Abstract
The overall goal of this work is to study the combined effects of Mini-B, a 34 residue synthetic analog of the lung surfactant protein SP-B, and cholesterol, a neutral lipid, on a model binary lipid mixture containing dipalmitolphosphatidylcholine (DPPC) and palmitoyl-oleoyl-phosphatidylglycerol (POPG), that is often used to mimic the primary phospholipid composition of lung surfactants. Using surface pressure vs. mean molecular area isotherms, fluorescence imaging and analysis of lipid domain size distributions; we report on changes in the structure, function and stability of the model lipid-protein films in the presence and absence of varying composition of cholesterol. Our results indicate that at low cholesterol concentrations, Mini-B can prevent cholesterol’s tendency to lower the line tension between lipid domain boundaries, while maintaining Mini-B’s ability to cause reversible collapse resulting in the formation of surface associated reservoirs. Our results also show that lowering the line tension between domains can adversely impact monolayer folding mechanisms. We propose that small amounts of cholesterol and synthetic protein Mini-B can together achieve the seemingly opposing requirements of efficient LS: fluid enough to flow at the air–water interface, while being rigid enough to oppose irreversible collapse at ultra-low surface tensions.
Keywords: Lung surfactants, Compressibility, Surface tension, Mini-B, Cholesterol, Line tension
1. Introduction
Lung surfactant [LS], produced by Type II epithelial cells, is a complex mixture of lipids and proteins present primarily in the alveolar lining of the lungs [1,2]. LS helps in lowering surface tension at the air–water interface with expiration, thereby reducing the energy needed for breathing and improving lung compliance [3,4]. They also form a line of defense against any foreign particle that is small enough to make its way through the air canal [5]. It has been firmly established that there is a lack of LS in cases of Neonatal Respiratory Distress Syndrome [NRDS] occurring in infants [6]. Currently NRDS is treated with a high success by using exogenous surfactant, referred to as Surfactant Replacement Therapy (SRT) [7]. In comparison to NRDS, a dysfunction/impairment of the surfactant may lead to Acute Respiratory Distress Syndrome (ARDS)/Acute Lung Injury (ALI). Each year, a staggering 50,000–190,000 case of ALI/ARDS is reported in USA itself [8]. While SRT has also been proposed for treating this condition as well, the success of SRT is currently debated, possibly because of a lack of complete understanding of the biophysical interactions between different LS components [9]. A lack of complete understanding of the biophysical role of the different constituents in combination has resulted in a lack of consensus on the composition of lung surfactants used in SRT, and forms the main motivation of this work.
Although native surfactants differ by species in their detailed composition, almost all contain about 90% by weight lipid and 10 wt.% of the surfactant specific proteins SP-A, SP-B, SP-C and SP-D [10,11]. The dominant phospholipid component is disaturated dipalmitoylphosphatidylcholine (DPPC, 30–70%), along with some unsaturated phosphatidylcholine (PC, 25–35%), anionic phospholipids like phosphatidylglycerol (PG), saturated fatty acids like palmitic acid, neutral lipids like cholesterol as well as minor fractions of phosphatidylethanolamine(PE) and sphingomyelin [12–14]. Although less in quantity, surfactant proteins play a crucial part. The hydrophobic SPB and SPC are involved in enhancing adsorption of LS to the air–water interface, whereas, the hydrophilic SPA and SPD mainly play a part in immune response [15].
Among the different constituents, the presence of cholesterol in SRT is highly disputed, primarily because its biophysical function in the proper functioning of the lung surfactant remains mostly unknown. Early experimental studies using Langmuir troughs and pulsating bubble surfactometer suggested that any amount of cholesterol is detrimental to the proper functioning of the lung, since the ability of the lung surfactant to reach a low surface tension [16–19] was inhibited in most of these studies. Cholesterol changes the morphology of DPPC domains even at low concentrations, which in turn can alter the surface tension lowering ability of DPPC [20].
On the other hand, it is important to note that cholesterol forms the major neutral lipid component of endogenous lung surfactants (3–10 wt.%), yet near zero surface tensions have been measured in the lung [21], suggesting that physiological amounts of cholesterol do not interfere with the proper functioning of the lung. Lessons learned from evolutionary studies on mammalian lung show that high amounts of cholesterol are present in more primitive animals with sac-like lungs [22]. The cholesterol content has been found to be 1.5 fold higher in hetero-thermic animals undergoing torpor [22]. Kim et al. showed that even small amounts (1 wt.%) can alter the surface viscosity of DPPC films, suggesting that addition of cholesterol to LS mixtures can be beneficial during intra-tracheal delivery of LS and enhance efficient spreading of synthetic LS, once it adsorbs to the interface. Interestingly, Gunasekara et al., found that physiological amount of cholesterol has no effect on the surface activity of a natural surfactant BLES (Bovine Lipid Extract Surfactant) and only concentrations as high as 20% by weight, imposed harmful effects on the function of this natural LS [23]. This suggests the highly plausible explanation that minor components in native LS mixtures can counter some of the deleterious effects of cholesterol that was observed for early studies. Therefore, in order to use cholesterol in the replacement mixtures, any deleterious effects must be countered with other components. For example, Gomez-Gil et al. showed that surfactant protein SP-C can counter the deleterious effects of cholesterol, if used in the right proportions, suggesting that controlled amounts of cholesterol and proteins should be considered while developing future surfactants.
SP-B is the only one among the four surfactant proteins that is essential for effective breathing. Therefore, in this paper, we focus on developing a biophysical understanding of protein interactions with the neutral lipid composition of the lung surfactant. Specifically, we report on biophysical properties of DPPC:POPG films containing different amounts of cholesterol, in the absence or presence of 1, 2.5 or 5 wt.% Mini-B. Mini-B, a synthetic analog of the surfactant protein SP-B, is a 34 amino acid residue of the full length native protein with enhanced in vivo and in vitro surfactant properties, where the predicted helices of the N and C terminals of the native protein are ether linked [24,25], while maintaining the net charge (+7) of the native protein [26]. Mini-B can also aid an artificial phospholipase-resistant surfactant, di-ether phosphonolipid (DEPN) in lowering surface tension efficiently [27], with possible application in treating ALI/ARDS. Since an elevated amount of cholesterol is also associated with impaired surfactant activity in ALI/ARDS [28], this study also aims to address if addition of Mini-B is sufficient to counter the negative effects of elevated amounts of cholesterol.
In addition to investigating the surface tension lowering ability of these model LS mixtures, we report on the changes in the compressibility modulus of the lipid films, and correlate these changes with changes in the lipid domain morphology and line tension. Domain morphology has an impact on the viscoelasticity and the compressibility of a monolayer [29], which in turn controls spreading, and the ability of these different monomolecular films to undergo reversible collapse. Reversible collapse is an important feature of LS, believed to be essential for efficient incorporation of material during the breathing cycle, by forming “surface-associated reservoirs” [30] at ultra-low surface tension. Further, some of us have also previously shown that SP-B causes an increase in the line tension of a clinical lung surfactant, an important biophysical property of phospholipid films at the air–water interface, which can control the overall energy of the lipid films [29]. By correlating high resolution atomic force microscopy (AFM) images to calculations of line tension changes in different binary lipid films, we have also recently shown that a change in line energy can be directly related to the tendency of a molecule to act as a line active species [31]. Further, previous research by McConnel and co-workers has established the line tension lowering ability of cholesterol [32]. However, quantitative information on how Mini-B and cholesterol alter the domain morphology and therefore the line tension between lipid domains is currently not available. Therefore, by applying our recently developed theory relating domain size distributions to changes in the excess free energy between lipid domain boundaries (line tension between domains), we also report on the line tension of lipid films due to Mini-B cholesterol interactions.
2. Materials and methods
2.1. Materials
The lipids, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (POPG) and cholesterol were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL) in powder form for cholesterol and at final concentrations of either 25 mg/ml or 5 mg/ml in organic mixtures of chloroform for the others. The synthetic protein Mini-B a mimic of the native surfactant protein SP-B [26,33] was supplied by Biopolymer Core Facility, LA BioMed at Harbor UCLA Medical Center, Torrance, California. Organic solvents, acetone, isopropanol and chloroform, were purchased from Thermo Fisher Scientific Inc. (Pittsburgh, PA). The fluorescent dye, 1, 2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt (TXR-DHPE), was obtained from Life Technologies (Invitrogen, Grand Island, NY) in dried form. The lipids were stored at −20 °C to prevent any evaporation of the organic mixture. Water, which served as a sub-phase as well as cleaning agent, was purified (resistivity of 18.2 MΩ cm) using Direct-Q 3 UV System purchased from EMD Millipore (Billerica, MA).
2.2. Methods
2.2.1. Sample preparation
Organic solutions of DPPC:POPG in high performance liquid chromatography (HPLC) grade chloroform were prepared in the ratio of 7:3 by weight as DPPC is the primary composition of LS. 1 wt.% dye was used for imaging purposes. Mini-B stock solutions were prepared in a 3:1 mixture of chloroform and methanol before being added to organic LS mixture. Multiple compositions of Mini-B (1, 2.5, 5 wt.%) and cholesterol (1, 2.5, 4, 5 wt.%, which corresponds to 2, 5, 7, 9 mol%) were used along with DPPC:POPG (7:3 M ratio).
2.2.2. Surface pressure vs. mean molecular area isotherm
Surface pressure vs. area isotherms were obtained using a Langmuir trough (Biolin Scientific Inc.), with an area of 166 cm2 and minimum area of 46 cm2. It uses a moveable ribbon that serves as barrier to control the available area per molecule, which thus mimics the compression and expansion process in the alveoli. Samples were added drop wise with a Hamilton glass syringe on an aqueous sub-phase and 20 min were given each time before running the experiment to allow the chloroform to evaporate. For isotherm experiments a compression rate of 125 cm2/min was chosen. Surface pressure was measured using Wilhelmy plate balance (filter paper). All experiments were repeated at least three times, to ensure reproducibility. All three sets of isotherms were found to overlap.
2.2.3. Fluorescence imaging
A Nikon Eclipse fluorescence microscope was used to visualize the lipid domain formation on the air–water interface. For imaging purposes a slower, quasi-static, rate of 7 cm2/min was selected. The images, at intervals of 1 to 5 mN/m (in the two-phase coexistence region), were captured using CCD camera (Andor LUCA). 5 frames were used for each image sequence and the representative images are presented in this report. Images were analyzed for calculations with ImageJ (NIH) software. Two neighboring images were analyzed for each image sequence for better statistical quality. This typically meant that at least 270 domains were analyzed to obtain the domain size distribution for each lipid composition. Furthermore, the circularity of the domains can also be measured using ImageJ. All final histograms and line tension graphs were generated using Origin 8.62 (OriginLab, Northampton, MA).
3. Theoretical analysis
3.1. Compressibility modulus
The compressibility modulus or modulus of elasticity β, of the film is often used to describe the properties of the monolayer film. It is defined as the ability of a film to store mechanical energy as stress under a compression force and may be mathematically defined as:
| (1) |
The inverse of β yields the isothermal compressibility κ of the film. Since β and κ are 2nd order derivatives of the surface free energy, G, (σ= −(∂G/∂A)T), discontinuities in these values confirm the presence of phase transitions. Experimentally, the phase transitions are represented as a blunt dip in the β-A profile rather than a sharp discontinuity.
The compressibility modulus is calculated from the raw isotherm data by calculating the slope m of the isotherm (Π-A) at each point using the formula
| (2) |
The derivatives can give rise to high-frequency, low-amplitude noise arising from fluctuations in the surface pressure reading as a result of discretization, which can be safely removed by a Fourier smoothing filter. The derivative and the Fourier smoothing filter are tools that are available as in-built functions through Origin 8.62, the graphing software used here for the isotherm data analysis.
3.2. Condensed area fraction
The condensed area fraction of domains provides us with an estimate of the fraction of the monolayer that forms ordered domain structures at a given surface pressure. Mathematically, the percentage area fraction is given by
| (3) |
3.3. Determining line tension from domain size distribution
The line tension (λ) between lipid domains depends on the difference in the height of the lipid chain, between the molecules in the liquid condensed (lo) and liquid expanded (ld) regions, and the interfacial tension of the hydrocarbon-air interface (γ) [i.e., λ = (lo − ld)γ]. On the other hand, the average dipole density (Δm2), which in turn is related to the electrostatic energy of the domains, depends on the packing density and composition of the lipid domains. The shape of the condensed domains in a monolayer is thus a function of the energy associated with the line tension and electrostatic repulsion. A method to simultaneously calculate the line tension and the dipole density moment from the distribution of circular phospholipid domains has been shown previously [29]. Using this method, we have shown that the line tension, λ can be related to the domain size distribution, CN, where CN is the number fraction of domains with N radius (i.e., ratio of mole fraction of molecules in N to the number of domains, N, given by XN/N) by the following equation:
| (4) |
| (5) |
The domain distribution, which is obtained experimentally, is then fitted with Eq. (4). Here we use CM, Δm2Ro/4εεokT, and Ro as the three fitting parameters. Eq. (5) is next used to determine the line tension.
4. Results
4.1. Isotherms
Fig. 1 presents the quasi-static surface pressure vs. mean molecular area isotherms of the different samples examined. Fig. 1A shows how the isotherm of a mixture of DPPC:POPG was modified by varying the concentration of Mini-B. The solid line represents the control curve for DPPC:POPG. At higher mean molecular areas, the monolayer is in the gas phase and therefore at 0 surface tension. However, the surface pressure increases with decrease in the mean molecular area, until finally the monolayer reached collapse pressures (~72 mN/m). With the addition of 1% Mini B to the system, we observed practically no change in the isotherm. However, when 5% Mini B was added to DPPC:POPG, given by the dotted line, the isotherm moved to higher values of surface pressure at any given mean molecular area. Fig. 1B represents the effect of varying amounts of cholesterol on the isotherm of DPPC:POPG, while Fig. 1C is a plot of DPPC:POPG mixed with 1% Mini-B and varying composition of cholesterol. The isotherm for Mini-B 1% and cholesterol 1%, given by the dashed curve, shows that the isotherm was pushed to higher values of surface pressure for the same mean molecular area. Increasing the concentration of cholesterol to 5% caused the surface pressure vs. area curve (dotted lines) to fall back close to the control. Fig. 1D represents the effect of 5% Mini-B with varying amounts of cholesterol on DPPC:POPG. The isotherms moved further to lower mean molecular areas indicating the need for higher area compression to achieve the same values of surface pressure.
Fig. 1.

Surface pressure vs. mean molecular area isotherms for different lung surfactant mixtures. (A) DPPC:POPG (7:3) with 1% and 5% Mini-B. (B) DPPC:POPG with 1% and 5% cholesterol. (C) DPPC:POPG with 1% Mini-B and varying concentration of cholesterol (D) DPPC:POPG with 5% Mini-B and different weightage of cholesterol.
4.2. Compressibility modulus
Fig. 2 presents the compressibility modulus as a function of mean molecular area of the samples tested. The data was processed using an FFT filter over 5 points, except near the collapse. Fig. 2A shows the compressibility modulus for varying composition of Mini-B with DPPC:POPG. In the case of the control DPPC:POPG sample, the compressibility modulus increased gradually till it reached around 31 mN/m after which there was a slight dip in the curve. The peak value of compressibility modulus before monolayer collapse was found to be 109 mN/m. In the case of DPPC:POPG monolayers containing 1% Mini-B, presented as dashed lines, there was almost no change in the compressibility modulus till 32 mN/m. In place of the dip in the curve beyond this value, like that of the control, there was a plateau until 46 Å2/molecule. The peak compressibility modulus was found to be around 98 mN/m. With 5% Mini-B, the peak compressibility modulus was measured to be 112 mN/m at 26 Å2/molecule. Fig. 2B shows the effect of different concentration of cholesterol on the compressibility modulus of DPPC:POPG. In the case of 1% cholesterol the peak compressibility modulus was found to be 97 mN/m and at a higher value of mean molecular area than that of the control DPPC:POPG film. DPPC:POPG films containing 5% cholesterol showed a gradual rise in the compressibility without a dip. The maximum compressibility dropped to 91 mN/m and also shifted to even higher mean molecular areas (29 Å2/molecule). Fig. 2C gives the compressibility modulus for systems containing both Mini-B and cholesterol. With Mini-B 1% and cholesterol 1% (dashed curve), there was an increase in the compressibility of the mixture. We noted the highest compressibility in this case, (137 mN/m) at a mean molecular area of 27 Å2/molecule. For 1% Mini-B and 5% cholesterol (dotted line), the curve increased gradually to a value of 92 mN/m at 27 Å2/molecule. Fig. 2D represents the compressibility modulus of DPPC:POPG along with 5% Mini-B and varying concentration of cholesterol. In the case of 5% Mini-B and 5% cholesterol, the peak was as low as 50 mN/m at 27 Å2/molecule, indicating a substantial change in the mechanical property due to Mini-B-cholesterol interactions.
Fig. 2.

Compressibility modulus for different lung surfactant mixtures. (A) DPPC:POPG (7:3) with 1% and 5% Mini-B. (B) DPPC:POPG with 1% and 5% cholesterol. (C) DPPC:POPG with fixed Mini-B percentage (1%) and varying weightage of cholesterol (D) DPPC:POPG with 5% Mini-B and different amounts of cholesterol.
4.3. Fluorescence images
To have a detailed understanding of the changes in the domain morphology in the monolayer, we used fluorescence microscopy imaging to monitor changes in lipid domains during the compression cycle. We present here images at surface pressures 20 mN/m. At 20 mN/m, the monolayer shows coexistence between two phases, namely the bright Liquid Expanded (LE) and the dark Liquid Condensed (LC) phases.
Fig. 3A shows the images for DPPC:POPG (7:3) at surface pressures 20 mN/m. In the case of the control, the monolayer is well packed agreeing with that found in literature [34]. The domains were more or less circular in shape at lower surface pressure. Fig. 3B shows the images for DPPC:POPG (7:3) with 1% cholesterol added to the system. Here we observed a drastic change in the domains. In place of the circular condensed regions of DPPC:POPG, we found protruding curls along the boundaries of the domains. The effect became more pronounced with the rise in the surface pressure. Fig. 3C shows the effect of 5% cholesterol, where the domain morphology had transitioned from circles to thin, but not continuous, stripes. A noticeable decrease in dark condensed domains was recorded, that we quantified in Fig. 5. Fig. 3D shows that adding 1% Mini-B to DPPC:POPG shows a slight decrease in the packing density of the dark domains, which increases with increasing protein concentration, as seen in Fig. 3G after addition of 5% Mini-B. Fig. 3E shows the monolayer consisting of both 1% Mini-B and 1% cholesterol, where an interesting new morphology of crescent shaped domains was observed. With 1% Mini-B and 5% cholesterol (Fig. 3F) the domains were completely transformed without getting packed at all. Fig. 3H shows the effect of 1% cholesterol along with 5% Mini-B. There were no appreciable changes in the shape of the domains when compared to DPPC:POPG film containing Mini-B only, showing that the higher concentration of Mini-B dominated over the lower percentage of cholesterol. Finally, at 5% Mini-B with the same concentration of cholesterol added to DPPC:POPG (Fig. 3I), the domains were smaller, but still circular in shape.
Fig. 3.

Fluorescence images of DPPC:POPG (7:3) along with varying concentrations of Mini-B and cholesterol. Images A, B, C, D, E, F, G, H and I were taken at 20 mN/m, which corresponds to the two-phase coexistence region. The first row represents samples without Mini-B, the next row represents samples with 1% Mini-B in them, and the last row contains sample with 5% Mini-B. Concentration of cholesterol increases from left to right. Scale bar indicates 50 μms.
Fig. 5.
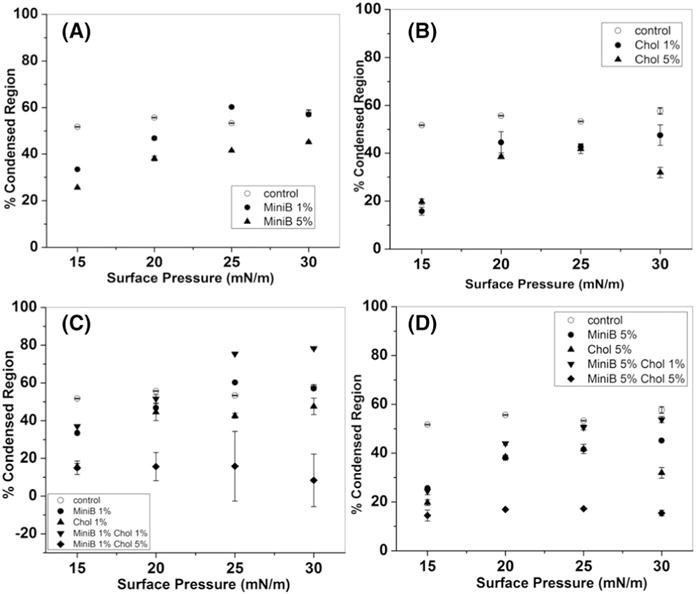
Percentage condensed domains for varying mixtures of DPPC:POPG (7:3). Percentage condensed domains due to addition of (A) different weights of Mini-B on DPPC:POPG (B) cholesterol (C) 1% Mini-B with different concentrations of cholesterol (D) 5% Mini-B along with different concentrations of cholesterol.
4.4. Ability to retain material during multiple compression–expansion cycles
When the monolayer is compressed to the limit of its stability, it transitions from its 2-dimensional existence to a 3-dimensional one. This phenomenon has been termed “monolayer collapse” and has been studied extensively by biophysicists due to its relevance to more efficient breathing [35,36]. The mechanism of collapse can either be reversible or irreversible in nature. Reversible collapse typically suggests formation of surface associated reservoirs, that enable quick and efficient re-adsorption of material during multiple compression-expansion cycles, and is believed to be desirable for efficient LS mixtures. The fluorescence-intense “streaks” seen in the images (Fig. 4A–E, indicated by arrows), which are found perpendicular to the direction of compression, are the reversibly collapsed regions, commonly known as “folds” or “collapse cracks” [36]. Here, the monolayer folds into multi-layers and upon expansion, the folded component respreads. Formation of giant folds, which extends a few microns laterally, indicates lower loss of material to the sub-phase. Monolayers lacking such folds, collapses irreversibly and solubilization is the likely mechanism when fluidizing agents, such as cholesterol, is present in the monolayer. Irreversible collapse due to solubilization of material is characterized by formation of vesicles, which are visible as bright specks in the images. It has been reported earlier that DPPC:POPG (7:3) on its own produces thin folds after collapse [37] and Fig. 4A shows similar results. Fig. 4B and C reveals that Mini-B helped DPPC:POPG to form fluorescence-intense giant folds suggesting reversible collapse. These features are very similar to that reported for KL4 [37]. With the addition of 1% cholesterol to 1% Mini-B (Fig. 4D) or 5% Mini-B (Fig. 4E), the monolayer collapsed reversibly. However, 5% cholesterol along with 5% Mini-B showed irreversible collapse (Fig. 4F).
Fig. 4.

Fluorescence images of DPPC:POPG and a combination of Mini-B and cholesterol, revealing collapse at higher surface pressures (A–F). Arrows indicate “collapse crack”. Isotherms with multiple compression cycles for the same samples (A′–F′). Mean and standard deviation of the area within the curve for each cycle for the same samples (A″–F″).
In order to further prove that the formation of these features correspond to better incorporation of material during multiple compression cycles, we also present the surface pressure vs. area isotherms for multiple cycles. In order to represent material loss, we also plot the area of the curve. An increase in the area of the isotherm suggests more material at the surface, while a significant drop in the area within the curve suggests loss of material from the surface. Fig. 4A′ shows multiple compression–expansion cycles for DPPC:POPG while 4A″ shows the corresponding area of the curve for the 1st and 3rd cycles. With multiple compressions, the isotherm shifted to lower fractional trough area. However, in the case of 1% and 5% Mini-B, this loss appeared to be reduced (Fig. 4B′, B″ and C′, C″). Higher concentration of Mini-B (5%) along with small concentration of cholesterol (1%) showed the least loss in material (Fig. 4E′, E″).
Furthermore, one-way-ANOVA revealed a significant difference (α = 0.05) in the area of the 1st cycle between the different samples (F6,14 = 9.036, p = 3.72 ∗ 10−4, R2 = 79.48%) as well as the area of the third cycle between the different samples (F6,14 = 15.22045, p = 2.062 ∗ 10−5, R2 = 86.71%).
4.5. Percentage condensed region
Fig. 5 shows the percentage of the monolayer that was condensed due to the addition of cholesterol and Mini-B to DPPC:POPG films. Surface pressures 15, 20, 25 and 30 mN/m were chosen for this analysis, since at higher surface pressures analysis of individual domains was no longer possible, and the entire field of view appeared dark. Fig. 5A shows the effect of Mini-B on DPPC:POPG. The condensed domain increased from 50% to about 55% with rise in surface pressure in the case of the model system. However, with the addition of Mini-B, at initial surface pressures there was a reduction in the condensed domains. The effect was more pronounced when higher percentage of Mini-B was added to the model mixture. Fig. 5B shows how cholesterol alters the formation of condensed domains in the model mixture. Even in this case, there is a decrease in the area of condensed domains with the addition of cholesterol. The next two figures reveal how Mini-B and cholesterol both act together in altering the area of the dark domains. Fig. 5C contains the data for 1% Mini B and varying concentrations of cholesterol. Here, with 1% of both put together, there was a steady increase in the domain area at 25 and 30 mN/m suggesting better domain packing. However, 1% Mini-B and 5% cholesterol showed a reduction in the area of the condensed domains. Fig. 5D shows the effect of varying amount of cholesterol on 5% Mini-B. In this case, 5% Mini-B was able to maintain the percent of condensed domains to almost that of the control system, even in the presence of 1 wt.% cholesterol. However, in the presence of higher cholesterol content, the area reduced drastically. This showed the dominance of higher concentration of cholesterol even with equal amount of Mini-B. The error in measurement was particularly high for the mixture with 1% Mini-B and 5% cholesterol mainly because of the difficulty in determining the condensed area owing to the small size of the domains, which made the analysis difficult. The error for the remaining mixtures was less than 10% of the total condensed area.
4.6. Line tension
The theoretical calculations relating line tension to domain size distribution, is only feasible for lipid domains that are circular in shape. Therefore, we were limited to calculating and presenting the line tension of the domains for the control, lipid mixture with both 1% and 5% Mini-B and the mixtures that had 5% Mini-B and varying concentration of cholesterol. Surface pressure 20 mN/m was selected for the ease of analysis. The line tension of DPPC:POPG (Fig. 6A) was around 2.4 × 10−2 pN. With the addition of 1% Mini-B, line tension increased to about 5 × 10−2 pN. Line tension in the case of 5% Mini-B was slightly higher than that of 1%. When 1%, 2.5% and 4% cholesterol was added to 5% Mini-B (Fig. 6B), no significant change in line tension was noted, even though cholesterol has been shown to reduce line tension [20]. However, increasing concentration of cholesterol to 5 wt.% showed a reduction in line tension.
Fig. 6.

Changes in line tension for different mixtures. Line tension between the domains in DPPC:POPG lipid films containing (A) different weights of Mini-B and SP-B, in the absence of cholesterol (B) 5% Mini-B with different cholesterol concentrations.
5. Discussion
This work is motivated by the desire to understand the biophysical interactions between cholesterol and synthetic surfactant protein Mini-B in a synthetic LS mixture. Kim et al. have shown that even 1 wt.% cholesterol is capable of reducing the surface viscosity of DPPC monolayer by an order of magnitude whereas 2 wt.% is capable of reducing the viscosity by two orders of magnitude [20]. This characteristic feature is valuable and is therefore a desirable property in synthetic surfactant because lowering of the surface viscosity can help in uniformly distributing the LS mixture throughout the lungs, as well as re-spreading of the surfactant with the expansion of lungs. However, as noted in the introduction, cholesterol is a highly debated component of LS and most of the commercial drugs used in SRT avoid cholesterol. Our own results confirm that not surprisingly, in the absence of protein Mini-B, even physiological amounts of cholesterol cannot maintain the desirable high surface pressures necessary during exhalation in the case of a synthetic binary LS mixture. Similarly, even 1% cholesterol prevent DPPC:POPG films from collapsing by forming surface associated reservoirs (reversible collapse), possibly by lowering the compressibility modulus of the film. On the other hand, 1 wt.% Mini-B alone is enough to significantly improve the ability of DPPC:POPG to undergo reversible collapse. Thus Mini-B and cholesterol demonstrate seemingly opposing biophysical characteristics, which when optimized, is expected to provide synthetic surfactant mixtures with enhanced therapeutic potential. Our results show that small quantities of cholesterol and Mini-B can together enhance the properties of LS. We discuss these results in further details in the following sub-sections.
5.1. Effect on mechanical properties
The surfactant mixture DPPC:POPG is efficient in lowering surface tension to near zero values owing to the presence of the disaturated phospholipid which has been reported previously [38]. Pressure vs. area isotherms suggests that higher amounts of Mini-B can cause early condensation of lipid domains, leading to higher surface pressures at the same area per molecule. On the other hand, cholesterol has a negative impact on the performance of DPPC:POPG films at higher surface pressures. Here, more area compression is needed to achieve the desired high surface pressure values. However, monolayers containing 1% of both the components, Mini-B and cholesterol together, are capable of enhancing the surface activity. Moreover, compressibility modulus of the system, which can be derived directly from the pressure vs. area isotherm [39], was found to be the highest for the above mixture. A highly compressible mixture can form well-packed structure, which again is supported by the fluorescence images. The compressibility modulus is much lower in the case of mixtures containing higher percentage of the two additives. Further, the isotherms for multiple cycles also show that these components together can improve the incorporation of material during multiple cycles.
5.2. Effect on line tension and collapse
Cholesterol and Mini-B alone have contrasting effects on the line tension of the LS films, as well as its ability to undergo reversible collapse. It is well known that cholesterol can lower the line tension between lipid domains to near zero values, as evidenced by the formation of spiral structures in the presence of cholesterol only [20]. On the other hand, our analysis of the domain size distribution shows that Mini-B increased the line tension between lipid domains. This finding is not surprising. Native SP-B was also found to increase the line tension between domains in a clinically relevant surfactant mixture [29]. We have previously attributed this increase in the line tension to the tendency of the positively charged protein to associate with the negatively charged POPG lipids that are in the fluid regions of the lipid membrane. It was interesting to note that in the presence of 5 wt.% Mini-B, 1–4 wt.% cholesterol did not lower the line tension between lipid domains, suggesting that interactions of the Mini-B with the negatively charged POPG lipid dominates over the protein-cholesterol interactions, or cholesterol’s tendency to associate with domain grain boundaries. Finally, the packing density between lipid domains was also high when both 1% Mini-B and 1% cholesterol were present. Further, this mixture was capable of collapsing reversibly, unlike the films containing cholesterol alone that were found to collapse irreversibly forming vesicles.
6. Conclusion
Based on our experimental observations, we conclude that any potential negative effects of low concentrations of cholesterol on the line tension, reversible collapse, and compressibility of synthetic LS mixtures, can be countered with synthetic protein Mini-B. However, higher concentration of cholesterol cannot be used as it has a greater negative impact on the model surfactant mixtures used in this study. For further exploration, smaller concentration of cholesterol along with 1% to 5% Mini-B can be tested in the case of SRT to allow the surfactant to be fluid enough to effectively cover the interface at inhalation, while resisting monolayer collapse at exhalation.
Supplementary Material
Acknowledgments
We would like to take this opportunity to thank our funding sources: NIH (P20GM103638), Inez Jay Award (from Higuchi Biosciences Center) and from Transportation Research Institute (University of Kansas). Without their support the project would not have been possible. We would also like to acknowledge Sadie Johnson and Carlie Copeland for their line tension data for SPB.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbamem.2016.01.008.
Footnotes
Transparency document
The Transparency document associated with this article can be found, in online version.
References
- 1.Robertson B, et al. Synthetic surfactants to treat neonatal lung disease. Mol Med Today. 2000;6(3):119–124. doi: 10.1016/s1357-4310(99)01656-1. [DOI] [PubMed] [Google Scholar]
- 2.Notter RH. Lung Surfactants: Basic Science and Clinical Applications. Vol. 149. CRC Press; 2000. [Google Scholar]
- 3.Zasadzinski JA, et al. The physics and physiology of lung surfactants. Curr Opin Colloid Interface Sci. 2001;6(5–6):506–513. [Google Scholar]
- 4.Dohm MT, et al. Biophysical mimicry of lung surfactant protein B by random nylon-3 copolymers. J Am Chem Soc. 2010;132(23):7957–7967. doi: 10.1021/ja909734n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuo YY, et al. Current perspectives in pulmonary surfactant — inhibition, enhancement and evaluation. Biochim Biophys Acta Biomembr. 2008;1778(10):1947–1977. doi: 10.1016/j.bbamem.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Clements J, et al. Lung surfactant and neonatal respiratory distress syndrome. Am J Respir Crit Care Med. 1998;157(4):S59–S66. doi: 10.1164/ajrccm.157.4.nhlb1-1. [DOI] [PubMed] [Google Scholar]
- 7.Stevens TP, et al. Sinkin, surfactant replacement therapy*. Chest. 2007;131(5):1577–1582. doi: 10.1378/chest.06-2371. [DOI] [PubMed] [Google Scholar]
- 8.Raghavendran K, et al. Surfactant therapy of ALI and ARDS. Crit Care Clin. 2011;27(3):525–559. doi: 10.1016/j.ccc.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willson DF, Thomas NJ. Surfactant composition and biophysical properties are important in clinical studies. Am J Respir Crit Care Med. 2010;181(7):762. doi: 10.1164/ajrccm.181.7.762. [DOI] [PubMed] [Google Scholar]
- 10.Goerke J. Pulmonary surfactant: functions and molecular composition. Biochim Biophys Acta (BBA) - Mol Basis Dis. 1998;1408(2–3):79–89. doi: 10.1016/s0925-4439(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 11.Veldhuizen R, et al. The role of lipids in pulmonary surfactant. Biochim Biophys Acta (BBA) - Mol Basis Dis. 1998;1408(2–3):90–108. doi: 10.1016/s0925-4439(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 12.Veldhuizen EJA, et al. The role of surfactant proteins in DPPC enrichment of surface films. Biophys J. 2000;79(6):3164–3171. doi: 10.1016/S0006-3495(00)76550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keough KMW, et al. Surface respreading after collapse of monolayers containing major lipids of pulmonary surfactant. Chem Phys Lipids. 1988;49(1–2):81–86. doi: 10.1016/0009-3084(88)90067-9. [DOI] [PubMed] [Google Scholar]
- 14.Gómez-Gil L, et al. Cholesterol modulates the exposure and orientation of pulmonary surfactant protein SP-C in model surfactant membranes. Biochim Biophys Acta Biomembr. 2009;1788(9):1907–1915. doi: 10.1016/j.bbamem.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Wright JR. Immunomodulatory functions of surfactant. Physiol Rev. 1997;77(4):931–962. doi: 10.1152/physrev.1997.77.4.931. [DOI] [PubMed] [Google Scholar]
- 16.Yu SH, Possmayer F. Dipalmitoylphosphatidylcholine and cholesterol in monolayers spread from adsorbed films of pulmonary surfactant. J Lipid Res. 2001;42:1421–1429. [PubMed] [Google Scholar]
- 17.Yu S, Harding PGR, Smith N, Possmayer F. Bovine pulmonary surfactant — chemical-composition and physical-properties. Lipids. 1983;18:522–529. doi: 10.1007/BF02535391. [DOI] [PubMed] [Google Scholar]
- 18.Taneva S, Keough KMW. Cholesterol modifies the properties of surface films of dipalmitoylphosphatidylcholine plus pulmonary surfactant-associated protein B or C spread or adsorbed at the air–water interface. Biochemistry. 1997;36:912–922. doi: 10.1021/bi9623542. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y. Effect of protein, cholesterol, and phosphatidylglycerol on the surface-activity of the lipid-protein complex reconstituted from Pig pulmonary surfactant. J Lipid Res. 1982;23:62–69. [PubMed] [Google Scholar]
- 20.C SQ, Kim KyuHan, et al. Effect of cholesterol nanodomains on monolayer morphology and dynamics. Proc Natl Acad Sci U S A. 2013;110(33):3054–3060. doi: 10.1073/pnas.1303304110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schurch S, Goerke J, Clements JA. Direct determination of surface-tension in lung. Proc Natl Acad Sci U S A. 1976;73(12):4698–4702. doi: 10.1073/pnas.73.12.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Codd JR, Slocombe NC, Daniels CB, Wood PG, Orgeig S. Periodic fluctuations in the pulmonary surfactant system in Gould’s wattled bat (Chalinolobus gouldii) Physiol Biochem Zool. 2000;73:605–612. doi: 10.1086/317745. [DOI] [PubMed] [Google Scholar]
- 23.Gunasekara L, et al. Pulmonary surfactant function is abolished by an elevated proportion of cholesterol. Biochim Biophys Acta Mol Cell Biol Lipids. 2005;1737(1):27–35. doi: 10.1016/j.bbalip.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Walther FJ, et al. Hydrophobic surfactant proteins and their analogues. Neonatology. 2007;91(4):303–310. doi: 10.1159/000101346. [DOI] [PubMed] [Google Scholar]
- 25.Walther FJ, et al. Critical structural and functional roles for the N-terminal insertion sequence in surfactant protein B analogs. PLoS One. 2010;5(1):e8672. doi: 10.1371/journal.pone.0008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waring AJ, et al. JA The role of charged amphipathic helices in the structure and function of surfactant protein B (SP-B) J Pept Res. 2005;66:364–374. doi: 10.1111/j.1399-3011.2005.00300.x. [DOI] [PubMed] [Google Scholar]
- 27.Walther FJ, et al. Dynamic surface activity of a fully synthetic phospholipase-resistant lipid/peptide lung surfactant. PLoS One. 2007;2(10):e1039. doi: 10.1371/journal.pone.0001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Rodriguez E, et al. Structure–function relationships in pulmonary surfactant membranes: from biophysics to therapy. Biochim Biophys Acta Biomembr. 2014;1838(6):1568–1585. doi: 10.1016/j.bbamem.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Dhar P, et al. Lipid-protein interactions alter line tensions and domain size distributions in lung surfactant monolayers. Biophys J. 2012;102(1):56–65. doi: 10.1016/j.bpj.2011.11.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipp ML, et al. Coexistance of buckled and flat monolayers. Phys Rev Lett. 1998;81:1650. [Google Scholar]
- 31.Chakraborty A, et al. Phospholipid compositions modulates carbon-induced alterations in phospholipid domain formation. Langmuir. 2015;31(18):5093–5104. doi: 10.1021/la504923j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McConnell HM. Structures and transitions in lipid monolayers at the air–water-interface. Annu Rev Phys Chem. 1991;42:171–195. [Google Scholar]
- 33.Sarker M, et al. Structure of mini-B, a functional fragment of surfactant protein B, in detergent micelle. Biochemistry. 2007;46:11047–11056. doi: 10.1021/bi7011756. [DOI] [PubMed] [Google Scholar]
- 34.Pocivavsek L, et al. Lateral stress relaxation and collapse in lipid monolayers. Soft Matter. 2008;4(10):2019–2029. doi: 10.1039/b804611e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ybert C, et al. Collapse of a monolayer by three mechanisms. J Phys Chem. 2002;106(8):2004–2008. [Google Scholar]
- 36.Lee KYC. Collapse mechanisms of Langmuir monolayers. Annu Rev Phys Chem. 2008;59:771–791. doi: 10.1146/annurev.physchem.58.032806.104619. [DOI] [PubMed] [Google Scholar]
- 37.Holten-Andersen N, et al. KL4 peptides induces reversible collapse structures on multiple length scales in model lung surfactants. Biophys J. 2011;101(12):2957–2965. doi: 10.1016/j.bpj.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diamant H, et al. Unstable topography of biphasic surfactant monolayers. Europhys Lett. 2000;52(2):171–177. [Google Scholar]
- 39.Dwivedi MV, et al. Size influences the effect of hydrophobic nanoparticles on lung surfactant model systems. Biophys J. 2014;106(1):289–298. doi: 10.1016/j.bpj.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


