Abstract
Objective
To evaluate if self-efficacy and financial incentives mediate the effect of health behavior on weight-loss in a group of overweight and obese nursing-home employees participating in a 16-week weight-loss intervention with 12 week follow-up.
Methods
99 overweight/obese (BMI>25) employees from four nursing-homes participated, with a mean age of 46.98 years and BMI of 35.33. Nursing-homes were randomized to receiving an incentive-based intervention (n=51) and no incentive (n=48). Participants’ health behaviors and eating and exercise self-efficacy were assessed at week 1, 16, and 28 using a self-reported questionnaire. Mediation and moderated mediation analysis assessed relationships among these variables.
Results
Eating self-efficacy and Exercise self-efficacy were significant mediators between health behaviors and weight-loss (p<0.05). Incentives significantly moderated the effects of self-efficacy (p=0.00) on weight-loss.
Conclusions
Self-efficacy and financial incentives may affect weight-loss and play a role in weight-loss interventions.
Introduction
Rising rates of obesity continues to be a public health crisis, currently affecting over one-third of American adults.1 Obesity is associated with chronic diseases including Type 2 Diabetes, cardiovascular disease and hypertension,2 and is currently a leading cause of morbidity and mortality in the U.S.1
It is well-established that regular engagement in physical activity can combat obesity, however the majority of the U.S. population is sedentary. Less than 5% of U.S. adults acquire the recommended amount of physical activity to maintain health.3 Despite widespread efforts to educate and encourage individuals to practice in healthy behaviors, these recommendations are not often heeded. Poor diet quality and physical inactivity are predictive of obesity and account for as much as 40% of premature deaths in the U.S.4
Human behavior remains the largest source of variances in health-related outcomes,5 warranting it a major area of interest in fighting obesity. Behavior change is complex and is influenced by a wide array of factors including physiological, psychological, environmental, and socioeconomic factors. Due to the complexity of variables involved in behavior, behavioral change is difficult to implement and sustain. Consequently, relapse is high in obesity interventions and typically most of the weight loss is regained within 6–18 months.6
Obesity and its comorbidities significantly drive healthcare spending. Obesity-related medical costs were estimated to have reached 146 billion dollars annually in 2008, accounting for 10% of all medical spending.7 If the obesity trends continue, it is estimated to reach 16–18% of all US healthcare expenditures annually by 2030.7 This include direct medical costs as well as indirect costs associated with absenteeism, disability, illness and premature death. With employers paying a significant share of these costs, there is growing interest in workplace wellness programs to improve employee health and workforce productivity, while lowering costs. A 2010 meta-analysis concluded that for every dollar spent on wellness programs, medical costs fall by approximately $3.27 and absenteeism costs fall by about $2.73.8 As workplaces have become more aware of how health affects efficiency and productivity through reductions in absenteeism and weight-related chronic conditions, the prevalence of workplace behavior-change interventions continues to increase.8
Workplace Weight-Loss Interventions for Nursing Home Employees
According to the Centers for Disease Control (CDC)’s National Nursing-Home Survey, there were 1.7 million nursing home beds in the United States in 2004.9 A total of 936,000 persons (registered nurses, licensed practical nurses, certified nursing assistants, nurse’s aides, and orderlies) provided nursing care to nursing-home residents.9 These facilities operate 24 hours a day, 365 days a year and often schedule employees to 12 hour shifts, instead of the typical 8 hours which is a common practice in other workplaces. To provide care for these patients, nursing-home employees are frequently on their feet during much of the workday, physically helping patients with activities of daily living. Strenuous physical effort and psychosocial strain is common among nursing-home employees. Despite the physical demands that nursing-home employees face while working, as a population, they are still at an overall heightened risk of being overweight and obese. A cross-sectional study conducted my Miranda et al published in 2015 analyzed associations between workplace stressors and health-related outcomes in nursing home employees. Of 1506 respondents, 20% reported having at least 3 physical workplace stressors, which were strongly associated with obesity and physical inactivity.10 Due to the demographic, social, and workforce characteristics of nursing home employees, this population is at increased risk for obesity and therefore the target of the present weight loss intervention. How to best encourage sedentary overweight individuals to be more confident and motivated to engage in health-promoting activities remains a challenge.
Self-Efficacy (SE)
The Social Cognitive Theory states that behavior is a function of past experiences, rewards/reinforcements, and expectations, and that the behavioral change learning process embodies a dynamic relationship between the individual, their physical environment and their behavior.11 Self-efficacy, referring to an individual’s perception of their ability to perform a behavior, has been shown to be one of the most powerful predictors of health behavior.12 Individuals with a greater self-efficacy are believed to have a stronger intention or motivation to act, put forth greater effort to achieve what they set out to do, and are able to overcome barriers.11
Self-efficacy has also been shown to be a key determinant and promising mediator of health related behaviors, such as dietary intake and physical activity.13–15 Additionally, higher weight-loss self-efficacy has been associated with better adherence and improved outcomes such as a greater likelihood to lose weight.16 Self-efficacy is a main predictor of physical activity maintenance and mediator of short-term weight control, dietary intake and physical activity, and appears to be a potentially effective strategy for promoting weight loss.17
Financial Incentives (FI)
Behavioral economics has also emerged as a potentially effective strategy for behavior modification, particularly for short-term weight-loss.18–21 FI provide people with immediate and tangible feedback that helps make it easier for them to do in the short term what is in their long-term best interest.18 FI have been associated with positive effects on promoting exercise initiation and adherence in previously sedentary adults.3 FI has also been linked to positive effects on healthy food purchases, consumption, and dietary behavior modification in short-term interventions.19, 22 Modest FI in workplace weight loss interventions have been shown to motivate overweight employees to lose weight23, improve health outcomes (weight loss) in the short-term, and incite some lifestyle and behavior modification.18 Additionally, FI have been linked to increased engagement and adherence to healthful behavioral change, perhaps even after the incentive is withdrawn,3,21 which may further lead to improved SE.18
However, some previous efforts to use FI for weight loss have resulted in substantial weight regain after the FI ceased.19 Despite widespread implementation of FI-based workplace wellness policies, the effects of FI on exercise or healthy diet initiation and maintenance and behavior change in adults remain unclear due to mixed findings. While most report FI to be effective, these results tend to be short lived,18, 22 and there is little evidence indicating FI lead to sustained weight loss maintenance.20 FI remain to be a potentially useful tool in aiding behavior modification, but further investigation is warranted.
In summary, a financial incentives approach is based on the idea that individual’s behavior is externally motivated, and that to change behavior, an external and tangible motivator will prompt the person to perform the recommended activity. Presently, there is controversy regarding what type of motivator works better (internal motivator such as self-efficacy or external motivator such as FI). Some suggest that if external motivators are used for behavior change, it may diminish the internal motivation in the longer-term. Some recommend using financial incentive to start a behavior change and as soon as the behavior is adopted, to then emphasize the internal motivators. Due to the detrimental health consequences of obesity, it is essential to determine which factors contribute to positive and/or sustainable behavior modifications and to identify effective strategies to treat and manage the obesity epidemic. Self-efficacy and FI appears to be promising factors associated with promoting behavior change and may facilitate making healthier lifestyle choices and weight loss.
Purpose
The primary purpose of the present study was to evaluate the effects of perceived self-efficacy and financial incentives on diet and exercise behaviors, weight loss, and weight maintenance in individuals with overweight and obesity participating in a 16-week workplace-based weight loss program with a 12-week follow-up. We tested the following hypotheses:
Hypothesis 1: Healthier eating behavior (as indicated by higher healthy eating score (HES)) at 16 weeks will be associated with higher Eat-SE at 16 weeks and greater weight loss from baseline to 16 weeks and incentives will moderate this relationship.
Hypothesis 2: Higher frequencies of mild, moderate and vigorous PA at 16 weeks will be associated with higher Ex-SE at 16 weeks and lower BMI at 16 weeks and incentives will moderate this relationship.
Hypothesis 3: Higher frequencies of mild, moderate and vigorous PA at 16 weeks will be associated with higher Ex-SE at 16 weeks and greater weight loss from 16 to 28 weeks and incentives will moderate this relationship.
Hypothesis 4: Higher frequencies of PA and higher HES at 28 weeks will predict higher SE at 28 weeks, which will further predict lower BMI at 28 weeks.
Methods
Design
This study was a randomized cluster design weight-loss intervention. Four nursing home facilities in the Northeastern United States with comparable size and characteristics were randomly assigned to incentivized participants (IP) (two nursing homes) or non-incentivized participants (NIP) (two nursing homes). Fifty-one employees participated as IP, and forty-eight participated as NIP. This was a 16-week workplace-based weight loss intervention with a 3-month follow-up (total program length was 28 weeks).
Prior to the beginning of the program, all participants received a personalized weight-loss consultation based on their reported physical activity habits and dietary preferences. This was meant to encourage each participant to adopt physical activities they enjoy, as well as identify their support system and to address barriers to their weight loss. Each participant received an action plan based on the National Diabetes Prevention Program (NDPP), which included diet and activity tracker encouraging them to reflect on their lifestyle and how they wish to improve it. It also provided information on safe weight loss, goal setting, healthy eating and increasing physical activity.24,25
Healthy weekly weight loss goals were set during this initial consultation, which consisted of losing 1 pound per week for those with a BMI between 25–30 kg/m2 and losing 1.5 pounds per week for those in obese category (BMI>30 kg/m2). Participants who met the total weight loss goal at the end of the intervention (week 16) were encouraged to continue losing weight and/or maintain their weight loss. IP were rewarded ten dollars per 1 pound or pound and half of weight loss during the 16-week intervention. For those who met their weight loss goal, this amounted to a total possible amount of 160 dollars, which was awarded at the end of intervention. Participants who met their weight loss goal and maintained the loss through the follow-up period (12 weeks) were then awarded an additional 100 dollars, for a maximum payment of 260 dollars. Trained health educators measured participants’ height and weight to calculate BMI.
Participants
Ninety-nine full or part-time employees of four long-term care facilities were screened to participate in the study. Participants were all individuals with overweight or obesity (BMI>25 kg/m2) who were at risk for Type 2 Diabetes, based on the CDC Diabetes risk score >8, indicating a high risk for diabetes.26 Participants had to be at least 18 years of age, but could be of any race, gender, education level, or salary level. Exclusion criteria included having any current or past history of heart disease, stroke, Type 1 Diabetes, or receiving radiation or chemotherapy for cancer treatment in past 5 years. Participants currently pregnant or lactating, taking weight loss supplements, who had lost 20 or more pounds in the last 6 months, or were planning to undergo weight loss surgery during the duration of the study were also excluded. All participants signed an informed consent form approved by the University Institutional Review Board (IRB) prior to data collection.
Questionnaire
Participants completed a self-reported questionnaire on their demographic information, and answered questions regarding their health, health-related behaviors and self-efficacy. Sum scores were generated for healthy eating score (HES), exercise self-efficacy (Ex-SE) and eating self-efficacy (Eat-SE).27,28 These scores were used to further assess the individuals’ lifestyle choices and health-related behavior patterns, including dietary intake and physical activity, as well as self-efficacy involving eating and exercise activities.
Measures
Body Mass Index (BMI)
Trained health educators measured height and weight. A calibrated Seca 700 physician balance beam scale was used to measure weight to the nearest 0.1 kg and height was measured the nearest mm. BMI was calculated as weight in kilograms divided by height in meters squared, and categorized based on CDC recommendations of overweight (25–29.99 kg/m2), obese class I (30–34.99 kg/m2), obese class II (35–39.99 kg/m2), and (> 40 kg/m2).1
The frequency-based Healthy Eating Score (HES) asked respondents to answer how often they consume certain unhealthy or healthy foods on a 4-point Likert scale. The Likert scale ranged from “never to <1 time/week (1), 1–4 times/week” (2), “5–7 times/week” (3), “2 times/day” (4). The scores were reverse coded when needed. A global scale was then generated with the highest possible score of 36 (9×4).29
Eating self-efficacy (Eat-SE), originally the Weight-loss Self-Efficacy Scale (WLSE) developed by Clark et al. (1991),29 was defined in terms of a summary score consisting of 20 questions, which asked participants to rate their confidence that they could motivate themselves to resist eating in certain situations, consistently, for at least six months. Rating was performed using a 4 point Likert-type scale ranging from “not confident” (1) to “somewhat confident” (2), “moderately confident” (3), and “very confident” (4). The situational factors consisted of: Negative Emotions (ex: eating when anxious/sad), Availability (ex: eating when food is readily available, such as at a party), Social Pressure (ex: eating food when others encourage eating), Physical Discomfort (eating when in pain or fatigued), and Positive Activities (ex: eating while watching television). The scale provides one global scale with the highest possible score of 80 (20×4).28
The frequency-based Physical Activity Scores were defined using three questions, which inquired how often participants engaged in mild, moderate, or vigorous physical activity for a 30-minute duration during a typical 7-day week. Individuals were provided with intervals of days for responses, including: 0 days, 1–2 days, 3–4 days, or 5 days or more.24,25
The confidence-based Exercise Self-Efficacy Score (Ex-SE) was defined in terms of a summary score consisting of 11 questions, which asked participants to rate their confidence that they could motivate themselves to keep up with certain exercise behaviors and activities, consistently, for at least six months. Rating was performed using a 4 point Likert-type scale, ranging from “not confident” (1) to “somewhat confident” (2), “moderately confident” (3), and “very confident” (4). The scale provides one global scale with the highest possible score of 44 (11×4).28
Data Analysis
To analyze descriptive statistics, frequency and means tests were run among different variables of the sample population using the Statistical Analysis Software program (SAS). These variables include gender, age, anthropometric measurements, race, and highest level of education. Descriptive results were compiled and analyzed to assess overall population health, participant characteristics, distributions of variables of interest (self-efficacy, stage of change, barriers to physical activity) and to compare ratios such as gender of participants and classes of obesity prior to and post-intervention. Also assessed were the general health of the population and the prevalence of chronic disease and conditions, such as hypertension, high cholesterol, and Diabetes. BMI normality was tested in SAS.
Latent variables were created for HES, Eat-SE and Ex-SE using sum scores, where higher scores were indicative of a more healthful diet and higher perceived SE. Missing values in the dataset were imputed using the Multivariate Imputation by Chained Equations package in R. Pearson’s bivariate correlation tests were run in the Statistical Package for Social Sciences. Mediation models and bootstrapping were run in the statistical program R Studio using the Mediation package.
Results
Descriptive Statistics
Demographic and anthropometric data are presented in Table 1. Majority of participants were obese, middle-aged, white or black females with at least 12 years’ education (equivalent to a high school diploma). BMI had a slightly non-normal distribution, as expected due to all participants being overweight or obese. There were no significant differences in characteristics between groups other than initial body weight, which was higher in IP (p=0.03). Comparisons between groups’ health behaviors and self-efficacy are presented in Table 2. Overall, IP lost an average of 5.05 pounds more than NIP (p=0.027), and reduced their BMI by an average of 1.73 kg/m2 more than NIP (p=0.043) at week 16. At week 28, IP lost an average of 5.17 pounds more than NIP (p=0.053), and reduced their BMI by an average of 1.05 kg/m2 more than NIP (p=0.308).
Table 1.
Demographic & Anthropometric Variables (n=99)
| Gender | Male | 9.09% (n=9) |
| Female | 88.89% (n=88) | |
| Age | Years ± SD | 46.98 ± 11.36 |
| Anthropometrics | Weight (lbs) ± SD | 203.84 ± 40.93 |
| BMI ± SD | 35.33 ± 6.91 | |
| Weight Classification | Overweight | 19.79% |
| Obese | 34.38% | |
| Severe Obesity | 22.92% | |
| Morbid Obesity | 17.71% | |
| Super Obesity | 5.21% | |
| Race | White | 48.48% |
| Black | 40.00% | |
| Hispanic | 5.05% | |
| Asian | 3.03% | |
| American Indian | 1.01% | |
| Education | 10 yrs (high school/secondary) | 4.12% |
| 11 yrs (high school/secondary) | 3.09% | |
| 12 yrs (high school/secondary) | 40.21% | |
| 13 yrs (college/professional) | 12.37% | |
| 14 yrs (college/professional) | 12.37% | |
| 15 yrs (college/professional) | 10.31% | |
| 16 yrs (college/professional) | 10.31% | |
| 17 (post-graduate) | 7.22% |
Table 2.
Comparisons between IP vs. NIP on PA, HES and SE at 1, 16, and 28 weeks
| Baseline | 16 weeks | 28 weeks | ||||
|---|---|---|---|---|---|---|
| IP | NIP | IP | NIP | |||
| Physical Activity | Mild | |||||
| 0 days | 24% | 0% | 0% | 0% | 0% | |
| 1–2 days | 36% | 33% | 35% | 55% | 58% | |
| 3–4 days | 26% | 67% | 65% | 45% | 42% | |
| >5 days | 11% | 0% | 0% | 0% | 0% | |
| Moderate | ||||||
| 0 days | 31% | 0% | 0% | 0% | 0% | |
| 1–2 days | 39% | 37% | 42% | 75% | 65% | |
| 3–4 days | 19% | 63% | 58% | 26% | 35% | |
| >5 days | 3% | 0% | 0% | 0% | 0% | |
| Vigorous | ||||||
| 0 days | 65% | 0% | 0% | 0% | 0% | |
| 1–2 days | 18% | 71% | 73% | 78% | 77% | |
| 3–4 days | 9% | 29% | 27% | 22% | 23% | |
| >5 days | 1% | 0% | 0% | 0% | 0% | |
| HES | <20 | 10% | 6% | 0% | 6% | 6% |
| 20–25 | 60% | 45% | 46% | 53% | 46% | |
| 26–30 | 26% | 20% | 29% | 33% | 33% | |
| Over 30 | 5% | 29% | 25% | 8% | 15% | |
| Self-Efficacy | Eating | |||||
| Not Confident | 0% | 4% | 0% | 0% | 0% | |
| Somewhat Confident | 4% | 37% | 2% | 10% | 13% | |
| Moderately Confident | 32% | 26% | 29% | 39% | 21% | |
| Very Confident | 64% | 33% | 69% | 51% | 67% | |
| Exercise | ||||||
| Not Confident | 0% | 4% | 0% | 0% | 0% | |
| Somewhat Confident | 13% | 14% | 25% | 33% | 15% | |
| Moderately Confident | 42% | 28% | 29% | 28% | 31% | |
| Very Confident | 44% | 55% | 46% | 39% | 54% |
Mediation Results
Hypothesis 1
(Figure 1) Higher HES at 16-weeks significantly predicts a higher Eat-SE at 16 weeks (β=1.19, p<0.00), and marginally significant greater weight loss from 1 to 16 weeks (β=0.136, p=0.07). Incentives significantly moderate this relationship (β=0.321, p=0.00), explaining 58.9% of the total effect for IP. When incentive is tested as mediator in place of Eat-SE, there is no longer a significant relation between HES and weight change at 16 weeks.
Figure 1.
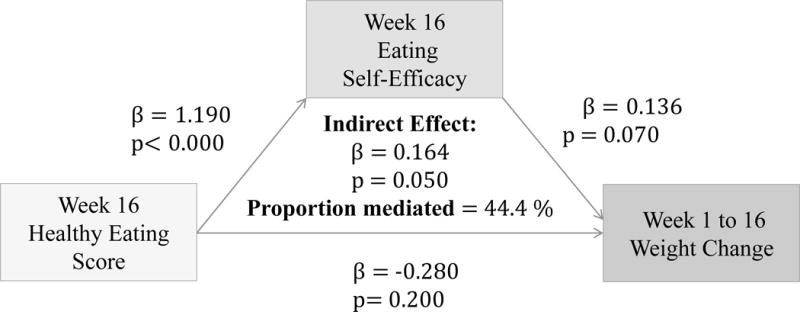
Associations between HES (week 16), Eat-SE (week 16) and weight change from 1 to 16 weeks
Hypothesis 2
(Figures 2–4) More frequent engagement in mild, moderate and vigorous PA at 16 weeks significantly predicts higher Ex-SE at 16 weeks (p<0.00). Higher Ex-SE at 16 weeks further predicts a lower BMI at week 16 (p<0.00) in all the PA models. FI significantly moderate this relationship in all PA models, explaining 51.6% of the total effect for IP (β= −1.115, p=0.00) at mild intensity PA. For moderate intensity PA, FI explain 223% of the total effect (β= −3.81, p=0.00) for IP. Finally, for vigorous PA, FI explain 94.57% of the total effect for IP (β= −1.5422, p=0.00).
Figure 2.
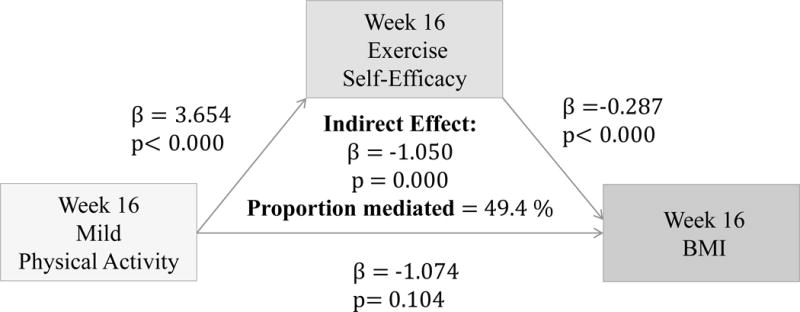
Associations between Mild PA (week 16), Ex-SE (week 16), and BMI at 16 weeks
Figure 4.
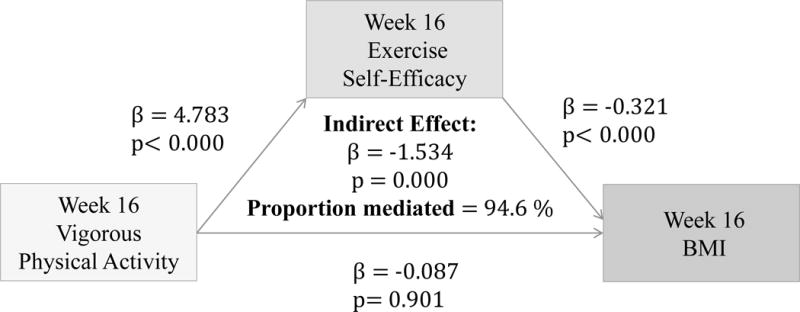
Associations between Vigorous PA (week 16), Ex-SE (week 16), and BMI at 16 weeks
Hypothesis 3
(Figures 5–7) More frequent engagement in mild, moderate and vigorous PA at 16 weeks significantly predict higher Ex-SE at 16 weeks (p<0.00). Higher Ex-SE at 16 weeks further predict a greater loss in BMI from 16 to 28 weeks (p=0.02) in all three PA intensities. FI significantly moderate this relationship in all three intensities, explaining 46.6% of the total effect for IP (β= −0.332, p=0.02) at mild intensity. For moderate intensity, incentives explain 113.4% of the total effect (β= −0.883, p=0.05) for IP. Finally, at vigorous intensity, FI explain 90.2% of the total effect for IP (β= −0.486, p=0.00).
Figure 5.
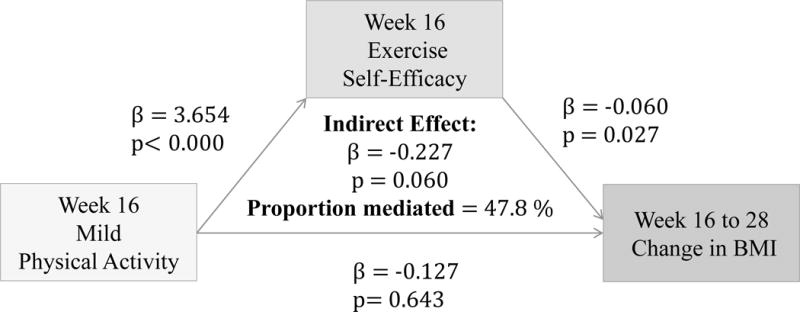
Associations between Mild PA (week 16), Ex-SE (week 16), and BMI change from 16 to 28 weeks
Figure 7.
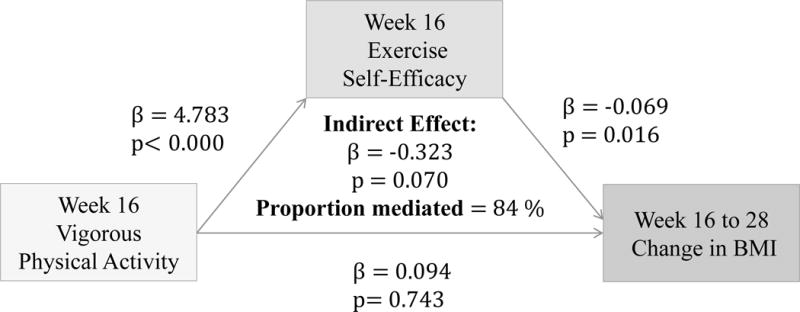
Associations between Vigorous PA (week 16), Ex-SE (week 16), and BMI change from 16 to 28 weeks
Hypothesis 4
(Figure 8–10) More frequent mild and moderate PA reported at 28 weeks significantly predict higher Ex-SE at 28 weeks (p=0.026, p=0.016). Higher Ex-SE at 28 weeks further predict a lower BMI at 28 weeks (p=0.03, p=0.04). More frequent vigorous PA at 28 weeks is also significantly predictive of higher Ex-SE at 28 weeks (p<0.00), as well as a lower BMI at 28 weeks (p=0.002). Higher HES at 28 weeks predicted higher Eat-SE at 28 weeks (B=0.3885, p=0.287), which further predicted lower BMI at 28 weeks (B=−0.1123, p=0.0113*). Eat-SE mediated 15.48% of the total effect of HES on BMI at 28 weeks (p=0.24).
Figure 8.
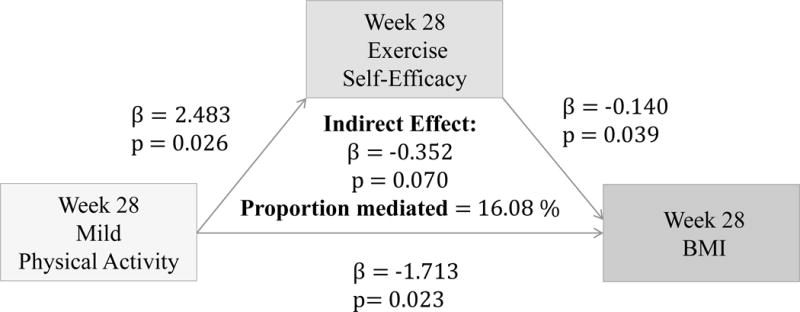
Associations between Mild PA (week 16), Ex-SE (week 16), and BMI at 28 weeks
Figure 10.
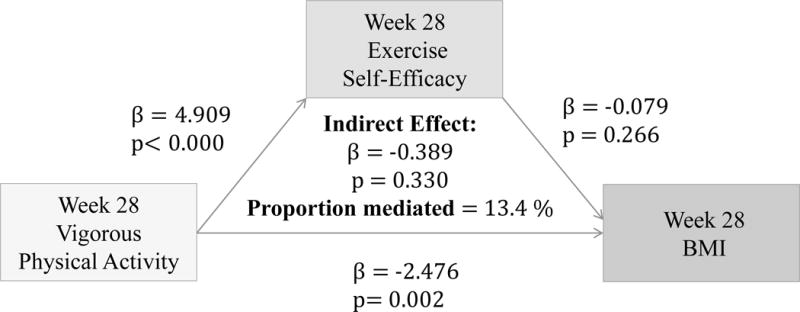
Associations between Vigorous PA (week 16), Ex-SE (week 16), and BMI at 28 weeks
Discussion
Healthy diet and regular physical activity have major impacts on health, such as metabolic improvement and weight management,30 with or without weight loss.17 With over 70% of U.S. adults currently overweight or obese,1 it is critical to design and implement evidenced-based interventions that will achieve sustained behavioral change, especially in individuals with overweight and obesity. In doing so, employee health can be improved while lowering healthcare spending, resulting in a greater return on investment for employers. Overall, our findings indicate that perceived self-efficacy regarding eating and exercise has notable influence on the relationship between health behavior and weight change, and that FI may help spur behavior change.
Results showed that higher HES at 16-weeks significantly predicted higher Eat-SE at 16 weeks and greater weight loss from 1 to 16 weeks (Figure 1). Incentives significantly moderated this relationship (β=0.321, p=0.00), explaining 58.9% of the total effect for incentivized participants. Previous literature on financial incentive for dietary behavior change have reported mixed findings. While most report incentives to be effective, these results tend to be short lived, and FI have not been shown to lead to sustained weight loss maintenance.20 Wall et al. (2006) conducted a systematic review of randomized control trials that measured the effectiveness of financial incentives in the modification of nutrition behavior. Wall’s findings demonstrated a positive effect of FI on food purchases, consumption, and/or weight loss in the short-term, supporting the notion that financial incentives as promising strategy in dietary behavior modification.20 In another systematic review, conducted by Purnell et al. (2014), eleven studies reported a positive link between financial incentives and dietary behavior change in the short-term which was not maintained at long-term follow ups.22 Although findings are mixed, financial incentives remain a potentially useful tool in aiding diet behavior modification.
Furthermore, our results show that more frequent PA at all intensity levels significantly predicted higher Ex-SE (p<0.00) and lower BMI (p<0.00) at 16 weeks, as predicted. Ex-SE significantly mediated these relationships at all intensity levels (p=0.00). Incentives also moderated the effect of SE in all PA models, as predicted. Participants who reported more frequent PA and higher Ex-SE at 16 weeks also had greater weight loss from week 16 to 28. This is perhaps due to the additional $100 that the IP were eligible to receive if they maintained their weight/losses at the follow-up. This finding agrees with previous research that incentives can improve adherence and sustain exercise/weight control for longer duration.3,18 Mitchell et al. conducted a systematic search of 15 databases in 2012 to compile RCTs which analyzed the use of financial incentives on exercise behaviors.3 Eleven studies were included with a total of 1,453 individuals, 50% of which were female. Pooled results significantly favored the incentive condition. Previously sedentary adults responded favorably to incentives 100% of the time.3 Eight studies showed incentives to have significant, positive effects on exercise. One of the studies determined that incentives can sustain exercise for longer periods (>1 year), and two studies found exercise maintenance to persist after withdrawal of the incentive. Findings from our study also agree that financial incentives may promote physical activity at all levels. We found that more frequent PA at 28 weeks predicted higher Ex-SE at 28 weeks, at all intensity levels. Higher Ex-SE further predicted a lower BMI in mild and moderate intensities, explaining 15–16% of the variability between PA and BMI. At the vigorous level of PA, the direct effect of PA on BMI at 28 weeks (p=0.002) is more significant than when mediated through SE.
From our results, it could be postulated that for both eating and exercise, while self-efficacy is an important internal factor, incentives may help initiate or encourage behavior change as an external motivator. However, incentives may not be an adequate motivator alone to spur behavior change and self-efficacy is needed to support long-term sustainable weight change.
Self-efficacy, self-regulation skills and autonomous motivation for physical activity have been associated with better weight control, adherence and improved health outcomes.17 Motivational factors such as self-efficacy may be more operative along the entire continuum, from adoption of behavior change and to maintenance.31 Increasing self-efficacy requires increasing knowledge regarding healthful behavior, building skills and changes in attitude, but these strategies may take time, which has potential to discourage individuals with overweight and obesity to participate in weight loss programs. In addition, individuals seeking weight loss tend to overestimate their ability to do so (overconfident self-efficacy),32 possibly indicating a lack of experience with the difficulty associated with such efforts.33 When these individuals fail to reach their goals, it can deject their motivation to continue. Incentives may be used to help overcome this barrier and keep the individual motivated to continue the behavior change. Financial incentives may encourage people to set goals by providing incitement and external motivation for achieving the goal and acting as a catalyst for initiating behavior change.22
In setting more challenging goals, people may put forth greater effort and interest in the task.34 This will lead to improved performance, greater skill on the task, and increased self-efficacy, which can improve the duration and intensity of effort, as well as developing strategies to achieve the goal or mastery the task.34 Financial incentives that are awarded directly upon performance have the ability to increase desirability of goal attainment, and may lead to improvements in motivation and performance.34
A notable finding in our study was that the NIP reported higher self-efficacy than the IP. This could perhaps be explained by the notion that self-efficacy and goal-setting can also be self-debilitating.35 Although NIP showed generally higher self-efficacy, they had less weight loss than IP. The IP were required to report their weight (goal attainment) to receive their incentive, whereas the NIP were not required to report their weight. Those that were required to report their goal and did not reach it, perhaps lost confidence in their ability, thus reporting lower SE at week 16 and 28. When individuals fail to fulfill their goals, they react self-critically.35 Consequently, self-satisfaction and self-efficacy plummet, which affects future performance and efficacy beliefs. In other words, the feedback provided by the incentive was perhaps self-debilitating to those who did not reach their goals. We found that IP continued to lose weight even after program was complete and they showed a significant drop in bodyweight from week 1–28. This could be due to the fact that the IP were eligible to receive the extra $100 if they maintained weight/losses at follow-up, indicating incentives may be effective for maintaining weight loss.
Finkelstein (2007) and Volpp (2008) also investigated if FI were effective in promoting weight loss among individuals with overweight or obesity. Findings from these studies both favored incentive groups and agreed that modest FI were an effective tool to motivate individuals with overweight or obesity to lose weight.18,23 Volpp found that IP weighed significantly less at 7 months than at baseline, whereas controls did not. However, the authors noted that longer-term use of incentives should be evaluated. John et al. (2011) conducted a 24-week (−1 lb/week) weight loss phase, followed by an 8-week maintenance phase. Results revealed that while the incentivized participants had lost more weight than non-incentive participants, at the 36 weeks follow-up, the lost weight was regained, making the net weight loss between groups no longer significant. This trial concluded that financial incentives were successful in producing significant weight loss during the intervention phase, which was not maintained post-intervention.19 Incentivized approaches to behavior change may initially provide participants with external motivation, however the extent to which these outcomes can be successfully sustained remains questionable.18,19
Intrinsic motivation such as self-efficacy is an important factor leading to success in changing behaviors, potentially providing the necessary power to face setbacks when undertaking challenging endeavors.35 Using constructive strategies to promote resilient self-efficacy long-term, in addition to utilizing modest FI as extra motivation in initial efforts, may improve and facilitate weight loss. FI appears to be an effective strategy to provide informative feedback that will not interfere with belief in one’s capability to succeed, rendering it a possible effective approach to incorporate in future weight loss interventions.36
Individuals with obesity tend to have a lower SE regarding health behaviors that their non-obese counterparts,30 thus indicating an even greater need for self-efficacy improvement in obese populations. Furthermore, individuals with obesity who complete the entirety of weight-loss interventions see improvements in their SE. If improvements in SE can improve chances of weight-loss as indicated, perhaps it is more effective to focus on increasing SE through the use of FI prior to and/or during treatment, as it would likely improve behavior change initiation and adherence in previously unmotivated individuals. Incentives may be useful in the early stages of behavioral adoption, while motivational factors such as self-efficacy may be more operative along the entire continuum, from adoption to maintenance.31 This may be particularly true in regards to physical activity, which has the potential to improve intervention outcomes. Initiation and adoption of regular physical activity is a critical step in the process of weight management, particularly for sustained success. Besides its direct contribution to energy expenditure, physical activity may also contribute to improved diet compliance through eating disinhibition and improved psychological well-being. Approaches which focus on improving SE through the use of FI, particularly in regards to physical activity, may be a useful strategy to encourage weight loss/maintenance.
Behavior modification, and comprehensive lifestyle interventions, in particular, are currently the first recommended step in obesity management.37 Findings from this study can direct practitioners and healthcare professionals in clinical settings to include time-efficient ways of assessing their patients’ perceived SE and barriers. Although these take longer than solely providing external motivation such as FI, it may be necessary to see lasting results. In addition, there is a need for further research that identifies causal predictors/mediators of sustained weight loss and weight control. Although it is unlikely that any single factor explains the variability in complex behaviors, testing of mediators of behavior modification is a critical step in improving future weight loss interventions by identifying variables responsible for desired health outcomes.
Limitations
A potential limitation of this study is the use of self-reported questionnaire items, which could be inaccurate due to errors in self-observation.38 Participants may have a skewed perception of their behavior or confidence, especially with knowledge of their upcoming participation in a weight-loss program, which could lead to an inflated sense of confidence about their ability to lose weight. The small sample size and short trial duration in this study also pose a limitation. Trials with longer duration and larger sample sizes are needed and should be evaluated, as well as within populations that are at high-risk of developing diet-related diseases.18,20 The majority of the sample were middle-aged females so there is not adequate evidence that our results can be applied to all obese populations. Lastly, mediation results over 100% reflect an issue likely relating to the small sample size or collinearity among predictor variables, rendering these results as non-robust.
Conclusion
In summary, the change our society needs to combat obesity and its related comorbidities must involve widespread, yet individualized strategies to induce behavior changes. In an effort to determine how internal (SE) and external motivators (FI) affect health behaviors and weight in an overweight population, the present study assessed the effects of perceived (dietary and exercise) SE and FI on health behaviors and weight status in an overweight and obese sample participating in a weight-loss program. Our results agree with current literature stating that SE and FI are likely factors contributing to weight-loss in obesity interventions. SE has been shown to further predict weight-loss maintenance and appears to be a stronger factor contributing to sustained weight control than financially incentivized approaches alone. Healthcare professionals aiding in weight loss pursuits may incorporate a focus on an individual’s self-efficacy to improve likelihood of overcoming barriers to weight loss and weight management. Identifying successful strategies to facilitate weight loss will help progress efforts toward achieving adequate physical activity and nutrition, and ultimately, improved weight status of our nation. FI may be used to encourage initiation of behavior change. Our research shows SE and FI as promising factors influencing weight loss. Further research is needed to investigate other causal mediators of weight change.
Figure 3.
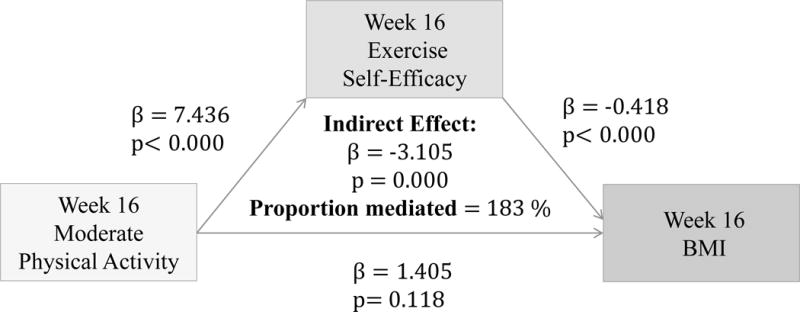
Associations between Moderate PA (week 16), Ex-SE (week 16), and BMI at 16 weeks
Figure 6.
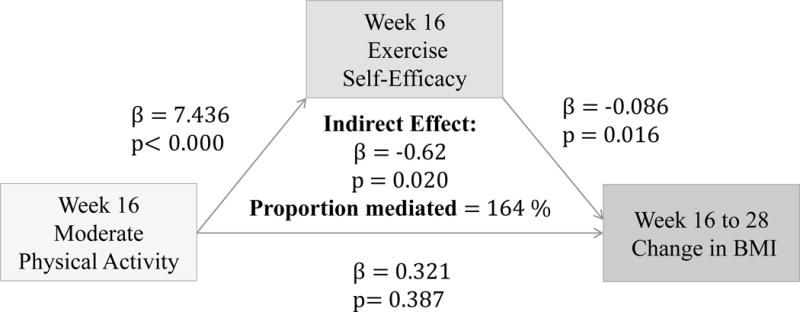
Associations between Moderate PA (16 weeks), Ex-SE (16 weeks), and BMI change from 16 to 28 weeks
Figure 9.
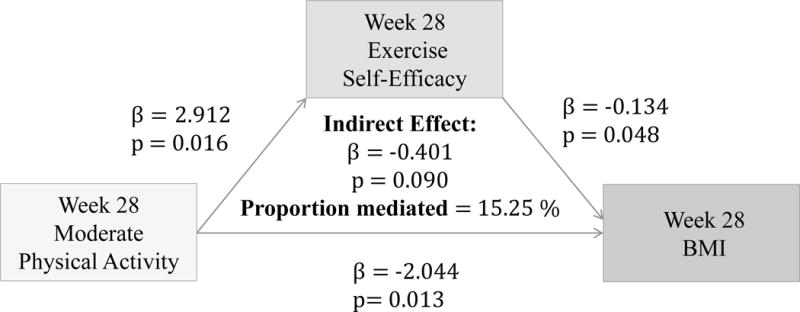
Associations between Moderate PA (week 16), Ex-SE (week 16), and BMI at 28 weeks
Acknowledgments
Funding Source:
This research was supported by the Center for Disease Control and Prevention grant (CDC, Grant TS-1444): Principal Investigator: Dr. Pouran Faghri.
Footnotes
Conflict of Interest:
The authors declare no relationships that would present a conflict of interest.
The authors acknowledge that content was previously published as an institutional requirement for a degree seeking candidate.39
References
- 1.Centers for Disease Control and Prevention. https://www.cdc.gov/obesity/data/adult.html. Accessed December 3, 2016.
- 2.Jokinen E. Obesity and cardiovascular disease. Minerva Pediatr. 2015 Mar;67(1):25–32. [PubMed] [Google Scholar]
- 3.Mitchell MS, Goodman JM, Alter DA, John LK, Oh PI, Pakosh MT, et al. Financial Incentives for Exercise Adherence in Adults: Systematic Review and Meta-Analysis. Am J Prev Med. 2013;45:5, 658–667. doi: 10.1016/j.amepre.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Volpp KG. Paying people to lose weight and stop smoking. LDI Issue Brief. 2009;14(3):1–4. [PubMed] [Google Scholar]
- 5.Ryan RM, Patrick H, Deci EL, Williams GC. Facilitating health behavior change and its maintenance: Interventions based on Self-Determination Theory. European Health Psychologist. 2008;10 [Google Scholar]
- 6.Wadden TA, West DS, Neiberg R, et al. One-Year Weight Losses in the Look AHEAD Study: Factors Associated with Success. Obesity (Silver Spring, Md) 2009;17(4):713–722. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Heart Association. http://www.heart.org/heartorg/. Accessed July 4, 2016.
- 8.Baicker K, Cutler D, Song Z. Workplace wellness programs can generate savings. Health Affairs. 2010;29(2):304–11. doi: 10.1377/hlthaff.2009.0626. [DOI] [PubMed] [Google Scholar]
- 9.Jones A, Dwyer L, Bercovitz A, Strahan G. The National Nursing Home Survey: 2004 Overview CDC Division of Health Care Statistics. (13).Vital and Health Statistics. 2009;(167) http://www.cdc.gov/nchs/data/series/sr_13/sr13_167.pdf. [PubMed]
- 10.Miranda H, Gore RJ, Boyer J, Nobrega S, Punnett L. Health Behaviors and Overweight in Nursing Home Employees: Contribution of Workplace Stressors and Implications for Worksite Health Promotion. The Scientific World Journal. 2015;915359 doi: 10.1155/2015/915359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandura A. Social cognitive theory. In: Vasta R, editor. Annals of child development Vol. 6. Six theories of child development. Greenwich, CT: JAI Press; 1989. pp. 1–60. [Google Scholar]
- 12.Bandura A. Self-Efficacy: The exercise of control. New York, NY: W. H. Freeman; 1997. [Google Scholar]
- 13.Annesi JJ. Supported Exercise Improves Controlled Eating and Weight through Its Effects on Psychosocial Factors: Extending a Systematic Research Program Toward Treatment Development. Perm J. 2012;16(1):7–18. doi: 10.7812/11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olander EK, Fletcher H, Williams S, Lou A, Turner A, French DP. What are the most effective techniques in changing obese individuals’ physical activity self-efficacy and behaviour: A systematic review and meta-analysis. Int J Behav Nutr Phys Act. 2013;10 doi: 10.1186/1479-5868-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dishman RK, Motl RW, Sallis JF. Self-Management Strategies Mediate Self-Efficacy and Physical Activity. Int J Behav Nutr Phys Act. 2005;29(1):10–18. doi: 10.1016/j.amepre.2005.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrne S, Barry D, Petry NM. Predictors of weight loss success: Exercise vs. dietary self-efficacy and treatment attendance. Appetite. 2012;95593 doi: 10.1016/j.appet.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teixeira PJ, Carraça EV, Marques MM, Rutter H, Oppert JM, De Bourdeaudhuij I, et al. Successful behavior change in obesity interventions in adults: a systematic review of self-regulation mediators. BMC Med. 2015;13:84. doi: 10.1186/s12916-015-0323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: A randomized trial. JAMA. 2008;300(22):2631–2637. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.John LK, Loewenstein G, Troxel AB, Norton L, Fassbender JE, Volpp KG. Financial incentives for extended weight loss: A randomized, controlled trial. J Gen Intern Med. 2011;26(6):621–626. doi: 10.1007/s11606-010-1628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wall J, Mhurchu CN, Blakely T, Rodgers A, Wilton J. Effectiveness of monetary incentives in modifying dietary behavior: A review of randomized, controlled trials. J Nutr Rev. 2006;64(12):518–31. doi: 10.1111/j.1753-4887.2006.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 21.Leahey TM, Subak LL, Fava J, Schembri M, Thomas G, Xu X, et al. Benefits of adding small financial incentives or optional group meetings to a web-based statewide obesity initiative. Obesity (Silver Spring) 2015;23(1):70–6. doi: 10.1002/oby.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purnell JQ, Gernes R, Stein R, Sherraden MS, Knoblock-Hahn A. A systematic review of financial incentives for dietary behavior change. J Acad Nutr Diet. 2014 Jul;114(7):1023–35. doi: 10.1016/j.jand.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finkelstein EA, Linnan LA, Tate DF, Birken BE. A pilot study testing the effect of different levels of financial incentives on weight loss among overweight employees. J Occup Environ Med. 2007;49(9):981–9. doi: 10.1097/JOM.0b013e31813c6dcb. [DOI] [PubMed] [Google Scholar]
- 24.Faghri P, Li R. Effectiveness of Financial Incentives in a Worksite Diabetes Prevention Program. Open Obes J. 2014;6:1–12. doi: 10.2174/1876823720140107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahiri S, Faghri P. Cost-effectiveness of a workplace-based incentivized weight loss program. J Occup Environ Med. 2012;54(3):371–377. doi: 10.1097/JOM.0b013e318247a394. [DOI] [PubMed] [Google Scholar]
- 26.Lindstrom J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care. 2003;26(3):725–731. doi: 10.2337/diacare.26.3.725. [DOI] [PubMed] [Google Scholar]
- 27.Sharafi M, Faghri P, Huedo-Medina T, Duffy V. Simple Liking Survey Associates with Weight Loss Success in a Worksite Intervention. FASEB J. 2015;905:29. 10. doi: 10.3390/nu13041338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallis JF, Pinski RB, Grossman RM, Patterson TL, Nader PR. The development of self-efficacy scales for health-related diet and exercise behaviors. Health Educ Res. 1988;3(3):283–292. [Google Scholar]
- 29.Clark MM, Abrams DB, Niaura RS, Eaton CA, Rossi JS. Self-efficacy in weight management. J Consult Clin Psychol. 1991 Oct;59(5):739–44. doi: 10.1037//0022-006x.59.5.739. [DOI] [PubMed] [Google Scholar]
- 30.Richman RM, Loughnan GT, Droulers AM, Steinbeck KS, Caterson ID. Self-efficacy in relation to eating behaviour among obese and non-obese women. Int J Obes Relat Metab Disord. 2001;25(6):907. doi: 10.1038/sj.ijo.0801606. [DOI] [PubMed] [Google Scholar]
- 31.D’Angelo ME, Pelletier LG, Reid RD, Huta V. The roles of self-efficacy and motivation in the prediction of short- and long-term adherence to exercise among patients with coronary heart disease. Health Psychol. 2014;33:1344–1353. doi: 10.1037/hea0000094. [DOI] [PubMed] [Google Scholar]
- 32.Foster GD, Wadden TA, Wogt RA, Brwer G. What is a reasonable weight loss? Patients’ expectations and evaluations of obesity treatment outcomes. J Consult Clin Psychol. 1997;65:79–85. doi: 10.1037//0022-006x.65.1.79. [DOI] [PubMed] [Google Scholar]
- 33.Martin PD, Dutton GR, Brantley PJ. Self-Efficacy as a Predictor of Weight Change in African-American Women. Obes Res. 2004;12(4):646–651. doi: 10.1038/oby.2004.74. [DOI] [PubMed] [Google Scholar]
- 34.Bonner SE, Sprinkle GB. The effects of monetary incentives on effort and task performance: theories, evidence, and a framework for research. Accounting, organizations and society. 2002;27:303–345. [Google Scholar]
- 35.Bandura A, Locke E. Negative self-efficacy and goal effects revisited. J Appl Psychol. 2003;88(1):87–99. doi: 10.1037/0021-9010.88.1.87. [DOI] [PubMed] [Google Scholar]
- 36.Teixeira PJ, Silva MN, Mata J, Palmeira AL, Markland D. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012;9:22. doi: 10.1186/1479-5868-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S139–S140. [Google Scholar]
- 38.Hawkshead J, Krousel-Wood MA. Techniques for measuring medication adherence in hypertensive patients in outpatient settings: advantages and limitations. Dis Manag Health Out. 2007;15(2):109–118. [Google Scholar]
- 39.Simon J. Perceived Self-Efficacy and Financial Incentives: Factors Affecting Health Behavior and Body Weight in Individuals with Overweight and Obesity. Master’s Theses. 2016;986 http://digitalcommons.uconn.edu/gs_theses/986. [Google Scholar]


