Abstract
Unilateral C2 spinal cord hemisection (C2Hx) interrupts bulbospinal respiratory pathways innervating ipsilateral phrenic motoneurons, resulting in cessation of ipsilateral diaphragm motor output. Plasticity within the spinal neural circuitry controlling the diaphragm can induce partial recovery of phrenic bursting which correlates with the time-dependent return of spinal serotonin (5-HT) immunoreactivity in the vicinity of phrenic motoneurons. The 5-HT2A receptor subtype is present on phrenic motoneurons and its expression is up-regulated after cervical spinal cord injury; however the functional role of these receptors following injury has not been clearly defined. The present study evaluated the functional role of 5-HT2A receptors by testing the hypothesis that pharmacologic blockade would attenuate diaphragm activity in rats with chronic cervical spinal cord injury. Bilateral diaphragm electromyography (EMG) was performed in vagal-intact and spontaneously breathing rats before and after intravenous administration of the 5-HT2A receptor antagonist Ketanserin (1 mg/kg). Intravenous ketanserin significantly attenuated ipsilateral diaphragm EMG activity in C2Hx animals but had no impact on diaphragm output in uninjured animals. We conclude that 5-HT2A receptor activation contributes to the recovery of ipsilateral phrenic motor output after chronic cervical spinal cord injury.
Keywords: cervical spinal cord injury, diaphragm, 5-HT, 5-HT2A receptor
1. Introduction
Unilateral hemisection of high cervical spinal cord (i.e., C2Hx) results in the immediate cessation of ipsilateral phrenic activity and paralysis of the hemidiaphragm. Phrenic motor output ipsilateral to the lesion gradually recovers over weeks to months post-injury due to activation of previously latent crossed spinal pathways (i.e., crossed phrenic phenomenon)(Lee and Hsu, 2017; Lee et al., 2014). Considerable evidence suggests a role for serotonin in this recovery. For example, Hadley et al. (1999) demonstrated that treatment with a serotonin (5-HT) depleting drug (para-chlorophenylalanine) prior to injury can block the expression of the crossed phrenic phenomenon evoked by asphyxia during the acute injury phase (Hadley et al., 1999a). Similarly, a study by Golder et al. (2001) showed that rats treated with the serotonin toxin 5,7-dihydroxytryptamine also demonstrated a reduced incidence of recovery of ipsilateral phrenic bursting (Golder et al., 2001). These results suggest that 5-HT is necessary for recovery of ipsilateral phrenic activity after cervical spinal cord injury. However, both the 5-HT depleting drug and 5-HT neurotoxin mentioned in above studies were applied prior to the spinal cord injury surgery. Thus it remains unclear whether the role of 5-HT is to induce activation of crossed spinal pathway and/or maintain the phrenic bursting after cervical spinal cord injury. Golder and Mitchell (2005) observed that phrenic burst amplitude after cervical spinal cord injury correlated with time-dependent return of spinal 5-HT immunoreactivity (Golder and Mitchell, 2005). Moreover, protein and mRNA expression of 5-HT2A receptor within the phrenic nucleus was elevated after cervical spinal hemisection (Fuller et al., 2005; Mantilla et al., 2012). Thus, we hypothesize that the recovery of 5-HT that occurs over several weeks after injury may act on 5-HT2A receptors to facilitate phrenic bursting during the chronic injury phase.
2. Methods
All experimental protocols were approved by the Institutional Animal Care and Use Committees at National Sun Yat-sen University. Nineteen male Sprague-Dawley rats at age of 7–8 weeks were purchased from BioLasco Taiwan Co., Ltd (Taiwan) and assigned to uninjured (n = 8) and C2Hx (n = 11) group.
2.1. Cervical spinal cord hemisection
At 9–10 weeks of age, rats in the C2Hx groups were anesthetized with xylazine (10 mg/kg, s.c., Rompun®, Bayer) and ketamine (140 mg/kg, i.p., Ketalar®, Pfizer) The C2 spinal cord was exposed by dorsal midline incision followed by C2 laminectomy. Using a microscalpel, the left C2 spinal cord was incised and a lesion cavity was created by gentle aspiration using a micropipette connected to a suction pump. The dura was sutured with 10-0 nylon (UNIK) sutures, and the overlying muscles and skin were closed with 4-0 chromic (UNIK) and 4-0 nylon sutures (UNIK) sutures, respectively. Animals were then given injections of yohimbine (1.2 mg/kg, s.c., Tocris) to reverse the effect of xylazine, lactated Ringer’s solution (5 ml, s.c., Nang Kuang Pharmacerutical Co., Ltd) to prevent dehydration, and buprenorphine (0.03 mg/kg, s.c., Shinlin Sinseng Pharmaceutical Co., Ltd) for analgesia. Oral supply of Nutri-cal (1 – 3 ml, EVSCO pharmaceuticals) and lactated Ringer’s solution injection (5 ml, s.c.) were administered daily until recovery of volitional eating and drinking.
2.2. Experimental preparation and protocol
At 8–9 weeks post-injury, terminal neurophysiology experiments were performed as previously described (Hsu and Lee, 2015). Briefly, animals were anesthetized with an injection of urethane (1.6 g/kg, i.p., Sigma). An adequate plane of anesthesia was confirmed by the absence of the toe-pinch withdrawal reflex. Rats were placed in a supine position and the rectal temperature was maintained at 37 ± 1 °C by a servo-controlled heating pad (model TC-1000, CWE Inc.). The trachea was cannulated with an endotracheal tube (PE-240, Clay Adams) and connected to a respiratory flow head (MLT1L, ADInstruments) and a spirometer (FE141, ADInstruments) for respiratory flow measurement. A hyperoxic gas mixture (50 % O2, balance N2; flow rate: 2 L/min) was delivered to the animal via a T-piece tube. The femoral artery and vein were catheterized for blood pressure measurement (Transducer: DTX-1; Amplifier: BPM-832, CWE Inc.) and drug administration, respectively.
The abdominal surface of the diaphragm were exposed by laparotomy, and the bipolar silver electrodes (coated silver wire with exposed tips, #786000, A-M system) threaded through 26 gauge needles were inserted into the medial costal region of bilateral diaphragm. The diaphragm EMG signals were amplified (1000 x) and band-pass filtered (0.3 – 10 kHz) by a differential A/C amplifier (Model 1700, A-M Systems), and processed with the rectified and smoothed function (time constant: 25 ms) by Spike2 software (Cambridge Electronic Design Limited). Physiological signals were digitized using the CED Power 1401 (Cambridge Electronic Design Limited) at sampling rate of 100 Hz (e.g., airflow and blood pressure) or 10K Hz (e.g., diaphragm EMG activity) and recorded in a computer by Spike 2 software.
After stable recording of the respiratory airflow and diaphragm EMG signals, a single bolus injection of ketanserin, a 5-HT2A receptor antagonist (1.0 mg/kg, 0.5 ml/kg, Tocris) was delivered intravenously to evaluate the functional role of 5-HT2A on the respiratory and cardiovascular parameters.
2.3. Data analyses
Respiratory frequency was calculated from the respiratory airflow trace. Tidal volume data were derived from the integrated inspiratory airflow using a Spike 2 script. The amplitude of inspiratory activity of the diaphragm was defined as the difference between the maximum and minimum values of the rectified and smoothed diaphragm EMG signals within a single breath. EMG data were expressed in arbitrary unit (a.u.) or as a percentage of the baseline activity (% BL; i.e., before ketanserin administration). All physiological parameters were averaged over 30 seconds prior to ketanserin administration (e.g. baseline) and over 10 seconds at ~ 1 min post-ketanserin administration. Student’s t-tests were used to compare the age and body weight between uninjured and C2Hx animals, and the amplitude of diaphragm EMG activity between the contralateral and ipsilateral sides. The influence of ketanserin administration on diaphragm output, respiratory frequency, and blood pressure was evaluated by a two-way mixed-design measures analysis of variance followed by the Dunnett post hoc test [factor one: group (uninjured vs. C2Hx); factor two: condition (before vs. after ketanserin administration)]. Differences were considered statistically significant when P < 0.05. Data are expressed as the mean ± standard error of the mean.
3. Results
3.1 Animals
Eight uninjured (age: 137 ± 4 days; weight: 588 ± 35 g) and eleven C2Hx animals (age: 137 ± 4 days; weight: 497 ± 17 g) were studied to examine the influence of intravenous ketanserin on the cardiorespiratory pattern in chronically injured rats. Age was similar between two groups; however, rats with chronic C2Hx weighed significantly less than uninjured rats at the time of terminal neurophysiology procedures (P < 0.05).
3.2 Cardiorespiratory pattern following chronic cervical spinal cord injury
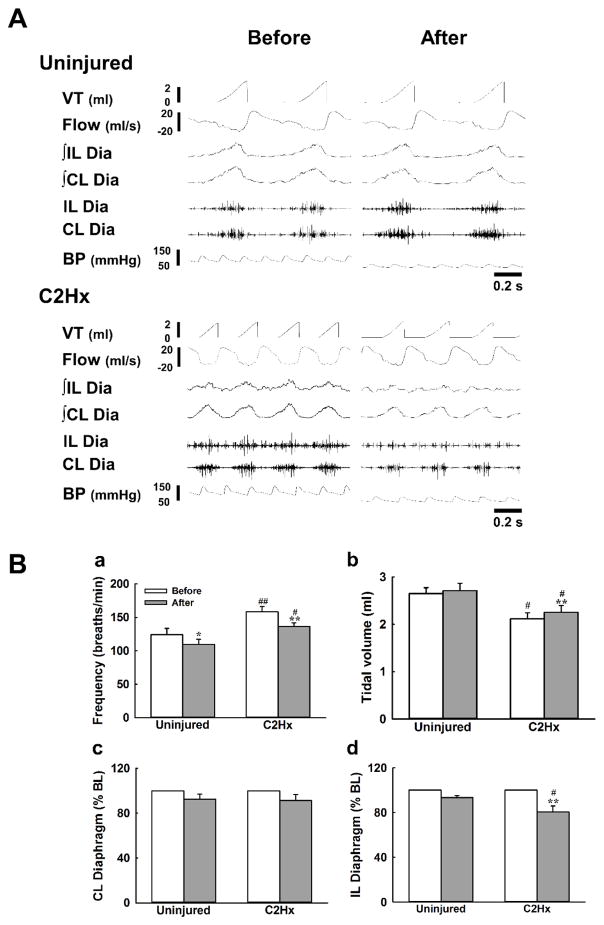
Representative examples of the cardiorespiratory pattern and diaphragm EMG activity were shown in Fig. 1A. Mean arterial pressure and heart rate were similar between uninjured (89 ± 4 mmHg; 363 ± 23 beats/min) and C2Hx rats (83 ± 6 mmHg; 381 ± 19 beats/min) at baseline; however, the pattern of respiratory efforts differed between groups. Specifically, in rats with C2Hx, respiratory frequency was higher and tidal volume was lower than uninjured animals (P <0.05, Fig. 1Ba and 1Bb). As shown in Fig 1A, robust bilateral diaphragm EMG output was recorded in uninjured animals at baseline. By comparison, although bilateral diaphragm output was evident in C2Hx animals, the amplitude of ipsilateral EMG signals was significantly attenuated (0.30 ± 0.13 a.u.) as compared to the contralateral diaphragm (0.81 ±0.14 a.u.)(P < 0.01).
Fig. 1. The effects of ketanserin on the respiratory pattern and bilateral diaphragm EMG activity.
Panel A presents the representative examples of the cardiorespiratory pattern and bilateral diaphragm EMG activity in response to intravenous administration of ketanserin. Panel B shows the averaged response of respiratory frequency (a), tidal volume (b) and bilateral diaphragm EMG activity (c and d) before (white bar) and after (grey bar) ketanserin injection. VT: tidal volume. ∫: the rectified and smoothed EMG signals. CL: contralateral; IL: ipsilateral. Dia: the raw signals of the diaphragm EMG. *: P < 0.05; **: P < 0.01 significant difference between the value before and after ketanserin administration. #: P < 0.01; ##: P < 0.01 significant difference between uninjured and C2Hx animals.
3.3 Alteration of cardiorespiratory pattern and diaphragm EMG activity following administration of ketanserin
Respiratory frequency was significantly reduced, from 124 ± 10 breaths/min to 110 ± 7 breaths/min after administration of ketanserin in uninjured animals (P < 0.05, Fig. 1Ba). Similarly, C2Hx animals exhibited a lower respiratory frequency following ketanserin injection (before vs. after: 158 ± 8 vs. 136 ± 6 breaths/min, P <0.01, Fig. 1Ba). tidal volume was not significantly affected by ketanserin delivery in spinal intact animals though it was slightly enhanced in C2Hx animals (before vs. after: 2.1 ± 0.1 vs. 2.3 ± 0.1 ml, P < 0.05, Fig. 1Bb). Mean arterial pressure was significantly decreased in response to ketanserin delivery in both spinal intact (from 89 ± 4 to 65 ± 4 mmHg) and C2Hx animals (from 83 ± 6 to 67 ± 5 mmHg)(P < 0.01). Intravenous injection of ketanserin also reduced heart rate in C2Hx animals (from 381 ± 19 to 352 ± 24 beats/min) (P < 0.05).
Intravenous administration did not significantly alter diaphragm EMG on either side in uninjured rats. Diaphragm EMG activity was maintained at 93 ± 4 % of BL and 93 ± 2 % of BL, respectively (Figs. 1Bc and 1Bd) for the contralateral (i.e., right side) and ipsilateral (i.e., left side) diaphragm. Similarly, intravenous ketanserin did not significantly alter contralateral diaphragm EMG activity in C2Hx rats, however it did attenuate the activity of the ipsilateral diaphragm to 80 ± 5 % BL (P < 0.01, Fig. 1Bd).
Discussion
The present study demonstrates that pharmacological blockade of 5-HT2A receptors attenuates diaphragm EMG activity ipsilateral to a unilateral cervical hemisection in spinal cord injured animals. However, the same dose of ketanserin did not significantly modulate contralateral diaphragm EMG activity in C2Hx animals nor did it have any impact on diaphragm motor output in uninjured animals. These results suggest that activation of 5-HT2A receptors contributes to the recovery of phrenic motor outputs following chronic spinal cord injury.
4.1 Critique of method
The dosage of ketanserin selected was based on a previous study (McGuire et al., 2004) which demonstrated that ketanserin can attenuate intermittent hypoxia-induced ventilatory long-term facilitation. Other studies have used a higher dose of ketanserin (2 mg/kg) to attenuate intermittent hypoxia-induced phrenic long-term facilitation and 5-HT2A/2C agonist-induced excitation of phrenic bursting in cervical spinal cord injured rats (Golder and Mitchell, 2005; Zhou et al., 2001). However, ketanserin also induce a substantial reduction in the blood pressure, which may confound the effect of ketanserin on the respiratory parameters. Accordingly, 1 mg/kg of ketanserin was used to evaluate the effect of ketanserin in the present study. In addition, ketanserin was injected intravenously in our experiments, therefore, alteration of cariodrespiratory pattern and diaphragm EMG activity could be attributed to blockade of supraspinal, spinal and peripheral 5-HT2A receptors. In addition, although ketanserin has a high affinity to 5-HT2A receptors, it may also block other G-protein coupled receptors (e.g., histamine type 1 and alpha-1 adrenergic receptors). Thus, the effect of ketanserin may represent a combinatorial interaction of monoaminergic systems.
4.2 Functional role of 5-HT2A receptors
Activation of phrenic motoneurons occurs primarily via the activation of glutamatergic premotor neurons in the rostral ventral respiratory group (Lee and Fuller, 2011). This excitatory respiratory drive is modulated by raphespinal inputs to phrenic motoneurons via 5-HT on several 5-HT receptor subtypes (e.g., 5-HT2A, 5-HT2B and 5-HT7) on the phrenic motoneurons. Our current data demonstrates that diaphragm EMG acitivty is not significantly altered by blockade of 5-HT2A receptors in the spinal intact rat, however, we found a significant impact of 5-HT2A receptor blockade on ipsilateral diaphragm activity following chronic C2Hx. Similarly,. Goshagarian and colleagues demonstrated that depletion of serotonin prior to injury attenuates recovery of phrenic bursting and cervcial hemisection-induced morphological changes in the phrenic nucleus at the acute injury phase (Hadley et al., 1999a, b). Together, these findings suggest that endogenous 5-HT is required for for recovery of ipsilateral phrenic motor output after injury. Our current results extend this observation by showing that even in the chronic post-injury phase, ipsilateral diaphragm EMG activity was attenuated by administration of ketanserin. These data suggested that 5-HT is involved not only in the early activationof crossed phrenic pathway but also in maintaining ipsilateral diaphragm activity in chronic cervical spinal cord injury.
Although the raphe-spinal pathway is usually damaged by cervical spinal cord injury, 5-HT innervation of phrenic nucleus can partially recover during the chronic injury phase (Golder and Mitchell, 2005). Moreover, Wienecke et al. (2014) observed that endogenous 5-HT can be also produced by intraspinal cells which expressed aromatic l-amino acid decarboxylase (the enzyme which can convert 5-HT precursor to 5-HT) after spinal cord injury (Wienecke et al., 2014). Prior studies also showed that expression of 5-HT2A receptors were up-regualted following cervical spinal cord injury (Fuller et al., 2005; Mantilla et al., 2012). Accordingly, recovery of ipsilateral phrenic bursting may be faciliated in part, by activation of 5-HT2A receptors thought action of the recovered 5-HT innervation from the raphe nucles and newly formed 5-HT from the spinal cord. Our recent report support this concept showing that exogenous application of 5-HT2 receptor agonist or 5-HT precursor can significantly enhance diaphragm EMG activity in C2Hx animals (Hsu and Lee, 2015).
Because the ipsilateral diaphragm EMG activity was not completely blocked by administration of ketanserin, it seems likely that other substrates are involved in the maintenance of ipsilataeral diaphragm motor output during the chronic post-injury phase. For example, Mantilla et al. (2012) demonstrated that mRNA expression of NMDA receptors increased after cervical hemisection, as well as 5-HT2A receptors, suggesting that the excitatory effect of glutamate may also be enhanced following C2Hx. In addition, recovery of motoneuron excitability after spinal cord injury can be also archived by constitutive activation of monoaminergic receptors (D’Amico et al., 2014). Thus, up-regulation of certain 5-HT receptors without action of 5-HT ligand may also augment phrenic motoneuron excitability and in turn modualte phrenic nerve activity.
Although ipsilateral diaphragm EMG activity was significantly decreased following k etanserin administration, tidal volume did not concomitantly reduce but rather slightly increase in spinal injured animals. Our previous data have demonstrated that ipsilateral phrenic motor outputs contribute to ~ 16 % of the tidal volume generation (Dougherty et al., 2012). Therefore, a 20 % reduction of ipsilateral diaphragmatic outputs following ketanserin treatment in the current study may not be able to cause a significant impact on the tidal volume. In addition, we have shown that increased ipsilateral diaphragm motor output, induced by either serotonin precursor (5-hydroxytryptophan) or 5-HT2 receptor agonist (TCB-2), is not associated with improved tidal volumes in C2Hx rats (Hsu and Lee, 2015). Moreover, Navarrete-Opazo et al. (2015) observed that functional recovery of the tidal volume induced by daily acute intermittent hypoxia was related to augmentation of the contralateral but not ipsilateral diaphragm activity in awake C2Hx rats (Navarrete-Opazo et al., 2015). These results lead us to speculate that alteration of ipsilateral diaphragmatic activity may be less correlated with tidal volume generation in cervical spinal cord injured animals. Notably, alteration of blood pressure following ketanserin administration may induce a ventilator baroreflex (Stewart et al., 2011), which can influence the tidal volume through modulation of other respiratory muscle activity (i.e., intercostal and abdominal muscles).
In summary, ouor results indicated that activation of 5-HT2A contributes to the spontaneous recovery of ipsilateral diaphragm EMG activity in rats with chronic cervical spinal cord injury. The development of therapeutic interventions targeted at restoring 5-HT and/or activating 5-HT2A receptors may potentially lead to improvement of respiratory function in patients with chronic cervical spinal cord injury.
Highlights.
Unilateral cervical hemisection impairs diaphragm EMG activity.
Diaphragmatic motor outputs partially recover during the chronic injury state.
Blockade of 5-HT2A receptors attenuates diaphragm EMG activity.
5-HT2A receptor activation contributes to phrenic recovery after spinal injury.
Acknowledgments
Support for this work was provided by grants from the (Most 102-2320-B-110-004-MY3 & 105-2628-B-110-002-MY3)(KZL), NSYSU-KMU Joint Research Project (106-I009)(KZL) and NIH/NICHD K12-HD055929 (EGR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- D’Amico JM, Condliffe EG, Martins KJ, Bennett DJ, Gorassini MA. Recovery of neuronal and network excitability after spinal cord injury and implications for spasticity. Front Integr Neurosci. 2014;8:36. doi: 10.3389/fnint.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty BJ, Lee KZ, Lane MA, Reier PJ, Fuller DD. Contribution of the spontaneous crossed-phrenic phenomenon to inspiratory tidal volume in spontaneously breathing rats. J Appl Physiol (1985) 2012;112:96–105. doi: 10.1152/japplphysiol.00690.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Baker-Herman TL, Golder FJ, Doperalski NJ, Watters JJ, Mitchell GS. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J Neurotrauma. 2005;22:203–213. doi: 10.1089/neu.2005.22.203. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J Neurosci. 2001;21:8680–8689. doi: 10.1523/JNEUROSCI.21-21-08680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley SD, Walker PD, Goshgarian HG. Effects of serotonin inhibition on neuronal and astrocyte plasticity in the phrenic nucleus 4 h following C2 spinal cord hemisection. Exp Neurol. 1999a;160:433–445. doi: 10.1006/exnr.1999.7238. [DOI] [PubMed] [Google Scholar]
- Hadley SD, Walker PD, Goshgarian HG. Effects of the serotonin synthesis inhibitor p-CPA on the expression of the crossed phrenic phenomenon 4 h following C2 spinal cord hemisection. Exp Neurol. 1999b;160:479–488. doi: 10.1006/exnr.1999.7240. [DOI] [PubMed] [Google Scholar]
- Hsu SH, Lee KZ. Effects of serotonergic agents on respiratory recovery after cervical spinal injury. J Appl Physiol (1985) 2015;119:1075–1087. doi: 10.1152/japplphysiol.00329.2015. [DOI] [PubMed] [Google Scholar]
- Lee KZ, Fuller DD. Neural control of phrenic motoneuron discharge. Respir Physiol Neurobiol. 2011;179:71–79. doi: 10.1016/j.resp.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Hsu SH. Compensatory function of the diaphragm following high cervical hemisection in the rat. J Neurotrauma. 2017 doi: 10.1089/neu.2016.4943. [DOI] [PubMed] [Google Scholar]
- Lee KZ, Huang YJ, Tsai IL. Respiratory motor outputs following unilateral midcervical spinal cord injury in the adult rat. J Appl Physiol (1985) 2014;116:395–405. doi: 10.1152/japplphysiol.01001.2013. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Bailey JP, Zhan WZ, Sieck GC. Phrenic motoneuron expression of serotonergic and glutamatergic receptors following upper cervical spinal cord injury. Exp Neurol. 2012;234:191–199. doi: 10.1016/j.expneurol.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Serotonin receptor subtypes required for ventilatory long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R334–341. doi: 10.1152/ajpregu.00463.2003. [DOI] [PubMed] [Google Scholar]
- Wienecke J, Ren LQ, Hultborn H, Chen M, Moller M, Zhang Y, Zhang M. Spinal cord injury enables aromatic L-amino acid decarboxylase cells to synthesize monoamines. J Neurosci. 2014;34:11984–12000. doi: 10.1523/JNEUROSCI.3838-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SY, Basura GJ, Goshgarian HG. Serotonin(2) receptors mediate respiratory recovery after cervical spinal cord hemisection in adult rats. J Appl Physiol (1985) 2001;91:2665–2673. doi: 10.1152/jappl.2001.91.6.2665. [DOI] [PubMed] [Google Scholar]