Abstract
Cancer/testis antigen 7 (CT7) is the most frequently and consistently expressed MAGE antigen in multiple myeloma (MM), exhibits tissue-restricted expression and is an independent negative prognostic factor for MM.
We sought to characterize CT7 protein expression in the bone marrow (BM) of MM patients undergoing allogeneic T cell-depleted hematopoietic stem cell transplantation (alloTCD-HSCT), and to examine the significance of CT7-specific cellular immune responses. We further aimed to determine CT7-derived immunogenic epitopes and their associated allelic restrictions.
CT7 protein expression in neoplastic CD138+ plasma cells was evaluated by immunohistochemistry in BM biopsies from 10 patients. CT7 was present in 8/10 patients. Longitudinal analyses of the 10 patients revealed an association between CT7 expression and prognosis.
Longitudinal monitoring of CT7-specific T cells revealed an association between increased frequencies of CT7-specific T cells and reductions in specific myeloma markers. Epitope-specific reactivity to the nonamer FLAMLKNTV was detected by intracellular IFN-γ assay in peripheral blood (PB) and BM-derived T-cells from HLA-A*0201+ patients. Serial monitoring of PB CT7-specific T cell frequencies in 4 HLA-A*0201+ patients by HLA-A*0201-CT7(1087-1095) tetramer staining revealed an association with disease course. Phenotypic analyses revealed BM enrichment for central memory CT7- specific T cells, while effector memory cells dominated the PB.
Together, these findings support the development of immunotherapeutic strategies that aim to enhance CT7-directed immune responses for the treatment of MM.
Keywords: CT7, MAGE-C1, immune responses, allogeneic stem cell transplant, multiple myeloma
Introduction
Cancer/testis antigen 7 (CT7, or melanoma-associated antigen (MAGE)-C1) is the most frequently and consistently expressed MAGE antigen by malignant plasma cells in multiple myeloma (MM) patients, and does not appear to be downregulated during the course of the disease [1-4]. Its expression correlates strongly with plasma cell proliferation index, paraprotein levels, and reduced time to relapse, rendering CT7 a significant and independent negative prognostic factor for MM [3]. Evidence suggests that CT7 may play a gatekeeper function in terms of MAGE co-expression in MM [5]. CT7 may also play central roles in anti-apoptosis, in the dysregulation of cell cycle control and tumor progression, and in the development of chemotherapy resistance [3, 4, 6, 7]. The high and consistent levels of CT7 expression, along with its tissue-restricted expression pattern, make CT7 a potential target for immunotherapeutic strategies against MM at all clinical stages, including minimal residual disease (MRD).
While much is known about the spontaneous development of cancer/testis antigens (CTA), and specifically CT7-specific immune responses in metastatic melanoma, there are very limited data available regarding their development in MM [8–12]. However, the detection of humoral and cellular immune responses against CTA in MM patients following allogeneic stem cell transplantation (alloSCT), combined with occurrences of donor lymphocyte infusion (DLI)-induced remissions in relapsed patients, suggest that strategies that boost anti-CTA responses might foster long lasting remissions [13, 14].
Specific T cell responses to CT7 have been examined in the bone marrow (BM) of only 4 MM patients. Minor frequencies of 0.04% and 0.06% CD3+ T cells were detected in the BM of 2 of these patients, following in vitro T cell expansion [15]. This study did not distinguish between CD8+ and CD4+ T cell reactivity, nor examine the specificity of CT7 reactivity in terms of epitope recognition. Peripheral blood (PB) T cell responses to CT7 have been described only in a single study of MM patients, in the context of CD4+ T cell reactivity. This study reported low frequencies of CT7-specific memory CD4+ T cells in 3/18 MM patients, following in vitro T cell expansion, but was unable to detect any CD8+ T cell reactivity [16]. Interestingly, those 3 patients that developed CD4+ T cell responses to CT7 had been shown in earlier studies to produce a humoral response to the protein, thereby demonstrating a potential concordance in the immune response to CT7, albeit in a very limited number of patients [17]. Analyses of the minimal epitopes of CT7 and their restriction elements conducted in monoclonal T cell lines expanded from these 3 MM patients identified 3 novel major histocompatibility (MHC) Class II-restricted epitopes: 971-990DDS presented by human leukocyte antigen (HLA)-DRB1*0701, 941-960RYT presented by HLA-DPB1*1901 (both contained within the MHD), and 491-510LLS presented by HLA-DP*0401 (located within the repetitive sequences of the 5′ end of the protein) [16]. Five additional immunogenic epitopes of CT7 have been reported in patients with metastatic melanoma and in healthy donors (HD) [9, 18]. As the immunodominant epitopes of CT7 that are recognized by HD and patients with metastatic melanoma and MM may differ, further research is required to: 1) determine whether the reported epitopes are capable of inducing immune responses in MM patients, 2) identify the MHC Class I-restricted MM-specific epitopes of CT7, and 3) evaluate the potential clinical benefit of targeting these epitopes in MM patients.
Spontaneous humoral responses against CTA have been documented in several studies, often in concordance with T cell responses [17, 19]. Antibody responses to CTA are detected frequently in allotransplant recipients, and occur in the absence of BM expression of the antigen, suggesting that they are derived from the allogeneic B cell responses [14]. In one study, CT7 appeared to elicit a dominant humoral response in MM patients [17]. The CT7 protein elicited heterogeneous responses in the sera, with several B cell epitopes of CT7 detected, and preferential targeting of the C-terminus of the CT7 protein (CT7522-1142) occurring.
In the present study we examined the significance of BM expression of CT7, and the development of CT7-specific cellular immune responses in patients undergoing allogeneic T cell-depleted hematopoietic stem cell transplantation (alloTCD-HSCT) and DLI for high-risk and multiply relapsed MM. In this study, we also sought to identify CT7-derived immunogenic epitopes and their associated allelic restrictions.
Our results demonstrate a correlation between CT7 protein levels in the BM of MM patients and disease outcome, supporting the prognostic value of CT7 in MM. Patients that developed marked populations of CT7-specific T cells had better disease outcomes, whereas patients that failed to expand CT7-specific T cells exhibited worse prognoses. We also identified a novel HLA-A*0201-restricted epitope of CT7, FLAMLKNTV, and demonstrate an association between CT7-specific T-cell frequencies and disease response.
Together, our data provide evidence to support the development of immunotherapeutic approaches targeting CT7 in patients with MM. Adoptive immunotherapy with CT7-specific T cells post alloTCD-HSCT may generate levels of myeloma-specific T cells capable of eradicating MRD and preventing relapse in MM.
Materials and Methods
Patients
After obtaining written informed consent, we obtained peripheral blood and bone marrow samples from patients pre- and post-alloTCD-HSCT as part of a study approved by the IRB of the Memorial Sloan-Kettering Cancer Center (MSKCC) (registered at http://clinicaltrials.gov, ID: NCT01131169). Patients with high-risk cytogenetics presenting with refractory or relapsed disease following autologous HSCT for MM were eligible for this study. After cytoreduction therapy with busulfan, melphalan, fludarabine, and antithymocyte globulin preparative therapy, patients received alloTCD-HSCT from human leukocyte antigen (HLA)-compatible donors. Patients did not receive immunosuppressive therapy after transplantation. DLI were generally administered not sooner than 5 months after alloTCD-HSCT, at 5×105 CD3/kg for the first and second infusions, with subsequent doses of 1×106 CD3/kg administered if required.
Response criteria
Clinical responses to alloTCD-HSCT and DLI were assessed according to criteria of the European Group for Blood and Marrow Transplantation (EBMT) [20].
Assessing CT7 protein expression in the bone marrow by immunohistochemistry
For morphological in situ protein expression analysis, immunohistochemical stains for CD138 and CT7 were performed. Single as well as double staining techniques were employed. Formalin-fixed paraffin-embedded samples of bone marrow biopsies were obtained from the archives of the Department of Pathology at MSKCC. Monoclonal antibody (mAb) CT7-33, previously generated by our group was used for the detection of CT7, and mAb MI-15 (DAKO, Carpenteria, CA) served as reagent for the analysis of CD138 [21].
Immunohistochemistry (IHC) was performed as described previously [22, 23]. For the single stain technique, slides were subjected to a heat based antigen retrieval procedure (30 minutes, 98°C) prior to the application of the primary antibody. Primary incubation with mAb CT7-33 or mAb MI-15 was done overnight at 4°C. A biotinylated horse-anti-mouse antibody (1:200; Vector, Burlingame, CA) was used as a secondary reagent, followed by an avidin-biotin-complex system using peroxidase as a reporter enzyme (ABC-elite kit, Vector Laboratories, Burlingame, CA); 3,3-diaminobenzidine tetrahydrochloride (liquid DAB, Biogenex, San Ramon, CA) served as chromogen. For double staining, a sequential staining technique was employed.
Double staining commenced with the detection of CD138. After completion of the first immunohistochemical staining step with mAb MI-15, a second round of antigen retrieval was performed, followed by an avidin-biotin blocking procedure (avidin-biotin-blocking-kit, Vector) to prevent cross-reactivity between the two detection steps. The second primary antibody CT7-33 to CT7 was then applied for one hour at 20°C. The biotinylated horse-anti-mouse antibody served as a secondary reagent, followed by an alkaline-phosphatase-linked-streptavidin (Boehringer Mannheim, Germany). A new fuchsin based chromogen (Vector Red, Vector Labs) was employed for the visualization of CT7. Morphological evaluation of antigen co-expression was based on the membranous expression pattern of CD138 and the cytoplasmic/nuclear presence of CT7 in myeloma cells. In order to control for appropriate double staining, each case was also subjected to a conventional staining protocol using anti-CT7 and anti-CD138 on separate slides. Appropriate negative controls omitting the primary reagent were included for each case. The extent of CT7 staining was estimated on the basis of CD138-positive tumor cells and graded as follows: Focal, approximately <5%; +, 5–25%; ++, >25–50%; +++, >50–75%; and ++++, >75%.
CT7 peptides
Overlapping peptides ranging from 20–26 amino acids in length and spanning the entire CT7 protein were purchased from Research Genetics (Bio-Synthesis Inc., Lewisville, TX). Peptides were manufactured to specifications of validity of sequence, 95% purity and sterility. A total pool of 110 synthetic pentadecapeptides spanning the entire 1142 amino acid sequence of the CT7 protein, with 11 amino acids overlaps, was prepared and stored. The nonamer FLAMLKNTV was manufactured to 98% purity and sterility (GenScript, Piscataway, NJ).
Quantitation of functional CT7- and FLAMLKNTV-specific T cells by intracellular IFN-γ analyses
The proportion of T cells producing IFN-γ in response to overnight stimulation with the CT7 total pool or the FLAMLKNTV peptide loaded onto autologous peripheral blood mononuclear cells (PBMC) were measured by FACS analysis, as previously described [24, 25].
Determination and characterization of CT7 peptide-specific frequencies by MHC-tetramer analyses
CD8+ CT7-specific T cell frequencies were quantified at multiple time points before and after transplantation in patients expressing HLA-A*0201 by staining with the HLA-A*0201-CT71087-1095 MHC-tetramer. The HLA-A*0201-CT71087-1095 MHC-tetramer complex was generated by the Lausanne branch of the Ludwig Institute and used as previously described [25–27]. Immunophenotyping was concurrently performed. PBMC and bone marrow mononuclear cells (BMMC) were stained with PE–labeled tetrameric complex, monoclonal anti-CD3 phycoerythrincyanin-7 (PE-Cy7), anti-CD8 PerCP, anti-CD45RA APC, and anti-CD62L FITC (all BD Bioscience, San Jose, CA) for 20 minutes at 4°C. Appropriate control stains with HLA-mismatched CMV tetramers were also performed.
Flow cytometric analyses
Data acquisition was performed with a FACSCalibur flow cytometer with triple lasers for 10-color capability using BD FACS Diva Software (BD Biosciences). Data analyses of T-cell frequencies were performed using FlowJo software (Tree Star Inc., Ashland, OR).
Correlating CT7-specific T-cell frequencies with disease status
The ability of CT7-specific T cells to mediate in vivo anti-myeloma cytotoxicity was measured indirectly by correlating T-cell emergence with myeloma markers, determined at diagnosis and followed in patients after alloTCD-HSCT. Absolute numbers of CT7- specific T cells/μL PB were calculated by multiplying the percentage of IFN-γ-producing or tetramer-positive (tet+) FLAMLKNTV-specific T cells by the absolute numbers of CD8+ and CD4+ T cells, derived from the absolute lymphocyte count (ALC). Levels of standard myeloma markers in the PB, M-Spike Gamma or IgA, were monitored and used to determine disease response or progression.
Results
CT7 expression by malignant plasma cells changes over the course of disease
While it has been shown that CT7 mRNA is expressed frequently in myeloma patients with advanced stage disease, the course of CT7 protein expression in individual patients has not been examined in detail. To evaluate the longitudinal expression of CT7 protein in the BM of MM patients, a dual staining IHC approach was developed to examine the expression of CT7 (clone CT7-133; red) in neoplastic CD138+ neoplastic plasma cells (clone MI15; brown). CT7 protein expression was monitored longitudinally in BM specimens from 10 myeloma patients, who were at least two years out from alloTCD-HSCT. Representative IHC stains of archival paraffin-embedded bone marrow specimens with varying levels of plasma cell infiltration are shown in Figure 1A–C.
Figure 1. CT7 is expressed in CD138+ plasma cells in the BM of myeloma patients and is associated with the disease course.
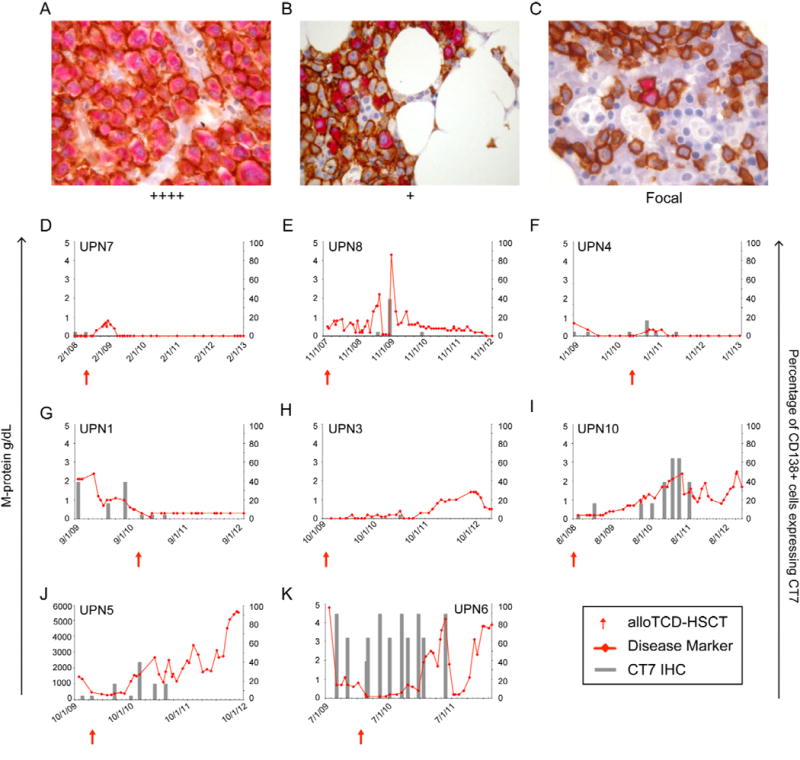
CT7 protein expression in CD138+ plasma cells was monitored longitudinally over the course of the disease in MM patients undergoing alloTCD-HSCT. Paraffin-fixed BM biopsies from MM patients were double stained with monoclonal antibodies to CD138 (MI15; DAB, brown) and CT7 (CT7-33; nFu, red). Immunohistochemical analysis of CT7 expression was performed and biopsies were graded negative, focal, +, ++, +++ or ++++ based on the percentage of CD138+ PC staining positive for CT7. Representative biopsy stainings are shown in Figures A–C. (A) ++++ >75% of CD138+ plasma cells stained positive for CT7; UPN6 (B) + >5–25% of CD138+ cells are CT7+; UPN5. (C) Focal, <5% of CD138+ cells are CT7+; UPN1 (20×). Longitudinal analyses of CT7 protein expression presented in panels D-K. The percentages of CD138+ cells expressing CT7 were compared to each patient’s relevant disease marker (D-I/K, M-Spike gamma g/dl; J, IgA mg/dl). (D) UPN7 (E) UPN8 (F) UPN4 (G) UPN1 (H) UPN3 (I) UPN10 (J) UPN5 (K) UPN6.
Overall, CT7 protein expression was detected in 8 of 10 patients. Pre-transplant biopsy specimens were available from 9 of the 10 patients, and CT7 expression was detected in 6 of those 9 patients’ pre-transplant specimens. Two of the ten patients were negative for CT7 expression at all time-points tested.
Fluctuations in CT7 protein expression levels in the BM were observed in each of the 8 CT7-expressing patients following alloTCD-HSCT and DLI. Durable reductions in BM CT7 protein expression were detected in 4 patients (UPN7/8/4/1), and coincided with the achievement of CR or VGPR (Figures 1D–G, respectively). UPN7 showed focal expression of CT7 protein in CD138+ plasma cells in the marrow prior to alloTCD-HSCT, but was negative for CT7 at all time-points tested thereafter (Figure 1D).
Increases in CT7 protein expression ultimately occurred in the remaining 4 patients (UPN3/10/5/6), with concurrent POD observed (Figures 1H-K, respectively). The expression of CT7 protein in >5% of CD138+ plasma cells (Grade + or higher) preceded disease relapse in several cases (Figures 1E and 1I-K) supporting previous reports that CT7 may serve as an early marker for MM relapse. The expression of CT7 protein was low (only 5% of the CD138+ MM cells) at the time point detected in UPN 3 (Figure 1H).
The maximum level of CT7 protein expression detected in the BM before or after transplant, as determined by IHC grading, appeared to be associated with disease outcome (Table 4.1). The maximum level of CT7 expression detected in the 4 patients who achieved durable CR in response to allotransplant and DLI was ++ (2 graded negative, 2 focal, 1 ++). One patient with maximal ++ disease achieved stable VGPR, but only upon reduction of CT7 protein expression to focal levels (<5% of CD138+ MM cells; UPN1, Figure 1G) following alloTCD-HSCT and DLI. Three patients with maximal CT7 disease ranging from ++ to ++++ achieved only PRs (UPN5/6) or VGPR (UPN10), persisting for only 2–3 months, when DLI were administered in addition to or following chemotherapy (Figures 1I-K, respectively). The short durations of these PRs suggests that responses to DLI are inferior in patients with higher levels of CT7 expression.
Table 1.
Maximal CT7 protein expression in the BM and disease outcome.
CR, complete remission; VGPR, very good partial response; PR, partial response; POD, progression of disease.
CT7 expression determined by immunohistochemical analysis. The extent of CT7 staining was estimated on the basis of CD138-positive tumor cells and graded as follows: Focal, approximately <5%; +, 5–25%; ++, >25–50%; +++, >50–75%; and ++++, >75%.
Patients received DLI in combination with or following chemotherapy, due to POD.
The persistent presence of the CT7 protein in a high percentage of CD138+ cells in UPN5 and UPN6 were eventually associated with POD (Figures 1J and 1K, respectively). Both patients ultimately succumbed to progressive myeloma disease.
The emergence of CT7-specific T cell frequencies following alloTCD-HSCT and DLI coincides with disease regression
To date, CT7-specific T cell responses have only been described in a very small number of MM patients, following in vitro T cell expansion methods. In this study, the development of CT7- specific T cell responses was evaluated following alloTCD-HSCT and DLI in all 10 patients in whom CT7 BM expression was examined. The clinical significance of the CT7-specific T-cell responses was further explored by correlating T cell emergence with myeloma disease load. Absolute numbers of CD8+ and CD4+ T cells specifically producing IFN-γ in response to overnight stimulation with the total pool of CT7 peptides were quantified in freshly isolated PBMC, and compared to each patient’s relevant marker of disease, either M-Spike Gamma or IgA (Figure 2).
Figure 2. CT7-specific T cell emergence coincides with the reduction and stabilization of the myeloma disease markers.
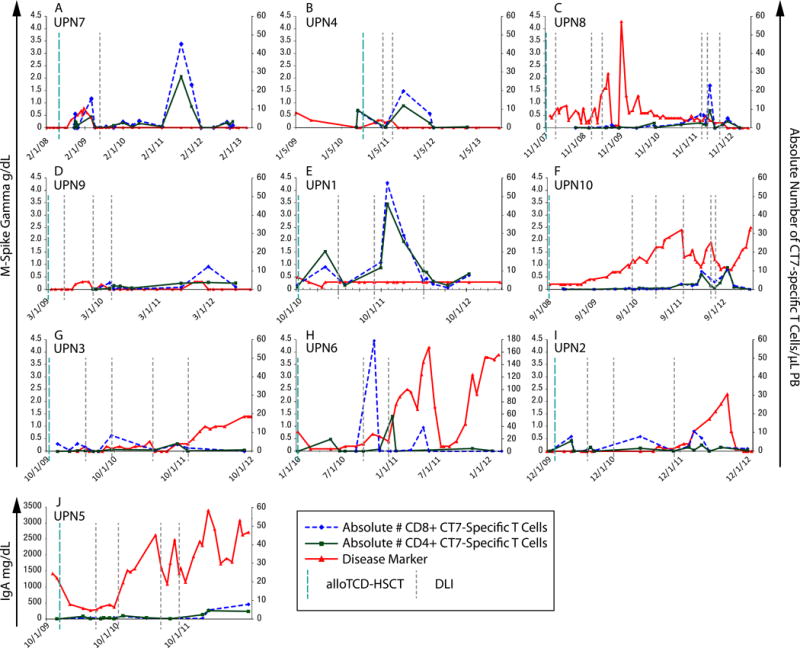
CT7-specific T-cell frequencies were quantified by intracellular IFN-γ assay in freshly isolated PBMCs. Absolute numbers of CT7-specific T cells were calculated from each patient’s ALC, and compared to the relevant clinical marker of myeloma disease, either M-Spike Gamma or IgA. Various increments in CT7-specific T cells were observed following DLI in individual patients, with fluctuations in T-cell frequencies observed over the course of the disease. (A) UPN7 (B) UPN4 (C) UPN8 (D) UPN9 (E) UPN1 (F) UPN10 (G) UPN3 (H) UPN6 (I) UPN2 (J) UPN5.
Increments in CT7-specific T cell frequencies were observed in all patients following DLI, and were associated with reductions or stabilization of myeloma load of varying durations. Clear temporal associations between peak CT7-specific T cells responses and disease regression were observed in 6 of the 10 patients monitored (UPN 7/4/8/9/10/6; Figures 2A/B/C/D/F/H, respectively). In UPN7, a marked T-cell response of 16 CT7-specific T cells/μL of blood occurred 8 weeks after the administration of the first therapeutic DLI, and coincided with disease regression to continuous CR (Figure 2A). Persistent low CT7-specific T cell frequencies were detected at several time-points thereafter and the patient developed a second peak of CT7-specific T-cell response at a later time-point as shown in Figure 2A. Findings from molecular chimerism studies conducted on isolated T cells 4 weeks after alloTCD-HSCT indicate that the CT7-specific T cells are of donor origin. A pronounced increase in the CT7-specific T cell population that occurred following UPN1’s second DLI was also associated with the stabilization of the M-protein in an otherwise very aggressive myeloma (Figure 2E).
Conversely, declining CT7-specific T cell populations were temporally associated with elevated myeloma markers and disease progression in several patients (UPN10/3/6/2; Figures 2F/G/H/I). Moreover, patients that failed to develop marked frequencies of CT7-specific T cells, despite repeated DLI, generally exhibited progressive disease courses (UPN10/3/5; Figures 2F/G/J).
Identification of a novel HLA-A*0201-restricted immunogenic epitope of CT7
To further evaluate the specificity of the CD8+ CT7-specific T cells detected in our MM patients, we sought to identify the immunogenic epitopes to which these CT7-specific T cells were responding. Reverse immunology was employed to predict the epitopes of CT7 that were most likely to have high affinity for, bind to, and be presented by the commonly expressed HLA allele A*0201. The top hit found in this search, the nonamer FLAMLKNTV, was located at position 1087 of the CT7 protein. A BLAST search revealed this nonamer to be unique to the CT7 protein. Reactivity to this protein was examined in 10 HLA-A*0201-expressing patients, who had undergone alloTCD-HSCT followed by DLI by intracellular IFN-γ assays. FLAMLKNTV-specific IFN-γ production was observed to varying degrees in all patients examined (mean, 3.174%; range 0.179–15.49% CD8+ T cells). Representative FACS plots from 7 patients are shown in Figure 3A-G. Overall, significantly greater IFN-γ production was observed in response to autologous PBMC targets pulsed with the FLAMLKNTV peptide compared to control non-pulsed autologous PBMC (p = 0.0156, Figure 3H).
Figure 3. FLAMLKNTV is an immunogenic epitope of CT7 that induces IFN-γ production by T cells isolated from HLA-A*0201+ MM patients.
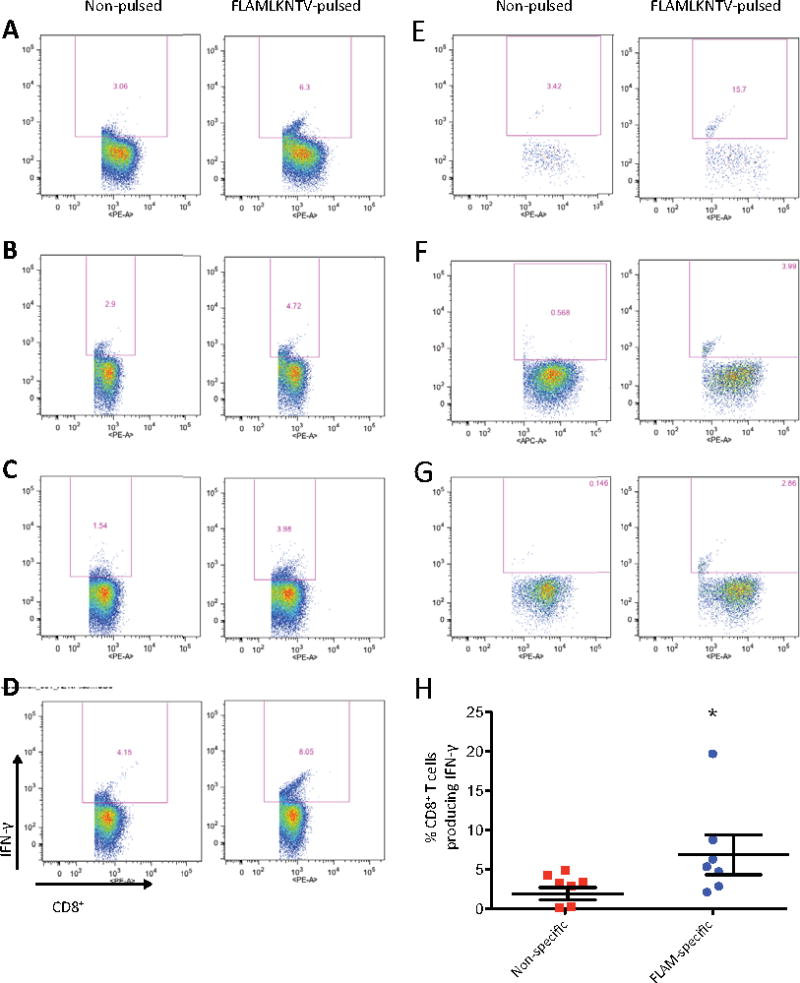
Intracellular IFN-γ production was measured in freshly isolated PBMC from 7 HLA-A*0201-expressing patients. Greater IFN-γ production by CD8+ T cells is observed in response to autologous PBMC pulsed with the HLA-A*0201-restricted immunogenic epitope FLAMLKNTV compared to non-pulsed control autologous PBMC targets. (A) UPN4 (B) UPN5 (C) UPN7 (D) UPN10 (E) UPN11 (F) UPN12 (G) UPN13. (H) FLAMLKNTV-induced IFN-γ production is significantly greater than the IFN-γ production to non-pulsed control targets (p = 0.0156, Wilcoxon matched pairs signed rank test).
Because of the consistent and reproducible detection of FLAMLKNTV-specific IFN-γ production in all HLA-A*0201+ patients, we proceeded to generate the cognate tetramer, HLA-A*0201-CT7-1087-1095. The generation of this tetramer facilitated the longitudinal monitoring of FLAMLKNTV-specific CD8+ T-cell frequencies in PB and BM samples from HLA-A*0201+ patients. Multiparametric analyses of samples co-stained with the tetramer and cell surface markers CD45RA and CD62L enabled phenotypic classification of the CT7-specific T cells generated. Figure 4 shows representative FACS plots of staining performed on PBMC and BMMC isolated from UPN7 ~3 years posttransplant, while the patient was in durable CR. Phenotypic analyses indicate that the accumulation of effector memory T cells (TEM) specific for CT7 in the PB, and the selective accumulation of central memory T cells (TCM) specific for CT7 in the BM.
Figure 4. Identification and characterization of CT7-specific T cells in the PB and BM of myeloma patients by MHC-tetramer analyses.
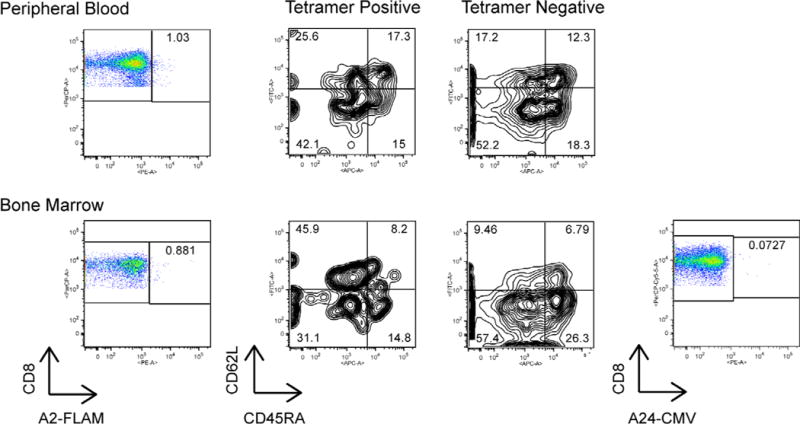
PBMC and BMMC were stained with the HLA-A*0201-CT71087-1095 or HLA-mismatched CMV-specific control tetramer, along with monoclonal antibodies specific for the surface markers CD45RA and CD62L for phenotypic characterization. Representative FACS plots from UPN7, performed ~3 years post alloTCD-HSCT, are shown. PB CT7-specific T cells are predominantly effector memory in phenotype, whereas CT7-specific T cells of central memory phenotype dominate the BM.
The clinical significance of the CT7-specific CD8+ T-cell frequencies detected by MHC-tetramer analyses post alloTCD-HSCT and DLI were again measured indirectly in four HLA-A*0201+ patients by correlating absolute numbers of PB-derived CT7-specific CD8+ tet+ T cells with each individual’s myeloma-specific disease marker. UPN7 experienced a disease recurrence ~7 months after alloTCD-HSCT that coincided with the loss of the TCM CT7-specific T cells in the PB. Shortly after the administration of the first DLI, an increase in TEM CT7-specific T cells occurred and the patient entered CR (Figure 5A). The elevated frequencies of CT7-specific T cells observed later in UPN7, following the achievement of durable CR, may reflect an immune response to subclinical levels of myeloma. Indeed, minimal residual disease was detected in UPN7 in later analyses. Emergence of CT7-specific TEM was also observed in UPN4 following the administration of the second DLI, and again coincided with the patient entering a durable CR (Figure 5B).
Figure 5. Kinetics of CT7-specific T cells post alloTCD-HSCT and DLI reveals an association with the disease response.
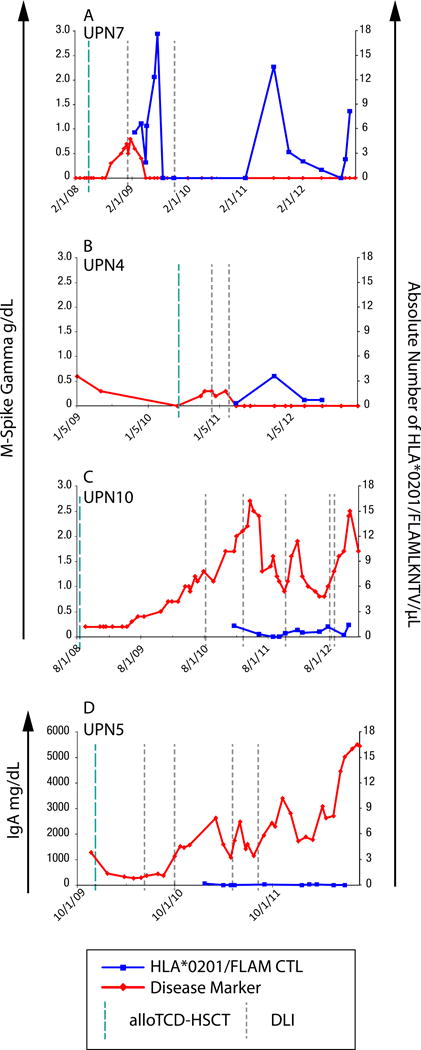
FLAMLKNTV-specific T-cell frequencies and their phenotypes were monitored longitudinally in the PB of HLA-A*0201+ patients by staining PBMC with the HLA-A*0201-CT71087-1095 tetramer. Absolute numbers of FLAMLKNTV-specific T cells were calculated and compared to each patient’s relevant clinical marker of disease, M-spike Gamma, (A) UPN7 (B) UPN4 (C) UPN10 or IgA, (D) UPN5.
In contrast, UPN10 and UPN5 (Figures 5C and 5D, respectively) failed to develop marked CT7-specific CD8+ T-cell frequencies, despite the administration of multiple DLI with and without preceding chemotherapy. Both patients exhibited progressive disease courses.
Discussion
CT7 is an X-linked CTA that was originally identified in melanoma cell lines by the technique of serological analysis of recombinant cDNA expression libraries (SEREX) and by representational difference analyses [29, 30]. Further investigation revealed that CT7 is additionally expressed in tumors of various histological types, including carcinomas of the liver, mammary glands and ovary, non-small cell lung carcinoma, metastatic melanoma and MM. Its expression in normal adult tissues is restricted to testicular germ cells, which are protected from T cell immunity by their lack of MHC Class I expression [4, 21, 31].
The high levels of CT7 expression observed in advanced stage disease, correlation between CT7 mRNA levels and disease burden, and evidence for CT7-mediated anti-apoptosis and cell cycle control, are suggestive of a role for CT7 in myeloma pathogenesis [3, 4, 7, 32].
Previous longitudinal studies demonstrated persistent CT7 mRNA expression by malignant plasma cells over the course of the disease, with CT7 mRNA levels paralleling BM plasma cell infiltration and paraprotein levels [3]. Our results extend on these findings by demonstrating a parallel relationship between CT7 expression at the protein level by IHC and disease course. Patients with low-level expression of or negative for CT7 entered CR or showed stable low-level disease. Furthermore, reductions in BM CT7 protein expression levels were observed in 4/8 measurable patients following alloTCD-HSCT and DLI, and coincided with the achievement of CR in 3 patients, and VGPR in the remaining patient. Increases in CT7 protein expression were observed in the remaining 4 patients, coincident with disease progression. Patients with high levels of CT7 protein experienced worse outcomes. Indeed, the two patients with persistently high levels of CT7 succumbed to progressive disease. The detection of CT7 protein both pre-transplant and after alloTCD-HSCT and DLI, provides further evidence that CT7 is a viable target for immunotherapeutic approaches at all clinical stages. Longitudinal monitoring of protein expression may provide a more reliable method of CT7 detection compared to techniques targeting mRNA levels. Weak levels of CTA mRNA expression have been reported, whereas CTA protein expression appears to be potent and stable [3]. While these data are limited by the small number of patients monitored longitudinally, they are suggestive of an association between CT7 expression in the BM and myeloma disease course. Further studies will be required to conclusively determine whether a true correlation exists between CT7 protein expression in the BM and the myeloma disease burden.
Very limited data exist regarding the development of cellular immune responses to CT7. In myeloma in particular, specific T cell responses to CT7 have only been examined in the PB of 18 patients and the BM of 4 patients, with minor responses detected following in vitro T cell expansion methods in 16.7% and 50% of patients, respectively [15]. In our studies, existing frequencies of CT7-specific T cells were identified in the PB and BM of all 10 MM patients examined, by intracellular IFN-γ and/or MHC-tetramer analyses. We were able to detect these CT7-specific T cells and quantify their absolute numbers in the PB without the requirement for in vitro T cell expansion.
Our studies did not reveal a correlation between the absolute number of CT7-specific T cells and disease burden, but instead demonstrated a temporal association between CT7-specific T-cell frequencies and myeloma disease. The minimum number of CT7-specific T cells required to induce a clinical response differs by patient. The ‘T cell threshold’ required to induce an anti-myeloma response is likely dependent on multiple factors, including but not limited to the extent of the myeloma disease, the amount of CT7 expressed by myeloma cells, and the host immune environment.
Longitudinal monitoring of CT7-specific T cell populations in the PB demonstrated the predominant presence of cells of an effector memory phenotype, and revealed an association with the myeloma disease course. The CT7-specific T cell responses described herein exhibited similar patterns of T-cell emergence, expansion, magnitude, and contraction as the WT1-specific T cell responses detected in these patients that we have reported previously [23].
Two patients in whom CT7 protein expression was not detected in the BM developed measurable CT7-specific T cell responses (UPN2 and UPN9). The CT7-specific T cell responses detected in these patients could possibly result from previous expression of CT7 protein in the bone marrow, which was present prior to our analyses. It is also possible that these patients expressed CT7 during the course of our studies, but we were unable to detect it. The BM aspirates obtained and analyzed reflect only a small percentage of the total BM compartment, which may contain low frequencies of malignant cells not captured within the aspirate sample.
We further sought to examine potential epitopes of CT7 to which the T cells detected in our MM patients may be responding. Reverse immunology predicted the HLA-A*0201-restricted epitope FLAMLKNTV, which induced peptide-specific IFN-γ production in all 10 HLA-A*0201+ patients examined. Generation of the novel HLA-A*0201-CT7-1087-1095 tetramer facilitated serial monitoring of the CT7-specific T-cell frequencies and their phenotypes over the course of the disease in 4 HLA-A*0201+ patients >2.5 years out from transplant. CT7 tetramer-positive T cells were detected in both the PB and BM of patients. Phenotypic analyses revealed the preferential targeting of central memory CT7-specific CD8+ T cells to the BM, whereas effector memory CT7-specific CD8+ T cells were predominantly found in the blood. While we did not have sufficient BM samples to assess a correlation between BM-derived CT7-specific T-cell frequencies and myeloma control/antigen suppression, we expect that such a correlation exists. Trafficking to the active site of disease, the BM, is likely required for CT7-specific T cells to elicit an anti-myeloma effect.
Longitudinal monitoring of CT7-specific T-cell frequencies by both intracellular IFN-γ and MHC-tetramer analyses showed that patients who developed marked frequencies of CT7-specific T cells after alloTCD-HSCT and DLI had better outcomes, often achieving CR or VGPR. However, those patients who failed to expand CT7-specific T cells, despite repeated DLI administered in combination with or following chemotherapy, experienced progressive disease courses and worse outcomes. These findings corroborate that which we have previously observed in terms of the emergence of T cells specific for WT1 [23].
CT7-specific T cell frequencies have also been monitored in a subset of MM patients prior to and following autologous SCT, in addition to the post alloTCD-HSCT and DLI analyses presented herein. Escalations in the frequencies of CT7-specific T cells populations occurred following autoSCT, suggesting that autologous transplantation may boost CT7-specific immune responses. The greatest frequencies of CT7-specific T cells were detected following allo TCD-HSCT and DLI, and were present at significantly higher levels than those detected prior to autoSCT and prior to DLI (data not shown).
Autologous and allogeneic transplantation may enhance adaptive cellular and humoral immune responses via numerous mechanisms. Firstly, chemotherapy applied prior to transplant and/or DLI likely promotes the release of tumor antigen upon tumor necrosis, making antigen available for uptake and processing by APC, and subsequent presentation to tumor-specific T cells [35]. The posttransplant lymphopenic host immune environment may further augment both types of immune responses via homeostatic proliferation of T cells and antibody-producing B cells. The elimination of suppressor cell subsets, removal of cytokine sinks, and greater availability and activation of APC may also promote antitumor immune responses in the lymphopenic setting [36]. It has also been postulated that the allogeneic immune environment itself may promote the persistence and efficacy of antitumor immune responses by providing “danger signals” that attenuate self-limiting immune mechanisms [14]. In addition to boosting ‘host’ immunity, these mechanisms also enhance the efficacy of adoptively transferred antigen-specific CD8+ T cells [37]. Conceivably, the presence of these mechanisms in the posttransplantation lymphopenic host environment promoted the expansion of CT7-specific precursors contained within the DLI products administered to some of our patients described herein, thereby generating or enhancing the graft-vs-myeloma (GVM) effect.
In order to eliminate MRD, CT7-specific T cells would need to target the putative multiple myeloma stem cell (MMSC), as well as the malignant plasma cells making up the bulk of the disease. Cancer stem cells are valid targets for immunotherapy due to their expression of CTA, and their lysis by both T cells in vitro, and by donor-derived T cells following allotransplant [38-45]. CT7 is expressed by both CD138+ myeloma cells and their clonogenic CD138-negative precursors, thought to represent the MMSC [6, 46, 47].
This study indicates that CT7 protein expression in the BM of MM patients is associated with the disease course, and may serve as a prognostic marker. The identification of a novel epitope of CT7 that is restricted by the HLA-A*0201 allele facilitated the development of a tetramer for serial monitoring of CT7-specific T-cell frequencies in MM patients. Longitudinal analyses of CT7-specific T-cell frequencies by IFN-γ and tetramer analyses revealed an association between T cell responses and disease outcome, with patients who developed marked populations of CT7-specific T cells entering CR, and those failing to expand CT7-specific T cells exhibiting progressive disease courses. These findings suggest that CT7-specific immune responses play a role in modulating myeloma disease, and support the development of immunotherapeutic approaches targeting CT7 for the treatment of MM.
Acknowledgments
The authors thank Lorna Barnett, Denise Frosina, and Megan Holz for their technical assistance, and Dr. Aisha Hasan for her input and support.
Grant Support
This work was funded in part by the Cancer Research Institute Pre-doctoral Fellowship in Tumor Immunology (EMT). GK is the recipient of a research grant from Otsuka Pharmaceuticals Inc. RJo’R receives grants CA023766-34, CA008748-48.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- 1.Dhodapkar MV, Osman K, Teruya-Feldstein J, Filippa D, Hedvat CV, Iversen K, et al. Expression of cancer/testis (CT) antigens MAGE-A1, MAGE-A3, MAGE-A4, CT-7, and NY-ESO-1 in malignant gammopathies is heterogeneous and correlates with site, stage and risk status of disease. Cancer immunity: a journal of the Academy of Cancer Immunology. 2003 Jul 23;3:9. [PubMed] [Google Scholar]
- 2.Tinguely M, Jenni B, Knights A, Lopes B, Korol D, Rousson V, et al. MAGE-C1/CT-7 expression in plasma cell myeloma: sub-cellular localization impacts on clinical outcome. Cancer science. 2008 Apr;99(4):720–5. doi: 10.1111/j.1349-7006.2008.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atanackovic D, Luetkens T, Hildebrandt Y, Arfsten J, Bartels K, Horn C, et al. Longitudinal analysis and prognostic effect of cancer-testis antigen expression in multiple myeloma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009 Feb 15;15(4):1343–52. doi: 10.1158/1078-0432.CCR-08-0989. [DOI] [PubMed] [Google Scholar]
- 4.Jungbluth AA, Ely S, DiLiberto M, Niesvizky R, Williamson B, Frosina D, et al. The cancer-testis antigens CT7 (MAGE-C1) and MAGE-A3/6 are commonly expressed in multiple myeloma and correlate with plasma-cell proliferation. Blood. 2005 Jul 1;106(1):167–74. doi: 10.1182/blood-2004-12-4931. [DOI] [PubMed] [Google Scholar]
- 5.de Carvalho F, Alves VL, Braga WM, Xavier CV, Jr, Colleoni GW. MAGE-C1/CT7 and MAGE-C2/CT10 are frequently expressed in multiple myeloma and can be explored in combined immunotherapy for this malignancy. Cancer immunology, immunotherapy: CII. 2012 Nov 22; doi: 10.1007/s00262-012-1376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atanackovic D, Hildebrandt Y, Jadczak A, Cao Y, Luetkens T, Meyer S, et al. Cancer-testis antigens MAGE-C1/CT7 and MAGE-A3 promote the survival of multiple myeloma cells. Haematologica. 2010 May;95(5):785–93. doi: 10.3324/haematol.2009.014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Carvalho F, Costa ET, Camargo AA, Gregorio JC, Masotti C, Andrade VC, et al. Targeting MAGE-C1/CT7 expression increases cell sensitivity to the proteasome inhibitor bortezomib in multiple myeloma cell lines. PLoS ONE. 2011;6(11):e27707. doi: 10.1371/journal.pone.0027707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991 Dec 13;254(5038):1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 9.Nuber N, Curioni-Fontecedro A, Matter C, Soldini D, Tiercy JM, von Boehmer L, et al. Fine analysis of spontaneous MAGE-C1/CT7-specific immunity in melanoma patients. Proc Natl Acad Sci U S A. 2010 Aug 24;107(34):15187–92. doi: 10.1073/pnas.1002155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaugler B, Van den Eynde B, van der Bruggen P, Romero P, Gaforio JJ, De Plaen E, et al. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med. 1994 Mar 1;179(3):921–30. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van den Eynde B, Peeters O, De Backer O, Gaugler B, Lucas S, Boon T. A new family of genes coding for an antigen recognized by autologous cytolytic T lymphocytes on a human melanoma. J Exp Med. 1995 Sep 1;182(3):689–98. doi: 10.1084/jem.182.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodyear O, Piper K, Khan N, Starczynski J, Mahendra P, Pratt G, et al. CD8+ T cells specific for cancer germline gene antigens are found in many patients with multiple myeloma, and their frequency correlates with disease burden. Blood. 2005 Dec 15;106(13):4217–24. doi: 10.1182/blood-2005-02-0563. [DOI] [PubMed] [Google Scholar]
- 13.Mielcarek M, Storb R. Non-myeloablative hematopoietic cell transplantation as immunotherapy for hematologic malignancies. Cancer Treat Rev. 2003 Aug;29(4):283–90. doi: 10.1016/s0305-7372(03)00004-5. [DOI] [PubMed] [Google Scholar]
- 14.Atanackovic D, Arfsten J, Cao Y, Gnjatic S, Schnieders F, Bartels K, et al. Cancer-testis antigens are commonly expressed in multiple myeloma and induce systemic immunity following allogeneic stem cell transplantation. Blood. 2007 Feb 1;109(3):1103–12. doi: 10.1182/blood-2006-04-014480. [DOI] [PubMed] [Google Scholar]
- 15.Lendvai N, Gnjatic S, Ritter E, Mangone M, Austin W, Reyner K, et al. Cellular immune responses against CT7 (MAGE-C1) and humoral responses against other cancer-testis antigens in multiple myeloma patients. Cancer immunity: a journal of the Academy of Cancer Immunology. 2010;10:4. [PMC free article] [PubMed] [Google Scholar]
- 16.Nuber N, Curioni-Fontecedro A, Dannenmann SR, Matter C, von Boehmer L, Atanackovic D, et al. MAGE-C1/CT7 spontaneously triggers a CD4(+) T-cell response in multiple myeloma patients. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2013 Feb 1; doi: 10.1038/leu.2013.31. [DOI] [PubMed] [Google Scholar]
- 17.Curioni-Fontecedro A, Knights AJ, Tinguely M, Nuber N, Schneider C, Thomson CW, et al. MAGE-C1/CT7 is the dominant cancer-testis antigen targeted by humoral immune responses in patients with multiple myeloma. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2008 Aug;22(8):1646–8. doi: 10.1038/leu.2008.43. [DOI] [PubMed] [Google Scholar]
- 18.Anderson LD, Jr, Cook DR, Yamamoto TN, Berger C, Maloney DG, Riddell SR. Identification of MAGE-C1 (CT-7) epitopes for T-cell therapy of multiple myeloma. Cancer immunology, immunotherapy: CII. 2011 Jul;60(7):985–97. doi: 10.1007/s00262-011-1009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholaou T, Ebert L, Davis ID, Robson N, Klein O, Maraskovsky E, et al. Directions in the immune targeting of cancer: lessons learned from the cancer-testis Ag NY-ESO-1. Immunology and cell biology. 2006 Jun;84(3):303–17. doi: 10.1111/j.1440-1711.2006.01446.x. [DOI] [PubMed] [Google Scholar]
- 20.Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. British journal of haematology. 1998 Sep;102(5):1115–23. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 21.Jungbluth AA, Chen YT, Busam KJ, Coplan K, Kolb D, Iversen K, et al. CT7 (MAGE-C1) antigen expression in normal and neoplastic tissues. International journal of cancer Journal international du cancer. 2002 Jun 20;99(6):839–45. doi: 10.1002/ijc.10416. [DOI] [PubMed] [Google Scholar]
- 22.Jungbluth AA, Chen YT, Stockert E, Busam KJ, Kolb D, Iversen K, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. International journal of cancer Journal international du cancer. 2001 Jun 15;92(6):856–60. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 23.Tyler EM, Jungbluth AA, O’Reilly RJ, Koehne G. WT1-specific T-cell responses in high-risk multiple myeloma patients undergoing allogeneic T cell-depleted hematopoietic stem cell transplantation and donor lymphocyte infusions. Blood. 2013 Jan 10;121(2):308–17. doi: 10.1182/blood-2012-06-435040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waldrop SL, Pitcher CJ, Peterson DM, Maino VC, Picker LJ. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. The Journal of clinical investigation. 1997 Apr 1;99(7):1739–50. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koehne G, Smith KM, Ferguson TL, Williams RY, Heller G, Pamer EG, et al. Quantitation, selection, and functional characterization of Epstein-Barr virus-specific and alloreactive T cells detected by intracellular interferon-gamma production and growth of cytotoxic precursors. Blood. 2002 Mar 1;99(5):1730–40. doi: 10.1182/blood.v99.5.1730. [DOI] [PubMed] [Google Scholar]
- 26.Marshall NA, Howe JG, Formica R, Krause D, Wagner JE, Berliner N, et al. Rapid reconstitution of Epstein-Barr virus-specific T lymphocytes following allogeneic stem cell transplantation. Blood. 2000 Oct 15;96(8):2814–21. [PubMed] [Google Scholar]
- 27.Busch DH, Pilip IM, Vijh S, Pamer EG. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 1998 Mar;8(3):353–62. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- 28.Gnjatic S, Old LJ, Chen YT. Autoantibodies against cancer antigens. Methods Mol Biol. 2009;520:11–9. doi: 10.1007/978-1-60327-811-9_2. [DOI] [PubMed] [Google Scholar]
- 29.Chen YT, Gure AO, Tsang S, Stockert E, Jager E, Knuth A, et al. Identification of multiple cancer/testis antigens by allogeneic antibody screening of a melanoma cell line library. Proc Natl Acad Sci U S A. 1998 Jun 9;95(12):6919–23. doi: 10.1073/pnas.95.12.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas S, De Smet C, Arden KC, Viars CS, Lethe B, Lurquin C, et al. Identification of a new MAGE gene with tumor-specific expression by representational difference analysis. Cancer research. 1998 Feb 15;58(4):743–52. [PubMed] [Google Scholar]
- 31.Lim SH, Bumm K, Chiriva-Internati M, Xue Y, Wang Z. MAGE-C1 (CT7) gene expression in multiple myeloma: relationship to sperm protein 17. Eur J Haematol. 2001 Nov-Dec;67(5–6):332–4. doi: 10.1034/j.1600-0609.2001.00552.x. [DOI] [PubMed] [Google Scholar]
- 32.Andrade VC, Vettore AL, Felix RS, Almeida MS, Carvalho F, Oliveira JS, et al. Prognostic impact of cancer/testis antigen expression in advanced stage multiple myeloma patients. Cancer immunity: a journal of the Academy of Cancer Immunology. 2008;8:2. [PMC free article] [PubMed] [Google Scholar]
- 33.Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading as memory cells. J Immunol. 2000 Aug 15;165(4):1733–7. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 34.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999 Aug;11(2):173–81. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 35.Jager E, Stockert E, Zidianakis Z, Chen YT, Karbach J, Jager D, et al. Humoral immune responses of cancer patients against “Cancer-Testis” antigen NY-ESO-1: correlation with clinical events. International journal of cancer Journal international du cancer. 1999 Oct 22;84(5):506–10. doi: 10.1002/(sici)1097-0215(19991022)84:5<506::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends in immunology. 2005 Feb;26(2):111–7. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005 Oct 3;202(7):907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004 Mar 15;103(6):2332–6. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Condomines M, Hose D, Raynaud P, Hundemer M, De Vos J, Baudard M, et al. Cancer/testis genes in multiple myeloma: expression patterns and prognosis value determined by microarray analysis. J Immunol. 2007 Mar 1;178(5):3307–15. doi: 10.4049/jimmunol.178.5.3307. [DOI] [PubMed] [Google Scholar]
- 40.Maloney DG, Molina AJ, Sahebi F, Stockerl-Goldstein KE, Sandmaier BM, Bensinger W, et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003 Nov 1;102(9):3447–54. doi: 10.1182/blood-2002-09-2955. [DOI] [PubMed] [Google Scholar]
- 41.Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood. 1996 Feb 1;87(3):1196–8. [PubMed] [Google Scholar]
- 42.Noonan K, Matsui W, Serafini P, Carbley R, Tan G, Khalili J, et al. Activated marrow-infiltrating lymphocytes effectively target plasma cells and their clonogenic precursors. Cancer research. 2005 Mar 1;65(5):2026–34. doi: 10.1158/0008-5472.CAN-04-3337. [DOI] [PubMed] [Google Scholar]
- 43.Dhodapkar MV, Krasovsky J, Olson K. T cells from the tumor microenvironment of patients with progressive myeloma can generate strong, tumor-specific cytolytic responses to autologous, tumor-loaded dendritic cells. Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):13009–13. doi: 10.1073/pnas.202491499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kroger N, Shimoni A, Zagrivnaja M, Ayuk F, Lioznov M, Schieder H, et al. Low-dose thalidomide and donor lymphocyte infusion as adoptive immunotherapy after allogeneic stem cell transplantation in patients with multiple myeloma. Blood. 2004 Nov 15;104(10):3361–3. doi: 10.1182/blood-2004-05-2031. [DOI] [PubMed] [Google Scholar]
- 45.Wen YJ, Min R, Tricot G, Barlogie B, Yi Q. Tumor lysate-specific cytotoxic T lymphocytes in multiple myeloma: promising effector cells for immunotherapy. Blood. 2002 May 1;99(9):3280–5. doi: 10.1182/blood.v99.9.3280. [DOI] [PubMed] [Google Scholar]
- 46.Costa FF, Le Blanc K, Brodin B. Concise review: cancer/testis antigens, stem cells, and cancer. Stem Cells. 2007 Mar;25(3):707–11. doi: 10.1634/stemcells.2006-0469. [DOI] [PubMed] [Google Scholar]
- 47.Cronwright G, Le Blanc K, Gotherstrom C, Darcy P, Ehnman M, Brodin B. Cancer/testis antigen expression in human mesenchymal stem cells: down-regulation of SSX impairs cell migration and matrix metalloproteinase 2 expression. Cancer research. 2005 Mar 15;65(6):2207–15. doi: 10.1158/0008-5472.CAN-04-1882. [DOI] [PubMed] [Google Scholar]


