Abstract
Objectives
Hemoglobin A1c levels <7.0% and systolic blood pressure (SBP) <140mmHg are each associated with lower risk of vascular complications in patients with diabetes mellitus. Association between combined A1c level and SBP categories and risk of mortality and morbidity in diabetic patients are not well characterized.
Methods
We examined 891,670 US diabetic veterans with baseline eGFR >60ml/min/1.73m2 (mean age 67±11 years, 97%males, 17% African-Americans). The association of mutually exclusive combined categories of A1c (<6.5, 6.5–6.9, 7.0–7.9, 8.0–8.9, 9.0–9.9, and ≥10%) and SBP (<100, 100–119, 120–139, 140–159, 160–179, and ≥180mmHg) with the risk of all-cause mortality and incident chronic kidney disease (CKD), coronary heart disease (CHD), and stroke was examined in Cox models adjusted for baseline characteristics using patients with concomitant A1c 6.5–6.9% and SBP of 120–139 mmHg as the referent group.
Results
A total of 221,529 (25%) patients died, and 178,588 (20%), 43,373 (5%) and 36,935 (4%) developed CKD, CHD and stroke, respectively, during a median follow-up of 7.4 years. SBP displayed a J-shaped association with each outcome except CKD risk that was linearly associated with SBP across all A1c categories. A1c above 7.0% was associated with monotonically worse outcomes for all end points in all SBP categories. Patients with the combined highest A1c and SBP levels experienced the worst outcomes.
Conclusion
Systolic BP >120–139mmHg and A1c >7.0% are associated with higher mortality and vascular complications in diabetic patients, independent of each other. Combined efforts to improve both glycemic and BP control may synergistically improve outcomes in patients with normal kidney function.
Keywords: Hemoglobin A1c, systolic blood pressure, morbidity, mortality
Introduction
Preventing vascular complications and reducing mortality is critical to the chronic management of diabetes mellitus in clinical practice. Comprehensive diabetes management programs should target the achievement of several goals [1]. Reducing glycated hemoglobin unequivocally diminishes the risk of microvascular complications of diabetes including the development of diabetic nephropathy [2–5]. On the other hand, the immediate macrovascular benefits of glycemic control were not as prominent across landmark diabetes trials [6] although recent reports demonstrated long-term cardiovascular benefits from intensive glycemic control in type 2 diabetes [7]. Despite vascular risk reduction, strict glycemic control had neutral or modest mortality benefits in some studies and harmful effects in the intensive arm of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial [6].
Hypertension is highly prevalent in patients with diabetes mellitus. Up to 75% of diabetics may have elevated blood pressure requiring pharmacological therapy [8]. The UK Prospective Diabetes Study (UKPDS) showed that blood pressure control in type 2 diabetes reduces the risk of vascular complications including cardiovascular disease and mortality [9]. The lower bound for optimal blood pressure reduction in diabetics was unclear until the ACCORD investigators showed that a systolic blood pressure target between 120–140 mmHg seems most optimal for the majority of patients [10]. Hence, it might be clinically strategic to target both hyperglycemia and hypertension in an attempt to achieve even better vascular and mortality outcomes.
Multifactorial risk factor modification in diabetes patients with microalbuminuria that simultaneously targets glycemia, blood pressure and lipids can offer additional cardiovascular morbidity and mortality benefits beyond the effects of individual risk factor modification [11, 12]. Observational analyses of the UKPDS suggested additive cardiovascular (CV) benefits from intensive control of both hyperglycemia and hypertension [13]. However, recent analyses from the interventional ACCORD study did not demonstrate additive cardiovascular benefits from a simultaneous strategy of both intensive glycemic and blood pressure control compared with the effects of each arm alone in type 2 diabetes patients with normal renal function [14]. In contrast, the subgroup analyses of the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial demonstrated that, compared with each arm alone, combination of routine blood pressure lowering and intensive glucose control produced additional reductions in new onset diabetic nephropathy risk and mortality [15].
With these variable findings in macrovascular and mortality outcomes across glycemic trials and trends towards additional vascular and mortality benefits from combined intensive glycemic and blood pressure control studies, it remains unknown as to whether there is a specific threshold at which glycemia and hypertension control would complement each other multiplicatively in chronic diabetes management, or if extremes of each risk factor might mitigate or enhance the benefits of controlling the other. The aim of this study was to investigate the impact of combined glycemic and blood pressure level on the risk of all-cause mortality, coronary heart disease, stroke, and chronic kidney disease in patients with normal kidney function at baseline.
Methods
Clinical and demographic measures
The institutional review committees at the Memphis and Long Beach Veterans Affairs Medical Centers approved the study. Our study utilized data from a cohort study examining risk factors in patients with incident chronic kidney disease (CKD) (Racial and Cardiovascular Risk Anomalies in CKD (RCAV) study) [16]. We used the national Veterans Affairs (VA) Labchem file to extract data on serum creatinine and to identify veterans with normal kidney function on the basis of estimated glomerular filtration rates (eGFRs) of ≥60 ml/min/1.73m2 [17]. eGFR was calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Equation [18]. We identified 3,582,478 patients with eGFR ≥60 ml/min/1.73m2 among a total of 4,444,699 patients with any available eGFR between October 1, 2004 and September 30, 2006. The algorithm for the cohort definition is shown in Figure 1. Diabetes mellitus was defined by ICD-9 codes, HbA1c >6.4% or based on the prescription of one or more hypoglycemic agents in the outpatient setting during the baseline period (October 1, 2004–September 30, 2006), based on information obtained from VA Pharmacy dispensation records. Hypertension was defined by ICD-9 codes during the baseline period. The final cohort included 891,670 patients who had both diabetes and hypertension.
Figure 1.
Flow chart of patients’ selection
Socio-demographic characteristics, comorbid conditions (Table S1) and laboratory characteristics were obtained as previously described [19–22]. Briefly, baseline information about age, gender, race, systolic blood pressure (SBP) and hemoglobin A1c (A1c) were obtained through the VA Corporate Data Warehouse (CDW) and from Medicare through the VA-Medicare data merge project. Comorbidity information was collected from the VA Inpatient and Outpatient Medical SAS Datasets using ICD-9-CM diagnostic and procedure codes during the baseline period. We divided patients into 36 a priori defined mutually exclusive categories based on combined baseline A1c and baseline SBP values: A1c levels <6.5, 6.5–6.9, 7.0–7.9, 8.0–8.9, 9.0–9.9, and ≥10% and SBP levels <100, 100–119, 120–139, 140–159, 160–179, and ≥180mmHg, respectively. The group with A1c level 6.5–6.9% and SBP level 120–139 mmHg was used as the reference group in our analysis.
Assessment of outcomes
We defined four different outcomes: 1) all-cause mortality, 2) incident coronary heart disease (CHD), 3) incident ischemic stroke, and 4) incident CKD. Data on all-cause mortality was obtained from the VA Vital Status Files (VSF), which contains dates of death or last medical/administrative encounter from all sources in the VA system with sensitivity and specificity of 98.3% and 99.8%, respectively, as compared to the National Death Index as gold standard [23]. Incident CHD was defined as the composite outcome of a first occurrence of an ICD-9-CM or CPT code for acute myocardial infarction, coronary artery bypass grafting, or percutaneous angioplasty after October 1, 2006 in patients without such diagnoses prior to this date. Incident stroke was defined as the first occurrence of ICD-9-CM codes for ischemic stroke after October 1, 2006 in patients without such diagnoses prior to this date (Table S2–S3). Incident CKD was defined as two consecutive eGFR levels <60 ml/min/1.73m2 separated by ≥90 days, and a >25% decrease from baseline eGFR [24].
The start of the follow-up period for mortality and incident CKD analyses was the date of the first eGFR ≥60 ml/min/1.73m2 during October 1, 2004–September 30, 2006. Patients were followed until death or development of incident CKD, or were censored at the date of last healthcare or administrative visit, or on July 26, 2013.
In incident CHD and stroke analyses, incident CHD and stroke events were identified in patients without such diagnoses prior to October 1, 2006; therefore, to avoid immortal time bias [25] the start of the follow-up period for these end points was October 1, 2006. Patients were followed until the first incident CHD/stroke event or were censored at the date of death, last healthcare or administrative visit, or on July 26, 2013.
Statistical Analysis
Data were summarized using proportions, means ± SD, or medians (interquartile ranges (IQR)) as appropriate. The associations between the combined A1c and SBP groups and outcomes were assessed using crude and multivariable adjusted Cox proportional hazard models. Models were adjusted for the following confounders based on a priori considerations: age, gender, race/ethnicity, baseline eGFR, various socio-economic parameters (income, marital status, service connection, adherence to medical interventions (defined as the presence of the ICD9-CM code V15.81 during any inpatient or outpatient encounter), comorbidities (cardiovascular disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, lung disease, malignancy and depression), body mass index (BMI) and statin treatment during baseline. We performed subgroup analyses by age and by race for all outcomes. Statistical analyses were performed using Stata MP version 12 (Stata Corporation, College Station, TX).
Results
Baseline Characteristics
The mean age of the cohort was 67±11 years, 97% were males, and 17% were African American. Baseline characteristics of the overall cohort categorized by A1c and SBP categories are shown in Table 1A–B. The mean A1c, SBP and BMI were 7.4±3%, 138±20 mmHg and 31.6±6 kg/m2, respectively. Patients with well-controlled diabetes defined by A1c of 6.5–6.9% were older and more likely to be married. During a median follow-up of 7.4 years, 221,529 (25%) patients died, and 178,588 (20%), 43,373 (5%) and 36,935 (4%) developed CKD, CHD and stroke, respectively.
Table 1A.
Baseline characteristics of individuals with diabetes stratified by hemoglobin A1c level
| Baseline | Total Cohort | Hemoglobin A1c categories, % (mmol/mol) | |||||
|---|---|---|---|---|---|---|---|
| Characteristics | <6.5 (<48) | 6.5–6.9 (48–52) | 7.0–7.9 (53–63) | 8.0–8.9 (64–74) | 9.0–9.9 (75–85) | >10 (>86) | |
| (N=891,670) | (N= 299,104) | (N= 165,117) | (N= 208,931) | (N=100,800) | (N=50,532) | (N=67,186) | |
| Age, mean (SD), year | 66.6 (11) | 67.6 (11) | 68.4 (11) | 67.4 (10) | 65.1 (10) | 62.9 (10) | 60.1 (11) |
| Gender, males, n (%) | 862,533 (97) | 288,557 (97) | 160,214 (97) | 203,089 (97) | 97,695 (97) | 48,786 (97) | 64,192 (96) |
| Race, n (%) | |||||||
| White | 636,765 (71) | 218,414 (73) | 118,217 (72) | 150,931 (72) | 71,367 (71) | 34,415 (68) | 43,421 (65) |
| African American | 1492,98 (17) | 46,001 (15) | 25,776 (16) | 32,201 (15) | 17,620 (18) | 10,375 (21) | 17,325 (26) |
| Married, n (%) | 518,654 (58) | 171,924 (58) | 101,093 (61) | 127,776 (61) | 58,288 (58) | 27,173 (54) | 32,400 (48) |
| eGFR, mean (SD), ml/min/1.73m2 | 82.1 (15) | 81.2 (15) | 80.4 (14) | 81.4 (14) | 83.3 (15) | 85.5 (16) | 87.4 (17) |
| BMI, mean (SD) (kg/m2) | 31.6 (6) | 31.1 (6) | 31.4 (6) | 31.8 (6) | 32.1 (6) | 32.3 (7) | 31.8 (7) |
| SBP, mean (SD), mmHg | 138.0 (20) | 137.4 (20) | 138.0 (19) | 138.3 (19) | 138.7 (20) | 138.8 (20) | 138.7 (20) |
| Per capita income (median and (IQR), USD | 23535 (12479–34287) | 23572 (12289–34471) | 24730 (13036–36540) | 24550 (13104–35497) | 23070 (12449–33283) | 21389 (11854–32234) | 19882 (11241–31572) |
| Service-connected, mean (SD) | 26.5 (37) | 25.9 (37) | 24.5 (36) | 26.2(36) | 28.8 (38) | 30.1 (38) | 28.5 (37) |
| CHF, n (%) | 64,819 (8) | 20,558 (8) | 10,803 (8) | 15,737 (8) | 8,389 (9) | 4,331 (9) | 5,001 (9) |
| Hypertension, n (%) | 659,445 (83) | 21,0278 (82) | 119,466 (83) | 164,102 (83) | 80,579 (83) | 39,665 (81) | 45,355 (79) |
| Cerebrovascular disease, n (%) | 705,75 (9) | 24,495 (10) | 12,582 (9) | 17,108 (9) | 8,086 (8) | 4,055 (8) | 4,249 (7) |
| PAD, n (%) | 76,583 (10) | 24,058 (9) | 13,450 (9) | 19,281 (10) | 9,776 (10) | 4,762 (10) | 5,256 (9) |
| Chronic lung disease, n (%) | 153,325 (19) | 54,438 (21) | 27,353 (19) | 35,510 (18) | 17,440 (18) | 8,713 (18) | 9,871 (17) |
| Depression, n (%) | 74,087 (9) | 25,794 (10) | 10,990 (8) | 15,803 (8) | 9,036 (9) | 5,304 (11) | 7,160 (13) |
| Cardiovascular disease, n (%) | 145,386 (18) | 44,147 (17) | 26,195 (18) | 37,523 (19) | 18,850 (19) | 9,037 (19) | 9,634 (17) |
| Malignancy, n (%) | 86,242 (11) | 32,834 (13) | 16,487 (12) | 20,241 (10) | 8,795 (9) | 3,897 (8) | 3,988 (7) |
| Non-compliant, n (%) | 89,177 (10) | 20,986 (7) | 11,454 (7) | 18,658 (9) | 13,558 (14) | 9,128 (18) | 15,393 (23) |
| Statin use, n (%) | 185,077 (21) | 61,431 (21) | 36,390 (22) | 45,467 (22) | 20,828 (21) | 9,639 (19) | 11,322 (17) |
Abbreviations: BMI: body mass index; CHF: congestive heart failure; eGFR, estimated glomerular filtration rate; IQR: interquartile range; SD, standard deviation; SBP, systolic blood pressure; PAD, peripheral artery disease
Table 1B.
Baseline characteristics of individuals with diabetes stratified by systolic blood pressure level
| Baseline | Total Cohort | SBP categories, mmHg | |||||
|---|---|---|---|---|---|---|---|
| Characteristics | <100 | 100–119 | 120–139 | 140–159 | 160–179 | ≥180 | |
| (N=891,670) | (N= 10,699) | (N= 124,349) | (N= 368,911) | (N=269,902) | (N=90,258) | (N= 27,551) | |
| Age, mean (SD), year | 66.6 (11) | 66.5 (11) | 65.6 (11) | 66.3 (11) | 67.0 (11) | 67.9 (10) | 67.6 (10) |
| Gender, males, n (%) | 862,533 (97) | 10,290 (96) | 119,160 (96) | 35,6657 (97) | 262,015 (97) | 87,768 (97) | 26,643 (97) |
| Race, n (%) | |||||||
| White | 636,765 (71) | 8,117 (76) | 90,927 (73) | 26,6179 (72) | 191,197 (71) | 62,361 (69) | 17,984 (65) |
| African American | 1492,98 (17) | 1,530 (14) | 18,954 (15) | 57,629 (16) | 46,951 (17) | 17,672 (20) | 6,562 (24) |
| Married, n (%) | 518,654 (58) | 5,627 (53) | 71,027 (57) | 21,8551 (59) | 157,969 (59) | 51,063 (57) | 14,417 (52) |
| eGFR, mean (SD), ml/min/1.73m2 | 82.1 (15) | 81.1 (16) | 82.2 (15) | 82.2 (15) | 82.1 (15) | 81.7 (15) | 81.5 (15) |
| BMI, mean (SD) (kg/m2) | 31.6 (6) | 29.4 (7) | 30.5 (6) | 31.5 (6) | 32.0 (6) | 32.1 (7) | 32.0 (7) |
| A1c, mean (SD), (%), mmol/mol | 7.4 (3) 57 | 7.3 (3) 56 | 7.4 (4) 57 | 7.4 (4) 57 | 7.4 (3) 57 | 7.4 (4) 57 | 7.5 (3) 58 |
| Per capita income (median and (IQR), USD | 23535 (12479–34287) | 21419 (11533–32252) | 23505 (12258–33613) | 24071 (12654–34824) | 23540 (12538–34467) | 22651 (12327–33923) | 20484 (11665–32185) |
| Service-connected, mean (SD) | 26.5 (37) | 28.5 (39) | 28.6 (38) | 27.0 (37) | 25.9 (36) | 24.3 (36) | 22.9 (35) |
| CHF, n (%) | 64,819 (8) | 2,023 (21) | 12,202 (11) | 24,327 (7) | 17,118 (7) | 6,598 (8) | 2,551 (10) |
| Hypertension, n (%) | 659,445 (83) | 6,659 (70) | 78,514 (71) | 25,9461 (78) | 214,088 (89) | 76,773 (95) | 23,950 (97) |
| Cerebrovascular disease, n (%) | 705,75 (9) | 1,243 (13) | 10,062 (9) | 26,706 (8) | 20,986 (9) | 8,327 (10) | 3,251 (13) |
| PAD, n (%) | 76,583 (10) | 1,416 (15) | 11,201 (10) | 29,444 (9) | 22,753 (9) | 8,651 (11) | 3,118 (13) |
| Chronic lung disease, n (%) | 153,325 (19) | 2,677 (28) | 24,452 (22) | 62,787 (19) | 44,438 (18) | 14,642 (18) | 4,329 (18) |
| Depression, n (%) | 74,087 (9) | 1,133 (12) | 12,429 (11) | 31,478 (10) | 20,675 (9) | 6,453 (8) | 1,919 (8) |
| Cardiovascular disease, n (%) | 145,386 (18) | 2,791 (29) | 23,923 (22) | 58,890 (18) | 41,134 (17) | 14,020 (17) | 4,628 (19) |
| Malignancy, n (%) | 86,242 (11) | 1,267 (13) | 12,454 (11) | 34,816 (11) | 26,292 (11) | 8,910 (11) | 2,503 (10) |
| Non-compliant, n (%) | 89,177 (10) | 1,168 (11) | 11,915 (10) | 33,964 (9) | 27,387 (10) | 10,480 (12) | 4,263 (16) |
| Statin use, n (%) | 185,077 (21) | 2,568 (24) | 27,166 (22) | 78,154 (21) | 54,708 (20) | 17,618 (20) | 4,863 (18) |
Abbreviations: BMI: body mass index; CHF: congestive heart failure; eGFR, estimated glomerular filtration rate; IQR: interquartile range; SD, standard deviation; SBP, systolic blood pressure; PAD, peripheral artery disease
Association of blood pressure and glucose levels with mortality
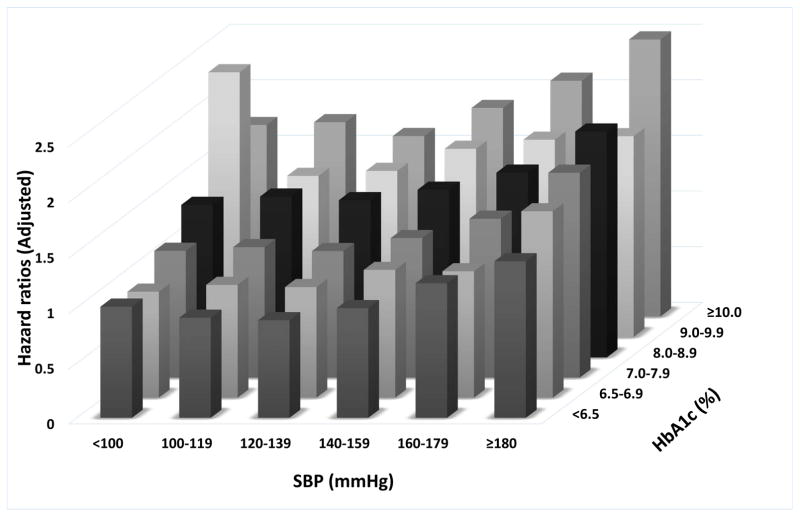
The adjusted mortality hazard ratios (HRs) associated with the combined A1c and SBP categories are shown in Figure 2. There was a J-shaped relationship between A1c and mortality in each category of SBP. Similar J-shaped associations were observed between SBP and mortality in each A1c category. The highest risk of death was observed in individuals with a combination of A1c of ≥10% and SBP of <100mmHg or >180mmHg with adjusted HRs of 2.27 (95% confidence interval (CI): 2.01–2.57) and 2.07 (95%CI: 1.91–2.23), respectively. In subgroup analyses, the combined effects of A1c and SBP on the risk of death were similar regardless of age or race (Supplementary Figure 1).
Figure 2.
Associations of systolic blood pressure and A1c categories with the risk of death from any cause.
The effects of the categories on the mortality risks were estimated from Cox proportional hazards models. Risk estimates were adjusted for the following factors at baseline: age, gender, race/ethnicity, baseline eGFR, various socio-economic parameters, service connection, adherence to medical interventions, comorbidities, body mass index and statin treatment. The adjusted hazard ratios are compared to the reference category of systolic blood pressure of 120–139mmHg and A1c of 6.5–7.0% taken as 1.0.
Association of blood pressure and glucose levels with incident macro- and microvascular events
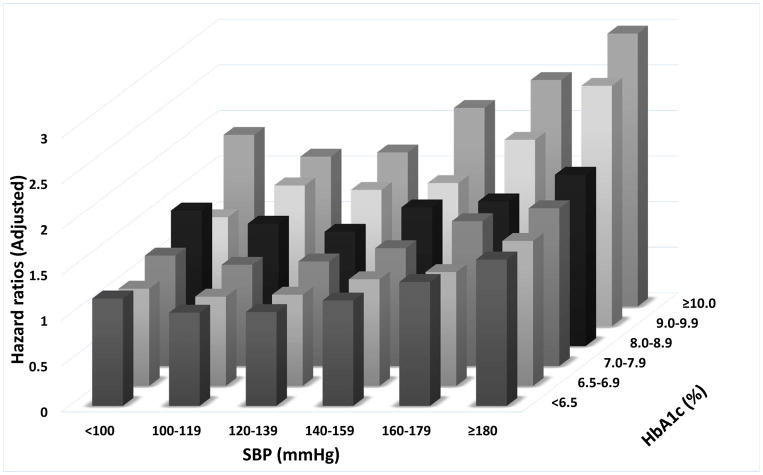
For both incident CHD and stroke, we found a J-shaped association with SBP levels across all A1c categories (Figure 3A–B and Supplementary Table 4). Conversely, higher A1c levels were associated with a monotonic increase in risk of CHD and stroke in each SBP category. The lowest CHD and stroke risks were observed with A1c of <6.5% combined with systolic BP of 100–139mmHg. The highest risk of both macrovascular events was observed with A1c >10% combined with SBP of >180 mmHg (adjusted HR: 2.99, 95%CI: 2.58–3.48 for stroke and adjusted HR: 2.49, 95%CI: 2.11–2.94 for CHD).
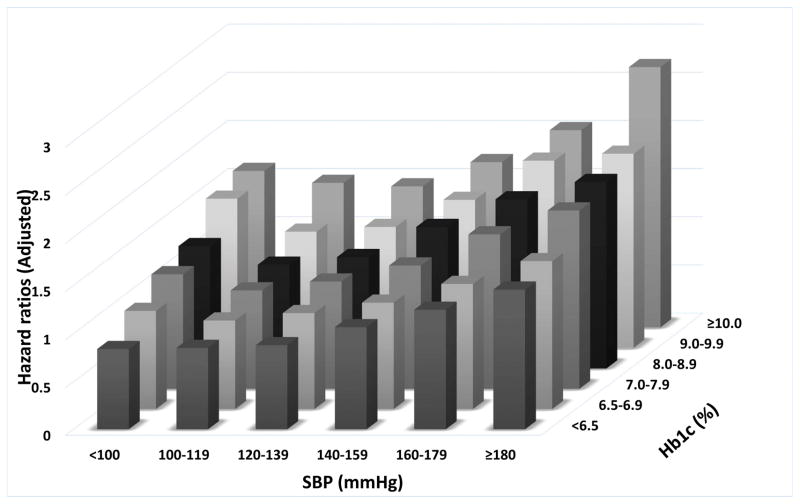
Figure 3.
Associations of blood pressure and A1c categories with the risk of cardiovascular disease (panel A), stroke (panel B) and chronic kidney disease (panel C).
The effects of the categories on the mortality risks were estimated from Cox proportional hazards models. Risk estimates were adjusted for the following factors at baseline: age, gender, race/ethnicity, baseline eGFR, various socio-economic parameters, service connection, adherence to medical interventions, comorbidities, body mass index and statin treatment. The adjusted hazard ratios are compared to the reference category of systolic blood pressure of 120–139mmHg and A1c of 6.5–7.0% taken as 1.0.
Higher A1c levels were associated with higher risk of incident CKD in all SBP categories (Figure 3C). In contrast, we observed J-shaped associations with SBP categories in patients with A1c levels ≥6.5–7.0%, and a linearly higher risk associated with higher SBP in patients with A1c <6.5%. The lowest risk of incident CKD was observed in patients with A1c <6.5% combined with SBP of <100 mmHg (adjusted HR: 0.84, 95%CI: 0.72–0.98). The highest risk of incident CKD was associated with A1c >10% combined with SBP of >180 mmHg (adjusted HR: 2.70, 95%CI: 2.39–3.06).
In subgroup analyses, the association of A1c and SBP combinations with the risk of CHD, stroke and CKD were consistent with the primary analyses regardless of age or ethnicity (Supplementary Figures 2–4).
Discussion
There is consensus that the majority of diabetic patients should achieve treatment target goals of A1c <7.0% with avoidance of hypoglycemia and SBP <140mmHg while refraining from reduction to <120mmHg [1]; however, no prior studies have ascertained the combined effects of inadequate glycemic and hypertension control on mortality and morbidity in diabetes patients. We observed that, in patients with diabetes and normal renal function, SBP below and above the recommended range of 120–139 mmHg was associated with an increased hazard of all-cause mortality and nonfatal vascular outcomes across all ranges of glycated hemoglobin. Our results also suggest that diabetes metrics of A1c and SBP above the recommended goals are associated with increased mortality and incident vascular events, and patients with diabetes may derive continuous and additive health benefits from optimizing both glycemic and blood pressure levels.
Many patients with diabetes are not achieving therapeutic targets that are proven to lower the incidence of vascular disease and are associated with reduction in mortality [26, 27]. Only about half of the patients with diabetes are able to achieve the benchmark goals of A1c and blood pressure control [27]. Although current success rates for A1c and blood pressure control have improved over the decades [26], there has been little reduction in the prevalence of stroke and end stage renal disease in the diabetes population [28]. With the marked economic burden of diabetes in society and the continuous search for opportunities to improve healthcare expenditures, recent evidence indicates that achieving LDL-cholesterol goals in addition to A1c management have even more profound clinical and economic benefits, compared to controlling either goal in isolation [29, 30]. To the best of our knowledge, there have been no similar attempts to assess the impact of combined glycemic and BP control in diabetics.
In our large cohort of the US veterans who were followed for about 7 years, we found a J-shaped association between mortality and A1c and SBP categories. These data are in accordance with the results of previous studies and expert recommendations that targeting glycemic and blood pressure to the levels below or above the suggested goals is associated with an increased risk of mortality and vascular morbidity [2, 31, 32]. Our findings demonstrate potential additive effects of uncontrolled glycemia and hypertension on the risk of all-cause mortality and vascular outcomes. Prior studies suggested that in healthy non-diabetic non-hypertensive men elevated baseline plasma glucose predicted development of future hypertension [33]. Whether more aggressive blood glucose lowering in patients with normotension or hypertension can result in better blood pressure control independent of use of anti-hypertensive agents deserves future investigation.
We showed higher risk of newly diagnosed CHD and stroke for those diabetes patients whose A1c and SBP control were below or above the recommended targets of 6.5–7.0% and 120–139 mmHg, respectively. While earlier analyses from the prospective observational UKPDS [13] and randomized controlled ADVANCE [15] trials suggested additive and independent effects of hyperglycemia and hypertension on mortality and microvascular complications, most recent reports from ACCORD did not support such interaction between A1c and blood pressure control on outcomes in long-term diabetes management [3, 14]. Our results demonstrate a continuous additive effect of A1c and SBP below and above the recommended goals on mortality and macrovascular complications in a large, unselected diabetic population with hypertension, and support current recommendations towards optimization of both parameters. In non-diabetic patients with high baseline cardiovascular risk, SPRINT (Systolic Blood Pressure Intervention Trial) investigators have addressed benefits of blood pressure lowering below accepted targets [34]. The trial was terminated earlier than planned due to significant reduction of fatal and non-fatal major cardiovascular events and death from any cause in the arm assigned to a systolic blood pressure lowering of less than 120 mmHg.
In our diabetes cohort with normal renal function, there was a linear association between glycemic and blood pressure categories and incident CKD. These findings could emphasize the importance of glycemic and hypertension control as a primary prevention measure for CKD. Conversely, the low incidence of CKD in the group with the lowest SBP could be affected by a competing risk of higher mortality associated with lower SBP, which was also shown in earlier results in veterans with established CKD [35, 36]. Future research should address the potential increased risks of mortality and macrovascular disease versus potential renal benefits in the process of achieving tight diabetic and blood pressure metrics.
It is noteworthy that the outcomes of our study reflect clinical behavior of providers who belong to a single payer system characterized by system-wide recommendations on achieving benchmark diabetes metrics. In our study of veterans with normal renal function, 299,104 (34%) and 165,117 (19%) veterans achieved A1c levels of <6.5% and 6.5–6.9%, respectively and 368,911 (41%) reached a SBP of 120–139 mmHg. Combined A1c <7.0% and SBP of 120–139 mmHg were seen in 193,485 (22%) of our cohort. The attainment of A1c goals in our cohort composed of mostly older men with significant numbers of co-morbid conditions mirrors nation-wide trends in improvement of glycemic control while our study findings regarding achievement of systolic blood pressure targets are slightly different compared to the recently published diabetes management metrics from community-based studies generally comprised of younger individuals, a greater proportion of women, and individuals with fewer comorbidities [26, 27].
With increasing prevalence of diabetes among the elderly, we performed subgroup analyses in patients who were 65 years old and older. We did not detect effect modification by age in the association of glycemic and blood pressure parameters with the risk of mortality and morbidity in diabetes. Also, several studies demonstrated ethnic/racial disparities in cardiovascular risk factor control in diabetes [27, 28, 37]. In our subgroup analyses, there was no difference in mortality risk and incidence of stroke, cardiovascular disease and CKD between white and black veterans. These results suggest that diabetes care received in the VA healthcare organization may potentially mitigate age or racial discordances in clinical outcomes that were reported based on the community patient care analyses [27, 37].
Our study is notable for its large sample size and for its representativeness of veterans from across the entire US. To the best of our knowledge, it is also the first study to ascertain the combined association of abnormal glycemic and hypertension levels on mortality and morbidity in diabetes patients from an unselected population enrolled in a large healthcare system. Several limitations of our study should be acknowledged. This being an observational study, only associations, but no cause-effect relationships can be established from it. Our cohort consisted of mostly men; hence, the findings may not be generalizable to women. We used multivariable adjusted analyses, but the presence of residual confounders cannot be excluded. In addition, not all veterans may have received all their health care in VA facilities resulting in missing events. We assessed association with observed levels of A1c and SBP, but we cannot ascertain if such levels were due to therapeutic interventions, or other factors such as acute or chronic illnesses. Finally, we do not have information about cause specific mortality.
Perspectives
Among patients with diabetes and normal renal function, abnormally elevated systolic blood pressure was associated with further increase in the risk of all-cause mortality and incidence of nonfatal cardiovascular outcomes independent of glycemic control. Conversely, higher A1c levels are associated with higher risk of mortality and vascular events independent of SBP levels. Our findings suggest that patients with diabetes may derive additional clinical benefits from control of both hyperglycemia and hypertension, if proven so in clinical trials.
Supplementary Material
Acknowledgments
Sources of funding.
This study is supported by grant 1R01DK096920 from the NIH to CPK and KKZ, and by resources from the US Department of Veterans Affairs. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
Footnotes
Conflict of Interests.
ARG reports personal fees from AstraZeneca and Janssen Pharmaceutical and grant support from Sanofi and Novo Nordisk paid to the UTHSC, Memphis. MZM reports support from Czech Health Research Council, outside the submitted work. KK-Z reports personal fees from Abbott, AbbVie, Amgen, Fresenius, Genentech, Genzyme–Sanofi, Hospira, Keryx, Shire, and Vifor, non-financial support from DaVita, and grants from the US National Institutes of Health (NIH), outside the submitted work. CPK reports personal fees from Amgen, NPS, Relypsa, ZS Pharma, and Sanofi-Aventis, royalties from UpToDate for a review article about metabolic acidosis, and grants from the NIH, AbbVie, Amgen, OPKO, and Shire, outside the submitted work. CPK and KK-Z are employees of the US Department of Veterans Affairs and their opinions expressed in this paper are those of the authors’ and do not necessarily represent the opinion of the Department of Veterans Affairs. The other authors declare no competing interests.
References
- 1.American Diabetes A. Standards of medical care in diabetes--2014. Diabetes care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 2.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 3.Ismail-Beigi F, Craven TE, O’Connor PJ, Karl D, Calles-Escandon J, Hramiak I, et al. Combined intensive blood pressure and glycemic control does not produce an additive benefit on microvascular outcomes in type 2 diabetic patients. Kidney Int. 2012;81(6):586–94. doi: 10.1038/ki.2011.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. The New England journal of medicine. 1993;329(5):304–9. doi: 10.1056/NEJM199307293290502. [DOI] [PubMed] [Google Scholar]
- 5.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. The New England journal of medicine. 1993;329(14):977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 6.Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Annals of internal medicine. 2009;151(6):394–403. doi: 10.7326/0003-4819-151-6-200909150-00137. [DOI] [PubMed] [Google Scholar]
- 7.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. The New England journal of medicine. 2008;359(15):1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 8.Colosia AD, Palencia R, Khan S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab Syndr Obes. 2013;6:327–38. doi: 10.2147/DMSO.S51325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. Bmj. 1998;317(7160):703–13. [PMC free article] [PubMed] [Google Scholar]
- 10.Group AS, Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. The New England journal of medicine. 2010;362(17):1575–85. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. The New England journal of medicine. 2008;358(6):580–91. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 12.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. The New England journal of medicine. 2003;348(5):383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 13.Stratton IM, Cull CA, Adler AI, Matthews DR, Neil HA, Holman RR. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75) Diabetologia. 2006;49(8):1761–9. doi: 10.1007/s00125-006-0297-1. [DOI] [PubMed] [Google Scholar]
- 14.Margolis KL, O’Connor PJ, Morgan TM, Buse JB, Cohen RM, Cushman WC, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes care. 2014;37(6):1721–8. doi: 10.2337/dc13-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zoungas S, de Galan BE, Ninomiya T, Grobbee D, Hamet P, Heller S, et al. Combined effects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes: New results from the ADVANCE trial. Diabetes care. 2009;32(11):2068–74. doi: 10.2337/dc09-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molnar MZ, Alhourani HM, Wall BM, Lu JL, Streja E, Kalantar-Zadeh K, et al. Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology. 2015;61(5):1495–502. doi: 10.1002/hep.27664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens PE, Levin A Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Annals of internal medicine. 2013;158(11):825–30. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovesdy CP, Norris KC, Boulware LE, Lu JL, Ma JZ, Streja E, et al. Association of Race With Mortality and Cardiovascular Events in a Large Cohort of US Veterans. Circulation. 2015;132(16):1538–48. doi: 10.1161/CIRCULATIONAHA.114.015124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu JL, Molnar MZ, Naseer A, Mikkelsen MK, Kalantar-Zadeh K, Kovesdy CP. Association of age and BMI with kidney function and mortality: a cohort study. The lancet Diabetes & endocrinology. 2015;3(9):704–14. doi: 10.1016/S2213-8587(15)00128-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gosmanova EO, Lu JL, Streja E, Cushman WC, Kalantar-Zadeh K, Kovesdy CP. Association of medical treatment nonadherence with all-cause mortality in newly treated hypertensive US veterans. Hypertension. 2014;64(5):951–7. doi: 10.1161/HYPERTENSIONAHA.114.03805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molnar MZ, Mucsi I, Novak M, Szabo Z, Freire AX, Huch KM, et al. Association of incident obstructive sleep apnoea with outcomes in a large cohort of US veterans. Thorax. 2015;70(9):888–95. doi: 10.1136/thoraxjnl-2015-206970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Population health metrics. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements. 2013;(3):136–50. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Weinhandl ED, Gilbertson DT, Collins AJ, St Peter WL. Issues regarding ‘immortal time’ in the analysis of the treatment effects in observational studies. Kidney international. 2012;81(4):341–50. doi: 10.1038/ki.2011.388. [DOI] [PubMed] [Google Scholar]
- 26.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes care. 2013;36(8):2271–9. doi: 10.2337/dc12-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010. The New England journal of medicine. 2013;368(17):1613–24. doi: 10.1056/NEJMsa1213829. [DOI] [PubMed] [Google Scholar]
- 28.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, et al. Changes in diabetes-related complications in the United States, 1990–2010. The New England journal of medicine. 2014;370(16):1514–23. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 29.Clarke PM, Gray AM, Briggs A, Stevens RJ, Matthews DR, Holman RR, et al. Cost-utility analyses of intensive blood glucose and tight blood pressure control in type 2 diabetes (UKPDS 72) Diabetologia. 2005;48(5):868–77. doi: 10.1007/s00125-005-1717-3. [DOI] [PubMed] [Google Scholar]
- 30.Shi L, Ye X, Lu M, Wu EQ, Sharma H, Thomason D, et al. Clinical and economic benefits associated with the achievement of both HbA1c and LDL cholesterol goals in veterans with type 2 diabetes. Diabetes care. 2013;36(10):3297–304. doi: 10.2337/dc13-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Action to Control Cardiovascular Risk in Diabetes Study G. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. The New England journal of medicine. 2008;358(24):2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrannini E, Cushman WC. Diabetes and hypertension: the bad companions. Lancet. 2012;380(9841):601–10. doi: 10.1016/S0140-6736(12)60987-8. [DOI] [PubMed] [Google Scholar]
- 33.Bjornholt JV, Erikssen G, Kjeldsen SE, Bodegard J, Thaulow E, Erikssen J. Fasting blood glucose is independently associated with resting and exercise blood pressures and development of elevated blood pressure. J Hypertens. 2003;21(7):1383–9. doi: 10.1097/00004872-200307000-00029. [DOI] [PubMed] [Google Scholar]
- 34.SPRINT Research Group. Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovesdy CP, Lu JL, Molnar MZ, Ma JZ, Canada RB, Streja E, et al. Observational modeling of strict vs conventional blood pressure control in patients with chronic kidney disease. JAMA Intern Med. 2014;174(9):1442–9. doi: 10.1001/jamainternmed.2014.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovesdy CP, Bleyer AJ, Molnar MZ, Ma JZ, Sim JJ, Cushman WC, et al. Blood pressure and mortality in U.S. veterans with chronic kidney disease: a cohort study. Annals of internal medicine. 2013;159(4):233–42. doi: 10.7326/0003-4819-159-4-201308200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parrinello CM, Rastegar I, Godino JG, Miedema MD, Matsushita K, Selvin E. Prevalence of and Racial Disparities in Risk Factor Control in Older Adults With Diabetes: The Atherosclerosis Risk in Communities Study. Diabetes care. 2015;38(7):1290–8. doi: 10.2337/dc15-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.