Abstract
Purpose
Dialysis access failure is a major cause of morbidity in patients with end-stage renal disease. The Fistula First Breakthrough Initiative (FFBI) dictates arteriovenous fistulae (AVFs) should be preferred over arteriovenous grafts (AVGs) as first line for surgically placed accesses. The purpose of this study was to compare patency rates of surgical dialysis accesses in our mature, urban population after the FFBI.
Methods
Current dialysis patients with accesses placed between 2006 and 2011 were included. Patient characteristics, access outcomes, interventions, and survival outcomes were analyzed.
Results
We report outcomes of 220 patients undergoing dialysis access. Of those 220, 75 received numerous accesses. All outcomes are evaluated as per access itself, that is, a patient may have numerous access types, each individually analyzed. Of the accesses, 138 were AVF and 190 were AVG. The average age of patients was 59.8 years. The groups were evenly matched in distribution of race and prevalence of hypertension, diabetes, coronary artery disease, and Peripheral Vascular Disease (PVD). Average number of complications requiring intervention per access were fewer with AVF than AVG (1.21 vs 1.72, P = .02). The AVF had greater rates of stenosis (51.4% vs 40.6%, P = .0182), whereas AVG had greater thrombosis rates (14.6% vs 31.9%, P < .001). Both AVF and AVG had similar primary patency (median: 186 vs 142 days, P = .1774) and 3-year secondary patency (59.2% vs 49.2%, P = .0945). Arteriovenous fistula in patients aged <60 years was found to have the greatest primary (P = .0078) and secondary patency (P = .0400). Outcomes did not differ between AVF and AVG in those aged >60 years.
Conclusions
Although complications requiring intervention are greater with AVG, primary and secondary patency rates are similar between AVF and AVG, except when considering AVF in patients aged <60 years.
Keywords: arteriovenous fistula, arteriovenous graft, dialysis, dialysis access
Introduction
The US Renal Data System estimates that 661 648 individuals were treated for end-stage renal disease (ESRD) in 2013.1 A total of 117 162 new cases of ESRD were diagnosed in that period, accounting for over US$50 billion in Medicare expenses. Of all, 88.4% (103 382) of those new patients began therapy with hemodialysis, 9.0% (10 562) with peritoneal dialysis, and 2.6%(3046) received a preemptive kidney transplant.
The 2001 Kidney Disease Outcome Quality Initiative guidelines advocated for the use of arteriovenous fistula (AVF) rather than arteriovenous graft (AVG) as the access of choice for those beginning dialysis.2 Subsequently, the Kidney Foundation, the Centers for Medicare and Medicaid Services, and other ESRD networks formed the National Vascular Access Improvement Initiative and promoted the Fistula First Breakthrough Initiative (FFBI).3 In 2005, 28.9% of patients had either an AV fistula or a maturing AV fistula. By 2013, that number had increased to 62.5%.1
Despite FFBI’s recommendations, debate continues as to the optimal access for first-time dialysis patients. Autogenous vascular access has been promoted for a number of reasons—lower infection rates compared to AVGs and catheters chief among them.1,4,5 Regarding the benefit of AVF over AVGs, critically assessing whether the literature supports AVFs in a diverse patient population is important. A lower prevalence of fistulas has been reported in females, blacks, the obese, the elderly patients, and those with peripheral vascular disease.4,6 Indeed, a 2008 manuscript published by Allon et al7 addressed potential selection bias in policy-defining studies bluntly: “Whereas a fistula may be the ideal access choice in a young white man without cardiovascular morbidity, it may be a poor choice in an older black woman with cardiovascular morbidity.”
Temple University Hospital is located in an area of North Philadelphia, Pennsylvania, that serves a mature, urban patient population. This area has a poor health status with increased rates of uninsured residents, poverty, and unemployment.8 Further, this population has high rates of hypertension, obesity, diabetes, and tobacco use, especially among its black population.8,9 This study sought to compare the outcomes of AVGs created between 2006 and 2011 with AVFs to determine whether AVFs are indeed superior in our patient population. We compared the outcomes of patients who received initial dialysis access at our hospital and received their treatment at dialysis centers in our area.
Methods
Our institution’s Division of Vascular Surgery, in conjunction with the Division of Nephrology, developed a database of dialysis accesses placed at our institution. The data elements included patient demographics and comorbidities, specifics about access placement, and any percutaneous or surgical interventions that the accesses required. The data were collected from Temple University Hospital medical records, the surrounding area hemodialysis centers, and the area’s interventional radiology database. The inclusion criteria for this study were consecutive patients with surgical dialysis accesses placed between January 1, 2006, and January 12, 2011, at Temple University Hospital and cared for by our institution’s nephrologists. Although all surgeons involved perform new fistula and redo access procedures at multiple hospitals, only those patients initially accessed at Temple University were included in this study. Access selection criteria were based upon surgeon preference and patient anatomy at the time of intervention. There was no defined commonality in surgical technique among the surgeons, but all accesses were performed according to acceptable surgical techniques previously described.10 Typically, the first choice of autogenous access was the radial artery to cephalic vein fistula, with the radial artery to forearm basilica vein transposition serving as a second option. All first-time interventions are reported upon, that is, new attempt at graft or fistula within an individual patient. Therefore, all analyses are performed on a per-access basis rather than a per-patient basis. All grafts used were Gore ProPaten and ePTFE (GORE-TEX1 Vascular Grafts; W. L. Gore & Associates, Inc, Flagstaff, Arizona). Patients were excluded if there was incomplete data. Our primary outcome of interest was comparative patency between AVF and AVG. Our secondary outcome of interest was patient survival. We adopted our definitions from Sidawy et al.11 Primary patency was defined as the interval from the time of access placement until any intervention was required to maintain or reestablish patency or access thrombosis. Secondary patency was defined as the interval from the time of access placement until access abandonment, thrombosis, or intervention designed to reestablish functionality in that access. Patency was assessed by physical examination and supplemented by ultrasound when necessary.
Patients receiving AVF were compared to those receiving AVG. Patient demographics and access complications were compared with t test for continuous data and χ2 or Fisher exact tests for discrete data. Kaplan-Meier method was used to describe the primary and secondary patency rates of the accesses. All analyses were performed with SAS 9.2 (SAS institute, Cary, North Carolina).
Results
Two hundred twenty patients were included in our analysis. Seventy-five of these patients had numerous accesses. These 220 patients received 138 new AVFs and 190 new AVGs placed by 8 surgeons, for a total of 328 new accesses. The median follow-up of patients with AVG was 16.6 months and that of AVF was 13.3 months.
Patient demographics are listed in Table 1. There were no differences between the AVG group and the AVF group in any of the characteristics listed. The majority of patients included in this study were black (82.3% of AVF accesses and 90.3% of AVG accesses). Ninety-five percent had hypertension; 64.6% and 54.8% of those undergoing AVF and AVG placement had diabetes; and 34.4% of those with AVF and 25% of those with AVG had coronary artery disease, whereas peripheral vascular disease had previously been diagnosed in 20.8% and 25.8% of those with AVF and AVG, respectively.
Table 1.
Patient Demographics of Those Receiving AVFs and AVGs.
| AVF | AVG | P Value | |
|---|---|---|---|
| No. of patients | 96 | 124 | |
| Age, years | 58.8 ± 13.5 | 60.6 ± 14.1 | .2487 |
| Female | 48 (50.0) | 65 (52.4) | .7218 |
| Race | .2308 | ||
| Black | 79 (82.3) | 112 (90.3) | |
| White | 5 (5.2) | 2 (1.6) | |
| Hispanic | 11 (12.4) | 8 (6.5) | |
| Asian | 1 (1.0) | 2 (1.6) | |
| Hypertension | 92 (95.8) | 118 (95.2) | .8124 |
| Diabetes | 62 (64.6) | 68 (54.8) | .1449 |
| Coronary artery disease | 33 (34.4) | 31 (25.0) | .1289 |
| Peripheral vascular disease | 20 (20.8) | 32 (25.8) | .3892 |
Abbreviations: AVFs, arteriovenous fistulae; AVGs, arteriovenous grafts.
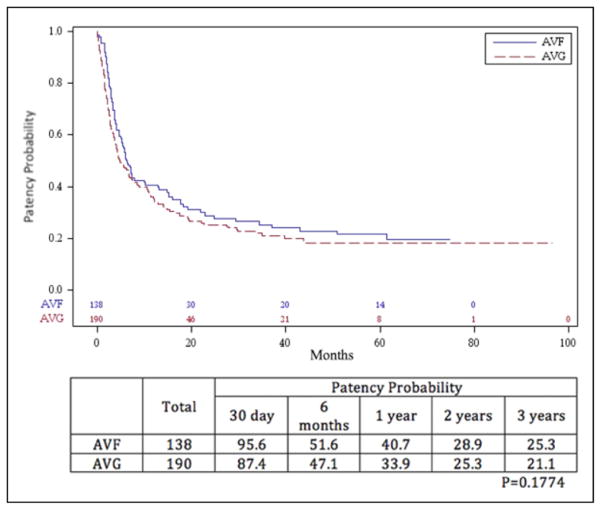
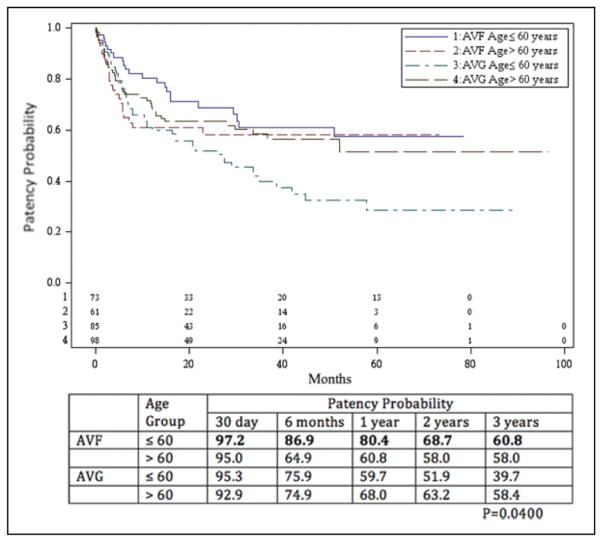
Primary patency rates are shown in Figures 1 and 2. The overall primary patency for AVF at 1, 2, and 3 years was 40.7%, 28.9%, and 25.3%, respectively. The overall primary patency for AVG was 33.9%, 25.3%, and 21.1%during that time period. The difference in patency between the 2 procedures, as determined by Kaplan-Meier method, was not significant (P = .177). An analysis of primary patency rates of AVF and AVG, stratified by age less than or equal to and greater than 60 years, revealed a benefit in AVF in those aged ≤60 years. The AVF primary patency rates for those aged ≤60 years at 1, 2, and 3 years was 54.9%, 40.9%, and 34.6%, respectively, and for those aged >60 years was 24.6%, 14.9%, and 14.9%, respectively. Primary patency for those aged ≤60 years with AVGs at 1, 2, and 3 years was 29.6%, 22.5%, and 18.0%, respectively, whereas that for those aged >60 years was 37.6%, 27.7%, and 23.8%, respectively. The patency rate of AVF in those aged ≤60 years was statistically better than those >60 years (P = .008), but there was no difference when comparing AVF in those aged >60 years and AVG in those less than or greater than 60 years.
Figure 1.
Primary patency probability of arteriovenous fistulae (AVFs) and arteriovenous grafts (AVGs). The difference in patency between the 2 procedures, as determined by Kaplan-Meier method, was not significant (P = .1774).
Figure 2.
Primary patency probability of arteriovenous fistulae (AVFs) and arteriovenous grafts (AVGs) stratified by age group. The patency rate of AVF in those aged ≤60 years was statistically better than those aged >60 years (P = .0078), but there was no difference when comparing AVF in those aged >60 years and AVG in those aged less than or greater than 60 years.
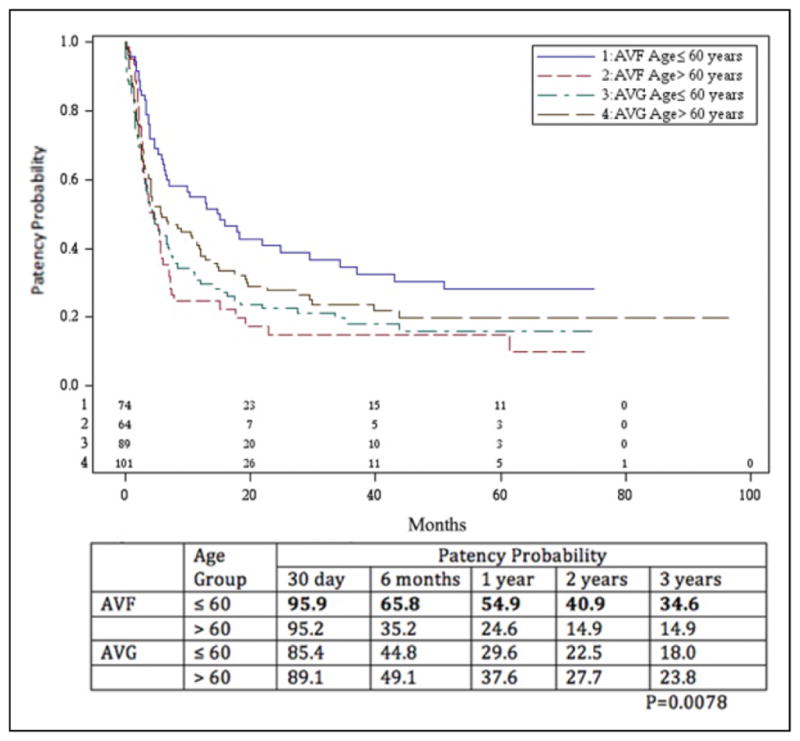
A total of 134 AVFs and 183 AVGs were assessed for secondary patency. Secondary patency rates are shown in Figures 3 and 4. The secondary patency for the AVF at 1, 2, and 3 years was 71.4%, 63.5%, and 59.2%, respectively. The secondary patency for AVGs was 64.0%, 57.7%, and 49.2% during that time period. The difference was not significant (P = .095). When subdivided by age group, analysis revealed that secondary patency rates for those aged ≤60 years with AVF at 1, 2, and 3 years were 80.4%, 68.7%, and 60.8%, respectively, and for those aged >60 years were 60.8%, 58.0%, and 58.0%, respectively. Secondary patency for those aged ≤60 years with AVGs was 59.7%, 51.9%, and 39.7%, whereas that for those aged >60 years was 68.0%, 63.2%, and 58.4%. The difference between those aged ≤60 years with AVFs and all others was significant (P = .040). There was actually a slight but statistically insignificant benefit improvement in secondary patency rates in favor of AVG in the >60 years age-group.
Figure 3.
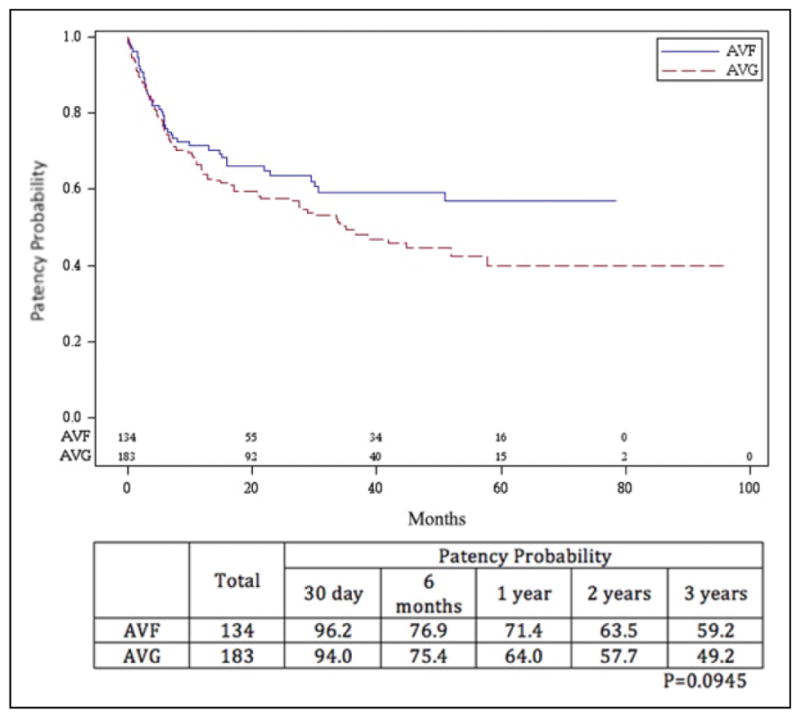
Secondary patency probability of arteriovenous fistulae (AVFs) and arteriovenous grafts (AVGs). The difference in patency between the 2 procedures, as determined by Kaplan-Meier method, was not significant (P = .0945).
Figure 4.
Secondary patency probability of arteriovenous fistulae (AVFs) and arteriovenous grafts (AVGs) stratified by age group. The patency rate of AVF in those aged ≤60 years was statistically better than those aged >60 years (P = .040), but there was no difference when comparing AVF in those aged >60 years and AVG in those less than or greater than 60 years.
Complication patterns differed between the 2 access methods (Table 2). Stenosis occurred significantly more often in patients with AVF than AVG (51.4% vs 40.6%, P = .018). Thrombosis was more common in AVG than in AVF (31.9% vs 14.6%, P = .00001). Neither infection (2.7% vs 5.0%) nor aneurysm rates (3.8% vs 1.5%) differed between the 2 groups.
Table 2.
Complication Patterns, Interventions, and Survival Outcomes in Patients Receiving AVFs and AVGs.
| Outcomes and Interventions | AVF | AVG | P Value |
|---|---|---|---|
| Complications | |||
| Stenosis | 95 (51.4) | 139 (40.6) | .0182 |
| Infection | 5 (2.7) | 17 (5.0) | .2140 |
| Aneurysm | 7 (3.8) | 5 (1.5) | .0881 |
| Thrombosis | 27 (14.6) | 109 (31.9) | <.0001 |
| Mean no. of complications per access | 1.21 ± 1.77 | 1.72 ± 2.13 | .0199 |
| Interventions | |||
| Stent | 7 (3.8) | 9 (2.6) | .4618 |
| Angioplasty | 88 (47.6) | 148 (43.3) | .3442 |
| Thrombolysis | 9 (4.9) | 69 (20.2) | <.0001 |
| Surgical revision | 25 (13.5) | 45 (13.2) | .9086 |
| New access placed | 36 (19.5) | 57 (16.7) | .4221 |
| Survival outcomes | .7592 | ||
| 1 year | 100 | 95.5 | |
| 2 years | 92.7 | 94.3 | |
| 3 years | 87.6 | 87.0 | |
Abbreviations: AVFs, arteriovenous fistulae; AVGs, arteriovenous grafts.
Boldface text indicates where statistically significant complication patterns differ between AVG and AVF.
The frequency of interventions required to maintain the function of the access was evaluated and shown in Table 2. Angioplasty was most commonly used to reestablish patency in both patient groups, required in 47.6% of those with AVFs and 43.3% of AVGs. Rates of stent placement (3.8% and 2.6%), surgical revision (13.5% and 13.2%), or the placement of new access (19.5% and 16.7%) did not differ between the 2 groups. Thrombolysis was used significantly more often in patients with AVG than AVF (20.2% vs 4.9%, respectively).
Median survival of patients dialyzed via AVF was 5.1 years, whereas that of those with AVG was 5.7 years (Table 2). One-, 2-, and 3-year survival in those with AVF access was 100%, 92.7%, and 87.6%, respectively, and in those with AVG was 95.5%, 94.3%, and 87%, respectively. The difference was not significant.
Discussion
Temple University Hospital serves a mature patient population in an urban, underserved area of Philadelphia. When this study was initiated in 2006, 14 (45%) of 31 access placements were AVFs. By 2011, that number had increased to 33 (55%) of 60, in line with recommendations in the years following the FFBI advocating for the increased use of autogenous vascular access due to superior patency rates and lower complication rates compared to AVGs once access has been established.12 This study sought to determine whether the transition to autogenous access in the Temple University population produced an improvement in our patient outcomes. Because many of our patients receive their dialysis primarily at centers served by our nephrology staff, we were able to follow them beyond their hospital stay.
In order to be successfully used for dialysis access, a newly formed fistula must mature and remain patent. An inability to successfully undergo either of these is termed suitability failure. Historically, failure of maturation rates is 18% to 53%, with failure to attain suitability up to 62%.13,14 Likewise, up to 75% of AVGs lose patency within 1 year.15–18
Our findings regarding primary patency suggested a slight but insignificant benefit in AVF over AVG. The benefits of AVF were marked in those aged ≤60 years, who were shown to receive significant benefit from an AVF at all time points (P = .008). The data showed no significant benefit in those aged >60 years.
Our analysis demonstrated no significant difference in patient survival between those accessed via AVG compared to those accessed via AVF. However, in a 2013 meta-analysis that compared clinical outcomes in hemodialysis access type in 586 227 participants, Ravani et al19 found that the relative risk of all-cause mortality in graft versus fistula was 1.18 (1.09–1.27, P < .01). This accounted for 18 to 54 additional deaths in those receiving AVGs for every 1000 persons each year compared to AVFs. Although the direction of risk of mortality was fairly consistent throughout the studies analyzed, there remained a general amount of heterogeneity in the significance of difference in mortality risk. Of the 17 studies analyzed that directly compared survival outcomes between AVGs and AVFs, only 8 found a significant mortality benefit for AVFs. Nine studies showed no or statistically insignificant mortality benefit of AVF over AVGs.
This questionable mortality benefit conferred by AVFs has led others to more closely assess whether the benefit is more nuanced, especially in an aging population that is increasingly requiring hemodialysis.20–23 Lazarides et al21 found that radial–cephalic AVF failed at significantly higher rates in the elderly patients compared to nonelderly adults at 1 year (odds ratio [OR]: 1.525; P = .001) and 2 years (OR: 1.357; P = .19). Lok et al22 reported that those older than 65 years had an OR of 2.23 for AVF failure to mature (95% confidence interval [CI]: 1.25–3.96) compared to those less than 65 years. On the other hand, Swindlehurst et al23 found no significant difference in both primary and secondary patency outcomes in AVF between those older and younger than 65 years. Their study showed that at 25.46 months (their mean follow-up), primary patency rates for AVFs were 70% in those older than 65 years and 68% in those less than 65 years (P = .75), whereas the secondary patency rates were 73% and 79% (P = .9) in those age groups, respectively. Although patency data for grafts were not recorded by year, the cumulative patency for polytetrafluoroethylene grafts was significantly greater in the >65 years age category than in the <65 years age category (94% vs 69%, P = .05). Death rates were also similar between the 2 age groups.
Care must be taken in comparing the data from Swindlehurst et al to that from our study. Although the majority of patients treated for vascular access at our institution are in their late 50s to early 60s and black, those assessed by Swindlehurst et al are elderly white men. Even the prevalence of diabetes in their study population, usually high in patients with ESRD,1 was significantly lower than that in our patient population (35% vs 59.7%). Comparatively, this is quite interesting. Diabetes mellitus is associated with higher rates of primary fistula patency loss.24 However, black patients older than 40 years undergoing hemodialysis survive longer than age-matched white patients.25,26 Unfortunately, black patients of any age are less likely to initiate hemodialysis with an AVF than are white patients.27
A recent study by Cui et al28 compared patient outcomes and access patency in patients older than 75 years. It demonstrated a higher primary failure rate for fistulas compared to grafts (OR: 2.89; P = .008) and a higher intervention rate for fistulas than grafts (31% vs 10%; P = .03) in patients older than 75 years. Survival rates were also similar between those with AVGs and AVFs. Similar to the patients seen by Swindlehurst et al, the patients studied by Cui et al are overwhelmingly white (>90%).
Other studies have gone so far as to suggest graft as the access of choice in the elderly patients. For years, support for the use of AVF in dialysis has arisen due to the fewer interventions needed in mature fistulae to maintain their patency and the fewer infectious complications of AVF.29 However, with decreased life expectancy in the elderly patients affected by numerous comorbidities, the benefits of AVF may not come to fruition.30–32 An analysis by DeSilva et al33 of 115 425 hemodialysis patients aged ≥67 years found no significant difference in mortality outcomes between those patients with a graft at first access and those with a fistula (Hazard Ratio = 1.05; 95% CI = 1.00–1.11; P = .06).
Our data do not provide quite as much support for the usage of grafts in the elderly patients, but it certainly questions the recommendations provided by the FFBI. Although not directly addressed by our study, the fact remains that too many patients, especially the elderly patients, are initiating dialysis with catheters. In 2012, 80% of patients aged 80 to 89 years and an astonishing 90% of patients aged >90 years initiated dialysis with a central venous catheter.27 The increased morbidity and mortality associated with catheter usage is well documented. Compared to grafts and fistulas, individuals receiving dialysis through catheters have higher risks of all-cause mortality, fatal infections, and cardiovascular events.13 This has led a number of groups to suggest modifications in the approach to dialysis. From “Catheter last, fistula not-so-first”34 to the 2012 amendment by the FFBI35 setting a goal of central venous catheter usage of less than 10% in patients undergoing hemodialysis for more than 90 days, the approach to dialysis is clearly shifting.
Our study had some limitations. The assessment of patency outcomes was determined retrospectively, which impaired our ability to assess maturity rates and time to dialysis access. Further, the vascular access procedures were performed by a number of surgeons, each with different experience and techniques. Nevertheless, our results raise concern for the generalizability of the FFBI.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Saran R, Li Y, Robinson B, Abbott KC, et al. US Renal Data System 2015 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2016;67(3 suppl 1):Svii, S1–S305. doi: 10.1053/j.ajkd.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.III NKF-K/DOQI. Clinical practice guidelines for vascular access: update 2000. Am J Kidney Dis. 2001;37(1 suppl 1):S137–S181. doi: 10.1016/s0272-6386(01)70007-8. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed February 7, 2016];Fistula First National Access Improvement Initiative. 2006 http://www.fistulafirst.org/
- 4.Sgroi MD, Patel MS, Wilson SE, Jennings WC, Blebea J, Huber TS. The optimal initial choice for permanent arteriovenous hemodialysis access. J Vasc Surg. 2013;58(2):539–548. doi: 10.1016/j.jvs.2013.04.058. [DOI] [PubMed] [Google Scholar]
- 5.Hemodialysis Adequacy 2006 Work Group. Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48(suppl 1):S2–S90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 6.Allon M, Ornt DB, Schwab SJ, et al. Factors associated with the prevalence of arteriovenous fistulas in hemodialysis patients in the HEMO study. Hemodialysis (HEMO) Study Group. Kidney Int. 2000;58(5):2178–2185. doi: 10.1111/j.1523-1755.2000.00391.x. [DOI] [PubMed] [Google Scholar]
- 7.Allon M, Robbin ML. Resolved: fistulas are preferred to grafts as initial vascular access for dialysis. Con J Am Soc Nephrol. 2008;19(9):1632–1633. [PubMed] [Google Scholar]
- 8.Philadelphia Department of Public Health. Philadelphia Adult (18+) Chronic Disease Indicators by Planning District, 2010. [Accessed February 29, 2016];2010 PHMC Household Health Survey. http://www.phila.gov/health/pdfs/Philadelphia_obesity%20and%20chronic%20disease%20health%20indicators_2010.pdf.
- 9.Philadelphia Department of Public Health. [Accessed February 29, 2016];Overview of Chronic Disease and Healthy Eating and Active Living Indicators for Philadelphia Adults and Children. 2011 http://www.phila.gov/health/pdfs/Philadelphia_obesity%20and%20chronic%20disease%20health%20indicators_2010.pdf.
- 10.Singh N, Starnes BW, Andersen C. Successful angioaccess. Surg Clin North Am. 87(5):1213–1228. doi: 10.1016/j.suc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Sidawy AN, Gray R, Besarab A, et al. Recommended standards for reports dealing with arteriovenous hemodialysis access. J Vasc Surg. 2002;35(3):603–610. doi: 10.1067/mva.2002.122025. [DOI] [PubMed] [Google Scholar]
- 12.Vascular Access Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(suppl 1):S248–S273. doi: 10.1053/j.ajkd.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 13.Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int. 2002;62(4):1109–1124. doi: 10.1111/j.1523-1755.2002.kid551.x. [DOI] [PubMed] [Google Scholar]
- 14.Dember LM, Beck GJ, Allon M, et al. Dialysis Access Consortium Study Group. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis. A randomized controlled trial. JAMA. 2008;299(18):2164–2171. doi: 10.1001/jama.299.18.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon BS, Beck GJ, Vazquez MA. Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med. 2009;360(21):2191–2201. doi: 10.1056/NEJMoa0805840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman JS, O’Connor TZ, Zhang JH, et al. Veterans Affairs Cooperative Study Group on Hemodialysis Access Graft Thrombosis. Randomized controlled trial of clopidogrel plus aspirin to prevent hemodialysis access graft thrombosis. J Am Soc Nephrol. 2003;14(9):2313–2321. doi: 10.1097/01.asn.0000081661.10246.33. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman J. Use of aspirin in hemodialysis patients. Semin Dial. 2001;14(1):72–73. doi: 10.1046/j.1525-139x.2001.00019-2.x. [DOI] [PubMed] [Google Scholar]
- 18.Mousa AY, Patterson W, Abu-Halimah S, et al. Patency in arteriovenous grafts in hemodialysis patients. Vasc Endovasc Surg. 47(6):438–443. doi: 10.1177/1538574413493678. [DOI] [PubMed] [Google Scholar]
- 19.Ravani P, Palmer SC, Oliver MJ, et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J Am Soc Nephrol. 2013;24(3):465–473. doi: 10.1681/ASN.2012070643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akoh JA. Prosthetic arteriovenous grafts for hemodialysis. J Vasc Access. 2009;10(3):137–147. doi: 10.1177/112972980901000301. [DOI] [PubMed] [Google Scholar]
- 21.Lazarides MK, Georgiadis GS, Antoniou GA, Staramos DN. A meta-analysis of dialysis access outcome in elderly patients. J Vasc Surg. 2007;45(2):420–426. doi: 10.1016/j.jvs.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 22.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D. Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I) J Am Soc Nephrol. 2006;17(11):3204–3212. doi: 10.1681/ASN.2006030190. [DOI] [PubMed] [Google Scholar]
- 23.Swindlehurst N, Swindlehurst A, Lumgair H, et al. Vascular access for hemodialysis in the elderly. J Vasc Surg. 2011;53(4):1039–1043. doi: 10.1016/j.jvs.2010.09.068. [DOI] [PubMed] [Google Scholar]
- 24.Ocak G, Rotmans JI, Vossen CY, et al. Type of arteriovenous vascular access and association with patency and mortality. BMC Nephrol. 2013;14:79. doi: 10.1186/1471-2369-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhee CM, Lertdumrongluk P, Streja E, et al. Impact of age, race and ethnicity on dialysis patient survival and kidney transplantation disparities. Am J Nephrol. 2014;39(3):183–194. doi: 10.1159/000358497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kucirka LM, Grams ME, Lessler J, et al. Association of race and age with survival among patients undergoing dialysis. JAMA. 2011;306(6):620–626. doi: 10.1001/jama.2011.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarkowsky DS, Arhuidese IJ, Hicks CW, et al. Racial/ethnic disparities associated with initial hemodialysis access. JAMA Surg. 2015;150(6):529–536. doi: 10.1001/jamasurg.2015.0287. [DOI] [PubMed] [Google Scholar]
- 28.Cui J, Steele D, Wenger J, et al. Hemodialysis arteriovenous fistula as first option not necessary in elderly patients. J Vasc Surg. 2016;63(5):1326–1332. doi: 10.1016/j.jvs.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 29.Praga M, Merello JI, Palomares I, et al. Type of vascular access and survival among very elderly hemodialysis patients. Nephron Clin Pract. 2013;124(1–2):47–53. doi: 10.1159/000355694. [DOI] [PubMed] [Google Scholar]
- 30.Tamura MK, Tan JC, O’Hare AM. Optimizing renal replacement therapy in older adults: a framework for making individualized decisions. Kidney Int. 2012;82(3):261–269. doi: 10.1038/ki.2011.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moist LM, Lok CE, Vachharajani TJ, et al. Optimal hemodialysis vascular access in the elderly patient. Semin Dial. 2012;25(6):640–648. doi: 10.1111/sdi.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keuter XH, De Smet AA, Kessels AG, van der Sande FM, Welten RJ, Tordoir JH. A randomized multicenter study of the outcome of brachial-basilic arteriovenous fistula and prosthetic brachial-antecubital forearm loop as vascular access for hemodialysis. J Vasc Surg. 2008;47(2):395–401. doi: 10.1016/j.jvs.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 33.DeSilva RN, Patibandla BK, Vin Y, et al. Fistula first is not always the best strategy for the elderly. J Am Soc Nephrol. 2013;24(8):1297–1304. doi: 10.1681/ASN.2012060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wish JB. Catheter last, fistula not-so-first. J Am Soc Nephrol. 2015;26(1):5–7. doi: 10.1681/ASN.2014060594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vassalotti J, Jennings WC, Beathard GA, et al. the Fistula First Breakthrough Initiative Community Education Committee. Fistula First Breakthrough Initiative: targeting catheter last in fistula first. Semin Dial. 2012;25(3):303–310. doi: 10.1111/j.1525-139X.2012.01069.x. [DOI] [PubMed] [Google Scholar]