Abstract
Background
It is not known to what extent improvement over time in breast cancer survival is related to earlier detection by mammography or to more effective treatments.
Methods
At our comprehensive cancer care center we conducted a retrospective cohort study of women ages 50–69 years diagnosed with invasive stage I–III breast cancer and followed over three time periods: 1990–1994, 1995–1999 and 2000–2007. Data was chart abstracted on detection method, diagnosis, treatment, and follow up for vital status in our breast cancer registry (n=2998). Method of detection was categorized as patient or physician (Pt/PhysD) or mammography detected (MamD). Cox proportional hazards models to estimate adjusted hazard ratios (HR) and 95% confidence intervals (CI) for five year disease specific survival (DSS) in relation to detection method and treatment factors, testing for differences in survival using the Kaplan-Meier method.
Results
58% of cases were MamD and 42% were Pt/PhysD with 56% stage I, 31% stage II and 13% stage III. Average length of follow up was 10.71 years. Combined five year DSS was 89% 1990–94, 94% 1995–99, and 96% in 2000–2007 (p<.001). In an adjusted model, mammography detection (HR=0.43, 95% CI 0.27–0.70), hormone therapy (HR=0.47, 95% CI 0.30–0.75), and taxane-containing chemotherapy (HR=0.61, 95% CI 0.37–0.99) were significantly associated with a decreased risk of disease-specific mortality
Conclusions
Better breast cancer survival over time is related to mammography detection, hormonal therapy and taxane-containing chemotherapy treatment. Treatment improvements alone are not sufficient to explain the observed survival improvements over time.
Keywords: breast cancer, survival, regression modeling, mammography, adjuvant chemotherapy
Introduction
Survival following a diagnosis of breast cancer has improved dramatically in the past 20 years with the development of early diagnosis tools and new treatment regimens. Between 1990 and 2010, there has been a 34% decrease in breast cancer mortality among women in the United States1. Modeling studies have indicated both screening mammography and treatment contribute to the survival improvement observed2, 3. However, international debate regarding the relative contribution of mammography screening programs versus treatment improvements to increased survival are ongoing4, 5, 6, 7. As no evidence exists to suggest a change in the biologic nature of breast cancer during this time period, it is important to examine how much improvement in primary breast cancer outcomes result from early diagnosis and treatment improvement.
In the United States breast cancer screening is largely opportunity based and dependent upon women and their physicians following published guidelines and recommendations. For the past twenty years guidelines have consistently recommended women age 50 and over have annual mammograms8, 9. In this situation a registry based study including both method of detection and treatment and validated by comparison to national statistics is an available method to evaluate factors influencing survival improvement over time10. In this study we evaluate factors related to breast cancer specific survival over time among breast cancer patients age 50–69 years at our comprehensive community cancer center to test the relative effect of breast cancer detection method and treatment adjusting for factors known to be related to survival.
Methods
Our institution is a comprehensive community cancer program that follows National Comprehensive Cancer Network (NCCN) clinical practice guidelines11. From our institutional breast cancer registry cohort we identified stage I – III patients age 50–69, 1990–2007 (n=2998). Cases with unknown detection method (n=51), non-surgical cases (n=2) or lost to follow up (n=2) were excluded. We confined our study to women age 50–69 years as this age group has had consistent USPSTF recommendations for screening over the study time period 12.
The breast cancer clinical data base registry is for all breast cancer patients seen at our institution with detailed information on method of diagnosis, patient characteristics, stage at diagnosis and follow up for disease and mortality outcomes. Clinical presentation characteristics including age, race, stage, and method of detection by patient (PtD), physician (PhysD) or mammography (MamD) were chart abstracted at time of diagnosis13.
Registry follow up is updated annually by a certified cancer registrar with information on recurrence, subsequent treatment and vital status, current through 2012. Vital and disease status information is obtained from chart review if the patient is still seen at our institution or through physician-directed follow up letter if follow up care is provided elsewhere. Patients not under the care of a managing physician are contacted by mail using an institutional review board (IRB) approved letter from their diagnosing physician. If no response is received, the institution’s cancer registry and the Surveillance Epidemiology and End Results (SEER) Seattle-Puget Sound Registry are reviewed for patient’s vital and disease status14.
IRB approved methods were used for patient data input and registry data are stored in a password protected HIPAA-compliant database. Analyses were conducted using de-identified data as per IRB and HIPAA guidelines. This project was reviewed and approved by the IRB at our community based regional cancer center.
Initial breast cancer (BC) detection method information was obtained by careful review of patient medical records by a certified cancer registrar. The three detection methods were mammography (MamD), physician exam (PhysD) or patient detection (PtD). A mammography-detected breast cancer refers to disease discovered by routine mammography in the absence of complaints or known physical findings or as a repeat mammogram to verify a previous equivocal mammography finding. Patient detection was assigned if the patient presented with personally detected breast symptoms, such as a palpable lump, pain, swelling, nipple discharge, or bleeding which prompted her to schedule a doctor visit. Patients with self-detected tumors may have subsequently had a mammogram or ultrasound done but would still be labeled as a patient-detected breast cancer. Physician detection is defined as initiation of work up for breast cancer by physical findings discovered by the physician at routine visit or visit for other problems. The detection method designation was only made when it was certain from the record. If the detection method was ambiguous or incomplete, the tumor detection method was marked as unknown and these patients were excluded from the analysis. Manually detected breast cancer, by the patient or physician, was combined into one group for the analysis.
We examined the distribution of variables to ensure they met the assumptions of the statistical tests employed and then compared breast cancer presentation and treatment characteristics by diagnosis year. Differences were assessed using the Pearson Chi-square test for categorical variables and analysis of variance for mean comparisons in SPSS version 21.015. The Kaplan-Meier method was used to estimate survival curves from breast cancer diagnosis. All P values are two-tailed.
To test treatment and detection method effect on five year invasive breast cancer specific survival (stage I–III), we used Cox regression analysis with breast cancer specific death as the outcome for disease specific survival (DSS). Our institutional cohort for the survival analysis was truncated at diagnosis year 2007 to allow for a minimum five years follow up through 2012 for mortality. Multivariate adjusted hazard ratios (HR) and 95% confidence intervals were estimated using Cox proportional hazards models to assess whether treatments and detection methods were associated with disease-specific survival. We modeled time from the incident breast cancer (time scale) with a left truncation of 90 days post-surgery (at risk date) to survival at 5 years as a function of exposure to chemotherapy and detection methods. All models were adjusted for confounders selected a priori, including age (50–59, 60–64, 65–69 years), tumor stage (I, II, III), diagnosis year (1990–1994, 1995–1999, 2000–2007), race (white, non-white), estrogen receptor status (negative, positive), radiotherapy (yes/no), adjuvant chemotherapy (yes/no), anthracycline chemotherapy, taxane chemotherapy and hormone therapy (yes/no) as categorical variables. From 1999–2007 14% of her2-neu tested patients were positive (282/2039) and of those 41% were treated with trastuzumab (117/282). Due to the recency of her2/neu testing and the low number treated, her2/neu and trastuzumab treatment were not included in the model as the estimates were unstable.
Proportional hazards assumptions were evaluated by testing the interaction between treatments and detection methods and the logarithm of follow-up time. There was no evidence suggesting a violation of the proportional hazards assumptions for all exposure-outcome pairs. Cox proportional hazard analyses were performed using Stata IC 1316.
We describe disease specific survival in our cohort using detailed registry-based information on disease-specific cause of death. We also report the relative survival from the Seattle-Puget Sound Cancer Surveillance System (CSS) for years 1990–2005 for comparison purposes17. CSS is a population-based Surveillance, Epidemiology and End Results (SEER) Program cancer registry18. Cancer survival reported by the SEER registry is approximated using relative survival, the ratio of the proportion of observed survivors (all causes of death) at 5 years of women diagnosed with breast cancer to the proportion of expected survivors in a cancer-free cohort calculated using survival life tables, assuming that cancer deaths are a negligible proportion of all deaths in the overall age-standardized population19, 20.
Results
Our cohort consisted of 2998 primary breast cancer patients age 50–69 (mean age 58.50) diagnosed between 1990 and 2007. Patients were predominantly white race with 58% of cases MamD and 42% Pt/PhysD (table 1). 56% were stage I, 31% stage II and 13% stage III. The majority of patients were estrogen receptor positive (83%) and the majority of those patients received hormone therapy (84%). Fifty percent of patients received adjuvant chemotherapy with 35% of those receiving chemotherapy getting an anthracycline containing regimen and 21% receiving a taxane containing regimen. Of patients receiving a non anthracycline chemotherapy treatment regimen, 17% received taxane therapy (n=84). Fifty two percent of patients (n=1044) had anthracycline or taxane therapy with 52% (n=548) receiving both. Forty nine percent of patients did not receive chemotherapy treatment (n=1458). Average length of follow up was 10.71 years, range 2 to 23 years with 14,524 person years of follow up.
Table 1.
Analysis by time interval stage I–III breast cancer patients age 50–69 (n=2998)
| 1990–2007 (n=2998) | 1990–1994 (n=492) | 1995–1999 (n=768) | 2000–2007 (n=1738) | Chi square | p value | |
|---|---|---|---|---|---|---|
| Variables | N (%) | N (%) | N (%) | |||
| Age | ||||||
| 50–59 years | 1726 (58%) | 244 (50%) | 443 (58%) | 1039 (60%) | 28.13 | <.001 |
| 60–64 years | 695 (23%) | 125 (25%) | 158 (21%) | 412 (24%) | ||
| 65–69 years | 577 (19%) | 123 (25%) | 167 (22%) | 287 (16%) | ||
| Race | ||||||
| White | 2645 (88%) | 451 (92%) | 707 (92%) | 1487 (86%) | 28.37 | <.001 |
| Non-white | 353 (12%) | 41 (8%) | 61 (8%) | 251 (14%) | ||
| TNM stage | ||||||
| I | 1678 (56%) | 263 (54%) | 427 (56%) | 988 (57%) | 7.24 | .124 |
| II | 935 (31%) | 148 (30%) | 246 (32%) | 541 (31%) | ||
| III | 385 (13%) | 81 (17%) | 95 (12%) | 209 (12%) | ||
| Estrogen Receptor status | ||||||
| Negative | 2448 (83%) | 97 (21%) | 136 (18%) | 269 (16%) | 7.29 | .026 |
| Positive | 502 (17%) | 375 (79%) | 616 (82%) | 1457 (84%) | ||
| Detection Method | ||||||
| Mammography | 1745 (58%) | 255 (52%) | 422 (55%) | 1068 (61%) | 19.09 | <.001 |
| Patient/Physician | 1253 (42%) | 237 (48%) | 346 (45%) | 670 (39%) | ||
| Hormone treatment* | * estrogen receptor positive patients only (n=2448) | |||||
| yes | 2061 (84%) | 283 (76%) | 511 (83%) | 1267 (87%) | 30.54 | <.001 |
| no | 387 (16%) | 92 (25%) | 105 (17%) | 190 (13%) | ||
| Chemotherapy | ||||||
| Yes | 1540 (51%) | 197 (40%) | 387 (50%) | 956 (55%) | 34.77 | <.001 |
| No | 1458 (49%) | 295 (60%) | 381 (50%) | 782 (45%) | ||
| Anthracycline treatment | ||||||
| anthracycline | 1044 (35%) | 96 (20%) | 288 (38%) | 660 (38%) | 68.69 | <.001 |
| Non-anthracycline | 496 (17%) | 101 (21%) | 99 (13%) | 296 (17%) | ||
| No chemotherapy | 1458 (49%) | 295 (60%) | 381 (49%) | 782 (45%) | ||
| Taxane treatment | ||||||
| Taxane regimen | 632 (21%) | 7 (1%) | 115 (15%) | 514 (30%) | 211.66 | <.001 |
| Non-taxane regimen | 908 (30%) | 194 (39%) | 272 (35%) | 442 (25%) | ||
| No chemotherapy | 1458 (49%) | 295 (60%) | 381 (49%) | 782 (45%) | ||
| Radiation treatment | ||||||
| Yes | 2459 (82%) | 386 (78%) | 671 (87%) | 1402 (81%) | 21.30 | <.001 |
| No | 539 (18%) | 106 (22%) | 97 (13%) | 336 (19%) | ||
| Died of disease | ||||||
| Yes | 261 (9%) | 91 (19%) | 80 (10%) | 90 (5%) | 89.37 | <.001 |
| No | 2737 (91%) | 401 (81%) | 688 (90%) | 1648 (95%) | ||
estrogen receptor positive patients only (n=2448)
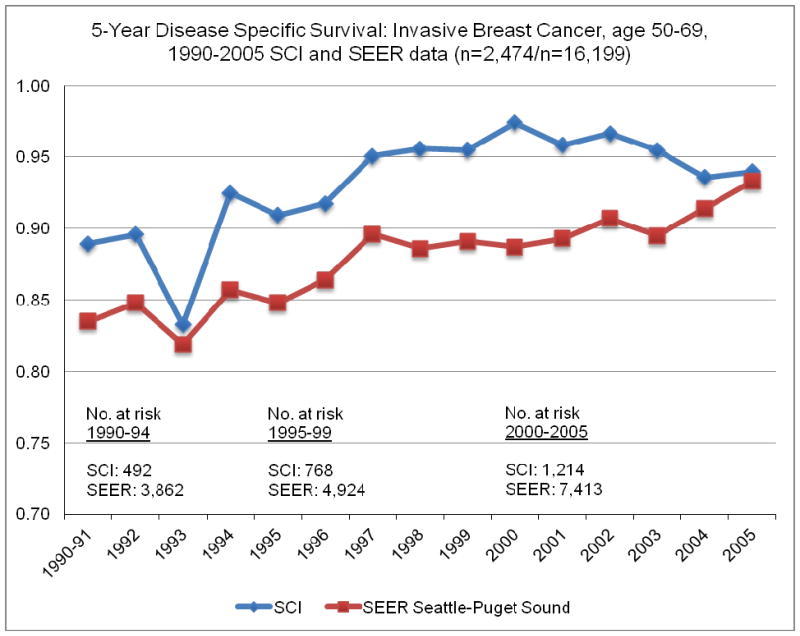
We compared the five year disease specific survival for our patient cohort (n=2998) and the SEER Puget Sound Seattle area five year relative survival from 1990–2005 (n= 16,199) for stage I–III breast cancer patients (figure 1). Statistically significant improvement occurred over time for both populations with time periods 1990–1994, 1995–1999 and 2000–2005 distinctively different. Based on the observed survival improvement over time we divided diagnosis year in our cohort into three time intervals 1990–1994, 1995–1999 and 2000–2007. The breast cancer specific survival (BCSS) for these three time periods in our cohort were statistically significantly different with five year DSS = 91% for 1990–1994, 96% for 1995–1999, and 97% for 2000–2007 (log rank test = 25.50, p<.001).
Figure 1.
Five Year Disease Specific Survival 1990–2005: SEER and SCI data
Statistically significant changes over time were observed for presentation, diagnostic and treatment variables (table 1). The cohort composition trended towards younger age, more non-white, and more mammography detection, hormone treatment, and chemotherapy treatment including increased anthracyline and taxane adjuvant therapy over other now non-standard therapies (p<.001 for each). Mammography detection increased from 52% of cases in 1990–94 to 61% in 2000–07. Over time treatment with hormone therapy increased 11%, chemotherapy treatment increased 15% and anthracycline treatment increased 18%. Adjuvant chemotherapy with taxane containing regimens did not begin until 1994. In the two time periods after 1994, taxane treatment increased 24% from 30% of chemotherapy treated patients in 1995–99 to 54% in 2000–07.
In Cox regression analysis we tested for the effect of detection method and treatment adjusted for age, race, diagnosis year (3 category), TNM stage, and ER status. Compared to women diagnosed with invasive BC in 1990–94, women diagnosed in later time intervals had significantly lower risk of five year mortality (1995–99 (HR=0.51, 95% CI 0.31–0.83), 2000–07 (HR=0.38, 95% CI, 0.24–0.62) P-trend <.001). Treatment variables included in the model were chemotherapy (yes/no), anthracycline therapy, taxane therapy, radiation, and hormone therapy (table 2). Detection method, taxane treatment and hormone therapy were all associated with improved invasive disease specific survival. By detection method, women with mammography detected breast cancer had significantly decreased risk of five-year disease specific death (HR=0.43, 95% CI, 0.27–0.70). Hormone therapy treatment and taxane containing therapy treatment were both associated with significant reduction in breast cancer mortality (hormone therapy: HR=0.47, 95% CI 0.30–0.75, taxane containing therapy: HR=0.61, 95% CI 0.37–0.99).
Table 2.
Risk of five-year breast cancer-specific mortality in relation to breast cancer detection method and treatments (n = 2998)
| Adjusted HR a | (95% CI) | p value | |
|---|---|---|---|
| Diagnosis Year | |||
| 1990–1994 | 1.0 | reference | |
| 1995–1999 | 0.51 | (0.31–0.83) | 0.007 |
| 2000–2007 | 0.38 | (0.24–0.62) | <0.001 |
| p-trend b | 0.001 | ||
| Detection Method | |||
| Patient/Physician | 1.0 | reference | |
| Mammography | 0.43 | (0.27–0.70) | 0.001 |
| Radiation treatment | |||
| No | 1.0 | reference | |
| Yes | 0.83 | (0.51–1.32) | 0.433 |
| Hormone treatment | |||
| No | 1.0 | reference | |
| Yes | 0.47 | (0.30–0.75) | 0.002 |
| Chemotherapy | |||
| No | 1.0 | reference | |
| Yes | 1.39 | 0.74–2.63 | 0.311 |
| Anthracyclines | |||
| Non-anthracycline-containing | 1.0 | reference | |
| Anthracycline-containing | 1.03 | (0.66–1.61) | 0.895 |
| Taxanes | |||
| Non-taxane-containing | 1.0 | reference | |
| Taxane-containing | 0.61 | (0.37–0.99) | 0.050 |
Abbreviations: HR, hazard ratio; CI, confidence interval
Risk estimates adjusted for TNM stage, age, race, and estrogen receptor status
p-trend is for HR of diagnosis year as a linear term
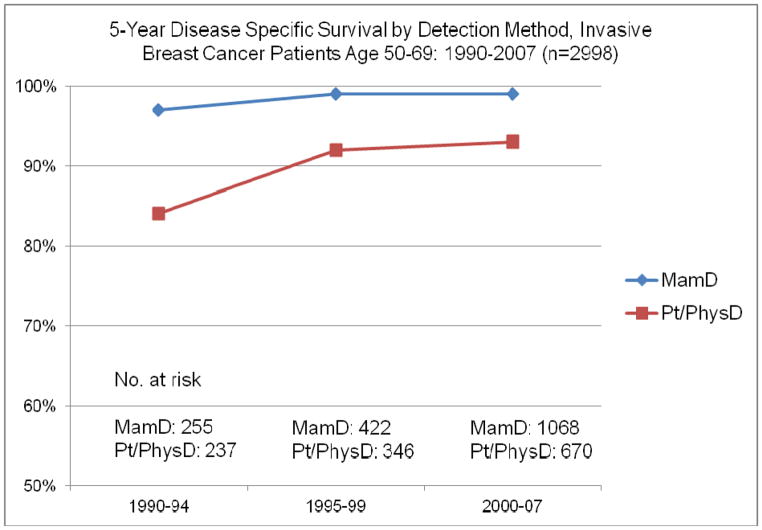
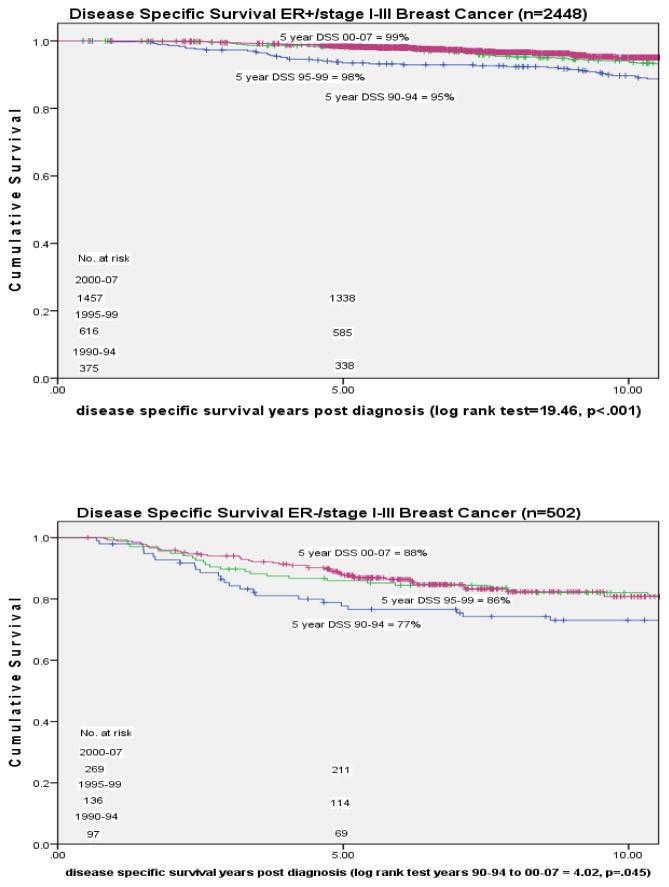
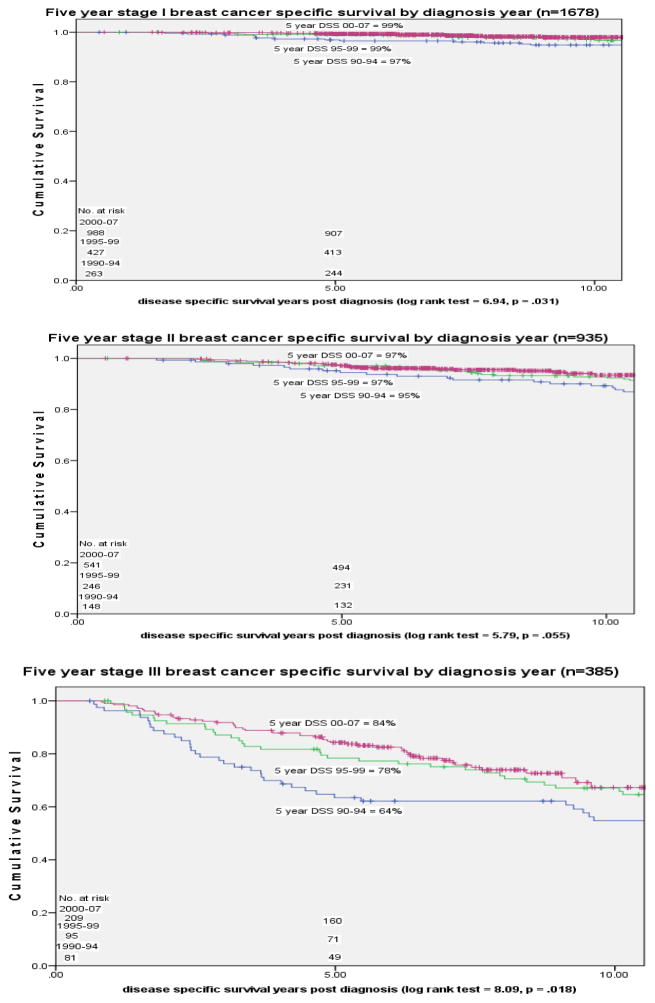
In an analysis of DSS by different presenting diagnostic characteristic categories we observed statistically significant change over time for patients in both detection method categories, MammD and Pt/PhysD, in each breast cancer stage, I–III, and in both estrogen receptor positive and negative tumor status patients (figures 2–4). For MammD and Pt/PhysD breast cancer patients from 1990–1994 to 2000–2007 there was a 9% improvement in DSS for the Pt/PhysD patients [84% to 93%, log rank test = 14.43, p <.001] and 2% improvement for mammography detected patients [97% to 99%, log rank test = 4.55, p = .033] (figure 2). The largest improvement over time in survival by stage was among stage III patients [stage I: 1990–94= 97%, 2000–07 = 99%, p = .031; stage II: 1990–94 = 95%, 2000–07 = 97%, p = .055; stage III: 1990–94 = 64%, 2000–07 = 84%, p = .018] (figure 3). The largest improvement over time in DSS by estrogen receptor status was among the estrogen receptor negative patients [ER positive: 1990–94 = 94%, 2000–07 = 99%, p<.001; ER negative: 1990–94 = 77%, 2000–2007 = 88%, p = .045] (figure 4).
Figure 2.
Five Year Disease Specific Survival by Detection Method 1990–2007
Figure 4.
Five year Disease Specific Survival by estrogen receptor status and diagnosis year
Figure 3.
Five Year Disease Specific Survival by stage and diagnosis year
Discussion
In our institutional cohort study of primary invasive breast cancer patients age 50–69 years we found both method of detection and changes in treatment were significant predictors of better five year disease specific survival in all time periods studied. Mammography detection was associated with the largest decrease in risk of breast cancer mortality (57%) with a similar but smaller reduction associated with hormone therapy (53%) and taxane treatment (39%). Correcting for stage at diagnosis, age, year diagnosed, radiation treatment and estrogen receptor status, we also found hormone therapy and to a lesser degree taxane therapy to be associated with better survival over time. Women diagnosed and treated in the most recent time period had the best survival. Our institutional cohort exhibited similar five year disease specific survival improvement as reported nationally and by direct comparison to the Seattle-Puget Sound population for the same time period. As both treatment and detection influenced disease specific survival over time, some of the improvement could be attributed to the simultaneous improvement of early detection with mammography and improved treatment of the earlier detected disease.
With regard to treatment effects, the two major changes that have occurred over this time period were the introduction of taxane adjuvant therapy and the standardized acceptance of adjuvant hormone treatment for estrogen receptor positive disease21, 22, 23, 24. The introduction of hormonal therapy and to a lesser extent taxane treatment appear to have had a significant favorable impact on survival as indicated by the favorable hazard ratios for disease specific mortality (mammography detection HR .43, hormone treatment HR .47, taxane treatment HR .61). We did not observe a significant change in outcome by anthracyclines during the time period studied, perhaps because they were already in widespread use prior to 1990. Five year disease specific survival improved over time for mammography and patient/physician detected breast cancers, stage I–III breast cancers and estrogen receptor positive and estrogen receptor negative breast cancers. Small improvements in survival among the most commonly diagnosed types of breast cancer, mammography detected, stage I/II, and estrogen receptor positive, contributed significantly to the overall improvement seen over time. Larger improvements in DSS were seen in the less common types of breast cancer i.e. patient/physician detected, stage III, and ER negative.
Survival improvement was almost equivalent between mammography detection and treatment (mammography detection HR .43, hormone treatment HR .47, taxane treatment HR .61). Mammography detection’s significant effect in a model corrected for both estrogen receptor status and stage may be due to the early detection profile of mammography detected breast cancer which includes estrogen receptor positive status, smaller tumor size, absence of lymph node involvement, fewer lymph nodes involved if there are positive nodes and possibly lower risk tumor biology25, 26, 27.
In the United States breast cancer mortality rates have continuously declined since 1990 but we have no organized screening in place to evaluate the effect of mammography screening in the U.S.28. Mortality measured by age adjusted years of potential life lost before age 75 from breast cancer has also dropped significantly from 1980 to 2005 with a total decline of 43% over 25 years29. Analyzing breast cancer mortality trends, van Schoor et al observed a 65% difference in the time period 1992–2008 between screened and unscreened women and trends in breast cancer mortality in France decreased annually 1990–2005 especially in areas where organized screening began in 1989–199130, 31. Pisani et al observed a decline in 3 year breast cancer mortality from 1982 to 1999 in Yorkshire associated with more favorable stage at diagnosis but not associated with systemic therapy32.
We restricted our study to patients age 50–69, the age group with the most consistent screening recommendations over the time period studied12. We also limited the analysis to invasive forms of breast cancer to avoid lead time bias which may be introduced with inclusion of in situ tumors. We do not have direct measures of improvements in surgical techniques or radiation therapy over time but published studies indicate improvements have occurred during this time period33, 34. As her-2/neu testing was nascent in 1999 and only became standard testing during the latter part of our study, her-2/neu status and trastuzumab treatment were not evaluated as factors of influence on survival35. However, the observed steady improvement in breast cancer mortality began prior to the regular use of trastuzumab in 199936. As approximately 18% of breast cancers demonstrate her-2/neu amplification the contribution of trastuzumab therapy would be modest relative to the overall improvement observed37, 38. To include the largest possible number of patients affected by improvement in both detection and treatment over time we restricted the reporting of our outcome of interest to five year DSS.
A limitation of our study is the degree to which the results are representative of the greater US population. Our institution is located in an area where the general population has above average education with readily available access to high quality care. The county in which our institution is located has relatively high access to mammography screening services, and more of our population may undergo screening than populations in other areas of the United States39. In this regard our cohort may not be representative of the US population at large. Although we do not know if patients participated in mammography screening programs, we do know from chart review the method by which breast cancers were detected.
In this study we evaluated the impact of treatment change over time on breast cancer mortality in a single institution cohort as well the impact of mammography detection compared to patient/physician detection. These results indicate improvements in breast cancer mortality resulted from improvements in both treatment and early detection by mammography with both contributing to the relative change observed over time. The closely equivalent association of detection method and treatment effect on disease specific mortality indicates treatment does not override the impact of mammography detection on survival. Further studies of breast cancer mortality change over time require the evaluation of method of breast cancer detection, participation in mammography screening programs and treatment. While large, long term randomized trials might answer these questions, it is likely that changes in both screening technology and treatment modalities will continue to occur too rapidly to make such studies practical. In order to continue to improve outcomes for breast cancer patients we will need to continue the development of better treatment options, continue promotion of participation in mammography screening programs and develop more accurate and sensitive means of detecting disease early in its clinical course.
Acknowledgments
This research was supported by the Kaplan Cancer Research Fund, the Cancer Surveillance System at the Fred Hutchinson Cancer Research System and NIH Cancer Prevention Training Grant in Nutrition, Exercise, and Genetics (R25CA094880)
Footnotes
The authors have no financial disclosures
Bibliography
- 1.DeSantis C, Ma J, Bryan L, Jemal A. Breast Cancer Statistics 2013. CA Cancer J Clin. 2014;64:52–63. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 3.Mandelblatt J, van Ravesteyn N, Schechter C, et al. Which strategies reduce breast cancer mortality most? Collaborative modeling of optimal screening, treatment and obesity prevention. Cancer. 2013;119(14):2541–2548. doi: 10.1002/cncr.28087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalager M, Zelen M, Langmark F, Adami HO. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363(13):1203–1210. doi: 10.1056/NEJMoa100727. [DOI] [PubMed] [Google Scholar]
- 5.Marmot MG. Sorting through the arguments on breast screening. JAMA. 2013;309(24):2553–2554. doi: 10.100/jama.2013.6822. [DOI] [PubMed] [Google Scholar]
- 6.Jatoi I. The impact of advances in treatment on the efficacy of mammography screening. Prev Med. 2011;53(3):103–104. doi: 10.1016/j.ypmed.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 8.American Cancer Society. [Accessed 3/26/2014];Chronological history of ACS recommendations for the early detection of cancer in people without cancer symptoms. http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/chronological-history-of-acs-recommendations.
- 9.Woolf SH. United States Preventive Services Task Force recommendations on breast cancer screening. Cancer. 1992;69(7 Suppl):1913–1918. doi: 10.1002/1097-0142(19920401)69:7+<1913::aid-cncr2820691707>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Von Euler-Chelpin M, Lynge E, Reboli M. Register-based studies of cancer screening effects. Scand J Public Health. 2011;39(7 Suppl):158–164. doi: 10.1177/1403494811401479. [DOI] [PubMed] [Google Scholar]
- 11.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Breast Cancer. Version 2.2014, 03/06/14 ©. National Comprehensive Care Network, Inc; 2014. [Accessed 3/25/2014]. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [Google Scholar]
- 12.US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–726. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 13.Edge SB, Burd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. 7. New York: Springer-Verlag; 2010. [Google Scholar]
- 14.Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute. Bethesda, Md: Cancer Surveillance System of the Fred Hutchinson Cancer Research Center, contract No. N01-CN-67009. [Google Scholar]
- 15.IBM Corp. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp; Released 2012. [Google Scholar]
- 16.Stata/IC Statistical Software Version 13. StataCorp LP; College Station, TX: [Google Scholar]
- 17.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data, Nov 2013 Sub (1973–2011) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, based on the November 2013 submission.
- 18.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2008, based on November 2012 SEER data submission, posted to the SEER web site. Bethesda, (MD): National Cancer Institute; 2012. [Accessed 2014 January 27]. Available from: http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 19.Cho H, Howlader N, Mariotto AB, Cronin KA. Technical Report #2011-01. Surveillance Research Program, National Cancer Institute; Estimating relative survival for cancer patients from the SEER program using expected rates based on Ederer I versus Ederer II method. http://surveillance.cancer.gov/reports/tech2011.01.pdf. [Google Scholar]
- 20.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 21.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21(6):976–83. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 22.Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23(16):3686–96. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 23.Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. Early Breast Cancer Trialists’ Collaborative Group. N Engl J Med. 1988;319(26):1661–92. doi: 10.1056/NEJM198812293192601. [DOI] [PubMed] [Google Scholar]
- 24.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet. 1998;351(9114):1145–67. [PubMed] [Google Scholar]
- 25.Heimann R, Munsell M, McBride R. Mammographically detected breast cancers and the risk of axillary lymph node involvement: is it just the tumor size? Cancer J. 2002;8(3):276–81. doi: 10.1097/00130404-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Drukker CA, Schmidt MK, Rutgers EJT, et al. Mammographic screening detects low-risk tumor biology breast cancers. Breast Cancer Res Treat. 2014;144:103–111. doi: 10.1007/s10549-013-2830-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malmgren JA, Parikh J, Atwood MK, Kaplan HG. Impact of mammography detection on the course of breast cancer in women aged 40–49 years. Radiology. 2012;262(3):797–806. doi: 10.1148/radiol.11111734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Cancer Institute. The surveillance, epidemiology, and end results (SEER) cancer statistics review 1975–2007. National Cancer Institute; Bethesda: 2012. [Google Scholar]
- 29.National Center for Health Statistics. Health, United States, 2013: With Special Feature on Prescription Drugs. Hyattsville, Maryland: 2014. http://www.cdc.gov/nchs/data/hus/hus13.pdf. [PubMed] [Google Scholar]
- 30.Van Schoor G, Moss SM, Otten JD, et al. Increasingly strong reduction in breast cancer mortality due to screening. Br J Cancer. 2011;104(6):910–14. doi: 10.1038/bjc.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molinie F, Vanier A, Woronoff AS, et al. Trends in breast cancer incidence and mortality in France 1990–2008. Breast Cancer Res Treat. 2014;147(1):167–75. doi: 10.1007/s10549-014-3073-9. [DOI] [PubMed] [Google Scholar]
- 32.Pisani P, Forman D. Declining mortality from breast cancer in Yorkshire, 1983–1998: extent and causes. Br J Cancer. 2004;90(3):652–6. doi: 10.1038/sj.bjc.6601614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10801 women in 17 randomized trials. Lancet. 2011;378:1707–16. doi: 10.1016/s0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal S, Pappas L, Neumayer L, Kokeny K, Agarwal J. Effect of breast conservation therapy vs. mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014;149(3):267–274. doi: 10.1001/jamasurg2013.3049. [DOI] [PubMed] [Google Scholar]
- 35.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 36.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressng metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 37.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5(1):63–69. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 38.Yaziji H, Goldstein LC, Barry TC, et al. HER-2 testing in breast cancer using parallel tissue-based methods. JAMA. 2004;291(16):1972–1927. doi: 10.1001/jama.291.16.1972. [DOI] [PubMed] [Google Scholar]
- 39.Elkin EB, Ishill NM, Snow JG, et al. Geographic access and the use of screening mammography. Med Care. 2010;48:349–356. doi: 10.1097/MLR.0b013e3181ca3ecb. [DOI] [PMC free article] [PubMed] [Google Scholar]