Abstract
A survivin-associated radio-adaptive response, characterized by increased radiation resistance or sensitization, was induced by exposure to 5 mGy of ionizing radiation and was correlated to the TP53 mutational status of exposed cells. Ten human cancer lines were investigated: colorectal carcinomas HCT116 and RKO [TP53 wild-type (WT)] and their respective TP53 null isogenic lines; breast adenocarcinomas MCF7 (TP53 WT) and MDA-MB-231 (TP53 Mut); lung carcinomas A549 (TP53 WT) and NCI-H1975 (TP53 Mut); and pancreatic carcinomas Hs766T (TP53 WT) and Panc-1 (TP53 Mut). Radiation induced (5 mGy) changes in the subsequent responses to 2 Gy in a multi-dose paradigm. Effects on radiation sensitivity were associated with changes in survivin’s intracellular translocation to the cytoplasm (TP53 WT) or nucleus (TP53 Mut). Survival responses were determined using a colony forming assay. Intracellular localization of survivin was determined by ELISA and correlated with survival response. Two 2 Gy doses had minimal effects on the intracellular translocation of survivin. When preceded 15 min earlier by a 5 mGy exposure, survivin translocated to the cytoplasm in all of the TP53 WT cell lines, and to the nuclei in the TP53 null and Mut cells. All TP53 WT cells were protected (P < 0.001) by 5 mGy exposures, while Mut cells were sensitized (P < 0.001). HCT116 and RKO TP53 WT cells were admixed with their respective isogenic TP53 null counterparts in different proportions: 75% to 25%, 50% to 50% and 25% to 75%, respectively. All mixed confluent cultures expressed enhanced radio-sensitization (P ≤ 0.047) characteristic of TP53 Mut cells, which could be inhibited by their exposure to the antioxidant N-acetyl-l-cysteine (NAC) indicating a role for intercellular signaling by reactive oxygen species (ROS). ROS signaling in propagating the survivin-mediated response is involved in both intra- and intercellular communication processes.
INTRODUCTION
We previously characterized and described a novel radio-adaptive response in which exposure of cells to a multi-fractionated irradiation protocol involving exposure to a very low radiation dose in the range of 5–100 mGy administered prior to or after irradiation with higher doses in the range of 2–5 Gy can result in an altered radiation response that is expressed as either an increase or decrease in the expected level of cell survival (1–3). This inducible phenomenon was initially characterized and specifically identified as a result of transfection experiments of cells with survivin siRNA and the use of a free radical scavenger N-acetyl-l-cysteine (NAC) as being an intracellular redox driven process mediated by the inhibitor of apoptosis protein (IAP) survivin (1, 3). The clinical significance of this novel radio-adaptive response relates to the routine use of very low dose imaging procedures used routinely to position and monitor the response of tumors undergoing image guided radiotherapy (IGRT) and the potential of altering therapeutic gain and expected outcomes (4). Survivin is a bifunctional protein found primarily in malignant cells that can exhibit different properties depending upon its intracellular location. Cytoplasmic localization of survivin is associated with enhanced cellular resistance to stress-inducing agents and increased survival through its ability to inhibit apoptosis (1). When survivin is localized in the nucleus it is not anti-apoptotic, but rather enhances apoptosis and is associated with regulation of both cellular proliferation and division (5–8). The mechanism underlying survivin’s transport from the nucleus to the cytoplasm has been well characterized and is known to be redox driven and highly regulated. Nuclear export of survivin occurs through the interaction with the redox sensitive chromosome region maintenance 1 nuclear export protein (CRM-1, exportin 1 or XPO1) and the Ras-related nuclear guanosine 5′-triphosphate binding protein (RanGTP) complex (8–10). Translocation of survivin from the cytoplasm into the nucleus does not involve an active transport process requiring a nuclear import protein complex, but rather occurs by simple diffusion (11–13). When located in the nucleus, survivin is susceptible to accelerated proteosomal degradation giving rise to a further loss of its cytoprotective properties (14).
In this study, we investigate the role of TP53 mutational status in the expression of the survivin-associated radio-adaptive response in homogeneous and mixed cultures comprised of both TP53 wild-type (WT) along with different proportions of corresponding isogenic TP53 null (−/−) mutant (Mut) cells. Since the functional loss of wild-type TP53 has been associated in a number of different cell lines with not only reduced expression of survivin (12, 13, 15, 16), but also with downstream ROS production and redox regulation (16–18), we have investigated ten human cancer models differing in TP53 mutational status: two TP53 WT and their isogenic TP53 null counterparts, and three TP53 WT and associated mutant tumor lines representing colon, lung, breast and pancreas. Tumor cells were evaluated under in vitro conditions utilizing a split dose radiation protocol of 2 Gy per fraction separated by 24 h with or without an additional 5 mGy dose administered 15 min prior to each 2 Gy exposure. This radiation protocol has been demonstrated to be required to demonstrate the expression of the survivin-mediated adaptive response as compared to the well characterized manganese superoxide dismutase (SOD2) adaptive response which requires a single very low-dose exposure to ionizing radiation followed at a later time by a much higher therapeutic level dose (1, 19, 20). The split dose irradiation protocol is also relevant to radiation therapy protocols currently in use and has been demonstrated to be propagated with each successive fractionated dose as demonstrated using multi-fractionated radiation exposure protocols of murine tumors exposed under in vivo conditions (3).
MATERIALS AND METHODS
Cells and Culture Conditions
Ten human cancer cell lines were investigated that included colorectal carcinomas TP53 WT HCT116 and RKO and their respective TP53 isogenic null mutant counterparts; breast adenocarcinomas MCF7 (TP53 WT) and MDA-MB-231 (TP53 Mut); lung carcinomas A549 (TP53WT) and NCI-H1975 (TP53 Mut); and pancreatic carcinomas Hs766T (TP53 WT) and Panc-1 (TP53 Mut). With the exception of HCT116 WT and its isogenic TP53 (−/−) Mut, which were supplied by ThermoFisher Scientific, Waltham, MA and given to us by Dr. Michael Spiotto in the Department of Radiation and Cellular Oncology, The University of Chicago, all of the cell lines were obtained from the American Type Culture Collection (ATCC, Manasses, VA). MDA-MB-231 cells have a p53 point mutation at base 839 from a G to A resulting in a protein change in amino acid 280 with a substitution of arginine for lysine (triple negative breast cancer panel, ATCC, Web Link: http://www.atcc.org/SearchCatalogs/LinkIn?Id=HTB-26); NCI-H1975 mutated at base 818 with a substitution of G to A resulting in a protein change at amino acid 248 with a substitution of arginine for leucine (Lung Cancer Panel, ATCC, web link: http://www.atcc.org/SearchCatalogs/LinkIn?Id=CRL-5908); and Panc-1 mutated at bases 815 from T to C and 818 from G to A resulting in protein changes at amino acid 272 of valine for alanine and amino acid 273 of arginine for histidine (Pancreatic Cancer p53 Hotspot Mutation Cell Panel, ATCC). HCT116, HCT116 TP53−/−, RKO, RKO TP53−/− and NCI-H1975 cells were cultured in RPMI1640 medium (ThermoFisher Scientific). MCF7 and MDA-MB-231 cells were cultured in Dulbecco’s modified Eagle medium (DMEM). A549 cells were cultured in F-12K medium (ThermoFisher Scientific), and Hs766T and Panc-1 cells were cultured in DMEM-HG. All cell cultures were supplemented with 10% fetal bovine serum (FBS, Denville Scientific Inc., Metuchen, NJ), penicillin and streptomycin (ThermoFisher Scientific).
For the HCT 116 TP53 WT and isogenic null mixed culture experiments, each cell line was trypsinized (ThermoFisher Scientific), counted and 106 total cells plated in 60-mm tissue culture dishes in the following proportions (75% TP53 WT and 25% TP53−/−, 50% TP53 WT and 50% TP53−/−, 25% TP53 WT and 75% TP53−/−). For the RKO TP53 WT and isogenic null mixed culture experiments, each cell line was trypsinized, counted and 5 × 105 total cells plated in 60-mm tissue culture dishes in the same proportions identified for HCT116 and its TP53 null isogenic line. All cell cultures were maintained at 37°C in a humidified environment containing 5% CO2, and all studies were performed with cells grown to confluence. The potential role of ROS-mediated intercellular communication between TP53 WT and isogenic Mut cells affecting the overall response of the mixed populations were investigated by exposing (50% WT/50% Mut) mixed cultures to 10 mM NAC 30 min prior to each of two 2 Gy doses or 5 mGy followed 15 min later by 2 Gy doses and then washed off immediately after each 2 Gy dose. To assess the possibility of a diffusible substance mediating this response, conditioned medium from TP53 WT HCT116 or RKO were added to their corresponding cultures of isogenic TP53 null cells and vice versa 30 min prior to each radiation exposure. Expression of the survivin-mediated adaptive response reflected by changes in cell survival was measured and contrasted to standard experimental conditions lacking exposure to NAC and conditioned medium.
Irradiation Conditions
Tumor cells were exposed to either two 2 Gy doses separated by 24 h or to 5 mGy doses given 15 min or less prior to each of the 2 Gy doses. All irradiations were performed using a Philips RT250 X-ray generator with a 1 mm copper filter operating at 15 mA and 250 kVp. Dosimetry was performed by a board-certified member of the Medical Physics faculty in the Department of Radiation and Cellular Oncology at The University of Chicago. Dosimetry procedures involved the use of TLD’s placed at various locations in the radiation field to calculate the dose rate under various exposure conditions. For the 2 Gy exposure the dose rate was 1.33 Gy/min and 0.05 Gy/min for 5 mGy exposures. Exposures were administered each day for two consecutive days. Twenty-four hours prior to the first irradiation all of the cell cultures were refed with fresh complete medium.
Cell Survival Assay
Immediately after each treatment protocol, cell survival was determined using an in vitro colony forming assay. Cells were counted, diluted and known numbers seeded into 100-mm tissue culture dishes to allow the development of 100–200 colonies per dish. Colonies were fixed and stained 14–21 days later. Each experiment was repeated three times.
Enzyme-Linked Immunosorbent Assay (ELISA)
Cytoplasmic and nuclear protein was prepared from cells according to a method described in detail elsewhere (21). Cell pellets were suspended in 400 µl ice-cold Buffer A [10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.9; 10 mM potassium chloride (KCl); 0.1 mM ethylenediaminetetraacetic acid (EDTA)] containing 1 mM dithiothreitol (DTT) (Sigma-Aldrich, St. Louis, MO); 0.5 mM phenylmethylsulfonyl fluoride (PMSF) (Sigma-Aldrich); 1 µg/ml leupeptin (Sigma-Aldrich) and 5 µg/ml aprotinin (Sigma-Aldrich) and allowed to swell on ice for 15 min. Fifty microliters of octylphenoxypolyethoxyethanol (IGEPAL CA-630) (Sigma-Aldrich) was added and the suspension vortexed on high for 30 s. Nuclei were pelleted by centrifugation at 25,000g for 1 min at 4°C and the supernatant containing cytoplasmic protein transferred to a 1.5 ml tube. The nuclei were re-suspended in 50 µl ice-cold Buffer B (20 mM HEPES, pH 7.9; 0.4 M NaCl; 1 mM EDTA; 25% glycerol) containing the same protease inhibitors in Buffer A and incubated on ice for 15 min. The nuclei were pelleted by centrifugation at 25,000g for 5 min at 4°C and the supernatant containing nuclear proteins transferred to a clean 1.5 ml tube. Protein was quantified by the Bradford method (22) and adjusted to 4 µg/µl. Survivin protein levels were determined using the Survivin BioAssay ELISA Kit (Human, cat. no. 144046) from USBiological Life Sciences (Salem, MA) according to the manufacturer’s instructions. The averages of duplicate wells for standards and samples were calculated, a standard curve generated, and the concentration of survivin protein in each of the samples determined.
Statistical Analysis
Means and standard errors were calculated for all data points from at least three independent experiments. Pairwise comparisons of survivin protein concentrations and cell survival between each of the experimental conditions were performed using a Student’s two-tailed t test (SigmaPlot software 11.0, SPSS, Chicago, IL).
RESULTS
Effect of 5 mGy Exposures on Human Tumor Cell Lines Differing in TP53 Mutational Status
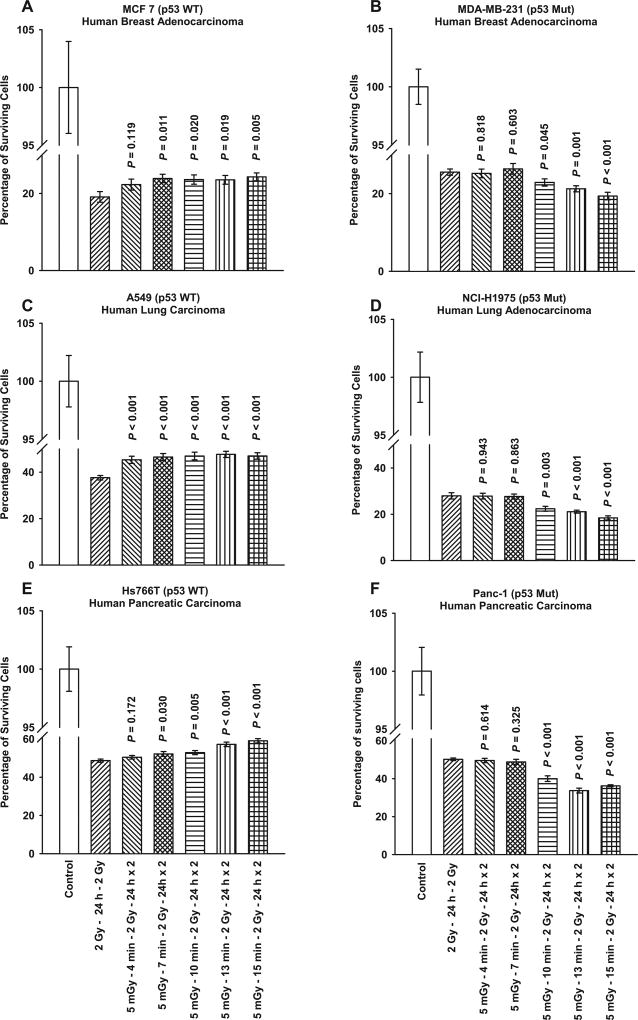
Figure 1 shows the time courses for the development of 5 mGy induced adaptive responses given prior to each of two 2 Gy doses separated by 24 h as a function of TP53 mutational status. Exposure of TP53 WT MCF7 breast (panel A), A549 lung (panel C), and Hs766T pancreatic (panel E) human cancer lines to 5 mGy 15 min prior to 2 Gy exposures induced a significant increase in survival as compared to that observed following exposure to two 2 Gy doses only. In contrast, when TP53 Mut MDA-MB-231 breast (panel B), NCI-H1975 lung (panel D), and Panc-1 pancreatic (panel F) human cancer lines were exposed to 5 mGy prior to the 2 Gy exposures a significant decrease in survival was observed compared to the two 2 Gy dosing protocol.
FIG. 1.
Time course for the development of very low-dose radiation-induced adaptive response in six human tumor cell lines differing in TP53 mutational status. P values were determined by comparing the survival of cells after two 2 Gy doses with those exposed to two 2 Gy doses along with the 5 mGy doses. Exposure to 5 mGy induced a significant elevation in survival in TP53 WT cells: (panel A) MCF7, (panel C) A549 and (panel E) Hs766T, and a significant reduction in survival in TP53 Mut cells: (panel B) MDA-MB-231, (panel D) NCI-H1975 and (panel F) Panc-1. Each experiment was repeated three times and error bars represent the standard error of the mean (SEM).
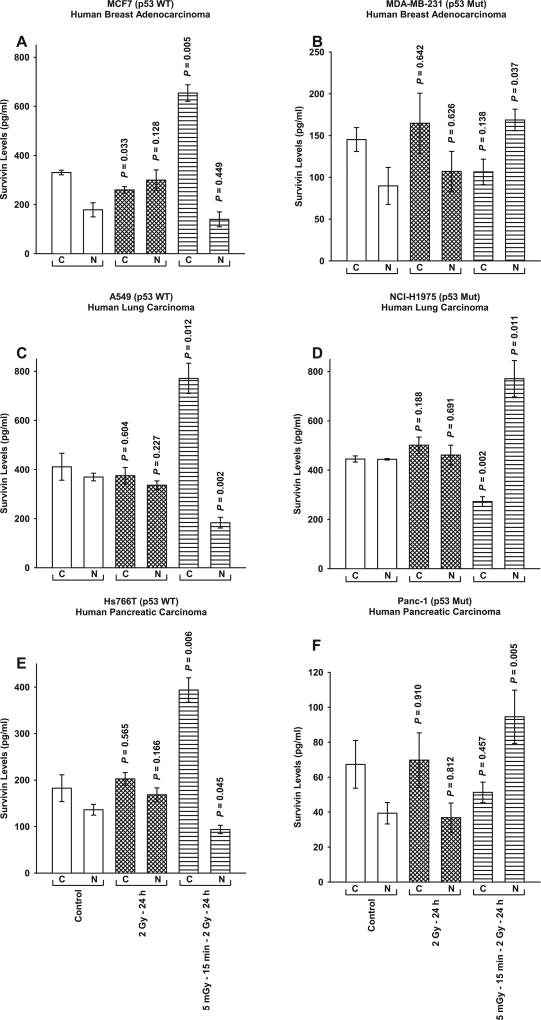
Accompanying the development of enhanced or diminished cell survival after 5 mGy exposures as a function of cellular TP53 mutational status was an associated intracellular redistribution of survivin as measured by ELISA (see Fig. 2). While two 2 Gy doses administered over 24 h had little or no effect on the intracellular localization of survivin, exposure of cells to 5 mGy 15 min prior to each of the two 2 Gy doses resulted in a highly significant (P ≤ 0.01) elevation of cytoplasmic as compared to nuclear survivin in the p53 WT cancer lines (see Fig. 2A, C and E). Conversely, an elevation in nuclear as compared to cytoplasmic survivin (P ≤ 0.04) was observed in each of the TP53 Mut cancer lines (see Fig. 2B, D and F).
FIG. 2.
Survivin protein levels in the cytoplasmic and nuclear fractions measured by ELISA as a function of treatment protocol in the six human tumor cell lines differing in TP53 mutational status. P values were determined by comparing the protein content in the unirradiated control cytoplasmic (C) and nuclear (N) fractions with their corresponding groups from the two radiation protocols: two 2 Gy doses separated by 24 h with or without 5 mGy exposures given 15 min prior. Each experiment was repeated three times and error bars represent the SEM.
Comparison of TP53 WT and Corresponding Isogenic TP53 Null HCT116 and RKO Human Colon Carcinoma Tumor Lines
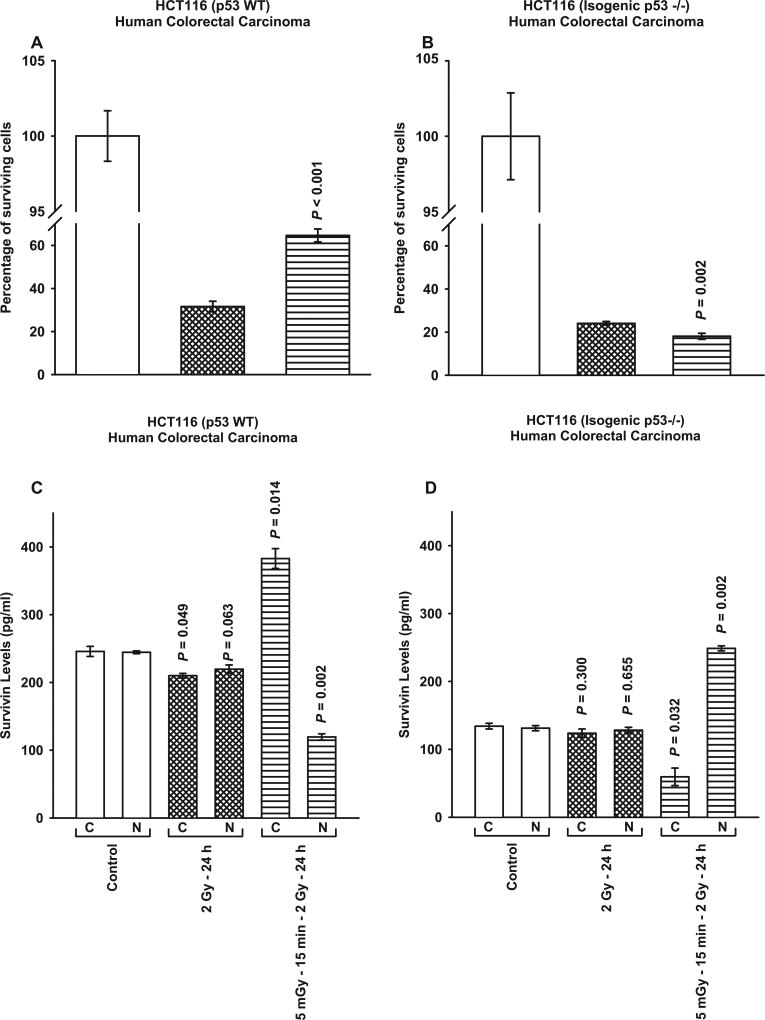
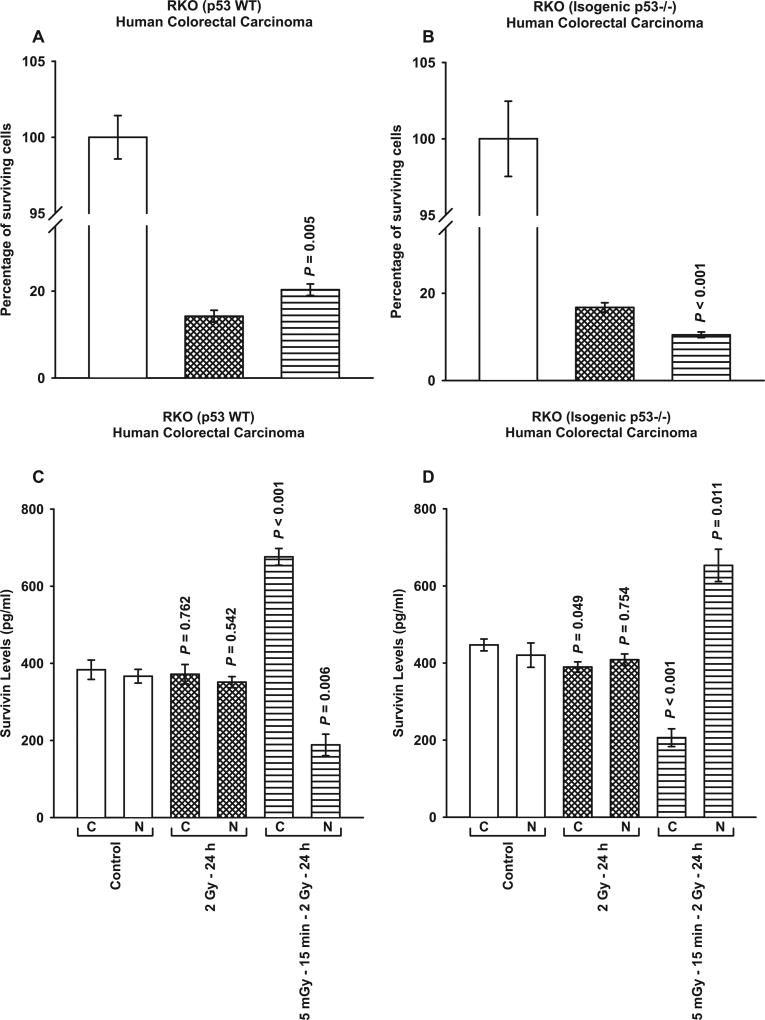
The three TP53 Mut human cancer lines investigated above all carry a point mutation in the DNA binding domain of the TP53 gene. It was therefore of interest to evaluate the survivin-mediated adaptive response in well characterized TP53 WT human tumor lines along with their associated isogenic TP53 null counterparts. Consistent with the data described in Figs. 1 and 2, HCT116 WT cells expressed an elevated cell survival and a corresponding enhanced cytoplasmic translocation and localization of survivin after exposure to 5 mGy doses given 15 min prior to each of two 2 Gy doses (P ≤ 0.002) (see Fig. 3A and C). Its isogenic TP53 null Mut, however, exhibited an increased radio-sensitization and a translocation of survivin to the nucleus (P ≤ 0.002) (see Fig. 3B and D). Similar responses to 5 mGy exposures and the expression of the survivin-mediated adaptive response as a function of TP53 mutational status along with the intracellular translocation of survivin were observed for both RKO TP53 WT and its isogenic TP53 null Mut (see Fig. 4).
FIG. 3.
Comparison of survivin-mediated radio-adaptive survival responses in HCT116 TP53 WT (panel A) and isogenic TP53 null cells (panel B). Cytoplasmic (C) and nuclear (N) survivin levels were determined by ELISA (panels C and D). P values were determined by comparing the survivin levels in each treatment group with its corresponding control. Each experiment was repeated three times and error bars represent SEM.
FIG. 4.
Comparison of survivin-mediated radio-adaptive survival responses in RKO TP53 WT (panel A) and isogenic TP53 null cells (panel B). Cytoplasmic (C) and nuclear (N) survivin levels were determined by ELISA (panels C and D). P values were determined by comparing the survivin levels in each treatment group with its corresponding control. Each experiment was repeated three times and error bars represent SEM.
The Effects of TP53 Genomic Heterogeneity on the Survivin-Mediated Radio-Adaptive Response
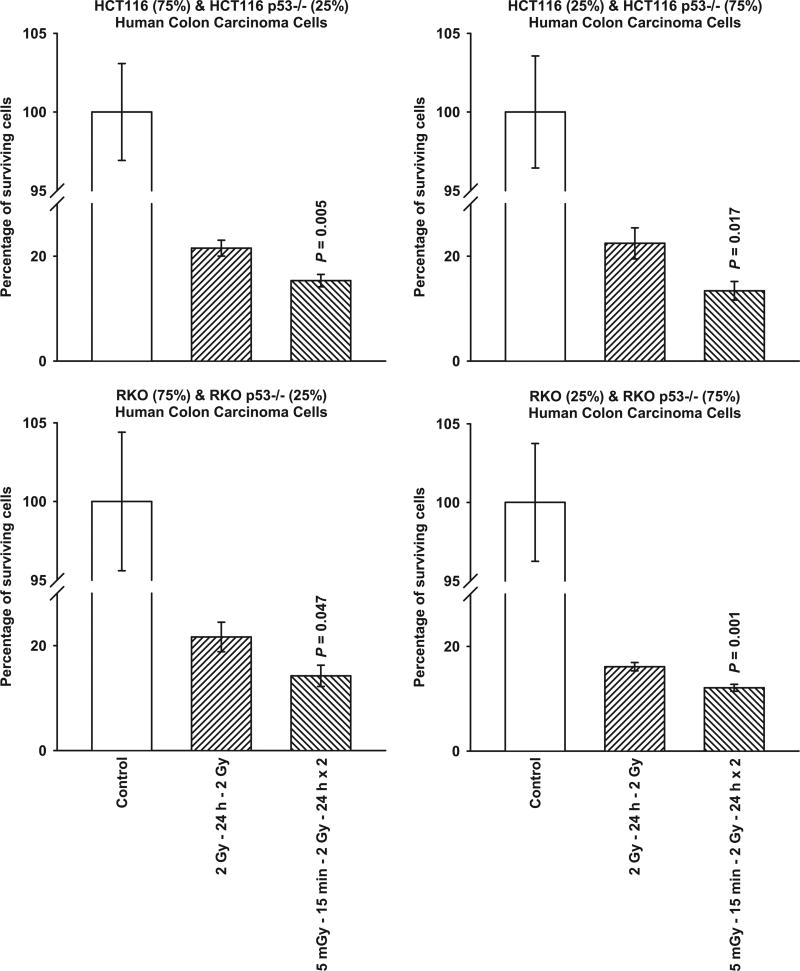
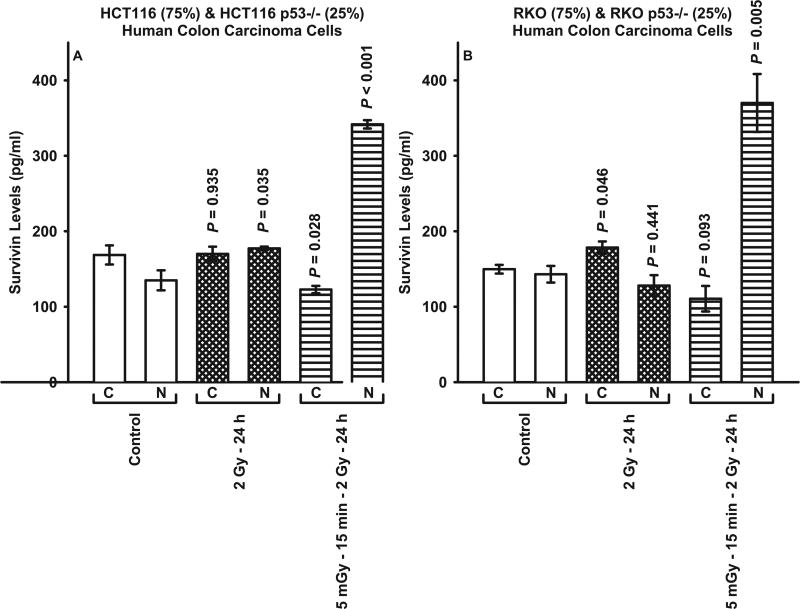
Through the use of mixed cultures, the effects of TP53 genomic heterogeneity on the survivin-mediated radio-adaptive response were investigated. TP53 WT HCT116 and its isogenic TP53 null Mut, and RKO WT with its isogenic TP53 null Mut were admixed in the following proportions: 75%:25% and 25%:75%, respectively. As shown in Fig. 5, regardless of the relative distributions of TP53 WT to TP53 null Mut tested for HCT116 and RKO cells, each of the admixed cultures exhibited a significant (P ≤ 0.05) radio-sensitization response consistent with that expressed by a 100% TP53 null Mut population of cells (see Figs. 3 and 4). Focusing on both the HCT116 and RKO mixed cultures representing 75% TP53 WT and 25% TP53 null Mut, 5 mGy exposures induced an intracellular translocation of survivin to the nucleus consistent with that observed in populations of 100% TP53 null Mut cells (see Fig. 6). A comparison of the cytoplasmic to nuclear survivin levels for each of the human cancer lines evaluated as a function of treatment are shown in Table 1. While the ratios for each of the cell lines evaluated clustered between 0.95 and 1.89 for both the untreated controls and cells exposed to two 2 Gy irradiations, the addition of a 5 mGy exposure 15 min prior to each of the 2 Gy irradiations resulted in ratios ranging from 3.2 to 4.7 for TP53 WT tumor cells indicating a significant cytoplasmic accumulation of survivin consistent with a radio-protective adaptive response. In contrast, in the TP53 Mut and mixed cultures the survivin ratios ranged from 0.24 to 0.63, representative of a nuclear accumulation of survivin and a corresponding survivin-mediated radio-sensitization adaptive response.
FIG. 5.
Comparison of survivin-mediated radio-adaptive survival responses in mixed cultures of HCT116 TP53 WT and isogenic TP53 null cells, and mixed cultures of RKO TP53 WT and isogenic TP53 null cells. Cells were mixed in the following proportions: 75% WT/25% null and 25% WT/75% null. P values were determined by comparing the survival of cells after exposure to two 2 Gy doses with those exposed to two 2 Gy doses along with the 5 mGy doses. Each experiment was repeated three times and error bars represent SEM.
FIG. 6.
Survivin protein levels in the cytoplasmic and nuclear fractions measured by ELISA in mixed cultures of HCT116 WT (75%) and isogenicTP53 null (25%), and RKO WT (75%) and isogenic TP53 null (25%). P values were determined by comparing the protein content in the unirradiated control cytoplasmic (C) and nuclear (N) fractions with their corresponding groups from the two radiation protocols. Each experiment was repeated three times and error bars represent the SEM.
TABLE 1.
Ratio of Cytoplasmic/Nuclear Survivin Levels
| Tumor | TP53 status |
Control | 2 Gy – 24 h |
5 mGy – 15 min −2 Gy – 24 h |
|---|---|---|---|---|
| HCT116 | WT | 1.01 | 0.96 | 3.20 |
| RKO | WT | 1.05 | 1.06 | 3.59 |
| MCF7 | WT | 1.85 | 0.87 | 4.67 |
| A549 | WT | 1.11 | 1.12 | 4.21 |
| Hs766T | WT | 1.34 | 1.20 | 4.20 |
| HCT116 | Isogenic null | 1.02 | 0.96 | 0.24 |
| RKO | Isogenic null | 1.06 | 0.95 | 0.32 |
| MDA-MB-231 | Mut | 1.62 | 1.54 | 0.63 |
| NCI-H1975 | Mut | 1.00 | 1.09 | 0.35 |
| Panc-1 | Mut | 1.71 | 1.89 | 0.54 |
| HCT116 | Mixed culturea | 1.25 | 0.96 | 0.36 |
| RKO | Mixed culturea | 1.05 | 1.39 | 0.30 |
75% TP53 WT cells/25% TP53 isogenic null cells.
Effects of NAC and Conditioned Medium Exposure on the Expression of the Survivin-Mediated Adaptive Response
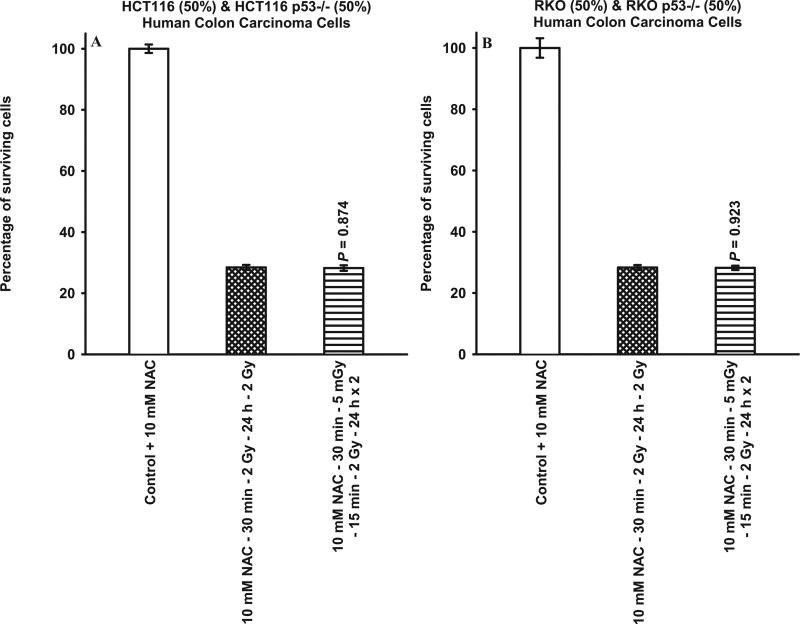
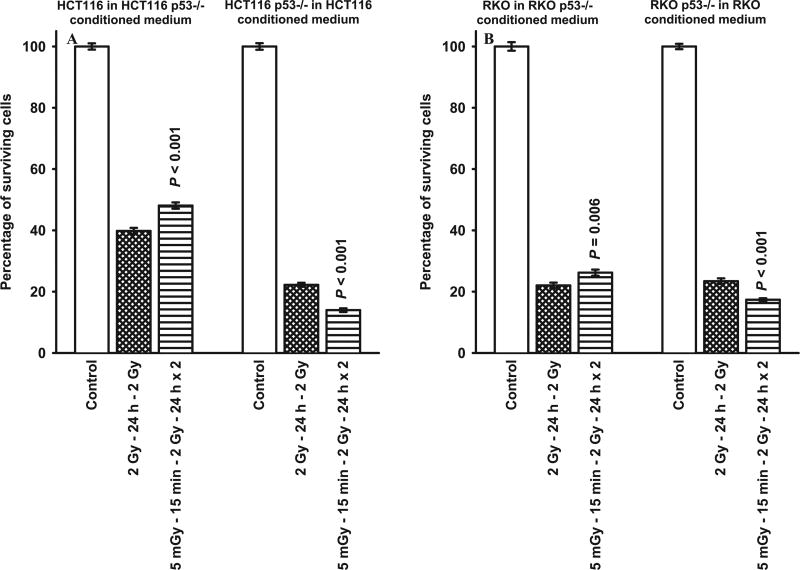
The exposure of (50%:50%) mixed cultures of HCT116 or RKO TP53 WT and null Mut to 10 mM NAC 30 min prior to each set of radiation doses resulted in an inhibition of the collective radio-sensitization expression of the survivin-mediated adaptive response as reflected by the absence in any change of radio-sensitivity induced by the 5 mGy exposures prior to each of the two 2 Gy exposures (see Fig. 7). In contrast, the addition of conditioned medium from TP53 WT cells onto TP53 isogenic null Mut cells or vice versa resulted in no observable effect on the expression of either the protective (TP53 WT) or sensitizing (TP53 Mut) survivin-mediated adaptive response (see Fig. 8).
FIG. 7.
Comparison of survivin-mediated radio-adaptive survival responses in mixed cultures of HCT116 TP53 WT (50%) and isogenic TP53 null (50%) cells, and mixed cultures of RKO TP53 WT (50%) and isogenic TP53 null (50%) cells treated with 10 mM NAC 30 min prior to radiation exposure. P values were determined by comparing the survival of cells after exposure to two 2 Gy doses with those exposed to two 2 Gy doses along with the 5 mGy doses. Each experiment was repeated three times and error bars represent SEM.
FIG. 8.
Comparison of survivin-mediated radio-adaptive survival responses in cultures of HCT116 TP53 WT and RKO TP53 WT cells irradiated in conditioned medium obtained from their corresponding isogenic TP53 null cell cultures, and HCT116 isogenic TP53 null and RKO isogenic TP53 null cells irradiated in conditioned medium obtained from their corresponding TP53 WT cell cultures. P values were determined by comparing the survival of cells after exposure to two 2 Gy doses with those exposed to two 2 Gy doses along with the 5 mGy doses. Each experiment was repeated three times and error bars represent SEM.
DISCUSSION
With the implementation of image guided radiotherapy and the growing dependence on very low dose imaging techniques to position and monitor the daily responses of tumors being treated with ionizing radiation, the potential for the development of very low radiation dose-induced adaptive responses capable of affecting treatment outcomes is becoming a concern. Recently we identified a survivin-associated radio-adaptive response that could be induced by doses ranging from 5 to 100 mGy administered before or after each therapeutic high dose in a multi-dosing protocol (1–3). The unique role of survivin in this phenomenon has been identified and characterized in both human and mouse tumor lines through the use of survivin siRNA transfection which uniformly resulted in complete inhibition of the adaptive response (1–3). Using a two 2 Gy dosing protocol separated by 24 h with or without the addition of very low doses simulating those associated with established imaging techniques, it was possible to induce either a radio-protective or -sensitizing response (2, 3). The two-dose radiation schedule was also found to be predictive for the propagation of these adaptive responses under in vivo conditions employing multiple additional irradiations (3). Survivin, an inhibitor of apoptosis protein, is a bifunctional protein whose effects are dependent upon its intracellular location (5–8). If localized within the cytoplasm and mitochondria, survivin exerts a prosurvival anti-apoptotic effect. In contrast, localization within the nucleus is associated with cell cycle and pro-apoptotic effects. Survivin has an active nuclear export signal in its linker region that associates with the ROS sensitive CRM1 nuclear transport factor, but lacks a nuclear import signal (5–9). Its movement into the nucleus is through simple diffusion. Survivin localized within the nucleus can also undergo proteosomal degradation, thus reducing the potential for expressing a cytoprotective adaptive response (14). This suggests that the underlying factor responsible for the expression of either a protective or sensitizing very low-dose radiation-induced adaptive response is due to the associated induced intracellular translocation of survivin.
Since malignant cells have the potential to express either a protective or sensitizing adaptive response after very low dose irradiation, the identification of a biomarker robust enough to predict which outcome will occur is needed to best exploit this phenomenon for use in clinical care. We recently demonstrated in homogeneous populations of p53 WT SA-NH and p53 Mut FSa murine tumor models that the survivin-mediated adaptive response is a redox driven process that can be inhibited by the free radical scavenger N-acetyl-l-cysteine (3). This inhibition encompassed both the complete loss of very low-dose radiation-induced effects on cell survival after subsequent challenge with higher doses but also the lack of any intracellular translocation of survivin protein between the nuclear and cytoplasmic sub-cellular fractions regardless of p53 mutational status (3). An important effector of intracellular oxidative stress and redox regulation in human cells is the tumor suppressor TP53 (16–18). Transcriptional targets of TP53 include a number of antioxidant genes such as glutathione peroxidase, manganese superoxide dismutase, and aldehyde dehydrogenase 4 (23). Intracellular oxidative stress is relatively lower in TP53 WT as compared to Mut cells (16), and is known to inhibit nuclear protein export suggesting that nuclear export of proteins such as survivin could be perturbed leading to a buildup of nuclear rather than cytoplasmic survivin (9). As described in Figs. 1 and 2, each of the TP53 WT human cancer cell lines expressed a radio-protective survivin-mediated adaptive response that was accompanied by a robust translocation of survivin into the cytoplasm. Conversely, TP53 Mut tumor cell lines when exposed to 5 mGy prior to each 2 Gy dose expressed a radio-sensitization phenotype accompanied by a nuclear accumulation of survivin. It is important to note that the time required for the development of the survivin-mediated adaptive response in these six tumor lines varied slightly, but by 15 min the effect was comparable in magnitude to that observed for all of the human and mouse tumor models evaluated thus far (1–3). This suggests that exploitation of the radio-sensitizing phenomenon will require a minimum of 15 min between exposure to a very low-imaging dose and irradiation with a higher therapeutic dose to allow for the effect to develop (see Fig. 1).
Three of the Mut human cancer lines evaluated in this study carried a mutation in the DNA binding domain of the TP53 gene. To further characterize the potential role of TP53 in this adaptive response process, two well characterized human colon carcinoma cell lines, HCT116 and RKO, along with their respective isogenic TP53 null counterparts were evaluated in the same manner. As demonstrated in Figs. 3 and 4, the mutational status of TP53 had predictive value regarding the expression of the survivin-mediated adaptive response along with the associated intracellular translocation of survivin. To address the issue of genomic heterogeneity regarding TP53 mutational status that can occur in tumors on the expression of an adaptive response, different proportions of HCT116 and RKO TP53 WT and corresponding isogenic null Mut cells were mixed and grown together. Both tumor models, regardless of the ratio of TP53 WT to null Mut, expressed radio-sensitization adaptive responses in magnitudes similar to those observed in populations that contained 100% TP53 null cells (see Fig. 5) demonstrating an intercellular communication process that suppressed the effects of TP53 WT cells allowing for the entire population to express the TP53 Mut associated radio-sensitization response. No growth advantage of TP53 null over their corresponding WT lines has been observed that could account for a skewed distribution resulting in an overpopulation of TP53 null cells (24, 25). The radio-sensitization effect expressed by this mixed population of cells was again associated with a nuclear translocation of survivin (see Fig. 6). These data suggest cell-to-cell signaling is involved in the development of the overall mixed TP53 population response. The ability of the free radical scavenger NAC to inhibit the survivin-mediated adaptive response reported elsewhere (3) indicates a role for ROS-mediated intracellular signaling in this process. This was further confirmed utilizing TP53 WT and isogenic null Mut mixed cultures. Exposure of mixed confluent cultures to NAC also resulted in an inhibition of changes in survival response induced by exposures to 5 mGy (see Fig. 7). Cells expressing WT TP53 exhibit lower oxidative stress than their isogenic TP53 null Mut counterparts (15). This suggests that TP53 null Mut cells in these mixed cultures may serve as sources of relatively high-oxidative stress to dominate ROS signaling to their TP53 WT counterparts resulting in the overall expression of a radio-sensitization adaptive response typical of populations of homogeneous TP53 Mut tumor cells, e.g., an intercellular ROS signaling process (see Figs. 1B, D and F; 3C; and 4C). The application of conditioned medium from TP53 WT to isogenic null Mut cells and vice versa failed to affect their respective expression of the survivin-mediated adaptive response suggesting that at least under the conditions evaluated intercell communication affecting expression of these responses is not due to a diffusible cellular factor but does require cell-to-cell contact (see Fig. 8).
The survivin-mediated radio-adaptive response, either protective in TP53 WT or sensitizing in TP53 Mut tumor cell populations, induced by very low doses of ionizing radiation has the potential to have a significant impact on overall tumor response. Data from both the homogeneous and mixed cultures of wild-type and isogenic null mutant cells demonstrate that these phenomena are under the control of both intra- and intercellular ROS signaling. The potential exists, therefore, for the development of adaptive responses capable of affecting clinical outcomes when imaging procedures are used routinely for tumor positioning and monitoring during the course of treatment. If TP53 Mut tumor cells are present even as a minority subpopulation, it is possible that they may serve as a source of oxidative stress capable of inducing a uniform adaptive response in a heterogeneous population comprised of both WT and Mut tumor cells. The relative TP53 mutational status of cells comprising complex heterogeneous tumors may thus serve as an indicator to predict prior to the initiation of radiation exposure, which of these two-disparate radio-adaptive responses will occur. Elevation or reduction in tumor cell survival of as little as 10% per fraction induced by imaging procedures, for example, can be amplified over a 30 fraction IGRT treatment protocol and significantly impact expected outcomes. The potential for this has been described using transplantable mouse tumor models and a tumor growth delay assay (3). When expanded to a five fraction 5 Gy per fraction daily protocol, the inclusion of 5 mGy doses 15 min prior to each 5 Gy dose resulted in highly significant alterations in the tumor response as compared to treatment protocols absent the administration of 5 mGy exposures (P ≤ 0.001). By including very low-dose exposures at least 15 min prior to the administration of therapeutic doses, the potential exists for the induction of a ROS initiated survivin-mediated radio-sensitization adaptive response in tumors containing at least 25% TP53 Mut cells that could enhance therapeutic outcomes.
Acknowledgments
The authors thank Dr. Michael Spiotto for the gift of the HCT116 TP53 WT and isogenic TP53 null Mut cells. Dr. Spiotto is a faculty member in the Department of Radiation and Cellular Oncology at The University of Chicago. This work was supported in part by the Department of Energy Low Dose Program/Project Grant (DE-SC0001271 to DJG); by the National Institutes of Health grant (R01-CA132998 to DJG); and the Daniel K. Ludwig Foundation for Metastasis Research (to RRW). R. R. Weichselbaum is a consultant to Reflexion, a radiotherapy company.
Footnotes
Disclosure of potential conflicts of interest: D. J. Grdina is a minority equity partner in Pinnacle Oncology LLC regarding the potential novel uses of amifostine. J. S. Murley has minority ownership interest in Pinnacle Oncology LLC.
References
- 1.Grdina DJ, Murley JS, Miller RC, Mauceri HJ, Sutton HG, Li JJ, et al. A survivin-associated adaptive response in radiation therapy. Cancer Res. 2013;73(14):4418–4428. doi: 10.1158/0008-5472.CAN-12-4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grdina DJ, Murley JS, Miller RC, Woloschak GE, Li JJ. NFκB and survivin-mediated radio-adaptive response. Radiat Res. 2015;183:391–407. doi: 10.1667/RR14002.1. [DOI] [PubMed] [Google Scholar]
- 3.Miller RC, Murley JS, Rademaker AW, Woloschak GE, Li JJ, Weichselbaum RR, et al. Very low doses of ionizing radiation and redox associated modifiers affect survivin-associated changes in radiation sensitivity. Free Radic Biol Med. 2016;99:110–119. doi: 10.1016/j.freeradbiomed.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon RG, Ogden K. Optimizing dose in computed tomographic procedures. Tech Vasc Interv Radiol. 2010;13(3):172–175. doi: 10.1053/j.tvir.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Li F, Yang J, Ramnath N, Javle MM, Tan D. Nuclear or cytoplasmic expression of survivin: What is the significance? Int J Cancer. 2005;114(4):509–512. doi: 10.1002/ijc.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stauber RH, Mann W, Knauer SK. Nuclear and cytoplasmic survivin: Molecular mechanism, prognostic, and therapeutic potential. Cancer Res. 2007;67(13):5999–6002. doi: 10.1158/0008-5472.CAN-07-0494. [DOI] [PubMed] [Google Scholar]
- 7.Knauer SK, Mann W, Stauber RH. Survivin’s dual role: An export’s view. Cell Cycle. 2007;6(5):518–521. doi: 10.4161/cc.6.5.3902. [DOI] [PubMed] [Google Scholar]
- 8.Johnson ME, Howerth EW. Survivin: A bifunctional inhibitor of apoptosis protein. Vet Pathol. 2004;41:599–607. doi: 10.1354/vp.41-6-599. [DOI] [PubMed] [Google Scholar]
- 9.Crampton N, Kodiha M, Shrivastava S, Umar R, Stochaj U. Oxidative stress inhibits nuclear protein export by multiple mechanisms that target FG nucleoporins and Crm1. Molec Biol of Cell. 2009;20:5106–5116. doi: 10.1091/mbc.E09-05-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodiha M, Stochaj U. Nuclear transport: A switch for oxidative stress-signaling circuit? J Signal Transduct. 2012;2012:1–18. doi: 10.1155/2012/208650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan KS, Wong CH, Huang YF, Li HY. Survivin withdrawal by nuclear export failure as a physiological switch to commit cells to apoptosis. Cell Death and Disease. 2010;1:e57, 1–9. doi: 10.1038/cddis.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen KT, Holloway MP, Altura RA. The CRM1 nuclear export protein in normal development and disease. Int J Biochem Molec Biol. 2012;3(2):137–151. [PMC free article] [PubMed] [Google Scholar]
- 13.Becskei A, Mattaj IW. The strategy for coupling the RanGTP gradient to nuclear protein export. PNAS. 2003;100(4):1717–1722. doi: 10.1073/pnas.252766999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connell CM, Colnaghi R, Wheatly SP. Nuclear survivin has reduced stability and is not cytoprotective. J Biol Chem. 2008;283(6):3289–3296. doi: 10.1074/jbc.M704461200. [DOI] [PubMed] [Google Scholar]
- 15.Boudreau HE, Casterline BW, Burke DJ, Leto TL. Wild-type and mutant p53 differentially regulate NADPH oxidase 4 in TGF-β-mediated migration of human lung and breast epithelial cells. Br J Cancer. 2014;110:2569–2582. doi: 10.1038/bjc.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pervin S, Tran L, Urman R, Braga M, Parveen M, Li SA, et al. Oxidative stress specifically downregulates survivin to promote breast tumour formation. Br J Cancer. 2013;108:848–858. doi: 10.1038/bjc.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maillet A, Pervaiz S. Redox regulation of p53, redox effectors regulated by p53: A subtle balance. Antiox Redox Sig. 2012;16(11):1285–1294. doi: 10.1089/ars.2011.4434. [DOI] [PubMed] [Google Scholar]
- 18.Liu B, Chen Y, St Clair DK. ROS and p53: Versatile partnership. Free Radic Biol Med. 2008;44(8):1529–1535. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grdina DJ, Murley JS, Miller RC, Mauceri HJ, Sutton HG, Thirman MJ, et al. A manganese superoxide dismutase (SOD2)-mediated adaptive response. Radiat Res. 2013;179:115–124. doi: 10.1667/RR3126.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin C, Qin L, Shi Y, Candas D, Fan M, Lu CL, et al. CDK4-mediated MnSOD activation and mitochondrial homeostasis in radioadaptive protection. Free Radic Biol Med. 2015;81:77–87. doi: 10.1016/j.freeradbiomed.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murley JS, Kataoka Y, Weydert CJ, Oberley LW, Grdina DJ. Delayed cytoprotection after enhancement of Sod2 (MnSOD) gene expression in SA-NH mouse sarcoma cells exposed to WR-1065, the active metabolite of amifostine. Radiat Res. 2002;158:101–109. doi: 10.1667/0033-7587(2002)158[0101:dcaeos]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1978;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The anti-oxidant function of the p53 tumor suppressor. Nat Med. 2005;11(12):1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov DE, Zeng Q, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes and Development. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma N, Ramachandran S, Bowers M, Yegappan M, Brown R, Azizs S, et al. Multiple factors other than p53 influence colon cancer sensitivity to paclitaxel. Cancer Chemother Pharmacol. 2000;46:329–337. doi: 10.1007/s002800000155. [DOI] [PubMed] [Google Scholar]