Abstract
Chronic heart failure (CHF) results in a greater cost of breathing and necessitates an elevated diaphragm blood flow (BF). Dietary nitrate (NO3−) supplementation lowers the cost of exercise. We hypothesized that dietary NO3− supplementation would attenuate the CHF-induced greater cost of breathing and thus the heightened diaphragm BF during exercise. CHF rats received either 5 days of NO3− -rich beetroot (BR) juice (CHF+BR, n=10) or a placebo (CHF, n=10). Respiratory muscle BFs (radiolabeled microspheres) were measured at rest and during submaximal exercise (20 m/min, 5% grade). Infarcted left ventricular area and normalized lung weight were not significantly different between groups. During submaximal exercise, diaphragm BF was markedly lower for CHF+BR than CHF (CHF+BR: 195±28; CHF: 309±71 mL/min/100g, p=0.04). The change in diaphragm BF from rest to exercise was less (p=0.047) for CHF+BR than CHF. These findings demonstrate that dietary NO3− supplementation reduces the elevated diaphragm BF during exercise in CHF rats thus providing additional support for this therapeutic intervention in CHF.
Keywords: respiratory muscles, intercostals, transversus abdominis, beetroot juice
1. Introduction
Over 6 million Americans are afflicted with chronic heart failure (CHF, Benjamin et al, 2017). CHF is characterized by impaired cardiac output and heightened sympathoexcitation constraining locomotor muscle blood flow (BF) and serving to limit exercise tolerance, the hallmark symptom of CHF (Poole et al, 2012). Furthermore, CHF is associated with respiratory muscle weakness (Dall’Argo et al, 2006) and pulmonary abnormalities such as increased physiological dead space, mild ventilation/perfusion mismatch, lower lung diffusion capacity, and obstructive-restrictive lung disorders (Poole et al, 2012). These pulmonary abnormalities result in a greater cost and work of breathing for a given rate of ventilation (Cross et al, 2012), which in concert with the exaggerated ventilatory response in CHF patients compared to healthy individuals (Sullivan et al, 1988; Agostoni et al, 2003; Myers et al, 1992) conflate to elevate diaphragm BF during submaximal exercise (Musch, 1993; Smith et al, 2017d). Furthermore, the elevated work of breathing in CHF results in the redistribution of cardiac output from the locomotor to the respiratory muscles during submaximal exercise further reducing locomotor O2 delivery and exercise tolerance (Miller et al, 2007; Olson et al, 2010; Musch, 1993; Smith et al, 2017d). Consequently, therapeutic interventions in CHF patients that alleviate this redistribution of cardiac output from the locomotor to respiratory muscles may be effective for improving exercise tolerance.
Recent findings present compelling evidence for dietary nitrate (NO3−) supplementation as an efficacious therapeutic intervention in CHF patients (Ferguson et al, 2016; Hirai et al, 2015; Coggan et al, 2015; Zamani et al, 2015, 2017; Eggebeen et al, 2016). Dietary NO3− supplementation has been found to improve exercise tolerance (Zamani et al, 2015, 2017; Eggebeen et al, 2016) and skeletal muscle contractile function (Coggan et al, 2015) in CHF patients. This latter finding is important in the context of the present study because CHF is often associated with diaphragm weakness (Dall’Argo et al, 2006) and compromised diaphragm contractile function (Howell et al, 1995; Supinski et al, 1994). Furthermore, in healthy humans, dietary NO3− supplementation has consistently lowered the oxygen cost of submaximal exercise (Larsen et al, 2007; Bailey et al, 2010; Bailey et al, 2009; Lansley et al, 2011) via reduced ATP cost of muscle force production (Bailey et al, 2010) and improved mitochondrial efficiency (Larsen et al, 2011). We reasoned that if dietary NO3− supplementation reduced the greater CHF-induced cost of diaphragm muscle contractions during submaximal exercise then diaphragm BF would be decreased. Therefore, the purpose of this study was to determine if dietary NO3− supplementation ameliorates the elevated diaphragm BF during submaximal exercise in CHF rats. We hypothesized that, during submaximal exercise in CHF rats, dietary NO3− supplementation via beetroot (BR) juice would attenuate the exacerbated diaphragm BF.
2. Methods
2.1 Ethical approval
The present study focuses on the effect of dietary NO3− supplementation on respiratory muscle (i.e. diaphragm, intercostal, and transversus abdominis) BF during submaximal exercise in CHF in the same animals used in a previously published investigation (Ferguson et al, 2016). In this previously published investigation, twenty young adult (~3 month old) male Sprague-Dawley rats received a myocardial infarction (MI) under aseptic conditions as previously described (Musch and Terrell, 1992; Ferguson et al., 2016) with surviving animals randomly assigned to one of two experimental groups. These groups consisted of rats that evidenced moderate CHF as indicated by heart morphometrics (see Table 1; Ferguson et al., 2016) and consisted of animals that received 5 days of NO3− -rich or NO3− -depleted BR. Consistent with Institutional Animal Care and Use Committee (IACUC) recommendations and the 3R’s (Refinement, Reduction, & Replacement) of animal research (Curzer et al., 2016) healthy non-MI sham-operated control rats were not included in our present investigation. This decision was made based on the fact that: 1) previous results from our laboratory have demonstrated the impact of dietary nitrate supplementation during exercise in a very similar cohort of healthy non-MI rats (Ferguson et al., 2013), 2) previous results from our laboratory have demonstrated that BF to the diaphragm is elevated during submaximal exercise in rats with CHF when compared to healthy non-MI counterparts (Musch, 1993), 3) the primary intent of the present investigation was to determine whether dietary nitrate supplementation would reduce BF to the diaphragm during submaximal exercise in CHF rats. This issue and the associated data were not addressed or presented previously. Rats were maintained at accredited animal facilities at Kansas State University on a 12:12 h light-dark cycle with food and water provided ad libitum. All procedures were approved by the IACUC of Kansas State University.
Table 1.
Heart morphometrics and plasma NO2−
| CHF | CHF+BR | |||||
|---|---|---|---|---|---|---|
| Body weight (g) | 494 | ± | 15 | 471 | ± | 18 |
| MI size (%) | 29 | ± | 3 | 33 | ± | 4 |
| LVEDP (mmHg) | 18 | ± | 2 | 18 | ± | 2 |
| LV dP/dt (mmHg/s) | 6,311 | ± | 259 | 6,438 | ± | 436 |
| RV wt/body wt (mg/g) | 0.66 | ± | 0.03 | 0.75 | ± | 0.06 |
| Lung wt/body wt (mg/g) | 5.43 | ± | 1.55 | 5.35 | ± | 0.80 |
| Plasma NO2− (nM) | 345 | ± | 59 | 569 | ± | 81* |
Values are mean±SE. MI, myocardial infarction; LVEDP, left ventricular end-diastolic volume; LV dp/dt, left ventricular developed pressure; RV, right ventricle; wt, weight; NO2−, nitrite;
significantly different than CHF
2.2 Myocardial infarction protocol
Rats were anesthetized with a 5% isoflurane-oxygen mixture, intubated, and connected to a rodent respirator. While being maintained on a 2% isoflurane-oxygen mixture a thoracotomy was performed, the pericardium was then opened, and the left main coronary artery was ligated with 6-0 Ti-cron suture. During lung hyperinflation the ribs were sutured back together using 2-0 gut. The muscles of the thorax along with the skin were sewn together using 4-0 gut and 3-0 silk suture. Each rat was given bupivacaine (1.5 mg/kg sc), buprenorphine (0.03 mg/kg im), and ampicillin (50 mg/kg im) to alleviate pain and reduce the risk of infection. Anesthesia was then removed and the rats were extubated followed by post-surgery monitoring (>6 h post-surgery). All rats were allowed >21 days of recovery for complete remodeling of necrotic myocardial tissue and development of CHF (Musch & Terrell, 1992).
All surviving CHF rats were familiarized with running on a motor-driven treadmill at a speed of 20 m/min, up a 5% grade for 5 consecutive days. Familiarization to treadmill running was limited to 5–10 min/day to ensure an exercise training effect was not produced in these animals (Musch & Terrell, 1992).
2.3 BR supplementation
As previously published (Ferguson et al, 2016) rats received 5 days of NO3− -rich (NO3− dose = 1mM/kg/day; Beet it Sport, James White Drinks, Ipswich UK; CHF+BR, n=10) or NO3− -depleted (Placebo Beet it Sport, James White Drinks, Ipswich UK; CHF, n=10) BR. Previous studies from our laboratory (Ferguson et al, 2013a, Ferguson et al, 2013b; Ferguson et al, 2014; Ferguson et al, 2015) have demonstrated that dosage used in the present investigation will elicit significant vascular effects in rats and that it is similar to the dosage used previously in humans (Bailey et al, 2009; Bailey et al, 2010), taking into account that the rat has higher resting metabolic rate when compared to human counterparts (~7 times greater than humans (Musch et al, 1988)). The dosage used in this investigation produced significant elevations of blood plasma NO2− concentrations (see Table 1; Ferguson et al., 2016) demonstrating the efficacy of the NO3− -supplementation.
2.4 Instrumentation and final experimental protocol
On the day of the final experimental protocol, rats were anesthetized initially with a 5% isoflurane-oxygen mixture and maintained on a 3% isoflurane-oxygen mixture. A midline incision was made on the anterior portion of the neck and the right carotid artery was isolated, exteriorized, and cannulated with a 2-Fr-catheter-tipped pressure micromanometer (model TC-510, Millar Instruments). Heart rate (HR) and the arterial pressure were measured and then the micromanometer was advanced in a retrograde manner into the left ventricle (LV) and LV end-diastolic pressure (LVEDP) and the derivative of the pressure wave form (LV dP/dt) were measured. Following the LVEDP and LV dP/dt measurements, the mircomanometer was retracted and removed and replaced by a catheter (PE-10 connected to PE-50, IntraMedic polyethylene tubing, Clay Adams, Becton, Dickinson, Sparks, MD) that was placed in the ascending aorta. This catheter was used for the measurements of MAP and HR (model 200, DigiMed BPA, Louisville, KY) and the infusion of radiolabeled microspheres. A second catheter was placed in the caudal (tail) artery for arterial blood sampling (Musch & Terrell, 1992). Both catheters were tunneled subcutaneously to the dorsal aspect of the neck, exteriorized, anesthesia was terminated, and the rats were given >60 min to recover (Flaim et al, 1984).
2.5 Determination of BF, Vascular Conductance, and LV infarct size
Following the completion of the final experimental protocol and resting measurements, rats were anesthetized with pentobarbital sodium (>50 mg/kg body wt.) via the right carotid artery catheter. The rats were then euthanized by performing a pneumothorax and removing the heart and lungs. Placement of each catheter was verified by anatomic dissection. The lungs were weighed and the right ventricle (RV) was then separated from the LV and septum, and both tissues weighed. The LV was cut open from base to apex, flattened, and under transillumination the necrotic zone of the LV produced by the MI was determined by planimetry and expressed as a percentage of the LV endocardial surface area. The diaphragm, intercostals, and transversus abdominis of each rat were dissected out.
Dissected tissues were blotted, weighed, and placed immediately into counting vials. The radioactivity of each tissue was determined with a gamma scintillation counter (model 5230, Auto Gamma Spectrometer, Packard, Downers Grove, IL). Taking into account the cross-talk fraction between the different isotopes, absolute muscle BF were calculated by the reference sample method (Ishise et al, 1980) and expressed in milliliters per min per 100g of tissue. Adequate mixing of the microspheres was verified for each microsphere infusion as demonstrated by <15% difference in BF to the right and left kidneys (Ferguson et al, 2016). Vascular conductance (VC) was then calculated by normalizing BF to MAP measured at the time of the microsphere infusion and expressed as mL/min/mmHg/100g.
2.6 Blood sampling and measurement of plasma [NO2−]
As previously described (Ferguson et al., 2016) blood was collected from CHF and CHF+BR rats for the measurement of plasma [NO2−]. Approximately 0.8 mL of blood was drawn from the caudal artery catheter and immediately centrifuged at 5,000 at 4°C for 6 min. The plasma was extracted and frozen at −80°C for subsequent analysis of [NO2−].
All measurements of plasma NO2− were performed within 30 min of thawing via chemiluminescence with an Ionic/Sievers NO analyzer (NOA 280i, Sievers Instruments, Boulder, CO). Potassium iodide in acetic acid was used as a reductant to obtain plasma NO2−, while avoiding potential reduction of NO3−. This reductant possesses the ability to reduce NO2− to NO but is incapable of reducing higher oxides of nitrogen, thus increasing the specificity of NO2−.
2.7 Statistical analyses
Values are reported as mean ± standard error (SE). All statistical analyses were performed by using SigmaStat 2.0 (Jandel Scientific, San Rafael, CA). Unpaired t-tests were used to compare body weight, MI size, heart morphometrics, plasma NO2−, arterial PCO2, and changes in respiratory muscle BF (from rest to submaximal exercise) between CHF and CHF+BR rats. MAP, HR and respiratory muscle (diaphragm, intercostal, and transversus abdominis) BFs and VCs were compared within (rest vs 20 m/min, 5% grade) and between (CHF vs CHF+BR) groups using mixed factorial analysis of variance and Student-Newman-Keuls post-hoc tests when appropriate. Statistical significance was set at p<0.05.
3. Results
3.1 Heart morphometrics and plasma NO2−
As previously reported (see Table 1; Ferguson et al., 2016) there were no differences in body weight (p=0.31), MI size (p=0.44), LVEDP (p=0.94), LV dP/dt (p=0.78), or normalized RV (p=0.17) or lung weight (p=0.96) between CHF+BR and CHF. However, by design, CHF+BR had an ~80% greater plasma [NO2−] than CHF rats.
3.2 Cardiovascular responses
As previously reported (Ferguson et al., 2016), MAP was not different between CHF+BR and CHF at rest (CHF+BR 118±3; CHF: 125±5 mmHg, p=0.20) or during submaximal exercise (CHF+BR 128±4; CHF: 131±3 mmHg, p=0.52). Furthermore, there were no differences in HR between CHF+BR and CHF at rest (CHF+BR 413±9; CHF: 409±12 beats/min, p=0.73) or during submaximal exercise (CHF+BR 507±5; CHF: 518±10 beats/min, p=0.39). There were no differences in arterial PCO2 (CHF+BR: 22±1; CHF: 21±1 mmHg, p=0.20) or [lactate] (CHF+BR: 2.1±0.2; CHF: 2.1±0.2 mM, p=0.41) between groups during submaximal exercise.
3.3 Respiratory muscle BFs
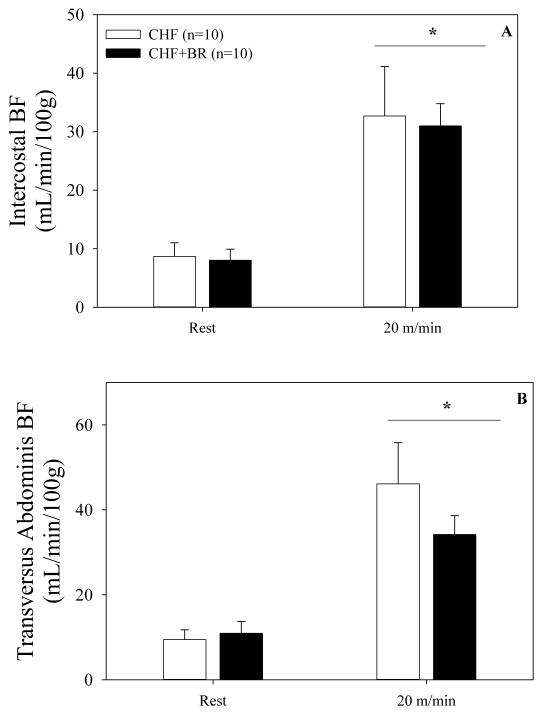
From rest to submaximal exercise, diaphragm BF significantly increased (p=0.01) in both CHF+BR and CHF (Figure 1). Diaphragm BF was not different at rest (p=0.99) between groups, but was ~38% lower (p=0.04) during submaximal exercise in CHF+BR compared to CHF. Furthermore, the change in diaphragm BF from rest to submaximal exercise was substantially less for CHF+BR compared to CHF (CHF+BR: 122±20; CHF: 237±68 mL/min/100g, p=0.047). Diaphragm VC was not different at rest (p=0.99), but was lower during submaximal exercise for CHF+BR compared to CHF (CHF+BR: 1.53±0.21; CHF: 2.33±0.53 mL/min/mmHg/100g, p=0.03). Intercostal and transversus abdominis BF increased (p<0.01) in both groups from rest to submaximal exercise (Figure 2A & 2B) but were not different at rest (p>0.86) or during submaximal exercise (p>0.14) between CHF+BR and CHF. In addition, the change in intercostal (p=0.91) and transversus abdominis (p=0.20) BF from rest to submaximal exercise was not different between groups. Neither intercostal nor transversus VC were different at rest (p>0.79) or during submaximal exercise (p>0.17) between CHF+BR and CHF.
Figure 1. Diaphragm BF at rest and during submaximal exercise.
Mean (A) and individual (B) diaphragm BF at rest and during submaximal exercise in CHF (white bar) and CHF+BR (black bar) rats. Diaphragm BF increased (p<0.01) from rest to submaximal exercise in both groups. Submaximal exercise diaphragm BF was lower (p=0.04) for CHF+BR than CHF. *, significantly different from rest. †, significantly different from CHF.
Figure 2. Intercostal and transversus abdominis BF at rest and during submaximal exercise.
Mean intercostal BF (2A) and transversus abdominis BF (2B) at rest and during submaximal exercise in CHF (white bar) and CHF+BR (black bar) rats. Intercostal and transversus abdominis BF increased (p<0.01) from rest to submaximal exercise in both groups. Intercostal and transversus abdominis BF was not different (p>0.14) between groups. *, significantly different from rest.
4. Discussion
The primary novel finding of the present investigation was that dietary NO3− supplementation via BR juice resulted in a ~38% reduction in diaphragm BF during submaximal exercise in rats with CHF. In contrast, intercostal and transversus abdominis BFs were not significantly decreased. These results have important implications as the diaphragm commands a higher BF during submaximal exercise in CHF at the expense of hindlimb BF (Musch, 1993; Smith et al, 2017d).
4.1 CHF and diaphragm BF
CHF is associated with pulmonary abnormalities resulting in a greater work and energetic cost of breathing (Cross et al, 2012) and thus, when compared with healthy animals, diaphragm BF is elevated in CHF during submaximal exercise (Musch, 1993; Smith et al, 2017d). Our submaximal exercise diaphragm BFs (i.e. 309 mL/min/100g) in CHF rats (placebo condition) are consistent with those recently reported in older CHF male rats during submaximal exercise (257 mL/min/100g; Smith et al, 2017d), yet lower than previously reported in young female CHF rats (356–483 mL/min/100g; Musch, 1993). This apparent discrepancy is explained by the lower speed and grade used in the present (20 m/min, 5% grade) versus the previous study in young female CHF rats (28 m/min, 20 % grade). Crucially, the CHF diaphragm BFs in the current study are substantially higher than those reported in healthy rats during submaximal exercise (124–136 mL/min/100g; Smith et al, 2017c). Furthermore, our CHF diaphragm BFs are in line with maximal exercise diaphragm BFs in healthy rats (283–304 mL/min/100g; Smith et al, 2017c) and also ponies (265–325 mL/min/100g; Manohar, 1986, 1990), while lower than those reported in healthy rats exercising supra-maximally (395 mL/min/100g; Poole et al, 2000) and maximal-intensity human knee-extensor BF (385 mL/min/100g; Richardson et al, 1993).
4.2 Dietary NO3− and diaphragm BF
As mentioned in the introduction, dietary NO3− supplementation lowers the oxygen cost of submaximal exercise (Bailey et al, 2009; Bailey et al, 2010; Lansley et al, 2011; Larsen et al, 2007) by a combination of decreased ATP cost of contraction (Bailey et al, 2010) and enhanced mitochondrial efficiency (Larsen et al, 2011). Importantly, dietary NO3− supplementation also improves contractile function (Coggan et al, 2015) and lowers the energetic cost of exercise (improved economy, ↓work rate/V̇O2, Zamani et al, 2017) in CHF patients. Taken together, we hypothesized that dietary NO3− supplementation would reduce the cost of diaphragmatic contractions during submaximal exercise subsequently lowering the CHF-heightened diaphragm BF. In the present study, we found that diaphragm BF was lower following BR juice compared to placebo supplementation (195 vs 309 mL/min/100g, respectively) suggesting that dietary NO3− supplementation reduced the oxygen cost of diaphragmatic contractions and/or improved the energetic efficiency of submaximal exercise in CHF. It is possible that this effect may have contributed to the BR-induced enhancement of exercise capacity demonstrated in CHF patients (Zamani et al, 2015, 2017).
What are the potential mechanisms by which dietary NO3− supplementation would decrease diaphragm BF? First, as above, dietary NO3− supplementation lowers the ATP cost of contraction (Bailey et al, 2010) and improves mitochondrial efficiency (Larsen et al, 2011). In this regard, it is pertinent that dietary NO3− supplementation has fiber type-specific effects in healthy humans and animals (Jones et al, 2016) including dietary NO3− supplementation-mediated improvements in O2 uptake kinetics (Bailey et al, 2015) and vascular control (Ferguson et al, 2013b) as well as calcium handling and contractile function (Hernandez et al, 2012) predominately in fast-twitch rather than slow-twitch muscles. However, in CHF rats, Ferguson et al (2016) found that dietary NO3− supplementation improved vascular control in both predominately fast-twitch and slow-twitch muscles during submaximal exercise providing evidence that dietary NO3− supplementation efficacy may lose its fiber type-specificity in CHF; possibly due to CHF-induced lowering of PO2 in the slow-twitch muscles (Behnke et al, 2004). Therefore, it is possible that dietary NO3− supplementation resulted in a lowered ATP cost and/or improved mitochondrial efficiency of diaphragm contraction. Second, the CHF-induced exaggeration of the ventilatory response during submaximal exercise (Sullivan et al, 1988; Agostoni et al, 2003; Myers et al, 1992) would heighten diaphragm energetic requirements and thus raise BF. With NO3− supplementation lowering the oxygen cost of submaximal exercise in healthy individuals (Bailey et al, 2009; Bailey et al, 2010; Lansley et al, 2011; Larsen et al, 2009) and CHF patients (Zamani et al, 2017) if PaCO2 is unaltered (as herein) V̇CO2 would fall necessitating a reduced ventilatory response in the CHF+BR and further reducing the diaphragm BF. Furthermore, blood lactate during submaximal exercise is lowered following dietary NO3− supplementation (Ferguson et al, 2013) also serving to reduce carotid body stimulation and V̇CO2 and thus ventilation. Third, it is possible that dietary NO3− supplementation-mediated increases in cardiac output (Zamani et al, 2015) and improved vascular control (Ferguson et al, 2016) in CHF resulted in increased hindlimb locomotor BF at the expense of diaphragm BF. However, this affect would presumably be opposed by improved NO3− -induced diaphragm vascular control; an effect that would act to oppose the lowered diaphragm BF demonstrated herein.
The findings of the present study have important implications as the derangements in the pulmonary system contribute to exercise intolerance in CHF. Specifically, unloading the respiratory muscles during submaximal exercise in CHF improves exercise tolerance (Mancini et al, 1997; O’Donnell et al, 1999; Borghi-Silva et al, 2008). High inspiratory muscle work and the concomitant accumulation of metabolites leads to the activation of the respiratory muscle metaboreflex resulting in sympathetically-mediated vasocontraction in health (Smith et al, 2016; Smith et al, 2017a; Smith et al, 2017b; St Croix et al, 2000) that is accentuated in CHF (Chiappa et al, 2008). During submaximal exercise, the dietary NO3− supplementation-mediated reduction in diaphragm BF (presumably via lower cost of breathing) in the present study in combination with the improvement in hindlimb BF previously reported (Ferguson et al, 2016) suggests that dietary NO3− supplementation partially alleviated the tonically active respiratory muscle metaboreflex. It is important to note that dietary NO3− supplementation has been found to reduce systemic vascular resistance and improve cardiac output during peak exercise in CHF patients with preserved ejection fraction (HFpEF; Zamani et al, 2015). If present herein, this mechanism could also contribute to an improved hindlimb BF during submaximal exercise in CHF rats.
4.3 Methodological considerations
Several methodological considerations may have influenced our results. First, a healthy control group was not included in the present study, which would have provided additional information as to the effect of dietary nitrate supplementation on diaphragm BF in healthy rats. Furthermore, a healthy control group would have provided a comparison to determine if dietary nitrate supplementation reduced diaphragm BF in CHF to those levels present in healthy rat diaphragms. Second, previous studies have found that the oxygen cost of exercise is consistently lower in healthy humans with dietary NO3− supplementation (Larsen et al, 2007; Bailey et al, 2010; Bailey et al, 2009; Lansley et al, 2011). To date, dietary NO3− supplementation has resulted in lowered oxygen cost of exercise in CHF patients in some (Zamani et al, 2017), but not all (Zamani et al, 2015; Eggebeen et al, 2016; Hirai et al, 2017) studies. Measuring the oxygen cost of exercise in the present study would have been valuable to determine whether it was indeed lower consistent with the findings in healthy humans.
4.4 Conclusions
This is the first study to investigate the effect of dietary NO3− supplementation on diaphragm BF during submaximal exercise in CHF rats. Five days of dietary NO3− supplementation resulted in a ~38% reduction in diaphragm BF in CHF during submaximal exercise. These results provide additional support for dietary NO3− supplementation as a therapeutic intervention in addition to exercise based cardiac rehabilitation programs. Future studies might usefully determine the mechanisms (i.e. lowered ATP cost of contraction, improved mitochondrial efficiency and/or attenuated ventilatory response) responsible for the dietary NO3− supplementation-mediated reduction in diaphragm BF in CHF.
Highlights.
Chronic heart failure (CHF) leads to greater diaphragm blood flow (BF)
Beetroot juice (BR) supplementation lowers the oxygen cost of exercise
We examined if BR supplementation attenuated the diaphragm BF response in CHF rats
Submaximal exercise diaphragm BF was lower after BR supplementation in CHF rats
Acknowledgments
Funding: National Institutes of Health, HL-2-108328 and American Heart Association Grant-in-Aid 10 GRANT 4350011.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agostoni P, Cattadori G, Bianchi M, Wasserman K. Exercise-induced pulmonary edema in heart failure. Circulation. 2010;108:2666–2671. doi: 10.1161/01.CIR.0000097115.61309.59. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong RB, Hayes DA, Delp MD. Blood flow distribution in rat muscles during preexercise anticipatory response. J Appl Physiol. 1989;67:1855–1861. doi: 10.1152/jappl.1989.67.5.1855. [DOI] [PubMed] [Google Scholar]
- 3.Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol. 2010;109(1):135–148. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 4.Bailey SJ, Varnham RL, DiMenna FJ, Breese BC, Wylie LJ, Jones AM. Inorganic nitrate supplementation improves muscle oxygenation, O2 uptake kinetics, and exercise tolerance at high, but not low pedal rates. J Appl Physiol. 2015;118:1396–1405. doi: 10.1152/japplphysiol.01141.2014. [DOI] [PubMed] [Google Scholar]
- 5.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, DiMenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol. 2009;107(4):1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 6.Behnke BJ, Delp MD, McDonough P, Spier SA, Poole DC, Musch TI. Effects of chronic heart failure on microvascular oxygen exchange dynamics in muscles of contrasting fiber type. Cardiovasc Res. 2004;61(2):352–332. doi: 10.1016/j.cardiores.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 Update: A report from the American Heart Association. Circulation. 135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borghi-Silva A, Carrascosa C, Oliveira CC, Barroco AC, Berton DC, Vilaca D, Lira-Filho EB, Ribeiro D, Nery LE, Neder JA. Effects of respiratory muscle unloading on leg muscle oxygenation and blood volume during high intensity exercise in chronic heart failure. Am J Physiol Heart Circ Physiol. 2008;294:H2465–H2472. doi: 10.1152/ajpheart.91520.2007. [DOI] [PubMed] [Google Scholar]
- 9.Chiappa GR, Roseguini BT, Vieira PJC, Alves CN, Tavares A, Winkelmann ER, Ferlin EL, Steine R, Ribeiro JP. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J Am Coll Cardiol. 2008;51:1663–1671. doi: 10.1016/j.jacc.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 10.Coggan AR, Leibowitz JL, Spearie CA, Kadkhodayan A, Thomas DP, Ramaurthy S, Mahmood K, Park S, Waller S, Farmer M, Peterson LR. Acute dietary nitrate intake improves muscle contractile function in patients with heart failure: a double-blind, placebo-controlled, randomized trial. Circ Heart Fail. 2015;8(5):914–920. doi: 10.1161/CIRCHEARTFAILURE.115.002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cross TJ, Sabapathy S, Beck KC, Morris NR, Johnson BD. The resistive and elastic work of breathing during exercise in patients with chronic heart failure. Eur Respir J. 2012;39:1449–1457. doi: 10.1183/09031936.00125011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curzer HJ, Perry G, Wallace MC, Perry D. The Three Rs of Animal Research: What they Mean for the Institutional Animal Care and Use Committee and Why. Sci Eng Ethics. 2016;22:549–565. doi: 10.1007/s11948-015-9659-8. [DOI] [PubMed] [Google Scholar]
- 13.Dall’Ago P, Chiappa GR, Guths H, Stein R, Ribeiro JP. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. J Am Coll Cardiol. 2006;47(4):757–763. doi: 10.1016/j.jacc.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 14.Eggebeen J, Kim-Shapiro DB, Haykowsky M, Morgan TM, Basu S, Brubaker P, Rejeski J, Kitzman DW. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2016;4(6):428–437. doi: 10.1016/j.jchf.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Dose dependent effects of nitrate supplementation on cardiovascular control and microvascular oxygenation dynamics in healthy rats. Nitric Oxide Biol Chem. 2014;39:51–58. doi: 10.1016/j.niox.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Effects of nitrate supplementation via beetroot juice on contracting rat skeletal muscle microvascular oxygen pressure dynamics. Resp Physiol Neurobiol. 2013a;187:250–255. doi: 10.1016/j.resp.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J Physiol. 2013b;591(2):547–557. doi: 10.1113/jphysiol.2012.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferguson SK, Holdsworth CT, Colburn TD, Wright JL, Craig JC, Fees A, Jones AM, Allen JD, Musch TI, Poole DC. Dietary nitrate supplementation: impact on skeletal muscle vascular control in exercising rats with chronic heart failure. J Appl Physiol. 2016;121(13):661–669. doi: 10.1152/japplphysiol.00014.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson SK, Holdsworth CT, Wright JL, Fees AJ, Allen JD, Jones AM, Musch TI, Poole DC. Microvascular oxygen pressure in muscles comprised of different fiber types: impact of dietary nitrate supplementation. Nitric Oxide Biol Chem. 2015;48:38–43. doi: 10.1016/j.niox.2014.09.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flaim SF, Nellis SH, Toggart EJ, Drexler H, Kanda K, Newman ED. Multiple simultaneous determinations of hemodynamics and flow distribution in conscious rats. J Pharmacol Methods. 1984;11:1–39. doi: 10.1016/0160-5402(84)90050-0. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez A, Schiffer TA, Ivarsson N, Cheng AJ, Bruton JD, Lundberg JO, Weitzberg E, Westerbad H. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle. J Physiol. 2012;590:3575–3583. doi: 10.1113/jphysiol.2012.232777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirai DM, Musch TI, Poole DC. Exercise training in chronic heart failure: improving skeletal muscle O2 transport and utilization. Am J Physiol Heart Circ Physiol. 2015;309:H1419-14-39. doi: 10.1152/ajpheart.00469.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirai DM, Zelt JT, Jones JH, Castanhas LG, Bentley RF, Earle W, Staples P, Tschakovsky ME, McCans J, O’Donnell DE, Neder JA. Dietary nitrate supplementation and exercise tolerance in patients with heart failure with reduced ejection fraction. Am J Physiol Regul Integr Comp Physiol. 2017;312(1):R13–R22. doi: 10.1152/ajpregu.00263.2016. [DOI] [PubMed] [Google Scholar]
- 24.Howell S, Maarek JM, Fournier M, Sullivan K, Zhan WZ, Sieck GC. Congestive heart failure: differential adaptation of the diaphragm and latissimus dorsi. J Appl Physiol. 1995;79(2):389–397. doi: 10.1152/jappl.1995.79.2.389. [DOI] [PubMed] [Google Scholar]
- 25.Ishise S, Pegram BL, Yamamoto J, Kitamura Y, Frohlich ED. Reference sample microsphere method: cardiac output and blood flows in conscious rat. Am J Physiol. 1980;239(4):H443–H449. doi: 10.1152/ajpheart.1980.239.4.H443. [DOI] [PubMed] [Google Scholar]
- 26.Jones AM, Ferguson SK, Bailey SJ, Vanhatalo A, Poole DC. Fiber type-specific effects of dietary nitrate. Exerc Sport Sci Rev. 2016;44(2):53–60. doi: 10.1249/JES.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 27.Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Gilchrist M, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol. 2011;110(3):591–600. doi: 10.1152/japplphysiol.01070.2010. [DOI] [PubMed] [Google Scholar]
- 28.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 30.Mancini D, Donchez L, Levine S. Acute unloading of the work of breathing extends exercise duration in patients with heart failure. J Am Coll Cardiol. 1997;29(3):590–596. doi: 10.1016/s0735-1097(96)00556-6. [DOI] [PubMed] [Google Scholar]
- 31.Manohar M. Vasodilator reserve in respiratory muscles during maximal exertion in ponies. J Appl Physiol. 1986;60(5):1571–1577. doi: 10.1152/jappl.1986.60.5.1571. [DOI] [PubMed] [Google Scholar]
- 32.Manohar M. Inspiratory and expiratory muscle perfusion in maximally exercised ponies. J Appl Physiol. 1990;68(2):544–548. doi: 10.1152/jappl.1990.68.2.544. [DOI] [PubMed] [Google Scholar]
- 33.Miller JD, Smith CA, Hemauer SJ, Dempsey JA. The effects of inspiratory intrathoracic pressure production on the cardiovascular response to submaximal exercise in healthy and chronic heart failure. Am J Physiol Heart Circ Physiol. 2007;292(1):H580–592. doi: 10.1152/ajpheart.00211.2006. [DOI] [PubMed] [Google Scholar]
- 34.Musch TI. Elevated diaphragmatic blood flow during submaximal in rats with chronic heart failure. Am J Physiol. 1993;265:H1721–H1726. doi: 10.1152/ajpheart.1993.265.5.H1721. [DOI] [PubMed] [Google Scholar]
- 35.Musch TI, Bruno A, Bradford GE, Vayonis A, Moore RL. Measurements of metabolic rate in rats: a comparison of techniques. J Appl Physiol. 1988;65:964–970. doi: 10.1152/jappl.1988.65.2.964. [DOI] [PubMed] [Google Scholar]
- 36.Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. Am J Physiol. 1992;31:H411–H419. doi: 10.1152/ajpheart.1992.262.2.H411. [DOI] [PubMed] [Google Scholar]
- 37.Myers J, Salleh A, Buchanan N, Smith D, Nuetel J, Bowes E, Froeclicher VF. Ventilatory mechanisms of exercise intolerance in chronic heart failure. Am Heart J. 124:710–719. doi: 10.1016/0002-8703(92)90282-z. [DOI] [PubMed] [Google Scholar]
- 38.O’Donnell DE, D’Arsigny C, Raj S, Abdollah H, Webb KA. Ventilatory assistance improves exercise endurance in stable congestive heart failure. Am J Respir Crit Care Med. 1999;160:1804–1811. doi: 10.1164/ajrccm.160.6.9808134. [DOI] [PubMed] [Google Scholar]
- 39.Olson TP, Joyner MJ, Dietz NM, Eisenach JH, Curry TB, Johnson BD. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J Physiol. 2010;588:2487–2501. doi: 10.1113/jphysiol.2009.186056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol. 2012;302:H1050–H1063. doi: 10.1152/ajpheart.00943.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poole DC, Sexton WL, Behnke BJ, Ferguson CS, Hageman KS, Musch TI. Respiratory muscle blood flows during physiological and chemical hyperpnea in the rat. J Appl Physiol. 2000;88:186–194. doi: 10.1152/jappl.2000.88.1.186. [DOI] [PubMed] [Google Scholar]
- 42.Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol. 1993;75(4):1911–1916. doi: 10.1152/jappl.1993.75.4.1911. [DOI] [PubMed] [Google Scholar]
- 43.Smith JR, Alexander AM, Hammer SM, Dider KD, Kurti SP, Broxterman RM, Barstow TJ, Harms CA. Cardiovascular consequences of the inspiratory muscle metaboreflex: effects of age and sex. Am J Physiol Heart Circ Physiol. 2017a;312(5):H1013–H1020. doi: 10.1152/ajpheart.00818.2016. [DOI] [PubMed] [Google Scholar]
- 44.Smith JR, Broxterman RM, Hammer SM, Alexander AM, Didier KD, Kurti SP, Barstow TJ, Harms CA. Sex differences in the cardiovascular consequences of the inspiratory muscle metaboreflex. Am J Physiol Regul Integr Comp Physiol. 2016;311(3):R574–R581. doi: 10.1152/ajpregu.00187.2016. [DOI] [PubMed] [Google Scholar]
- 45.Smith JR, Didier KD, Hammer SM, Alexander AM, Kurti SP, Copp SW, Barstow TJ, Harms CA. Effect of cyclooxygenase inhibition on the inspiratory muscle metaboreflex-induced cardiovascular consequences in men. J Appl Physiol. 2017b doi: 10.1152/japplphysiol.00165.2017. [DOI] [PubMed] [Google Scholar]
- 46.Smith JR, Hageman KS, Harms CA, Poole DC, Musch TI. Respiratory muscle blood flow during exercise: effects of sex and ovarian cycle. J Appl Physiol. 2017c;122(4):918–924. doi: 10.1152/japplphysiol.01007.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith JR, Hageman KS, Harms CA, Poole DC, Musch TI. Effect of chronic heart failure in older rats on respiratory muscle and hindlimb blood flow during submaximal exercise. Resp Physiol Neurobiol. 2017d;243:20–26. doi: 10.1016/j.resp.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol. 2000;529:493–504. doi: 10.1111/j.1469-7793.2000.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan MJ, Higginbotham MB, Cobb FR. Increased exercise ventilation in patients with chronic heart failure: intact ventilatory control despite hemodynamic and pulmonary abnormalities. Circulation. 1988;77:552–559. doi: 10.1161/01.cir.77.3.552. [DOI] [PubMed] [Google Scholar]
- 50.Supinski G, DiMarco A, Dibner-Dunlap M. Alterations in diaphragm strength and faigability in congestive heart failure. J Appl Physiol. 1994;76(6):2707–2713. doi: 10.1152/jappl.1994.76.6.2707. [DOI] [PubMed] [Google Scholar]
- 51.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC, Chirinos JA. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–380. doi: 10.1161/CIRCULATIONAHA.114.012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zamani P, Tan V, Soto-Calderon H, Beraun M, Brandimarto JA, Trieu L, Varakantam S, Doulias PA, Townshend RR, Chittams J, Margulies KB, Cappola TP, Poole DC, Ischiropoulos H, Chirinos JA. Pharmacokinetics and pharmacodynamics of inorganic nitrate in heart failure with preserved ejection fraction. Circ Res. 2017;120:1151–1161. doi: 10.1161/CIRCRESAHA.116.309832. [DOI] [PMC free article] [PubMed] [Google Scholar]