Abstract
Background
There is a paucity of data on the association of aldosterone and plasma renin activity (PRA) with incident cardiovascular disease (CVD) or all-cause mortality (mortality) among community-dwelling African Americans (AAs).
Objective
We examined the association of aldosterone and PRA with incident CVD (composite endpoint of coronary heart disease (CHD), stroke and heart failure) and mortality among AAs in the Jackson Heart Study.
Methods
A total of 4,985 AA adults, aged 21–94 years, were followed for 12 years. Aldosterone, PRA, and cardiovascular risk factors were collected at baseline (2000–2004). Incident events included CHD and stroke (assessed from 2000–2011) and heart failure (assessed from 2005–2011). Cox models were used to estimate hazard ratios (HR) for incident CVD and mortality adjusting for age, sex, education, occupation, current smoking, physical activity, dietary intake and body mass index.
Results
Among 4,160 participants without prevalent CVD over a median follow-up of 7 years, there were 322 incident CVD cases. In adjusted analyses, each 1-unit standard deviation (SD) increase in log-aldosterone and log-PRA were associated with HRs of 1.26 (95% CI: 1.14, 1.40) and 1.16 (95% CI: 1.02, 1.33) for incident CVD, respectively. Over a median of 8 years, 513 deaths occurred among 4,985 participants. In adjusted analyses, each 1-unit SD increase in log-aldosterone and log-PRA were associated with HRs of 1.13 (95% CI: 1.04, 1.23) and 1.12 (95% CI: 1.01, 1.24) for mortality, respectively.
Conclusions
Elevated aldosterone and PRA may play a significant role in the development of CVD and all-cause mortality among AAs.
Keywords: Aldosterone, Plasma Renin Activity, Cardiovascular Disease, All-Cause Mortality
Introduction
Cardiovascular disease (CVD) remains the most common cause of morbidity and mortality in the United States among African Americans (AA) (1). The renin-angiotensin-aldosterone system (RAAS) plays an important role in the pathogenesis of hypertension, a major risk factor for CVD (2). Inappropriate RAAS activation has been hypothesized to explain some of the racial disparities observed in the incidence of hypertension, left ventricular hypertrophy, and heart failure (HF) (3). In particular, the higher rate of hypertension-related complications among AAs, including HF and death, may be attributed to greater RAAS activity (3).
To date, studies evaluating the association of aldosterone and incident or recurrent CVD and mortality predominantly among NHWs revealed positive associations in participants with acute myocardial infarction (4), coronary artery disease (5–7), advanced HF (8, 9) and among community-dwelling adults (10). In contrast, a multi-ethnic study of individuals with chronic renal insufficiency revealed an association between aldosterone and incident HF, but not with atherosclerotic events or mortality (11). Prospective studies assessing the association of renin with CVD and mortality, mainly including NHW, have yielded inconsistent results (12–15).
The association of components of the RAAS (aldosterone and plasma renin activity [PRA]) with incident CVD or all-cause mortality in AAs remains unclear. We examined these associations among community-dwelling AAs in the Jackson Heart Study (JHS).
Methods
Study Participants
The JHS is a prospective cohort study of 5,301 AA adults, aged 21–94 years from the tri-county area of metropolitan Jackson, Mississippi. The baseline examination was performed between 2000–2004, with two subsequent follow-up examinations between 2005–2008 and 2009–2013. The design of the study has been described elsewhere (16). The JHS was approved by the institutional review boards of the participating institutions and informed consent was obtained from all participants. For this analysis, participants were excluded if they had missing data on exposures, outcomes or important covariates including aldosterone (n=52), systolic blood pressure (n=17), diabetes status (n=61), education (n=20), smoking status (n=40), alcohol use (n=26), body-mass index (n=5), waist circumference (n=2), adiponectin (n=83) and lost to follow-up (n=57, incident CVD and n=65, all-cause mortality analysis). Participants with supra-physiologic serum aldosterone > 2,774 pmol/L (100 ng/dL) (n=2) were excluded. In the incident CVD analysis, participants with CVD at baseline were excluded (n=776). After these exclusions, 4,160 and 4,985 participants were included in the incident CVD and all-cause mortality longitudinal analyses, respectively.
Exposure: Aldosterone and PRA
Fasting blood samples were drawn in the supine position and processed using a standardized protocol, plasma and serum were prepared from samples by sedimentation in a refrigerated centrifuge within two hours of blood collection, stored at −70°C and sent to central laboratories (University of Minnesota) (16, 17). Serum aldosterone was measured by radioimmunoassay (Siemens) and the intra-assay coefficients of variation were 8.7% and 6.2% for low and high concentrations. PRA was measured at baseline using immunoradiometric assays of PRA in ng/ml/hr (n=2,252) with intra-assay coefficient of variation of 8.0%.
Outcomes
The outcomes were incident CVD (CHD, stroke and HF) and all-cause mortality. Methods for ascertaining cardiovascular events and deaths in the JHS cohort have been described previously (18). Briefly, CVD events were ascertained through a combination of active and passive surveillance. Annual follow-up included interviews with participants and next of kin to ascertain health events, such as cardiac events, hospitalizations or death, through questionnaires completed by physicians and medical examiners or coroners and reviewed by the medical record abstraction unit to generate diagnosis information. These diagnoses were reviewed and adjudicated by trained medical personnel. Cardiovascular illness hospitalizations were identified and adjudicated as described previously (18). Hospitalization data was obtained from the hospital discharge index from all catchment area hospitals and annual follow-up data; and data from non-catchment area hospitals were obtained after patient consent. Death certificates from state vital statistics offices were surveyed for potential CVD events. The self-reported data from annual follow-up were reconciled with the hospital discharge index data. The primary diagnoses based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were reviewed and adjudicated by trained medical personnel. We assessed the CHD and stroke occurrence between 2000 and December 30, 2011 and HF hospitalizations between 2005 and December 30, 2011.
Covariates
The covariates included demographics, occupation (management/professional versus not), level of education (≥Bachelor’s degree versus <Bachelor’s degree), tobacco use (current smoking versus not), alcohol use (in the past 12 months versus not), medical conditions and current prescription medication usage. Body mass index (BMI) was calculated as weight (kilograms)/height2 (meters). Hypertension was defined as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mmHg, or use of antihypertensive therapy.
Physical activity and dietary intake were categorized according to the American Heart Association 2020 CV health guidelines as poor, intermediate or ideal health, as described previously (19, 20). Daily sodium intake in milligrams/day was assessed using a validated food frequency questionnaire (21).
Glucose and glycosylated hemoglobin (HbA1c) concentrations were measured as previously described (20). Diabetes was defined based on 2010 American Diabetes Association guidelines (HbA1c ≥ 6.5%, fasting glucose ≥ 126 mg/dL, taking diabetes medications or self-reported physician diagnosis) (22). Fasting serum low-density lipoprotein (LDL: mg/dL) was assayed using standard techniques (17). Estimated glomerular filtration rate (eGFR) was derived using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (ml/min per 1.73 m2) (23).
Statistical Analysis
Due to their non-normal distribution, aldosterone and PRA were log-transformed. To evaluate for dose-response relationships, aldosterone and PRA were divided into medians. Baseline characteristics of participants are presented by medians of log-transformed aldosterone using one-way analysis of variance for normally distributed continuous variables, Mann-Whitney and Kruskal-Wallis test for non-normally distributed continuous variables and the Chi-square test for categorical variables.
Time of incident CVD or all-cause mortality was defined based on the adjudicated date. We censored data for participants at the time of study participation drop out or the end of study follow-up (December 30, 2011). Cox proportional hazards modeling was utilized to estimate hazard ratios (HR) with inclusion of exposures as continuous (each unit increase in SD) or categorical variables (medians). Based on prior analyses covariates were selected a priori (10, 20), multivariable modeling was performed with sequential adjustment as follows:
Model 1 adjusted for age, sex, education, current occupation status, smoking, physical activity, dietary intake, alcohol use and body-mass index (kg/m2),
Model 2: Model 1 + systolic blood pressure, LDL, hemoglobin A1c, and
Model 3: Model 2 + eGFR.
We assessed the shape of the association of aldosterone and PRA with incident CVD and mortality using cubic spline regression with 4 knots, to explore potential nonlinear relationships. We performed a series of sensitivity analyses by (1) limiting the analyses to participants not taking medications that antagonize the RAAS including angiotensin converting enzyme- inhibitors (ACE-inhibitors), angiotensin receptor blockers (ARBs), mineralocorticoid receptor blockers and statins; (2) examining the association of log-aldosterone and log-PRA by individual incident CVD outcome (CHD, stroke and HF). We tested for effect modification by age, sex, hypertension, diabetes and BMI by inserting multiplicative interaction term models and using the likelihood ratio test. The proportional hazards assumption was assessed using Schoenfeld residuals and no significant violations were noted. Statistical significance was defined as two-sided alpha <0.05. Analyses were performed using Stata 13.1 (Statacorp, College Station, TX).
Results
During a median follow-up period of 7.3 years, 322 cases of incident CVD (among 4,160 participants without prevalent CVD at baseline) and 513 deaths (among 4,985 participants with and without CVD at baseline) occurred. Table 1 shows the profile of participants across medians of baseline log-aldosterone. Participants in upper median aldosterone had a more adverse cardiovascular risk profile including higher baseline BMI, waist circumference, systolic blood pressure, diastolic blood pressure, glucose, HbA1c, and lower eGFR compared to participants in lower median aldosterone category. Incidence rates of CHD, Stroke, HF and combined CVD were higher in the upper vs. lower median (all comparisons p<0.05).
Table 1.
Characteristics of Participants in the Jackson Heart Study by Medians of log-Aldosterone at Baseline
| All | Lower Median | Upper Median | p-value* | |
|---|---|---|---|---|
| Baseline Characteristics* | n=4,985 | n=2,544 | n=2,441 | |
| Age (years) | 55.2 (12.8) | 55.3 (12.9) | 55.1 (12.8) | 0.591 |
| Female, sex (%) | 63.2 | 67.1 | 59.2 | <0.001 |
| Education ≥ Bachelor’s Degree (%) | 32.6 | 32.2 | 33.0 | 0.534 |
| Occupation, Management/Professional (%) | 35.7 | 36.1 | 35.3 | 0.550 |
| Current Smoking (%) | 13.1 | 13.1 | 13.2 | 0.915 |
| Current Alcohol Intake (%) | 46.2 | 45.4 | 47.0 | 0.238 |
| Poor AHA Physical Activity† (%) | 49.0 | 49.0 | 48.9 | 0.162 |
| Poor AHA Dietary Intake† (%) | 60.6 | 62.1 | 59.0 | 0.080 |
| Dietary Sodium Intake Total (mg/day) ‡ | 3447 (1521) | 3433 (1507) | 3462 (1536) | 0.527 |
| Body-mass Index (kilograms/meter2) | 31.8 (7.2) | 31.4 (7.4) | 32.1 (7.1) | 0.001 |
| Waist circumference (cm) | 100.7 (16.2) | 99.2 (16.1) | 102.3 (16.0) | <0.001 |
| Systolic blood pressure (mmHg) | 127 (18) | 126 (18) | 128 (19) | <0.001 |
| Diastolic blood pressure (mmHg) | 79 (11) | 78 (10) | 80 (11) | <0.001 |
| Hypertension (%) § | 60.0 | 51.9 | 68.4 | <0.001 |
| Glucose (mg/dL) (4,976) | 102 (38) | 99 (33) | 105 (42) | <0.001 |
| Hemoglobin A1c (4,899) (%) | 6.0 (1.3) | 5.9 (1.2) | 6.1 (1.4) | <0.001 |
| Type 2 Diabetes Mellitus (%) ‖ | 21.6 | 19.5 | 23.9 | <0.001 |
| Estimated Glomerular Filtration Rate (CKD-EPI) mL/min per 1.73 m2 (n=4,976) | 94 (22) | 98 (20) | 91 (23) | <0.001 |
| Low-density Lipoprotein (mg/dL) (n=4,584) | 127(36) | 124 (35) | 129 (37) | <0.001 |
| Non-parametric Variables | Median (Interquartile Range) | |||
| Aldosterone (ng/dl) | 4.4 (2.6, 7.2) | 2.6 (1.9, 3.5) | 7.3 (5.7, 10.1) | 0.001 |
| Plasma Renin Activity (PRA) (ng/mL/hour) ¶ | 0.5 (0.2, 1.1) | 0.4 (0.2, 0.8) | 0.6 (0.3, 1.7) | 0.001 |
| Adiponectin (ng/mL) | 4234 (2685, 6748) | 4658 (2923, 7219) | 3814 (2435, 6118) | 0.001 |
| Crude Incidence Rates per 1000 person-years (95% Confidence Interval) | ||||
| Incidence of Coronary Heart Disease # | 5.1 (4.4, 5.8) | 4.2 (3.4, 5.2) | 6.0 (5.0, 7.2) | 0.019 |
| Incidence of Stroke ** | 3.6 (3.1, 4.3) | 2.7 (2.1, 3.5) | 4.6 (3.8, 5.7) | 0.002 |
| Incidence of Heart Failure †† | 6.5 (5.5, 7.5) | 5.5 (4.3, 6.9) | 7.5 (6.1, 9.2) | 0.042 |
| Incidence of Combined CVD ‡‡ | 10.9 (9.8, 12.2) | 8.5 (7.2, 10.1) | 13.5 (11.7, 15.5) | <0.001 |
| Incidence of All-Cause Mortality §§ | 12.5 (11.4, 13.6) | 11.6 (10.2, 13.2) | 13.4 (11.9, 15.1) | 0.13 |
Mean (SD) or percentages are listed, p-values calculated using chi-square (categorical variables), ANOVA (parametric continuous variables) and Kruskal-Wallis test (non-parametric continuous variables)
AHA = American Heart Association, Ideal physical activity and dietary intake recommendations were defined by AHA “2020” guidelines. Physical Activity was considered ideal if participant achieved ≥ 150 minutes/week moderate intensity or ≥75 min/week vigorous intensity physical activity. Dietary Intake was considered ideal if participant met 4–5 out of 5 of the following recommendations: Fruits and vegetables ≥4.5 cups/day; fish ≥two 3.5 oz. servings per week (preferably oily fish); fiber-rich whole grains ≥three 1 oz.-equivalent servings/day; sodium <1500 mg/day; sugar-sweetened beverages ≤450 kcal (36 oz.)/week (19).
n = 4,507 participant with dietary sodium intake calculated from Jackson Heart Study Food Frequency Questionnaire. (Lower and Upper Halves: n=2,296, n=2,211)
Hypertension was defined as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mmHg, or use of antihypertensive therapy.
Diabetes was defined based on 2010 American Diabetes Association guidelines (HbA1c ≥ 6.5%, fasting blood glucose ≥ 126 mg/dL, taking diabetes medications or with a self-reported physician diagnosis) (22)
n = 2,250 participants with plasma renin activity at baseline (log-Aldosterone Lower and Upper 50%: n=1,118, n=1,132)
n= 4,642 participants, incident rate per 1000 person-years (Overall 192 coronary heart disease events, log-Aldosterone Lower 50%: n=80 events, Upper 50% n=112 events)
n = 4,770 participants, incident rate per 1000 person-years (Overall 142 stroke events, log-Aldosterone Lower 50%: n= 54 events, Upper 50% n=88 events)
n=4,120 participants, incident rate per 1000 person-years (Overall 162 heart failure events, log-Aldosterone Lower 50%: n= 69 events, Upper 50% n=93 events)
n= 4,160 participants, incident rate per 1000 person-years (Overall 322 cardiovascular events, log-Aldosterone Lower 50%: n= 129 events, Upper 50% n=193 events)
incident rate per 1000 Person-years (Overall 513 deaths, Aldosterone Lower 50%: n= 244 deaths, Upper 50% n=269 deaths)
Association of Aldosterone and PRA with Incident Cardiovascular Disease (Table 2)
Table 2.
The Association of log-Aldosterone and log-Renin with Incident Cardiovascular Disease (CHD, Stroke, Heart Failure) over 7 years
| Lower Median | Upper Median | Continuous (per 1-unit SD) | |
|---|---|---|---|
| log-Aldosterone | n=2131 | n=2029 | n=4,160 |
| Median Aldosterone ng/dl (Interquartile Range) | 2.6 (1.9, 3.4) | 7 (5.5, 9.6) | 4.3 (2.5, 6.9) |
| Cardiovascular Disease Cases | 129 | 193 | 322 |
| Rate per 1000 person-years | 8.5 (7.2, 10.1) | 13.5 (11.7, 15.5) | 10.9 (9.8, 12.2) |
| Unadjusted hazard ratio (95% CI) *† | 1.00 (referent) | 1.55 (1.24, 1.94) | 1.25 (1.12, 1.38) |
| Model 1 hazard ratio (95% CI) | 1.00 (referent) | 1.59 (1.27, 1.98) | 1.26 (1.14, 1.40) |
| Model 2 hazard ratio (95% CI) | 1.00 (referent) | 1.41 (1.11, 1.81) | 1.18 (1.05, 1.32) |
| Model 3 hazard ratio (95% CI) | 1.00 (referent) | 1.31 (1.02, 1.68) | 1.13 (1.00, 1.27) |
| log-Plasma Renin Activity | n= 918 | n=901 | n=1,819 |
| Median Plasma Renin Activity ng/ml/hour Interquartile Range) | 0.2 (0.2, 0.3) | 1 (0.6, 2.0) | 0.4 (0.2, 1.0) |
| Cardiovascular Disease Cases | 86 | 105 | 191 |
| Rate per 1000 person-years | 11.9 (9.7, 14.8) | 15.0 (12.4, 18.1) | 13.4 (11.7, 15.5) |
| Unadjusted hazard ratio (95% CI) *† | 1.00 (referent) | 1.18 (0.88, 1.56) | 1.15 (1.00, 1.31) |
| Model 1 hazard ratio (95% CI) | 1.00 (referent) | 1.24 (0.93, 1.65) | 1.16 (1.02, 1.33) |
| Model 2 hazard ratio (95% CI) | 1.00 (referent) | 1.27 (0.92, 1.76) | 1.20 (1.03, 1.39) |
| Model 3 hazard ratio (95% CI) | 1.00 (referent) | 1.21 (0.87, 1.67) | 1.16 (0.99, 1.35) |
Cox-Proportional Hazards Model —Hazard ratio is per 1-unit standard deviation increase in log-aldosterone or log-plasma renin activity for continuous analysis.
Unadjusted: (log-aldosterone n=4,160 with 322 cases of incident CVD: log-plasma renin activity n=1,819 with 191 cases of incident CVD)
Model 1: age, sex, education, current occupation status, smoking, physical activity (American Heart Association-Life’s Simple 7), dietary intake (American Heart Association-Life’s Simple 7), alcohol use and body-mass index (kg/m2) (log-aldosterone n=4,160 with 322 cases of incident CVD: log-plasma renin activity n=1,819 with 191 cases of incident CVD)
Model 2: Model 1 + systolic blood pressure, low-density lipoprotein and hemoglobin A1c (log-aldosterone n=3,805 with 267 cases of incident CVD: log-plasma renin activity n=1,665 with 162 cases of incident CVD)
Model 3: Model 2 + estimated glomerular filtration rate (CKD-EPI) (log-aldosterone n=3,798 with 266 cases of incident CVD: log-plasma renin activity n=1,661 with 161 cases of incident CVD)
Every 1-unit SD increase in log-aldosterone was associated with a 26% (95% CI: 1.14, 1.40) higher risk of CVD, after adjustments for socioeconomic variables and cardiovascular risk factors including BMI. This association remained significant with full adjustment for cardiovascular risk factors including LDL, systolic blood pressure, HbA1c and eGFR (Model 3). When examined as a categorical variable, the upper median of aldosterone was associated with a 59% higher risk of CVD compared to the lower median (Model 1) and remained significant after full adjustment (Model 3). The shape of the association of aldosterone with incident CVD unadjusted and fully-adjusted using cubic splines revealed a non-linear association (Figure 2A+B). Log-aldosterone was additionally positively associated with the individual outcomes of incident stroke, CHD and HF (Supplemental Tables 1–3).
Figure 2. Associations of Plasma Renin Activity, Examined as a Continuous Variable, with All-Cause Mortality.
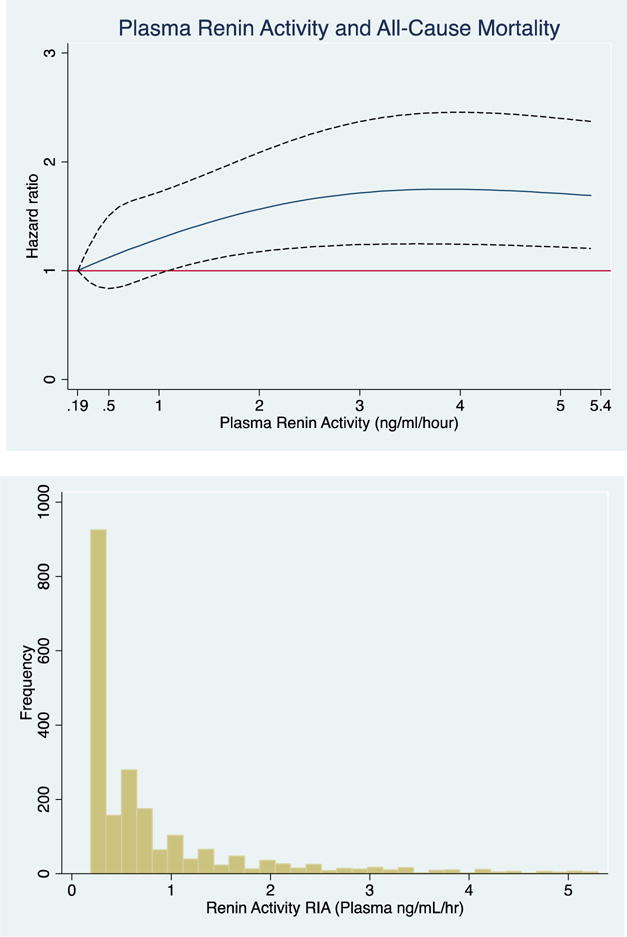
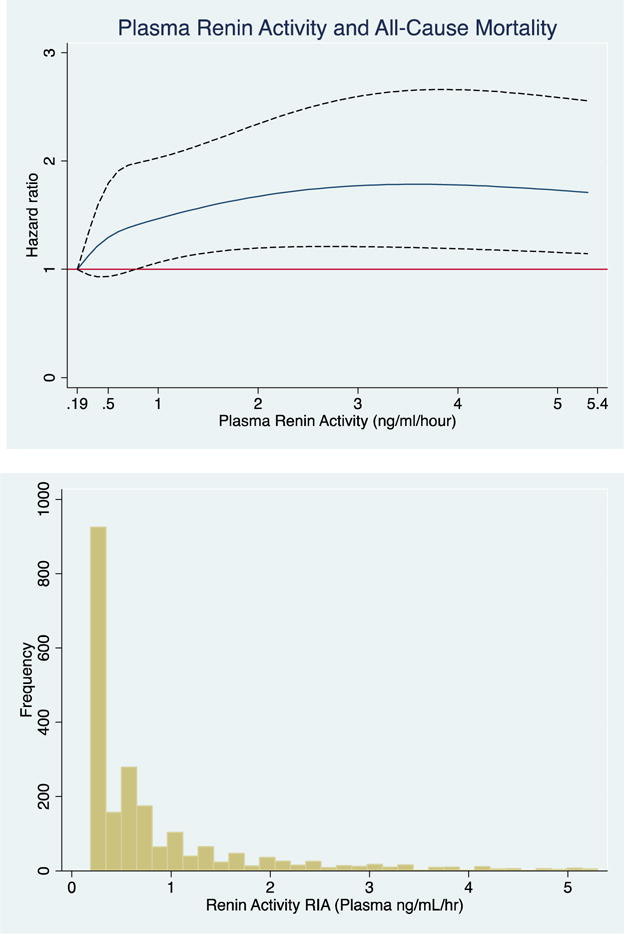
The cubic spline regression with 4 knots (referent) estimates the hazard ratio of plasma renin activity (ng/ml/hour) up to 95th percentile with all-cause mortality. Dotted lines represent 95% confidence intervals. Below each spline is the histogram of the distribution of plasma renin activity. A) unadjusted B) fully adjusted (age, sex, education, current occupation status, smoking, physical activity, dietary intake, alcohol use and body mass index, systolic blood pressure, low-density lipoprotein, HbA1c and eGFR.
Similar findings were seen for log-PRA, each 1-unit SD increase in log-PRA was associated with an 16% higher risk of incident CVD, full adjustments for cardiovascular risk factors modestly attenuated the significance of this finding (Model 3: HR 1.16 (95% CI: 0.99, 1.35)). When examined as a categorical variable, findings were in a similar direction but were non-significant.
Excluding participants on RAAS antagonists including ACE-I, ARBs, mineralocorticoid receptor antagonists and statins strengthened the continuous and categorical positive associations with log-aldosterone and incident cardiovascular disease, but attenuated the association of log-PRA with incident CVD (Supplemental Table 4).
Association of Aldosterone and PRA with All-Cause Mortality (Table 3)
Table 3.
The Association of log-Aldosterone and log-Plasma Renin Activity with All-Cause Mortality over 8 years
| Lower Median | Upper Median | Continuous (per 1-unit SD) | |
|---|---|---|---|
| log-Aldosterone | n=2,544 | n=2,441 | n=4,985 |
| Median Aldosterone ng/dl (Interquartile Range) | 2.6 (1.9, 3.5) | 7.3 (5.7, 10.1) | 4.4 (2.6, 7.2) |
| All-Cause Mortality Cases | 244 | 269 | 513 |
| Rate per 1000 person-years | 11.6 (10.2, 13.2) | 13.4 (11.9, 15.1) | 12.5 (11.4, 13.6) |
| Unadjusted hazard ratio (95% CI) *† | 1.00 (referent) | 1.14 (0.96, 1.36) | 1.12 (1.03, 1.22) |
| Model 1 hazard ratio (95% CI) | 1.00 (referent) | 1.18 (0.99, 1.41) | 1.13 (1.04, 1.23) |
| Model 2 hazard ratio (95% CI) | 1.00 (referent) | 1.11 (0.91, 1.34) | 1.09 (0.99, 1.20) |
| Model 3 hazard ratio (95% CI) | 1.00 (referent) | 1.01 (0.83, 1.24) | 1.04 (0.94, 1.14) |
| log-Plasma Renin Activity | n=1232 | n=1018 | n=2,250 |
| Median Plasma Renin Activity ng/ml/hour (Interquartile Range) | 0.2 (0.2, 0.3) | 1.2 (0.8, 2.6) | 0.5 (0.2, 1.1) |
| All-Cause Mortality Cases | 137 | 160 | 297 |
| Rate per 1000 person-years | 12.3 (10.4, 14.6) | 17.7 (15.2, 20.7) | 14.7 (13.2, 16.5) |
| Unadjusted hazard ratio (95% CI) *† | 1.00 (referent) | 1.41 (1.13, 1.78) | 1.14 (1.02, 1.26) |
| Model 1 hazard ratio (95% CI) | 1.00 (referent) | 1.40 (1.11, 1.76) | 1.12 (1.01, 1.24) |
| Model 2 hazard ratio (95% CI) | 1.00 (referent) | 1.59 (1.22, 2.06) | 1.17 (1.04, 1.32) |
| Model 3 hazard ratio (95% CI) | 1.00 (referent) | 1.53 (1.18, 1.99) | 1.14 (1.01, 1.29) |
Cox-Proportional Hazards Model — Hazard ratio is per 1-unit standard deviation increase in log-aldosterone or log-plasma renin activity for continuous analysis
Unadjusted: (log-aldosterone n=4,985 with 513 deaths: log-plasma renin activity n=2,250 with 297 deaths)
Model 1: age, sex, education, current occupation status, smoking, physical activity (American Heart Association-Life’s Simple 7), dietary intake (American Heart Association-Life’s Simple 7), alcohol use and body-mass index (kg/m2) (log-aldosterone n=4,985 with 513 deaths: log-plasma renin activity n=2,250 with 297 deaths)
Model 2: Model 1 + systolic blood pressure, low-density lipoprotein and hemoglobin A1c (log-aldosterone n=4,508 with 414 deaths: log-plasma renin activity n=2,036 with 249 deaths)
Model 3: Model 2 + estimated glomerular filtration rate (CKD-EPI) (log-aldosterone n=4,499 with 412 deaths: log-plasma renin activity n=2,030 with 247 deaths)
A 1-unit SD increase in log-aldosterone was associated with a 13% higher risk of mortality with model 1 adjustments, was mildly attenuated after adjustment for systolic blood pressure, LDL and HbA1c and fully attenuated after additional adjustment for eGFR. Similar findings were seen for categorical results, although non-significant.
A 1-unit SD increase in log-PRA was associated with a 12% higher risk of all-cause mortality, which remained significant after full-adjustment. The upper median of log-PRA was associated with a 53% higher risk of all-cause mortality in the fully adjusted model. Evaluation of the continuous association of PRA with all-cause mortality unadjusted and fully-adjusted using cubic splines revealed a similar positive association (Figure 2A+B).
Excluding participants on RAAS antagonists including ACE-I, ARBs, mineralocorticoid receptor antagonists and statins did not significantly alter these findings (Supplemental Table 5). We found no evidence of effect modification by age, sex, hypertension, diabetes or BMI, in the association of log-aldosterone with incident CVD or all-cause mortality (Supplemental Table 6).
Discussion
In this prospective study of AAs, higher aldosterone and PRA were non-linearly associated with higher risks of CVD and all-cause mortality, independently of established cardiovascular risk factors. The use of RAAS antagonists did not affect the relationship of aldosterone with CVD or aldosterone and PRA with all-cause mortality. We found no evidence of effect modification by age, sex, hypertension, diabetes or BMI. These results suggest that aldosterone and PRA are important in the pathophysiology of CVD and all-cause mortality among AAs. Our findings combined with those of prior studies indicate that the relative value of aldosterone and PRA may be more important than the absolute value among AAs. Among participants without treated hypertension in the Multi-Ethnic Study of Atherosclerosis (MESA) aldosterone and PRA were significantly lower in AA vs. NHWs (24), but lower aldosterone:renin ratio was associated with lower blood pressures among only NHWs (24). This finding of a low renin state among AAs is consistent with other studies (25, 26), whereas results for aldosterone, have been mixed with AA normotensive subjects favoring lower aldosterone (27–29) and AA hypertensive subjects favoring elevated aldosterone (29–31). Consistent with these findings, a study of AAs and NHWs including aldosterone, PRA activity and blood pressure measurements (during childhood and adulthood), found that although levels of PRA and aldosterone were lower in AAs, blood pressure was positively associated with plasma aldosterone in AAs, but not NHWs. Furthermore, administration of 9-α fludrocortisone (synthetic steroid with mineralocorticoid receptor activity) increased blood pressure in AAs but not NHWs (32).
Aldosterone, CVD and Mortality
To our knowledge, the current report is the largest community-based study of the effects of components of RAAS. Our study presents novel findings of a positive association of aldosterone with stroke, CHD, HF and a composite of CVD. These results extend the previous positive association of aldosterone with all-cause mortality seen among community-dwelling NHWs (10). Our results align with previous studies of predominantly NHW participants with known CVD (high-risk), reporting positive associations of aldosterone with cardiovascular events and mortality (4–9). The Chronic Renal Insufficiency Cohort (CRIC), included 3,866 participants with chronic kidney disease of which 1,606 were AA; revealing a positive association of aldosterone with incident HF among AAs but no association with atherosclerotic events or death (11). However, the CRIC population may not be representative of the general population as CKD is a CVD risk factor and a CHD risk equivalent for all-cause mortality (33), hence people with CKD may develop events through pathways that could be independent of aldosterone, especially those in CKD stage IV and V. Our cohort is similar to prior studies with a high prevalence of diabetes (19%) and hypertension (55%), but importantly differed in that greater than 80% of individuals were without CVD at baseline, hence participants had less burden of CVD at baseline compared to the studies of NHW participants with CVD at baseline and later stage CKD patients.
PRA, CVD and Mortality
Our investigation of the association of PRA with CVD and all-cause mortality is the largest ever conducted among AAs; thus, having an adequate size to robustly assess the relation of PRA to CVD and all-cause mortality. Our results extend previous findings in smaller, predominantly NHW cohorts, with discrepant findings (12–15). A multi-ethnic study of 1,717 hypertensive subjects (64% NHW) found an increased risk of CHD with higher renin profile levels (PRA divided by urinary excretion of sodium), but not stroke or mortality (12). These findings were driven by the significant association among NHWs, as the incident event rates for non-whites was similar for the normal and high renin groups. A study of 803 NHW males, selected independent of blood pressure, found no association between renin and incident CVD (13). In the Framingham Offspring study (majority NHW), there was an association between renin and greater short-term but not long-term mortality nor incident CVD (14). Lastly, in a study of patients referred for coronary angiography (high risk) there was an association between renin and higher cardiovascular mortality over 10 years among NHWs (15).
Mechanisms: Blood Pressure Dependent and Independent RAAS Effects
The observed effects of aldosterone and renin, may be mediated through blood pressure or by more direct effects on the vasculature, myocardium and central nervous system (34). Aldosterone via activation of the mineralocorticoid receptor may exert deleterious effects on the vasculature by decreasing nitric oxide bioavailability, inflammation, smooth muscle proliferation, fibrosis and calcification leading to remodeling and atherosclerosis independent of blood pressure (35, 36). In the myocardium formation of reactive oxygen species, inflammation and coronary vasoconstriction leading to decreased contractility and fibrosis, promoting heart failure and arrhythmic generation (37, 38). Through central nervous system neural actions, aldosterone may promote sodium retention and hypertension (39). In humans, plasma aldosterone is an independent predictor of progression of carotid plaque, left ventricular hypertrophy and atrial fibrillation, potentially resulting from the deleterious effects on the vasculature, myocardium and biological effects of aldosterone and renin including sodium and water retention and hypertension (10, 36).
In preclinical models and human trials, the deleterious end organ effects of aldosterone depend upon co-existent high dietary sodium intake (20, 40). Recently, high-salt diet was shown to potentiate the negative effects of aldosterone via activation of the Rho family member Rac1 (41). AAs may be more impacted by the effects of the RAAS system due to a combination of a high sodium intake (>85% with intake exceeding 2,300 mg/day, mean 3,500 mg/day in JHS) and high prevalence of salt-sensitivity (73% of hypertensive and 36% of normotensive AAs) (20, 42, 43). Similar to the preclinical model Dahl Salt-Sensitive rat which acutely suppress aldosterone in response to salt loads, but with paradoxical elevation over time (44); Inappropriate activation of the RAAS in AAs combined with a maladaptive inability to lower aldosterone levels in response to high sodium intake, may be a mediator of both the hypertension-mediated effects and the direct effects of aldosterone on end-organs including the heart (45). On the contrary, high plasma renin and aldosterone levels with very low sodium intake is not associated with cardiovascular disease, as seen in Yanomamo Indians (46).
Strengths and Limitations
There are potential imitations to our study. First, the participants in the JHS are from a single metropolitan area in the southeastern United States, possibly limiting the generalizability to other AA populations. Second, aldosterone and PRA levels were measured at baseline and we were not able to account for changes during follow-up. Third, we did not have a measure of 24-hour urinary sodium in the full cohort and thus were unable to assess the impact of dietary sodium intake on aldosterone and PRA. Fourth, the association of PRA with CVD was significantly attenuated after exclusion of participants on RAAS antagonists. This may have been the result of ACE-I, ARBs, mineralocorticoid receptor antagonists causing elevation in PRA levels (47), which potentially could lead to type 1 error in our primary analysis. Fifth, statistical associations were interpreted without correction for multiple comparisons, as typical multiple comparison corrections assume tests are independent, and are too conservative for correlated hypotheses as have been performed. Therefore, some caution is warranted in the interpretation of our study results. Lastly, we may have underestimated the relation of RAAS elements with cardiovascular disease, as individuals with peripheral vascular disease only, may have remained undetected.
Strengths of the current investigation include the examination of a large, socioeconomically diverse, community-dwelling sample of AAs with over a decade of follow-up, standardized assessments of biomarkers, adjudicated outcomes of CVD and mortality, and adjustment for several key factors known to influence both serum aldosterone, PRA and cardiovascular disease.
Conclusion
Our study reveals a novel association of aldosterone and PRA with incident CVD and all-cause mortality among community-dwelling AAs. It suggests that the RAAS should be considered in the identification of high-risk individuals, to optimize therapeutic or preventive strategies for CVD and mortality in AAs, through a more personalized approach. Further research and clinical trials exploring the impact of current and novel RAAS antagonists in the prevention of CVD, over and above blood pressure and cardiac remodeling in AAs (stroke, glucose metabolism, etc.) is paramount given the potential to improve cardiovascular health and reduce racial/ethnic disparities in CVD and mortality.
Supplementary Material
Figure 1. Associations of Aldosterone, Examined as a Continuous Variable, with Cardiovascular Events.
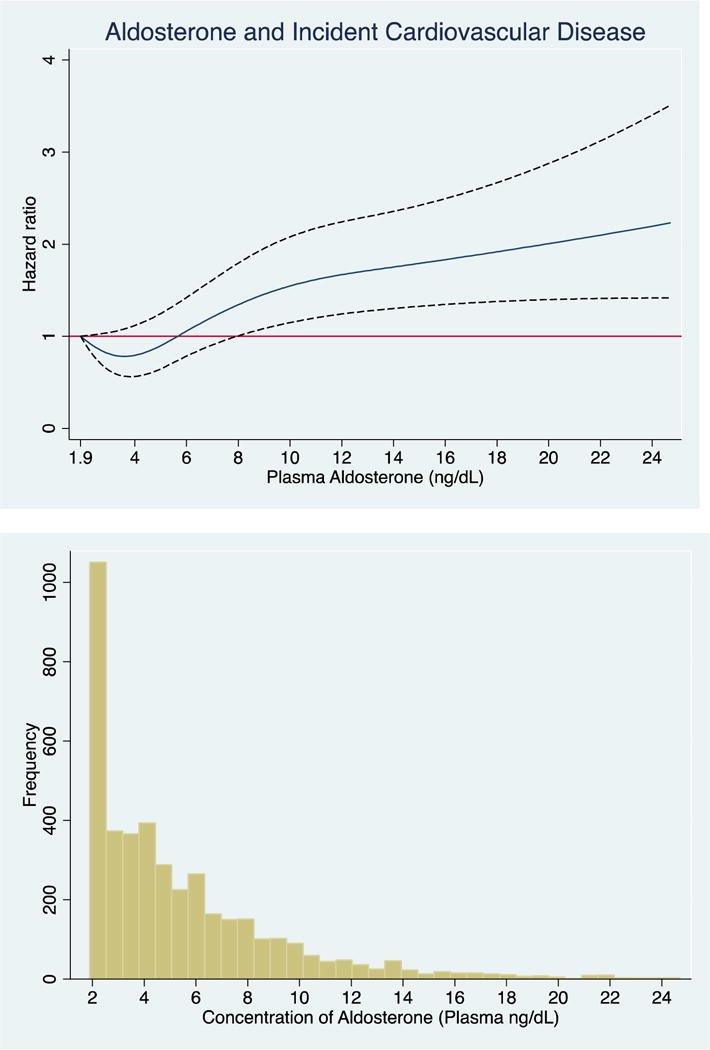
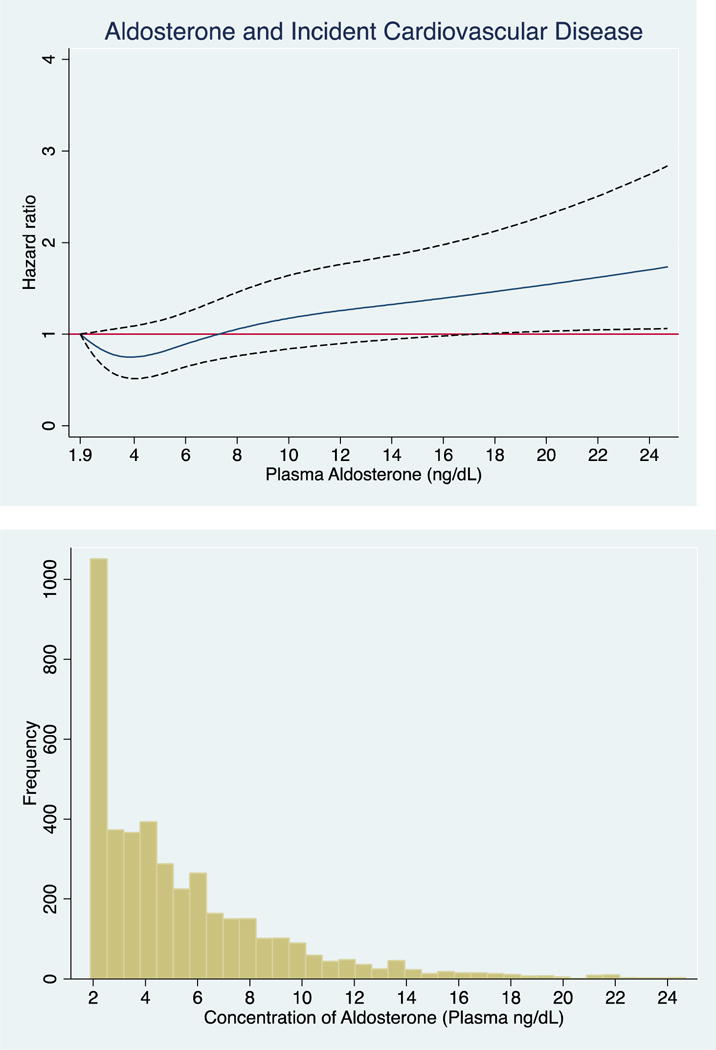
The cubic spline regression with 4 knots (referent 1.9) estimates the hazard ratio of serum aldosterone up to 99th percentile with incident CVD. Dotted lines represent 95% confidence intervals. Below each spline is the histogram of the distribution of serum aldosterone concentration. A) unadjusted B) fully adjusted (age, sex, education, current occupation status, smoking, physical activity, dietary intake, alcohol use and body mass index, systolic blood pressure, low-density lipoprotein, HbA1c and eGFR.
PERSPECTIVES.
Competency in Medical Knowledge: Higher levels of aldosterone and plasma renin activity are associated with incident cardiovascular disease and all-cause mortality among AAs.
Translational Outlook 1: Further research on the assessment of the renin-angiotensin-aldosterone system in risk prediction models for cardiovascular disease and mortality among African Americans at all risk strata is warranted.
Translational Outlook 2: Current guidelines (JNC 8) recommend initiation of renin-angiotensin aldosterone antagonist (ACE-inhibitors and ARBs) after thiazide diuretics or calcium channel blockers among African Americans. Current and novel RAAS antagonists warrant further study in the blood pressure dependent and independent effects on cardiovascular disease and mortality among African Americans.
Acknowledgments
The authors thank the other investigators, the data collection staff, and the participants of the JHS for their valuable contributions. The JHS is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. Joshua Joseph was supported by an institutional training grant from the National Institute of Diabetes, Digestive, and Kidney Diseases (T32 DK062707). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the NIH; or the U.S. Department of Health and Human Services.
Abbreviations
- CVD
Cardiovascular disease
- RAAS
renin-angiotensin-aldosterone system
- AA
African American
- NHW
non-Hispanic white
- HF
heart failure
- PRA
plasma renin activity
- LDL
low-density lipoprotein
- eGFR
estimated glomerular filtration rate
- HbA1c
hemoglobin A1c
- BMI
body mass index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors have no relationships with industry to disclose.
References
- 1.Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002;347:1585–1592. doi: 10.1056/NEJMsa012979. [DOI] [PubMed] [Google Scholar]
- 2.Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13:S9. doi: 10.18553/jmcp.2007.13.s8-b.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michel FS, Norton GR, Majane OHI, et al. Contribution of Circulating Angiotensinogen Concentrations to Variations in Aldosterone and Blood Pressure in a Group of African Ancestry Depends on Salt Intake. Hypertension. 2012;59:62–69. doi: 10.1161/HYPERTENSIONAHA.111.181230. [DOI] [PubMed] [Google Scholar]
- 4.Beygui F, Collet J-P, Benoliel J-J, et al. High Plasma Aldosterone Levels on Admission Are Associated With Death in Patients Presenting With Acute ST-Elevation Myocardial Infarction. Circulation. 2006;114:2604–2610. doi: 10.1161/CIRCULATIONAHA.106.634626. [DOI] [PubMed] [Google Scholar]
- 5.Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, Marz W. Plasma aldosterone levels are associated with increased cardiovascular mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Eur Heart J. 2010;31:1237–1247. doi: 10.1093/eurheartj/ehq019. [DOI] [PubMed] [Google Scholar]
- 6.Tomaschitz A, Pilz S, Ritz E, et al. Association of Plasma Aldosterone With Cardiovascular Mortality in Patients With Low Estimated GFR: The Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Am J Kidney Dis. 2011;57:403–414. doi: 10.1053/j.ajkd.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 7.Ivanes F, Susen S, Mouquet F, et al. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur Heart J. 2012;33:191–202. doi: 10.1093/eurheartj/ehr176. [DOI] [PubMed] [Google Scholar]
- 8.Latini R. The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in Val-HeFT. Eur Heart J. 2004;25:292–299. doi: 10.1016/j.ehj.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Guder G, Bauersachs J, Frantz S, et al. Complementary and Incremental Mortality Risk Prediction by Cortisol and Aldosterone in Chronic Heart Failure. Circulation. 2007;115:1754–1761. doi: 10.1161/CIRCULATIONAHA.106.653964. [DOI] [PubMed] [Google Scholar]
- 10.Buglioni A, Cannone V, Cataliotti A, et al. Circulating Aldosterone and Natriuretic Peptides in the General Community Relationship to Cardiorenal and Metabolic Disease. Hypertension. 2015;65:45–53. doi: 10.1161/HYPERTENSIONAHA.114.03936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deo R, Yang W, Khan AM, et al. Serum Aldosterone and Death, End-Stage Renal Disease, and Cardiovascular Events in Blacks and Whites Findings From the Chronic Renal Insufficiency Cohort (CRIC) Study. Hypertension. 2014;64:103–110. doi: 10.1161/HYPERTENSIONAHA.114.03311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alderman MH, Madhavan S, Ooi WL, Cohen H, Sealey JE, Laragh JH. Association of the Renin-Sodium Profile with the Risk of Myocardial Infarction in Patients with Hypertension. N Engl J Med. 1991;324:1098–1104. doi: 10.1056/NEJM199104183241605. [DOI] [PubMed] [Google Scholar]
- 13.Meade TW, Cooper JA, Peart WS. Plasma Renin Activity and Ischemic Heart Disease. N Engl J Med. 1993;329:616–619. doi: 10.1056/NEJM199308263290905. [DOI] [PubMed] [Google Scholar]
- 14.Parikh NI, Gona P, Larson MG, et al. Plasma renin and risk of cardiovascular disease and mortality: the Framingham Heart Study. Eur Heart J. 2007;28:2644–2652. doi: 10.1093/eurheartj/ehm399. [DOI] [PubMed] [Google Scholar]
- 15.Tomaschitz A, Pilz S, Ritz E, et al. Associations of plasma renin with 10-year cardiovascular mortality, sudden cardiac death, and death due to heart failure. Eur Heart J. 2011;32:2642–2649. doi: 10.1093/eurheartj/ehr150. [DOI] [PubMed] [Google Scholar]
- 16.Taylor HA, Jr, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15:S6–4. [PubMed] [Google Scholar]
- 17.Carpenter MA, Crow R, Steffes M, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328:131–144. doi: 10.1097/00000441-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Keku E, Rosamond W, Taylor HA, Jr, et al. Cardiovascular disease event classification in the Jackson Heart Study: methods and procedures. Ethn Dis. 2005;15:S6–62. [PubMed] [Google Scholar]
- 19.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction: The American Heart Association’s Strategic Impact Goal Through 2020 and Beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 20.Joseph JJ, Echouffo-Tcheugui JB, Kalyani RR, et al. Aldosterone, Renin, and Diabetes Mellitus in African Americans: The Jackson Heart Study. J Clin Endocrinol Metab. 2016;101:1770–1778. doi: 10.1210/jc.2016-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carithers T, Dubbert PM, Crook E, et al. Dietary assessment in African Americans: methods used in the Jackson Heart Study. Ethn Dis. 2005;15:S6-49-55. [PubMed] [Google Scholar]
- 22.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, Young BA, Fülöp T, et al. Effects of Serum Creatinine Calibration on Estimated Renal Function in African Americans: The Jackson Heart Study. Am J Med Sci. 2015;349:379–384. doi: 10.1097/MAJ.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rifkin DE, Khaki AR, Jenny NS, et al. Association of Renin and Aldosterone With Ethnicity and Blood Pressure: The Multi-Ethnic Study of Atherosclerosis. Am J Hypertens. 2014;27:801–810. doi: 10.1093/ajh/hpt276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy SB, Lilley JJ, Frigon RP, Stone RA. Urinary kallikrein and plasma renin activity as determinants of renal blood flow. The influence of race and dietary sodium intake. J Clin Invest. 1977;60:129–138. doi: 10.1172/JCI108749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitas JA, Holle R, Levy SB, Stone RA. Racial analysis of the volume-renin relationship in human hypertension. Arch Intern Med. 1979;139:157–160. [PubMed] [Google Scholar]
- 27.He FJ, Markandu ND, Sagnella GA, MacGregor GA. Importance of the renin system in determining blood pressure fall with salt restriction in black and white hypertensives. Hypertension. 1998;32:820–824. doi: 10.1161/01.hyp.32.5.820. [DOI] [PubMed] [Google Scholar]
- 28.Pratt JH, Jones JJ, Miller JZ, Wagner MA, Fineberg NS. Racial differences in aldosterone excretion and plasma aldosterone concentrations in children. N Engl J Med. 1989;321:1152–1157. doi: 10.1056/NEJM198910263211703. [DOI] [PubMed] [Google Scholar]
- 29.Grim CE, Cowley AW, Hamet P, et al. Hyperaldosteronism and Hypertension: Ethnic Differences. Hypertension. 2005;45:766–772. doi: 10.1161/01.HYP.0000154364.00763.d5. [DOI] [PubMed] [Google Scholar]
- 30.Drayer JI, Weber MA, Sealey JE, Laragh JH. Low and high renin essential hypertension: a comparison of clinical and biochemical characteristics. Am J Med Sci. 1981;281:135–142. doi: 10.1097/00000441-198105000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Griffing GT, Wilson TE, Melby JC. Alterations in aldosterone secretion and metabolism in low renin hypertension. J Clin Endocrinol Metab. 1990;71:1454–1460. doi: 10.1210/jcem-71-6-1454. [DOI] [PubMed] [Google Scholar]
- 32.Tu W, Eckert GJ, Hannon TS, et al. Racial differences in sensitivity of blood pressure to aldosterone. Hypertension. 2014;63:1212–1218. doi: 10.1161/HYPERTENSIONAHA.113.02989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Briasoulis A, Bakris GL. Chronic Kidney Disease as a Coronary Artery Disease Risk Equivalent. Curr Cardiol Rep. 2013;15 doi: 10.1007/s11886-012-0340-4. Available at: http://link.springer.com/10.1007/s11886-012-0340-4. Accessed January 27, 2016. [DOI] [PubMed] [Google Scholar]
- 34.Williams SF. African Americans, hypertension and the renin angiotensin system. World J Cardiol. 2014;6:878. doi: 10.4330/wjc.v6.i9.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pruthi D, McCurley A, Aronovitz M, Galayda C, Karumanchi SA, Jaffe IZ. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler Thromb Vasc Biol. 2014;34:355–364. doi: 10.1161/ATVBAHA.113.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Rita O, Hackam DG, Spence JD. Effects of Aldosterone on Human Atherosclerosis: Plasma Aldosterone and Progression of Carotid Plaque. Can J Cardiol. 2012;28:706–711. doi: 10.1016/j.cjca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Fujita M, Minamino T, Asanuma H, et al. Aldosterone Nongenomically Worsens Ischemia Via Protein Kinase C-Dependent Pathways in Hypoperfused Canine Hearts. Hypertension. 2005;46:113–117. doi: 10.1161/01.HYP.0000171184.84077.80. [DOI] [PubMed] [Google Scholar]
- 38.Chai W, Garrelds IM, de Vries R, Batenburg WW, van Kats JP, Jan Danser AH. Nongenomic Effects of Aldosterone in the Human Heart: Interaction With Angiotensin II. Hypertension. 2005;46:701–706. doi: 10.1161/01.HYP.0000182661.98259.4f. [DOI] [PubMed] [Google Scholar]
- 39.Geerling JC, Kawata M, Loewy AD. Aldosterone-sensitive neurons in the rat central nervous system. J Comp Neurol. 2006;494:515–527. doi: 10.1002/cne.20808. [DOI] [PubMed] [Google Scholar]
- 40.du Cailar G, Fesler P, Ribstein J, Mimran A. Dietary Sodium, Aldosterone, and Left Ventricular Mass Changes During Long-Term Inhibition of the Renin-Angiotensin System. Hypertension. 2010;56:865–870. doi: 10.1161/HYPERTENSIONAHA.110.159277. [DOI] [PubMed] [Google Scholar]
- 41.Shibata S, Mu S, Kawarazaki H, et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor—dependent pathway. J Clin Invest. 2011;121:3233–3243. doi: 10.1172/JCI43124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svetkey LP, McKeown SP, Wilson AF. Heritability of salt sensitivity in black Americans. Hypertension. 1996;28:854–858. doi: 10.1161/01.hyp.28.5.854. [DOI] [PubMed] [Google Scholar]
- 43.Jackson SL, King SM, Zhao L, Cogswell ME. Prevalence of excess sodium intake in the United States—NHANES, 2009–2012. MMWR Morb Mortal Wkly Rep. 2016;64:1393–7. doi: 10.15585/mmwr.mm6452a1. [DOI] [PubMed] [Google Scholar]
- 44.Morizane S, Mitani F, Ozawa K, et al. Biphasic time course of the changes in aldosterone biosynthesis under high-salt conditions in Dahl salt-sensitive rats. Arterioscler. Thromb Vasc Biol. 2012;32:1194–1203. doi: 10.1161/ATVBAHA.111.242719. [DOI] [PubMed] [Google Scholar]
- 45.Catena C, Colussi G, Nait F, Martinis F, Pezzutto F, Sechi LA. Aldosterone and the Heart: Still an Unresolved Issue? Front Endocrinol. 2014;5 doi: 10.3389/fendo.2014.00168. Available at: http://journal.frontiersin.org/article/10.3389/fendo.2014.00168/abstract. Accessed March 25, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation. 1975;52:146–151. doi: 10.1161/01.cir.52.1.146. [DOI] [PubMed] [Google Scholar]
- 47.Funder JW, Carey RM, Fardella C, et al. Case Detection, Diagnosis, and Treatment of Patients with Primary Aldosteronism: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


