Abstract
Sialylglycopeptide (SGP) is a complex bi-antennary N-glycan bearing a short peptide fragment that can be isolated from the yolk of hen eggs. This natural product has gained popularity as a starting material for the semi-synthesis of N-glycans. We have found that current isolation methods provide a glycopeptide contaminated with several related structures, one being a glycopeptide having a hexose directly attached to peptide backbone, most like through the hydroxyl containing side chain of the threonine moiety. Furthermore, current methods employ fresh egg yolks that need to be lyophilized and involve several tedious purification steps. Herein, we report a convenient method for the isolation of gram quantities of homogeneous SGP from commercially available egg yolk powder using solid/liquid extraction and HILIC-HPLC purification.
Keywords: Sialylglycopeptide, N-glycan, Isolation, Egg yolk powder, HILIC-HPLC
1. Introduction
Isolation of advanced glycans from natural sources can provide attractive starting materials for the semi-synthesis of complex glycans of biological importance. Sialylglycopeptide (SGP, Fig. 1A), having an A2G2S2 structure, where A stands for GlcNAc, G stands for Gal, S stands for Neu5Ac, is abundant in the yolk of hen eggs [1] and is commonly employed as a starting material for the preparation N-glycans by enzymatic or chemical manipulations [2–6]. Initially, SGP was isolated from fresh egg yolks [1] through phenol extraction followed by an elaborate purification process involving six size-exclusion and ion-exchange columns. In 2012, an improved purification procedure was reported employing porous graphite carbon (PGC) instead of size-exclusion column chromatography [7]. Further optimization of the SGP isolation procedure involved the removal of the lipids via diethyl ether extraction, thereby eliminating problems caused by emulsification. Additionally, an aqueous acetone solution was used for SGP extraction, followed by an active carbon column to provide gram quantities of SGP (Fig. 1B) [8].
Fig. 1.
A The structure of sialylglycopeptide (SGP). B Previously reported SGP isolation protocols. C Newly developed strategy for SGP isolation. Legend:
 GlcNAc;
GlcNAc;
 Man;
Man;
 Gal;
Gal;
 Neu5Ac; ○ Hex.
Neu5Ac; ○ Hex.
Despite the greatly improved protocol for SGP isolation [9–12], several drawbacks remain. To obtain gram amounts of SGP, kilogram quantities of dried egg yolk are need, which requires cracking, separating, and lyophilization of hundreds of fresh eggs. Furthermore, the large volume of diethyl ether for lipid extraction poses a safety concern. We have found that the current isolated protocol provides SGP contaminated with several closely related structures. To address these issues, we have developed a convenient extraction and purification procedure that gives homogenous SGP employing commercially available egg yolk powder using aqueous ethanol as the extracting solvent, and hydrophilic-interaction-chromatography (HILIC) for further purification (Fig. 1C).
2. Results and discussion
2.1. Commercially available egg yolk powder as a source for SGP
To find an alternative source of SGP in lieu of fresh hen eggs, we have examined commercially available egg yolk powder [13] that is routinely used by the food industry as an emulsifying agent and a source of high quality protein. One kilogram of egg yolk powder is equivalent to 100–150 egg yolks and is available in large quantities for an economical price ($4.45/kg) [14]. Using bulk egg yolk powder has a concern that the preparation procedure involves “spray drying” in which atomized liquid egg is sprayed into a hot air stream well above 100 °C to evaporate water [13] which may compromise the integrity of the glycopeptide.
When egg yolk powder was extracted with water and analyzed by ESI-MS, despite significant emulsification resulting from the presence of lipids, we detected a signal at m/z 1431 (2−) corresponding to SGP indicating that egg yolk powder may be an appropriate source of the glycopeptide. Approximately, 60% of the weight of egg yolk powder is of lipids [15], and its emulsifying properties cause difficulties in SGP extraction. We investigated several organic solvents commonly used in the food industry for lipid extraction including acetone [16], diethyl ether [17], hexanes [18], chloroform/methanol mixtures [19,20], and ethanol [21]. Egg yolk powder was stirred in each solvent for 30 min, filtered, and the insoluble material was dried in vacuo to determine the weight loss due to the removal of the lipids (Table 1). The combination of chloroform and methanol (entry 1), also known as the Folch method [19,20], gave the best result with a weight reduction of 61.9%. However, due to the toxicity of chloroform, it is not a preferred solvent for large-scale extraction. Acetone (entry 2) and hexanes (entry 3) extractions resulted in a weight reduction of 44.7% and 46.3%, respectively. Diethyl ether (entry 4), which was used in the previous reported isolation method [8], and ethanol (entry 5) resulted in a 50.8% and 51.2% reduction in weight, respectively. Among the solvents tested, ethanol proved to be the best choice due to its low toxicity and affordability. We found that performing a second extraction with ethanol resulted in an additional 20% reduction in weight which (entry 6) making this protocol as efficient as the chloroform/methanol mixture (entry 1). After removal of the lipids, the remaining solid became off-white powder that was easy to handle.
Table 1.
Removal of the lipids in egg yolk powder using different solvents.
| Entry | Solvent | Extracted lipid/whole weight (%) |
|---|---|---|
| 1 | Chloroform: Methanol, 2: 1 | 61.9 |
| 2 | Acetone | 44.7 |
| 3 | Hexanes | 46.3 |
| 4 | Diethyl ether | 50.8 |
| 5 | Ethanol | 51.2 |
| 6 | Ethanol, twice | 61.5 |
2.2. Optimizing the conditions for SGP extraction
Next, we focused on extracting SGP from the lipid-free material to identify a solvent mixture amenable to large-scale preparations that would extract the highest quantity of SGP while limiting the amount of undesired egg yolk components. Several different aqueous solution mixtures were examined including acetone, acetonitrile, methanol, and ethanol (Fig. 2A), which were analyzed by HILIC-ESI-MS (see later section for details). The LC-MS chromatograms showed two peaks having retention times of 27.9 and 25.3 min corresponding to the desired symmetrical SGP and an asymmetric derivative lacking a sialic acid and galactosyl moiety. It was found that all aqueous solutions could extract SGP, however, subtle differences were observed. An impurity was detected in the acetone/water extracts near the peak of SGP at 24.3 min, while it was absent in the other extracts. The chromatogram of the methanol and ethanol extractions were quite similar, and showed fewer impurities compared to acetonitrile. Due to the lower toxicity of ethanol compared to methanol, we decided to use aqueous ethanol as the solvent for SGP extraction.
Fig. 2.
A LC-MS chromatogram of the crude extracts from different solvents. B The relative amount of SGP in the crude extracts using aqueous ethanol solutions with different concentrations.
Next, we investigated the effect of ethanol concentration on SGP extraction efficiency. Fig. 2B shows the relative amount of SGP detected by LC-MS in each extraction mixture, normalized against the highest value. This data shows that SGP was absent from extractions with ethanol concentrations greater than 80% (v/v), but present starting at 70%, albeit in low yield. The amount of extracted product appeared to be fairly consistent from 10% → 60% aqueous ethanol. However, when the aqueous content was high (10–20% ethanol), a substantial amount of undesired protein was extracted which caused problems in further purification steps. From a practical point of view, 40% ethanol was chosen for large-scale extraction.
2.3. Optimized procedure for SGP isolation from egg yolk powder
Having successfully optimized the solvent conditions for lipid removal and SGP extraction from egg yolk powder, we set out to improve the carbon column chromatography purification protocol. The fine particle size of active carbon creates significant back pressure resulting in a slow flow rate, and using this procedure, it was not uncommon that a single run took several days. To increase the flow rate, the ratio of active carbon/Celite was decreased from 2/1 to 1/1 (wt/wt), which reduced the back pressure resulting in faster elution flow rates. The bulk of impurities could be removed by using a gradient of 0 → 15% acetonitrile/water (0.1% TFA v/v) mixture while desired SGP could be eluted from the column using 25% acetonitrile in water (0.1% TFA v/v). Miscellaneous salts could be removed by P-2 size exclusion column chromatography. The NMR and mass spectrometry data of the purified SGP matched previous reported data and on average 0.8 mg SGP/g egg yolk powder was isolated.
2.4. Heterogeneity of isolated SGP
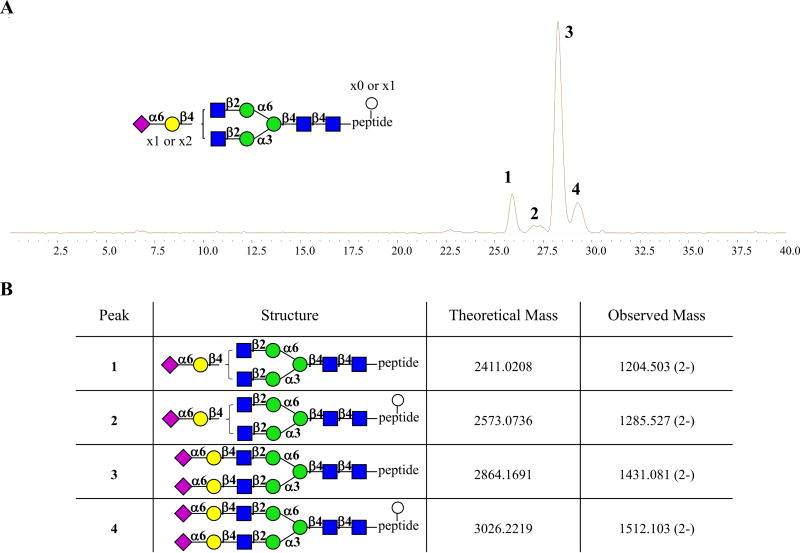
While our optimized purification method greatly facilitated the isolation of SGP from egg yolk powder, we noticed that regardless of the source or isolation method, the recovered SGP was not homogenous, and contained ~10% impurities due to the presence of several closely related structures. The use of SGP as starting material for N-glycan syntheses requires homogenous SGP, and therefore several HPLC methods were considered for further purification. It was found that both reverse phase and porous graphitized carbon (PGC) chromatography could not separate the individual components. Gratifyingly, hydrophilic interaction chromatography (HILIC) [22], a technique used to separate highly polar compounds [23], showed an incredible ability to fractionate SGP into homogeneous constituents (Fig. 3A).
Fig. 3.
HILIC-MS analysis of isolated SGP. A Analytic LC-MS chromatogram of isolated SGP showing four components. B Assignment for the peaks.
Four peaks in the LC-MS chromatogram were observed with the major component (peak 3), eluting at 28.2 min having a mass of 2864.17 Da matching the expected mass of the desired symmetrical glycopeptide A2G2S2. Peak 1 had a mass of 2411.02 Da, corresponding to the glycopeptide bearing an A2G1S1 glycoform. Interestingly, peak 2 and 4 had a mass of 2573.07 and 3026.22 Da, respectively corresponding to peak 1 and 3 with an increase in mass of 162 Daltons, indicating the presence of an additional hexose (Fig. 3B). To determine if the hexose was linked to the glycan or the peptide backbone, SGP was treated with pronase, which is a commercially available mixture of various amidases that cleaves the aglycon peptide to a single asparagine (Fig. 4A). The resulting LC-MS chromatogram of the digested product showed only the presence of 5, A2G1S1-Asn and 6, A2G2S2-Asn, indicating that the hexose is directly linked the peptide (Fig. 4B). To support this finding, we pooled the four peaks and treated the mixture with PNGase F (Fig. 4A), which allowed analysis of the intact peptide. As expected, the ESI-MS chromatogram showed signals at 659 and 821 Da corresponding to peptide 7 and peptide 8 bearing a hexose, respectively (Fig. 4C).
Fig. 4.
Determining the position of the hexose on the isolated SGP. A The isolated SGP could be treated with pronase (top) to trim the peptide down to Asn or PNGase F (bottom) to remove the peptide completely. B The LC-MS chromatogram of pronase treated SGP, showing disappearance of the additional hexose. C ESI of the cleaved peptide from isolated SGP with PNGase F. The peak at 821 corresponds to the peptide with hexose.
Preparative HILIC-HPLC was used to obtain homogenous SGP in gram quantities and NMR analysis further confirmed the identity of the resulting compound (Fig. 5A). When using the SGP for N-glycan synthesis, we found it is beneficial to treat the product after the carbon/Celite column with a general neuraminidase and galactosidase to afford the A2 glycan 10 (Scheme 1). Further treatment with pronase furnished a homogeneous product 11, A2-Asn, that could be purified by preparative HILIC-HPLC to obtain a highly pure product (Fig. 5B).
Fig. 5.
NMR spectra of compounds purified by semi-preparative HPLC using a HILIC column. A SGP. B A2-Asn.
Scheme 1.
Trimming SGP using neuraminidase, galactosidase and pronase.
It may be possible that during the spray drying process, amino acids of SGP epimerize including the asparagine moiety harboring the N-glycan. We carefully compared the 1H (900 MHz) and 13C NMR (225 MHz) spectral data of 11 with previously reported data of the same asparagine-linked oligosaccharide obtained from fresh egg yolks [25,26]. Gratifying, no differences were detected, and furthermore careful analysis of key 1H and 13C signals suggesting the absence of an epimeric compound. Thus, the methylene group at the β position of Asn appeared as two doublets of doublets. One proton appears at δ 2.80 (J = 17.2, 4.2 Hz), the other was found at δ 2.73 (J = 17.2, 6.9 Hz) with a corresponding 13C signal at δ 34.74. The Asn α-proton at δ 3.85 (J = 6.9, 4.2 Hz) appeared as a doublet of doublets with a corresponding carbon signal is at δ 50.73 ppm. The anomeric proton of GlcNAc-1 was observed as a doublet at δ 4.93 (J = 9.8 Hz) with a corresponding 13C signal at δ 77.95. We did not observe additional proton/carbon signals of the reducing anomeric GlcNAc or the Asn further supporting the absence of epimers (Figs. S1–S8).
3. Conclusion
We have developed a convenient method for isolating gram quantities of homogeneous SGP from commercially available egg yolk powder. Ethanol was chosen as the solvent to remove lipids from the bulk egg yolk powder, and a 40% aqueous ethanol solution for extracting SGP. The choice of solvent is amenable to large-scale extractions and the optimized ratio of carbon/Celite allowed for faster elution of the desired glycopeptide. Analytical HILIC-HPLC analysis provided important insights into the heterogeneity of the isolated SGP. Treatment of the heterogeneous mixture with exoglycosidases and either pronase or PNGaseF resulted in homogeneous material that could undergo a final HPLC purification by preparative HILIC chromatography to yield an analytically pure substance. This new method of purifying SGP from readily available egg yolk powder will aid the synthesis of complex N-glycans needed in the field of glycobiology and analytical chemistry.
4. Experimental section
4.1. Materials and reagents
Chemicals and solvents were purchased from Sigma-Aldrich and used without further purification. Active carbon, NORIT™ SA 2, decolorizing grade, catalog 40403 and Celite®545 was purchased from Acros. Egg yolk powder was purchased from Natural Foods Inc, Toledo, OH (item 40504) and was stored at 4 °C.
Pronase (from Streptomyces griseus) was purchased from Sigma-Aldrich, #P5147. Neuraminidase (Clostridium perfringens), #P0720 and PNGase F (Flavobacterium meningosepticum), #P0704 were from New England Biolabs. Galactosidase (Aspergillus niger) was purchased from Megazyme, #E-BGLAN.
4.2. LC-MS analysis
LC-MS was performed on a Shimadzu LC-ESI-IT-TOF with a Waters XBridge BEH, Amide column, 2.5 µm, 130 Å, 2.1 × 150 mm using ESI as detector at a flow rate of 0.16 mL/min. Mobile phase A was 100 mM ammonium formate in water, adjusted to pH 3.43 with formic acid; mobile phase B was 100% aceteonitrile.
| Time (min) | A (%) | B (%) |
|---|---|---|
| 0 | 35 | 65 |
| 25 | 50 | 50 |
| 40 | 50 | 50 |
| 45 | 60 | 40 |
| 50 | 60 | 40 |
| 60 | 35 | 65 |
4.3. Optimization of lipid removal
Organic solvent (40 mL) was added to egg yolk powder (10 g) in a round bottom flask. The suspension was stirred at room temperature for 2 h and then filtered and dried to yield an off-white powder.
4.4. Optimization of SGP extraction
Ethanol solutions (2 mL) (Fig. 2B) were added to egg yolk powder (1 g), which has been treated with 40% ethanol for lipid removal, in a 15 mL centrifuge tube. The mixture was vortexed for 1 h at room temperature, centrifuged, and the top layer was decanted and used for LC-MS analysis.
4.5. Large-scale SGP extraction
Egg yolk powder (2.27 kg, 5 lb) was suspended in ethanol (4 L of 95%) and mechanically stirred for 2 h, twice, to extract lipids from the bulk starting material. The suspension was filtered and dried via suction filtration to yield an off-white, dry powder; the filtrate was discarded. To extract the SGP, the off-white powder was twice suspended in 3 L of 40% ethanol and mechanically stirred for 2 h. The filtrates were pooled and concentrated under reduced pressure at 40 °C. It is important to stress that during each filtration, the solid should be washed thoroughly with plenty of extraction solvent to ensure high recovery, since the long filtration time from the large scale would lead to evaporation of the solvent in the solid. Cold aqueous ethanol (40%, 100 mL) was added to the concentrated solution to precipitate proteins which were be removed by centrifugation (3000 rpm, 10 min). The resulting solution was concentrated and purified by an active carbon/Celite column chromatography (500 g of active carbon and 500 g of Celite). The column was eluted with 3 L of water (0.1% TFA), 3 L of 5% acetonitrile in water (0.1% TFA v/v), and 10% acetonitrile in water (0.1% TFA v/v). SGP was released from the column by eluting with 25% acetonitrile in water (0.1% TFA v/v). Fractions of 250 mL were collected and analyzed by ESI-MS. Fractions containing the desired product were concentrated in vacuo and subjected to size chromatography (Bio-Rad P-2, fine particle size 45–90 µm, column dimensions 5.0 cm × 80 cm) and eluted with a 0.1 M ammonium bicarbonate solution. SGP containing fractions were lyophilized to yield a white, fluffy powder (1.82 g, or 0.8 mg SGP/g of egg yolk powder).
4.6. Neuraminidase, galactosidase trimming and pronase treatment on SGP
Isolated SGP (225 mg) was dissolved in a sodium acetate buffer (50 mM, pH 5.5, 5 mL) with CaCl2 (5 mM). To the mixture was added neuraminidase (20 µL, Clostridium perfringens) The reaction was incubated at 37 °C with shaking overnight until ESI indicated all the sialic acid had been removed.
The pH of the reaction mixture was adjusted to 4.5 with acetic acid. To the mixture BSA (3 mg) and β-galactosidase (150 µL, Aspergillus niger) were added. The reaction was incubated at 37 °C with shaking. After overnight incubation, another 100 µL of galactosidase were added, and the reaction was monitored until ESI indicated all the galactose had been removed. The protein in the mixture was removed by centrifugation using a 10K MWCO filter.
The pH of the filtrate was adjusted to 8.0 with sodium hydroxide (1 M), and additional CaCl2 (50 µL, 500 mM) was added. To this mixture was added pronase (100 mg, Streptomyces griseus). The reaction was incubated at 37 °C with shaking for 5 days. When ESI indicated the reaction had completed, pronase was removed using a centrifuge filter (10K MWCO). The filtrate was lyophilized and further purified via size chromatography (Bio-Rad P-2, fine particle size 45–90 µm, 2 × 80 cm), eluting with a 0.1 M ammonium bicarbonate solution. The fractions with the product were collected, lyophilized to provide the compound 11, A2-Asn (75 mg).
4.7. Semi-preparative HILIC-LC-MS protocol
Semi-preparative HILIC-LC was performed on a Shimadzu LC-ESI-IT-TOF with a Waters XBridge BEH, Amide column, 5 µm, 10 × 250 mm at a flow rate of 3.8 mL/min, with 1/% of the flow is diverted to the ESI-MS detector using a splitter. Mobile phase A was 100 mM ammonium formate in water, adjusted to pH 3.43 with formic acid; mobile phase B was 100% aceteonitrile. A general condition for separation is as follows:
| Time (min) | A (%) | B (%) |
|---|---|---|
| 0 | 35 | 65 |
| 40 | 50 | 50 |
| 45 | 60 | 40 |
| 50 | 60 | 40 |
| 60 | 35 | 65 |
Supplementary Material
Acknowledgments
This research was supported by the National Institute of General Medical Sciences (U01GM120408, R01GM090269 and P01GM107012 to G.J.B.), the National Heart, Lung, and Blood Institute (P01HL107150 to G.J.B.), and the National Cancer Institute (F31CA180478 to A.R.P.) from the US National Institutes of Health (NIH). The research benefitted from instrumentation provided by NIH Grant S10RR027097. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors would like to thank Drs. Chantelle J. Capicciotti and Xiuru Li for their assistance with separating egg yolks from the whole eggs at the initial stage of this project.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.carres.2017.10.001.
References
- 1.Seko A, Koketsu M, Nishizono M, Enoki Y, Ibrahim HR, Juneja LR, Kim M, Yamamoto T. Biochim. Biophys. Acta - Gen. Subj. 1997;1335:23. doi: 10.1016/s0304-4165(96)00118-3. [DOI] [PubMed] [Google Scholar]
- 2.Kajihara Y, Suzuki Y, Yamamoto N, Sasaki K, Sakakibara T, Juneja LR. Chem. - Eur. J. 2004;10:971. doi: 10.1002/chem.200305115. [DOI] [PubMed] [Google Scholar]
- 3.Huang W, Wang D, Yamada M, Wang L-X. J. Am. Chem. Soc. 2009;131:17963. doi: 10.1021/ja9078539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang W, Giddens J, Fan S-Q, Toonstra C, Wang L-X. J. Am. Chem. Soc. 2012;134:12308. doi: 10.1021/ja3051266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nycholat CM, Peng W, McBride R, Antonopoulos A, de Vries RP, Polonskaya Z, Finn MG, Dell A, Haslam SM, Paulson JC. J. Am. Chem. Soc. 2013;135:18280. doi: 10.1021/ja409781c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng W, de Vries RP, Grant OC, Thompson AJ, McBride R, Tsogtbaatar B, Lee PS, Razi N, Wilson IA, Woods RJ, Paulson JC. Cell Host Microbe. 2017;21:23. doi: 10.1016/j.chom.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou Y, Wu Z, Chen L, Liu X, Gu G, Xue M, Wang PG, Chen M. J. Carbohydr. Chem. 2012;31:436. [Google Scholar]
- 8.Sun B, Bao W, Tian X, Li M, Liu H, Dong J, Huang W. Carbohydr. Res. 2014;396:62. doi: 10.1016/j.carres.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 9.A number of patents describe extraction/purification methods of SGP including the use of delipidated egg yolk powder. These methods employ protease digestion [10], aqueous extraction of delipidated egg yolk powders followed by either ethanol precipitation [11], and addition of a deproteinizing agent [12]. The large amount of undesired proteins in egg yolks makes these separation quite tedious.
- 10.Fukae K. US 7955819 B2. US patent: Process for producing sugar chain asparagine derivative. 2011
- 11.Sugawara S, Osumi K. US 8809496 B2. US patent: Production method of 11-sugar sialylglycopeptide. 2014
- 12.Miyauchi T. US 20160168204 A1. US patent: Method for purifying oligosaccharide peptide. 2016
- 13.Zeidler G. In: Commercial Chicken Meat and Egg Production. Bell DD, Weaver WD, editors. Springer US; Boston, MA: 2002. p. 1163. [Google Scholar]
- 14.U.S. Department of agriculture. Agricultural Marketing Service Livestock, Poultry & Grain Market News. 2017 https://www.ams.usda.gov/mnreports/pybshellegg.pdf.
- 15.Juneja L. In: Hen Eggs, Their Basic and Applied Science. Yamamoto T, Juneja LR, Hatta H, Kim M, editors. CRC Press; 1996. p. 73. [Google Scholar]
- 16.Borges SV, Martucci ET, Müller CO. LWT - Food Sci. Technol. 1996;29:687. [Google Scholar]
- 17.Wu P, Pan Y, Yan J, Huang D, Li S. Molecules. 2016;21:106. doi: 10.3390/molecules21010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furusawa N, Ozaki A, Nakamura M, Morita Y, Okazaki K. J. Chromatogr. A. 1999;830:473. doi: 10.1016/s0021-9673(98)00873-5. [DOI] [PubMed] [Google Scholar]
- 19.Folch J, Ascoli I, Lees M, Meath JA, LeBaron FN. J. Biol. Chem. 1951;191:833. [PubMed] [Google Scholar]
- 20.Folch J, Lees M, Stanley GHS. J. Biol. Chem. 1957;226:497. [PubMed] [Google Scholar]
- 21.Aro H, Järvenpää EP, Könkö K, Sihvonen M, Hietaniemi V, Huopalahti R. Eur. Food Res. Technol. 2009;228:857. [Google Scholar]
- 22.Huang Y, Nie Y, Boyes B, Orlando R. J. Biomol. Tech. 2016;27:98. doi: 10.7171/jbt.16-2703-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buszewski B, Noga S. Anal. Bioanal. Chem. 2012;402:231. doi: 10.1007/s00216-011-5308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto N, Ohmori Y, Sakakibara T, Sasaki K, Juneja LR, Kajihara Y. Angew. Chem. Int. Ed. 2003;42:2537. doi: 10.1002/anie.200250572. [DOI] [PubMed] [Google Scholar]
- 26.Sato H, Kajihara Y. Carbohydr. Res. 2005;340:469. doi: 10.1016/j.carres.2004.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.