Abstract
The threat of exposure to ionizing radiation from a nuclear reactor accident or deliberate terrorist actions is a significant public health concern. The lung is particularly susceptible to radiation-induced injury from external sources or inhalation of radioactive particles from radioactive fallout. Radiation-induced lung disease can manifest with an acute radiation pneumonitis and/or delayed effects leading to pulmonary fibrosis. As prior warning of radiation exposure is unlikely, medical countermeasures (MCMs) to mitigate radiation-induced lung disease that can be given in mass-casualty situations many hours or days postirradiation are needed to prevent both early and late lung damage. In this study, KL4 surfactant (lucinactant) was evaluated as a radiation mitigator in a well-characterized mouse model of targeted thoracic radiation exposure, for its effect on both early (several weeks) and late (18 weeks) lung damage. Here, 120 mg/kg total phospholipid of KL4 surfactant was administered twice daily intranasally, (enabling intrapulmonary inhalation of drug) to C57BL/6 mice 24 h after a single 13.5 Gy dose of thoracic irradiation (LD50 dose). Both early and chronic phase (2 and 4 weeks and 18 weeks postirradiation, respectively) assessments were performed. Mice were evaluated for evidence of reduced arterial blood oxygenation and early and chronic lung and systemic inflammation, lung fibrosis and oxidative stress. Analysis was done by performing lung function/respiration dynamics and measuring cellular protein content of bronchoalveolar lavage fluid (BALF), and levels of cytokines, 8-iso-prostaglandin F2α, hydroxyproline in lung and plasma, along with evaluating lung histology. The results of this study showed that intranasal delivery of KL4 surfactant was able to preserve lung function as evidenced by adequate arterial oxygen saturation and reduced lung inflammation and oxidative stress; total white count and absolute neutrophil count was decreased in BALF, as were plasma pro-inflammatory cytokine levels and biomarker of oxidative stress. KL4 surfactant is a promising MCM for mitigation of lung tissue damage after targeted, thoracic irradiation and has the potential to be developed as a broad-spectrum, multi-use MCM against chemical, biological, radiological or nuclear threat agents with potential to cause lung injury.
INTRODUCTION
Exposure to ionizing radiation from an unpredictable nuclear reactor accident, a nuclear attack or deliberate terrorist actions, including the detonation of a radiological dispersal device (RDD), represents a significant public health concern. Radiological and nuclear threats are complex, since the radiation source, duration and extent of exposure all contribute to the nature and effect of radiation. Unprotected individuals in close proximity to the event, as well as inadequately protected early-response rescue workers will likely be affected by radiation exposure (1–3).
The lung is particularly susceptible to injury from exposure to external radiation sources, as well as from inhaled radioactive particles from nuclear radioactive fallout. As described by many investigators, radiation-induced lung disease refers to a continuing process triggered by exposure to radiation, radiation pneumonitis, an early inflammatory response that involves alveolar cell destruction and inflammatory cell influx in the interstitial and alveolar space, which may be followed by an intermediate exudative phase and a late phase whereby radiation-induced fibrosis develops. Radiation-induced fibrosis is an irreversible process characterized by fibroblast proliferation, collagen accumulation and destruction of normal lung architecture (4).
Currently, treatment of radiation-induced lung disease remains primarily supportive and usually includes administration of oxygen, positive airway pressure, mechanical ventilation, bronchodilators and steroids, which, in addition, may lead to a rebound effect once treatment has stopped. There is an urgent and unmet need to develop medical countermeasures (MCMs) to prevent radiation-induced lung disease. An ideal MCM can be efficiently administered in a mass casualty situation within hours, if not days after exposure, since radiation accidents or attacks are likely to occur without warning. There is also a need for the development of MCMs that can be administered once early signs or symptoms of early/chronic injury begin to manifest to prevent progressive lung destruction/late fibrosis.
The primary role of naturally occurring pulmonary surfactant is to facilitate alveolar expansion, thus permitting normal gas exchange. Surfactants also regulate lung immune response and clearance of foreign particles, debris and inflammatory material (5). Decreased production of endogenous surfactant by alveolar type II cells and increased degradation of surfactant both occur in acute lung injury (ALI), leading to surfactant insufficiency, alveolar collapse and poor health outcomes (6). In radiation-induced lung disease, the role of endogenous pulmonary surfactant has not been fully elucidated. Significant fluctuations in natural pulmonary surfactant have been described, including reports of early surfactant insufficiency, or transient early rises that have been attributed to a pseudo-physiological response of alveolar type II pneumocytes, which are particularly sensitive to ionizing radiation injury (7–13). Alternately, radiation-induced membrane lipid peroxidation and membrane permeabilization may lead to the leakage of surfactant from lamellar bodies within these cells. There is also limited data on the functionality and composition of endogenous surfactant after irradiation. Most importantly, exogenous surfactant therapies have not been evaluated to treat radiation-induced lung disease, either alone or in combination with other mitigating agents.
KL4 surfactant (lucinactant), a peptide containing synthetic surfactant used as an endogenous surfactant replacement therapy (14, 15), can function locally in an inflammatory milieu in the lung and can also modulate the immune response, including limiting cytokine activation (16–21), as we have previously shown in other reported models of acute lung injury (22, 23). Since KL4 surfactant can function in an inflammatory milieu, and reduces cytokine activation and neutrophil influx into the lungs, it is a potentially promising therapeutic approach for mitigating radiation-induced lung disease. Therefore, we have been exploring these mechanisms of action associated with KL4 surfactant as they relate to mitigation of radiation-induced lung damage. In our current study, we evaluated the effectiveness of KL4 surfactant as a novel approach to mitigate both early radiation-induced pneumonitis and the delayed manifestations of lung injury (lung fibrosis) using the well-characterized C57BL/6 thoracic-irradiation mouse model (24–27). Our underlying premise is that KL4 surfactant, via its immune-modulatory properties (20, 21, 28), should prevent activation of, and/or downregulate the cascade of multiple inflammatory mediators that have been implicated in pathophysiology of radiation-induced lung injury.
METHODS
Animal Protocol
For these studies, female C57BL/6 mice were used (n =15–30 mice per group depending on assessments to be done at specific time points). This mouse strain is well characterized in the field of pulmonary radioprotection (29–31). Female mice were selected for this initial study to determine drug dosing, administration method and treatment regimen, because they are more susceptible to radiation-induced lung disease (32). Subsequent studies will extend to evaluation of male mice as well.
Mice were obtained from Charles River (Wilmington, MA) and exposed to 13.5 Gy thoracic irradiation at 6–8 weeks of age, an acceptable age for such studies (33) under animal protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. Animals were housed in conventional cages under standardized conditions with controlled temperature and humidity and a 12:12 h light-dark schedule. Animals had access to water and standard mouse chow ad libitum. Mice were randomized following a routine strategy as previously described by Suresh (34). We eliminated confounding covariates such as animal age and weight. In the current study of 450 mice, we ensured that mice at study start were within 1 g weight difference and only 1–2 weeks age difference. Assignment of mice into cohorts was then less complicated and followed a simple randomization method.
Intranasal Delivery of KL4 Surfactant
KL4 surfactant (Windtree Therapeutics Inc., Warrington, PA) as a liquid instillate at a concentration of 30 mg total phospholipid (TPL) per ml, or normal saline as vehicle control was delivered via intranasal administration [mice are obligate nasal breathers and effectiveness of delivery method has been previously established (35)] twice daily beginning 24 h postirradiation and lasting for 2 weeks (Fig. 1). Methods for intranasal instillation have been previously described elsewhere (35). Briefly, mice were placed in an induction chamber and lightly anesthetized with isoflurane. Once anesthetized, mice were removed from the induction chamber and placed in a supine position and 100 μl of instillate (total dose) delivered drop-wise to the nares using a pipette. Approximate 120 mg/kg TPL of KL4 surfactant was delivered per dose, a dose that has been shown to be effective in the ALI and respiratory distress syndrome models in restoring lung function and modulating an immune response (19, 21, 22). After treatment, mice were allowed to recover in a warm environment under observation prior to being returned to their home cage.
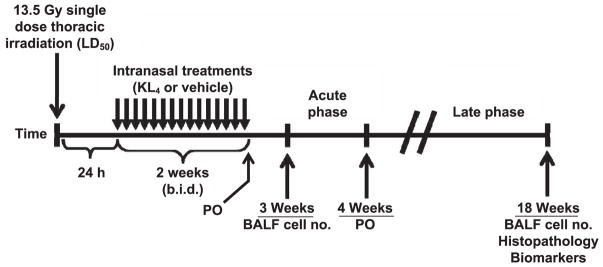
FIG. 1.
Experimental plan. KL4 surfactant (total phospholipid of 120 mg/kg) was administered intranasally to C57BL/6 mice (n =approximately 15–30 per group depending on assessments to be done at specific time points) 24 h after a single 13.5 Gy dose of thoracic irradiation. Mice were evaluated for evidence of reduced blood oxygenation and lung inflammation at 2 and 4 weeks postirradiation and for lung fibrosis, chronic pneumonitis, oxidative stress and local and system inflammation at 18 weeks postirradiation.
Irradiation Procedure
Animals were irradiated on the Small Animal Radiation Research Platform (SARRP), (Xstrahl Ltd., Camberley, UK) using a custom-made beam collimator, as previously described elsewhere (36). Briefly, this system uses an X-ray tube (model no. NDI-225-22 kV; Varian Medical Systems Inc., Palo Alto, CA) mounted on a gantry that rotates between 0 and 120 degrees. The custom collimator creates a 12.5-cm circular field with well-defined borders and with animals arranged in a circular, “head in” arrangement using a single central shield that provides uniform, simultaneous exposure to the thoracic region of multiple mice. This setup consists of a single, anterior 225-kV, 15 mA X-ray beam with 0.15-mm copper filter at an SSD of 35 cm, which is designed to accurately reproduce the internal radiation dose distribution in mice that were used in previously reported studies (25, 26, 37). The dosimetry and shielding of this system is routinely tested based on the description provided by Baumann et al. (38). Briefly, a 1-mm nylon fiducial bead was affixed to Gafchromic™ EBT dosimetry exposure film (International Specialty Products, Wayne, NJ) and CT scanned using the SARRP. The center of the nylon fiducial marker was chosen as the isocenter for a 5 × 5-mm square collimator that was used to deliver a 13.5 Gy dose of radiation to the film. The exposed area of film was evaluated to confirm that the center of the irradiated field corresponded to the marked isocenter. Radiation was delivered in a single dose via single anterior-posterior approach. A 13.5 Gy dose was delivered (roughly corresponding to LD50/120) as described in our previously published work (25–27). Nonirradiated animals (untreated, KL4 surfactant-treated or vehicle-treated) were also included in the experiment to serve as appropriate controls for respective irradiated mouse cohorts.
All mouse cohorts were injected subcutaneously (s.c.) with 1 cc of saline daily for the first 2 weeks postirradiation. In addition, mice were treated topically with triple antibiotic ointment, containing 5,000 units of polymyxin B sulfate, 400 units of bacitracin zinc and 3.5 mg of neomycin sulfate and 1% silver sulfadiazine cream to treat radiation dermatitis in accordance with veterinarian recommendations.
Evaluation of Cardiopulmonary Function Parameters
Prior to sacrifice at 2, 4 or 18 weeks postirradiation, pulse oximetry was performed, as previously described elsewhere (39) on conscious mice (n = approximately 5–10 mice per group) using a MouseOX noninvasive vital signs monitor (STARR® Life Sciences Corp., Oakmont, PA). A mouse collar sensor was used to obtain measurements for arterial oxygen saturation (SpO2), pulse distension, respiratory rate and heart rate, as previously described (36). To minimize stress and maintain body temperature, mice were placed on a heating pad. Continuous readings over 3 min were taken from each mouse. Data are reported as mean ± standard error of mean (SEM) after removing occasional readings that reported error codes.
Bronchoalveolar Lavage Fluid Analysis
Mice were euthanized using ketamine (100 mg/ml) and xylazine (20 mg/ml) at 3 and 18 weeks (Figs. 3 and 4, respectively) after thoracic irradiation. Bronchoalveolar lavage (BAL) was then performed as previously described elsewhere (25–27). Briefly, bronchoalveolar lavage fluid (BALF) was obtained using a 20g angiocatheter (BD Pharmingen™, San Diego, CA), with the intratracheal instillation of 1 ml phosphate-buffered saline (PBS) containing an anti-protease cocktail (Sigma-Aldrich® LLC, St. Louis, MO) and 5 mM EDTA given in sequential 0.5 ml increments (25, 26, 40). An aliquot was immediately separated to measure total leukocyte cell counts (cells/ml BALF) using a Coulter Cell and Particle Counter (Beckman Coulter® Inc., Miami, FL). The remaining lavage fluid was centrifuged at 1,200 rpm for 10 min and the cell-free supernatant was frozen at −80°C for cytokine determination and evaluation of oxidative stress. The pelleted total leukocytes were then spun on a Shandon Cytospin-3 cell preparation system (Thermo Electron/Thermo Fisher Scientific™ Inc., Waltham, MA) at 1,500 rpm for 10 min and stained with a standard Diff-Quick® (Hemacolor®) protocol from EM Diagnostic Systems, Inc. (Gibbstown, NJ) as described previously (41). The concentration of BALF neutrophils, macrophages, eosinophils and lymphocytes was also determined. Analysis was performed in a blinded fashion.
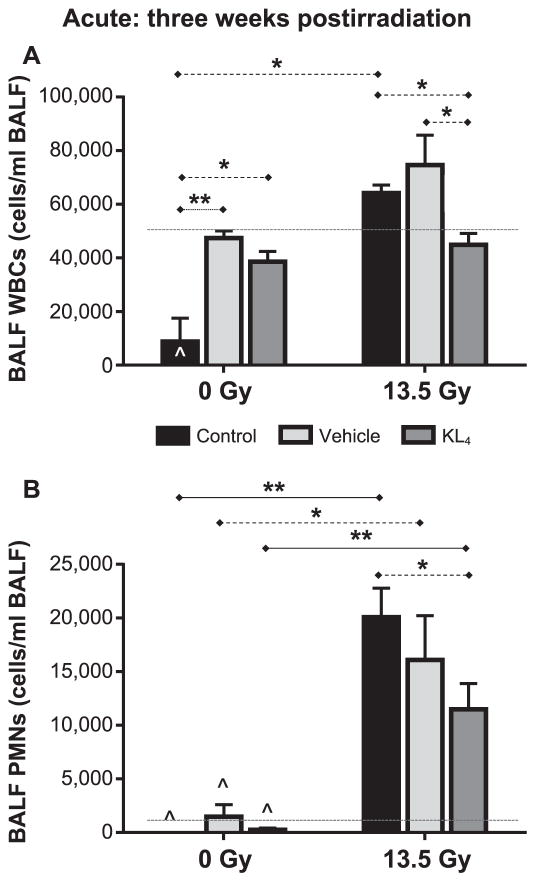
FIG. 3.
Evaluation of lung inflammation in mice at 3 weeks after 13.5 Gy thoracic irradiation. Cell counts in BALF collected at 3 weeks postirradiation (n = 4 for the following treatment groups: control, vehicle, KL4, vehicle + irradiation, KL4 + irradiation; n = 3 for the irradiation group). WBC (panel A) and PMN (panel B) counts are shown. The upper reference ranges in healthy, nonirradiated mice for WBC (50,000/ml) and PMN (1,500/ml), based on our previous observations, are shown as dashed lines. Statistically significant differences in the data are represented graphically (*P < 0.05 as dashed horizontal lines and **P < 0.01 as solid horizontal lines). Data points falling below the lower limit of detection are indicated by the hat symbol “^”.
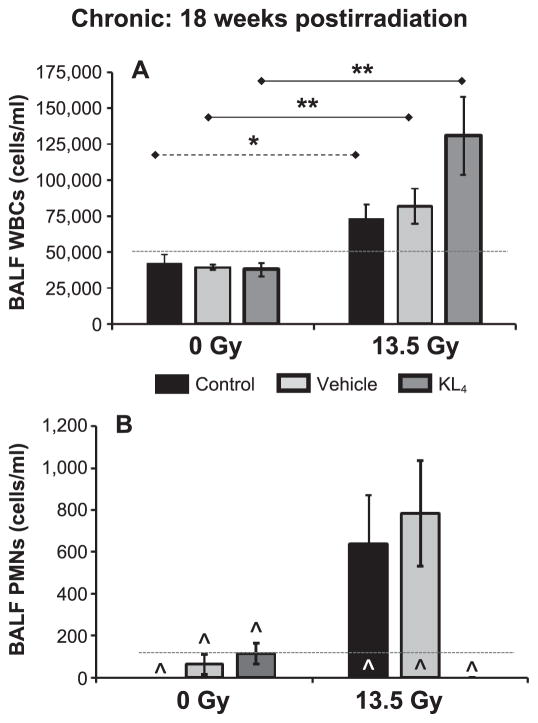
FIG. 4.
Determination of total PMN cells in BALF. Cell counts in BALF collected at 18 weeks postirradiation (n = 5 mice per group). Upper reference range is shown for WBCS, but all PMN data are under this limit (see Fig. 3 legend). Statistically significant differences in the data are represented graphically (*P < 0.05 as dashed horizontal lines and **P < 0.01 as solid horizontal lines). Data points falling below the lower limit of detection are indicated by the hat symbol “^”.
Analysis of 8-Iso-Prostaglandin F2α Levels in Bronchoalveolar Fluid
Levels of 8-iso-prostaglandin F2α (8-IsoP), metabolites of tissue phospholipid oxidation and a biomarker of oxidative stress and antioxidant deficiency in BALF and mouse plasma were determined using an 8-IsoP enzyme-linked immunosorbent assay (ELISA) kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s protocol. The levels of 8-IsoP were determined in BALF and plasma at 18 weeks after thoracic irradiation. BALF and plasma samples were run undiluted and the data are reported as the concentration (pg/ml) of 8-iso-prostaglandin F2α in the BALF and plasma.
Multiplexed Cytokine Analysis of Bronchoalveolar Fluid
Bronchoalveolar lavage fluid (BALF) cytokine concentrations were determined, as previously described (24), using the Mouse Cytokine 20-Plex Panel (Invitrogen™, Carlsbad, CA), according to the manufacturer’s protocol. This multiplex panel permits simultaneous quantification of multiple cytokines in solution by capturing them onto antibody-coated spectrally distinct fluorescent microspheres and measuring fluorescence intensity using the BioPlex 200 (Bio-Rad® Laboratories Inc., Hercules, CA) system. The assay was performed according to the manufacturer’s protocol. All samples were run in duplicate. The detection limit of this kit is in pg/ml for all the included cytokines.
Tissue Harvesting and Histopathological Evaluation of Lung
Radiation experiments were terminated at 18 weeks postirradiation, corresponding to a time point when radiation-induced pulmonary fibrosis is readily detectable in our model using both biochemical assays and histopathological evaluation. For histological studies, the lungs prior to removal from the animal were instilled with 0.75 ml of buffered formalin through a 20g angiocatheter placed in the trachea, immersed in buffered formalin overnight and processed for conventional paraffin histology. Sections were stained with hematoxylin and eosin (H&E) and examined by light microscopy. Paraffin-embedded lungs were sectioned and processed for routine immunohistochemistry as described earlier (27).
Evaluation of radiation pneumonitis/lung injury
Radiation pneumonitis and lung injury score was prepared from evaluation of H&E-stained murine lung sections, and was based on five histological findings: 1. neutrophils in the alveolar space; 2. neutrophils in the interstitial space; 3. hyaline membranes; 4. proteinaceous debris filling the alveolar space; and 5. alveolar septal thickening, graded using a three-tiered schema (grade 0, 1, 2) described by Matute-Bello et al. (42). Instead of normalizing scores to the number of fields evaluated, scores were normalized to the percentage of lung area involved by the injury.
Evaluation of pulmonary fibrosis
Pulmonary fibrosis evaluation was done by scoring Masson’s trichrome-stained lung sections. Briefly, a lung pathologist blinded to treatment groups evaluated lung sections using a scale of 0–4, with 4 signifying maximal fibrosis. A score of 0 = normal lung; 1 = fibrosis involving less than 5% of the lung (predominantly the subpleural areas); scores of 2, 3 or 4 = fibrosis involving 5–20%, 21–50% or >50%, respectively, of lung (involving subpleural and peribroncheal areas).
Collagen content of murine lung tissue was evaluated quantitatively by determining the hydroxyproline content using acid hydrolysis according to Woessner et al. (43). Data are expressed as micrograms of hydroxyproline/whole lung.
Statistical Analyses
Results are presented as mean ± SEM of two independent experiments, both in text and in graphs. Statistical differences among groups were determined using one-way analysis of variance (ANOVA) with GraphPad Prism version 6.00 for Windows (Graph-Pad Software Inc., La Jolla, CA). Any statistically significant differences in the data are represented graphically (*P < 0.05 as dashed horizontal lines and **P < 0.01 as solid horizontal lines). Any data points falling below the lower limit of detection for that particular detection method were set, for convenience, to 0, and specific data groupings having one or more readings less than the lower limit of detection are indicated by the hat symbol “^” shown in the figures.
RESULTS
The potential benefits of the KL4 surfactant for preservation of pulmonary function after irradiation were assessed both at an early phase (2 and 4 weeks) and a later phase (18 weeks) after a single 13.5 Gy dose of targeted thoracic irradiation (Fig. 1). KL4 treatment was compared with both irradiated vehicle (phosphate-buffered saline) and irradiated untreated controls. Pulse oximetry data were collected at 2 weeks postirradiation (immediately after discontinuing twice-daily KL4 treatment) and at 4 weeks postirradiation to assess the influence on mitigating early pneumonitis, as well as prior to sacrifice at 18 weeks postirradiation to assess preservation of lung function and mitigation of late radiation fibrosis (Fig. 1). Samples for all other evaluations were collected after sacrifice at the times indicated.
KL4 Surfactant Improves Arterial Oxygen Saturation
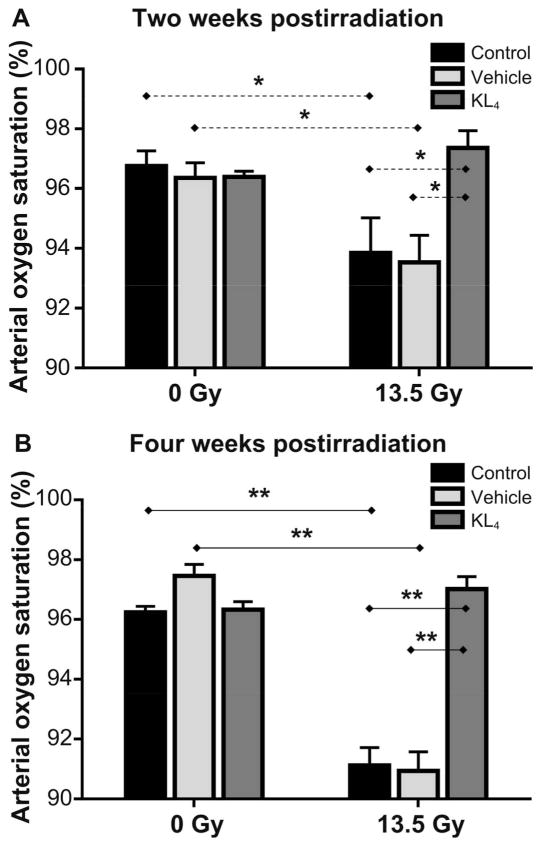
Preservation of pulmonary function in vivo can be assessed from relevant metrics, including arterial oxygen saturation (SpO2), pulse distension, respiratory rate and heart rate, all of which can be gathered with a pulse oximetry system. One of the most direct metrics for therapeutic benefit in preservation of pulmonary function is the level of arterial oxygen saturation during the early postirradiation phase. Not surprisingly, irradiation without any treatment reduces arterial oxygen saturation by an absolute 3% at two weeks postirradiation [from 96.8 ± 0.5% for nonirradiated untreated control mice to 93.8 ± 0.9 for irradiated untreated control mice, P = 0.05 (Fig. 2A)], and similar effects were observed with vehicle-treated mice (96.4 ± 0.5 vs. 93.5 ± 0.9, P = 0.03) showing modest oxygen desaturation. In contrast, at 2 weeks postirradiation, oxygenation levels of the KL4-treated mice remained unchanged (96.4 ± 0.2 for KL4-treated nonirradiated mice vs. 97.4 ± 0.6 for KL4-treated irradiated mice), suggesting a mitigation (sparing) of early lung injury. At 4 weeks postirradiation (Fig. 2B), a more clinically significant desaturation was observed in untreated and vehicle-treated irradiated animals (96.2 ± 0.3 vs. 91.1 ± 0.6, P < 0.001 for untreated controls; 97.5 ± 0.5 vs. 90.9 ± 0.6, P < 0.001 for vehicle), whereas KL4-treated mice showed preservation in lung function (96.3 ± 0.3 for KL4, nonirradiated vs. 97.0 ± 0.4 for KL4, irradiated, P = 0.17).
FIG. 2.
Evaluation of blood oxygenation levels and lung injury in mice at 2 and 4 weeks postirradiation. Pulse oximetry measurements taken at 2 and 4 weeks postirradiation. Panel A: Measurements at 2 weeks postirradiation (n = 5 mice per group), immediately after KL4 treatment was discontinued. Panel B: Measurements at 4 weeks postirradiation [n = 5 for control, n = 10 for vehicle, n = 10 for KL4, n = 10 for irradiated (13.5 Gy), n = 10 for vehicle + irradiation, n = 9 for KL4 + irradiation]. Statistically significant differences in the data are represented graphically (*P < 0.05 as dashed horizontal lines and **P < 0.01 as solid horizontal lines).
Over the long term, arterial oxygen saturation was also significantly preserved compared to irradiated untreated controls and vehicle-treated mice, indicating preservation of lung function and mitigation of late lung damage and impairment.
KL4 Surfactant Reduces Radiation-Induced Lung Inflammation
Total leukocyte [white blood cell (WBC)] counts in BALF, collected immediately after sacrifice, are valuable indicators of the level of the localized lung inflammatory response to radiation damage. In acute radiation syndrome (ARS), it is not surprising to find elevated WBC and polymorphonuclear leukocyte (PMN) levels in the lungs of irradiated mice, as we observed (Fig. 3). KL4-treated mice had statistically significantly lower levels of WBCs in the BALF (45 ± 4 ×103 WBC; Fig. 3A) than the untreated (64 ± 3, P = 0.018) and vehicle-treated (75 ± 11, P = 0.047) irradiated mice. PMN cell levels also trended lower (11.5 ± 2.4 vs. 20.1 ± 2.7 for irradiated KL4-treated vs. irradiated untreated control mice, respectively, P = 0.064; Fig. 3B).
BALF cell analyses were also performed at 18 weeks postirradiation (Fig. 4). In all three paired comparisons, a statistically significant (P < 0.05) increase in WBC count (Fig. 4A) was observed for the irradiated cohorts compared to the nonirradiated cohorts. Although KL4-treated irradiated mice had the highest observed WBC counts (a trend vs. untreated control, P = 0.08), KL4-treated mice had undetectable PMN counts (n = 5; Fig. 4B).
KL4 Surfactant Protected against Radiation-Induced Late Lung Injury
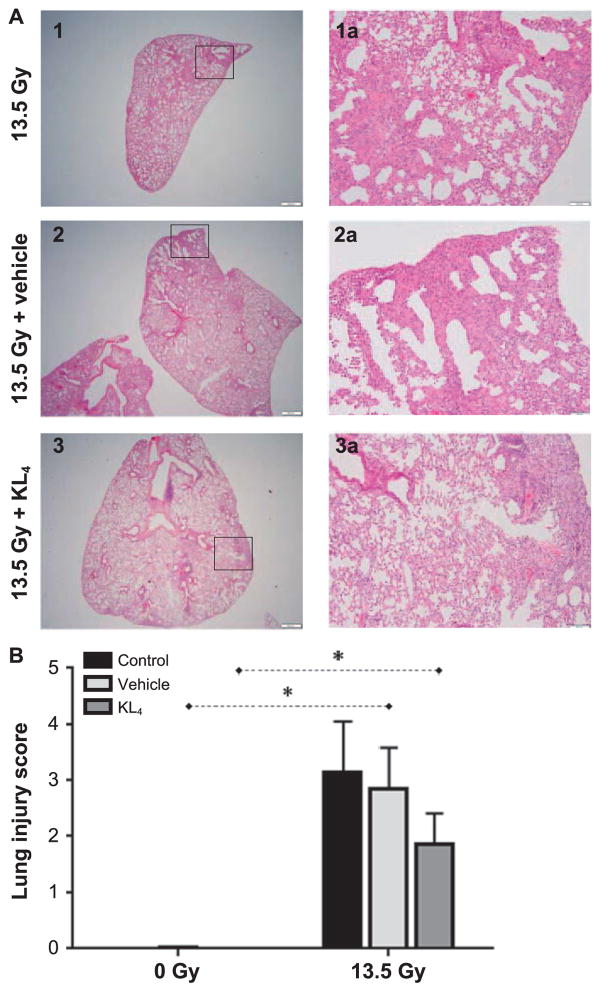
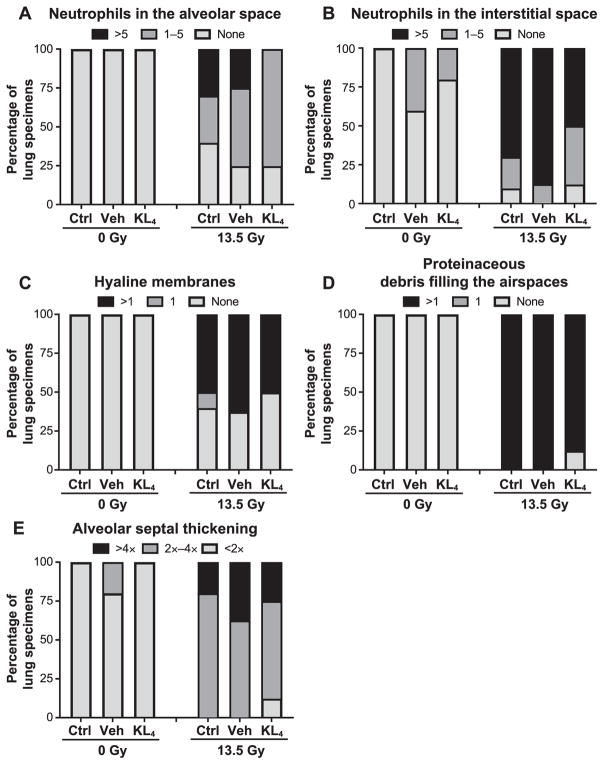
At 18 weeks postirradiation, irradiated cohorts displayed an increased lung injury score determined according to the American Thoracic Society (ATS) scoring system for lung injury (Fig. 5A and B) and is comprised of findings related to the presence of neutrophils in the alveolar space (Fig. 6A), neutrophils in the interstitial space (Fig. 6B), hyaline membranes (Fig. 6C), proteinaceous debris filling the airspaces (Fig. 6D) and alveolar septal thickening (Fig. 6E). Importantly, compared to irradiated untreated control-and vehicle-treated mice, the lung injury score was lower among irradiated mice treated with KL4 (3.14 ± 0.92 and 2.86 ± 0.72 compared to 1.88 ± 0.54, respectively).
FIG. 5.
Determination of lung injury in murine lungs at 18 weeks after 13.5 Gy thoracic irradiation. Severity of pneumonitis derived from histopathology examination of lung tissue at 18 weeks postirradiation (n = 2 for control, n =5 for vehicle, n = 10 for irradiation, n =8 for vehicle + irradiation, n = 5 for KL4, n = 8 for KL4 + irradiation). Representative images (panel A) allow visual comparison of the amount of involved in pneumonitis. Such images were used to quantitate the extent of pneumonitis (panel B). Statistically significant differences in the data are represented graphically (*P < 0.05 as dashed horizontal lines).
FIG. 6.
Assessment of pneumonitis in murine lungs at 18 after 13.5 Gy thoracic irradiation. Severity of pneumonitis derived from histopathology examination of lung tissue at 18 weeks postirradiation [n = 2 for control (Ctrl), n =5 for vehicle (Veh), n = 5 for KL4, n = 10 for irradiation, n =8 for vehicle + irradiation, n =8 for KL4 + irradiation], following the ATS scoring system for lung injury. Panel A: Presence of neutrophils in the alveolar space. Panel B: Neutrophils in the interstitial space. Panel C: Hyaline membranes. Panel D: Proteinaceous debris filling the airspaces. Panel E: Alveolar septal thickening.
Late Fibrosis after a Single Dose of Thoracic Irradiation
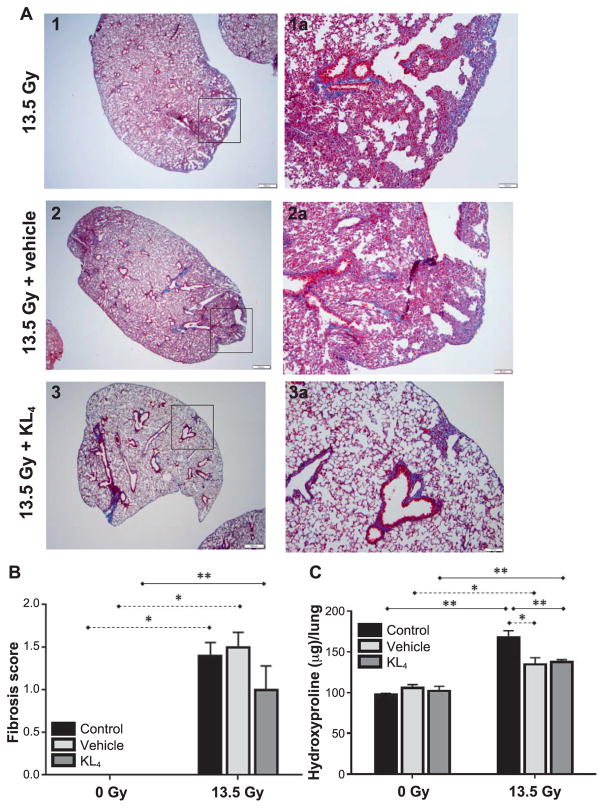
Histopathology of the lung tissue (sacrifice at 18 weeks postirradiation) was performed to assess the degree of late lung injury and subsequent fibrosis induced by radiation, as well as effectiveness of KL4 surfactant in mitigating such effects. The extent of fibrosis was based on assessment of the amount of stained lung tissue on visual inspection of trichrome staining (19) under microscopy (Fig. 7A), as well as a quantitative assessment (Fig. 7B). In the irradiated cohort, fibrosis appears reduced by KL4 surfactant vs. vehicle-treated mice (1.0 ± 0.3, n = 8 vs. 1.5 ± 0.2), although statistical differences are not achieved (P = 0.15), reflecting the wide range in lung fibrosis in the untreated control cohort. Surprisingly, in the vehicle-treated cohort, a reduction in levels of fibrosis over irradiated untreated control mice was also observed, and may reflect some benefit from improved mucus clearance and removal of inflammatory mediates from the lungs with administration of saline into the lungs (see Discussion).
FIG. 7.
Histological evaluation of lung fibrosis at 18 weeks after 13.5 Gy thoracic irradiation. Histopathological evaluation of lung tissue for examination of the extent of both fibrosis and oxidative damage at 18 weeks postirradiation. Panel A: Trichrome and H&E staining allows direct visualization of the extent of fibrosis, with representative images shown. Darker staining indicates more severe fibrosis, with the irradiated but no therapy (image 1) being most intense, slightly reduced by vehicle treatment (image 2) and further reduced by KL4 treatment (image 3). Panel B: Quantitative assessment of fibrosis (n = 2 for control, n =5 for vehicle, n =5 for KL4, n = 10 for irradiation, n = 8 for vehicle + irradiation, n = 8 for KL4 + irradiation). Panel C: Hydroxyproline levels indicate the local extent of oxidative damage (n =2 for control, n =5 for vehicle, n =5 for KL4, n = 9 for irradiation, n = 8 for vehicle + irradiation, n = 7 for KL4 + irradiation). Statistically significant differences in the data are represented graphically (*P < 0.05 as dashed horizontal lines and **P < 0.01 as solid horizontal lines). Data points falling below the lower limit of detection are indicated by the hat symbol “^”.
Similar results can be seen from measurement of hydroxyproline levels (Fig. 7C). In this case, very tight data ranges in all six of the groups leads to strong statistical significance (P < 0.02) for all meaningful pairwise comparisons (all 3 of the 0 Gy vs. 13.5 Gy pairings, as well as KL4 and vehicle compared to untreated controls within the irradiated mice). While we note the potential benefits of the vehicle, the vehicle alone is not sufficient to deliver the significant therapeutic benefit of KL4 for actual functional benefit (arterial oxygenation levels) or reducing inflammation (BALF cell count) as discussed above.
KL4 Surfactant Mitigated Lung Oxidative Tissue Damage and Pro-inflammatory Cytokine Release Induced by a Single Dose of Thoracic Irradiation
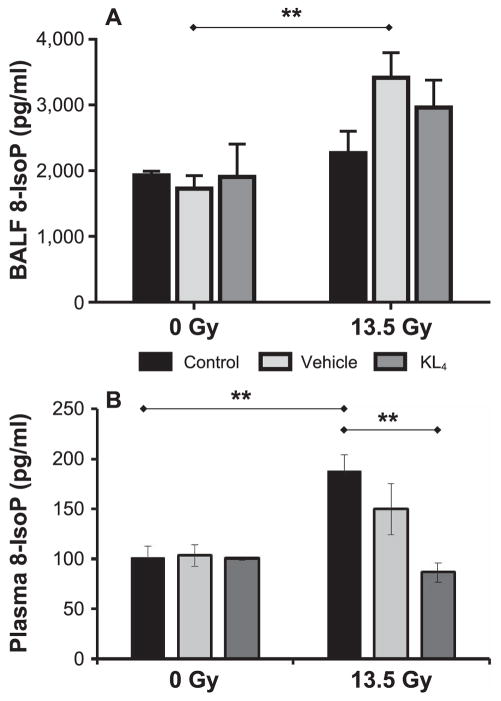
As an indication of the extent of oxidative stress, we measured 8-IsoP in both BALF and plasma (Fig. 8). In BALF, both KL4 and vehicle lead to slight increases in 8-IsoP vs. untreated control in irradiated mice (3.0 ± 0.4 ng/ml for KL4, 3.4 ± 0.4 for vehicle and 2.3 ± 0.3 for untreated control), with the wide range of data reducing statistical significance (untreated control vs. vehicle, P = 0.053; untreated control vs. KL4, P = 0.229). While this represents a counterintuitive effect of the treatment, discussed below, all nonirradiated groups showed similar 8-IsoP levels, indicating that the therapeutic delivery method in itself is not a root cause.
FIG. 8.
Oxidative stress in lung tissues and systemic circulation 18 weeks after 13.5 Gy thoracic irradiation. 8-iso-prostaglandin F2α (8-IsoP) concentrations determined in BALF (n = 5 mice per group) (panel A) and plasma (n = 5 mice per group) (panel B) at 18 weeks postirradiation are a surrogate biomarker for proximal and systemic oxidative stress levels. Statistically significant differences in the data are represented graphically (**P < 0.01 as solid horizontal lines).
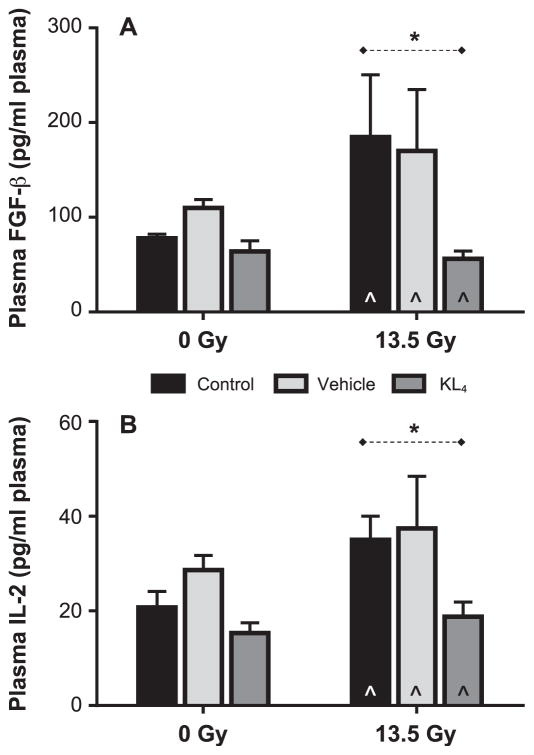
We also evaluated plasma and BALF levels of a panel of cytokines with both early and late phases. Although a panel of 20 cytokines was evaluated, only basic fibroblast growth factor (FGF-β) and interleukin-2 (IL-2) showed a significant change from nonirradiated cohorts in response to radiation. KL4 treatment resulted in clear reduction of plasma levels of these two pro-inflammatory cytokines (FGF-β and IL-2; Fig. 9). In both cases, at 18 weeks postirradiation, the two weeks of KL4 treatment resulted in essentially baseline levels of both cytokines, while untreated control and vehicle-treated irradiated mice both clearly showed increase over baseline. The wide variability in levels is consistent with other reported studies (44). For IL-2, it is notable that only the irradiated untreated control and vehicle-treated mice had levels outside of normal reference ranges (44). In fact, despite the variability in the data, reduction in KL4 vs. untreated control for IL-2 was statistically significant (19 ± 3 pg/ml vs. 35 ± 5, P = 0.02) and KL4 treatment returned IL-2 levels to the normal range.
FIG. 9.
Plasma cytokine levels in mice 18 weeks after 13.5 Gy thoracic irradiation. Plasma levels of basic fibroblast growth factor (FGF-β, panel A) and interleukin-2 (IL-2, panel B) at 18 weeks postirradiation (n =5 for control, n =5 for vehicle, n =5 for KL4, n = 6 for irradiation, n = 7 for vehicle + irradiation, n = 6 for KL4 + irradiation) are surrogate biomarkers that represent systemic proin-flammatory status. Statistically significant differences in the data are represented graphically (*P < 0.05 as dashed horizontal lines). Data points falling below the lower limit of detection are indicated by the hat symbol “^”. (n = 2 for IR (13.5 Gy), n = 1 for vehicle + irradiation, n = + irradiation for FGF-β and n = 4 for irradiation, n =2 for vehicle + irradiation, n = 3 for KL4 irradiation for IL-2).
Interestingly, although KL4 treatment mitigated inflammation, oxidative lung damage and improved oxygenation over the vehicle-treated control, we did not detect a significant survival benefit compared to untreated control, irradiated (P = 0.2358) or vehicle-treated, irradiated (P = 0.2457) mice at the time of sacrifice at 18 weeks postirradiation.
DISCUSSION
Exposure to dangerous levels of ionizing radiation, although a relatively small risk in everyday life, continues to be a persistent source of major concern in public health and biodefense. This is largely based on the relatively recent Fukushima incident, as well as general global unrest amid “dirty bomb” terror threats. Early effects of radiation exposure typically result in acute lung injury with resultant decrease in pulmonary function, which can resolve over time if individuals survive. Chronic lung damage, characterized by pulmonary fibrosis, can contribute to poor survival (4). In this study, we evaluated the therapeutic benefit of a surfactant, KL4, introduced into the lungs after targeted irradiation to assess the effect of this treatment in mitigating the severity of both early and chronic lung tissue damage. Mice were irradiated with a single 13.5 Gy dose (the LD50) followed by KL4 treatments twice daily over 2 weeks starting at 24 h postirradiation. The study included matched cohorts that were untreated or treated with saline (the KL4 delivery vehicle) in both irradiated and nonirradiated mice. Our data show that intranasal delivery of KL4 (as a means to deliver the drug into the lungs as mice are obligate nasal breathers) early after irradiation preserves oxygenation, reduces local lung and systemic inflammation and reduces oxidative injury to lung tissue. Of note, the current study was limited to only female mice due to their known susceptibility to radiation-induced lung damage compared to male mice (32). In future studies, KL4 surfactant will be evaluated in both genders. These data support the potential for KL4 surfactant to be used as a medical countermeasure for mitigation of damage to lungs after severe radiation exposure.
One obvious goal of effective radiotherapy is to preserve normal arterial blood oxygen levels. Indeed, our pulse oximetry data (Fig. 2) shows that intranasal KL4 treatments help maintain physiological levels of arterial oxygen saturation at 2 and 4 weeks postirradiation, as well as in the late phase, when lung damage would result in abnormal lung function and decreased oxygen saturation. Preservation of oxygenation potentially would eliminate the need for respiratory support of exposed individuals, allowing patients to survive through the early phase, and for caregivers to focus efforts toward addressing other effects of acute radiation exposure on other organ systems.
We observed a reduction in lung inflammatory response, suggesting reduced tissue damage, although we consistently saw treatment-induced WBC increase in the nonirradiated mice. We hypothesize that the delivery method, intranasal liquid introduction, might represent a mild, but localized, physiologic insult to the lungs, even for the nonirradiated mice; the observed WBC increase suggests a low-level inflammatory response, but PMN levels remain low, suggesting that the response is localized and mild. With the effect of the vehicle in mind, WBC levels in the nonirradiated, vehicle-treated mice should be used to assess any inflammation-reducing benefits of KL4. Indeed, the WBC counts are similar for KL4-treated mice in the irradiated vs. nonirradiated groups. By contrast, all irradiated mice showed an expected increase in early-phase PMN counts, indicating radiation-induced tissue damage. KL4 treatment reduces PMN count by approximately 50%, suggesting that it was able to mitigate the extent of radiation damage and accordingly, decrease the localized inflammatory response. Vehicle treatment alone may introduce a partial reduction in PMN, suggesting the possibility that the introduction of saline into the lung might have improved mucus clearance of inflammatory mediators and WBCs, but this benefit is only partial (in both WBC and PMN levels) compared to the more significant benefits of the KL4 surfactant, especially on examination for chronic lung damage.
We believe that completely eliminating the inflammatory response is not an achievable therapeutic goal in a real-world accidental or terrorist exposure scenarios, which would produce unpredictable radiation exposure, and treatment would not be available immediately. Indeed, the natural and immediate inflammatory response will likely proceed until therapy can be introduced (24 h postirradiation in our experiments). Moreover, any therapy (KL4 or otherwise) must not reduce the body’s ability to heal such damage. Elevated PMN levels in all groups early after irradiation suggest that a natural response in tissue is occurring as part of the repair process. During the early phase, KL4 treatment results in the lowest PMN counts and during the chronic phase, the KL4-treated group showed undetectable PMN levels in BALF analysis, confirmed by histopathological evaluation of PMN in alveolar spaces in lung sections. As detected in BALF analysis of WBC in late-phase lungs and confirmed by histopathology (interstitial inflammatory cells), KL4 treatment did not decrease overall WBC. This suggests that treatment may either need to be given over a longer duration (i.e., more than just two weeks) or re-administered repeatedly during a later phase of the evolving injury.
Histopathology data further support the potential benefits of KL4 in minimizing chronic lung tissue damage. Specifically, fibrosis and pneumonitis were both lowest in the KL4-treated mice, an indication that later chronic pulmonary injury could be minimized, if not eliminated (a focus of our ongoing research). Although investigating the mechanism of reduced lung damage by KL4 treatment is outside of the scope of this current study per se, the interesting observation of reduced fibrosis and pneumonitis, not only in the nonirradiated KL4- but also in vehicle-treated groups, suggests that intranasally introduced lavage, even with saline, could improve mucus clearance of cytokines and inflammatory mediators that are likely responsible for development of acute lung injury and subsequent late lung damage, as discussed above.
Levels of local (lung tissue) and systemic biochemical markers of inflammation, oxidative stress and tissue damage further support KL4 surfactant benefit. Lung tissue markers included assessment of hydroxyproline levels and staining on histopathology, as well as 8-iso-prostaglandin F2α levels in BALF. Systemic markers included plasma levels of 8-IsoP, FGF-β and IL-2. 8-IsoP is a marker of oxidative stress (45). Hydroxyproline has been used previously as a marker of lung fibrosis. Although known as a pro-inflammatory cytokine, FGF-β has also been associated with normal repair processes in ALI (46), and IL-2 has been correlated with lung injury and disease (47, 48). In our study, KL4 reduced lung hydroxyproline (Fig. 7C), although not down to the nonirradiated cohort levels, suggesting partial mitigation of lung injury. In this pilot study, we did not evaluate higher doses of drug or continued or later treatment with onset of late fibrosis, which we are currently exploring in ongoing experiments. BALF 8-IsoP (Fig. 8A) data are counterintuitive, in that levels are only elevated with KL4 or vehicle, and only in the irradiated mice, which indicates that the delivery of drug (or vehicle) itself is not the cause. This aspect of the data will be evaluated in our future research. Circulating markers of oxidative stress (Figs. 8B and 9) unambiguously indicated KL4 benefits: in all cases, KL4 treatment reduced radiation-elevated levels down to the level of the nonirradiated cohort. Albeit from a limited set of 3 markers, the data indicate that KL4 treatment helped to reduce propagation of the severe localized lung damage into wider (and likely more life threatening) systemic responses.
When inflammation is a hallmark of the underling pathophysiology, such as in radiation-induced lung disease, it is essential to employ a fully functional surfactant that is resistant to inactivation or inhibition. Although exogenous surfactants have been studied extensively for prevention and treatment of multifactorial ALI/acute respiratory distress syndrome with limited success (6), currently available exogenous surfactant replacement therapies, which are of animal origin, have only been approved for treating neonatal respiratory distress syndrome. Treatment with KL4 surfactant, a completely synthetic peptide-based product, avoids the complications observed with animal-derived surfactants, and is thus an ideal candidate MCM for mitigating radiation-induced lung disease. Among KL4’s benefits are: 1. The KL4-peptide (sinapultide) mimics human surfactant protein B, which is essential for alveolar expansion and can serve as a substitute for surfactant protein B if depleted after degradation of the protein or its production with damage of type II pneumocytes (14, 15); 2. It is more resistant to inactivation by plasma proteins and oxidants that would typically be present in the inflamed injured lung (16–19, 49); 3. It modulates key inflammatory processes that accompany lung injury (20, 21, 28); 4. It can be effectively aerosolized (50, 51); and 5. Its chemical composition and properties allow for development of a lyophilized formulation, which could produce a product with extended self-life/storage that could also be rapidly reconstituted for ease of use in the field.
In our model of radiation-induced lung damage, the early phase is just 2–3 weeks postirradiation, where we record neutrophilic influx in lungs and reproducible lung edema/leakiness. Similarly, robust lung fibrosis is observed as early as 16–18 weeks postirradiation (24, 52), which is likely due to the use of younger mice (6–8 weeks old) instead of slightly older mice (10–12 weeks old) in the thorax irradiations. Importantly, thoracic exposure models provide useful and much needed information on partial exposure scenarios where lung only is exposed or when radionuclides are inhaled. Single organ injuries after irradiation comprise the first step to evaluate a potential mitigator such as the one studied here. More complex scenarios such as combined injuries (radiation plus trauma, hemorrhagic shock, burn injury, sepsis or viral infection) (53, 54) or underlying comorbidities such as diabetes, obesity, cardiopathy or viral infections are also valuable but further complicate the interpretation of data. Total-body irradiation (TBI) models (55) provide much more realistic exposure models for developing MCM agents, although, multiorgan injury models are complex. We believe that such a TBI model would be the next step in the validation of KL4 surfactant as an effective MCM agent.
CONCLUSION
The goal in developing a therapy to mitigate radiation-induced damage to the lungs caused by direct exposure or inhalation of dispersed ionizing radiation, is to reduce both early and chronic pulmonary complications [e.g., ARS/delayed effects of acute radiation exposure (DEARE)], ideally doing so in an easily administered form suitable for emergency mass-casualty situations. KL4 surfactant, in its liquid form, demonstrates both short-term and long-term benefits when provided 24 h after exposure to LD50 levels of radiation. This indicates that KL4 has practical utility as a potential therapeutic after irradiation, rather than requiring prophylactic dosing, which would not be feasible or practical in real-world unpredictable exposure scenarios. KL4 treatment could provide early benefits by reducing respiratory distress caused by surfactant dysfunction and increased capillary permeability, alveolar edema and protein leak into the alveolar space. Preservation of normal lung function could substantially reduce the need for intubation and supplemental oxygenation and the early postirradiation treatment burden.
Most important, a reduction in radiation-induced chronic lung injury was observed in this study using KL4 surfactant treatment. Previously published literature suggests that the cytokine cascade plays a likely role in both the early phase of radiation lung injury and subsequent DEARE (10, 39, 56). KL4 surfactant has been shown to reduce cytokine levels. A plausible hypothesis thus exists as to the mechanism of how KL4 surfactant may reduce not only ARS, but also DEARE. Research is ongoing to further evaluate KL4 surfactant as a mitigation agent using an alternate and more practical delivery method, namely aerosol delivery, which could potentially circumvent the need for intratracheal administration of the drug for intrapulmonary delivery.
Acknowledgments
This work was funded in large part by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIH/NIAID award no. R44AI102308 to RS). The contents herein are solely the responsibility of the authors and do not necessarily represent the official views of the NIH/NIAID.
References
- 1.Cerveny TJ. Treatment of internal radionuclide contamination. In: Walker RI, Cerveny TJ, editors. Medical consequences of nuclear war. Washington, D.C: TMM Publications, Office of the Surgeon General; 1988. [Google Scholar]
- 2.Durakovic A. Internal contamination with medically significant radionuclides. In: Conklin JJ, Walker RI, editors. Military radiobiology. Cambridge, MA: Academic Press; 1987. [Google Scholar]
- 3.NCRP Report No. 65. Bethesda, MD: National Council on Radiation Protection and Measurements; 1980. Management of persons accidentally contaminated with radionu-clides. [Google Scholar]
- 4.Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys. 2006;66:1281–93. doi: 10.1016/j.ijrobp.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 5.Notter RH. Lung surfactants: basic science and clinical applications. Boca Raton, FL: CRC Press; 2000. [Google Scholar]
- 6.Willson DF, Notter RH. The future of exogenous surfactant therapy. Respir Care. 2011;56:1369–88. doi: 10.4187/respcare.01306. [DOI] [PubMed] [Google Scholar]
- 7.Rubin P, Shapiro DL, Finklestein JN, Penney DP. The early release of surfactant following lung irradiation of alveolar type II cells. Int J Radiat Oncol Biol Phys. 1980;6:75–7. doi: 10.1016/0360-3016(80)90206-0. [DOI] [PubMed] [Google Scholar]
- 8.Rubin P, Siemann DW, Shapiro DL, Finkelstein JN, Penney DP. Surfactant release as an early measure of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1983;9:1669–73. doi: 10.1016/0360-3016(83)90420-0. [DOI] [PubMed] [Google Scholar]
- 9.Harris RA, Popov KM, Zhao Y, Kedishvili NY, Shimomura Y, Crabb DW. A new family of protein kinases–the mitochondrial protein kinases. Adv Enzyme Regul. 1995;35:147–62. doi: 10.1016/0065-2571(94)00020-4. [DOI] [PubMed] [Google Scholar]
- 10.Movsas B, Raffin TA, Epstein AH, Link CJ., Jr Pulmonary radiation injury. Chest. 1997;111:1061–76. doi: 10.1378/chest.111.4.1061. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro DL, Finkelstein JN, Penney DP, Siemann DW, Rubin P. Sequential effects of irradiation on the pulmonary surfactant system. Int J Radiat Oncol Biol Phys. 1982;8:879–82. doi: 10.1016/0360-3016(82)90092-x. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro DL, Finkelstein JN, Rubin P, Penney DP, Siemann DW. Radiation induced secretion of surfactant from cell cultures of type II pneumocytes: an in vitro model of radiation toxicity. Int J Radiat Oncol Biol Phys. 1984;10:375–8. doi: 10.1016/0360-3016(84)90057-9. [DOI] [PubMed] [Google Scholar]
- 13.Hallman M, Maasilta P, Kivisaari L, Mattson K. Changes in surfactant in bronchoalveolar lavage fluid after hemithorax irradiation in patients with mesothelioma. Am Rev Respir Dis. 1990;141:998–1005. doi: 10.1164/ajrccm/141.4_Pt_1.998. [DOI] [PubMed] [Google Scholar]
- 14.Cochrane CG, Revak SD. Pulmonary surfactant protein B (SP-B): structure-function relationships. Science. 1991;254:566–8. doi: 10.1126/science.1948032. [DOI] [PubMed] [Google Scholar]
- 15.Revak SD, Merritt TA, Hallman M, Heldt G, La Polla RJ, Hoey K, et al. The use of synthetic peptides in the formation of biophysically and biologically active pulmonary surfactants. Pediatr Res. 1991;29:460–5. doi: 10.1203/00006450-199105010-00010. [DOI] [PubMed] [Google Scholar]
- 16.Amirkhanian JD, Merritt TA. Inhibitory effects of oxyradicals on surfactant function: utilizing in vitro Fenton reaction. Lung. 1998;176:63–72. doi: 10.1007/pl00007592. [DOI] [PubMed] [Google Scholar]
- 17.Manalo E, Merritt TA, Kheiter A, Amirkhanian J, Cochrane C. Comparative effects of some serum components and proteolytic products of fibrinogen on surface tension-lowering abilities of beractant and a synthetic peptide containing surfactant KL4. Pediatr Res. 1996;39:947–52. doi: 10.1203/00006450-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Manalo E, Merritt TA, Amirkhanian JD, Kheiter A. Characterization of surfactant subtypes of beractant and a synthetic peptide containing surfactant KL4 following surface area cycling and addition of fibrinogen. Lung. 1997;175:225–33. doi: 10.1007/pl00007569. [DOI] [PubMed] [Google Scholar]
- 19.Merritt TA, Amirkhanian JD, Helbock H, Halliwell B, Cross CE. Reduction of the surface-tension-lowering ability of surfactant after exposure to hypochlorous acid. Biochem J. 1993;295(Pt 1):19–22. doi: 10.1042/bj2950019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahuja A, Oh N, Chao W, Spragg RG, Smith RM. Inhibition of the human neutrophil respiratory burst by native and synthetic surfactant. Am J Respir Cell Mol Biol. 1996;14:496–503. doi: 10.1165/ajrcmb.14.5.8624255. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, Miller TL, Chidekel A, Shaffer TH. KL4-surfactant (Lucinactant) protects human airway epithelium from hyperoxia. Pediatr Res. 2008;64:154–8. doi: 10.1203/PDR.0b013e318175dd14. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann AM, Roberts KD, Lampland AL, Meyers PA, Worwa CT, Plumm B, et al. Improved gas exchange and survival after KL-4 surfactant in newborn pigs with severe acute lung injury. Pediatr Pulmonol. 2010;45:782–8. doi: 10.1002/ppul.21252. [DOI] [PubMed] [Google Scholar]
- 23.Ramstedt U, Serhan CN, Nicolaou KC, Webber SE, Wigzell H, Samuelsson B. Lipoxin A-induced inhibition of human natural killer cell cytotoxicity: studies on stereospecificity of inhibition and mode of action. J Immunol. 1987;138:266–70. [PubMed] [Google Scholar]
- 24.Thomas JA, AD, Benitez Quintana Bosch MA, Coll De Pena A, Aguilera E, Coulibaly A, et al. Identification of essential genes in the salmonella phage SPN3US reveals novel insights into giant phage head structure and assembly. J Virol. 2016;90:10284–98. doi: 10.1128/JVI.01492-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JC, Kinniry PA, Arguiri E, Serota M, Kanterakis S, Chatterjee S, et al. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat Res. 2010;173:590–601. doi: 10.1667/RR1522.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JC, Krochak R, Blouin A, Kanterakis S, Chatterjee S, Arguiri E, et al. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol Ther. 2009;8:47–53. doi: 10.4161/cbt.8.1.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machtay M, Scherpereel A, Santiago J, Lee J, McDonough J, Kinniry P, et al. Systemic polyethylene glycol-modified (PEGy-lated) superoxide dismutase and catalase mixture attenuates radiation pulmonary fibrosis in the C57/bl6 mouse. Radiother Oncol. 2006;81:196–205. doi: 10.1016/j.radonc.2006.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinniry P, Pick J, Stephens S, Jain D, Solomides CC, Niven R, et al. KL4-surfactant prevents hyperoxic and LPS-induced lung injury in mice. Pediatr Pulmonol. 2006;41:916–28. doi: 10.1002/ppul.20468. [DOI] [PubMed] [Google Scholar]
- 29.Babu US, Wiesenfeld PW, Collins TF, Sprando R, Flynn TJ, Black T, et al. Impact of high flaxseed diet on mitogen-induced proliferation, IL-2 production, cell subsets and fatty acid composition of spleen cells from pregnant and F1 generation Sprague-Dawley rats. Food Chem Toxicol. 2003;41:905–15. doi: 10.1016/s0278-6915(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 30.Sharplin J, Franko AJ. A quantitative histological study of strain-dependent differences in the effects of irradiation on mouse lung during the intermediate and late phases. Radiat Res. 1989;119:15–31. [PubMed] [Google Scholar]
- 31.Sharplin J, Franko AJ. A quantitative histological study of strain-dependent differences in the effects of irradiation on mouse lung during the early phase. Radiat Res. 1989;119:1–14. [PubMed] [Google Scholar]
- 32.Das SK, Chen S, Deasy JO, Zhou S, Yin FF, Marks LB. Combining multiple models to generate consensus: application to radiation-induced pneumonitis prediction. Med Phys. 2008;35:5098–109. doi: 10.1118/1.2996012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dabjan MB, Buck CM, Jackson IL, Vujaskovic Z, Marples B, Down JD. A survey of changing trends in modelling radiation lung injury in mice: bringing out the good, the bad, and the uncertain. Lab Invest. 2016;96:936–49. doi: 10.1038/labinvest.2016.76. [DOI] [PubMed] [Google Scholar]
- 34.Suresh K. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4:8–11. doi: 10.4103/0974-1208.82352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Southam DS, Dolovich M, O’Byrne PM, Inman MD. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol. 2002;282:L833–9. doi: 10.1152/ajplung.00173.2001. [DOI] [PubMed] [Google Scholar]
- 36.Pietrofesa R, Turowski J, Tyagi S, Dukes F, Arguiri E, Busch TM, et al. Radiation mitigating properties of the lignan component in flaxseed. BMC Cancer. 2013;13:179. doi: 10.1186/1471-2407-13-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christofidou-Solomidou M, Tyagi S, Tan KS, Hagan S, Pietrofesa R, Dukes F, et al. Dietary flaxseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice. BMC Cancer. 2011;11:269. doi: 10.1186/1471-2407-11-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inga A, Storici F, Darden TA, Resnick MA. Differential transactivation by the p53 transcription factor is highly dependent on p53 level and promoter target sequence. Mol Cell Biol. 2002;22:8612–25. doi: 10.1128/MCB.22.24.8612-8625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christofidou-Solomidou M, Tyagi S, Pietrofesa R, Dukes F, Arguiri E, Turowski J, et al. Radioprotective role in lung of the flaxseed lignan complex enriched in the phenolic secoisolariciresinol diglucoside (SDG) Radiat Res. 2012;178:568–80. doi: 10.1667/RR2980.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JC, Bhora F, Sun J, Cheng G, Arguiri E, Solomides CC, et al. Dietary flaxseed enhances antioxidant defenses and is protective in a mouse model of lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2008;294:L255–65. doi: 10.1152/ajplung.00138.2007. [DOI] [PubMed] [Google Scholar]
- 41.Pietrofesa RA, Velalopoulou A, Arguiri E, Menges CW, Testa JR, Hwang WT, et al. Flaxseed lignans enriched in secoisolariciresinol diglucoside prevent acute asbestos-induced peritoneal inflammation in mice. Carcinogenesis. 2016;37:177–87. doi: 10.1093/carcin/bgv174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44:725–38. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 44.Stenina MA, Krivov LI, Voevodin DA, Savchuk VI, Kovalchuk LV, Yarygin VN. Cytokine profile of the blood in mice with normal and abnormal heart rhythm. Bull Exp Biol Med. 2012;152:692–5. doi: 10.1007/s10517-012-1608-9. [DOI] [PubMed] [Google Scholar]
- 45.Lim PS, Chang YM, Thien LM, Wang NP, Yang CC, Chen TT, et al. 8-iso-prostaglandin F2alpha as a useful clinical biomarker of oxidative stress in ESRD patients. Blood Purif. 2002;20:537–42. doi: 10.1159/000066962. [DOI] [PubMed] [Google Scholar]
- 46.Cross LJ, Matthay MA. Biomarkers in acute lung injury: insights into the pathogenesis of acute lung injury. Crit Care Clin. 2011;27:355–77. doi: 10.1016/j.ccc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vitale J, Convers KD, Goretzke S, Guzman M, Noyes B, Parkar N, et al. Serum IL-12 and soluble IL-2 receptor levels as possible biomarkers of granulomatous and lymphocytic interstitial lung disease in common variable immunodeficiency: a case report. J Allergy Clin Immunol Pract. 2015;3:273–6. doi: 10.1016/j.jaip.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 48.Barnett N, Ware LB. Biomarkers in acute lung injury–marking forward progress. Crit Care Clin. 2011;27:661–83. doi: 10.1016/j.ccc.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersson S, Kheiter A, Merritt TA. Oxidative inactivation of surfactants. Lung. 1999;177:179–89. doi: 10.1007/pl00007639. [DOI] [PubMed] [Google Scholar]
- 50.Finer NN, Merritt TA, Bernstein G, Job L, Mazela J, Segal R. An open label, pilot study of Aerosurf(R) combined with nCPAP to prevent RDS in preterm neonates. J Aerosol Med Pulm Drug Deliv. 2010;23:303–9. doi: 10.1089/jamp.2009.0758. [DOI] [PubMed] [Google Scholar]
- 51.Wolfson MR, Malone DJ, Wu J, Gregory T, Mazela J, Shaffer TH. Aerosurf(TM) reduces lung inflammation, improves lung mechanics and preserves lung histomorphology in spontaneously breathing CPAP-supported preterm lambs (Poster symposia. Neonatal respiratory) Arch Dis Child. 2008;93(Suppl 2):69. [Google Scholar]
- 52.Kaminski MD, Lee SD, Magnuson M. Wide-area decontamination in an urban environment after radiological dispersion: A review and perspectives. J Hazard Mater. 2016;305:67–86. doi: 10.1016/j.jhazmat.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 53.DiCarlo AL, Ramakrishnan N, Hatchett RJ. Radiation combined injury: overview of NIAID research. Health Phys. 2010;98:863–7. doi: 10.1097/HP.0b013e3181a6ee32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DiCarlo AL, Hatchett RJ, Kaminski JM, Ledney GD, Pellmar TC, Okunieff P, et al. Medical countermeasures for radiation combined injury: radiation with burn, blast, trauma and/or sepsis. Radiat Res; Report of an NIAID Workshop; March 26–27, 2007; 2008. pp. 712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Medhora M, Gao F, Wu Q, Molthen RC, Jacobs ER, Moulder JE, et al. Model development and use of ACE inhibitors for preclinical mitigation of radiation-induced injury to multiple organs. Radiat Res. 2014;182:545–55. doi: 10.1667/RR13425.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morgan GW, Breit SN. Radiation and the lung: a reevaluation of the mechanisms mediating pulmonary injury. Int J Radiat Oncol Biol Phys. 1995;31:361–9. doi: 10.1016/0360-3016(94)00477-3. [DOI] [PubMed] [Google Scholar]