Abstract
To determine whether or not petrosal sinus sampling is useful to distinguish patients with mild or intermittent Cushing disease from normal subjects and from individuals with pseudo-Cushing states, we performed bilateral inferior petrosal sinus sampling for adrenocorticotropin (ACTH) before and after the administration of corticotropin-releasing hormone (CRH) in 7 eucortisolemic volunteers, 8 hypercortisolemic patients with pseudo-Cushing states, and 40 patients with ACTH-dependent Cushing disease whose urine free cortisol excretion was within the range found in patients having pseudo-Cushing states (<1000nmol/d [<360 μg/d]). The ACTH level, the ratio of the inferior petrosal sinus ACTH to the peripheral vein ACTH concentration (the IPS:P ratio) and the greater ratio of the right to left, or left to right, petrosal sinuses (the R:L ratio) were compared in patients with and without Cushing disease.
Maximal petrosal ACTH values were significantly elevated in patients with Cushing disease compared to patients with pseudo-Cushing states before administration of CRH (p<0.001), but not after CRH. Maximal petrosal plasma ACTH values after the administration of CRH as high as 808 pmol/L (3670 pg/mL) and 469 pmol/L (2130 pg/mL) were found in patients with pseudo-Cushing states and in normal volunteers, respectively, whereas maximal petrosal ACTH levels as low as 10 pmol/L (46 pg/mL) were observed in patients with surgically-proven Cushing disease. Maximal IPS:P ratio and minimal IPS:P ratio were significantly greater in patients with Cushing disease than in subjects without Cushing disease before, but not after, CRH. R:L ratios did not differ among groups either before, or after, CRH. All of the subjects without Cushing disease showed large R:L gradients, consistent with the notion of one dominant petrosal sinus containing a greater percentage of pituitary effluent. The ACTH concentrations, the IPS:P ratios, and the R:L ratios exhibited great overlap between those with and without Cushing disease, which resulted in a diagnostic accuracy of 81% at best for the diagnosis of Cushing disease.
We conclude that petrosal sinus sampling is of limited usefulness in distinguishing either normal individuals or patients with pseudo-Cushing states from those with mild Cushing disease. This limited usefulness must be recognized when interpreting the results of petrosal sinus sampling in patients with mild or intermittent hypercortisolism who may have a pseudo-Cushing state. Because of these limitations, petrosal sinus sampling should be reserved for patients with clear clinical and biochemical evidence of Cushing syndrome.
Introduction
Simultaneous bilateral, inferior petrosal sinus sampling for adrenocorticotropin (ACTH) either without1–10 or with11–21 corticotropin-releasing hormone (CRH) stimulation is useful in the differential diagnosis of Cushing syndrome. In one series of 281 patients,21 petrosal sinus sampling with CRH distinguished 100% of patients with an ACTH-secreting pituitary microadenoma from those with other causes of Cushing syndrome when the ratio of the plasma ACTH concentration in one of the inferior petrosal sinuses to the level in peripheral blood (the IPS:P ratio) was greater than or equal to 3.3. In contrast, all patients with ectopic ACTH syndrome had a maximal IPS:P ratio of less than or equal to 2.3. Even without administration of CRH, an IPS:P ratio greater than or equal to 2.0 was 100% specific for the diagnosis of Cushing disease. Thus, the inferior petrosal sinus sampling test has a higher sensitivity and specificity for the diagnosis of Cushing disease than either the standard low-dose-high-dose dexamethasone suppression test22 or the peripheral CRH test.23
The diagnostic accuracy of petrosal sinus sampling has been demonstrated only in patients with clear clinical and laboratory evidence for sustained hypercortisolism. However, the utility of petrosal sinus sampling in patients whose normal pituitary corticotrophs may be incompletely suppressed by mild or intermittent hypercortisolism is unknown. Such patients include individuals with subtle clinical and mild laboratory manifestations of Cushing syndrome, episodic (periodic) hormonogenesis alternating with eucortisolemic (or even hypocortisolemic) intervals,24,25 or recent adrenosuppressive therapy. The inferior petrosal sinus ACTH levels of such patients might be elevated above peripheral levels because of the contribution from the normal corticotrophs, thus suggesting the presence of Cushing disease, regardless of the true etiology. However, if the petrosal sinus sampling results of most patients with proven Cushing disease could be shown to be sufficiently different from normal individuals or from patients with pseudo-Cushing states, even when the patients with Cushing disease were mildly hypercortisolemic or eucortisolemic (with episodic hormonogenesis), criteria for interpretation of the results of petrosal sinus sampling might be developed so that early diagnosis and treatment of such patients might be possible.
We hypothesized that the results of petrosal sinus sampling in patients with Cushing disease would differ in three ways from petrosal sinus sampling performed in individuals without Cushing disease. First, the maximal ACTH concentrations in one petrosal sinus and the IPS:P ratios would usually be greater in patients with Cushing disease than in normal individuals or in those with pseudo-Cushing states. Second, patients with Cushing disease would demonstrate greater differences between the ACTH levels in the two petrosal sinuses. Third, after administration of CRH, patients with Cushing disease would have lower IPS:P ratios for one petrosal sinus and higher IPS:P ratios for the contralateral petrosal sinus. To evaluate these hypotheses, we performed bilateral, simultaneous inferior petrosal sinus sampling before and after CRH administration in patients with Cushing disease, in individuals with pseudo-Cushing states, and in normal volunteers.
Subjects and Methods
Patients
A total of 48 patients were referred to the National Institute of Child Health and Human Development between December 15, 1989 and August 1, 1991 for diagnosis and treatment of hypercortisolism who had mild-to-moderate (45) or episodic (3) hypercortisolism, as shown by multiple urine free cortisol measurements < 1000 nmol/d (360 μg/d). Forty of these patients had a definitive diagnosis of Cushing disease, and 8 patients were shown to have pseudo-Cushing states. Seven normal volunteers also participated (Table 1).
Table 1.
Demographics of study participants
| Cushing Disease | Pseudo-Cushing States | Normal Volunteers | |
|---|---|---|---|
| Sample size | 40 | 8 | 7 |
| Age (y) | 38.6 ± 15.8 | 42.3 ± 12.7 | 30.0 ± 4.2 |
| Height (cm) | 165.0 ± 14.8 | 163.1 ± 6.5¶ | 170.5 ± 5.0 |
| Weight (kg) | 82.9 ± 23.9 | 79.2 ± 20.2 | 70.4 ± 11.3 |
| Body Mass Index m/kg2 | 31.5 ± 7.0 | 29.8 ± 7.8 | 24.2 ± 3.0* |
| Sex: | |||
| % female | 85% | 75% | 43%* |
| % male | 15% | 25% | 57% |
| Urine Free Cortisol, nmol/d, normal < 248) | 920 ± 1590# | 652 ± 258 | 142 ± 55 |
Data are presented as mean ± SD.
p<0.05, pseudo-Cushing states versus normal volunteers,
p<0.05, Cushing disease versus normal volunteers;
p <0.001, Cushing disease versus normal volunteers.
The initial diagnosis of Cushing disease was based on clinical features, a blunted diurnal variation in plasma cortisol, a cortisol or ACTH response to CRH, and increased urine free cortisol (>248 nmol/d [90 μg/d]).29 The diagnosis of Cushing disease was confirmed by remission (eucortisolism or hypocortisolism) after pituitary surgery or by ACTH staining of a pituitary adenoma.
The diagnosis of a pseudo-Cushing state required a mildly or moderately elevated urine free cortisol (270–1000 nmol/d), preservation of the diurnal cortisol rhythm, and minimal Cushingoid features without progression over a minimum of 1 year (median, 28 months, range 1–5 years). None of these patients had anorexia or bulimia nervosa, but all had psychiatric diagnoses including substance abuse withdrawal (1), depression (4), undifferentiated somatoform disorder (2), and avoidant personality disorder (1). Magnetic resonance imaging of the sella with Gd-DTPA enhancement was negative for a pituitary adenoma in each.
Normal volunteers had normal urine free cortisol excretion, normal hepatic, renal, and thyroid function, no history of psychiatric disease, and Beck Depression Inventory scores in the non-depressed range (all <8).
The protocol for the use of ovine CRH with petrosal sinus sampling was approved by the NICHD institutional review board, and informed consent was obtained from all subjects before the procedure. None of the petrosal sinus sampling results from these patients have been reported previously.
Methods
Petrosal sinus sampling was performed previously described.26 Briefly, after systemic anticoagulation with heparin and sedative administration, catheters were advanced to the petrosal sinuses under fluoroscopic guidance, and their position verified by contrast injections. Blood was obtained simultaneously from each petrosal sinus and a peripheral vein 5 and 1 minutes before, and 3, 5, and 10 minutes after, intravenous injection of ovine CRH (1 μg/kg). Because the standard practice at the NIH is to determine the ACTH concentrations obtained at the 5 and 10 minute times only in patients with Cushing disease who have inconclusive results using the basal and the 3 minute samples, the results described in this paper are limited to the ACTH levels obtained from the two basal sets of samples and the set obtained 3 minutes after CRH. Retrograde venography was performed at the conclusion of sampling to verify proper catheter placement. Radiation exposure, determined by dosimetry, was 7.91 rem to limited areas of the skin, and was approved by the NIH Radiation Safety Committee.
Assays
ACTH was measured by radioimmunoassay.27 The limit of detection for ACTH ranged from 0.7 to 1.1 pmol/L. The mean intraassay and interassay coefficients of variation were 3.8% and 7.2%, respectively. All samples from each subject were analyzed in the same assay. These results were used to calculate the ratio of the ACTH concentration in each inferior petrosal sinus to the concurrent peripheral venous ACTH concentration (termed the IPS:P ratio) at each time point. The greatest of the IPS:P ratios (whether from the right or the left petrosal sinus) was termed the maximal IPS:P ratio and was calculated separately for the baseline samples and for the 3-minute samples alone. The lowest of the IPS:P ratios, determined in an analogous manner, was termed the minimal IPS:P ratio. The gradient of ACTH between the right and left petrosal sinuses (i.e. the greater of the right-to-left or left-to-right ratio, hereafter termed the R:L ratio) was also calculated.
Statistics
Statistical analysis was performed using Superanova and Statview II (Abacus Concepts, Inc., Berkeley, CA) on a Macintosh IIfx. Analysis of variance of log-transformed data was followed by post-hoc Fisher LSD comparisons. Receiver operating characteristics of each variable for the diagnosis of Cushing disease were then determined using the RuleMaker program (Digital Medicine, Inc., Hanover, New Hampshire). Sensitivity, specificity, and diagnostic accuracy were calculated for each variable at multiple criteria. The variables and criteria with the highest sensitivity, given 100% specificity for the diagnosis of Cushing disease, were identified and used for between-test comparisons. Basal and post-CRH results of the following variables were examined: maximal and minimal petrosal ACTH; peripheral ACTH; maximal and minimal IPS:P ratio; and R:L ratio. The criteria obtained from each variable were also combined factorially to determine if increased sensitivity for the diagnosis of Cushing disease resulted.
Results
Both inferior petrosal sinuses were catheterized in all subjects except one normal volunteer (inability to enter one sinus because it was a venous plexus connected with the basilar plexus of the clivus) and one pseudo-Cushing patient (difficulty catheterizing the femoral veins because of previous bilateral inguinal surgery). The results of petrosal sinus sampling were similar in normal volunteers and patients with pseudo-Cushing states but were significantly different in patients with Cushing disease and those without the disorder (the normal volunteers and individuals with pseudo-Cushing states).
Plasma ACTH
Before CRH
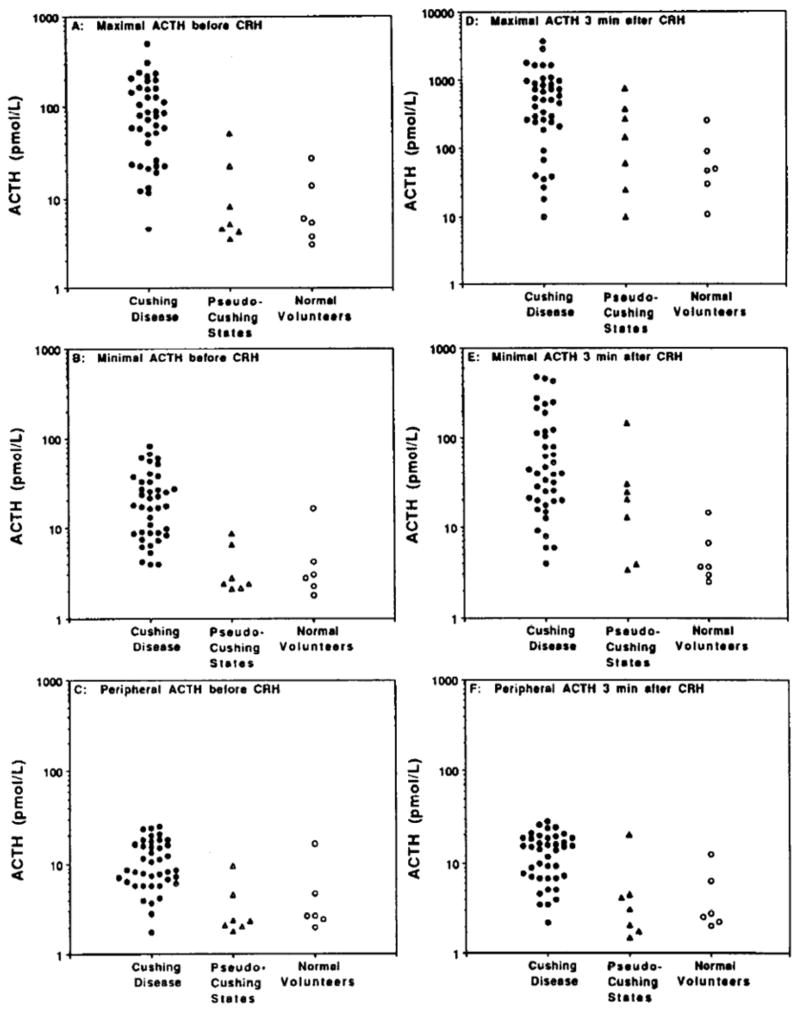
The maximal basal petrosal sinus ACTH level (i.e., the highest of the 4 basal samples, 2 from each petrosal sinus) was significantly higher in patients with Cushing disease (122 ± 173 pmol/L, mean ± SD, p<.0001) than in patients with pseudo-Cushing states (14 ± 18 pmol/L) or in normal volunteers (10 ± 10 pmol/L). However, the individual ACTH concentrations exhibited considerable overlap between the groups (Fig. 1A). A petrosal ACTH level > 51 pmol/L (233 pg/mL) in any basal sample excluded all individuals without Cushing disease, but failed to detect 33% of patients with Cushing disease (100% specificity, 67% sensitivity, and 75% diagnostic accuracy). Similar results were obtained using the lowest of the basal petrosal ACTH values (Fig. 1B). While the minimal petrosal ACTH was significantly (p<0.001) greater in patients with Cushing disease (24 ± 20 pmol/L) than in either patients with pseudo-Cushing states (4 ± 3 pmol/L) or in normal volunteers (5 ± 5 pmol/L), a minimal petrosal ACTH value > 16 pmol/L (74 pg/mL), chosen for 100% specificity, had only 60% sensitivity and 70% diagnostic accuracy for Cushing disease. A combination rule for the diagnosis of Cushing disease requiring any basal petrosal ACTH > 51 pmol/L, or all 4 basal petrosal ACTH levels > 16 pmol/L had 100% specificity, 75% sensitivity, and 81% diagnostic accuracy, significantly better than either criterion alone (p<0.05).
Figure 1.
ACTH concentrations obtained during petrosal sinus sampling.
A: ACTH in petrosal sinus with highest value before administration of CRH;
B: ACTH in petrosal sinus with lowest value before CRH;
C: ACTH in peripheral vein with higher value before CRH;
D: ACTH in petrosal sinus with higher value 3 minutes after CRH;
E: ACTH in petrosal sinus with lower value 3 minutes after CRH;
F: ACTH in peripheral vein 3 minutes after CRH.
Basal ACTH levels in peripheral blood were also elevated significantly (p<0.001) in patients with Cushing disease (26 ± 96 pmol/L) compared to normal volunteers (4 ± 3 pmol/L) and patients with pseudo-Cushing states (5 ± 6 pmol/L). No subject without Cushing disease had a basal peripheral ACTH in excess of 16 pmol/L (74 pg/mL) (Fig. 1C). This criterion had 100% specificity, 22% sensitivity and 41% diagnostic accuracy for the diagnosis of Cushing disease, and did not add diagnostic accuracy to the combination rule based on basal petrsoal ACTH.
After CRH
After CRH, the highest petrosal ACTH levels (Fig. 1D) were significantly greater in patients with Cushing disease (732 ± 808 pmol/L), compared to the normal volunteers (80 ± 88 pmol/L, p < 0.005) but not to those with pseudo-Cushing states (235 ± 267 pmol/L, p > 0.06). Maximal petrosal sinus ACTH values after CRH were as high as 808 pmol/L (3670 pg/mL) in the pseudo-Cushing group and 469 pmol/L (2130 pg/mL) in the normal volunteer group, and were as low as 10 pmol/L (46 pg/mL) in patients with Cushing disease. The minimal petrosal sinus ACTH value after CRH in Cushing disease was significantly elevated, compared to normal volunteers (103 ± 147 pmol/L vs 6 ± 5 pmol/L, p < 0.001), but not to pseudo-Cushing states (34 ± 50 pmolL, p > 0.05). After CRH, the peripheral ACTH levels (Fig. 1F) of patients with Cushing disease (31 ± 96 pmol/L) were significantly (p < 0.05) elevated compared to the normal volunteers (5 ± 4 pmol/L) or those with pseudo-Cushing states (5 ± 7 pmol/L). The addition of criteria based upon the CRH-stimulated ACTH levels did not improve the diagnostic accuracy using basal petrosal sinus ACTH levels.
IPS:P and R:L ratios
Before CRH
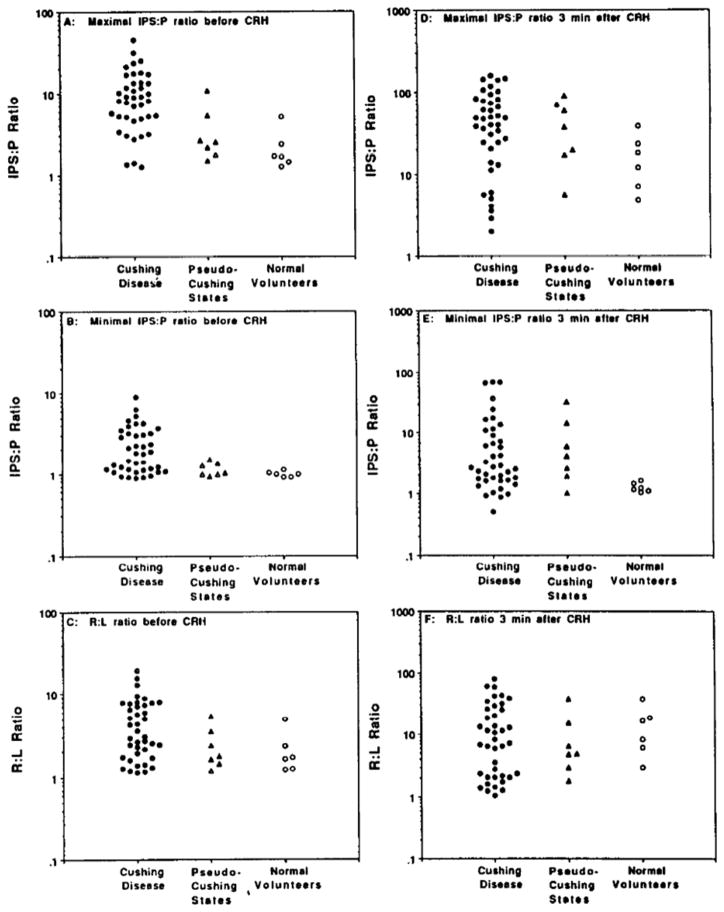
Before administration of CRH, patients with Cushing disease had significantly elevated maximal IPS:P ratios (Fig. 2A) when compared to all other groups (p<0.0001). A basal IPS:P ratio > 10.8 achieved 100% specificity for the diagnosis of Cushing disease, at the expense of sensitivity (37%) and diagnostic accuracy (53%). The minimal IPS:P ratios before CRH did not differ between the three groups. All normal volunteers, and all patients with pseudo-Cushing states, had minimal IPS:P ratios < 1.5. A minimal IPS:P ratio > 1.5 had 100% specificity, 51% sensitivity, and 64% diagnostic accuracy for the diagnosis of Cushing disease. Before CRH, R:L ratios were similar in all groups. Surprisingly, R:L ratios > 1.5 were present in all patients with pseudo-Cushing states and in all normal volunteers (Fig. 2C). A R:L ratio > 5.4 had 100% specificity, but only 37% sensitivity and 53% diagnostic accuracy for the diagnosis of Cushing disease.
Figure 2.
IPS:P and R:L ratios obtained during petrosal sinus sampling.
A: Maximal C:P ratio before administration of CRH;
B: Minimal C:P ratio before CRH;
C: R:L ratio before CRH;
D: Maximal C:P ratio 3 minutes after CRH;
E: Minimal C:P ratio 3 minutes after CRH;
F: R:L ratio 3 minutes after CRH.
After CRH
After CRH, neither the maximal (Fig. 2D) nor the minimal (Fig. 2E) IPS:P ratios of patients with Cushing disease were significantly different from the other groups. A maximal IPS:P ratio > 91 was required to achieve 100% specificity for the diagnosis of Cushing disease (20% sensitivity, 40% diagnostic accuracy). All of the normal volunteers and 3/7 patients with pseudo-Cushing states had minimal IPS:P ratios < 3.0. A minimal IPS:P ratio > 33 after CRH had 100% specificity, but only 12% sensitivity, and 34% diagnostic accuracy for Cushing disease. Similarly, the R:L ratios after CRH were not statistically different among the groups (p > 0.2). R:L ratios in Cushing disease ranged from 1 to 79, and exceeded 1.5 in each patient without Cushing disease. The addition of the post CRH data to the basal results did not increase diagnostic accuracy.
Combining ACTH and IPS:P ratio criteria
To find rules that might better identify individuals with Cushing disease, criteria with 100% specificity were combined. IPS:P ratios and R:L ratios, whether from basal or post CRH samples, did not increase the diagnostic accuracy of rules based solely on the basal ACTH concentrations.
Discussion
While petrosal sinus sampling can distinguish Cushing disease from the syndrome of ectopic ACTH,21 petrosal sinus sampling does not reliably differentiate normal volunteers or individuals with pseudo-Cushing states from patients with mild or episodic Cushing disease. The diagnostic accuracy of petrosal sinus sampling to detect Cushing disease in this study, at best, 81%, is no better than that reported for less invasive tests, such as the low-dose dexamethasone test, the peripheral ovine CRH test, or the insulin tolerance test.28,29
The findings of this study reinforce the need for patients to have biochemical and physical evidence of sustained hypercortisolism before petrosal sinus sampling. Because both normal volunteers and people with pseudo-Cushing states can have petrosal sinus sampling results that are indistinguishable from those in Cushing disease, physicians should take special care to ensure that patients not be evaluated with petrosal sinus sampling if they are eucortisolemic, have recently received therapy to reverse hypercortisolism, or are suspected of a pseudo-Cushing state.
ACTH concentrations in the inferior petrosal sinuses have previously been evaluated as predictors of the location of a pituitary microadenoma that causes Cushing disease. Since each petrosal sinus receives the venous drainage from the ipsilateral side of the pituitary,30–32 a tumor with a fairly lateral location should secrete ACTH mostly into the corresponding petrosal sinus. In contrast, because the ACTH secretion of the normal pituitary corticotrophs is inhibited by hypercortisolism, the petrosal sinus receiving blood from the other side of the pituitary would be expected to contain plasma ACTH levels similar to those obtained in peripheral veins. In one study, when the R:L ratio was ≥ 1.4 and the minimal IPS:P ratio was ≤ 3.0, the side of the pituitary gland found to contain the microadenoma was correctly predicted by the petrosal with the higher ACTH level in 84% of patients.21
Based on these observations, we predicted minimal differences between the two petrosal ACTH levels in patients without Cushing disease, since neither half of the pituitary would be suppressed by hypercortisolism. However, these subjects had marked asymmetry in their petrosal sinus ACTH levels. All had basal R:L ratios >1.2, and post CRH R:L ratios >1.8 and minimal basal IPS:P ratio < 1.5. 9/13 had minimal IPS:P ratios < 3.0 even after administration of CRH. Variation in catheter location at the time of collection, variation in petrosal sinus anatomy or amount of non-pituitary blood, and right-to-left variation in functional activity of the pituitary corticotroph cells could each cause differences in the amount of ACTH found in the two petrosal sinuses. Other venous sampling procedures have found similar right-to-left differences in measured hormone concentrations. For example, right-to-left concentration gradients of parathyroid hormone levels are found in normocalcemic patients, depending upon the proximity of the catheter tip to the normal parathyroid gland and upon the size of the sampled vein.33 Perhaps each individual has a dominant petrosal sinus that contains a much higher percentage of the total pituitary ACTH. The concept of a dominant petrosal sinus could explain why, even when there is a large R:L ratio, the R:L ratio does not always correctly predict the side on which a small corticotropinoma will be found.21 If one petrosal sinus is more likely to contain a higher concentration of pituitary ACTH, then the dominant petrosal may contain the higher ACTH concentration, regardless of the tumor’s location.
In sum, neither normal volunteers nor patients with pseudo-Cushing states can be discriminated reliably from patients with Cushing disease on the basis of their petrosal sinus ACTH concentrations, their IPS:P ratios, or their R:L ratios. We conclude that petrosal sinus sampling should be reserved for patients with clear clinical and laboratory evidence of ACTH-dependent hypercortisolism.
References
- 1.Doppman JL, Oldfield EH, Krudy AG, et al. Petrosal sinus sampling for Cushing syndrome: anatomical and technical considerations. Radiology. 1984;150:99–103. doi: 10.1148/radiology.150.1.6316418. [DOI] [PubMed] [Google Scholar]
- 2.Doppman JL, Krudy AG, Girton ME, Oldfield EH. Basilar venous plexus of the posterior fossa: a potential source of error in petrosal sinus sampling. Radiology. 1985;155:375–8. doi: 10.1148/radiology.155.2.2984718. [DOI] [PubMed] [Google Scholar]
- 3.Oldfield EH, Chrousos GP, Schulte HM, et al. Preoperative lateralization of ACTH secreting pituitary microadenomas by bilateral and simultaneous inferior petrosal sinus sampling. N Engl J Med. 1985;312:100–3. doi: 10.1056/NEJM198501103120207. [DOI] [PubMed] [Google Scholar]
- 4.Grant SJB, Stiel JN, Sorby WA, Henniker AJ. Venous ACTH sampling in Cushing’s syndrome. Med J Aust. 1983;1:226–7. doi: 10.5694/j.1326-5377.1983.tb136113.x. [DOI] [PubMed] [Google Scholar]
- 5.Manni A, Latshaw RF, Page R, Santen RJ. Simultaneous bilateral venous sampling for adrenocorticotropin in piutuitary dependent Cushing’s disease: evidence for lateralization of pituitary venous drainage. J Clin Endocrinol Metab. 1983;57:1070–3. doi: 10.1210/jcem-57-5-1070. [DOI] [PubMed] [Google Scholar]
- 6.Snow RP, Patterson RH, Jr, Horwith M, Saint Louis L, Fraser RA. Usefulness of preoperative inferior petrosal vein sampling in Cushing’s disease. Surg Neurol. 1988;29:17–21. doi: 10.1016/0090-3019(88)90117-6. [DOI] [PubMed] [Google Scholar]
- 7.McCance DR, McIlrath E, McNeill A. Bilateral inferior petrosal sinus sampling as a routine procedure in ACTH-dependent Cushing’s syndrome. Clin Endocrinol. 1989;30:157–166. doi: 10.1111/j.1365-2265.1989.tb03737.x. [DOI] [PubMed] [Google Scholar]
- 8.Crock PA, Pestell RG, Calenti AJ, Gilford EJ, Henderson JK, Best JD, Alford FP. Multiple pituitary hormone gradients from inferior petrosal sinus sampling in Cushing’s disease. Acta Endocrinologica (Copenh) 1988;119:75–80. doi: 10.1530/acta.0.1190075. [DOI] [PubMed] [Google Scholar]
- 9.Boolell M, Gilford E, Arnott R, McNeill P, Cummins J, Alford F. An overview of bilateral synchronous inferior petrosal sinus sampling (BSIPSS) in the pre-operative assessment of Cushing’s disease. Aust NZ J Med. 1990;20:765–770. doi: 10.1111/j.1445-5994.1990.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 10.Lombardi G, Merola B, Colao A, et al. Comparison of anterior pituitary hormone levels in the inferior petrosal sinuses and peripheral blood in various pituitary disorders during perihypophyseal phlebography. Acta Endocrinologica (Copenh) 1991;124:258–266. doi: 10.1530/acta.0.1240258. [DOI] [PubMed] [Google Scholar]
- 11.Chrousos GP, Schuermeyer TH, Doppman J, et al. Clinical applications of corticotropin-releasing factor. Ann Intern Med. 1985;102:344–58. doi: 10.7326/0003-4819-102-3-344. [DOI] [PubMed] [Google Scholar]
- 12.Landolt AM, Valavanis A, Girard J, Eberle AN. Corticotropin-releasing factor test used with bilateral simultaneous inferior petrosal sinus blood sampling for the diagnosis of pituitary-dependent Cushing’s disease. Clin Endocrinol (Oxf) 1986;25:687–96. doi: 10.1111/j.1365-2265.1986.tb03624.x. [DOI] [PubMed] [Google Scholar]
- 13.Schulte HM, Allolio B, Gunther RW, Benker G, Winkelmann W, Ohnhaus E, Reinwein D. Selective bilateral and simultaneous catheterization of the inferior petrosal sinus: CRF stimulates prolactin secretion from ACTH-producing microadenomas in Cushing’s disease. Clin Endocrinol. 1988;28:289–295. doi: 10.1111/j.1365-2265.1988.tb01215.x. [DOI] [PubMed] [Google Scholar]
- 14.Allolio B, Gunther RW, Benker G, Reinwein D, Winkelmann W, Schulte H. A multihormonal response to corticotropin-releasing hormone in inferior petrosal sinus blood of patients with Cushing’s disease. J Clin Endocrinol Metab. 1990;71:1195–1201. doi: 10.1210/jcem-71-5-1195. [DOI] [PubMed] [Google Scholar]
- 15.Findling JW, Kehoe ME, Shaker JL, Raff H. Routine inferior petrosal sinus sampling in the differential diagnosis of adrenocorticotropin (ACTH) dependent Cushing’s syndrome: early recognition of the occult ectopic ACTH syndrome. J Clin Endocrinol Metab. 1991;73:408–413. doi: 10.1210/jcem-73-2-408. [DOI] [PubMed] [Google Scholar]
- 16.Vignati F, Berselli ME, Scialfa G, Boccardi E, Loli P. Bilateral and simultaneous venous sampling of inferior petrosal sinuses for ACTH and PRL determination: Preoperative localization of ACTH-secreting microadenomas. J Endocrinol Invest. 1989;12:235–238. doi: 10.1007/BF03349972. [DOI] [PubMed] [Google Scholar]
- 17.Sgoutas DS, Sgoutas SA, Clark RV. Parallel assays of β-endorphin and ACTH in Cushing’s patients undergoing petrosal sinus sampling. Clin Biochem. 1990;23:321–326. doi: 10.1016/0009-9120(90)80063-o. [DOI] [PubMed] [Google Scholar]
- 18.Tabarin A, Greselle JF, San-Galli F, Leprat F, Caille JM, Latapie JL, Guerin J, Roger P. Usefulness of the corticotropin-releasing hormone test during bilateral inferior petrosal sinus sampling for the diagnosis of Cushing’s disease. J Clin Endocrinol Metab. 1991;73:53–59. doi: 10.1210/jcem-73-1-53. [DOI] [PubMed] [Google Scholar]
- 19.Loli P, Vignati F, Scialfa G, Boccardi E, Branca V, Berselli ME. Inferior petrosal sinus sampling: Evidence of a stimulatory effect of oCRH on GH secretion in Cushing’s disease. Acta Endocrinol (Copenh) 1990;123:238–242. doi: 10.1530/acta.0.1230238. [DOI] [PubMed] [Google Scholar]
- 20.Nussey SS, Page SR, Peterson DB, et al. Corticotrophin releasing hormone (CRH1-41) stimulates the secretion of adrenocorticotrophin, vasopressin and oxytocin but not adrenocorticotrophin precursors: evidence from petrosal sinus sampling in man. Clin Endocrinol. 1991;34:51–56. doi: 10.1111/j.1365-2265.1991.tb01735.x. [DOI] [PubMed] [Google Scholar]
- 21.Oldfield EH, Doppman JL, Nieman LK, et al. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing’s syndrome. N Engl J Med. 1991;325:897–905. doi: 10.1056/NEJM199109263251301. [DOI] [PubMed] [Google Scholar]
- 22.Liddle GW. Tests of pituitary-adrenal suppressability in the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 1960;20:1539–60. doi: 10.1210/jcem-20-12-1539. [DOI] [PubMed] [Google Scholar]
- 23.Nieman LK, Chrousos GP, Oldfield EH, Avgerinos PC, Cutler GB, Jr, Loriaux DL. The ovine corticotropin-releasing hormone stimulation test and the dexamethasone suppression test in the differential diagnosis of Cushing’s syndrome. Ann Intern Med. 1986;105:862–7. doi: 10.7326/0003-4819-105-6-862. [DOI] [PubMed] [Google Scholar]
- 24.Sakiyama R, Ashcraft MW, Van Herle AJ. Cyclic Cushing’s Syndrome. Am J Med. 1984;77:944–946. doi: 10.1016/0002-9343(84)90547-3. [DOI] [PubMed] [Google Scholar]
- 25.Kuchel O, Bolte E, Chretien M, et al. Cyclical edema and hypokalemia due to occult episodic hypercorticism. J Clin Endocrinol Metab. 1987;64:170–174. doi: 10.1210/jcem-64-1-170. [DOI] [PubMed] [Google Scholar]
- 26.Miller DL, Doppman JD. Petrosal sinus sampling: Technique and rationale. Radiology. 1991;178:37–47. doi: 10.1148/radiology.178.1.1845785. [DOI] [PubMed] [Google Scholar]
- 27.Chrousos GP, Schulte HM, Oldfield EH, Gold PW, Cutler GB, Jr, Loriaux DL. The corticotropin-releasing factor stimulation test: an aid in the evaluation of patients with Cushing’s syndrome. N Engl J Med. 1984;310:622–6. doi: 10.1056/NEJM198403083101004. [DOI] [PubMed] [Google Scholar]
- 28.Gold PW, Loriaux DL, Roy A, et al. Responses to corticotropin releasing hormone in the hypercortisolism of depression and Cushing’s disease. N Engl J Med. 1986;314:1329–35. doi: 10.1056/NEJM198605223142101. [DOI] [PubMed] [Google Scholar]
- 29.Crapo L. Cushing’s syndrome: a review of diagnostic tests. Metabolism. 1979;9:955–77. doi: 10.1016/0026-0495(79)90097-0. [DOI] [PubMed] [Google Scholar]
- 30.Green HT. The venous drainage of the human hypophysis cerebri. Am J Anat. 1957;100:435–469. doi: 10.1002/aja.1001000307. [DOI] [PubMed] [Google Scholar]
- 31.Page RB. Directional pituitary blood flow: a microcinephotographic study. Endocrinology. 1983;112:157–165. doi: 10.1210/endo-112-1-157. [DOI] [PubMed] [Google Scholar]
- 32.Xuerub GP, Prichard MML, Daniel PM. The arterial supply and venous drainage of the human hypophysis cerebri. Q J Exp Physiol. 1954;39:199–217. doi: 10.1113/expphysiol.1954.sp001072. [DOI] [PubMed] [Google Scholar]
- 33.Shimkin PM, Doppman JL, Pearson KD, Powell D. Anatomic considerations in parathyroid venous sampling. Am J Roentgenology. 1973;118:654–662. doi: 10.2214/ajr.118.3.654. [DOI] [PubMed] [Google Scholar]