Abstract
Hepatic ischemia/reperfusion injury (IRI), an inevitable antigen-independent inflammation response in cadaveric liver transplantation, correlates with poor early graft function, rejection episodes, and contributes to donor organ shortage. Sirtuin 1 (SIRT1) is a histone deacetylase that may regulate inflammatory cell activity and manage liver function in IRI, though its functional role and clinical relevance remains to be elucidated. We investigated the efficacy of SIRT1 activation in a murine liver IRI model and verified the concept of putative SIRT1-mediated hepatoprotection in clinical liver transplantation. In the experimental arm, mice were subjected to 90 minutes of liver partial warm ischemia followed by 6 hours of reperfusion with or without adjunctive SIRT1 activation in vivo (resveratrol [Res]). In parallel, bone marrow–derived macrophage (BMDM) or spleen lymphocyte cultures were treated with Res. In the clinical arm, liver biopsies from 21 adult primary liver transplant patients (2 hours after reperfusion) were divided into “low” (n = 11) versus “high” (n = 10) SIRT1 expression groups, assessed by Western blots. Treatment with Res attenuated murine liver IRI while up-regulating SIRT1, suppressing leukocyte infiltration, and decreasing proinflammatory cytokine programs. SIRT1 silencing (small interfering RNA) in BMDM cultures enhanced inflammatory cytokine programs, whereas addition of Res decreased proinflammatory response in a SIRT1-dependent manner. In addition, Res decreased interferon γ production in liver-infiltrating and spleen lymphocyte cultures. Human liver transplants with high SIRT1 levels showed improved hepatocellular function and superior survival (P = 0.04), accompanied by lower proinflammatory cytokine profile. In conclusion, our translational study is the first to identify SIRT1 as a regulator of hepatocellular function in human liver transplant recipients under ischemia/reperfusion stress. By targeting innate and adaptive immune activation, manipulation of SIRT1 signaling should be considered as a novel means to combat inflammation in liver transplantation.
Liver transplantation has become the standard care for patients with end-stage liver disease and those with hepatic malignancies, whereas organ shortage remains the biggest challenge. Liver ischemia/reperfusion injury (IRI), an inevitable event during graft procurement, preservation, and reperfusion, correlates with poor early graft function and rejection crises. Thus, targeting hepatic IRI is necessary to improve clinical outcomes and expand the organ donor pool. However, despite its importance, mechanisms that account for liver IRI are only partially understood and optimal protective strategies have not yet been established.(1)
Liver IRI represents an exogenous antigen-independent inflammatory process, which includes proinflammatory cytokine release and hepatocyte death. Macrophages play a critical role in this inflammatory innate immune cascade triggered by toll-like receptor 4 signaling.(2) Because T-lymphocytes also mediate ischemia/reperfusion (IR)–liver inflammation,(3) regulating macrophage and T-lymphocyte activation interface is a critical step in developing novel therapeutic means against IRI in transplant recipients.(3)
Sirtuin 1 (SIRT1) is a member of the class III histone/protein deacetylase involved in cellular senescence, inflammation, and stress resistance.(4) Previous studies have shown that SIRT1 induction not only suppressed macrophage activation(5) but also inhibited CD4+ T cell T helper 1 (Th1) cytokine production,(6) suggesting SIRT1 activation as a promising strategy to protect the liver against IR stress. Although carbon monoxide inhalation(7) or calorie starvation(8) protect mice against liver IR insult while increasing SIRT1 levels, the mechanistic insights and clinical relevance of these findings remains debatable. Recently, pharmacological SIRT1 activation (2-ME2,(9) SIRT1720(10,11)) or adenoviral SIRT1 overexpression(11) was shown to mitigate liver IRI. No studies on human hepatic SIRT1 expression or liver transplant clinical outcomes were reported to date.
Resveratrol (Res; 3,4,5-trihydroxystilbene) is a natural polyphenolic phytoalexin present in plant species, including grape and peanut.(12) While screening for small molecule SIRT1 activators, Howitz et al. identified 21 different SIRT1-activating molecules, with Res being the most potent compound among them.(13) Previous preclinical studies have shown Res-mediated beneficial biological effects, including life-span extension, insulin-resistance, cancer-prevention, and anti-inflammatory functions.(14,15) On the basis of a plethora of clinical trials, it is becoming evident that Res may exert health benefits in humans, including cardiovascular protection.(16) Because Res mediates the majority of key target molecules by SIRT1-depending manner, including adenosine monophosphate–activated protein kinase and peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC-1α),(17) SIRT1 activation is considered a key feature of Res treatment benefits,(18) while some SIRT1-independent mechanisms are also reported.(17) Despite previous observational studies on protective effects of Res treatment in rat liver IRI models,(19,20) the function of SIRT1 in Res-mediated liver protection remains to be elucidated.
Our present in vivo and in vitro results from well-defined mouse models confirm protective effects of Res against liver IRI, accompanied by SIRT1 up-regulation and macrophage/T-lymphocyte inhibition. Concomitant evaluation of postreperfusion human liver transplant biopsies (bxs) demonstrated, for the first time, that increased SIRT1 expression associated with a depressed proinflammatory cytokine program, improved hepatocellular function and superior posttransplant survival. Our findings validate a novel concept of SIRT1 activation in clinical liver transplantation.
Materials and Methods
CLINICAL LIVER TRANSPLANT STUDY/SAMPLE COLLECTION
Twenty-one adult primary orthotopic liver transplantation (OLT) recipients were recruited under institutional review board protocol (13-000143) between May 10, 2013 and April 6, 2015. Patients provided informed consent prior to their participation in the study. The demographic data and clinical parameters of recipients and donors are shown in Supporting Table 1. Routine standard of care and immunosuppressive therapy were administered, as specified by University of California, Los Angeles (UCLA) liver transplant protocols. Study data were collected and managed using REDCap electronic data capture tools hosted at UCLA. All donor organs, procured from donation after brain death with standardized techniques, were perfused with and stored in cold University of Wisconsin solution. Cold ischemia time was defined as the time from the perfusion of the donor with preservation solution to the removal of the liver from cold storage. Recipient blood was collected prior to the transplant and at postoperative day (POD) 1–14. Liver function was evaluated with standard of care tests, including serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Protocol Tru-Cut needle bxs were obtained intraoperatively from the left lobe approximately 2 hours after portal reperfusion (prior to surgical closing of abdomen) and snap-frozen. Early allograft dysfunction (EAD) was defined by the presence of 1 or more of the following: total bilirubin ≥ 10 mg/dL (171 μmol/L) or international normalized ratio ≥ 1.6 on day 7, and ALT/AST > 2000 IU/L within the first 7 PODs.(21)
ANIMALS
Male wild-type mice (C57BL/6; 6–8 weeks of age), purchased from The Jackson Laboratory (Bar Harbor, ME), were housed in the UCLA facility under specific pathogen-free conditions. All animals received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 86-23, revised 1985).
REAGENTS
Res, lipopolysaccharide (LPS), and concanavalin A (ConA) were purchased (Sigma Aldrich, St. Louis, MO). Gene-specific small interfering RNA (siRNA) against SIRT1 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse macrophage cell line RAW 264.7 (RAW cell) was from the American Type Culture Collection (Manassas, VA).
MOUSE LIVER IRI MODEL
We used an established mouse model of partial hepatic warm IRI.(22,23) Briefly, animals were anesthetized, injected with heparin (100 U/kg), and an atraumatic clip was used to interrupt artery/portal venous blood supply to the left/middle liver lobes. After 90 minutes of ischemia, the clamp was removed and mice were killed at 6 hours of reperfusion. Res (25 mg/kg) or vehicle (VHC; 15% ethanol) was administrated intraperitoneally (IP) at 1 hour prior to ischemia. Sham-operated mice underwent the same procedure without vascular occlusion.
STATISTICS
Statistical tests were described in each figure/table legend. Survival time was defined from the date of transplantation until the date of death. The cumulative survival was analyzed by Kaplan-Meier method. The cutoff value of human graft SIRT1 expression was determined using receiver operating characteristics (ROC) curve and Youden index on the basis of best accuracy in relation to an outcome (recipient death). P < 0.05 was considered statistically significant. JMP for Windows 8.0 (SAS Institute, Cary, NC) was used for statistical analyses (see Supporting Materials for additional methods).
Results
RES AMELIORATES LIVER IRI
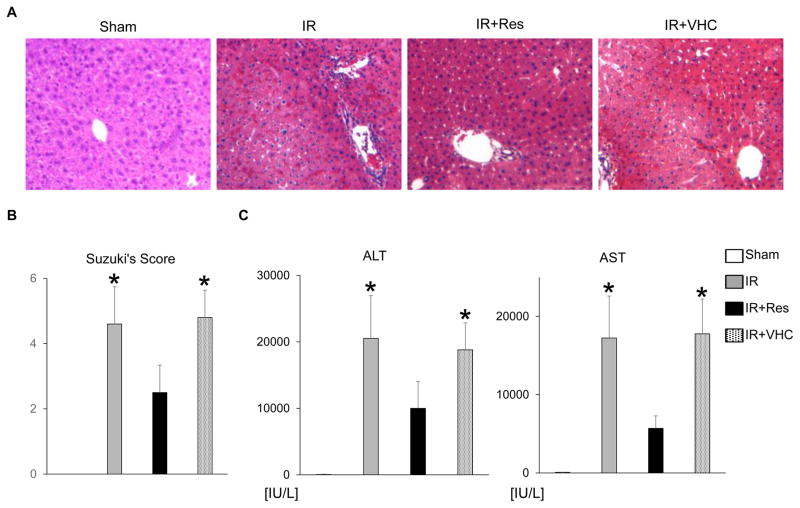
We first examined whether pretreatment with Res (25 mg/kg IP 1 hour) may affect IRI in a murine model of partial liver warm ischemia (90 minutes). The hematoxylin-eosin staining at 6 hours of reperfusion showed well-preserved hepatic architecture (minimal sinusoidal congestion, lobular edema, vacuolization, or necrosis) in the IR+Res group as compared with livers in IR or IR+VHC–treated controls (Fig. 1A). These data correlated with the Suzuki histological grading of the severity of liver IRI (IR+Res = 2.5 ± 0.8 versus IR = 4.6 ± 1.1 or IR+VHC = 4.8 ± 0.8; n = 5–6/group; P < 0.05; Fig. 1B). Treatment with Res improved the hepatocellular function, assessed by serum ALT and AST levels (ALT—IR+Res = 10,000 ± 4040 versus IR = 20,500 ± 6430 or IR+VHC = 18,800 ± 4050 IU/L; AST—IR+Res = 5700 ± 1590 versus IR = 17,200 ± 5370 or IR+VHC = 17,800 ± 4430 IU/L; n = 5–6/group; P < 0.05; Fig. 1C). Thus, Res pretreatment protected mouse livers against IR stress.
FIG. 1.
Res attenuates hepatic IRI. Groups of mice (C57/BL6) were subjected to 90 minutes of partial liver warm ischemia followed by 6 hours of reperfusion. Some animals were pretreated with Res (25 mg/kg IP) or VHC (15% ethanol) at 1 hour of the ischemia insult. (A) Representative liver histology (original magnification, ×100). (B) Suzuki’s histological grading of liver IRI. (C) Hepatocellular function evaluated by serum ALT and AST levels (IU/L); n = 5–6/group; data are presented as mean ± SD. *P < 0.05 versus IR+Res (1-way ANOVA).
RES ATTENUATES THE HEPATOCELLULAR DEATH AND INFLAMMATORY CELL TRAFFICKING
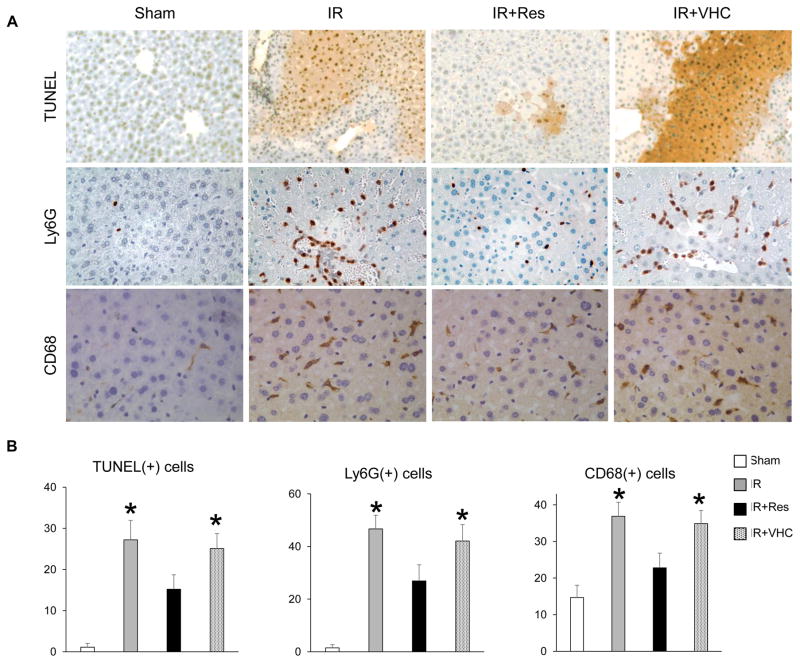
As shown in Fig. 2A,B, the immunohistochemistry evaluation revealed a lower frequency of terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL)+ cells in the IR+Res treatment group as compared with IR or IR+VHC controls (IR+Res = 15.2 ± 3.5 versus IR = 27.2 ± 4.7 or IR+VHC =25.1 ±3.6/high-power field [HPF]; n =4/group; P < 0.05). Depressed numbers of neutrophils (lymphocyte antigen 6 complex locus G [Ly6-G]) and macrophages (CD68) were detected in Res-conditioned IR-stressed livers (Ly6-G—IR+Res = 26.9 ± 6.1 versus IR = 46.7 ± 5.2 or IR+VHC = 42.1 ± 6.2/HPF; CD68—IR+Res = 22.8 ± 4.0 versus IR = 36.9 ± 3.8 or IR +VHC = 34.9 ± 3.6/HPF; n = 4/group; P < 0.05). Thus, Res treatment ameliorated hepatocellular death and mitigated inflammatory leukocyte homing in IR-stressed livers.
FIG. 2.
Res mitigates apoptosis/inflammatory cell trafficking in IR-stressed liver. (A) Representative immunohistochemical staining of TUNEL (apoptosis), Ly6-G (neutrophil), and CD68 (macrophage; original magnification, ×400). (B) Quantification of positive cells/HPF; n = 4/group; data are presented as mean ± SD. *P < 0.05 versus IR+Res (1-way ANOVA).
RES UP-REGULATES SIRT1 AND SUPPRESSES PROINFLAMMATORY CYTOKINES IN LIVER IRI
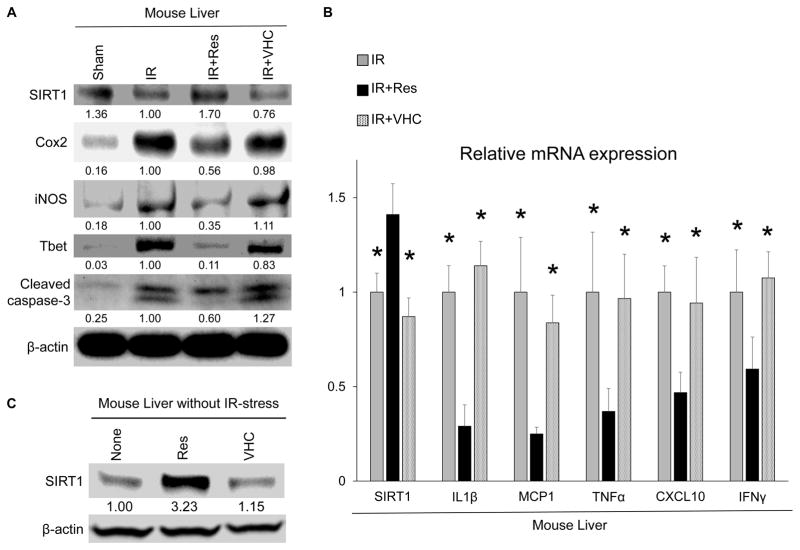
Western blot analysis (Fig. 3A) showed that pretreatment with Res, but not VHC, increased hepatic SIRT1 protein expression, while decreasing leukocyte activation markers (cyclooxygenase 2 [COX-2], inducible nitric oxide synthase [iNOS]). Real-time polymerase chain reaction (RT-PCR) analysis demonstrated that Res, but not VHC, suppressed messenger RNA (mRNA) levels coding for interleukin (IL) 1β, monocyte chemoattractant protein 1 (MCP1), tumor necrosis factor α (TNF-α), and chemokine (C-X-C motif) ligand 10 (CXCL10) with increased level of SIRT1 mRNA (n = 4/group; P < 0.05; Fig. 3B). In parallel, Res decreased T-box transcription factor (Tbet) protein (Th1 master transcription factor) and interferon γ (IFNγ) mRNA levels (Fig. 3A,B). Thus, Res treatment suppressed proinflammatory cytokine program and up-regulated SIRT1 expression in IR-stressed livers. Notably, Res increased SIRT1 protein level not only in IR-stressed but also nonstressed naïve mouse liver (Fig. 3C).
FIG. 3.
Res up-regulates SIRT1 and suppresses inflammatory cytokine production in IR-stressed liver. (A) Protein levels of SIRT1, COX-2, iNOS, Tbet, and cleaved caspase 3 were evaluated by Western blotting. The intensity of the bands was quantified with ImageJ and normalized by dividing the target band intensity by that of housekeeping β-actin. The values under the bands represent the relative ratio of normalized intensity as compared with that of IR. Representations of 3 experiments are shown. (B) Quantitative RT-PCR–assisted detection of mRNA coding for SIRT1, IL1β, MCP1, TNF-α, CXCL10, and IFNγ. Expression levels were normalized to HPRT expression, then normalized to expression of IR group (*P < 0.05 versus IR+Res; n = 4; 1-way ANOVA). Data are presented as mean ± SD. (C) Liver samples without IR stress were harvested from wild-type naïve mice 8 hours after treatment with Res (25 mg/kg IP) or VHC. The values under the bands represent the relative ratio of normalized intensity compared with that of untreated mice. Representations of 3 experiments are shown.
SIRT1 INHIBITS MACROPHAGE ACTIVATION IN VITRO
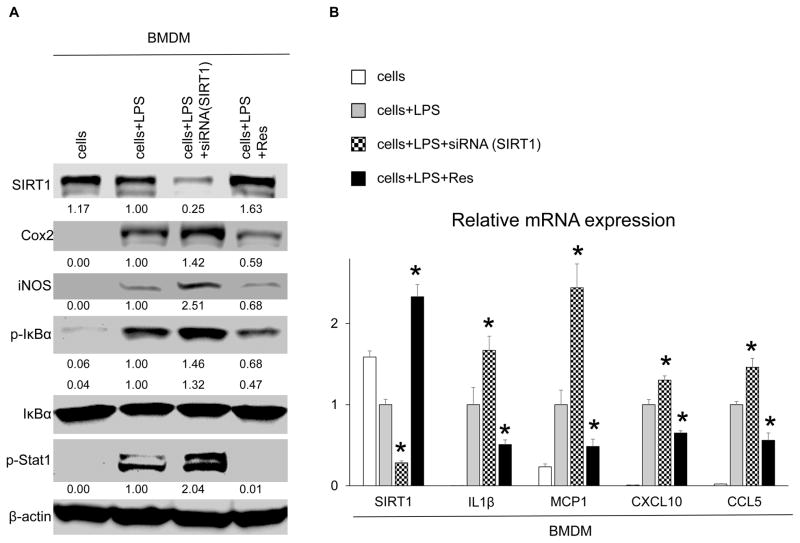
As macrophages play a key role in controlling IR-induced inflammation, we examined LPS-stimulated murine (C57/BL6) bone marrow–derived macrophages (BMDM) cultures. To investigate the function of SIRT1 in macrophage activation, BMDMs were pretreated with siRNA against SIRT1 or Res. As shown in Fig. 4A,B, SIRT1 silencing triggered enhanced expression of macrophage activation markers (COX-2, iNOS, phospho I kappa B alpha [p-IκBα], phospho signal transducer and activator of transcription 1 [p-Stat1]) and increased levels of mRNA coding for IL1β, MCP1, CXCL10, and C-C motif chemokine ligand 5 (CCL5). These data document that SIRT1 depressed macrophage activation in vitro. Meanwhile, Res supplementation increased SIRT1 levels while exhibiting opposite effects to those after SIRT1 silencing, implying that Res inhibited macrophage activation via SIRT1 signaling. We confirmed regulatory effects of Res supplementation in the RAW cell culture system (Supporting Fig. 1).
FIG. 4.
SIRT1 suppresses macrophage activation in vitro. BMDM cultures were pretreated with SIRT1-siRNA or Res (100 μM, 12 hours), and then stimulated with LPS (100 ng/mL, 6 hours). (A) Western blot–assisted detection of SIRT1, COX-2, iNOS, p-IκBα, IκBα, and p-Stat1. The intensity of the bands was normalized by dividing the target band intensity by that of β-actin. The values under the bands represent the relative ratio of normalized intensity compared with that of cells+LPS. Representatives of at least 3 experiments are shown. (B) Quantitative RT-PCR–assisted detection of mRNA coding for SIRT1, IL1β, MCP1, CXCL10, and CCL5. Expression levels were normalized to HPRT expression, then normalized to expression of cells+LPS (*P < 0.05 versus cells+LPS; n = 4; 1-way ANOVA). Data are presented as mean ± SD.
RES DEPRESSES LIVER INFILTRATION BY IFNγ+T CELLS
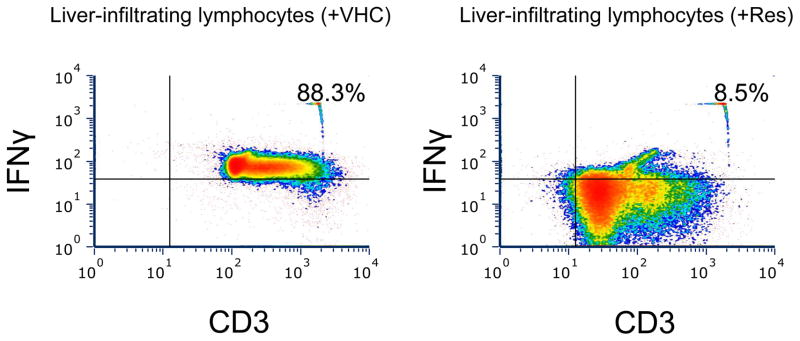
To analyze regulatory effects of Res on IFNγ production in vivo, we assessed the frequency of liver-infiltrating IFNγ+ T-lymphocytes by fluorescence-activated cell sorting (FACS). As shown in Fig. 5, Res treatment abolished liver infiltration by IFNγ+ T cells (CD3+) as compared with VHC-treated controls (Res, 8.5% versus VHC, 88.3%).
FIG. 5.
Res decreases the number of liver-infiltrating IFNγ+ T cells. Groups of mice (C57/BL6) were injected with Res (25 mg/kg IP) or VHC (15% ethanol). Eight hours later, lymphocytes isolated from liver samples were stained with anti-CD3 Ab and anti-IFNγ Ab and analyzed by FACS. Representations of 2 experiments are shown.
RES SUPPRESSES Th1 CYTOKINE PRODUCTION IN VITRO
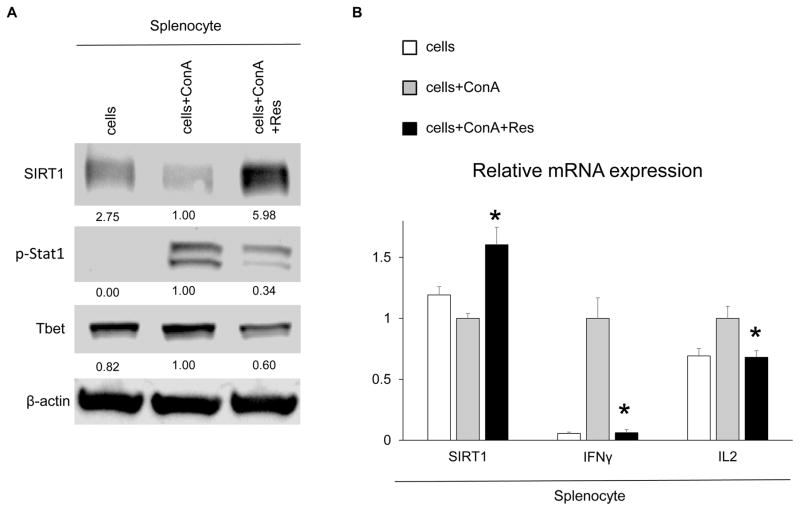
We employed Con A-activated spleen lymphocyte cultures to analyze how Res may affect Th1 cytokine program in vitro. As shown in Fig. 6A,B, addition of Res increased SIRT1 expression and suppressed master transcription regulators (p-Stat1 and Tbet) along with a Th1-type cytokine program (IFNγ and IL2), indicating that Res inhibited Th1 lymphocyte activation and cytokine production.
FIG. 6.
Res increases SIRT1 and mitigates Th1 cytokine production in vitro. Spleen lymphocytes were stimulated with ConA (5 μg/mL) with or without Res (100 μM, 12 hours). (A) Western blot–assisted detection of SIRT1, p-Stat1, and Tbet. The intensity of the bands was normalized by dividing the target band intensity by that of β-actin. The values under the bands represent the relative ratio of normalized intensity compared with that of cells+ConA. Representations of 3 experiments are shown. (B) Quantitative RT-PCR–assisted detection of mRNA coding for SIRT1, IFNγ, and IL2. Expression levels were normalized to HPRT and normalized to the expression of cells+ConA (*P < 0.05 versus cells+ConA; n = 4; 1-way ANOVA). Data are presented as mean ± SD.
SIRT1 POSTREPERFUSION LEVELS IN HUMAN LIVER TRANSPLANTATION
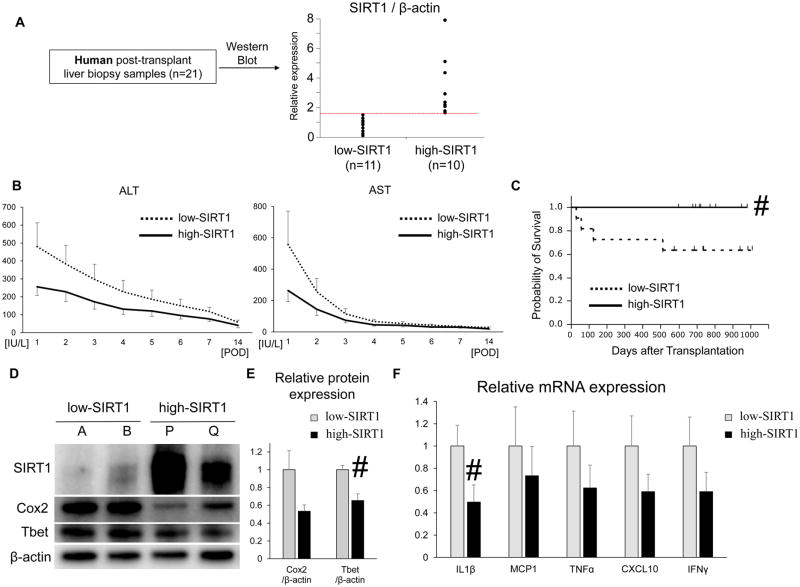
We assessed putative regulatory function of SIRT1 signaling in human liver transplants. Liver bxs were obtained intraoperatively (ca. 2 hours after reperfusion) in 21 patients. Posttransplant relative SIRT1 expression was measured by Western blots. According to the ROC curve and Youden index, the graft SIRT1 cutoff value of 1.53 was determined to be optimal (area under the curve = 0.79), and based on the cutoff value, liver bxs were divided into “low” versus “high” SIRT1 expression groups (n = 11 and 10, respectively, Fig. 7A,D). There was no correlation between SIRT1 levels and donor/recipient background, including cold ischemia time, Model for End-Stage Liver Disease score, age, sex, or body mass index (BMI; Supporting Table 1). Postreperfusion high-SIRT1 expression levels correlated with lower ALT/AST levels as compared with low-SIRT1 expression in human OLT, although values did not reach statistical significance (Fig. 7B). To assess whether local SIRT1 levels may correlate with short-term and longterm outcomes, we analyzed EAD and cumulative posttransplant survival rates. The median follow-up period of 21 patients was 712 days (range, 27–1009 days). None of those individuals underwent secondary liver transplant. Among 21 recipients, 27.3% of the low-SIRT1 bx recipients were diagnosed as EAD, whereas none of the high-SIRT1 bx recipients were within the criterion (P = 0.12). Noteworthy, cumulative OLT survival with high-SIRT1 bx levels was significantly (P = 0.04) longer as compared with the low-SIRT1 bx recipient group (Fig. 7C). Moreover, high-SIRT1 bx demonstrated lower levels of COX-2 and Tbet transcripts (Western blot, Fig. 7D,E) and proinflammatory cytokines (IL1β, MCP1, TNF-α, CXCL10, and IFNγ; RT-PCR, Fig. 7F). Hence, postreperfusion SIRT1 expression levels in human OLT bx correlated with inflammatory response, liver function, and clinical outcomes.
FIG. 7.
Posttransplant hepatic SIRT1 levels and OLT patient outcomes. (A) Twenty-one posttransplant human liver bxs were divided, based on the relative SIRT1 expression level in OLT, ie, “low” (n = 11) versus “high” (n = 10), as measured by Western blots. (B) Serum ALT and AST levels at POD 1–14. The solid line indicates the high SIRT1 patient groups while the dotted line indicates the low SIRT1 patient group. (C) The cumulative probability of survival after liver transplantation, analyzed by Kaplan-Meier method. Solid line indicates high-SIRT1, and dotted line indicates low-SIRT1 patient groups. #P < 0.05 versus low-SIRT1 bx group (log-rank test). (D) Representative Western blot–assisted expression of SIRT1, COX-2, Tbet, and β-actin in bx samples (A/B: low-SIRT1; P/Q: high-SIRT1). (E) Western blot–assisted protein quantification of COX-2 and Tbet. Relative expressions were calculated by dividing the target band intensity by that of β-actin, then normalized to expression of low-SIRT1 bx. Mean ± SEM are shown. #P < 0.05 low (n = 11) versus high (n = 10) SIRT1 bx group (Mann-Whitney U test). (F) Quantitative RT-PCR–assisted detection of mRNA coding for IL1β, MCP1, TNF-α, CXCL10, and IFNγ. Expression levels were first normalized to GAPDH and then normalized to the expression of low-SIRT1 bx group. Mean ± SEM are shown. #P < 0.05 low (n = 11) versus high (n = 10) SIRT1 bx group (Mann-Whitney U test).
Discussion
The present study is the first to link postreperfusion SIRT1 expression in human liver transplants with clinical outcomes, including hepatocellular function, proinflammatory cytokine pattern, and patients’ survival. By integrating liver transplant clinical data with results from murine liver IRI and in vitro cell culture systems, our findings provide a rationale for further investigating SIRT1 activation in liver transplantation. Indeed, high-SIRT1 postreperfusion levels correlated with depressed proinflammatory response (COX-2/IL1β/TNF-α/CXCL10 and Tbet/IFNγ), preservation of liver function (ALT/AST), and improved patient survival.
Because inflammation is a key feature in the mechanism of liver IRI,(1,3) the current study focused on the anti-inflammatory role of SIRT1. However, SIRT1 is also known as an antiapoptotic mediator, consistent with a recent report by Yan et al. where SIRT1 silencing increased susceptibility of HepG2 cells to hypoxia/reoxygeneration-induced apoptosis.(24) In our study, treatment of mice with Res or increased SIRT1 expression in human liver grafts correlated with diminished hepatocellular damage, evidenced by serum enzyme levels (Figs. 1C and 7B, respectively), and depressed frequency of TUNEL+ cells in IR-stressed livers (Fig. 2A,B). Thus, SIRT1-mediated hepatoprotection results not only from anti-inflammatory effects but also from its direct antiapoptotic function. Cui et al. reported that hepatocyte-specific SIRT1 knockout mice exhibited enhanced nuclear factor kappa B (NF-κB) activity and resistance to apoptosis in an endotoxemic liver injury model.(25) As hepatocyte SIRT1 function against apoptosis may be disease- and/or microenvironment-dependent, future studies with cell-specific SIRT1 mutant mice are warranted. Despite these shortcomings, however, our study presents evidence that global SIRT1 activation was accompanied by reduced liver IRI.
SIRT1 is often referred to as a “longevity gene.” Endogenous SIRT1 in human vascular smooth muscle cells is lost with age,(26) whereas SIRT1 expression in mouse livers declined with age at both transcriptional and translational levels.(27) Meanwhile, SIRT1 also relates functionally to human metabolic disorders. Indeed, Nascimento et al. reported that SIRT1 mRNA levels were lower in adipose tissue of obese patients compared with normal-weight controls.(28) In line with these data, we had expected certain correlations between high-SIRT1 graft and younger/lower-BMI donors, though we failed to find significant associations. This may be due to the limited number of patients in our study (n = 21). Although others have identified a single-nucleotide polymorphism that may change SIRT1 protein expression and anti-oxidant function,(29) future trials on putative factors influencing human SIRT1 responses are warranted.
To induce SIRT1, we used Res, a natural nonflavonoid polyphenol, identified originally as a small molecule SIRT1 activator with antioxidant, cardioprotective, and anticancer properties.(16) Being aware of putative SIRT1-independent Res off-target effects,(17) we applied the siRNA silencing approach to confirm that our findings in BMDM cultures were indeed SIRT1 dependent. We focused on SIRT1 signaling in BMDM because of the following:
Circulating monocytes are instrumental in the mechanism of liver IRI.(1,30)
Adoptive transfer of BMDM recreated hepatocellular damage in otherwise IRI-resistant CD11b-DTR mice.(30)
Circulating monocytes generate self-renewing Kupffer cells, the resident macrophages in the liver.(31)
Consistent with the prominent role of necrosis rather than apoptosis in the mechanism of IR-hepatocellular death,(32) pan caspase inhibitors Z-Asp-2,6-dichlorobenzoyloxymethylketone (Z-Asp-cmk)(32) or Z-Val-Ala-Asp(OMe)-fluoromethylketone (Z-VD-fmk)(33) failed to protect livers against IRI. However, necrosis and apoptosis are interdependent phenomena resulting from activation of shared signaling pathways(34) and apoptosis precedes necrosis in the pathogenesis of liver IRI.(32,33) Although some pan caspase inhibitors paradoxically increased necrosis,(35) necroptosis,(36) or autophagic cell death,(37) it is noteworthy that a novel caspase inhibitor IDN-6556 reduced liver IRI in a murine model(38) and offered local therapeutic protection in a phase 2 trial in human liver transplantation.(39) We reported that hepatoprotection after treatment with 7-Cl-O-Nec-1 necrosis inhibitor (Nec-1s) was lost when Kupffer cells were deleted by clodronate, indicating that Nec-1s failed to mitigate liver cell death in an IR-stressed liver.(40) Thus, consistent with beneficial effects of Res, accompanied by decreased cleaved caspase 3 (Fig. 3A), targeting hepatocellular apoptosis is required to ameliorate liver IRI.
SIRT1, a member of the class III histone deacetylase (HDAC) family, may deacetylate not only histone but also nonhistone proteins. Previous studies focused on deacetylation function to nonhistone target proteins, including NF-κB,(25) PGC-1α,(41) and sterol regulatory element binding protein-1c (SREBP1c).(42) Although cytoprotective and organ protective effect of HDAC inhibitors were documented in some animal models,(43,44) little is known of how histone deacetylation may affect an IR-stressed liver. Indeed, butyrate (class I/II HDAC inhibitor) was shown to alleviate rat liver IRI,(45) whereas valproic acid (pan-HDAC inhibitor) failed to protect rat liver against IR stress.(46) Further evaluation of HDAC inhibitors, especially those readily available in daily medical practice, is warranted.
We acknowledge the limitations of our study. First, we are aware Res may produce SIRT1-independent effects, eg, antioxidative function in cultured renal proximal tubular cells(47); autophagy by directly inhibiting mammalian target of rapamycin in human cervical cancer cell line (HeLa cells)(48); or inhibition of insulin-induced mitogen-activated protein kinase/Akt phosphorylations in human embryonic kidney 293 cells.(49) Hence, hepatoprotection after adjunctive Res might result from off-target effects rather than up-regulation of SIRT1 signaling per se. Second, although our murine and human transplant bx data consistently demonstrated correlations between SIRT1 expression and the outcomes, we cannot document the causality, because of the aforementioned off-target effects and IR stress itself decreasing SIRT1 expression in the liver (Fig. 3A). Future studies in SIRT1 mutant animals are needed to confirm our findings. Third, as timing of postoperative blood collection varied between clinical subjects, the evaluation of pretransplant/posttransplant liver function may be not totally accurate. Fourth, with clinical bx samples from a limited number of patients evaluated retrospectively, significant differences between the high-versus low-SIRT1 expression groups were detected in some parameters only (cumulative survival; Tbet/IL1β OLT expression). Although other differences did not reach statistical significance, low-SIRT1 bx samples tended to associate with “older” recipient age and increased intraoperative “blood loss” (Supporting Table 1). As we cannot eliminate the possibility of inferior clinical outcomes influenced by these factors, studies in larger patient cohorts merit further research.
In conclusion, while the experimental arm of our study confirmed the beneficial anti-inflammatory functions of SIRT1 induction in murine IRI, our clinical data are the first to identify SIRT1 as an important regulator of hepatocellular function in human liver transplants under IR stress. By targeting innate (macrophage) and adaptive (T cell) immune activation, SIRT1 manipulation should be considered as a novel therapeutic means to combat inflammation and tissue damage in liver transplantation.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants: R01 DK102110, R01 DK107533, and R01 DK062357-11 (to Jerzy W. Kupiec-Weglinski).
We thank Ko Takanashi at UCLA-TPCL for immunohistochemical assistance. Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility.
Abbreviations
- Ab
antibody
- ALT
alanine aminotransferase
- ANOVA
analysis of variance
- AST
aspartate aminotransferase
- BMDM
bone marrow–derived macrophages
- BMI
body mass index
- bx
biopsy
- CCL5
c-c motif chemokine ligand 5
- ConA
concanavalin A
- COX-2
cyclooxygenase 2
- CXCL10
chemokine (C-X-C motif) ligand 10
- EAD
early allograft dysfunction
- FACS
fluorescence-activated cell sorting
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HDAC
histone deacetylase
- HeLa cells
human cervical cancer cell line
- HPF
high-power field
- HPRT
hypoxanthine-guanine phosphoribosyltransferase
- IFNγ
interferon γ
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IP
intraperitoneally
- IR
ischemia/reperfusion
- IRI
ischemia/reperfusion injury
- LPS
lipopolysaccharide
- Ly6-G
lymphocyte antigen 6 complex locus G
- MCP1
monocyte chemoattractant protein 1
- mRNA
messenger RNA
- Nec-1s
7-Cl-O-Nec-1 necrosis inhibitor
- NF-κB
nuclear factor kappa B
- OLT
orthotopic liver transplantation
- PGC-1α
peroxisome proliferator-activated receptor-gamma coactivator 1α
- p-IκBα
phospho I kappa B alpha
- POD
postoperative day
- p-Stat1
phospho signal transducer and activator of transcription 1
- RAW cell
RAW 264.7
- Res
resveratrol
- ROC
receiver operating characteristics
- RT-PCR
real-time polymerase chain reaction
- SD
standard deviation
- SEM
standard error of the mean
- siRNA
small interfering RNA
- SIRT1
sirtuin 1
- SREBP1c
sterol regulatory element binding protein-1c
- Tbet
T-box transcription factor
- TNF-α
tumor necrosis factor α
- Th1
T helper 1
- TUNEL
terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling
- UCLA
University of California, Los Angeles
- VHC
vehicle
- Z-Asp-cmk
Z-Asp-2,6-dichlorobenzoyloxymethylketone
- Z-VD-fmk
Z-Val-Ala-Asp(OMe)-fluoromethylketone.
Footnotes
View this article online at wileyonlinelibrary.com.
Potential conflict of interest: Nothing to report.
Additional supporting information may be found in the online version of this article.
References
- 1.Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation – from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ke B, Shen XD, Ji H, Kamo N, Gao F, Freitas MC, et al. HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury: regulation of TLR4 innate responses through PI3K/PTEN signaling. J Hepatol. 2012;56:359–366. doi: 10.1016/j.jhep.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchida Y, Ke B, Freitas MC, Yagita H, Akiba H, Busuttil RW, et al. T-cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in mice in a TLR4-dependent manner. Gastroenterology. 2010;139:2195–2206. doi: 10.1053/j.gastro.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Lee SM, Shannon S, Gao B, Chen W, Chen A, et al. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J Clin Invest. 2009;119:3048–3058. doi: 10.1172/JCI38902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HJ, Joe Y, Yu JK, Chen Y, Jeong SO, Mani N, et al. Carbon monoxide protects against hepatic ischemia/reperfusion injury by modulating the miR-34a/SIRT1 pathway. Biochim Biophys Acta. 2015;1852:1550–1559. doi: 10.1016/j.bbadis.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Rickenbacher A, Jang JH, Limani P, Ungethüm U, Lehmann K, Oberkofler CE, et al. Fasting protects liver from ischemic injury through Sirt1-mediated downregulation of circulating HMGB1 in mice. J Hepatol. 2014;61:301–308. doi: 10.1016/j.jhep.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Cho HI, Seo MJ, Lee SM. 2-Methoxyestradiol protects against ischemia/reperfusion injury in alcoholic fatty liver by enhancing sir-tuin 1-mediated autophagy. Biochem Pharmacol. 2017;131:40–51. doi: 10.1016/j.bcp.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Khader A, Yang WL, Godwin A, Prince JM, Nicastro JM, Coppa GF, Wang P. Sirtuin 1 stimulation attenuates ischemic liver injury and enhances mitochondrial recovery and autophagy. Crit Care Med. 2016;44:e651–e663. doi: 10.1097/CCM.0000000000001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biel TG, Lee S, Flores-Toro JA, Dean JW, Go KL, Lee MH, et al. Sirtuin 1 suppresses mitochondrial dysfunction of ischemic mouse livers in a mitofusin 2-dependent manner. Cell Death Differ. 2016;23:279–290. doi: 10.1038/cdd.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Udenigwe CC, Ramprasath VR, Aluko RE, Jones PJ. Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr Rev. 2008;66:445–454. doi: 10.1111/j.1753-4887.2008.00076.x. [DOI] [PubMed] [Google Scholar]
- 13.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 14.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 15.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomé-Carneiro J, Larrosa M, González-Sarrías A, Tomás-Barberán FA, García-Conesa MT, Espín JC. Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr Pharm Des. 2013;19:6064–6093. doi: 10.2174/13816128113199990407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Côté CD, Rasmussen BA, Duca FA, Zadeh-Tahmasebi M, Baur JA, Daljeet M, et al. Resveratrol activates duodenal Sirt1 to reverse insulin resistance in rats through a neuronal network. Nat Med. 2015;21:498–505. doi: 10.1038/nm.3821. [DOI] [PubMed] [Google Scholar]
- 19.Gedik E, Girgin S, Ozturk H, Obay BD, Ozturk H, Buyukbayram H. Resveratrol attenuates oxidative stress and histological alterations induced by liver ischemia/reperfusion in rats. World J Gastroenterol. 2008;14:7101–7106. doi: 10.3748/wjg.14.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassan-Khabbar S, Cottart CH, Wendum D, Vibert F, Clot JP, Savouret JF, et al. Postischemic treatment by trans-resveratrol in rat liver ischemia/reperfusion: a possible strategy in liver surgery. Liver Transpl. 2008;14:451–459. doi: 10.1002/lt.21405. [DOI] [PubMed] [Google Scholar]
- 21.Deschênes M, Belle SH, Krom RA, Zetterman RK, Lake JR. Early allograft dysfunction after liver transplantation: a definition and predictors of outcome. National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database Transplantation. 1998;66:302–310. doi: 10.1097/00007890-199808150-00005. [DOI] [PubMed] [Google Scholar]
- 22.Zhai Y, Shen XD, O’Connell R, Gao F, Lassman C, Busuttil RW, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Shen XD, Yue S, Zhu J, Gao F, Zhai Y, et al. Adoptive transfer of heme oxygenase-1 (HO-1)-modified macrophages rescues the nuclear factor erythroid 2-related factor (Nrf2) antiinflammatory phenotype in liver ischemia/reperfusion injury. Mol Med. 2014;20:448–455. doi: 10.2119/molmed.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan H, Jihong Y, Feng Z, Xiaomei X, Xiaohan Z, Guangzhi W, et al. Sirtuin 1-mediated inhibition of p66shc expression alleviates liver ischemia/reperfusion injury. Crit Care Med. 2014;42:e373–e381. doi: 10.1097/CCM.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 25.Cui X, Chen Q, Dong Z, Xu L, Lu T, Li D, et al. Inactivation of Sirt1 in mouse livers protects against endotoxemic liver injury by acetylating and activating NF-kB. Cell Death Dis. 2016;7:e2403. doi: 10.1038/cddis.2016.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson AM, Wagner R, Rzucidlo EM. Age-related loss of SirT1 expression results in dysregulated human vascular smooth muscle cell function. Am J Physiol Heart Circ Physiol. 2014;307:H533–H541. doi: 10.1152/ajpheart.00871.2013. [DOI] [PubMed] [Google Scholar]
- 27.Gong H, Pang J, Han Y, Dai Y, Dai D, Cai J, Zhang TM. Age-dependent tissue expression patterns of Sirt1 in senescence-accelerated mice. Mol Med Rep. 2014;10:3296–3302. doi: 10.3892/mmr.2014.2648. [DOI] [PubMed] [Google Scholar]
- 28.Nascimento AF, Ip BC, Luvizotto RA, Seitz HK, Wang XD. Aggravation of nonalcoholic steatohepatitis by moderate alcohol consumption is associated with decreased SIRT1 activity in rats. Hepatobiliary Surg Nutr. 2013;2:252–259. doi: 10.3978/j.issn.2304-3881.2013.07.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilic U, Gok O, Bacaksiz A, Izmirli M, Elibol-Can B, Uysal O. SIRT1 gene polymorphisms affect the protein expression in cardiovascular diseases. PloS One. 2014;9:e90428. doi: 10.1371/journal.pone.0090428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji H, Liu Y, Zhang Y, Shen XD, Gao F, Busuttil RW, et al. T-cell immunoglobulin and mucin domain 4 (TIM-4) signaling in innate immune-mediated liver ischemia/reperfusion injury. Hepatology. 2014;60:2052–2064. doi: 10.1002/hep.27334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun. 2016;7:10321. doi: 10.1038/ncomms10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia/reperfusion in rats: apoptosis or necrosis? Hepatology. 2001;33:397–405. doi: 10.1053/jhep.2001.22002. [DOI] [PubMed] [Google Scholar]
- 33.Yang M, Antoine DJ, Weemhoff JL, Jenkins RE, Farhood A, Park BK, Jaeschke H. Biomarkers distinguish apoptotic and necrotic cell death during hepatic ischemia/reperfusion injury in mice. Liver Transpl. 2014;20:1372–1382. doi: 10.1002/lt.23958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43(suppl 1):S31–S44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 35.Lemaire C, Andréau K, Souvannavong V, Adam A. Inhibition of caspase activity induces a switch from apoptosis to necrosis. FEBS Lett. 1998;425:266–270. doi: 10.1016/s0014-5793(98)00252-x. [DOI] [PubMed] [Google Scholar]
- 36.Galluzzi L, Kroemer G. Necroptosis: a specialized pathway of programmed necrosis. Cell. 2008;135:1161–1163. doi: 10.1016/j.cell.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Vandenabeele P, Vanden Berghe T, Festjens N. Caspase inhibitors promote alternative cell death pathways. Sci STKE. 2006;2006:pe44. doi: 10.1126/stke.3582006pe44. [DOI] [PubMed] [Google Scholar]
- 38.Hoglen NC, Anselmo DM, Katori M, Kaldas M, Shen XD, Valentino KL, et al. A caspase inhibitor, IDN-6556, ameliorates early hepatic injury in an ex vivo rat model of warm and cold ischemia. Liver Transpl. 2007;13:361–366. doi: 10.1002/lt.21016. [DOI] [PubMed] [Google Scholar]
- 39.Baskin-Bey ES, Washburn K, Feng S, Oltersdorf T, Shapiro D, Huyghe M, et al. Clinical trial of the pan-caspase inhibitor, IDN-6556, in human liver preservation injury. Am J Transplant. 2007;7:218–225. doi: 10.1111/j.1600-6143.2006.01595.x. [DOI] [PubMed] [Google Scholar]
- 40.Yue S, Zhou H, Wang X, Busuttil RW, Kupiec-Weglinski JW, Zhai Y. Prolonged ischemia triggers necrotic depletion of tissue-resident macrophages to facilitate inflammatory immune activation in liver ischemia/reperfusion injury. J Immunol. 2017;198:3588–3595. doi: 10.4049/jimmunol.1601428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 42.Ponugoti B, Kim DH, Xiao Z, Smith Z, Miao J, Zang M, et al. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem. 2010;285:33,959–33,970. doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cianciolo Cosentino C, Skrypnyk NI, Brilli LL, Chiba T, Novitskaya T, Woods C, et al. Histone deacetylase inhibitor enhances recovery after AKI. J Am Soc Nephrol. 2013;24:943–953. doi: 10.1681/ASN.2012111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levine MH, Wang Z, Bhatti TR, Wang Y, Aufhauser DD, McNeal S, et al. Class-specific histone/protein deacetylase inhibition protects against renal ischemia/reperfusion injury and fibrosis formation. Am J Transplant. 2015;15:965–973. doi: 10.1111/ajt.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu B, Qian J, Wang Q, Wang F, Ma Z, Qiao Y. Butyrate protects rat liver against total hepatic ischemia/reperfusion injury with bowel congestion. PloS One. 2014;9:e106184. doi: 10.1371/journal.pone.0106184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruess DA, Probst M, Marjanovic G, Wittel UA, Hopt UT, Keck T, Bausch D. HDACi valproic acid (VPA) and suberoylanilide hydroxamic acid (SAHA) delay but fail to protect against warm hepatic ischemia/reperfusion injury. PloS One. 2016;11:e0161233. doi: 10.1371/journal.pone.0161233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitada M, Kume S, Imaizumi N, Koya D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes. 2011;60:634–643. doi: 10.2337/db10-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park D, Jeong H, Lee MN, Koh A, Kwon O, Yang YR, et al. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci Rep. 2016;6:21772. doi: 10.1038/srep21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J. Resveratrol inhibits insulin responses in a SirT1-independent pathway. Biochem J. 2006;397:519–527. doi: 10.1042/BJ20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.