Abstract
The AMP-activated protein kinase (AMPK) is a sensor of cellular energy status that regulates cellular and whole body energy balance. A recent crystal structure has illuminated the complex regulatory mechanisms by which AMP and ADP cause activation of AMPK, involving phosphorylation by the upstream kinase, LKB1. Once activated by falling cellular energy status, AMPK activates catabolic pathways that generate ATP, while inhibiting anabolic pathways and other cellular processes consuming ATP.
AMPK is implicated in many human diseases. Mutations in the γ2 subunit cause heart disease due to excessive glycogen storage in cardiac myocytes, leading to ventricular pre-excitation. AMPK-activating drugs reverse many of the metabolic defects associated with insulin resistance, and recent studies suggest that the insulin-sensitizing effects of the widely used anti-diabetic drug, metformin, are mediated by AMPK. The upstream kinase LKB1 is a tumor suppressor, and AMPK may exert many of its anti-tumor effects. AMPK activation promotes the oxidative metabolism typical of quiescent cells, rather than the aerobic glycolysis observed in tumor cells and cells involved in inflammation, explaining in part why AMPK activators have both anti-tumor and anti-inflammatory effects. Salicylate (the major in vivo metabolite of aspirin) activates AMPK, and this could be responsible for at least some of the anti-cancer and anti-inflammatory effects of aspirin. In addition to metformin and salicylates, novel drugs that modulate AMPK are likely to enter clinical trials soon. Finally, AMPK is implicated in viral infection: down-regulation of AMPK during hepatitis C virus infection appears to be essential for efficient viral replication.
Keywords: metformin, cancer, type 2 diabetes mellitus, Wolff-Parkinson-White syndrome, cell biology, biochemistry
Introduction
The AMP-activated protein kinase (AMPK) was originally discovered as apparently distinct protein kinase activities that phosphorylated and inactivated two key enzymes of lipid biosynthesis, i.e. acetyl-CoA carboxylase, involved in fatty acid synthesis [1], and 3-hydroxy-3-methylglutaryl-CoA reductase, involved in sterol synthesis [2]. In 1987 the author’s laboratory reported that these were functions of a single protein kinase that was activated allosterically by AMP, as well as by phosphorylation by a distinct upstream kinase [3]; we named it the AMP-activated protein kinase (AMPK) at that time [4].
Since these early studies it has become clear that AMPK has dozens of physiological targets, and is a crucial regulator of energy balance, both at the cellular and at the whole body levels. Given the crucial nature of energy balance in the function of cells and organisms, it is not surprising that the AMPK system has many implications in human health, with roles in diverse disorders such as heart disease, diabetes, cancer, inflammatory disorders and viral infection. Some drugs that are already widely used, including metformin and salicylates, now appear to act in part by activating AMPK, while several novel AMPK-activating drugs are under development. The purpose of this review is to discuss the implications of recent discoveries about the AMPK system in the development and treatment of human disease.
AMPK –regulation by adenine nucleotides and calcium ions
AMPK appears to exist throughout eukaryotes as heterotrimeric complexes composed of a catalytic α subunit and regulatory β and γ subunits [5] (Fig. 1, top). Each subunit occurs in mammals as multiple isoforms (α1, α2; β1, β2; γ1, γ2, γ3) encoded by distinct genes. The site that upstream kinases phosphorylate to cause AMPK activation was identified within the rat α2 isoform as Thr172 [6]. Phosphorylation of this site, and the critical site on the downstream target acetyl-CoA carboxylase (Ser79 in rat ACC1 [7]), both usually monitored using phosphospecific antibodies, are now widely used as biomarkers for AMPK activation.
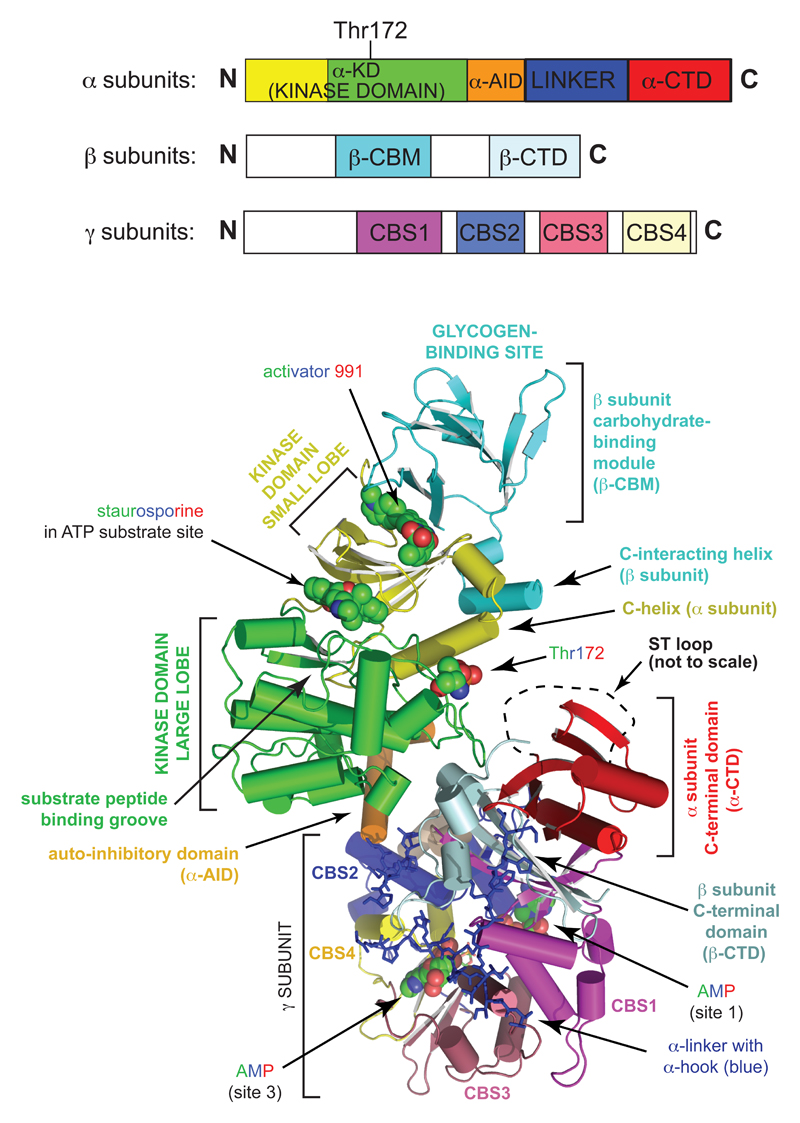
Figure 1. Structure of the heterotrimeric AMPK complex.
The linear layout of the domains is shown at the top, and a three dimensional model of a human α2β1γ1 complex (created in MacPyMol from the RCSB ProteinDataBank entry 4CFE [22]) at the bottom, using similar color coding. From the view at the bottom, the complex can be seen to be divided into two rather separate regions, the “catalytic module” at top left, and the “nucleotide-binding module” at bottom right, with Thr172 partially exposed in the narrow cleft between them. The activators AMP (only shown in sites 1 and 3) and 991, the inhibitor staurosporine, and the side chain of Thr172 are in “sphere” view with C atoms in green, O in red, and N in blue (H omitted). The extended linker that connects the α-AID and the α-CTD, which wraps around one face of the γ subunit, is in “stick” view in deep blue color. All other domains are in “cartoon” view with α-helices represented as cylinders and β-strands as ribbons. The “ST loop” a regulatory region referred to in the text, was not resolved in this structure, but its approximate location is shown by a black dashed line.
What is the physiological significance of activation of AMPK by AMP? The major source of AMP in the cell is the reaction catalyzed by adenylate kinase (2ADP ↔ ATP + AMP), which appears to be maintained close to equilibrium in most eukaryotic cells. In unstressed cells, catabolism maintains the ATP:ADP ratio at around 10:1, and this drives the adenylate kinase reaction towards ADP, so that AMP is maintained at low levels. However, if the cells experience a metabolic stress that causes the ATP:ADP ratio to fall, the adenylate kinase reaction will tend to be displaced towards AMP. Because it starts at such a low level, the changes in AMP concentration in stressed cells are always much larger than the changes in ATP or ADP [8]. Cellular AMP concentrations are thus sensitive indicators of energy stress.
The principal upstream kinase phosphorylating Thr172 was identified in 2003 to be a complex containing the protein kinase LKB1 [9–11]. This was an exciting discovery because LKB1 had previously been identified as the product of a tumor suppressor gene mutated in an inherited susceptibility to cancer, Peutz-Jeghers syndrome [12], although its downstream targets had not been identified. These findings introduced the first clear link between AMPK and cancer, which is discussed further below.
Binding of AMP to AMPK causes >10-fold allosteric activation, and also promotes net phosphorylation of Thr172, both by stimulating phosphorylation by LKB1, and by inhibiting dephosphorylation by protein phosphatases [8]. The latter effect of AMP, but not the other two, is mimicked by ADP [13], albeit only at 10-fold higher concentrations [8]. All three effects are antagonized by ATP, but concentrations of AMP observed in stressed cells cause allosteric activation and promote phosphorylation even in the presence of physiological concentrations of ATP [8].
Thr172 can also be phosphorylated by the Ca2+/calmodulin-dependent kinase kinases, especially CaMKKβ [14–16], which represents an alternate upstream pathway by which AMPK can be activated by increases in intracellular Ca2+ in the absence of changes in AMP. There are conflicting findings concerning whether phosphorylation by CaMKKβ is promoted by AMP [8, 17], but since AMP binding inhibits Thr172 dephosphorylation, low concentrations of the two signals can act in an additive manner in any case [18]. Activation of AMPK by CaMKKβ is now known to occur in many physiological situations, including hippocampal neurons subject to depolarization [14], T cells activated by antigen [19], and cells treated with agonists that activate G protein-coupled receptors linked (via Gq/G11) to release of intracellular inositol trisphosphate (IP3) and hence Ca2+. The latter include thrombin acting via protease-activated receptor-1 (PAR-1) in endothelial cells [20], and ghrelin acting via growth hormone secretagogue receptor-1 (GHSR1) in hypothalamic neurons [21].
To conclude this section, binding of AMP to AMPK promotes its activation by three complementary mechanisms: (i) allosteric activation; (ii) promoting phosphorylation of Thr172 by LKB1; (iii) inhibiting dephosphorylation of Thr172 by protein phosphatases, with only the third of these being mimicked by ADP. Phosphorylation of Thr172 can also occur in response to an increase in intracellular Ca2+. In the next section, I will discuss how structural studies on AMPK have illuminated this complex regulatory mechanism.
Structure of the AMPK complex
A crystal structure of a complete human α2β1γ1 complex was recently reported [22]. The complex had been phosphorylated at Thr172 and crystallized in the presence both of the classical allosteric activator AMP and a novel pharmacological activator called “991”, and was therefore in a fully active conformation, although the non-specific kinase inhibitor staurosporine was also included during crystallization. The layout of all of the major globular domains was clear, although some linking peptides were not fully resolved (Fig. 1).
The catalytic kinase domain (α-KD) is located at the N-terminal end of the α subunit and has the archetypal kinase domain structure, with small and large lobes joined by a hinge. As expected, the inhibitor staurosporine was located in the cleft between these lobes, where it occupies the site used by ATP during catalysis. Although a peptide substrate was not present in this structure, previous biochemical studies had suggested that the peptide sequence N-terminal to the phosphorylated residue on a substrate binds in a groove in the surface of the large lobe, placing the phosphoacceptor adjacent to the γ-phosphate of bound ATP [23]. The α-KD is immediately followed by a small auto-inhibitory domain (α-AID), so-called because α-KD:α-AID constructs are much less active than those containing the α-KD alone [24]; reorientation of the position of the α-AID relative to the α-KD is likely to be involved in the activation mechanism [22]. The C-terminal end of the α-AID marks the boundary between what can be termed the “catalytic module” (top left in the lower part of Fig. 1) and the rather discrete “nucleotide-binding module” (bottom right in Fig. 1), with the later containing the C-terminal domains of the α and β subunits (α- and β-CTD) as well as the entire γ subunit. The critical phosphorylation site, Thr172, lies in the cleft between these two modules, and in the active conformation shown in Fig. 1 access of protein phosphatases to Thr172 would be restricted by the close proximity of the α-CTD.
The β subunits contain two conserved globular domains, the carbohydrate-binding module (β-CBM) and the previously mentioned β-CTD. The β-CTD acts as the core of the heterotrimeric αβγ complex, bridging the α-CTD to the γ subunit. Although the connections between the β-CBM and the β-CTD are not fully resolved, the former lies on the opposite side of the kinase domain to the latter, with 991 and A-769662 binding in the cleft between the β-CBM and the small lobe of the kinase domain. These two compounds are synthetic ligands derived from high-throughput screens designed to detect AMPK activators, and their binding causes both allosteric activation and inhibition of Thr172 dephosphorylation. The effects of their binding may be transmitted to the kinase domain via the “C-interacting helix” on the β subunit, which immediately follows the β-CBM and which interacts with the C-helix on the small lobe of the kinase domain. On the opposite side of the β-CBM from the 991-binding site is the glycogen-binding site. Although this is known to be responsible for binding of AMPK to the surface of glycogen particles [25, 26], its exact physiological role remains uncertain.
The γ subunits contain an N-terminal region involved in interaction with the β-CTD, and then four tandem repeats (CBS1-CBS4) of a sequence motif known as a cystathione β-synthase (CBS) repeat, each represented in a different color in Fig. 1. CBS repeats occur, usually as just two tandem repeats, in other human proteins where they often bind ligands containing adenosine [27]. Intriguingly, mutations in conserved residues within CBS repeats disrupt ligand binding, and several of these (including those in the AMPK-γ2 subunit, see below) cause inherited diseases in humans [27]. The four CBS repeats in the AMPK-γ subunits assemble in a pseudosymmetrical manner to generate a flattened disk, with four clefts in the centre where ligands might bind. However, only three of these appear to be utilized, and these represent the sites where the regulatory ligands, AMP, ADP and ATP bind in competition with each other. Interestingly, the α-AID and the α-CTD are connected by an extended linker peptide, which in the active conformation in Fig. 1 wraps around one face of the γ subunit, forming a structure called the “α hook” that contacts AMP in site 3. A previously proposed model [13] suggests that binding of AMP or ADP, but not ATP, in site 3 would allow interaction with the α hook. The consequent conformational change in the α-linker peptide when ATP binds was proposed to cause the nucleotide-binding and catalytic modules to move apart, allowing protein phosphatases better access to Thr-172 and thus explaining the ability of AMP and ADP binding to inhibit Thr172 dephosphorylation.
Downstream effects of AMPK activation
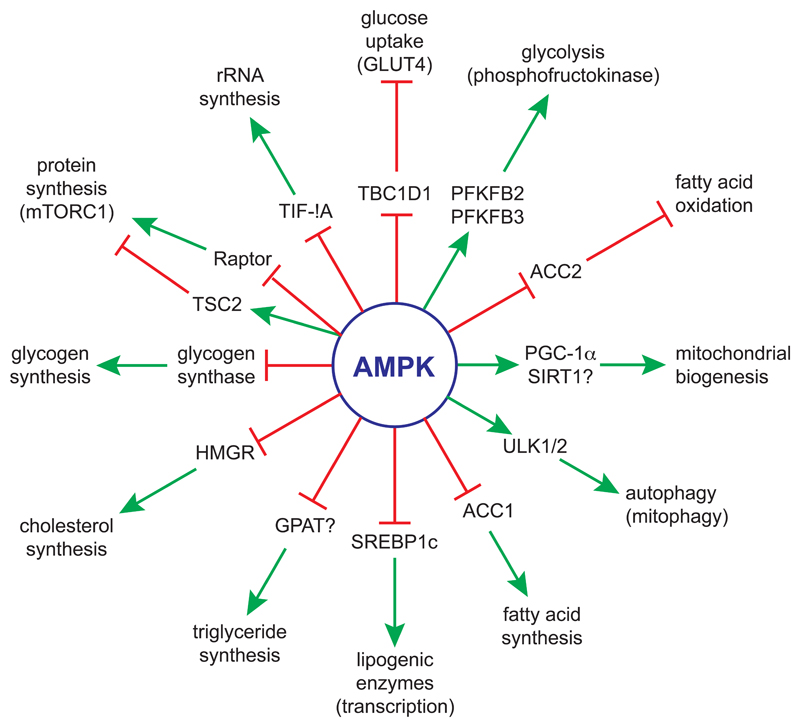
A full discussion of the known downstream targets for AMPK is beyond the scope of this review, and readers are referred to a previous review [5]. However, a summary of some of these, and the pathways they regulate, is shown in Figure 2. Consistent with the findings that it is switched on by ATP depletion, and has a key role in maintaining cellular energy balance, AMPK promotes catabolic pathways that generate ATP, while inhibiting anabolic pathways involved in cell growth, and other processes that consume ATP. It also causes a cell cycle arrest in G1 phase [28], in part by phosphorylating MDM4 (MDMX), part of the E3 ubiquitin ligase complex that regulates p53 turnover [29]. Cell cycle arrest by AMPK makes sense, since DNA replication (during S phase) and mitosis (M phase) are both energy-requiring processes. In general, AMPK achieves its effects both acutely via direct phosphorylation of metabolic enzymes or regulatory proteins involved in the process being regulated, and in the longer term via effects on gene expression. I will discuss here just a few examples of downstream effects of AMPK that will become relevant later in the review:
-
1)
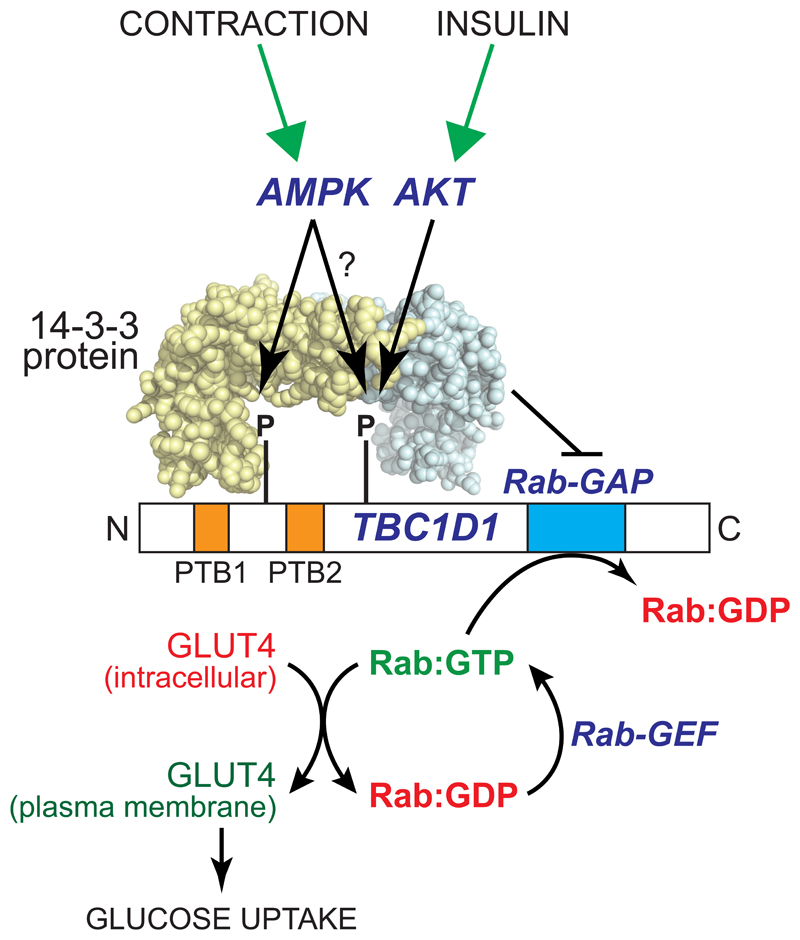
AMPK accelerates glucose uptake to drive catabolism of glucose during muscle contraction, which it does, at least in part, by phosphorylating the Rab-GTPase activator protein (Rab-GAP), TBC1D1 (Fig. 3). TBC1D1 is a member of the same family as TBC1D4 (also known as AS160), whose phosphorylation by the insulin-activated kinase PKB/Akt plays a key role in insulin-stimulated glucose uptake [30]. While TBC1D4 appears to be the predominant form in adipocytes, TBC1D1 is the predominant form in most muscle types [31]. Both TBC1D1 and TBC1D4 bind to intracellular vesicles containing the glucose transporter GLUT4, and their Rab-GAP domains promote the GTPase activity of members of the Rab family of small G proteins, thus maintaining them in their inactive, GDP-bound state. Phosphorylation of TBC1D1 at two sites, Ser237 and Thr596, promotes its binding to 14-3-3 proteins, which appears to repress its Rab-GAP activity. Rabs are thus converted to their active GTP-bound forms, and they then promote trafficking of the GLUT4-containing intracellular vesicles to the plasma membrane and thus increased glucose uptake [32–34]. While AMPK can phosphorylate both Ser237 and Thr596 in cell-free assays, available evidence suggests that only Ser237 may be phosphorylated by AMPK in intact cells, with Thr596 being phosphorylated by other kinases including the insulin-stimulated kinase, Akt [32].
-
2)
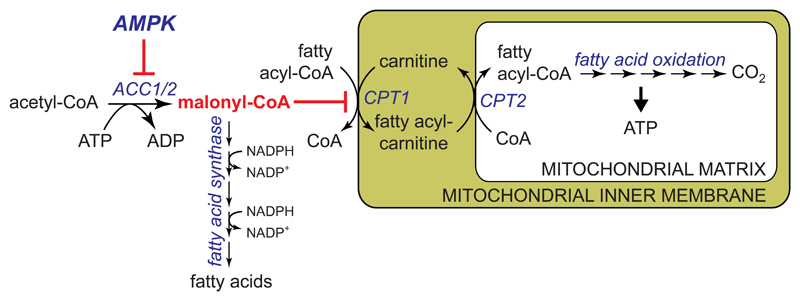
AMPK phosphorylates and inactivates both isoforms of acetyl-CoA carboxylase, ACC1 and ACC2, lowering the concentration of their reaction product, malonyl-CoA. Since malonyl-CoA is both an intermediate in fatty acid synthesis and an inhibitor of fatty acid uptake into mitochondria via the transport system involving carnitine:palmitoyl transferase-1 (CPT1), this has the dual effect of inhibiting fatty acid synthesis and enhancing fatty acid oxidation (Fig. 4) [35, 36].
-
3)
AMPK enhances mitochondrial biogenesis by effects on the transcriptional co-activator PGC-1α. This may involve direct phosphorylation of PGC-1α [37], or activation of the lysine deacetylase SIRT1, which deacetylates and activates PGC-1α [38].
-
4)
As well as its direct effects on acetyl-CoA carboxylase, AMPK inhibits fatty acid synthesis in the longer term by phosphorylating SREBP-1c, inhibiting its ability to promote transcription of lipogenic enzymes [39]. AMPK also inhibits cholesterol and phospholipid/triacylglycerol synthesis, the former by phosphorylating and inactivating HMG-CoA reductase (HMGR) [35], and the latter by inactivating glycerol phosphate acyl transferase (GPAT; it remains unclear whether this is via direct phosphorylation) [40].
-
5)
AMPK inhibits two other major biosynthetic pathways required for cell growth, i.e. protein and rRNA synthesis. It inhibits the former mainly by inhibiting the mechanistic target-of-rapamycin complex-1 (mTORC1), by phosphorylating both the upstream regulator TSC2 [41] and the mTORC1 subunit, Raptor [42] (Fig. 5). AMPK also inhibits rRNA synthesis by phosphorylating TIF-1A, a transcription factor for RNA polymerase-1 [43].
Figure 2. Summary of selected protein targets and processes downstream of AMPK.
A green arrow signifies activation, and a red line with a cross-bar signifies inhibition. Note that if AMPK inhibits a protein that in turn inhibits a downstream process, (two successive red lines with cross-bars) then the overall process (e.g. glucose uptake, fatty acid oxidation) will be activated. A question mark next to a protein signifies that it is not certain that the protein is a direct target for AMPK.
Figure 3. Model for acute activation of glucose transport in muscle by AMPK.
In the unphosphorylated form, the protein TBC1D1 retains GLUT4 at intracellular sites because its Rab-GAP domain promotes the inactive GDP-bound state of members of the Rab family of small G proteins. AMPK phosphorylates TBC1D1 at Ser237 near the PTB1 domain, while Akt (and perhaps also AMPK?) phosphorylates Thr596 near PTB2. This dual phosphorylation promotes the binding of 14:3:3 proteins, abundant dimeric proteins containing two symmetrical pockets that bind to phosphorylated peptides, which is proposed to inhibit the Rab-GAP activity of TBC1D1. The functions of the two phosphotyrosine-binding (PTB) domains of TBC1D1 remain unclear.
Figure 4. Acute activation of fatty acid oxidation and inhibition of fatty acid synthesis by AMPK.
AMPK phosphorylates both isoforms ACC1 and ACC2 at equivalent sites (Ser80 and Ser221 in human ACC1 and ACC2 respectively), causing their inactivation. This lowers malonyl-CoA, a key intermediate in fatty acid synthesis that is also an inhibitor of carnitine palmitoyl transferase-1 (CPT1). CPT1 is involved in uptake of fatty acids into mitochondria, where they are oxidized to generate ATP. It was thought that ACC1 produced the pool of malonyl-CoA involved in fatty acid synthesis, and ACC2 a separate pool of malonyl-CoA that regulates CPT1 [117], but recent results suggest that these two pools cannot be completely distinct [77].
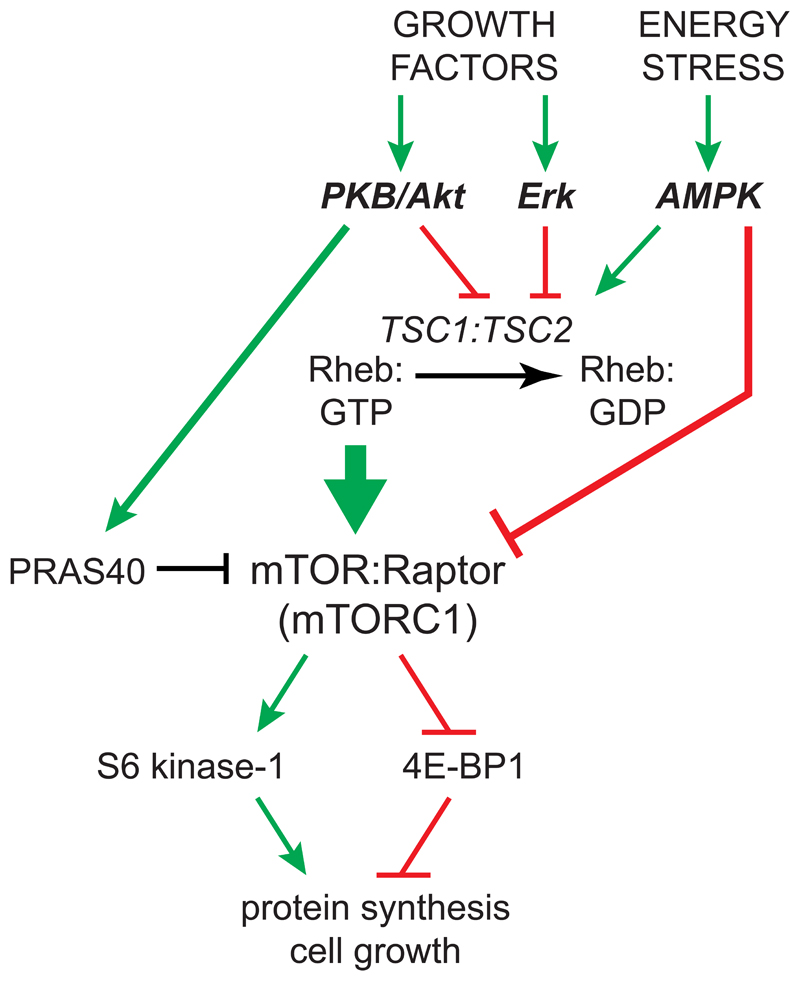
Figure 5. Growth factors activate, whereas energy stress and AMPK inactivate, the mechanistic target-of-rapamycin complex 1 (mTORC1).
mTORC1 is a large multiprotein complex containing mTOR and Raptor, which phosphorylates and activates S6 kinase-1, an activator of protein synthesis, and phosphorylates and inactivates initiation factor 4E-binding protein-1 (4E-BP1), an inhibitor of protein synthesis. Binding of the active GTP-bound form of the small G protein Rheb recruits mTORC1 to the lysosome, where it is activated. Growth factors activate the protein kinase B/Akt pathway and the Erk pathway, and both of those protein kinases phosphorylate the TSC2 component of the TSC1:TSC2 complex, inhibiting its Rheb-GAP activity and thus activating mTORC1. PKB/Akt also phosphorylates PRAS40, relieving its inhibitory effect on mTORC1. On the other hand AMPK, activated in response to energy stress, phosphorylates TSC2, enhancing its Rheb-GAP activity, as well as Raptor, with both effects inhibiting mTORC1.
Role of AMPK in hypertrophic cardiomyopathy
The only disease-causing mutations currently identified in AMPK genes are in PRKAG2 (encoding the γ2 isoform of the nucleotide-binding subunit), which cause a form of the heart disease Wolff-Parkinson-White syndrome [44]. This syndrome is characterized by ventricular pre-excitation (a premature excitation of the ventricles, readily detected by electrocardiogram), but when caused by PRKAG2 mutations it is usually, although not always [45], accompanied by cardiac hypertrophy. More than ten different mutations in PRKAG2 have been identified, all of which cause amino acid substitutions within the CBS repeats of γ2 [44]. Most are inherited in a dominant manner and are thus frequent in the affected families, although some (e.g. R384T in CBS2 [46] and R531Q in CBS4 [47]) cause a more severe form of disease associated with death during infancy, and are therefore only found as de novo mutations not present in the parents. Many of the substitutions (R302Q, H383R, R384T, R531G, R531Q) affect basic side chains that occur at similar positions in CBS repeats 1, 2 and 4, and structural studies show that these positively charged side chains bind the negatively charged phosphate groups of the bound adenine nucleotides [48]. These substitutions not only reduce allosteric activation, but also the enhanced net Thr172 phosphorylation caused by AMP binding [27, 46, 47, 49]. Other substitutions (e.g. T400N and N488I), although not appearing to affect residues directly involved in nucleotide binding, still negatively affect AMP binding. Reduced AMP activation is clearly a loss-of-function effect, which is difficult to reconcile with dominant inheritance of the mutations. However, since the mutations affect binding of the inhibitory nucleotide, ATP, as well as the activating nucleotide, AMP [27], the most likely explanation is that they reduce binding of ATP and thus increase basal Thr172 phosphorylation and AMPK activity, a gain-of-function effect. This has been directly demonstrated with the R531G and R531Q substitutions [47, 50], while the effects of mutations equivalent to T400N and N488I made in the γ subunit ortholog in budding yeast also suggest a gain-of-function [51]. In addition, elevated basal AMPK activity was observed in transgenic mice over-expressing the N488I mutation in the heart [52].
One feature of the cardiac myocytes of these patients, based on post mortem analysis, is the presence of large vacuole-like structures containing glycogen, which appear to disrupt the normal myofibrillar structure [51]. The hearts of children bearing the R531Q and R384T mutations displayed gross hypertrophy and also contained up to 10 times the normal glycogen content [46, 47]. Transgenic mice over-expressing γ2 from a cardiac-specific promoter with R302Q [53], N488I [52] or R531G [54] mutations, but not wild type γ2, also displayed gross cardiac hypertrophy, ventricular pre-excitation and elevated cardiac glycogen. Thus, the γ2 mutations appear to cause a glycogen storage disorder, but why should this lead to ventricular pre-excitation? The atria and ventricles of normal adult hearts are separated by a fibrous ring containing collagen (the annulus fibrosis), which ensure that electrical excitation passes from the atria to the ventricles only through the atrio-ventricular node (where a short delay is imposed), and cannot then return to the atria. Without a fully intact annulus fibrosis, ventricular pre-excitation and potentially fatal arrhythmias can occur. Studies of transgenic mice over-expressing the N488I mutation showed that the fibrous rings were disrupted by glycogen-filled myocytes [52], which may account for the ventricular pre-excitation exhibited by these mice.
The elevated glycogen content can therefore potentially explain the cardiac arrhythmias, but why do the mutations cause high glycogen? The most likely explanation is that the elevated basal Thr172 phosphorylation and AMPK activity enhances GLUT4 translocation and glucose uptake. Evidence in favor of this idea comes from studies of the transgenic mice over-expressing the N488I mutation [55], which have elevated cardiac glucose uptake, glycogen synthesis and glucose-6-phosphate content (the latter a key allosteric activator of glycogen synthase), as well as increased ACC phosphorylation and fatty acid oxidation. Interestingly, when the N488I over-expressing mice were crossed with mice carrying a point mutation in the GYS1 gene that rendered its product, glycogen synthase, resistant to glucose-6-phosphate activation, both the glycogen storage and the ventricular pre-excitation were almost completely reversed [56]. These results suggest that the increased basal AMPK activity causes enhanced glucose uptake into cardiac myocytes but that, in the absence of a genuine increased demand for glucose, glucose-6-phosphate concentrations rise thus driving flux of glucose carbon into glycogen synthesis rather than glycolysis and oxidation. The cells are, in effect, behaving as if they were short of energy even though they are not. Tellingly, however, although the glycogen storage and ventricular pre-excitation phenotypes of the N488I mice were reversed by crossing with the GYS1 mutants, their cardiac hypertrophy was not, showing that the latter has a distinct origin. The authors suggested that this was due to enhanced insulin sensitivity of the cardiac myocytes, leading to hyper-activation of the mTORC1 pathway. Consistent with this, the hypertrophy could be largely reversed by treatment with the mTORC1 inhibitor, rapamycin [56].
The γ2 isoform is expressed most abundantly in the heart, although also expressed elsewhere [57]. Similar mutations have not been reported to occur in the γ1 isoform, but they have been found in γ3, which is expressed almost exclusively in skeletal muscle [57]. An R200Q substitution in γ3 in domestic pigs (equivalent to the R302Q substitution in human γ2) is associated with a high glycogen content in skeletal muscle, which adversely affects meat quality [58]. Intriguingly, in a screen of candidate genes in 1500 lean and obese humans, a heterozygous mutation causing an R225W substitution in γ3 (R225 aligns with R302 in human γ2 and R200 in pig γ3) was found in two unrelated individuals. In these subjects, skeletal muscle glycogen content and basal AMPK activity were 2-fold higher, while intramuscular triglyceride was 30% lower than in matched controls. In both the pigs and the humans, there was no obvious clinical problem associated with the γ3 mutation, and it is interesting to speculate that some endurance athletes might carry γ3 mutations, because a high muscle glycogen content would be expected to confer an advantage in endurance events. Relevant to this, it is interesting to note that the AMPK activator 5-aminoimidazole-4-carboxamide riboside (AICAR), which increases muscle glycogen content [59] and improves endurance on treadmills when administered to sedentary mice over several weeks [60], has been banned for use in sport by the World Anti-Doping Agency.
Role of AMPK in type 2 diabetes
Type 2 diabetes occurs when increased release of insulin from the β cells of the pancreas can no longer compensate for insulin resistance, with the latter being strongly associated with increased triacylglycerol content, particularly in liver and skeletal muscle. One hypothesis is that insulin resistance occurs in obese individuals when their capacity to store triacylglycerols in adipose tissue is exceeded, leading to their accumulation in liver and muscle. This is supported by studies of humans with lipodystrophy, who lack adipose tissue and store triacylglycerols instead in liver and muscle, which then become severely insulin resistant [61]. Despite the association between insulin resistance and triacylglycerols, the real culprit may be diacylglycerols whose levels are elevated at the same time. Diacylglycerols activate novel isoforms of protein kinase C (PKCθ in muscle and PKCε in liver) that then appear to down-regulate the insulin signaling pathway [61]. This represents one explanation of the insulin resistance associated with obesity, although other factors such as increased inflammation [62] or endoplasmic reticulum stress [63] may also play a part. However, AMPK activation may be able to reverse both of these as well, since it has anti-inflammatory effects (discussed below) while inhibition of protein synthesis by AMPK has the potential to relieve endoplasmic reticulum stress.
By promoting the oxidation of fatty acids and inhibiting synthesis of fatty acids and triacylglycerols, treatments that activate AMPK would be expected to reduce lipid stores in liver and skeletal muscle and hence improve insulin sensitivity. By promoting glucose uptake by skeletal muscle [36, 64] and inhibiting gluconeogenesis in the liver [65, 66], they might also be expected to ameliorate the hyperglycemia associated with type 2 diabetes more directly. Indeed, pharmacological agents that activate AMPK do have many of these effects in vivo. The first to be tested was AICAR, an adenosine analogue that is taken up into cells via adenosine transporters and converted to the equivalent nucleotide, ZMP, which mimics all of the activating effects of AMP on AMPK [35]. AICAR treatment was found to reverse many metabolic abnormalities in animal models of obesity and insulin resistance, such as fa/fa rats [67, 68], ob/ob mice [69], and rats fed a high-fat diet [70]. At about the same time, it was reported that the biguanide drug metformin, currently the first choice drug for type 2 diabetes that is prescribed to >100 million patients worldwide, also activated AMPK both in intact cells and in vivo [71]. Metformin activates AMPK indirectly by inhibiting the mitochondrial respiratory chain [50], but A-769662, a direct AMPK activator that binds at the same site as 991 (Fig. 1), also has similar in vivo effects in animals; it increases fatty acid oxidation in rats, and decreases plasma glucose, body weight gain, plasma and liver triacylglycerols, and hepatic expression of gluconeogenic and lipogenic enzymes in ob/ob mice [72]. Similarly, the natural product berberine used in traditional Chinese medicine, which activates AMPK by inhibiting the respiratory chain in a similar manner to metformin [50], also reduces body weight and improves glucose tolerance in db/db mice, while reducing body weight and plasma triacylglycerols, and improving insulin sensitivity, in rats fed a high-fat diet [73].
Since (with the exception of A-769662) these agents activate AMPK indirectly, it has been important to establish whether their favorable metabolic effects are indeed mediated by AMPK. Arguing against this, the ability of AICAR or metformin to reduce glucose production by hepatocytes in vitro, or of metformin to improve glucose tolerance after short-term (30 minute) treatment in vivo, was unaffected by a knockout of both isoforms of the AMPK catalytic subunit (α1 and α2) in the liver [74]. The AMPK-independent effects of AICAR may be due to effects of its intracellular metabolite, ZMP, to directly inhibit the gluconeogenic enzyme fructose-1,6-bisphosphatase [75] and/or to lower cyclic AMP (which promotes transcription of gluconeogenic enzymes) due to inhibition of adenylate cyclase [76], while the AMPK-independent effects of metformin in part may a consequence of ATP depletion, which was more severe in cells lacking AMPK [74]. Despite these negative findings, there is good evidence that the longer term insulin-sensitizing effects of metformin are mediated by AMPK. This comes from recent studies of “double knock-in” (DKI mice) in which both endogenous genes encoding acetyl-CoA carboxylase (ACC1 and ACC2) were replaced by genes encoding single amino acid substitutions that eliminate the AMPK sites [77]. As expected, the ACC1/ACC2 activities, and the cellular content of the product malonyl-CoA, were elevated in the livers of these mice, and fatty acid oxidation was reduced. This was in turn associated with increased levels of hepatic di- and tri-acylglycerols and increased PKCε activity, while the mice had increased fasting blood glucose and insulin, were glucose-intolerant and insulin-resistant, and insulin was less effective at suppressing hepatic glucose production. Even more interesting was the effect of feeding a high-fat diet, which obliterated the metabolic differences between the wild type and DKI mice. However, while long-term (6 week) treatment with metformin improved metabolic parameters such as fasting blood glucose, insulin suppression of hepatic glucose production and gluconeogenic enzyme expression in the fat-fed wild type animals, all of these effects were absent in the DKI mice. These results suggest that the insulin-sensitizing effects of metformin can be almost entirely attributed to its effects on fatty acid metabolism, mediated by AMPK.
Role of AMPK in cancer
The first link between AMPK and cancer came with the finding that LKB1 is the upstream kinase required for AMPK activation in response to energy stress and biguanide drugs [9–11]. Mutations in the gene encoding LKB1 were already known to cause Peutz-Jeghers syndrome in humans. Humans with this syndrome are heterozygous for mutations that cause a loss of kinase activity, and they develop frequent benign intestinal tumors (polyps) that in mouse models appears to be due to haploinsufficiency [78]. They also have an increased risk of developing malignant cancers at other sites, which in mice appears to be due to loss-of-heterozygosity in somatic cells [79]. LKB1 is therefore a classical tumor suppressor, raising the question as to whether its tumor suppressing effects are mediated by AMPK. LKB1 is also required for phosphorylation and activation of a family of twelve “AMPK-related kinases” that have kinase domains closely related to AMPK [80]. While one or more of these might also contribute to the tumor suppressor effects of LKB1, AMPK remains the best candidate because of its ability to inhibit mTORC1 and almost all biosynthetic pathways required for cell growth, and to cause cell cycle arrest. In addition, tumor cells and other rapidly proliferating cells tend to have greatly elevated rates of glucose uptake and glycolytic metabolism (termed the Warburg effect or aerobic glycolysis), this metabolic switch being required in part to provide precursors for biosynthesis [81]. In the longer-term, AMPK activation tends to promote the more energy-efficient oxidative metabolism utilized by quiescent cells, thus opposing the switch to the aerobic glycolysis observed in many tumor cells.
Consistent with the idea that AMPK is a tumor suppressor are results showing that the AMPK activators metformin, phenformin and A-769662 all delay tumor onset in tumor-prone mice [82], and that a whole body knock-out of AMPK-α1 (the only catalytic subunit expressed in lymphocytes) accelerates the development of lymphomas in transgenic mice over-expressing the Myc oncogene in B cells [83]. In the latter case, this was accompanied by a shift towards aerobic glycolysis, which (as argued above) might be opposed if AMPK was present. This shift was dependent on hypoxia-inducible factor-1α, a transcription factor that up-regulates expression of glucose and lactate transporters and glycolytic enzymes [83]. Translation of HIF-1α mRNA is up-regulated by mTORC1 [84], a signaling pathway that is switched off by AMPK (Fig. 5).
The original finding that the tumor suppressor LKB1 lay on the same pathway as AMPK inspired epidemiological studies showing that diabetics treated with metformin had a lower incidence of cancer than those on other medications [85], a finding reproduced in many subsequent studies [86]. There are several possible explanations for this association, and it is not yet certain that the effect is mediated by AMPK, nor that it is an effect of metformin on the tumor cells themselves. There is some evidence from a mouse xenograft model that it may be mediated in part by metformin acting on the liver, lowering plasma glucose and insulin, with the latter being thought to trigger increased tumorigenesis in insulin-resistant states [87]. There is also evidence from mouse models that metformin and the related biguanide phenformin prolong survival by triggering more apoptosis in tumor cells that have lost LKB1, because the lack of a functional AMPK pathway in such cells makes them more sensitive to ATP depletion caused by the biguanide [87, 88]. If this mechanism operates in humans, then biguanides might be most effective in treating cancers where the LKB1-AMPK has been lost, which is quite a frequent occurrence (see next paragraph). It might also make sense for phenformin rather than metformin to be used for cancer treatment, because being more hydrophobic and less dependent on OCT1 for cellular uptake [50], it can probably enter tumor cells more readily.
If the LKB1-AMPK axis is indeed a tumor suppressing pathway that restrains tumor growth, you would expect tumor cells to be under selection pressure to down-regulate it. Consistent with this, biallelic loss-of-function mutations in LKB1 are common in human cancers, occurring in up to 30% of non-small cell lung cancers [89, 90], 20% of cervical cancers [91], and 10% of melanomas [92]. Loss of LKB1 leads to failure of AMPK activation during energy stress [9]. Mutations in the subunits of AMPK itself seem to be much less common in human cancer, possibly because (unlike LKB1) each subunit is encoded by more than one gene. However, down-regulation of expression of AMPK-α2 is relatively frequent in hepatocellular carcinoma, and is associated with poor prognosis [93]. Finally, the growth-promoting protein kinase B/Akt pathway is hyper-activated in many human cancers, either by activating mutations in phosphatidylinositide 3-kinase (PI3K) or receptors upstream of it, or loss-of-function mutations in PTEN, which is the tumor suppressor that degrades PIP3, the PI3K product and second messenger for protein kinase B (also known as Akt) [94]. It was shown in 2006 [95] that PKB/Akt phosphorylates Ser485 on rat AMPK-α1, and that this inhibited subsequent phosphorylation of the activating site, Thr172, by LKB1. The author’s group has recently confirmed that Akt phosphorylates the equivalent site (Ser487) on human AMPK-α1 (although not the equivalent site on AMPK-α2, Ser491, which is modified instead by autophosphorylation) [96]. These sites lie within a loop of about 50 residues that the author terms the “ST loop” due to its serine/threonine-rich nature. In the structure shown in Fig. 1 this loop was not resolved, but it would loop out of the rear side of the α-CTD (shown with a dashed line in Fig. 1). Because the complex may not be phosphorylated on the ST loop after expression in bacteria it is likely to be mobile within the crystals, but we have provided evidence that, after phosphorylation on Ser487 and possibly other sites, the ST loop interacts with the C-helix on the kinase domain, thus blocking access of upstream kinases to Thr172 [96]. We also showed that Ser487 was phosphorylated in three different human tumor cell lines where Akt was hyperactivated due to loss of PTEN. In these cells, AMPK was rather resistant to Thr172 phosphorylation and activation, but this could be rescued either by addition of an Akt inhibitor or by re-expressing PTEN, both of which triggered net dephosphorylation of Ser487 [96]. These results show that a previously unrecognized effect of Akt hyper-activation in tumor cells is that it down-regulates the AMPK pathway, thus reducing its restraining influence on cell growth and division. Since Ser487 phosphorylation inhibits but does not totally block Thr172 phosphorylation, they also suggest that this down-regulation might be overcome using AMPK-activating drugs, indicating a potential therapeutic role for the latter in tumors where the Akt pathway is hyper-activated.
Role of AMPK in inflammatory disease
There is increasing evidence that AMPK has anti-inflammatory effects, and that this may be mediated by its metabolic actions [97]. Unactivated cells of the immune system, including dendritic cells, neutrophils and T cells, utilize mainly oxidative metabolism (including fatty acid oxidation) to generate ATP. However, once activated, they usually switch to aerobic glycolysis, analogous to the metabolic changes that occur in tumor cells. In dendritic cells this switch is associated with reduced AMPK activation, is inhibited by pharmacological activation of AMPK, and is promoted by AMPK down-regulation [98]. Similar effects are observed in macrophages: classically activated (M1) macrophages with a pro-inflammatory role utilize aerobic glycolysis, whereas alternatively activated (M2) macrophages, more involved in the resolution of inflammation, tend to utilize oxidative metabolism instead. Studies using macrophages where AMPK has been down-regulated suggest that AMPK normally attenuates production of inflammatory cytokines [99, 100]. The idea that the anti-inflammatory actions of AMPK are due to its metabolic actions was strengthened by studies with mouse knockouts of AMPK-β1, the predominant β subunit isoform expressed in macrophages. AMPK-β1 deficient macrophages displayed reduced ACC phosphorylation and mitochondrial content, and reduced rates of fatty acid oxidation that promoted the accumulation of pro-inflammatory diacylglycerols. This triggered M1 skewing in vivo in macrophages derived from both bone marrow and adipose tissue. Activation of AMPK with A-769662 increased fatty acid oxidation in wild type but not β1-deficient macrophages, and the effects of A-769662 to suppress inflammation were impaired if fatty acid oxidation was blocked [101]. These findings suggest that a major component of the anti-inflammatory action of AMPK is mediated via its effects on fatty acid oxidation. The role of AMPK in macrophages has also been studied using mice with global or myeloid-specific knockouts of AMPK-α1, the catalytic subunit isoform expressed in macrophages. Regeneration of muscle in response to muscle injury was defective in these mice, and this was associated with reduced skewing towards M2 macrophages [102].
Interestingly, the author’s laboratory has shown that AMPK is directly activated by salicylate, the natural product from which aspirin (acetyl salicylate) was derived [103]. Salicylate and A-769662 appear to bind to the same site on AMPK; both are selective activators of β1-containing complexes, and they increase whole body fatty acid oxidation in wild type but not β1 knockout mice, showing that increased fat oxidation is mediated by AMPK activation in vivo. Although AMPK is not directly activated by aspirin itself [103], aspirin is rapidly broken down to salicylate. Indeed, following oral administration of aspirin the plasma half-life and peak concentration of its breakdown product, salicylate, are orders of magnitude greater than those of aspirin itself. Although blockade of prostanoid biosynthesis, via inhibition of cyclo-oxygenases, has been considered to be the main mechanism of action of aspirin, there has always been a paradox in that, while aspirin and salicylate have similar anti-inflammatory potencies, salicylate is a very poor inhibitor of cyclo-oxygenase [104, 105]. Although significant AMPK activation requires at least 1 mM salicylate, such concentrations are reached in humans taking high doses of aspirin or another salicylate derivative, salsalate, for rheumatoid arthritis. This raises the intriguing possibility that at least some of the anti-inflammatory effects of salicylate-based drugs may be mediated by AMPK. Intriguingly, the regular use of aspirin is also associated with reduced incidence of cancer [106], although whether this effect is mediated by AMPK remains uncertain.
Role of AMPK in viral infection
Hepatitis C virus is a positive-sense, single-stranded RNA virus with a lipid envelope, and it is estimated that an infected hepatocyte produces up to 50 viral particles per day. Because this requires synthesis of viral protein and lipids as well as RNA, one might imagine that it would cause increased ATP turnover and hence AMPK activation, which might then restrain further viral replication. Paradoxically, however, AMPK activation is down-regulated in a cell culture model of hepatitis C virus infection [107]. This appears to be because the viral protein NS5A binds to and activates PI3K, thus switching on the PKB/Akt pathway [108]. PKB/Akt would promote protein and lipid synthesis and cell survival, but would also down-regulate AMPK via phosphorylation AMPK-α1 at Ser487 within the ST loop (see section on cancer above). Indeed, experiments involving transfection of an S487A mutant suggested that phosphorylation of Ser487 is required for efficient viral replication [107]. Although the role of AMPK in viral infection is a relatively new area, there are indications that other viruses also interact with the AMPK system [109].
The potential application of novel AMPK-modulating drugs
An important consideration in the development of novel AMPK activating drugs is that they would have to be significantly more effective than existing agents such as metformin or salicylates, which are both inexpensive and have a good safety record. One potential shortcoming with metformin is that its effects appear to be largely confined to the liver. Since it is a cation that is not readily cell-permeable, the uptake of metformin into cells requires membrane transporters of the organic cation transporter family, such as OCT1 [110]. Because hepatocytes are exposed to higher concentrations of orally delivered metformin via the portal vein than most other cells [71], and also express high levels of OCT1, effects of metformin in vivo may be largely restricted to the liver [110]. In particular, the drug may not cause much activation of AMPK in skeletal muscle because of low peripheral drug concentrations and low OCT1 expression. The doses of metformin that can be administered to humans are limited by gastrointestinal side effects, which are likely to be AMPK-independent and may be caused by inhibition of the respiratory chain and consequent lactate production in the liver or gut [111, 112]. The more hydrophobic biguanide phenformin is more cell-permeable than metformin, and therefore much less dependent on OCT1 for cellular uptake [50]. Although formerly used for treatment of Type 2 diabetes, it was withdrawn in most countries in the 1970s due to cases where persons taking the drug developed lactic acidosis (with hindsight, an unsurprising side effect since both biguanides inhibit the respiratory chain and that is how they activate AMPK [50]). However, the frequency of lactic acidosis with phenformin, while life-threatening and around 20-fold more frequent than with metformin, was still rare (≈60 cases per 100,000 patient-years) [112]. This small risk might be more acceptable if the drug was being used to treat cancer, rather than diabetes [88].
The indirect AMPK activator AICAR, which is converted into the AMP mimetic ZMP within the cell [35], has the generic drug name acadesine and has been tested in humans [113]. However, it is not orally available and has to be injected and, as discussed above, has clear “off-target” AMPK-independent effects in mice, so its potential as an AMPK-activating drug in humans seems limited. New direct AMPK activators that are readily cell-permeable, exemplified by 991 and A-769662 [22], may have additional benefits over metformin by activating AMPK and promoting glucose uptake in organs other than the liver, especially skeletal muscle. These compounds are being developed by pharmaceutical companies, and studies of their potential toxicity are not yet available within the public domain. However, one caveat with their use is that increased glucose uptake into skeletal and cardiac muscle, in the absence of a demand for increased catabolism, would be expected to increase their glycogen content, as observed with the γ2 mutations that increase basal AMPK activity (see above). Nevertheless, while it is clear that increased glycogen content is harmful if it occurs during fetal development of the heart, it is not yet certain that it would be a problem in adults. Whether AMPK-activating drugs would also cause cardiac hypertrophy, which seems to be a secondary consequence of the activating γ2 mutations that is independent of their effects on glycogen accumulation [56], also remains unclear at present. However, adverse cardiac side effects would be a potential issue with any novel AMPK activators that would have to be carefully monitored, and the development of AMPK-activating drugs targeting isoform combinations not expressed in the heart, such as γ3 [57], might be advantageous.
Are there any indications for the development of inhibitors of AMPK? These might, of course, be useful for the treatment of the heart disease associated with γ2 mutations; studies using transgenic mice expressing the γ2 N488I mutation in the heart from a promoter switched off by tetracycline administration revealed that the clinical signs of the disease were at least partially reversible in mice [114]. A larger market for AMPK inhibitors might lie with treatment of those cancers where the LKB1-AMPK pathway, rather than being down-regulated as discussed above, has remained fully functional. There is evidence from mouse models that tumors with a functional LKB1-AMPK pathway are protected against cell death induced by addition of metformin or phenformin in vivo, compared with tumors lacking the pathway [87, 88]. In these cases, the biguanides may be acting as a kind of cytotoxic drug that acts by depleting cellular ATP, which is less harmful when AMPK is available to mount a response. It seems possible that the existence of a functional LKB1-AMPK pathway within tumor cells may also protect then against the effects of other cytotoxic drugs used for cancer treatment, in which case the latter might be usefully combined with an AMPK inhibitor. At present the only widely available AMPK inhibitor is compound C [71] (also known as dorsomorphin [115]), but that compound is in fact a rather non-selective kinase inhibitor [116], and much more specific inhibitors are needed.
Conclusions and perspectives
It should be clear from the discussion above that AMPK plays an important role in several diseases that have a high prevalence within most human populations, including type 2 diabetes, cancer and inflammatory diseases. It also appears to be involved in one disorder (hepatitis C) that, while less common amongst the whole population, is nevertheless prevalent in certain subgroups. The fact that widely used drugs that have been in human use for fifty years (metformin) or even longer (salicylates) may work, at least in part, by activating AMPK suggests that AMPK activators are fertile ground for the development of novel drugs. There are many recent patents for such activators, and it seems likely that some of these may enter clinical trials soon. However, one caveat has emerged from the studies of mutations in AMPK-γ2 that cause excessive glycogen storage and ventricular pre-excitation in cardiac muscle. The adverse consequences of these mutations suggest that there could be some situations (e.g. in cardiac myocytes) where the activation of AMPK in the absence of real energy deficit may be undesirable.
Acknowledgements
Studies in the author’s laboratory are supported by Senior Investigator Award (097726) from the Wellcome Trust and by a Programm Grant (C37030/A15101) from Cancer Research UK.
Footnotes
This article may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for Self-Archiving.
References
- 1.Carlson CA, Kim KH. Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. J Biol Chem. 1973;248:378–80. [PubMed] [Google Scholar]
- 2.Beg ZH, Allmann DW, Gibson DM. Modulation of 3-hydroxy-3-methylglutaryl coenzyme: A reductase activity with cAMP and with protein fractions of rat liver cytosol. Biochem Biophys Res Comm. 1973;54:1362–9. doi: 10.1016/0006-291x(73)91137-6. [DOI] [PubMed] [Google Scholar]
- 3.Carling D, Zammit VA, Hardie DG. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987;223:217–22. doi: 10.1016/0014-5793(87)80292-2. [DOI] [PubMed] [Google Scholar]
- 4.Hardie DG, Carling D, Sim ATR. The AMP-activated protein kinase - a multisubstrate regulator of lipid metabolism. Trends Biochem Sci. 1989;14:20–3. [Google Scholar]
- 5.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver, and identification of threonine-172 as the major site at which it phosphorylates and activates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–87. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 7.Davies SP, Sim AT, Hardie DG. Location and function of three sites phosphorylated on rat acetyl-CoA carboxylase by the AMP-activated protein kinase. Eur J Biochem. 1990;187:183–90. doi: 10.1111/j.1432-1033.1990.tb15293.x. [DOI] [PubMed] [Google Scholar]
- 8.Gowans GJ, Hawley SA, Ross FA, Hardie DG. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 2013;18:556–66. doi: 10.1016/j.cmet.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawley SA, Boudeau J, Reid JL, et al. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woods A, Johnstone SR, Dickerson K, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 11.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–35. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alessi DR, Sakamoto K, Bayascas JR. Lkb1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–63. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 13.Xiao B, Sanders MJ, Underwood E, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–3. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawley SA, Pan DA, Mustard KJ, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Woods A, Dickerson K, Heath R, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmoldulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–6. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 17.Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S, Kemp BE. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332:1433–5. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- 18.Fogarty S, Hawley SA, Green KA, Saner N, Mustard KJ, Hardie DG. Calmodulin-dependent protein kinase kinase-beta activates AMPK without forming a stable complex - synergistic effects of Ca2+ and AMP. Biochem J. 2010;426:109–18. doi: 10.1042/BJ20091372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamas P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, Cantrell DA. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–70. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stahmann N, Woods A, Carling D, Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta. Mol Cell Biol. 2006;26:5933–45. doi: 10.1128/MCB.00383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao B, Sanders MJ, Carmena D, et al. Structural basis of AMPK regulation by small molecule activators. Nature Commun. 2013;4:3017. doi: 10.1038/ncomms4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott JW, Norman DG, Hawley SA, Kontogiannis L, Hardie DG. Protein kinase substrate recognition studied using the recombinant catalytic domain of AMP-activated protein kinase and a model substrate. J Mol Biol. 2002;317:309–23. doi: 10.1006/jmbi.2001.5316. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Jiao ZH, Zheng LS, Zhang YY, Xie ST, Wang ZX, Wu JW. Structural insight into the autoinhibition mechanism of AMP-activated protein kinase. Nature. 2009;459:1146–9. doi: 10.1038/nature08075. [DOI] [PubMed] [Google Scholar]
- 25.Hudson ER, Pan DA, James J, et al. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Current Biol. 2003;13:861–6. doi: 10.1016/s0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- 26.Polekhina G, Gupta A, van Denderen BJ, Feil SC, Kemp BE, Stapleton D, Parker MW. Structural basis for glycogen recognition by AMP-activated protein kinase. Structure (Camb) 2005;13:1453–62. doi: 10.1016/j.str.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Scott JW, Hawley SA, Green KA, et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–84. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5'-AMP activated protein kinase activator, 5-aminoimidazole- 4-carboxamide-1-beta-d- ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun. 2001;287:562–7. doi: 10.1006/bbrc.2001.5627. [DOI] [PubMed] [Google Scholar]
- 29.He G, Zhang YW, Lee JH, et al. AMP-activated protein kinase induces p53 by phosphorylating MDMX and inhibiting its activity. Mol Cell Biol. 2014;34:148–57. doi: 10.1128/MCB.00670-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S, Wasserman DH, MacKintosh C, Sakamoto K. Mice with AS160/TBC1D4-Thr649Ala knockin mutation are glucose intolerant with reduced insulin sensitivity and altered GLUT4 trafficking. Cell Metab. 2011;13:68–79. doi: 10.1016/j.cmet.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor EB, An D, Kramer HF, et al. Discovery of TBC1D1 as an Insulin-, AICAR-, and Contraction-stimulated Signaling Nexus in Mouse Skeletal Muscle. J Biol Chem. 2008;283:9787–96. doi: 10.1074/jbc.M708839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, Mackintosh C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J. 2008;409:449–59. doi: 10.1042/BJ20071114. [DOI] [PubMed] [Google Scholar]
- 33.Pehmoller C, Treebak JT, Birk JB, et al. Genetic disruption of AMPK signaling abolishes both contraction- and insulin-stimulated TBC1D1 phosphorylation and 14-3-3 binding in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2009;297:E665–E75. doi: 10.1152/ajpendo.00115.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatakeyama H, Kanzaki M. Regulatory mode shift of Tbc1d1 is required for acquisition of insulin-responsive GLUT4-trafficking activity. Mol Biol Cell. 2013;24:809–17. doi: 10.1091/mbc.E12-10-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside: a specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–65. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 36.Merrill GM, Kurth E, Hardie DG, Winder WW. AICAR decreases malonyl-CoA and increases fatty acid oxidation in skeletal muscle of the rat. Am J Physiol. 1997;273:E1107–E12. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 37.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1{alpha} Proc Natl Acad Sci USA. 2007;104:12017–22. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canto C, Jiang LQ, Deshmukh AS, et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–9. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Xu S, Mihaylova MM, et al. AMPK phosphorylates and Inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–88. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muoio DM, Seefeld K, Witters LA, Coleman RA. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J. 1999;338:783–91. [PMC free article] [PubMed] [Google Scholar]
- 41.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 42.Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoppe S, Bierhoff H, Cado I, Weber A, Tiebe M, Grummt I, Voit R. AMP-activated protein kinase adapts rRNA synthesis to cellular energy supply. Proc Natl Acad Sci USA. 2009;106:17781–6. doi: 10.1073/pnas.0909873106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circ Res. 2007;100:474–88. doi: 10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- 45.Gollob MH, Seger JJ, Gollob TN, Tapscott T, Gonzales O, Bachinski L, Roberts R. Novel PRKAG2 mutation responsible for the genetic syndrome of ventricular preexcitation and conduction system disease with childhood onset and absence of cardiac hypertrophy. Circulation. 2001;104:3030–3. doi: 10.1161/hc5001.102111. [DOI] [PubMed] [Google Scholar]
- 46.Akman HO, Sampayo JN, Ross FA, et al. Fatal infantile cardiac glycogenosis with phosphorylase kinase deficiency and a mutation in the gamma-2 subunit of AMP-activated protein kinase. Pediatr Res. 2007;62:499–504. doi: 10.1203/PDR.0b013e3181462b86. [DOI] [PubMed] [Google Scholar]
- 47.Burwinkel B, Scott JW, Buhrer C, et al. Fatal congenital heart glycogenosis caused by a recurrent activating R531Q mutation in the g2 subunit of AMP-activated protein kinase (PRKAG2), not by phosphorylase kinase deficiency. Am J Hum Genet. 2005;76:1034–49. doi: 10.1086/430840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao B, Heath R, Saiu P, et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 49.Daniel TD, Carling D. Functional analysis of mutations in the γ2 subunit of AMP-activated protein kinase associated with cardiac hypertrophy and Wolff-Parkinson-White syndrome. J Biol Chem. 2002;277:51017–24. doi: 10.1074/jbc.M207093200. [DOI] [PubMed] [Google Scholar]
- 50.Hawley SA, Ross FA, Chevtzoff C, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–65. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arad M, B DW, Perez-Atayde AR, et al. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. 2002;109:357–62. doi: 10.1172/JCI14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arad M, Moskowitz IP, Patel VV, et al. Transgenic mice overexpressing mutant PRKAG2 define the cause of Wolff-Parkinson-White syndrome in glycogen storage cardiomyopathy. Circulation. 2003;107:2850–6. doi: 10.1161/01.CIR.0000075270.13497.2B. [DOI] [PubMed] [Google Scholar]
- 53.Sidhu JS, Rajawat YS, Rami TG, et al. Transgenic Mouse Model of Ventricular Preexcitation and Atrioventricular Reentrant Tachycardia Induced by an AMP-Activated Protein Kinase Loss-of-Function Mutation Responsible for Wolff-Parkinson-White Syndrome. Circulation. 2004 doi: 10.1161/01.CIR.0000151291.32974.D5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davies JK, Wells DJ, Liu K, et al. Characterization of the role of gamma2 R531G mutation in AMP-activated protein kinase in cardiac hypertrophy and Wolff-Parkinson-White syndrome. Am J Physiol Heart Circ Physiol. 2006;290:H1942–H51. doi: 10.1152/ajpheart.01020.2005. [DOI] [PubMed] [Google Scholar]
- 55.Luptak I, Shen M, He H, et al. Aberrant activation of AMP-activated protein kinase remodels metabolic network in favor of cardiac glycogen storage. J Clin Invest. 2007;117:1432–9. doi: 10.1172/JCI30658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim M, Hunter RW, Garcia-Menendez L, et al. Mutation in the gamma-2 subunit of AMPK stimulates cardiomyocyte proliferation and hypertrophy independent of glycogen storage. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.114.302364. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheung PCF, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase γ subunit isoforms and their role in AMP binding. Biochem J. 2000;346:659–69. [PMC free article] [PubMed] [Google Scholar]
- 58.Milan D, Jeon JT, Looft C, et al. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science. 2000;288:1248–51. doi: 10.1126/science.288.5469.1248. [DOI] [PubMed] [Google Scholar]
- 59.Holmes BF, Kurth-Kraczek EJ, Winder WW. Chronic activation of 5'-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol. 1999;87:1990–5. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]
- 60.Narkar VA, Downes M, Yu RT, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–15. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–77. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15:623–34. doi: 10.1016/j.cmet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 64.O'Neill HM, Maarbjerg SJ, Crane JD, et al. AMP-activated protein kinase (AMPK) {beta}1{beta}2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc Natl Acad Sci USA. 2011;108:16092–7. doi: 10.1073/pnas.1105062108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lochhead PA, Salt IP, Walker KS, Hardie DG, Sutherland C. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes. 2000;49:896–903. doi: 10.2337/diabetes.49.6.896. [DOI] [PubMed] [Google Scholar]
- 66.Koo SH, Flechner L, Qi L, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–14. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 67.Bergeron R, Previs SF, Cline GW, Perret P, Russell RR, 3rd, Young LH, Shulman GI. Effect of 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes. 2001;50:1076–82. doi: 10.2337/diabetes.50.5.1076. [DOI] [PubMed] [Google Scholar]
- 68.Buhl ES, Jessen N, Pold R, et al. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes. 2002;51:2199–206. doi: 10.2337/diabetes.51.7.2199. [DOI] [PubMed] [Google Scholar]
- 69.Song XM, Fiedler M, Galuska D, et al. 5-Aminoimidazole-4-caboxamide ribonucleoside treatment improves glucose homeostasis in insulin-resistant diabetic (ob/ob) mice. Diabetologia. 2002;45:56–65. doi: 10.1007/s125-002-8245-8. [DOI] [PubMed] [Google Scholar]
- 70.Iglesias MA, Ye JM, Frangioudakis G, et al. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002;51:2886–94. doi: 10.2337/diabetes.51.10.2886. [DOI] [PubMed] [Google Scholar]
- 71.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cool B, Zinker B, Chiou W, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–16. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 73.Lee YS, Kim WS, Kim KH, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–64. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 74.Foretz M, Hebrard S, Leclerc J, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–69. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vincent MF, Marangos PJ, Gruber HE, Van den Berghe G. Inhibition by AICA riboside of gluconeogenesis in isolated rat hepatocytes. Diabetes. 1991;40:1259–66. doi: 10.2337/diab.40.10.1259. [DOI] [PubMed] [Google Scholar]
- 76.Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494:256–60. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fullerton MD, Galic S, Marcinko K, et al. Single phosphorylation sites in ACC1 and ACC2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19:1649–54. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miyoshi H, Nakau M, Ishikawa TO, Seldin MF, Oshima M, Taketo MM. Gastrointestinal hamartomatous polyposis in LKB1 heterozygous knockout mice. Cancer Res. 2002;62:2261–6. [PubMed] [Google Scholar]
- 79.Nakau M, Miyoshi H, Seldin MF, Imamura M, Oshima M, Taketo MM. Hepatocellular carcinoma caused by loss of heterozygosity in LKB1 gene knockout mice. Cancer Res. 2002;62:4549–53. [PubMed] [Google Scholar]
- 80.Lizcano JM, Göransson O, Toth R, et al. LKB1 is a master kinase that activates 13 protein kinases of the AMPK subfamily, including the MARK/PAR-1 kinases. EMBO J. 2004;23:833–43. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang X, Wullschleger S, Shpiro N, et al. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412:211–21. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- 83.Faubert B, Boily G, Izreig S, et al. AMPK Is a negative regulator of the Warburg effect and suppresses tumor growth In vivo. Cell Metab. 2012;17:113–24. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thomas GV, Tran C, Mellinghoff IK, et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12:122–7. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- 85.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PloS one. 2012;7:e33411. doi: 10.1371/journal.pone.0033411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Algire C, Amrein L, Bazile M, David S, Zakikhani M, Pollak M. Diet and tumor LKB1 expression interact to determine sensitivity to anti-neoplastic effects of metformin in vivo. Oncogene. 2011;30:1174–82. doi: 10.1038/onc.2010.483. [DOI] [PubMed] [Google Scholar]
- 88.Shackelford DB, Abt E, Gerken L, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23:143–58. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanchez-Cespedes M, Parrella P, Esteller M, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–62. [PubMed] [Google Scholar]
- 90.Ji H, Ramsey MR, Hayes DN, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–10. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 91.Wingo SN, Gallardo TD, Akbay EA, et al. Somatic LKB1 mutations promote cervical cancer progression. PLoS ONE. 2009;4:e5137. doi: 10.1371/journal.pone.0005137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu W, Monahan KB, Pfefferle AD, et al. LKB1/STK11 inactivation leads to expansion of a prometastatic tumor subpopulation in melanoma. Cancer Cell. 2012;21:751–64. doi: 10.1016/j.ccr.2012.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee CW, Wong LL, Tse EY, et al. AMPK promotes p53 acetylation via phosphorylation and inactivation of SIRT1 in liver cancer cells. Cancer Res. 2012;72:4394–404. doi: 10.1158/0008-5472.CAN-12-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Horman S, Vertommen D, Heath R, et al. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281:5335–40. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 96.Hawley SA, Ross FA, Gowans GJ, Tibarewal P, Leslie NR, Hardie DG. Phosphorylation by Akt within the ST loop of AMPK-α1 down-regulates its activation in tumour cells. Biochem J. 2014 doi: 10.1042/BJ20131344. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Steinberg GR, Dandapani M, Hardie DG. AMPK: mediating the metabolic effects of salicylate-based drugs? Trends Endocrinol Metab. 2013;24:481–7. doi: 10.1016/j.tem.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Krawczyk CM, Holowka T, Sun J, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–9. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5'-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–41. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage {alpha}1-AMP-activated protein kinase ({alpha}1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285:19051–9. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Galic S, Fullerton MD, Schertzer JD, et al. Hematopoietic AMPK beta1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J Clin Invest. 2011;121:4903–15. doi: 10.1172/JCI58577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mounier R, Theret M, Arnold L, et al. AMPKalpha1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 2013;18:251–64. doi: 10.1016/j.cmet.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 103.Hawley SA, Fullerton MD, Ross FA, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–22. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Amann R, Peskar BA. Anti-inflammatory effects of aspirin and sodium salicylate. Eur J Pharmacol. 2002;447:1–9. doi: 10.1016/s0014-2999(02)01828-9. [DOI] [PubMed] [Google Scholar]
- 105.April P, Abeles M, Baraf H, et al. Does the acetyl group of aspirin contribute to the antiinflammatory efficacy of salicylic acid in the treatment of rheumatoid arthritis? Sem Arth Rheum. 1990;19:20–8. [PubMed] [Google Scholar]
- 106.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 107.Mankouri J, Tedbury PR, Gretton S, et al. Enhanced hepatitis C virus genome replication and lipid accumulation mediated by inhibition of AMP-activated protein kinase. Proc Natl Acad Sci USA. 2010;107:11549–54. doi: 10.1073/pnas.0912426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Street A, Macdonald A, Crowder K, Harris M. The Hepatitis C virus NS5A protein activates a phosphoinositide 3-kinase-dependent survival signaling cascade. J Biol Chem. 2004;279:12232–41. doi: 10.1074/jbc.M312245200. [DOI] [PubMed] [Google Scholar]
- 109.Mankouri J, Harris M. Viruses and the fuel sensor: the emerging link between AMPK and virus replication. Reviews Med Virol. 2011;21:205–12. doi: 10.1002/rmv.687. [DOI] [PubMed] [Google Scholar]
- 110.Wang DS, Jonker JW, Kato Y, Kusuhara H, Schinkel AH, Sugiyama Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J Pharmacol Exp Ther. 2002;302:510–5. doi: 10.1124/jpet.102.034140. [DOI] [PubMed] [Google Scholar]
- 111.Bailey CJ, Wilcock C, Day C. Effect of metformin on glucose metabolism in the splanchnic bed. Br J Pharmacol. 1992;105:1009–13. doi: 10.1111/j.1476-5381.1992.tb09093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang DS, Kusuhara H, Kato Y, Jonker JW, Schinkel AH, Sugiyama Y. Involvement of organic cation transporter 1 in the lactic acidosis caused by metformin. Mol Pharmacol. 2003;63:844–8. doi: 10.1124/mol.63.4.844. [DOI] [PubMed] [Google Scholar]
- 113.Cuthbertson DJ, Babraj JA, Mustard KJ, et al. 5-aminoimidazole-4-carboxamide 1-beta-D-ribofuranoside acutely stimulates skeletal muscle 2-deoxyglucose uptake in healthy men. Diabetes. 2007;56:2078–84. doi: 10.2337/db06-1716. [DOI] [PubMed] [Google Scholar]
- 114.Wolf CM, Arad M, Ahmad F, et al. Reversibility of PRKAG2 glycogen-storage cardiomyopathy and electrophysiological manifestations. Circulation. 2008;117:144–54. doi: 10.1161/CIRCULATIONAHA.107.726752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–6. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]