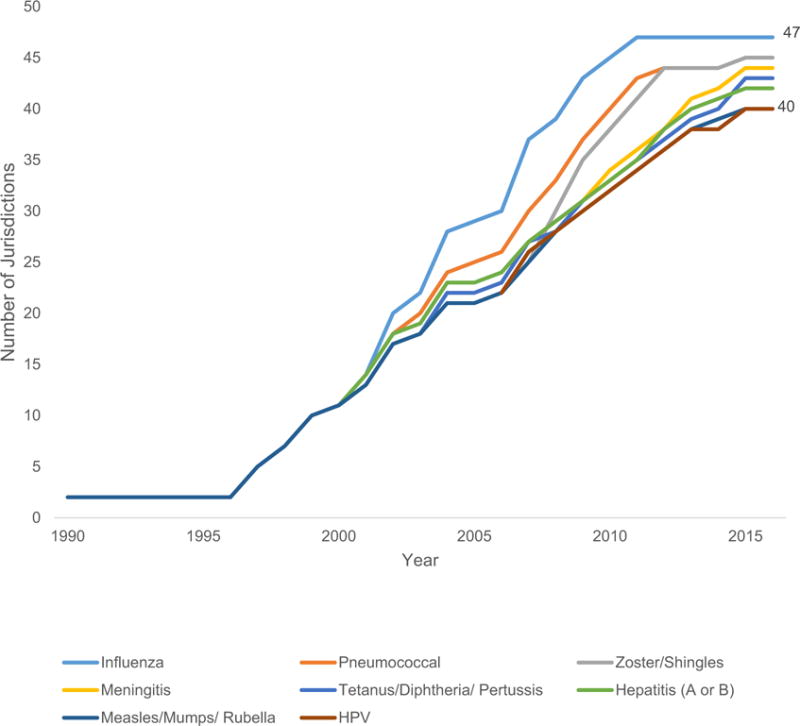
Numbers of jurisdictions authorizing specific vaccines for pharmacist administrations by vaccine type, 1990–2016.
Recently, many jurisdictions have removed vaccine-identifying language in favor of language that authorizes any ACIP-recommended vaccine or does not expressly limit the administration of any vaccine. In 2016, 8 states authorized the administration of ACIP-recommended vaccines (CT, FL, ID, MD, ME, NC, OH, SC), 10 states had laws with language indicating that there are no restrictions on vaccine types (AR, AZ, HI, IA, IN, MN, ND, NM, NV, RI), and 21 states did not have language in statutes or regulation to indicate restrictions on vaccine types (AK, CA, CO, DC, DE, IL, KS, KY, LA, MI, MT, NE, NJ, OK, OR, PA, TX, UT, VA, VT, WI).
Discussion
Our results show when states have substantively changed their pharmacist vaccination authority laws, they have overwhelmingly expanded the vaccination authority. Moreover, the only 3 states that were found to restrict pharmacists’ vaccination authority (CA, NH, and NC) subsequently expanded the authority beyond the initial restriction. These cumulative expansions of pharmacist vaccination authority represent several national trends in pharmacists’ vaccination authority: reductions in required physician oversight, reductions in minimum patient age requirements, and addition of new vaccines that can be administered.
Many state authorizations that originally required patient-specific authorizations have been repealed, amended, or replaced in favor of laws permitting pharmacists to vaccinate multiple patients under a single third-party prescriber authorization, such as a standing order. More recently, states are going further by authorizing pharmacists to independently prescribe and administer vaccines. Given these trends to authorize vaccination with less direct prescriber oversight, it is possible that future changes to pharmacist vaccination laws will further expand pharmacists’ authority to independently prescribe and administer vaccines.
We have also seen a drop in minimum patient age restrictions for pharmacist vaccination services. Given some physicians’ reservations relating to pharmacist-administered vaccinations, high minimum patient age requirements may have once reflected safety concerns relating to inexperienced providers providing injections to children. However, pharmacists now have a growing track record as vaccination providers,3 and the decrease in patient age restrictions might represent lawmakers’ growing confidence in pharmacists’ competence in vaccinating young patients. Or it could also represent an increasing alignment with vaccine recommendations and public health goals. For example, ACIP recommends that 11–13-year-old adolescents receive HPV vaccinations, yet many states that authorized pharmacists to administer HPV vaccine initially limited it to adult patients only. Some of those states subsequently expanded the authorizations to allow pharmacists to administer HPV vaccine for the recommended patient ages.
We also identified a significant trend showing states changing their laws to authorize pharmacists to administer new vaccines. Although some of this trend is likely due to the advent, approval, or recommendation of new vaccines (e.g., for HPV), many state law changes reflected authorizations to administer existing vaccines. Most recently, states have begun to adopt laws that authorize pharmacists to administer any vaccine that is recommended by ACIP, creating flexibility in the state authorities. It is conceivable that jurisdictions prefer laws that tie vaccine restrictions to ACIP recommendations–which are updated regularly to reflect advances and new evidence–to continually undergoing tedious legislative and rulemaking processes to update existing authorized vaccine lists.
Impact of state law on vaccination rates
Numerous studies have identified the importance of state laws to pharmacist-administered vaccinations.2,3,17,19,34 We are aware of no other study of state pharmacist vaccination laws that measures more state law requirements (more than 200) over a greater time period. We make this data publicly available in recognition that future research is critical to understanding the types of laws that best facilitate increased vaccination rates.20 However, we note that this research reveals a number of important preliminary findings relating to the impact of state law on vaccination rates.
Vaccine and patient age restrictions
ACIP establishes vaccination schedules to assist vaccination providers in deciding when to recommend certain vaccines to their patients. Many existing state laws prevent pharmacists from administering vaccines as recommended. There are 2 primary dimensions to following ACIP recommendations: limitations on specific vaccine types and minimum patient age restrictions. On one hand, pharmacists cannot administer a recommended vaccine without the legal authority to administer it. On the other hand, a pharmacist cannot administer a vaccine as recommended if minimum patient age restrictions prevent administration at the recommended time.
Minimum patient age restrictions are a significant barrier to meeting vaccination coverage goals. Even if pharmacists could administer any vaccine, the 9 states that prevented pharmacists from vaccinating patients under 18 years of age in 2016 effectively bar pharmacists from administering any of the vaccines listed in the 2016 ACIP child immunization schedules as recommended35; the 2 states permitting vaccination of patients as young as 14 years of age allowed pharmacists to administer only the recommended booster for meningococcal and annual influenza vaccines for children; the 15 states with minimum patient age restrictions from 7 to 12 years allowed pharmacists only to adhere to ACIP recommendations for 4 vaccines on the 2016 schedule for children (meningococcal, tetanus–diphtheria–acellular pertussis, HPV, and influenza [annual]); pharmacists in the 7 states with minimum patient age restrictions from 3 to 6 years could administer the final dose of 3 more vaccines during the recommended range (inactivated poliovirus, measles-mumps-rubella, diphtheria–tetanus–acellular pertussis, and influenza [annual]). Consequently, laws with minimum age restrictions by themselves continue to pose significant barriers to the timely administration of recommended vaccines by pharmacists.
Third-party prescriber authorization requirements
Advocates of pharmacist vaccination laws argue that increased access to vaccination services will promote greater vaccination rates through improved convenience.2,6 However, many state laws still require patient-specific third-party pre-scriber authorizations to administer some vaccines. Patients in those states must first go to a prescribing practitioner to get the authorization before going to a pharmacist for vaccination. This is obviously less convenient than obtaining the vaccination from the original prescriber. To a lesser extent, we would also anticipate that the transaction costs associated with a pharmacist obtaining a general authorization from a third party, such as a standing order or collaborative practice agreement, could inhibit some pharmacists from providing vaccines permitted by law. In contrast, state laws that permit pharmacists to administer ACIP-recommended vaccines without a third-party prescriber authorization pose fewer barriers to vaccination access and could have a greater likelihood of improving vaccination rates.2
Broad versus specific laws
We identified 47 jurisdictions with statutes and regulations that specifically address pharmacists’ vaccination authority. Four states (AL, MS, TN, and WA) did not have statutes or regulations that specifically address pharmacist-administered vaccines. These states have broadly worded laws that authorize drug administration. Still, these states do allow pharmacists to administer vaccines, and according to Stewart et al., these states all permit pharmacists to administer vaccines on their “own authority” (as opposed to a standing order).17 Given that state agencies can issue informal guidance that can interpret (but not contradict) statutes and regulations, these states with broadly worded laws appear to be interpreting pharmacists’ authority broadly.
Other states might also be liberally interpreting broadly worded statutes and regulations. Four states (CO, CT, MA, and MI) authorized pharmacists to administer vaccines pursuant to a third-party prescriber authorization but did not specifically address whether the authorizations can cover more than 1 patient. However, at least 1 study suggests that these states are interpreting these laws as permitting vaccination under a general authorization as opposed to a more conservative interpretation (i.e., requiring patient-specific authorization).17 In addition, Nebraska’s laws do not address third-party pre-scriber authorizations or specifically grant pharmacists’ prescriptive authority; however, Stewart et al. describe Nebraska’s laws as permitting pharmacists to administer vaccines on their “own authority.”17 Accordingly, these 5 states also might be interpreting their laws broadly (e.g., using informal guidance from executive agencies).
There are advantages to using liberal legal interpretations of broadly worded laws. For example, informal guidance is easier to change than statutes or regulations, so it is easier to keep pace with changing evidence and recommendations. However, there is also less long-term stability, and agency interpretations are vulnerable to judicial review. More research is needed to investigate whether broadly worded laws, such as those in Alabama, Mississippi, Tennessee, and Washington, are more effective at promoting vaccination coverage than state laws that are specific but grant expansive vaccination authority.
Laws before 1990
The literature indicates that the first significant pharmacist vaccination programs occurred in the mid-1990s. Our data include laws in effect before the 1990s. These laws do not expressly mention vaccine administration. However, they do contain language that could be interpreted to authorize vaccination. For example, many laws authorize the administration of “drugs,” which are often legally defined so broadly (e.g., substances that prevent disease or “affect the structure or any function of the body”) that vaccines could reasonably be included. These laws in effect before the first major pharmacist vaccination programs in the 1990s were included for completeness.
Limitations
This study evaluates only state statutes and regulations relating to pharmacists’ vaccination authority. Although statutes and regulations form the foundation of pharmacists’ legal authority, they are not the only relevant authorities that impact variations in pharmacist practice between jurisdictions (see above). Moreover, states with broadly worded laws could provide expansive interpretations of the law through agency guidance. For example, Washington was among the first states to permit pharmacists to administer vaccines, but it is 1 of the 4 states that does not have express vaccination language (it authorizes drug administration). State agencies have the authority to provide pharmacists guidance on how to interpret laws, but this informal guidance cannot contradict the state statutes or regulations. Similarly, expert organizations (e.g., ACIP) provide recommendations on vaccine administration, but state statutes and regulations ultimately limit pharmacists’ ability to follow these recommendations as well.
Conclusion
Collectively, the laws in the 50 states and DC paint a clear picture: the scope of pharmacists’ vaccination authority is expanding. Jurisdictions are allowing pharmacists to administer more vaccines to younger patients with less direct prescriber oversight. This expansion supports patients seeking greater convenience and access to vaccination services outside of traditional clinical settings and hours.
This clear expansion of pharmacist vaccination authority stands in contrast to the reservations previously expressed by some physician groups for pharmacists as vaccination providers.15 Legal provisions in some jurisdictions might address some provider-pharmacist communication and documentation concerns by instituting requirements for reporting and recordkeeping for pharmacists who administer vaccines. One thing appears certain: state laws support a new norm for pharmacists as alternate vaccination providers.
Supplementary Material
Key Points.
Background
Since the 1990s, states have passed laws giving pharmacists the authority to administer vaccines to improve immunization access.
Limitations of pharmacists’ legal vaccination authority include restrictions on types of vaccines, patient age requirements, and third-party prescriber requirements.
New CDC data on pharmacist vaccination laws will facilitate research on how specific types of laws affect vaccination rates.
Findings
Trends in state vaccination laws show pharmacists are administering more vaccines to younger patients with less physician oversight.
As of 2016, 10 jurisdictions permitted pharmacists to independently administer a vaccine.
State laws with restrictions on vaccine types and minimum patient ages remain a substantial barrier to pharmacists administering vaccines as recommended by the Advisory Committee on Immunization Practices.
Acknowledgments
The authors thank Allison Reddick, JD, MPH, who assisted with data collection and validation; Tara Ramanathan, JD, MPH, and Abby Ferrell, JD, MPA, attorneys with PHLP who assisted with manuscript review; and Lindsay Culp, JD, MPH, an attorney with the CDC Public Health Associate Program who assisted with assessment design and validation. No additional compensation was provided to these acknowledged individuals for their contributions. PHLP cannot provide legal advice on any issue and cannot represent any individual or entity in any matter. PHLP recommends seeking the advice of an attorney or other qualified professional with questions regarding the application of law to a specific circumstance. The findings and conclusions in this summary are those of the authors and do not necessarily represent the official views of CDC.
Funding: C.D.S. was supported by the Oak Ridge Institute for Science and Education and the Office of State, Tribal, and Local Support, the Centers for Disease Control and Prevention.
Footnotes
Disclosure: The authors have no other conflicts of interest, including relevant financial interests, activities, relationships, and affiliations.
Previous presentation: Portions of this research were presented at the Public Health Law Conference, Washington, DC, September 15, 2016.
Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.japh.2017.07.001.
Contributor Information
Cason D. Schmit, Research Assistant Professor, Department of Health Policy and Management; HIPAA Compliance Officer, School of Public Health, Texas A&M University, College Station, TX.
Matthew S. Penn, Director, PHLP, OSTLTS, CDC, Atlanta, GA.
References
- 1.Centers for Disease Control and Prevention. Ten Great public health achievements–United States, 1900–1999. MMWR Morb Mortal Wkly Rep. 1999;48(12):241–243. [PubMed] [Google Scholar]
- 2.Goad JA, Taitel MS, Fensterheim LE, Cannon AE. Vaccinations administered during off-clinic hours at a national community pharmacy: implications for increasing patient access and convenience. Ann Fam Med. 2013;11(5):429–436. doi: 10.1370/afm.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogue MD, Grabenstein JD, Foster SL, Rothholz MC. Pharmacist involvement with immunizations: a decade of professional advancement. J Am Pharm Assoc. 2003;46(2):168–179. doi: 10.1331/154434506776180621. [DOI] [PubMed] [Google Scholar]
- 4.Grabenstein J, Bonasso J. Health-system pharmacists’ role in immunizing adults against pneumococcal disease and influenza. Am J Heal Pharm. 1999;56(17 Suppl 2):S3–S22. doi: 10.1093/ajhp/56.suppl_2.S3. [DOI] [PubMed] [Google Scholar]
- 5.Grabenstein J. Pharmacists as vaccine advocates: roles in community pharmacies, nursing homes, and hospitals. Vaccine. 1998;16(18):1705–1710. doi: 10.1016/s0264-410x(98)00131-5. [DOI] [PubMed] [Google Scholar]
- 6.Papastergiou J, Folkins C, Li W, Zervas J. Community pharmacist–administered influenza immunization improves patient access to vaccination. Can Pharm J (Ott) 2014;147(6):359–365. doi: 10.1177/1715163514552557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurley LP, Wortley P, Allison MA, et al. Seasonal influenza vaccination in adults: practice and attitudes about collaborative delivery with community vaccinators. Vaccine. 2011;29:8649–8655. doi: 10.1016/j.vaccine.2011.08.126. [DOI] [PubMed] [Google Scholar]
- 8.Weitzel KW, Goode J-V. Implementation of a pharmacy-based immunization program in a supermarket chain. J Am Pharm Assoc. 2000;40(2):252–256. doi: 10.1016/s1086-5802(16)31066-x. [DOI] [PubMed] [Google Scholar]
- 9.Bearden DT, Holt T. Statewide impact of pharmacist-delivered adult influenza vaccinations. Am J Prev Med. 2005;29(5):450–452. doi: 10.1016/j.amepre.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Wong-Beringer A, Brodetsky E, Quist R. Pneumococcal vaccination in hospitalized elderly patients: role of the pharmacist. Pharmacotherapy. 2003;23(2):199–208. doi: 10.1592/phco.23.2.199.32085. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Ford LJ, Wingate L, et al. Effect of pharmacist intervention on herpes zoster vaccination in community pharmacies. J Am Pharm Assoc. 2013;53(1):46–53. doi: 10.1331/JAPhA.2013.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Amburgh JA, Waite NM, Hobson EH, Migden H. Improved influenza vaccination rates in a rural population as a result of a pharmacist-managed immunization campaign. Pharmacotherapy. 2001;21(9):1115–1122. doi: 10.1592/phco.21.13.1115.34624. [DOI] [PubMed] [Google Scholar]
- 13.Taitel M, Cohen E, Duncan I, Pegus C. Pharmacists as providers: targeting pneumococcal vaccinations to high risk populations. Vaccine. 2011;29(45):8073–8076. doi: 10.1016/j.vaccine.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 14.Higginbotham S, Stewart A, Pfalzgraf A. Impact of a pharmacist immunizer on adult immunization rates. J Am Pharm Assoc. 2012;52(3):367–371. doi: 10.1331/JAPhA.2012.10083. [DOI] [PubMed] [Google Scholar]
- 15.Hurley LP, Bridges CB, Harpaz R, et al. U.S. physicians’ perspective of adult vaccine delivery. Ann Intern Med. 2014;160(3):161–170. doi: 10.7326/M13-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blake EW, Blair MM, Couchenour RL. Perceptions of pharmacists as providers of immunizations for adult patients. Pharmacotherapy. 2003;23(2):248–254. doi: 10.1592/phco.23.2.248.32083. [DOI] [PubMed] [Google Scholar]
- 17.Stewart AM, Lindley MC, Cox MA. State Law and Standing Orders for Immunization Services. Am J Prev Med. 2016;50(5):e133–e142. doi: 10.1016/j.amepre.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothholz MC. Pharmacist-provided immunization compensation and recognition: white paper summarizing APhA/AMCP stakeholder meeting: American Pharmacists Association and Academy of Managed Care Pharmacy. J Am Pharm Assoc. 2011;51(6):704–712. doi: 10.1331/JAPhA.2011.11544. [DOI] [PubMed] [Google Scholar]
- 19.Brewer NT, Chung JK, Baker HM, Rothholz MC. Pharmacist authority to provide HPV vaccine: novel partners in cervical cancer prevention. Gynecol Oncol. 2014;132:S3–S8. doi: 10.1016/j.ygyno.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Schmit C, Reddick A. Pharmacist vaccination laws. Policy Surveillance Program. 2017 Available at: http://lawatlas.org/datasets/pharmacist-vaccination. Accessed August 4, 2017.
- 21.Anderson ED, Tremper C, Thomas S, Wagenaar AC. Measuring statutory law and regulations for empirical research. In: Wagenaar AC, Burris S, editors. Public Health Law Research: Theory and Methods. San Francisco: John Wiley and Sons; 2013. pp. 237–260. [Google Scholar]
- 22.Burris S, Hitchcock L, Ibrahim J, Penn M, Ramanathan T. Policy surveillance: a vital public health practice comes of age. J Health Polit Policy Law. 2016;41(6):1151–1173. doi: 10.1215/03616878-3665931. [DOI] [PubMed] [Google Scholar]
- 23.Immunization Action Coalition. States authorizing pharmacists to vaccinate. 2016 Available at: http://www.immunize.org/laws/pharm.asp. Accessed November 1, 2016.
- 24.American Pharmacists Association. Pharmacist administered vaccines. 2013 Available at: http://www.pharmacist.com/sites/default/files/PharmacistIZAuthority.pdf. Accessed May 25, 2017.
- 25.N.H. Code Admin. R. Ph 1304.01.; 2009.
- 26.N.H. Rev. Stat. § 318:16-B.; 2008.
- 27.N.H. Code Admin. R. Ph 1304.01.; 2012.
- 28.N.C. Admin. Code Tit. 21, R. 32U.0101.; 2009.
- 29.N.C.G.S.A. §90-85.15B.
- 30.Ala. Code 1975 § 34-23-1(5).; 2016.
- 31.Miss. Code Ann. § 73-21-73(i).; 2016.
- 32.T. C. A. § 63-10-204(16).; 2016.
- 33.RCWA 18.64.011(11).; 2016.
- 34.McIntosh J, Sturpe DA, Khanna N. Human papillomavirus vaccine and cervical cancer prevention: practice and policy implications for pharmacists. J Am Pharm Assoc. 2008;48(1):e1–e16. doi: 10.1331/JAPhA/2008.07032. [DOI] [PubMed] [Google Scholar]
- 35.National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years United States, 2016. 2016 Available at: https://www.cdc.gov/vaccines/schedules/downloads/past/2016-child.pdf. Accessed April 12, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


