Abstract
Summary
We report long-term outcomes on 1002 patients treated to 8640 cGy for localized prostate cancer using IMRT. High dose-escalation results in excellent tumor control with minimal toxicity when implementing strict dose constraints. Dose-escalation beyond 80 Gy is feasible and safe, and should be considered for select men with localized prostate cancer.
Purpose
To report the long-term survival and toxicity outcomes with the use of high dose intensity modulated radiation therapy (IMRT) to 86.4 Gy for patients with localized prostate cancer.
Materials and Methods
Between August 1997 and December 2008, 1002 patients were treated to a dose of 86.4 Gy using a 5–7 field IMRT technique. Patients were stratified into prognostic risk groups based on the National Comprehensive Cancer Network risk classification criteria. Five hundred eighty-seven patients (59%) were treated with neoadjuvant and concurrent androgen deprivation therapy (ADT). The median follow-up for the entire cohort was 5.5 years (range, 1–14 yrs).
Results
For low-, intermediate- and high-risk groups the 7-year biochemical relapse-free survival outcomes were 98.8%, 85.6%, and 67.9% (p<0.001), and distant metastasis-free survival rates of 99.4%, 94.1%, and 82.0% (p<0.001), respectively. On multivariate analysis T-stage (p<0.001), Gleason score (p<0.001), and >50% of initial biopsy cores positive (p=0.001) were predictive for DM. No prostate cancer related deaths were observed in the low-risk group. The 7-year prostate cancer–specific mortality (PCSM) using competing risk analysis for intermediate- and high-risk groups was 3.3% and 8.1%, respectively (p=0.008). On multivariate analysis Gleason score (p=0.004), percent biopsy core positivity (p=0.003), and T-stage (p=0.033) were predictive for PCSM. Actuarial 7-year grade 2 or higher late gastrointestinal and genitourinary toxicities were 4.4% and 21.1%, respectively. Late grade 3 gastrointestinal and genitourinary toxicity was experienced by 7 patients (0.7%) and 22 patients (2.2%), respectively. Of the 427 men with full potency at baseline, 317 men (74%) retained sexual function at time of last follow-up.
Conclusions
This represents the largest cohort of patients treated with high dose radiation to 86.4 Gy using IMRT for localized prostate cancer with the longest follow-up to date. Our findings indicate that this treatment results in excellent clinical outcomes with acceptable toxicity.
Introduction
External beam radiation therapy (EBRT) is a primary treatment modality for prostate cancer. An early obstacle in improving outcomes with EBRT was the inability to deliver doses beyond 70 Gy with standard two-dimensional treatment planning techniques secondary to the tolerance of bowel to moderate dose levels of irradiation. Resistant clones were not eliminated with these low doses in the 60 Gy range, and due to this limitation, biopsy proven local relapse rates after definitive EBRT have been reported between 19% and 65%.1 Local relapse from prostate cancer has been shown to be associated with an increased risk of metastatic disease and resultant survival deterioration.2
Five randomized phase III studies have compared standard doses of EBRT of 64–70 Gy with dose escalated doses of 74–80 Gy.3–7 Of these, only the Proton Radiation Oncology Group and the Groupe d’Etude des Tumeurs Uro-Génitales (GETUG) trials prescribed the dose to the prostate volume.7,8 The other trials prescribed the dose to the isocenter which correlates to a 5–7% dose falloff when compared to prescribing to the prostate volume. Regardless, all of these studies found improvements in biochemical control. Recently, M.D. Anderson’s dose escalation trial has reported improvements in distant failure rates.4 Based upon these trials and others, a recent meta-analysis demonstrated linear improvements in biochemical control with dose up to 80 Gy, suggesting that biochemical improvements is expected to continue past 80 Gy.9,10
In our prospective phase I dose escalation study for patients with localized prostate cancer we escalated dose levels to as high as 86.4 Gy. This dose level has become the standard dose level employed at our institution for patients when conventional fractionation is used.11 We have previously published preliminary toxicity and biochemical data on a cohort of 476 patients treated to dose levels of 86.4 Gy.12 We report here the long-term survival and toxicity outcomes of 1002 patients treated with EBRT using IMRT to a dose of 86.4 Gy.
Materials and Methods
Between August 1997 and December 2008, 1002 consecutive patients with localized prostate cancer were treated with definitive IMRT to a dose of 86.4 Gy. We previously reported the first 50 high-risk patients treated on our phase I dose escalation protocol.11 Standard follow-up was every 3 months from the time of completion of treatment for the first year, followed by every 6 months for the next 5 years and yearly thereafter. The median follow-up for the entire cohort was 5.5 years (range, 1–14 years). The median age was 70 years (range, 46–93 years). All patients had a histologic diagnosis of prostate adenocarcinoma from a transrectal biopsy reviewed by a urologic pathologist at our institution. The median number of cores collected at time of biopsy was 9 (interquartile range, 6–12). Pretreatment diagnostic evaluations were performed as previously described.12 Endorectal coil-based MRI was performed pretreatment to evaluate for the presence of extra-prostatic extension and pelvic lymph node metastases. MRI was used for prostate volumetric assessment. If unavailable, either ultrasound or CT information were utilized.
Baseline demographics and clinical characteristics for the cohort are shown in Table 1. Based upon current National Comprehensive Center Network (NCCN) prognostic risk groupings, which include the new ‘very low risk group’, patients were stratified by risk group (www.nccn.org).
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | Number (N=1002) |
% | |
|---|---|---|---|
| Age (year) | |||
| Median (range) | 70 (46–93) | ||
| Tumor Stage | |||
| ≤T1c, T2a | 719 | 72 | |
| T2b–T2c | 141 | 14 | |
| ≥T3a | 120 | 12 | |
| Unknown (Tx) | 22 | 2 | |
| Gleason Score | |||
| 2 – 6 | 306 | 30.5 | |
| 7 | 490 | 48.9 | |
| 8 – 10 | 206 | 20.6 | |
| PSA | |||
| ≤10 ng/mL | 668 | 66.7 | |
| >10 ng/mL | 334 | 33.3 | |
| Risk group | |||
| Very Low risk | 96 | 9.6 | |
| Low risk* | 100 | 10 | |
| Low risk‡ | 196 | 19.6 | |
| Intermediate risk | 462 | 46.1 | |
| High risk | 344 | 34.3 | |
| ADT use | |||
| Yes | 589 | 58.8 | |
| Duration ≤6 months | 277 | 47 | |
| Duration >6 months | 312 | 53 | |
| Percent Core Involvement | |||
| Median (range) (%) | 37.5 (4–100) | ||
| IPSS | |||
| Total Available | 640 | 64 | |
| Median (range) | 8 (0 – 35) | ||
| ≤15 | 542 | 85 | |
| >15 | 98 | 15 | |
| Baseline Sexual Function | |||
| Full Potency | 427 | 42.7 | |
| Partial Potency | 277 | 27.6 | |
| Impotent | 267 | 26.6 | |
| Unknown | 31 | 3.1 | |
Note: Risk grouping based upon the National Comprehensive Cancer Network (NCCN) risk classification.
Abbreviations: ADT, androgen-deprivation therapy; IPSS, International Prostate Symptom Score; PSA, prostate-specific antigen
New NCCN low-risk group, excluding very-low-risk patients
Classic NCCN low risk group that combines the new very low risk and low risk groups together
All patients were treated using a five- to seven-field IMRT plan with 15 MV photons. The detailed techniques used have been described previously.12 The entire cohort received 86.4 Gy in 48 fractions of 180 cGy. The PTV consisted of the prostate, entire seminal vesicles and a 1 cm margin in all directions except posteriorly, where the margin was reduced to 0.6 cm to decrease dose at the prostate-rectal interface. Patient position was verified with weekly port films. Dose constraints were placed on the large and small intestine, rectal and bladder walls, and PTV. Maximum point dose was limited to 60 Gy and 53 Gy respectively for the large and small intestine. Rectal and bladder walls were limited to a V47 <53% and V75.6<30%. In the region of PTV and rectum overlap, the maximum point dose to the rectal wall was limited to 99% of the prescription dose (8550 cGy). Maximum dose to the PTV was 110%, and typically greater than 87% of the PTV volume received the prescribed dose of 86.4 Gy or more (V100). The rectal-prostatic interface was the primary region where dose reduction occurred in order to meet the rectal dose constraint.
Androgen deprivation therapy (ADT) was prescribed at the discretion of the treating physician. In general a 6-month course of ADT (3 months neoadjuvantly and 3 month concurrently) was used for select low- and intermediate-risk patients, and 6 months to 2 years was utilized for high-risk patients. A total of 587 (59%) patients were treated with ADT in our cohort. ADT use by risk group was 54 (27.5%), 221 (47.8%), and 314 (91.3%) for low-, intermediate-, and high-risk groups, respectively.
The database was closed for analysis in December of 2011. All endpoints were calculated from the radiation completion date. Biochemical failure was defined using the Phoenix consensus definition of the nadir PSA plus 2 ng/mL. The cause of death was recorded for all patients who died during the analysis period. Prostate cancer specific death was denoted if there was clear evidence death was secondary to prostate cancer, or by having metastatic disease with an elevated PSA at time of death. Greater than 50% core involvement was determined by dividing the total number of positive biopsy cores by the total cores.
Genitourinary (GU) and gastrointestinal (GI) acute and late toxicities were collected. Acute toxicities were defined as from the beginning of treatment until 3 months post-RT, and late toxicities thereafter. Toxicity grading was performed based upon the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE). A strict definition of the grading system was used for Grade 1 – 4. Grade 1 was defined as minimal side effects not affecting activities of daily living (ADL); Grade 2 – side effects requiring medications for symptom management (or increase in dose of pre-existing medication) with symptoms affecting ADL, Grade 3 – side effects that are severe or medically significant, but not life-threatening, or necessitate procedures (i.e., endoscopy, cauterization, catheterization, blood transfusions); Grade 4 – life threatening and urgent treatment/intervention needed.
To capture urinary dysfunction, baseline International Prostate Symptom Score (IPSS) and quality-of-life questionnaires were collected prior to treatment and at each follow-up. Complete IPSS information was available for 642 patients as the IPSS was not implemented for routine use prior to 2002. Erectile function was assessed using a three-tier grading system at baseline and last follow-up; Full potency - ability to have full erections adequate for penetration; partial potency - ability to achieve penetration but either aids are needed or the patient reports difficulty in doing so; impotent - inability to achieve an erection adequate for penetration. The International Index of Erectile Function (IIEF) questionnaires were utilized to aid in the grading of erectile function in addition to each office visit clinic note. Similarly to the IPSS score, the IEFF was not routinely collected prior to 2002; however, sexual function details were collected at each visit.
Actuarial likelihood estimates were determined using the Kaplan-Meier method for biochemical relapse-free survival (bRFS), distant metastasis-free survival (DMFS), and GI/GU toxicity and compared using the log-rank test. Univariate hazard ratios and 95% confidence intervals (95% CI) for bRFS and DMFS were calculated using a Cox proportional hazards model. Multivariate models for bRFS, DMFS, and toxicity outcomes were constructed using a stepwise Cox regression analysis. Erectile function multivariate analysis was performed using logistical regression. Prostate cancer–specific mortality (PCSM) was estimated using the cumulative incidence method with competing risk analysis with the Fine and Gray multivariable regression. Two-sided p values ≤0.05 were considered statistically significant. Statistical analysis was performed using R version 2.14.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Biochemical control
Freedom from biochemical recurrence for low-, intermediate-, and high-risk patients at 7-years based on the nadir plus 2 ng/mL definition was 98.8%, 85.6%, and 67.9%, respectively (p<0.001, Fig 1a). When separating the classic low-risk group into the new definitions of very-low risk and low-risk, the 7-year bRFS was 100% and 97.7%, respectively (p=.11). Using the multivariate model, age <65 years old (p=.03, HR=.68 [.48–.96]), T-stage (p=.009, HR=1.34 [1.08–1.67]), Gleason score (p<.001, HR=1.79 [1.34–2.39]), pretreatment PSA (p=.027, HR=1.71 [1.06–2.76]), and >50 % core involvement (p<.001, HR=2.33 [1.61–3.37]) predicted for bRFS. Use of ADT (p=0.79) and PSA density (p=.26) did not predict for biochemical failure.
Fig. 1.
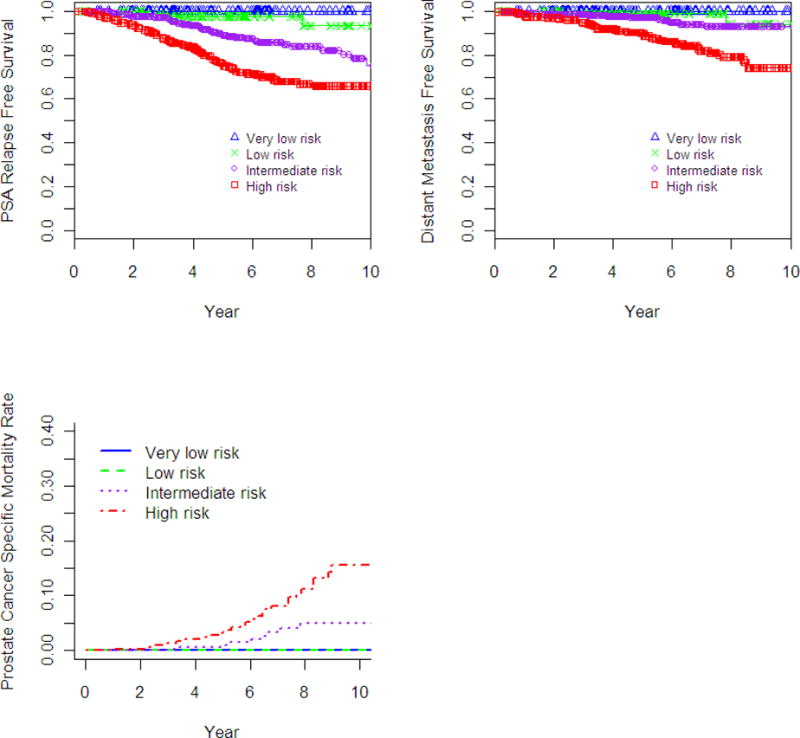
Estimated survival-time curves for biochemical relapse-free survival (bRFS), distant metastasis-free survival (DMFS), and prostate cancer-specific mortality (PCSM). a) bRFS, unadjusted; b) DMFS, unadjusted; c) PCSM, unadjusted using Fine and Gray’s test.
Distant metastasis-free survival
The 7-year actuarial DMFS for low-, intermediate- and high-risk groups was 99.4%, 94.1%, and 82.0%, respectively (p<0.001, Fig 1b). When separating the classic low-risk into very-low and low-risk groups, the 7-year DMFS was 100%, and 98.9%, respectively (p=.18). As shown in Table 2, on multivariate analysis T-stage (p<0.001), Gleason score (p<0.001), and percent core positivity >50% (p=0.001) were predictive for distant metastases. ADT use was significant on univariate analysis (p=0.03), and in the multivariate model (MVA) (p=0.05, HR=0.51 [0.26–1.01]). Pretreatment PSA (p=0.05) and PSA density (p=0.003) were predictive for DMFS on univariate analysis, but not on multivariate analysis.
Table 2.
Univariate and Multivariate Analysis of DMFS
| Univariate
|
Multivariate
|
|||
|---|---|---|---|---|
| P-Value | HR (95% CI) | P-Value | HR (95% CI) | |
| Age >65 vs ≤65 | 0.37 | 0.79 (0.49–1.31) | – | – |
| T-stage | <0.001 | 2.27 (1.74–2.99) | <0.001 | 1.72 (1.27–2.32) |
| Gleason score | <0.001 | 3.17 (2.19–4.61) | <0.001 | 2.661 (1.69–4.18) |
| PSA | 0.052 | 1.61 (1.00–2.61) | 0.49 | 1.25 (0.66–2.39) |
| ADT (yes vs no) | 0.03 | 1.842 (1.061–3.20) | 0.054 | 0.51 (0.26–1.01) |
| % Core Positivity (>50% vs ≤50%) | <0.0001 | 4.24 (2.57–6.99) | 0.001 | 2.55 (1.50–4.32) |
| PSA density (>0.25 vs ≤0.25) | 0.003 | 2.07 (1.27–3.36) | 0.42 | 1.31 (0.69–2.48) |
Abbreviations: ADT, androgen-deprivation therapy; CI, confidence interval; HR, hazard ration; PSA, prostate-specific antigen
Prostate cancer-specific mortality
No prostate cancer related deaths were observed in the very-low or low-risk groups. The 7-year PCSM using competing risk analysis for intermediate- and high-risk groups was 3.3% and 8.1%, respectively (p=0.008). As shown in Table 3, on multivariate analysis only Gleason score (p<0.004), and percent core positivity >50% (p=0.003) were predictive for prostate cancer death. ADT use was associated on univariate analysis with a detriment in PCSM (p=0.021, HR=2.83) as was PSA density >0.25 ng/mL/g (p=0.017, HR=2.25), however both were non-significant on multivariate analysis and was excluded from the final model due to the limited number of events.
Table 3.
PCSM Unadjusted Univariate and Adjusted Multivariate Fine and Gray Analysis
| Univariate
|
Multivariate
|
|||
|---|---|---|---|---|
| P-Value | HR (95% CI) | P-Value | HR (95% CI) | |
| Age >65 vs ≤65 | 0.21 | 0.65 (0.34–1.26) | – | – |
| T-stage | <0.001 | 1.40 (1.2–1.64) | 0.033 | 1.22 (1.02–1.45) |
| Gleason score | <0.001 | 1.98 (1.58–2.49) | 0.004 | 1.51 (1.14–2.01) |
| PSA | 0.4 | 1.01 (0.98–1.01) | – | – |
| ADT (yes vs no) | 0.021 | 2.83 (1.17–6.83) | – | – |
| % Core Positivity (>50% vs ≤50%) | <0.001 | 4.87 (2.41–9.85) | 0.003 | 3.15 (1.49–6.67) |
| PSA density (>0.25 vs ≤0.25) | 0.017 | 2.25 (1.15–4.37) | – | – |
Abbreviations: ADT, androgen-deprivation therapy; CI, confidence interval; HR, hazard ration; PSA, prostate-specific antigen
Genitourinary and Gastrointestinal Toxicity
Figure 2 shows late GI and GU toxicity rates. Actuarial 7-year grade 2 or higher late gastrointestinal and genitourinary toxicities were 4.4% and 21.1%. Late grade 3 gastrointestinal and genitourinary toxicity was experienced in 7 patients (0.7%) and 22 patients (2.2%), respectively. The late Grade 3 toxicities consisted of urethral strictures and hemorrhagic cystitis. The late Grade 3 rectal toxicities primarily related to rectal bleeding. One patient experienced full GI incontinence, and another developed a rectal stricture requiring sphincterotomy. Table 4 shows the multivariate analysis for predictors of Grade 2 or higher late GU and GI toxicity. Both pre-RT baseline >15 IPSS (p=0.001), and acute GU toxicity (p<0.001) predicted for late GU toxicity. Only acute GI toxicity predicted for Grade 2 or higher late GI toxicity (p=0.03).
Fig. 2.
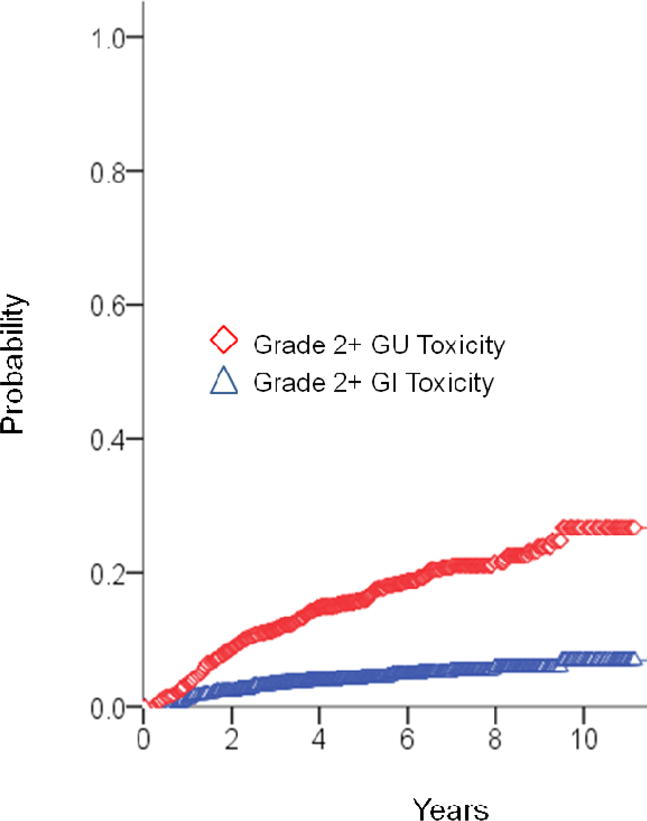
Late grade ≥2 gastrointestinal and genitourinary toxicity actuarial outcomes.
Table 4.
Multivariate for Late Toxicity
| Late Toxicity | Late GU ≥ Grade 2 | Late GI ≥ Grade 2 | Impotence |
|---|---|---|---|
| Age >65 vs ≤65 | NS | NS | p<0.001 |
| Pre-RT IPSS >15 vs ≤15 | p=0.001 | NS | – |
| Diabetes mellitus | NS | NS | NS |
| ADT (yes vs no) | NS | NS | NS |
| Acute GU Toxicity | p=<0.001 | NS | p<0.001 |
| Acute GI Toxicity | NS | p=0.03 | NS |
Abbreviations: ADT, androgen-deprivation therapy; GI, gastrointestinal; GU, genitourinary; IPSS, International Prostate Symptom Score; NS, non-significant; RT, radiation therapy; PSA, prostate-specific antigen
Erectile function
Of the 1002 men at baseline, 427 (42.6%) patients had full potency, 277 (27.6%) had partial potency, 267 (26.6%) were impotent, and 31 (3%) had unknown pre-RT baseline sexual function. Of the 427 men with full potency at baseline, 317 men (74%) retained sexual function at time of last follow-up. On multivariate analysis seen in Table 4, age greater than 65 years old (p<0.001, HR=2.3), and acute GU toxicity (p<0.001, HR=1.64) predicted for the development of impotency.
Discussion
Dose escalation beyond 70 Gy improves biochemical control for patients with clinically localized prostate cancer.4–8 The highest radiation doses utilized in currently published randomized prospective trials has been 79 to 80 Gy,7,8 and the highest EBRT dose reported in non-randomized clinical trials has been to dose levels of 86.4 Gy.12,13 With the current level of EBRT dose-escalation commonly reported at 75–81 Gy, post-treatment positive biopsy rates are in excess of 15%–20%.13 Therefore, greater dose intensification may be required to achieve an improved likelihood of local tumor control, especially for patients with moderate to large volume of intra-prostatic disease. Eade et al. noted that doses of ≥80 Gy were associated with a greater likelihood for improved local control and fewer distant failures compared to <80 Gy. The authors estimated that for each additional 1 Gy there is a decrease in the risk of biochemical recurrence by 2.2%.14
Overall survival and PCSM are beginning to be reported as follow-up of early studies mature. Kuban et al. published an update to their phase III trial and reported a benefit in cause-specific survival in the dose-escalated arm with marginal significance (99% vs 95%, p=0.06).4 Also, recently published data from Pahlajani et al. has shown that there appears to be an overall survival advantage with dose-escalation up to 84 Gy compared to doses less than 80 Gy in high-risk patients.15
To more rigorously examine the effect of dose-escalation, a meta-analysis of seven randomized dose-escalated trials of 2812 patients was conducted by Viani et al., showing biochemical control benefits for all risk groups.9 In addition, they performed a meta-regression analysis to analyze the relative benefit of dose escalation from doses of 64 Gy to 81 Gy on biochemical control. For all risk groups, a linear benefit was shown with dose-escalation. Results from the regression analysis also suggested that further dose-escalation would continue to provide improvements in biochemical control, with no indication that the slope of the dose response curve is trending downward or demonstrating a plateau with higher doses beyond 80 Gy. Likewise, our institution assessed the dose response curve in relation to positive biopsy cores post-treatment rather than biochemical failure to more accurately assess local tumor eradication. The analysis suggested a TCP50, the dose to have a local control rate of 50%, of 70.5 Gy.16 The model also showed linear improvements in dose approaching 85 Gy before the slope began downward trending. We believe these data suggest that while radiation doses of 78–80 Gy may be sufficient for low tumor volume and/or low-risk disease, further dose escalation may be necessary for higher tumor volume or higher-risk disease.
The outcomes reported with combination brachytherapy and supplemental EBRT for intermediate- and higher-risk disease also are consistent with improved tumor control rates with greater intensification of the intraprostatic radiation dose. Estimates of biological equivalent dose (BED) levels with brachytherapy with or without supplemental EBRT have been in the range of 90 to ≥180 Gy depending on the prescription dose and dose rate. Stone et al. demonstrated that the application of even higher BED dose levels with such treatment modalities have been associated with improved biochemical tumor control outcomes.17 Nevertheless, dose-escalation through brachytherapy is not feasible for all patients, and in such patients dose-escalation via EBRT alone may be needed for those with significant extraprostatic T3 disease, large prostate volumes or those with more significant pretreatment urinary symptoms.
Dose-escalation using IMRT to dose levels of 81 Gy compared to 3D-CRT has been associated with reduced rectal bleeding18 yet there is concern for increased risk of late normal tissue toxicity when dose levels would be escalated beyond 81 Gy even in the setting of IMRT. Our data presented in this report do not appear to justify this concern.12,19 We present here excellent late toxicity rates using IMRT with only ~4% of patients experiencing a Grade 2 or higher late GI toxicity at 7-years post-treatment. Toxicity rates in this report were comparable to our previous study which comprised 772 patients and we observed Grade 2 or higher late GI toxicity at 4%.11 We have attributed the relatively low rates of treatment-related toxicities to careful adherence to dose constraints in the treatment plan, and reducing the volume of normal rectum, bladder and bowel exposed to the high dose of irradiation. We may also observe a lessening in late toxicities with the current routine use of image-guidance with fiducial-based treatment, and real-time prostate tracking methods, as PTV expansion margins have decreased. In fact, in a cohort of patients treated with IGRT to 86.4 Gy, Zelefsky et al. has recently reported that IGRT based dose-escalated treatment for localized prostate cancer reduced the risk of late grade 2 or higher GU toxicity from 20% to 10.4% at 3 years.20
In the current report we note excellent biochemical tumor control outcomes with high dose IMRT. Randomized trials will be necessary to evaluate the role of additional dose escalation beyond 80 Gy as questions of safety continue to be posed. RTOG 94–06 demonstrated an excellent toxicity profile with dose-escalation up to 79.2 Gy with the use of 3D-CRT with ≤3% experiencing a Grade 3 GI or GU acute toxicity, and ~85% of patients experienced either no late toxicity or Grade 1 toxicity. Based upon this trial, the maximally tolerated dose (MTD) was attempted to be calculated, however the MTD was not reached. RTOG began another dose-escalation trial, RTOG 01–26. This trial is closed and maturing, and compares 82.3 Gy to 72.9 Gy prescribed to the isocenter, and allows the use of IMRT. The data from this relatively modern trial will be important to confirm the safety and efficacy of further dose-escalation.
As this study is retrospective in nature, is it subject to biases of this study methodology. The majority of our patients were prospectively captured to help minimize bias. However, selection bias inherently exists as patients have the option to undergo other treatment modalities at our institution. As with all retrospective studies, grading of toxicity is limited to the questionnaires and documentation performed during initial consultation and follow-up visits. Further limitations include the lack of routine biopsies to accurately assess local tumor control. High-risk patients at our institution commonly receive 1 to 2 years of ADT despite randomized studies often prescribing ≥3 years, as the optimum duration necessary in the setting of high dose EBRT is unclear.
Conclusion
Our findings indicate that high dose IMRT to 86.4 Gy in a large cohort of 1002 patients results in excellent tumor control and rare grade 3 toxicity. Strict adherence to dose constraints is critical in the delivery of such high doses to minimize toxicity and not negate the gained therapeutic advantage. Results from ongoing dose-escalation trials will continue to provide understanding toward the role of dose-escalation in localized prostate cancer. The long-term results of other dose intensification approaches like supplemental brachytherapy and hypofractionated stereotactic radiosurgery will need to be evaluated to determine if these achieve more durable tumor control outcomes than conventionally fractionated high-dose IMRT.
Acknowledgments
The authors wish to thank Eve S. Ferdman for her editorial assistance on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This was submitted in part to the Annual ASTRO meeting in Boston 2012, and has not been submitted for publication elsewhere.
The authors have no financial disclosures or conflicts of interest to report.
References
- 1.Rhamy RK, Wilson SK, Caldwell WL. Biopsy-proved tumor following definitive irradiation for resectable carcinoma of the prostate. J Urol. 1972;107:627–630. doi: 10.1016/s0022-5347(17)61099-1. [DOI] [PubMed] [Google Scholar]
- 2.Zelefsky MJ, Reuter VE, Fuks Z, et al. Influence of local tumor control on distant metastases and cancer related mortality after external beam radiotherapy for prostate cancer. J Urol. 2008;179:1368–1373. doi: 10.1016/j.juro.2007.11.063. discussion 1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubray BM, Beckendorf V, Guerif S, et al. Does short-term androgen depletion add to high-dose radiotherapy (80 Gy) in localized intermediate-risk prostate cancer? Intermediate analysis of GETUG 14 randomized trial (EU20503/ NCT00104741) J Clin Oncol. 2011;29(suppl. 15):4521. [Google Scholar]
- 4.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 5.Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24:1990–1996. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 6.Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8:475–487. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 7.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from Proton Radiation Oncology Group/American College of Radiology 95–09. J Clin Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beckendorf V, Guerif S, Le Prise E, et al. The GETUG 70 Gy vs. 80 Gy randomized trial for localized prostate cancer: feasibility and acute toxicity. Int J Radiat Oncol Biol Phys. 2004;60:1056–1065. doi: 10.1016/j.ijrobp.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 9.Viani GA, Stefano EJ, Afonso SL. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys. 2009;74:1405–1418. doi: 10.1016/j.ijrobp.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 10.Hanks GE, Hanlon AL, Epstein B, et al. Dose response in prostate cancer with 8–12 years’ follow-up. Int J Radiat Oncol Biol Phys. 2002;54:427–435. doi: 10.1016/s0360-3016(02)02954-1. [DOI] [PubMed] [Google Scholar]
- 11.Zelefsky MJ, Fuks Z, Hunt M, et al. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53:1111–1116. doi: 10.1016/s0360-3016(02)02857-2. [DOI] [PubMed] [Google Scholar]
- 12.Cahlon O, Zelefsky MJ, Shippy A, et al. Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys. 2008;71:330–337. doi: 10.1016/j.ijrobp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Zelefsky MJ, Fuks Z, Hunt M, et al. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001;166:876–881. [PubMed] [Google Scholar]
- 14.Eade TN, Hanlon AL, Horwitz EM, et al. What dose of external-beam radiation is high enough for prostate cancer? Int J Radiat Oncol Biol Phys. 2007;68:682–689. doi: 10.1016/j.ijrobp.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pahlajani N, Ruth KJ, Buyyounouski MK, et al. Radiotherapy doses of 80 gy and higher are associated with lower mortality in men with Gleason score 8 to 10 prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:1949–1956. doi: 10.1016/j.ijrobp.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levegrun S, Jackson A, Zelefsky MJ, et al. Risk group dependence of dose-response for biopsy outcome after three-dimensional conformal radiation therapy of prostate cancer. Radiother Oncol. 2002;63:11–26. doi: 10.1016/s0167-8140(02)00062-2. [DOI] [PubMed] [Google Scholar]
- 17.Stock RG, Stone NN, Tabert A, et al. A dose-response study for I-125 prostate implants. Int J Radiat Oncol Biol Phys. 1998;41:101–108. doi: 10.1016/s0360-3016(98)00006-6. [DOI] [PubMed] [Google Scholar]
- 18.Zelefsky MJ, Fuks Z, Happersett L, et al. Clinical experience with intensity modulated radiation therapy (IMRT) in prostate cancer. Radiother Oncol. 2000;55:241–249. doi: 10.1016/s0167-8140(99)00100-0. [DOI] [PubMed] [Google Scholar]
- 19.Michalski JM, Bae K, Roach M, et al. Long-term toxicity following 3D conformal radiation therapy for prostate cancer from the RTOG 9406 phase I/II dose escalation study. Int J Radiat Oncol Biol Phys. 2010;76:14–22. doi: 10.1016/j.ijrobp.2009.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zelefsky MJ, Kollmeier M, Cox B, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012 Feb 11; doi: 10.1016/j.ijrobp.2011.11.047. epub ahead of print. [DOI] [PubMed] [Google Scholar]


