Abstract
Background
Accurate diagnosis and subsequent treatment of latent tuberculosis infection (LTBI) is essential for TB elimination. However, the absence of a gold standard test for diagnosing LTBI makes assessment of the true prevalence of LTBI and the accuracy of diagnostic tests challenging. Bayesian latent class models can be used to make inferences about disease prevalence and the sensitivity and specificity of diagnostic tests using data on the concordance between tests. We performed the largest meta-analysis to date aiming to evaluate the performance of tuberculin skin test (TST) and interferon-gamma release assays (IGRAs) for LTBI diagnosis in various patient populations using Bayesian latent class modelling.
Methods
Systematic search of PubMeb, Embase and African Index Medicus was conducted without date and language restrictions on September 11, 2017 to identify studies that compared the performance of TST and IGRAs for LTBI diagnosis. Two IGRA methods were considered: QuantiFERON-TB Gold In Tube (QFT-GIT) and T-SPOT.TB. Studies were included if they reported 2x2 agreement data between TST and QFT-GIT or T-SPOT.TB. A Bayesian latent class model was developed to estimate the sensitivity and specificity of TST and IGRAs in various populations, including immune-competent adults, immune-compromised adults and children. A TST cut-off value of 10 mm was used for immune-competent subjects and 5 mm for immune-compromised individuals.
Findings
A total of 157 studies were included in the analysis. In immune-competent adults, the sensitivity of TST and QFT-GIT were estimated to be 84% (95% credible interval [CrI] 82–85%) and 52% (50–53%), respectively. The specificity of QFT-GIT was 97% (96–97%) in non-BCG-vaccinated and 93% (92–94%) in BCG-vaccinated immune-competent adults. The estimated figures for TST were 100% (99–100%) and 79% (76–82%), respectively. T-SPOT.TB has comparable specificity (97% for both tests) and better sensitivity (68% versus 52%) than QFT-GIT in immune-competent adults. In immune-compromised adults, both TST and QFT-GIT display low sensitivity but high specificity. QFT-GIT and TST are equally specific (98% for both tests) in non-BCG-vaccinated children; however, QFT-GIT is more specific than TST (98% versus 82%) in BCG-vaccinated group. TST is more sensitive than QFT-GIT (82% versus 73%) in children.
Conclusions
This study is the first to assess the utility of TST and IGRAs for LTBI diagnosis in different population groups using all available data with Bayesian latent class modelling. Our results challenge the current beliefs about the performance of LTBI screening tests, and have important implications for LTBI screening policy and practice. We estimated that the performance of IGRAs is not as reliable as previously measured in the general population. However, IGRAs are not or minimally affected by BCG and should be the preferred tests in this setting. Adoption of IGRAs in settings where BCG is widely administered will allow for a more accurate identification and treatment of LTBI.
Introduction
Reliable detection of latent tuberculosis infection (LTBI) is a priority as this will help direct appropriate use of limited resources for tuberculosis (TB) control. One-third of the world’s population have LTBI with 10% of these individuals eventually developing active TB [1]. The risk of progression from LTBI to active TB is considerably higher in the presence of predisposing factors such as immune-compromised conditions [2]. Treatment costs of TB, particularly multi-drug-resistant infection are high [3]. Cases with pulmonary TB disease are the source of ongoing transmission in the community.
Diagnosis of LTBI suffers from the absence of a gold standard test. The tuberculin skin test (TST) remains the most widely used principally due to its low cost. However, it is substantially affected by cross-reactivity with non-tuberculous mycobacterial proteins found in the Bacillus Calmette-Guérin (BCG) vaccine, causing false-positive test results [4]. Interferon-gamma release assays (IGRAs), including the commercially available assays QuantiFERON-TB Gold In Tube (QFT-GIT; Qiagen, Hilden, Germany), and the T-SPOT.TB (Oxford Immunotec, Oxfordshire, UK), are used as alternatives to TST in settings where higher test acquisition costs can be supported. IGRAs are thought to be more specific than TST as they measure interferon-gamma released by T-cells after stimulation with Mycobacterium tuberculosis-specific antigens absent in BCG and most non-tuberculosis mycobacteria [5].
The diagnostic performance of IGRAs for LTBI in clinical practice has been evaluated in a number of studies in immune-competent adults, which largely show that these tests have higher specificity than TST [6,7]. The data on the reliability of IGRAs for the diagnosis of LTBI in immune-compromised adults and children have not been resolved with certainty. Without a gold standard, the true prevalence of disease and accuracy of diagnostic tests are difficult to measure reliably. Many studies have instead compared the performance of IGRAs against TST by evaluating the agreement between these tests.
Bayesian latent class models can be used to make inferences about disease prevalence and the sensitivity and specificity of diagnostic tests using data on the concordance between tests [8–10]. This approach is based on the notion that the observed results of various imperfect diagnostic tests for the same disease are influenced by an underlying unobserved (i.e. latent) variable, the true disease status [8–10]. In this study, we used the Bayesian latent class modelling approach to evaluate the diagnostic performance of IGRAs (QFT-GIT and T-SPOT.TB) and TST for the diagnosis of LTBI in various population groups.
Methods
Search strategy and selection criteria
A systematic literature search of PubMed, Embase and African Index Medicus databases was conducted on September 11, 2017 to identify original studies that evaluated the concordance between TST and QFT-GIT or T-SPOT.TB for the diagnosis of LTBI in human subjects. The search included the following Medical Subject Headings (MeSH) terms or text key words: (tuberculin[mesh]) OR “TST” OR “Mantoux”) and (“interferon gamma release assay” OR “interferon gamma assay” OR “QuantiFero*” OR “IGRA” OR “T-SPO*” OR “TSPO*” OR “Elispot” OR CFP10 OR ESAT6) and (tuberculosis[mesh]). No restrictions on date, language, or type of studies were applied. The full search strategy is described in S1 Text. Secondary searching of the reference lists of relevant articles and reviews was also performed for saturation. Titles and abstracts were screened by three authors (TD, AS, and GS) to remove articles that were not relevant to our study. After this initial screening, full-texts of potentially relevant studies were obtained and reviewed independently by at least two of the authors (TD, DE, AS, and GS). Articles were included in this study if they met the following data criteria: 2x2 agreement tables or sufficient information that allowed the construction of such tables between TST and QFT-GIT or T-SPOT.TB; used a TST cut-off value of 5 mm or 10 mm; included IGRAs that were commercial versions using a mixture of the synthetic peptides ESAT-6 and CFP-10; and that the tests were used for the diagnosis of LTBI. This study was reported in accordance with the PRISMA Statement [11]. The review protocol was registered with the International prospective register of systematic reviews (PROSPERO) (CRD42017060705).
Data synthesis and analysis
Data from each eligible study were extracted by two independent reviewers. Discrepancies between the two reviewers were resolved by consensus or by consultation with a third reviewer (DE) if consensus could not be reached. The following variables were extracted: year of publication, country of origin, population group, BCG vaccination rate, TST cut-off value, methods of IGRAs, age range and mean/median where available, proportion of participants on immunosuppressive therapy, and 2x2 test agreement data (TST+/IGRA+, TST+/IGRA-, TST-/IGRA+, TST-/IGRA-). If separate agreement tables were available for different subgroups of patients, these data were included separately [6]. Authors were contacted for further information where appropriate. The QUADAS-2 checklist for the quality assessment of diagnostic accuracy studies was used for quality assessment of the included studies [12]. A description of the QUADAS-2 items can be found in S2 Text.
The primary outcome was the diagnostic performance, i.e. sensitivity, specificity, positive predictive value and negative predictive value, of TST, QFT-GIT and T-SPOT.TB in immune-competent adults aged 15 years or above. For studies to be included in this primary analysis, the prevalence of immune-compromised conditions had to be less than 5% [6]. Subgroup analyses investigating the diagnostic performance of TST and QFT-GIT were performed on immune-competent children (≤ 14 years of age) and immune-compromised adults. Subgroup analyses on these population groups were not performed with T-SPOT.TB due to insufficient data. In accordance with international guidelines [13–15] and real-life clinical practice, we used a TST cut-off value of 10 mm for immune-competent subjects and 5 mm for immune-compromised individuals. We allowed for factors that could potentially lead to variability of diagnostic test performance between studies including BCG vaccination rate and immune status.
Bayesian latent class model
We developed a Bayesian latent class model to describe the observed 2x2 data to estimate the true prevalence (π) of LTBI in the population, and the sensitivity (S1, S2) and specificity (C1, C2) of TST (test 1, T1) and IGRA (test 2, T2). Let D be the unknown (latent) true disease status, the prevalence, sensitivity and specificity can be formally expressed as follows:
| (1) |
The observed data follow a multinomial distribution where each probability of the four combinations of the results of the two tests can be expressed in terms of π, S1, S2, C1 and C2 as follows:
| (2) |
In the latent class model in Eq 2, π, S1, S2, C1 and C2 were the unknown model parameters to be estimated. A Bayesian approach was used to make inferences about these unknown parameters. This approach combines the observed data, i.e. 2x2 table, and prior knowledge about the parameters formally expressed as a prior probability distribution, to obtain a posterior probability distribution of the unknown parameters. We assumed a beta(α,β) distribution for the priors of the sensitivity and specificity. Beta distribution was chosen because its region of positive density ranges from 0 to 1, matching the range of these parameters [8]. It also has the advantage of being flexible, allowing a wide variety of the shapes of the distribution to be determined by selecting different choices of α and β [8]. The α and β parameters of the beta distributions of the sensitivity and specificity of T1 and T2 were determined by equating the midpoint of the range reported in the literature to the mean (μ) of the beta distribution, and equating one quarter of the range to the standard deviation (δ) of the beta distribution [10]. The mean, standard deviation and the parameters of a beta distribution were given by the following equations:
| (3) |
For TST (T1), the sensitivity reported in the literature ranged from 57% to 95% [16,17], while the specificity ranged from 55% to 100% [18,19]. Using Eq 3, these corresponded to beta(14.6, 4.6) and beta(9.9, 2.88) for S1 and C1, respectively. The sensitivity of IGRAs reported in the literature ranged from 55% to 93% [18,20], and their specificity ranged from 89% to 100% [21,22]. These were converted into beta(15.04, 5.28) and beta(64, 3.7) for S2 and C2, respectively. A uniform(0, 0.9) was used for the priors of LTBI prevalence (π), knowing that the highest prevalence rate reported in the literature was 90% [23]. This distribution assigns equal weights to all possible values from 0 to 0.9 to allow LTBI prevalence to vary freely within this range among studies (i.e. populations). A separate estimate of prevalence for each population was performed.
We also estimated the effect of BCG on the specificity of the tests as follows:
| (4) |
where C1i is the specificity of a test in the current (ith) population, p is the proportion of individuals in that population who is vaccinated, and EBCG is the effect of BCG on the specificity of the test in that population.
Positive predictive value (PPV) and negative predictive value (NPV) were also estimated using the following formulae. S3 Text describes how these formulae were derived.
| (5) |
Bayesian inferences with the Gibbs sampler algorithm was used to estimate the model parameters. For each parameter, three Markov chains were constructed, each chain with different initial values. Convergence of the Markov chains was assessed by visual inspection of the density plots of parameter estimates and by examining the Gelman-Rubin statistics [24]. A Gelman-Rubin value of less than 1.1 was considered convergence [24]. We ran each chain with 70,000 iterations and a burn-in period of 10,000. For each parameter, median estimates and their 95% credible interval (CrI) were reported. The log-odds ratio check (LORC) method was used for assessment of conditional independence between the two test observations [25]. Briefly, the LORC investigates how well a model describes a particular dataset by comparing the empirical pairwise log-odds ratios with the pairwise predicted log-odds ratios [25]. The difference between the observed and expected log-odds ratios is expressed by a z-score. A z-score within the ±1.96 range indicates that the assumption of conditional independence is valid [25]. All analyses were performed in WinBUGS (version 1.4, Imperial College & Medical Research Council, UK). As this study used data from published literature, ethics approval was not required.
Results
A total of 2,195 articles were identified from the initial searches. After assessment of titles and abstracts, 480 articles were assessed as potentially relevant and their full-texts were reviewed. Of these, 157 articles met the a priori inclusion criteria [26–182]. These studies comprised 170 agreement tables. The earliest and latest years of publication were 2006 and 2017, respectively. Of the included studies, four were published in languages other than English (one Polish, three Spanish); however, the full-texts of these studies were already translated into English by the journal. Fig 1 outlines how the final sample size was reached.
Fig 1. Flowchart of study selection.
TB, tuberculosis; TST, tuberculin skin test.
The characteristics of the included studies are shown in Table 1. Eighty seven percent (137/157) of the included studies reported rates of BCG vaccination. The majority (132/157, 84%) of the studies were conducted in adults (≥15 years of age). Twenty five percent (39/157) of the studies were conducted in patients selected because of altered immunity due to the presence of HIV/AIDS, solid organ transplantation, stem cell transplantation, immune-mediated inflammatory diseases, end-stage kidney disease or malignancy. QFT-GIT was the most common IGRA, used in 87% (137/157) of the included studies. T-SPOT.TB was used in 15/157 studies; all of which included only immune-competent adults. The remaining studies (5/150) used both methods.
Table 1. Characteristics of the included studies.
| Reference | Country | Population | Age range (years) | BCG rate (%) | 2x2 data* |
|---|---|---|---|---|---|
|
Immune-competent, IGRA = QFT-GIT |
|||||
| Diel et al. (2006) [26] | Germany | Contacts | Any age | 50.8 | 25, 39, 6, 239 |
| Nakaoka et al. (2006) [27] | Nigeria | Contacts | 1–14 | 90 | 40, 14, 8, 93 |
| Tsiouris et al. (2006) [28] | South Africa | Students | 5–15 | 72.3 | 51, 29, 10, 94 |
| Adetifa et al. (2007) [29] | Gambia | Contacts | ≥15 | 43 | 69, 16, 33, 57 |
| Arend et al. (2007) [30] | Netherlands | Unvaccinated | ≥17 | 0 | 74, 186, 7, 518 |
| Dogra et al. (2007) [31] | India | Contacts | 1–12 | 82 | 8, 2, 3, 92 |
| Franken et al. (2007) [32] | Netherlands | Military personnel | ≥18 | 12.6 | 19, 120, 2, 535 |
| Silverman et al. (2007) [33] | Canada | Contacts | ≥18 | 100 | 3, 10, 0, 9 |
| Chun et al. (2008) [34] | Korea | Contacts | ≤13 | 100 | 9, 12, 1, 47 |
| Healthy controls | ≤14 | 100 | 1, 41, 0, 23 | ||
| Mirtskhulava et al. (2008) [35] | Georgia | HCW | 18–74 | 92 | 133, 44, 26, 62 |
| Petrucci et al. (2008) [36] | Nepal | Contacts | ≤15 | 84.9 | 65, 9, 5, 58 |
| Brazil | Contacts | ≤15 | 84.9 | 33, 2, 12, 63 | |
| Baker et al. (2009) [37] | USA | Refugees | 1–81 | NR | 85, 23, 20, 67 |
| Bianchi et al. (2009) [38] | Italy | Contacts, Immigrants | ≤16 | 51.5 | 33, 21, 27, 253 |
| Fox et al. (2009) [39] | Israel | HCW | ≥18 | 34 | 9, 22, 8, 52 |
| Herrmann et al. (2009) [40] | France | HCW | 24–53 | 100 | 4, 9, 2, 4 |
| Kik et al. (2009) [41] | Netherlands | Contacts | ≥16 | NR | 142, 97, 10, 33 |
| Kim et al. (2009) [42] | Korea | Immune-competent | 19–98 | 100 | 17, 8, 7, 53 |
| Lien et al. (2009) [43] | Vietnam | HCW | 20–58 | 32 | 114, 49, 21, 71 |
| Lighter et al. (2009) [44] | USA | Mixed | ≤18 | 36 | 27, 88, 4, 85 |
| Machado et al. (2009) [45] | Brazil | Contacts | Any age | 76 | 100, 44, 17, 94 |
| Ringshausen et al. (2009) [46] | Germany | HCW | 20–62 | 51 | 7, 22, 6, 108 |
| Saracino et al. (2009) [47] | Italy | Immigrants | Any age | NR | 49, 23, 58, 149 |
| Torres Costa et al. (2009) [48] | Portugal | HCW | ≥16 | 100 | 371, 532, 26, 289 |
| Tripodi et al. (2009) [49] | France | HCW | 20–60 | 100 | 23, 74, 5, 46 |
| Vinton et al. (2009) [50] | Australia | HCW | 20–66 | 78 | 16, 98, 5, 222 |
| Zhao et al. (2009) [51] | USA | HCW | ≥18 | NR | 10, 10, 0, 20 |
| Adetifa et al. (2010) [52] | Gambia | Contacts | 0.5–14 | 59 | 43, 14, 29, 127 |
| Costa et al. (2010) [53] | Portugal | HCW | ≥16 | 100 | 525, 792, 33, 332 |
| Grare et al. (2010) [54] | France | Contacts | ≥18 | 45.4 | 5, 10, 0, 22 |
| Huang et al. (2010) [55] | Taiwan | Contacts | Any age | 89 | 12, 24, 3, 39 |
| Jong Lee et al. (2010) [56] | Korea | HCW | 22–53 | 100 | 10, 21, 9, 42 |
| Katsenos et al. (2010) [57] | Greece | Army recruits | 18–35 | 100 | 11, 85, 2, 31 |
| Lee et al. (2010) [58] | Korea | Contacts | 16–70 | 67.2 | 97, 29, 11, 48 |
| Torres Costa et al. (2010) [59] | Portugal | HCW | ≥18 | 63.7 | 525, 792, 33, 332 |
| Thomas et al. (2010) [60] | Bangladesh | Mixed | 11–15.3 | 79 | 72, 16, 35, 105 |
| Tsolia et al. (2010) [61] | Greece | Mixed | ≥15 | NR | 58, 70, 4, 16 |
| Caglayan et al. (2011) [62] | Turkey | HCW | Any age | 87 | 33, 32, 1, 12 |
| Diel et al. (2011) [63] | Germany | Contacts | 1–62 | 52 | 138, 104, 60, 652 |
| Kasambira et al. (2011) [64] | South Africa | Contacts | ≤16 | 95 | 48, 7, 27, 154 |
| Kus et al. (2011) [65] | Poland | Healthy | ≥18 | 100 | 85, 140, 41, 186 |
| Legesse et al. (2011) [66] | Ethiopia | General | 18–70 | 17.4 | 151, 16, 76, 28 |
| Moon et al. (2011) [67] | Korea | HCW | 22–67 | 100 | 18, 34, 14, 90 |
| Moyo et al. (2011) [68] | South Africa | Contacts | ≤3 | 100 | 57, 13, 11, 295 |
| Pavic et al. (2011) [69] | Croatia | Contacts | 0–5 | 100 | 14, 11, 4, 112 |
| Rafiza et al. (2011) [70] | Malaysia | HCW | 19–56 | 99.7 | 11, 45, 2, 37 |
| Shanaube et al. (2011) [71] | Zambia, South Africa | Contacts | ≥15 | NR | 577, 148, 570, 508 |
| Talebi-Taher et al. (2011) [72] | Iran | HCW | 23–59 | 100 | 14, 91, 3, 92 |
| Torres Costa et al. (2011) [73] | Portugal | HCW | ≥18 | 68.2 | 850, 1252, 103, 679 |
| Torres Costa et al. (2011) [74] | Portugal | HCW | ≥16 | 98.6 | 153, 344, 8, 67 |
| Weinfurter et al. (2011) [75] | USA | Mixed | ≥13 | 36 | 167, 155, 64, 1267 |
| Yassin et al. (2011) [76] | Ethiopia | Contacts | ≥15 | 52 | 87, 39, 24, 59 |
| Healthy controls | ≥15 | 52 | 6, 10, 12, 86 | ||
| Bergot et al. (2012) [77] | France | Contacts | 12–97 | 20.4 | 28, 50, 7, 62 |
| Di Renzi et al. (2012) [78] | Italy | Staff of homeless shelter | 25–71 | 6.5 | 22, 0, 2, 27 |
| Healthy controls | ≥18 | 66 | 16, 12, 3, 10 | ||
| He et al. (2012) [79] | Mongolia | HCW | 18–72 | 26 | 350, 89, 288, 190 |
| Jeong et al. (2012) [80] | Korea | X-ray healed TB | 36–88 | 42.6 | 79, 10, 48, 26 |
| Jo et al. (2012) [81] | Korea | Contacts | Any age | 78.2 | 34, 14, 20, 33 |
| Jung da et al. (2012) [82] | Korea | Medical students | ≥18 | 86.3 | 6, 17, 2, 128 |
| Larcher et al. (2012) [83] | Italy | HCW | 19–64 | 38 | 57, 103, 24, 365 |
| Onur et al. (2012) [84] | Turkey | Outpatient paediatric clinic | ≤14 | 87.6 | 33, 18, 4, 36 |
| Pattnaik et al. (2012) [85] | India | Contacts | ≥15 | 40.7 | 64, 24, 1, 11 |
| Zwerling et al. (2012) [86] | Canada | HCW | ≥18 | 36.1 | 7, 15, 17, 348 |
| Jo et al. (2013) [87] | Korea | HCW | ≥20 | 81 | 54, 127, 31, 281 |
| Serrano-Escobedo et al. (2013) [88] | Mexico | Contacts | ≥18 | 87 | 31, 11, 20, 61 |
| Whitaker et al. (2013) [89] | Georgia | HCW | ≥18 | 89 | 68, 38, 9, 39 |
| Zwerling et al. (2013) [90] | Canada | HCW | ≥18 | 61.6 | 3, 10, 10, 234 |
| Alvarez et al. (2014) [91] | Canada | High risk groups | Any age | 73 | 46, 40, 4, 166 |
| Charisis et al. (2014) [92] | Greece | HCW | ≥20 | 68 | 30, 179, 2, 32 |
| de Souza et al. (2014) [93] | Brazil | HCW | ≥18 | 86.4 | 114, 138, 58, 322 |
| Erkens et al. (2014) [94] | Netherlands | Mixed | Any age | 40 | 870, 1777, 66, 639 |
| Garazzino et al. (2014) [95] | Italy | General | ≤2 | NR | 0, 10, 9, 463 |
| Garcell et al. (2014) [96] | Qatar | HCW | ≥18 | NR | 10, 9, 1, 182 |
| Goodwin et al. (2014) [97] | USA | Army recruits | 17–36 | 1 | 1, 13, 5, 2062 |
| Mathad et al. (2014) [98] | India | Pregnant women | ≥18 | NR | 46, 12, 79, 206 |
| Ribeiro-Rodrigues et al. (2014) [99] | Brazil | Contacts | 0.5–87 | 77.3 | 159, 36, 14, 100 |
| Sauzullo et al. (2014) [100] | Italy | HCW | 25–60 | 3.1 | 34, 29, 0, 126 |
| Song et al. (2014) [101] | Korea | Contacts | 11–19 | 61 | 231, 430, 86, 2219 |
| Adams et al. (2015) [102] | South Africa | HCW | ≥18 | 92 | 293, 112, 24, 53 |
| El-Sokkary et al. (2015) [103] | Egypt | HCW | ≥18 | 92.4 | 26, 52, 12, 42 |
| Gao et al. (2015) [104] | China | Mixed | ≥5 | 50.6 | 2933, 2945, 1013, 13587 |
| Goebel et al. (2015) [105] | Australia | Contacts | Any age | 84 | 160, 194, 18, 91 |
| He et al. (2015) [106] | Mongolia | HCW | 19–77 | 36.4 | 122, 45, 276, 422 |
| Howley et al. (2015) [107] | Vietnam, Philippines, Mexico | Migrants to USA | 2–14 | 100 | 111, 553, 31, 1812 |
| Jones-Lopez et al. (2015) [108] | Uganda | Contacts | ≥10 | 2 | 182, 19, 15, 36 |
| Lucet et al. (2015) [109] | France | HCW | ≥18 | 97.4 | 95, 348, 18, 343 |
| Ferrarini et al. (2016) [110] | Brazil | Contacts | ≤15 | 98.3 | 31, 3, 3, 4 |
| Al Hajoj et al. (2016) [111] | Saudi Arabia | HCW | ≥18 | 90.6 | 227, 275, 172, 921 |
| Biraro et al. (2016) [112] | Uganda | Contacts | 0–30 | 78 | 62, 7, 92, 76 |
| Bozkanat et al. (2016) [113] | Turkey | HCW | ≥18 | 94.1 | 7, 21, 0, 6 |
| Grare et al. (2010) [114] | France | Children | NR | 41 | 5, 7, 0, 32 |
| Lowenthal et al. (2016) [115] | USA | Immigrants | 2–14 | NR | 142, 523, 3, 48 |
| Marco Mourino et al. (2011) [116] | Spain | Prisoners | 19–66 | 17 | 27, 13, 10, 99 |
| Marquez et al. (2016) [117] | Uganda | Children | 0–5 | 94 | 10, 114, 10, 343 |
| Miramontes et al. (2015) [118] | USA | General | ≥6 | NR | 127, 158, 176, 5603 |
| Mostafavi et al. (2016) [119] | Iran | HCW | ≥20 | 86 | 13, 26, 29, 176 |
| Nienhaus et al. (2011) [120] | Germany, Portugal, France | HCW | ≥18 | NR | 409, 654, 41, 523 |
| Oren et al. (2016) [121] | USA | Migrant farmers | ≥48 | 74 | 16, 8, 12, 32 |
| Pavic et al. (2015) [122] | Croatia | Contacts | <5 | 98.8 | 18, 13, 8, 132 |
| Reechaipichitkul et al. (2015) [123] | Thailand | Contacts | NR | 86 | 15, 24, 5, 56 |
| Rose et al. (2015) [124] | Canada | Contacts | 0–17 | 42 | 27, 16, 4, 47 |
| Salinas et al. (2015) [125] | Spain | Immigrants | 12–18 | 26.75 | 140, 103, 2, 34 |
| Sharma et al. (2017) [126] | India | Contacts | 1–65 | 76 | 540, 187, 377, 394 |
| Yoo et al. (2016) [127] | Korea | Contacts | NR | 84 | 92, 71, 40, 241 |
| Anibarro et al. (2011) [128] | Spain | Contacts | ≥18 | 36 | 68, 14, 5, 50 |
| Diel et al. (2008) [129] | Germany | Contacts | 1–56 | 46 | 62, 181, 4, 354 |
| Ferreira et al. (2015) [130] | Brazil | Contacts | ≥18 | 86.7 | 19, 5, 9, 27 |
| Nienhaus et al. (2008) [131] | Germany | HCW | 18–67 | 37.5 | 15, 48, 10, 188 |
|
Immune-competent, IGRA = T-SPOT.TB |
|||||
| Porsa et al. (2006) [132] | USA | Prisoners | ≥18 | NR | 9, 28, 13, 359 |
| Arend et al. (2007) [30] | Netherlands | Unvaccinated | ≥17 | 0 | 103, 151, 39, 466 |
| Rangaka et al. (2007) [133] | South Africa | Mixed | Any age | 71 | 40, 21, 5, 8 |
| Bienek & Chang (2009) [134] | USA | Unvaccinated | 18–41 | 3 | 2, 0, 6, 318 |
| Janssens et al. (2008) [135] | Switzerland | Contacts | 16–83 | 80.6 | 78, 65, 37, 100 |
| Leung et al. (2008) [136] | Hong Kong | Silicosis | ≥18 | 1.5 | 72, 20, 14, 28 |
| Soysal et al. (2008) [137] | Turkey | Healthy | Any age | 83 | 7, 18, 0, 21 |
| Girardi et al. (2009) [138] | Italy | HCW | ≥18 | 37.4 | 37, 24, 5, 49 |
| Hansted et al. (2009) [139] | Lithuania | Contacts | 10–17 | 100 | 7, 20, 1, 17 |
| Low risk | 10–17 | 100 | 3, 31, 2, 16 | ||
| Kik et al. (2009) [41] | Netherlands | Contacts | ≥16 | NR | 154, 85, 14, 29 |
| Adetifa et al. (2010) [52] | Gambia | Contacts | 0.5–14 | 59 | 43, 14, 27, 129 |
| Leung et al. (2010) [140] | Hong Kong | Silicosis | ≥18 | 3.5 | 168, 35, 36, 69 |
| Borkowska et al. (2011) [141] | Poland | HCW | 27–73 | 100 | 7, 4, 0, 6 |
| Zhao et al. (2011) [142] | China | Students | 17–24 | 0 | 11, 26, 16, 103 |
| Larcher et al. (2012) [83] | Italy | HCW | 19–64 | 38 | 24, 51, 35, 282 |
| Nkurunungi et al. (2012) [143] | Uganda | Healthy | ≤5 | 100 | 17, 6, 51, 218 |
| Adams et al. (2015) [102] | South Africa | HCW | ≥18 | 92 | 249, 126, 20, 55 |
| Leung et al. (2015) [144] | Hong Kong | Contacts | 5–64 | 66 | 254, 228, 89, 478 |
| Spicer et al. (2015) [145] | USA | Mixed | 0.3–16 | 72.5 | 5, 18, 0, 71 |
| Non-TB diseases | 25–63 | 100 | 0, 3, 1, 26 | ||
|
Immune-compromised, IGRA = QFT-GIT |
|||||
| Mendez-Echevarria et al. (2011) [146] | Spain | IMID | ≥18 | 5.6 | 4, 3, 5, 37 |
| Moon et al. (2011) [67] | Korea | Stem cell transplant | 35–55 | 82 | 9, 24, 31, 146 |
| Takahashi et al. (2007) [147] | USA | HIV | 22–79 | 7.4 | 2, 5, 7, 259 |
| Aichelburg et al. (2014) [148] | Austria | HIV | ≥18 | NR | 24, 3, 13, 195 |
| Balcells et al. (2008) [149] | Chile | HIV | 21–71 | 88 | 9, 2, 8, 90 |
| Bourgarit et al. (2015) [150] | France | HIV | ≥18 | 60.6 | 20, 42, 14, 316 |
| Casas et al. (2011) [151] | Spain | IMID | NR | 26 | 43, 19, 13, 210 |
| Casas et al. (2011) [152] | Spain | ESRD | NR | 31.6 | 34, 10, 9, 42 |
| Chkhartishvili et al. (2013) [153] | Georgia | HIV | ≥18 | 94 | 25, 16, 44, 148 |
| Gogus et al. (2010) [154] | Turkey | IMID | 20–70 | 100 | 8, 17, 1, 12 |
| Hanta et al. (2012) [155] | Turkey | IMID | ≥18 | 92 | 24, 32, 10, 24 |
| Hsia et al. (2012) [156] | Worldwide | IMID | All age | 34.2 | 59, 150, 101, 1931 |
| James et al. (2014) [157] | India | HIV | ≥18 | 100 | 10, 16, 4, 18 |
| Jones et al. (2007) [158] | USA | HIV | All age | 2 | 5, 8, 6, 172 |
| Karadag et al. (2010) [159] | Turkey | IMID | All age | 100 | 19, 34, 2, 39 |
| Khawcharoenporn et al. (2015) [160] | Thailand | HIV | 17–65 | 73 | 8, 16, 12, 114 |
| Kim et al. (2014) [161] | Korea | IMID | All age | 70.7 | 56, 77, 12, 269 |
| Kim et al. (2013) [162] | Korea | IMID | All age | NR | 102, 133, 81, 408 |
| Kim et al. (2015) [163] | Korea | IMID | All age | NR | 52, 67, 26, 271 |
| Latorre et al. (2014) [164] | Spain | IMID | ≥18 | NR | 1, 6, 11, 81 |
| Manuel et al. (2007) [165] | Canada | Liver transplant | ≥18 | 82 | 18, 9, 16, 98 |
| Matulis et al. (2008) [166] | Switzerland | IMID | ≥18 | 83 | 10, 34, 5, 60 |
| Minguez et al. (2012) [167] | Spain | IMID | ≥18 | 5.6 | 4, 3, 5, 37 |
| Moon et al. (2013) [168] | Korea | Stem cell transplant | 35–55 | 82 | 9, 24, 31, 146 |
| Papay et al. (2011) [169] | Austria | IMID | NR | 100 | 6, 20, 9, 157 |
| Ramos et al. (2013) [170] | Spain | IMID | 16–82 | 19 | 13, 30, 2, 107 |
| Ramos et al. (2012) [171] | Spain | HIV | 15–85 | 15.8 | 21, 25, 8, 40 |
| Sauzullo et al. (2010) [172] | Italy | IMID | 18–80 | 8.7 | 27, 26, 5, 11 |
| Talati et al. (2009) [173] | USA | HIV | 22–79 | 7.4 | 2, 5, 7, 259 |
| Vassilopoulos et al. (2011) [174] | Greece | IMID | ≥18 | 76 | 17, 41, 15, 82 |
| Hoffmann et al. (2010) [175] | Switzerland | Haemodialysis | 30–87 | 18 | 5, 2, 4, 21 |
| Mariette et al. (2012) [176] | France | IMID | All age | 65.7 | 24, 114, 15, 239 |
| Ponce de Leon et al. (2008) [177] | Peru | IMID | All age | 80.2 | 21, 6, 24, 50 |
| Scrivo et al. (2012) [178] | Italy | IMID | 18–80 | 5.8 | 2, 11, 3, 82 |
| Cho et al. (2016) [179] | Korea | IMID | NR | 77.9 | 19, 16, 19, 148 |
| Kurti et al. (2015) [180] | Hungary | IMID | 18–30 | 100 | 7, 28, 5, 126 |
| Kussen et al. (2016) [181] | Brazil | HIV | ≥18 | 78 | 9, 4, 12, 115 |
| Palomar et al. (2011) [182] | Spain | Haemodialysis | NR | 42.6 | 7, 9, 3, 26 |
* TST+/IGRA+, TST+/IGRA-, TST-/IGRA+, TST-/IGRA-.
ESRD, end stage renal disease; IGRA, interferon gamma release assay; IMID, immune-mediated inflammatory disease; HCW, healthcare worker; NR, not reported; QFT-GIT, QuantiFERON-TB Gold In Tube; TB, tuberculosis.
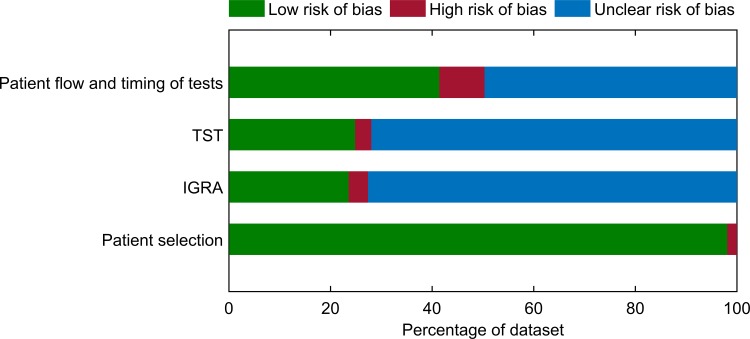
The results of the quality assessment of the included studies are summarised in Fig 2 and presented for each individual study in S1 Table. Many studies did not report all the information that could be used to fully assess the quality of the study. For the “patient selection” domain, most studies (154/157, 98%) were deemed to have low risk of bias (Fig 2). The remaining 2% were considered to have high risk of bias because these studies used a case-control study design in which the status of LTBI were known prior to the test. For the “diagnostic test domains”, risk of bias could not be assessed for the majority of studies because it was unknown whether the results of one test were interpreted without knowledge of the results of the other test (Fig 2). Nine percent (14/157) of the studies were deemed to have high risk of bias for the “patient flow and timing of tests domain” because there were participants excluded from the analysis without explanation given (Fig 2). There was unclear risk of bias for this domain for 50% (78/157) of the studies because the interval between the two tests was not reported (Fig 2).
Fig 2. Summary of quality assessment results.
Risk of Bias of each QUADAS-2 domain presented as percentages across the 157 included studies. IGRA; interferon-gamma release assay; TST, tuberculin skin test.
Table 2 shows the estimated sensitivity and specificity of TST, QFT-GIT and T-SPOT.TB in different populations. In immune-competent non-BCG-vaccinated adults, TST has better sensitivity (84% versus 52%) and slightly better specificity (100% versus 97%) than QFT-GIT. BCG vaccination significantly reduces the specificity of TST, from 100% in non-vaccinated subjects to 79% in BCG-vaccinated subjects; whereas the effect of BCG on the specificity of QFT-GIT is modest (Table 2). T-SPOT.TB has comparable specificity (97% for both tests) and better sensitivity (68% versus 52%) than QFT-GIT in immune-competent adults. In immune-compromised adults, QFT-GIT is less sensitive than TST (46% versus 71%) whereas the specificity of both tests is comparable (97% versus 99% in non-BCG-vaccinated adults, 93% for both tests in BCG-vaccinated adults) (Table 2). QFT-GIT and TST have comparable specificity in non-BCG-vaccinated children; however the former is less sensitive than the latter (Table 2). The specificity of QFT-GIT in BCG-vaccinated children is not affected by BCG and is substantially better than that of TST (98% versus 82%) (Table 2).
Table 2. Estimated sensitivity and specificity of TST and IGRAs in different population groups.
| Parameter | Diagnostic test | Immune-competent adults* median (95% CrI) |
Immune-compromised adults† median (95% CrI) |
Immune-competent children* median (95% CrI) |
|---|---|---|---|---|
| Sensitivity (%) | QFT-GIT | 52 (50–53) | 46 (43–49) | 73 (70–76) |
| TST | 84 (82–85) | 71 (66–75) | 82 (79–84) | |
| Specificity (%) | QFT-GIT (non-BCG) | 97 (96–97) |
97 (96–98) | 98 (97–99) |
| QFT-GIT (BCG) | 93 (92–94) | 93 (92–95) | 98 (97–99) | |
| TST (non-BCG) |
100 (99–100) | 99 (97–100) | 98 (96–99) | |
| TST (BCG) | 79 (76–82) | 93 (91–96) | 82 (81–83) |
*TST cut-off value = 10 mm
†TST cut-off value = 5 mm
BCG, Bacillus Calmette-Guérin; CrI, credible interval; QFT-GIT, QuantiFERON-TB Gold In Tube; TST, tuberculin skin test.
The mean prevalence of LTBI among the populations where the studies were performed was estimated to be 49% (standard deviation ± 27%). The relationship between prevalence and predictive values is shown in Fig 3. In a high-prevalence setting (prevalence > 50%), QFT-GIT has a PPV of at least 88% and a NPV value of at most 69%. The PPV of TST is around 100% in non-BCG-vaccinated and at least 73% in BCG-vaccinated subjects. The NPV of TST was estimated to be 71% and 61% in these populations, respectively.
Fig 3. Relationship between prevalence and predictive value in immune-competent adults.
BCG, Bacillus Calmette-Guérin; LTBI, latent tuberculosis infection; NPV, negative predictive value; PPV, positive predictive value; QFT-GIT, QuantiFERON-TB Gold In Tube; TB, tuberculosis; TST, tuberculin skin test.
Discussion
Accurate identification and subsequent treatment of LTBI is essential to TB control and elimination. The lack of a gold standard for diagnosing LTBI means that the true prevalence of the disease is unknown, and the estimations of the sensitivity and specificity of diagnostic tests are unreliable. This study represents the most comprehensive Bayesian latent class analysis of published data on the performance of TST and IGRAs for the diagnosis of LTBI. We have confirmed that IGRAs have high specificity but that these tests have considerably lower sensitivity than TST in immune-competent populations than had previously been demonstrated [6,7,183]. A meta-analysis by Pai et al.[7] estimated the pooled sensitivity of QFT and TST to be 70% and 77%, respectively; the specificity of QFT to be 96–99%; and the specificity of TST in non-BCG-vaccinated and BCG-vaccinated populations to be 97% and 59%, respectively. Our estimate of the sensitivity of QFT-GIT is lower than that of Pai et al. [7]; however it should be noted that the sensitivity of QFT in Pai et al. [7] was estimated in patients with active TB as a surrogate for LTBI. It is plausible that the cellular immune response, which is the measure of QFT, is different between LTBI and active TB disease, being higher with the latter [5]. Using a similar latent class modelling approach, Sadatsafavi et al. [6] estimated the sensitivity and specificity of QFT in immune-competent adults to be 64.2% and 99.6%, respectively. However, methodological differences make comparison between our results and those of Sadatsafavi et al. [6] challenging. Sadatsafavi et al. [6], conducted in 2008, is nearly a decade old and only included a very limited number (nineteen) of studies. Since then, a great amount of new studies that compared the diagnostic performance of IGRAs and TST in this setting have been published. Indeed, our search has found that since the study of Sadatsafavi et al. [6] was conducted, there have been 132 new studies that are included in our analysis. Sadatsafavi et al. [6] combined all versions of QFT in their analysis, assuming no difference between these tests; whereas our study included only the latest QFT-GIT version, which replaced the discontinued older QFT versions. In addition, Sadatsafavi et al. [6] only included immune-competent adults; whereas we included not only immune-competent adults but also children and immune-compromised individuals. The study of Sadatsafavi et al. [6] is limited to a single database and to studies in English language only. Single database and English-only language restrictions are likely to result in an incomplete coverage of the literature and biased estimates.
Conventional meta-analysis of diagnostic tests simply entails pooling of data to provide pooled estimates of test sensitivity and specificity. Simple pooling of data may cause serious bias due to confounding of disease prevalence in the contributing studies [184]. Our latent class modelling approach accounts for the imperfect nature of the tests; and allows us to estimate not only diagnostic parameters (i.e. sensitivity, specificity, predictive values), but also disease prevalence. Unlike conventional meta-analysis, Bayesian latent class modelling incorporates prior information on sensitivity, specificity and disease prevalence, improving the precision of model estimates for these parameters. It also allows for the quantification of the effect of BCG on the performance of the tests, which otherwise is impossible to measure in conventional epidemiological studies and meta-analysis. Before our study, there had been no formal quantification of the effect of BCG on the specificity of IGRAs; even though it is generally thought that such effect, if any, is modest based on the biological mechanism of the tests, rather than on empirical data [185]. Our study is the first to quantify the effect of BCG on the specificity of IGRAs. We have found that such effect is minimal, confirming this hypothesis. We have also been able to quantify the decrement in specificity of TST in BCG-vaccinated subjects. To date, studies that investigated the impact of BCG on TST have only reported such effect as relative risk or odds ratio of having positive TST results between subjects with and without BCG [22,23,167]. We have found that BCG negatively affects the performance of TST, reducing the specificity of the test by 21% in the general population. In contrast, QFT-GIT has reasonable sensitivity and superior specificity in BCG-vaccinated subjects, supporting the recommendation that QFT-GIT should be the preferred diagnostic test of LTBI in this setting [177,178]. Of note, the effect of BCG on the specificity of the tests was inferred in our model based on the rates of BCG vaccination. We did not take into account other factors that are known to potentially affect the diagnostic performance of TST including age at vaccination and time since vaccination because of the lack of data [179]. An important assumption underlying Bayesian latent class models is the assumption of conditional independence between the two test observations [25]. Using the LORC method, we estimated the z-score to be 0.8, falling within the ± 1.96 range, indicating no violation of the conditional independence assumption. To explore the potential effects that studies deemed to be of high risk of bias may have on the results, we performed an analysis in which these studies were excluded. We found that our results were robust to the inclusion (or exclusion) of these studies (S2 Table).
Immune-compromised patients have an increased risk of LTBI reactivation [5]. Screening for LTBI is therefore required prior to commencement of immunosuppressive therapies [5]. To date, data on the performance of diagnostic tests for LTBI in immune-compromised subjects are limited and the few published studies evaluating the performance of TST and QFT-GIT show conflicting results [5,186]. We have found that both tests are specific but have suboptimal sensitivity in immune-compromised patients. We believe that more data on the performance of TST and QFT-GIT in this population group are required.
The limitations of our study must be considered. Our results are derived from studies where the estimates of LTBI prevalence vary widely. This is due to the heterogeneity in study settings, populations and methodology of the included studies. Bayesian analysis requires prior information on model parameters. One criticism of Bayesian latent class models is that they may be sensitive to the choice of prior information. This may particularly be the case when there are limited observed data. When the number of observed data are large, as in our study, these begin to dominate any prior information. We believe that we have used the most informative priors obtained from the literature. Furthermore, we performed sensitivity analysis and found that our results are not sensitive to choice of prior (S3 Table).
In conclusion, our study represents the most comprehensive Bayesian latent class analysis of the diagnostic accuracy of TST and IGRAs derived from all published agreement data. Our results challenge the current beliefs about the performance of LTBI screening tests and provide important information to guide choice of tests for LTBI screening that will enhance the millennium goals for elimination of TB. Our findings show that IGRAs may be inferior to TST for diagnosing LBTI in non-BCG-vaccinated populations. For BCG-vaccinated individuals, IGRAs appear to be a more favourable choice. IGRAs will therefore allow physicians and TB controllers to better understand the background prevalence of LTBI for targeted preventive therapy in settings where BCG vaccination is widely administered. QFT-GIT and TST have suboptimal sensitivity in immune-compromised patients and results should be interpreted with caution. A combination of both tests could potentially overcome the problems of false-positives in this setting. Considerations regarding cost-effectiveness, logistics, availability for clinicians and patient acceptability should be taken into account to decide which test to use for the diagnosis of LTBI.
Supporting information
(PDF)
(PDF)
(PDF)
IGRA, interferon gamma release assay; N, No; Q, Question; TST, tuberculin skin test; U, Unclear; Y, Yes.
(PDF)
*Results are for immune-competent adults. BCG, Bacillus Calmette-Guérin; CrI, credible interval; QFT-GIT, QuantiFERON-TB Gold In Tube; TB, tuberculosis; TST, tuberculin skin test.
(PDF)
*Results are for immune-competent adults. BCG, Bacillus Calmette-Guérin; CrI, credible interval; QFT-GIT, QuantiFERON-TB Gold In Tube; TB, tuberculosis; TST, tuberculin skin test.
(PDF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
There was no funding for this work.
References
- 1.World Health Organization. Tuberculosis fact sheet. 2017. http://www.who.int/mediacentre/factsheets/fs104/en/ (accessed March 18, 2017).
- 2.Vikram HR and Kusne S. Mycobacterium tuberculosis infection in immunocompromised hosts: a diagnostic challenge. Liver Transpl. 2009; 15: 834–7. doi: 10.1002/lt.21787 [DOI] [PubMed] [Google Scholar]
- 3.Laurence YV, Griffiths UK, Vassall A. Costs to health services and the patient of treating tuberculosis: a systematic literature review. PharmacoEconomics. 2015; 33: 939–55. doi: 10.1007/s40273-015-0279-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sollai S, Galli L, de Martino M, Chiappini E. Systematic review and meta-analysis on the utility of interferon-gamma release assays for the diagnosis of Mycobacterium tuberculosis infection in children: a 2013 update. BMC Infect Dis. 2014; 14: S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong SH, Gao Q, Tsoi KK, et al. Effect of immunosuppressive therapy on interferon gamma release assay for latent tuberculosis screening in patients with autoimmune diseases: a systematic review and meta-analysis. Thorax. 2016; 71: 64–72. doi: 10.1136/thoraxjnl-2015-207811 [DOI] [PubMed] [Google Scholar]
- 6.Sadatsafavi M, Shahidi N, Marra F, Fitzgerald MJ, Elwood KR, Guo N, et al. A statistical method was used for the meta-analysis of tests for latent TB in the absence of a gold standard, combining random-effect and latent-class methods to estimate test accuracy. J Clin Epidemiol. 2010; 63: 257–269. doi: 10.1016/j.jclinepi.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 7.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008; 149: 177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph L, Gyorkos TW, Coupal L. Bayesian estimation of disease prevalence and the parameters of diagnostic tests in the absence of a gold standard. Am J Epidemiol. 1995; 141: 263–272. [DOI] [PubMed] [Google Scholar]
- 9.Ang M, Wong WL, Li X, Chee SP. Interferon gamma release assay for the diagnosis of uveitis associated with tuberculosis: a Bayesian evaluation in the absence of a gold standard. Br J Ophthalmol. 2013; 97: 1062–1067. doi: 10.1136/bjophthalmol-2012-302199 [DOI] [PubMed] [Google Scholar]
- 10.Ling DI, Pai M, Schiller I, Dendukuri N. A Bayesian framework for estimating the incremental value of a diagnostic test in the absence of a gold standard. BMC Med Res Methodol. 2014; 14: 67 doi: 10.1186/1471-2288-14-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009; 339: b2535 doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155: 529–536. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society and the Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000; 161: S221–47. doi: 10.1164/ajrccm.161.supplement_3.ats600 [DOI] [PubMed] [Google Scholar]
- 14.Government of Canada. Canadian tuberculosis standards 7th edition: 2014 –Diagnosis of latent tuberculosis infection. https://www.canada.ca/en/public-health/services/infectious-diseases/canadian-tuberculosis-standards-7th-edition/edition-16.html#a5_3 (accessed September 27, 2017).
- 15.Queensland Health (Australia). Management of latent tuberculosis in adults 2016. https://www.health.qld.gov.au/__data/assets/pdf_file/0023/444425/latent-tb-adult.pdf (accessed September 27, 2017).
- 16.Kobashi Y, Abe M, Mouri K, Obase Y, Miyashita N, Oka M. Usefulness of tuberculin skin test and three interferon-gamma release assays for the differential diagnosis of pulmonary tuberculosis. Intern Med. 2012; 51: 1199–1205. [DOI] [PubMed] [Google Scholar]
- 17.Dominguez J, Ruiz-Manzano J, De Souza-Galvao M, Latorre I, Mila C, Blanco S, et al. Comparison of two commercially available gamma interferon blood tests for immunodiagnosis of tuberculosis. Clin Vaccine Immunol. 2008; 15: 168–171. doi: 10.1128/CVI.00364-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Detjen AK, Keil T, Roll S, Hauer B, Mauch H, Wahn U, et al. Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clin Infect Dis. 2007; 45: 322–328. doi: 10.1086/519266 [DOI] [PubMed] [Google Scholar]
- 19.Center for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States. Morb Mortal Wkly Rep. 2010; 59: 1–25. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5905a1.htm (accessed March 18, 2017). [PubMed] [Google Scholar]
- 20.Ferrara G, Losi M, Meacci M, Meccugni B, Piro R, Roversi P, et al. Routine hospital use of a new commercial whole blood interferon-gamma assay for the diagnosis of tuberculosis infection. Am J Respir Crit Care Med. 2005; 172: 631–635. doi: 10.1164/rccm.200502-196OC [DOI] [PubMed] [Google Scholar]
- 21.Brock I, Munk ME, Kok-Jensen A, Andersen P. Performance of whole blood IFN-gamma test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int J Tuberc Lung Dis. 2001; 5: 462–467. [PubMed] [Google Scholar]
- 22.Palazzo R, Spensieri F, Massari M, Fedele G, Frasca L, Carrara S, et al. Use of whole-blood samples in in-house bulk and single-cell antigen-specific gamma interferon assays for surveillance of Mycobacterium tuberculosis infections. Clin Vaccine Immunol. 2008; 15: 327–337. doi: 10.1128/CVI.00342-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adjoh K, Wateba IM, Tidjani O. Prevalence of latent TB infection in HIV infected persons in the Sylvanus Olympio teaching hospital of Lome. Int J Mycobacteriol. 2013; 2: 26–8. doi: 10.1016/j.ijmyco.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 24.Link WA. Bayesian inference. 1st ed. London: Elsevier; 2010. [Google Scholar]
- 25.Subtil A, de Oliveira R, Goncalves L. Conditional dependence diagnostic in the latent class model: a simulation study. Stat Prob Lett. 2012; 82: 1407–12. [Google Scholar]
- 26.Diel R, Nienhaus A, Lange C, Meywald-Walter K, Forssbohm M, Schaberg T. Tuberculosis contact investigation with a new, specific blood test in a low-incidence population containing a high proportion of BCG-vaccinated persons. Respir Res. 2006; 7: 77 doi: 10.1186/1465-9921-7-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakaoka H, Lawson L, Squire BS, Coulter B, Ravn P, Brock I, et al. Risk for tuberculosis among children. Emerg Infect Dis. 2006; 12: 1383–1388. doi: 10.3201/eid1209.051606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsiouris SJ, Austin J, Toro P, Coetzee D, Weyer K, Stein Z, et al. Results of a tuberculosis-specific IFN-gamma assay in children at high risk for tuberculosis infection. Int J Tuberc Lung Dis. 2006; 10: 939–941. [PubMed] [Google Scholar]
- 29.Adetifa IM, Lugos MD, Hammond A, Jeffries D, Donkor S, Adegbola RA, et al. Comparison of two interferon gamma release assays in the diagnosis of Mycobacterium tuberculosis infection and disease in The Gambia. BMC Infect Dis. 2007; 7: 122 doi: 10.1186/1471-2334-7-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arend SM, Thijsen SF, Leyten EM, Bouwman JJ, Franken WP, Koster BF, et al. Comparison of two interferon-gamma assays and tuberculin skin test for tracing tuberculosis contacts. Am J Respir Crit Care Med. 2007; 175: 618–627. doi: 10.1164/rccm.200608-1099OC [DOI] [PubMed] [Google Scholar]
- 31.Dogra S, Narang P, Mendiratta DK, Chaturvedi P, Reingold AL, Colford JM, et al. Comparison of a whole blood interferon-gamma assay with tuberculin skin testing for the detection of tuberculosis infection in hospitalized children in rural India. J Infect. 2007; 54: 267–276. doi: 10.1016/j.jinf.2006.04.007 [DOI] [PubMed] [Google Scholar]
- 32.Franken WP, Timmermans JF, Prins C, Slootman EJ, Dreverman J, Bruins H, et al. Comparison of Mantoux and Quanti-FERON TB gold tests for diagnosis of latent tuberculosis infection in army personnel. Clin Vaccine Immunol. 2007; 14: 477–480. doi: 10.1128/CVI.00463-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silverman MS, Reynolds D, Kavsak PA, Garay J, Daly A, Davis I. Use of an interferon-gamma based assay to assess bladder cancer patients treated with intravesical BCG and exposed to tuberculosis. Clin Biochem. 2007; 40: 913–915. doi: 10.1016/j.clinbiochem.2007.04.006 [DOI] [PubMed] [Google Scholar]
- 34.Chun JK, Kim CK, Kim HS, Jung GY, Lee TJ, Kim KH, et al. The role of a whole blood interferon-gamma assay for the detection of latent tuberculosis infection in Bacille Calmette-Guerin vaccinated children. Diagn Microbiol Infect Dis. 2008; 62: 389–394. doi: 10.1016/j.diagmicrobio.2008.08.022 [DOI] [PubMed] [Google Scholar]
- 35.Mirtskhulava V, Kempker R, Shields KL, Leonard MK, Tsertsvadze T, del Rio C, et al. Prevalence and risk factors for latent tuberculosis infection among health care workers in Georgia. Int J Tuberc Lung Dis. 2008; 12: 513–519. [PMC free article] [PubMed] [Google Scholar]
- 36.Petrucci R, Abu Amer N, Gurgel RQ, Sherchand JB, Doria L, Lama C, et al. Interferon gamma, interferon-gamma-induced-protein 10, and tuberculin responses of children at high risk of tuberculosis infection. Pediatr Infect Dis J. 2008; 27: 1073–1077. doi: 10.1097/INF.0b013e31817d05a3 [DOI] [PubMed] [Google Scholar]
- 37.Baker CA, Thomas W, Stauffer WM, Peterson PK, Tsukayama DT. Serial testing of refugees for latent tuberculosis using the QuantiFERON-gold in-tube: effects of an antecedent tuberculin skin test. Am J Trop Med Hyg. 2009; 80: 628–633. [PubMed] [Google Scholar]
- 38.Bianchi L, Galli L, Moriondo M, Veneruso G, Becciolini L, Azzari C, et al. Interferon-gamma release assay improves the diagnosis of tuberculosis in children. Pediatr Infect Dis J. 2009; 28: 510–514. [DOI] [PubMed] [Google Scholar]
- 39.Fox BD, Kramer MR, Mor Z, Preiss R, Rusanov V, Fuks L, et al. The QuantiFERON-TB-GOLD assay for tuberculosis screening in healthcare workers: a cost-comparison analysis. Lung. 2009; 187: 413–419. doi: 10.1007/s00408-009-9182-2 [DOI] [PubMed] [Google Scholar]
- 40.Herrmann JL, Simonney N, Bergeron A, Ducreux-Adolphe N, Porcher R, Rouveau M, et al. IFN-gamma and antibody responses among French nurses during a tuberculosis contact tracing investigation. Pathol Biol (Paris). 2009; 57: e49–53. [DOI] [PubMed] [Google Scholar]
- 41.Kik SV, Franken WP, Arend SM, Mensen M, Cobelens FG, Kamphorst M, et al. Interferon-gamma release assays in immigrant contacts and effect of remote exposure to Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2009; 13: 820–828. [PubMed] [Google Scholar]
- 42.Kim EY, Lim JE, Jung JY, Son JY, Lee KJ, Yoon YW, et al. Performance of the tuberculin skin test and interferon-gamma release assay for detection of tuberculosis infection in immunocompromised patients in a BCG-vaccinated population. BMC Infect Dis. 2009; 9: 207 doi: 10.1186/1471-2334-9-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lien LT, Hang NT, Kobayashi N, Yanai H, Toyota E, Sakurada S, et al. Prevalence and risk factors for tuberculosis infection among hospital workers in Hanoi, Viet Nam. PloS One. 2009; 4: e6798 doi: 10.1371/journal.pone.0006798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lighter J, Rigaud M, Eduardo R, Peng CH, Pollack H. Latent tuberculosis diagnosis in children by using the QuantiFERON-TB Gold In-Tube test. Pediatrics. 2009; 123: 30–37. doi: 10.1542/peds.2007-3618 [DOI] [PubMed] [Google Scholar]
- 45.Machado A Jr, Emodi K, Takenami I, Finkmoore BC, Barbosa T, Carvalho J, et al. Analysis of discordance between the tuberculin skin test and the interferon-gamma release assay. Int J Tuberc Lung Dis. 2009; 13: 446–453. [PubMed] [Google Scholar]
- 46.Ringshausen FC, Schlosser S, Nienhaus A, Schablon A, Schultze-Werninghaus G, Rohde G. In-hospital contact investigation among health care workers after exposure to smear-negative tuberculosis. J Occup Med Toxicol. 2009; 4: 11 doi: 10.1186/1745-6673-4-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saracino A, Scotto G, Fornabaio C, Martinelli D, Faleo G, Cibelli D, et al. QuantiFERON-TB Gold In-Tube test (QFT-GIT) for the screening of latent tuberculosis in recent immigrants to Italy. The New Microbiologica. 2009; 32: 369–376. [PubMed] [Google Scholar]
- 48.Torres Costa J, Sa R, Cardoso MJ, Silva R, Ferreira J, Ribeiro C, et al. Tuberculosis screening in Portuguese healthcare workers using the tuberculin skin test and the interferon-gamma release assay. Eur Respir J. 2009; 34: 1423–1428. doi: 10.1183/09031936.00053809 [DOI] [PubMed] [Google Scholar]
- 49.Tripodi D, Brunet-Courtois B, Nael V, Audrain M, Chailleux E, Germaud P, et al. Evaluation of the tuberculin skin test and the interferon-γ release assay for TB screening in French healthcare workers. J Occup Med Toxicol. 2009; 4: 30 doi: 10.1186/1745-6673-4-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vinton P, Mihrshahi S, Johnson P, Jenkin GA, Jolley D, Biggs BA. Comparison of QuantiFERON-TB Gold In-Tube Test and tuberculin skin test for identification of latent Mycobacterium tuberculosis infection in healthcare staff and association between positive test results and known risk factors for infection. Infect Control Hosp Epidemiol. 2009; 30: 215–221. doi: 10.1086/595695 [DOI] [PubMed] [Google Scholar]
- 51.Zhao X, Mazlagic D, Flynn EA, Hernandez H, Abbott CL. Is the QuantiFERON-TB blood assay a good replacement for the tuberculin skin test in tuberculosis screening? a pilot study at Berkshire Medical Center. Am J Clin Pathol. 2009; 132: 678–686. doi: 10.1309/AJCPUHC34NBDGKKL [DOI] [PubMed] [Google Scholar]
- 52.Adetifa IM, Ota MO, Jeffries DJ, Hammond A, Lugos MD, Donkor S, et al. Commercial interferon gamma release assays compared to the tuberculin skin test for diagnosis of latent Mycobacterium tuberculosis infection in childhood contacts in the Gambia. Pediatr Infect Dis J. 2010; 29: 439–443. doi: 10.1097/INF.0b013e3181cb45da [DOI] [PubMed] [Google Scholar]
- 53.Costa JT, Silva R, Sa R, Cardoso MJ, Ribeiro C, Nienhaus A. Comparison of interferon-gamma release assay and tuberculin test for screening in healthcare workers. Rev Port Pneumol. 2010; 16: 211–221. [PubMed] [Google Scholar]
- 54.Grare M, Derelle J, Dailloux M, Laurain C. QuantiFERON-TB Gold In-Tube as help for the diagnosis of tuberculosis in a French pediatric hospital. Diagn Microbiol Infect Dis. 2010; 66: 366–372. doi: 10.1016/j.diagmicrobio.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 55.Huang YW, Shen GH, Lee JJ, Yang WT. Latent tuberculosis infection among close contacts of multidrug-resistant tuberculosis patients in central Taiwan. Int J Tuberc Lung Dis. 2010; 14: 1430–1435. [PubMed] [Google Scholar]
- 56.Jong Lee K, Ae Kang Y, Mi Kim Y, Cho SN, Wook Moon J, Suk Park M, et al. Screening for latent tuberculosis infection in South Korean healthcare workers using a tuberculin skin test and whole blood interferon-gamma assay. Scand J Infect Dis. 2010; 42: 672–678. doi: 10.3109/00365548.2010.485575 [DOI] [PubMed] [Google Scholar]
- 57.Katsenos S, Nikolopoulou M, Konstantinidis AK, Gartzonika C, Gogali A, Margelis I, et al. Interferon-gamma release assay clarifies the effect of bacille Calmette-Guerin vaccination in Greek army recruits. Int J Tuberc Lung Dis. 2010; 14: 545–550. [PubMed] [Google Scholar]
- 58.Lee SH, Lew WJ, Kim HJ, Lee HK, Lee YM, Cho CH, et al. Serial interferon-gamma release assays after rifampicin prophylaxis in a tuberculosis outbreak. Respir Med. 2010; 104: 448–453. doi: 10.1016/j.rmed.2009.10.006 [DOI] [PubMed] [Google Scholar]
- 59.Torres Costa J, Silva R, Sa R, Cardoso MJ, Nienhaus A. Results of five-year systematic screening for latent tuberculosis infection in healthcare workers in Portugal. J Occup Med Toxicol. 2010; 5: 22 doi: 10.1186/1745-6673-5-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas TA, Mondal D, Noor Z, Liu L, Alam M, Haque R, et al. Malnutrition and helminth infection affect performance of an interferon gamma-release assay. Pediatrics. 2010; 126: e1522–1529. doi: 10.1542/peds.2010-0885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsolia MN, Mavrikou M, Critselis E, Papadopoulos NG, Makrinioti H, Spyridis NP, et al. Whole blood interferon-gamma release assay is a useful tool for the diagnosis of tuberculosis infection particularly among Bacille Calmette Guerin-vaccinated children. Pediatr Infect Dis J. 2010; 29: 1137–1140. doi: 10.1097/INF.0b013e3181ebfe8a [DOI] [PubMed] [Google Scholar]
- 62.Caglayan V, Ak O, Dabak G, Damadoglu E, Ketenci B, Ozdemir M, et al. Comparison of tuberculin skin testing and QuantiFERON-TB Gold-In Tube test in health care workers. Tuberk Toraks. 2011; 59: 43–47. [PubMed] [Google Scholar]
- 63.Diel R, Loddenkemper R, Niemann S, Meywald-Walter K, Nienhaus A. Negative and positive predictive value of a whole-blood interferon-gamma release assay for developing active tuberculosis: an update. Am J Respir Crit Care Med. 2011; 183: 88–95. doi: 10.1164/rccm.201006-0974OC [DOI] [PubMed] [Google Scholar]
- 64.Kasambira TS, Shah M, Adrian PV, Holshouser M, Madhi SA, Chaisson RE, et al. QuantiFERON-TB Gold In-Tube for the detection of Mycobacterium tuberculosis infection in children with household tuberculosis contact. Int J Tuberc Lung Dis. 2011; 15: 628–634. doi: 10.5588/ijtld.10.0555 [DOI] [PubMed] [Google Scholar]
- 65.Kus J, Demkow U, Lewandowska K, Korzeniewska-Kosela M, Rabczenko D, Siemion-Szczesniak I, et al. Prevalence of latent infection with Mycobacterium tuberculosis in Mazovia Region using interferon gamma release assay after stimulation with specific antigens ESAT-6 and CFP-10. Pneumonologia i Alergologia Polska. 2011; 79: 407–418. [PubMed] [Google Scholar]
- 66.Legesse M, Ameni G, Mamo G, Medhin G, Bjune G, Abebe F. Community-based cross-sectional survey of latent tuberculosis infection in Afar pastoralists, Ethiopia, using QuantiFERON-TB Gold In-Tube and tuberculin skin test. BMC Infect Dis. 2011; 11: 89 doi: 10.1186/1471-2334-11-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moon HW, Kim H, Hur M, Yun YM, Lee A. Latent tuberculosis infection screening for laboratory personnel using interferon-gamma release assay and tuberculin skin test in Korea: an intermediate incidence setting. J Clin Lab Anal. 2011; 25: 382–388. doi: 10.1002/jcla.20479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moyo S, Isaacs F, Gelderbloem S, Verver S, Hawkridge AJ, Hatherill M, et al. Tuberculin skin test and QuantiFERON(R) assay in young children investigated for tuberculosis in South Africa. Int J Tuberc Lung Dis. 2011; 15: 1176–1181. doi: 10.5588/ijtld.10.0770 [DOI] [PubMed] [Google Scholar]
- 69.Pavic I, Topic RZ, Raos M, Aberle N, Dodig S. Interferon-gamma release assay for the diagnosis of latent tuberculosis in children younger than 5 years of age. Pediatr Infect Dis J. 2011; 30: 866–870. doi: 10.1097/INF.0b013e318220c52a [DOI] [PubMed] [Google Scholar]
- 70.Rafiza S RK, Tahir A. Prevalence and risk factors of latent tuberculosis infection among health care workers in Malaysia. BMC Infect Dis. 2011; 11: 19 doi: 10.1186/1471-2334-11-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shanaube K, Hargreaves J, Fielding K, Schapp A, Lawrence KA, Hensen B, et al. Risk factors associated with positive QuantiFERON-TB Gold In-Tube and tuberculin skin tests results in Zambia and South Africa. PloS One. 2011; 6: e18206 doi: 10.1371/journal.pone.0018206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Talebi-Taher M, Javad-Moosavi SA, Entezari AH, Shekarabi M, Parhizkar B. Comparing the performance of QuantiFERON-TB Gold and Mantoux test in detecting latent tuberculosis infection among Iranian health care workers. Int J Occup Med Environ Health. 2011; 24: 359–366. doi: 10.2478/s13382-011-0046-7 [DOI] [PubMed] [Google Scholar]
- 73.Torres Costa J, Silva R, Ringshausen FC, Nienhaus A. Screening for tuberculosis and prediction of disease in Portuguese healthcare workers. J Occup Med Toxicol. 2011; 6: 19 doi: 10.1186/1745-6673-6-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Torres Costa J, Silva R, Sa R, Cardoso M, Nienhaus A. Serial testing with the interferon-gamma release assay in Portuguese healthcare workers. Int Arch Occup Environ Health. 2011; 84: 461–469. doi: 10.1007/s00420-010-0571-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weinfurter P, Blumberg HM, Goldbaum G, Royce R, Pang J, Tapia J, t al. Predictors of discordant tuberculin skin test and QuantiFERON(R)-TB Gold In-Tube results in various high-risk groups. Int J Tuberc Lung Dis. 2011; 15: 1056–1061. doi: 10.5588/ijtld.10.0650 [DOI] [PubMed] [Google Scholar]
- 76.Yassin MA, Petrucci R, Garie KT, Harper G, Arbide I, Aschalew M, et al. Can interferon-gamma or interferon-gamma-induced-protein-10 differentiate tuberculosis infection and disease in children of high endemic areas? PloS One. 2011; 6: e23733 doi: 10.1371/journal.pone.0023733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bergot E, Haustraete E, Malbruny B, Magnier R, Salaun MA, Zalcman G. Observational study of QuantiFERON(R)-TB gold in-tube assay in tuberculosis contacts in a low incidence area. PloS One. 2012; 7: e43520 doi: 10.1371/journal.pone.0043520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Di Renzi S, Tomao P, Martini A, Capanna S, Rubino L, D’Amico W, et al. Screening for tuberculosis among homeless shelter staff. Am J Infect Control. 2012; 40: 459–461. doi: 10.1016/j.ajic.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 79.He GX, Wang LX, Chai SJ, Klena JD, Cheng SM, Ren YL, et al. Risk factors associated with tuberculosis infection among health care workers in Inner Mongolia, China. Int J Tuberc Lung Dis. 2012; 16: 1485–1491. doi: 10.5588/ijtld.12.0193 [DOI] [PubMed] [Google Scholar]
- 80.Jeong YJ, Yoon S, Koo HK, Lim HJ, Lee JS, Lee SM, et al. Positive tuberculin skin test or interferon-gamma release assay in patients with radiographic lesion suggesting old healed tuberculosis. J Korean Med Sci. 2012; 27: 761–766. doi: 10.3346/jkms.2012.27.7.761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jo KW, Jeon K, Kang YA, Koh WJ, Kim KC, Kim YH, et al. Poor correlation between tuberculin skin tests and interferon-gamma assays in close contacts of patients with multidrug-resistant tuberculosis. Respirology. 2012; 17: 1125–1130. doi: 10.1111/j.1440-1843.2012.02218.x [DOI] [PubMed] [Google Scholar]
- 82.Jung da H, Jo KW, Shim TS. Prevalence of latent tuberculosis infection among medical students in South Korea. Tuberc Resp Dis. 2012; 73: 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Larcher C, Frizzera E, Pretto P, Lang M, Sonnleitner N, Huemer HP. Immunosurveillance for Mycobacterium tuberculosis of health care personnel in a third level care hospital. Med Lav. 2012; 103: 26–36. [PubMed] [Google Scholar]
- 84.Onur H, Hatipoglu S, Arica V, Hatipoglu N, Arica SG. Comparison of quantiferon test with tuberculin skin test for the detection of tuberculosis infection in children. Inflammation. 2012; 35: 1518–1524. doi: 10.1007/s10753-012-9466-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pattnaik S, John KR, Shalini E, Michael JS. Agreement between skin testing and QuantiFERON-TB Gold In-Tube assay (QFT-TB) in detecting latent tuberculosis infection among household contacts in India. Indian J Tuberc. 2012; 59: 214–218. [PubMed] [Google Scholar]
- 86.Zwerling A, Cojocariu M, McIntosh F, Pietrangelo F, Behr MA, Schwartzman K, et al. TB screening in Canadian health care workers using interferon-gamma release assays. PloS One. 2012; 7: e43014 doi: 10.1371/journal.pone.0043014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jo KW, Hong Y, Park JS, Bae IG, Eom JS, Lee SR, et al. Prevalence of latent tuberculosis infection among health care workers in South Korea: a multicenter study. Tuberc Resp Dis. 2013; 75: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Serrano-Escobedo CJ, Enciso-Moreno JA, Monarrez-Espino J. Performance of tuberculin skin test compared to QFT-IT to detect latent TB among high-risk contacts in Mexico. Arch Med Res. 2013; 44: 242–248. doi: 10.1016/j.arcmed.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 89.Whitaker JA, Mirtskhulava V, Kipiani M, Harris DA, Tabagari N, Kempker RR, et al. Prevalence and incidence of latent tuberculosis infection in georgian healthcare workers. PloS One. 2013; 8: e58202 doi: 10.1371/journal.pone.0058202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zwerling A, Benedetti A, Cojocariu M, McIntosh F, Pietrangelo F, Behr MA, et al. Repeat IGRA testing in Canadian health workers: conversions or unexplained variability? PloS One. 2013; 8: e54748 doi: 10.1371/journal.pone.0054748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alvarez GG, Van Dyk DD, Davies N, Aaron SD, Cameron DW, Desjardings M, et al. The feasibility of the interferon gamma release assay and predictors of discordance with the tuberculin skin test for the diagnosis of latent tuberculosis infection in a remote Aboriginal community. PloS One. 2014; 9: e111986 doi: 10.1371/journal.pone.0111986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Charisis A, Tatsioni A, Gartzonika C, Gogali A, Archimandriti D, Katsanos C, et al. Value of adding an IGRA to the TST to screen for latent tuberculous infection in Greek health care workers. Int J Tuberc Lung Dis. 2014; 18: 1040–1046. doi: 10.5588/ijtld.14.0018 [DOI] [PubMed] [Google Scholar]
- 93.de Souza FM, do Prado TN, Pinheiro Jdos S, Peres RL, Lacerda TC, Loureiro RB, et al. Comparison of interferon-gamma release assay to two cut-off points of tuberculin skin test to detect latent Mycobacterium tuberculosis infection in primary health care workers. PloS One. 2014; 9: e102773 doi: 10.1371/journal.pone.0102773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Erkens CG, Dinmohamed AG, Kamphorst M, Toumanian S, van Nispen-Dobrescu R, Alink M, et al. Added value of interferon-gamma release assays in screening for tuberculous infection in the Netherlands. Int J Tuberc Lung Dis. 2014; 18: 413–420. doi: 10.5588/ijtld.13.0589 [DOI] [PubMed] [Google Scholar]
- 95.Garazzino S, Galli L, Chiappini E, Pinon M, Bergamini BM, Cazzato S, et al. Performance of interferon-gamma release assay for the diagnosis of active or latent tuberculosis in children in the first 2 years of age: a multicenter study of the Italian Society of Pediatric Infectious Diseases. Pediatr Infect Dis J. 2014; 33: e226–231. doi: 10.1097/INF.0000000000000353 [DOI] [PubMed] [Google Scholar]
- 96.Guanche Garcell H, Crespo Ramirez E, Kindelan Contreras A, Gutierrez Garcia F. Latent tuberculosis infection in healthcare workers at a community hospital in Qatar. J Infect Public Health. 2014; 7: 356–359. doi: 10.1016/j.jiph.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 97.Goodwin DJ, Mazurek GH, Campbell BH, Bohanon J, West KB, Bell JJ, et al. Automation of an interferon-gamma release assay and comparison to the tuberculin skin test for screening basic military trainees for Mycobacterium tuberculosis infection. Mil Med. 2014; 179: 333–341. doi: 10.7205/MILMED-D-13-00364 [DOI] [PubMed] [Google Scholar]
- 98.Mathad JS, Bhosale R, Sangar V, Mave V, Gupte N, Kanade S, et al. Pregnancy differentially impacts performance of latent tuberculosis diagnostics in a high-burden setting. PloS One. 2014; 9: e92308 doi: 10.1371/journal.pone.0092308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ribeiro-Rodrigues R, Kim S, Coelho da Silva FD, Uzelac A, Collins L, Palaci M, et al. Discordance of tuberculin skin test and interferon gamma release assay in recently exposed household contacts of pulmonary TB cases in Brazil. PloS One. 2014; 9: e96564 doi: 10.1371/journal.pone.0096564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sauzullo I, Mastroianni CM, Mengoni F, Ermocida A, Mascia C, Salotti A, et al. Long-term IFN-gamma and IL-2 response for detection of latent tuberculosis infection in healthcare workers with discordant immunologic results. J Immunol Methods. 2014; 414: 51–57. doi: 10.1016/j.jim.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 101.Song SE, Yang J, Lee KS, Kim H, Kim YM, Kim S, et al. Comparison of the tuberculin skin test and interferon gamma release assay for the screening of tuberculosis in adolescents in close contact with tuberculosis TB patients. PloS One. 2014; 9: e100267 doi: 10.1371/journal.pone.0100267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adams S, Ehrlich R, Baatjies R, van Zyl-Smit RN, Said-Hartley Q, Dawson R, et al. Incidence of occupational latent tuberculosis infection in South African healthcare workers. Eur Respir J. 2015; 45: 1364–1373. doi: 10.1183/09031936.00138414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.El-Sokkary RH, Abu-Taleb AM, El-Seifi OS, Zidan HE, Mortada EM, El-Hossary D, et al. Assessing the Prevalence of Latent Tuberculosis among Health Care Providers in Zagazig City, Egypt Using Tuberculin Skin Test and QuantiFERON-TB Gold In-Tube Test. Cent Eur J Public Health. 2015; 23: 324–330. doi: 10.21101/cejph.a4101 [DOI] [PubMed] [Google Scholar]
- 104.Gao L, Lu W, Bai L, Wang X, Xu J, Catanzaro A, et al. Latent tuberculosis infection in rural China: baseline results of a population-based, multicentre, prospective cohort study. Lancet Infect Dis. 2015; 15: 310–319. doi: 10.1016/S1473-3099(14)71085-0 [DOI] [PubMed] [Google Scholar]
- 105.Goebel KM, Tay EL, Denholm JT. Supplemental use of an interferon-gamma release assay in a state-wide tuberculosis contact tracing program in Victoria: a six-year review. Commun Dis Intell Q Rep. 2015; 39: E191–196. [DOI] [PubMed] [Google Scholar]
- 106.He G, Li Y, Zhao F, Wang L, Cheng S, Guo H, et al. The prevalence and incidence of latent tuberculosis infection and its associated factors among village doctors in China. PloS One. 2015; 10: e0124097 doi: 10.1371/journal.pone.0124097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Howley MM, Painter JA, Katz DJ, Graviss EA, Reves R, Beavers SF, et al. Evaluation of QuantiFERON-TB gold in-tube and tuberculin skin tests among immigrant children being screened for latent tuberculosis infection. Pediatr Infect Dis J. 2015; 34: 35–39. doi: 10.1097/INF.0000000000000494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jones-Lopez EC, White LF, Kirenga B, Mumbowa F, Ssebidandi M, Moine S, et al. Cough aerosol cultures of Mycobacterium tuberculosis: insights on TST / IGRA discordance and transmission dynamics. PloS One. 2015; 10: e0138358 doi: 10.1371/journal.pone.0138358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lucet JC, Abiteboul D, Estellat C, Roy C, Chollet-Martin S, Tubach F, et al. Interferon-gamma release assay vs. tuberculin skin test for tuberculosis screening in exposed healthcare workers: a longitudinal multicenter comparative study. Infect Control Hosp Epidemiol. 2015; 36: 569–574. doi: 10.1017/ice.2015.19 [DOI] [PubMed] [Google Scholar]
- 110.Ferrarini MA, Spina FG, Weckx LY, Lederman HM, De Moraes-Pinto MI. Rate of tuberculosis infection in children and adolescents with household contact with adults with active pulmonary tuberculosis as assessed by tuberculin skin test and interferon-gamma release assays. Epidemiol Infect. 2016; 144: 712–723. doi: 10.1017/S0950268815001727 [DOI] [PubMed] [Google Scholar]
- 111.Al Hajoj S, Varghese B, Datijan A, Shoukri M, Alzahrani A, Alkhenizan A, et al. Interferon gamma release assay versus tuberculin skin testing among healthcare workers of highly diverse origin in a moderate tuberculosis burden country. PLoS One. 2016; 11: e0154803 doi: 10.1371/journal.pone.0154803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Biraro IA, Kimuda S, Egesa M, Cose S, Webb EL, Joloba M, et al. The use of interferon gamma inducible protein 10 as a potential biomarker in the diagnosis of latent tuberculosis infection in Uganda. PLoS One. 2016; 11: e0146098 doi: 10.1371/journal.pone.0146098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bozkanat E, Kaya H, Sezer O, Caliskan T, Kilic E, Ciftci F, et al. Comparison of tuberculin skin test and quantiferon-TB gold in tube test for diagnosis of latent tuberculosis infection in health care workers: A cross sectional study. J Pak Med Assoc. 2016; 66: 270–4. [PubMed] [Google Scholar]
- 114.Grare M, Derelle J, Dailloux M, Laurain C. Difficulties of TB diagnosis in children: QuantiFERON TB Gold In-Tube as useful tool. Arch Pediatr. 2010; 17: 77–85. doi: 10.1016/j.arcped.2009.07.021 [DOI] [PubMed] [Google Scholar]
- 115.Lowenthal P, Barry PM, Flood J. High discordance between pre-US and post-US entry tuberculosis test results among immigrant children: is it time to adopt interferon gamma release assay for pre-entry tuberculosis screening? Pediatr Infect Dis J. 2016; 35: 231–6. doi: 10.1097/INF.0000000000000986 [DOI] [PubMed] [Google Scholar]
- 116.Marco Mourino A, Orcau Palau A, Jane Galliga R, Escribano Ibanez M, Cayla Buqueras JA, Sole Zapata N, et al. Concordance of tuberculin tests and Interferon gamma release assays in the prison population. Rev Esp Sanid Penit. 2011; 13: 15–20. [DOI] [PubMed] [Google Scholar]
- 117.Marquez C, Chamie G, Achan J, Luetkemeyer A, Kyohere M, Okiring J, et al. Tuberculosis infection in early childhood and the association with HIV-exposure in HIV-uninfected children in rural uganda. Pediatr Infect Dis J. 2016; 35: 524–9. doi: 10.1097/INF.0000000000001062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miramontes R, Hill AN, Woodruff RSY, Lambert LA, Navin TR, Castro KG, et al. Tuberculosis infection in the United States: prevalence estimates from the National Health and Nutrition Examination Survey, 2011–2012. PLoS One. 2015; 10: e0140881 doi: 10.1371/journal.pone.0140881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mostafavi E, Nasehi M, Shahraki AH, Esmaeili S, Ghaderi E, Sharafi S, et al. Comparison of the tuberculin skin test and the QuantiFERON-TB Gold test in detecting latent tuberculosis in health care workers in Iran. Epidemiol Health. 2016; 38: e2016032 doi: 10.4178/epih.e2016032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nienhaus A, Schablon A, Tripodi D, Torres Costa J. The prevalence of latent tuberculosis infections among health-care workers—a three-country comparison. Pneumologie. 2011; 65: 726–9. doi: 10.1055/s-0031-1291392 [DOI] [PubMed] [Google Scholar]
- 121.Oren E, Fiero MH, Barrett E, Anderson B, Nunez M, Gonzalez-Salazar F. Detection of latent tuberculosis infection among migrant farmworkers along the US-Mexico border. BMC Infect Dis. 2016; 16: 630 doi: 10.1186/s12879-016-1959-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pavic I, Katalinic-Jankovic V, Cepin-Bogovic J, Resic A, Dodig S. Discordance between tuberculin skin test and interferon-gamma release assay in children younger than 5 years who have been vaccinated with Bacillus Calmette-Guerin. Lab Med. 2015; 46: 200–6. doi: 10.1309/LMCQLO8PG0IZ5APX [DOI] [PubMed] [Google Scholar]
- 123.Reechaipichitkul W, Pimrin W, Bourpoern J, Prompinij S, Faksri K. Evaluation of the QuantiFERON-TB Gold In-Tube assay and tuberculin skin test for the diagnosis of Mycobacterium tuberculosis infection in northeastern Thailand. Asian Pac J Allergy Immunol. 2015; 33: 236–44. doi: 10.12932/AP0576.33.3.2015 [DOI] [PubMed] [Google Scholar]
- 124.Rose W, Read SE, Bitnun A, Rea E, Stephens D, Pongsamart W. Relating tuberculosis (TB) contact characteristics to QuantiFERON-TB-Gold and tuberculin skin test results in the Toronto pediatric TB clinic. J Pediatric Infect Dis Soc. 2015; 4: 96–103. doi: 10.1093/jpids/piu024 [DOI] [PubMed] [Google Scholar]
- 125.Salinas C, Ballaz A, Diez R, Aguirre U, Anton A, Altube L. Tuberculosis screening program for undocumented immigrant teenagers using the QuantiFERON((R))-TB Gold In-Tube test. Med Clin (Barc). 2015; 145: 7–13. [DOI] [PubMed] [Google Scholar]
- 126.Sharma SK, Vashishtha R, Chauhan LS, Sreenivas V, Seth D. Comparison of TST and IGRA in diagnosis of latent tuberculosis infection in a high TB-burden setting. PLoS One. 2017; 12: e0169539 doi: 10.1371/journal.pone.0169539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yoo JW, Jo KW, Park GY, Shim TS. Comparison of latent tuberculosis infection rate between contacts with active tuberculosis and non-contacts. Respir Med. 2016; 111: 77–83. doi: 10.1016/j.rmed.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 128.Anibarro L, Trigo M, Feijoo D, Rios M, Palomares L, Pena A, et al. Tuberculin skin test and interferon-gamma release assay show better correlation after the tuberculin 'window period' in tuberculosis contacts. Scand J Infect Dis. 2011; 43: 424–9. doi: 10.3109/00365548.2011.558912 [DOI] [PubMed] [Google Scholar]
- 129.Diel R, Loddenkemper R, Meywald-Walter K, Niemann S, Nienhaus A. Predictive value of a whole blood IFN-gamma assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2008; 177: 1164–70. doi: 10.1164/rccm.200711-1613OC [DOI] [PubMed] [Google Scholar]
- 130.Ferreira TF, da Fonseca Silva Matsouka P, dos Santas AM, Caldas de Jesus Mendes. Diagnosis of latent Mycobacterium tuberculosis infection: tuberculin test versus interferon-gamma release. Rev Soc Bras Med Trop. 2015; 48: 724–30. doi: 10.1590/0037-8682-0258-2015 [DOI] [PubMed] [Google Scholar]
- 131.Nienhaus A, Schablon A, Le Bacle C, Siano B. Evaluation of the interferon-gamma release assay in healthcare workers. Int Arch Occup Environ Health. 2008; 81: 295–300. doi: 10.1007/s00420-007-0212-1 [DOI] [PubMed] [Google Scholar]
- 132.Porsa E, Cheng L, Seale MM, Delclos GL, Ma X, Reich R, et al. Comparison of a new ESAT-6/CFP-10 peptide-based gamma interferon assay and a tuberculin skin test for tuberculosis screening in a moderate-risk population. Clin Vaccine Immunol. 2006; 13: 53–8. doi: 10.1128/CVI.13.1.53-58.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rangaka MX, Wilkinson KA, Seldon R, Van Cutsem G, Meintjes GA, Morroni G, et al. Effect of HIV-1 infection on T-Cell-based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med. 2007; 175: 514–20. doi: 10.1164/rccm.200610-1439OC [DOI] [PubMed] [Google Scholar]
- 134.Bienek DR and Chang CK. Evaluation of an interferon-gamma release assay, T-SPOT.TB, in a population with a low prevalence of tuberculosis. Int J Tuberc Lung Dis. 2009. 13: 1416–21. [PubMed] [Google Scholar]
- 135.Janssens JP, Lombard PR, Perneger T, Metzger M, Vivien R, Rochat T. Contribution of a IFN-gamma assay in contact tracing for tuberculosis in a low-incidence, high immigration area. Swiss Med Wkly. 2008; 138: 585–93. [DOI] [PubMed] [Google Scholar]
- 136.Leung CC, Yam WC, Yew WW, Ho PL, Tam CM, Law WS, et al. Comparison of T-Spot.TB and tuberculin skin test among silicotic patients. Eur Respir J. 2008; 31: 266–72. doi: 10.1183/09031936.00054707 [DOI] [PubMed] [Google Scholar]
- 137.Soysal A, Torun T, Efe S, Gencer H, Tahaoglu K, Bakir M. Evaluation of cut-off values of interferon-gamma-based assays in the diagnosis of M. tuberculosis infection. Int J Tuberc Lung Dis. 2008; 12: 50–6. [PubMed] [Google Scholar]
- 138.Girardi E, Angeletti C, Puro V, Sorrentino R, Magnavita N, Vincenti D, et al. Estimating diagnostic accuracy of tests for latent tuberculosis infection without a gold standard among healthcare workers. Euro Surveill. 2009; 14. [PubMed] [Google Scholar]
- 139.Hansted E, Andriuskeviciene A, Sakalauskas R, Kevalas R, Sitkauskiene B. T-cell-based diagnosis of tuberculosis infection in children in Lithuania: a country of high incidence despite a high coverage with bacille Calmette-Guerin vaccination. BMC Pulm Med. 2009; 9: 41 doi: 10.1186/1471-2466-9-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Leung CC, Yam WC, Yew WW, Ho PL, Tam CM, Law WS, et al. T-Spot.TB outperforms tuberculin skin test in predicting tuberculosis disease. Am J Respir Crit Care Med. 2010; 182: 834–40. doi: 10.1164/rccm.200912-1875OC [DOI] [PubMed] [Google Scholar]
- 141.Borkowska D, Zwolska Z, Michałowska-Mitczuk D, Korzeniewska-Koseła M, Zabost A, Napiórkowska A, et al. Interferon-gamma assays T-SPOT.TB for the diagnosis of latent tuberculosis infection. Pneumonol Alergol Pol. 2011; 79: 264–71. [PubMed] [Google Scholar]
- 142.Zhao J, Wang Y, Wang H, Jiang C, Liu Z, Meng X, et al. Low agreement between the T-SPOT(R).TB assay and the tuberculin skin test among college students in China. Int J Tuberc Lung Dis. 2011; 15: 134–6. [PMC free article] [PubMed] [Google Scholar]
- 143.Nkurunungi G, Lutangira JE, Lule SA, Akurut H, Kizindo R, Fitchett JR, et al. Determining Mycobacterium tuberculosis infection among BCG-immunised Ugandan children by T-SPOT.TB and tuberculin skin testing. PLoS One. 2012; 7: e47340 doi: 10.1371/journal.pone.0047340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Leung CC, Yam WC, Ho PL, Yew WW, Chan CK, Law WS, et al. T-Spot.TB outperforms tuberculin skin test in predicting development of active tuberculosis among household contacts. Respirology. 2015; 20: 496–503. doi: 10.1111/resp.12483 [DOI] [PubMed] [Google Scholar]
- 145.Spicer KB, Turner J, Wang SH, Koranyi K, Powell DA. Tuberculin skin testing and T-SPOT.TB in internationally adopted children. Pediatr Infect Dis J. 2015; 34: p. 599–603. doi: 10.1097/INF.0000000000000680 [DOI] [PubMed] [Google Scholar]
- 146.Mendez-Echevarria A, Gonzalez-Munoz M, Mellado MJ, Baquero-Artigao F, Vecino R, Perez E. Optimizing interpretation of the tuberculin test using an interferon-gamma release assay as a reference standard. Pediatr Infect Dis J. 2011; 30: 426–8. doi: 10.1097/INF.0b013e3182001294 [DOI] [PubMed] [Google Scholar]
- 147.Takahashi H, Shigehara K, Yamamoto M, Suzuki C, Naishiro Y, Tamura Y, et al. Interferon gamma assay for detecting latent tuberculosis infection in rheumatoid arthritis patients during infliximab administration. Rheumatol Int. 2007; 27: 1143–8. doi: 10.1007/s00296-007-0361-2 [DOI] [PubMed] [Google Scholar]
- 148.Aichelburg MC, Mandorfer M, Tittes J, Breitenecker F, Reiberger T, Rieger A, et al. The association of smoking with IGRA and TST results in HIV-1-infected subjects. Int J Tuberc Lung Dis. 2014; 18: 709–16. doi: 10.5588/ijtld.13.0813 [DOI] [PubMed] [Google Scholar]
- 149.Balcells ME, Perez CM, Chanqueo L, Lasso M, Villanueva M, Espinoza M, et al. A comparative study of two different methods for the detection of latent tuberculosis in HIV-positive individuals in Chile. Int J Infect Dis. 2008; 12: 645–52. doi: 10.1016/j.ijid.2008.03.005 [DOI] [PubMed] [Google Scholar]
- 150.Bourgarit A, Baron G, Breton G, Tattevin P, Katlama C, Allavena C, et al. Latent tuberculosis infection screening and 2-year outcome in antiretroviral-naive HIV-infected patients in a low-prevalence country. Ann Am Thorac Soc. 2015; 12: 1138–45. doi: 10.1513/AnnalsATS.201412-600OC [DOI] [PubMed] [Google Scholar]
- 151.Casas S, Andreu A, Juanola X, Bordas X, Alcaide F, Moure R, et al. Diagnosis of tuberculosis infection by tuberculin skin test and a whole-blood interferon-gamma release assay in patients considered for anti-tumor necrosis factor-alpha therapy. Diagn Microbiol Infect Dis. 2011; 71: 57–65. doi: 10.1016/j.diagmicrobio.2010.12.020 [DOI] [PubMed] [Google Scholar]
- 152.Casas S, Muñoz L, Moure R, Castellote J, Guerra MR, Gonzalez L, et al. Comparison of the 2-step tuberculin skin test and the quantiFERON-TB Gold In-Tube Test for the screening of tuberculosis infection before liver transplantation. Liver Transpl. 2011; 17: 205–11. [DOI] [PubMed] [Google Scholar]
- 153.Chkhartishvili N, Kempker RR, Dvali N, Abashidze L, Sharavdze L, Gabunia P, et al. Poor agreement between interferon-gamma release assays and the tuberculin skin test among HIV-infected individuals in the country of Georgia. BMC Infect Dis. 2013; 13: 513 doi: 10.1186/1471-2334-13-513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gogus F, Gunendi Z, Karakus R, Erdogan Z, Hizel K, Atalay F. Comparison of tuberculin skin test and QuantiFERON-TB gold in tube test in patients with chronic inflammatory diseases living in a tuberculosis endemic population. Clin Exp Med. 2010; 10: 173–7. doi: 10.1007/s10238-009-0082-9 [DOI] [PubMed] [Google Scholar]
- 155.Hanta I, Ozbek S, Kuleci S, Seydaoglu G, Ozyilmaz E. Detection of latent tuberculosis infection in rheumatologic diseases before anti-TNFalpha therapy: tuberculin skin test versus IFN-gamma assay. Rheumatol Int. 2012; 32: 3599–603. doi: 10.1007/s00296-011-2243-x [DOI] [PubMed] [Google Scholar]
- 156.Hsia EC, Schluger N, Cush JJ, Chaisson RE, Matteson EL, Xu S, et al. Interferon-gamma release assay versus tuberculin skin test prior to treatment with golimumab, a human anti-tumor necrosis factor antibody, in patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis. Arthritis Rheum. 2012; 64: 2068–77. doi: 10.1002/art.34382 [DOI] [PubMed] [Google Scholar]
- 157.James PM, Ganaie FA, Kadahalli RL. The performance of quantiferon-TB gold in-tube (QFT-IT) test compared to tuberculin skin test (TST) in detecting latent tuberculosis infection (LTBI) in the presence of HIV coinfection in a high TB-burden area with BCG-vaccinated population. J Int Assoc Provid AIDS Care. 2014; 13: 47–55. doi: 10.1177/2325957412469687 [DOI] [PubMed] [Google Scholar]
- 158.Jones S, de Gijsel D, Wallach FR, Gurtman AC, Shi Q, Sacks H. Utility of QuantiFERON-TB Gold in-tube testing for latent TB infection in HIV-infected individuals. Int J Tuberc Lung Dis. 2007; 11: 1190–5. [PubMed] [Google Scholar]
- 159.Karadag O, Aksu K, Sahin A, Zihni FY, Sener B, Inanc N, et al. Assessment of latent tuberculosis infection in Takayasu arteritis with tuberculin skin test and Quantiferon-TB Gold test. Rheumatol Int. 2010; 30: 1483–7. doi: 10.1007/s00296-010-1444-z [DOI] [PubMed] [Google Scholar]
- 160.Khawcharoenporn T, Apisarnthanarak A, Phetsuksiri B, Rudeeaneksin J, Srisungngam S, Mundy LM. Tuberculin skin test and QuantiFERON-TB Gold In-tube Test for latent tuberculosis in Thai HIV-infected adults. Respirology. 2015; 20: 340–7. doi: 10.1111/resp.12442 [DOI] [PubMed] [Google Scholar]
- 161.Kim HC, Jo KW, Jung YJ, Yoo B, Lee CK, Kim YG, et al. Diagnosis of latent tuberculosis infection before initiation of anti-tumor necrosis factor therapy using both tuberculin skin test and QuantiFERON-TB Gold In Tube assay. Scand J Infect Dis. 2014; 46: 763–9. doi: 10.3109/00365548.2014.938691 [DOI] [PubMed] [Google Scholar]
- 162.Kim JH, Cho SK, Han M, Choi CB, Kim TH, Jun JB, et al. Factors influencing discrepancies between the QuantiFERON-TB gold in tube test and the tuberculin skin test in Korean patients with rheumatic diseases. Semin Arthritis Rheum. 2013; 42: 424–32. doi: 10.1016/j.semarthrit.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 163.Kim JH, Won S, Choi CB, Sung YK, Song GG, Bae SC. Evaluation of the usefulness of interferon-gamma release assays and the tuberculin skin test for the detection of latent Mycobacterium tuberculosis infections in Korean rheumatic patients who are candidates for biologic agents. Int J Rheum Dis. 2015; 18: 315–22. doi: 10.1111/1756-185X.12515 [DOI] [PubMed] [Google Scholar]
- 164.Latorre I, Carrascosa JM, Vilavella M, Díaz J, Prat C, Domínguez J, et al. Diagnosis of tuberculosis infection by interferon-gamma release assays in patients with psoriasis. J Infect. 2014; 69: 600–6. doi: 10.1016/j.jinf.2014.07.026 [DOI] [PubMed] [Google Scholar]
- 165.Manuel O, Humar A, Preiksaitis J, Doucette K, Shokoples S, Peleg AY, et al. Comparison of quantiferon-TB gold with tuberculin skin test for detecting latent tuberculosis infection prior to liver transplantation. Am J Transplant. 2007; 7: 2797–801. doi: 10.1111/j.1600-6143.2007.02011.x [DOI] [PubMed] [Google Scholar]
- 166.Matulis G, Jüni P, Villiger PM, Gadola SD. Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases: performance of a Mycobacterium tuberculosis antigen-specific interferon gamma assay. Ann Rheum Dis. 2008; 67: 84–90. doi: 10.1136/ard.2007.070789 [DOI] [PubMed] [Google Scholar]
- 167.Mínguez S, Latorre I, Mateo L, Lacoma A, Diaz J, Olivé A, et al. Interferon-gamma release assays in the detection of latent tuberculosis infection in patients with inflammatory arthritis scheduled for anti-tumour necrosis factor treatment. Clin Rheumatol. 2012; 31: 785–94. doi: 10.1007/s10067-012-1938-z [DOI] [PubMed] [Google Scholar]
- 168.Moon SM, Lee SO, Choi SH, Kim YS, Woo JH, Yoon DH, et al. Comparison of the QuantiFERON-TB Gold In-Tube test with the tuberculin skin test for detecting latent tuberculosis infection prior to hematopoietic stem cell transplantation. Transpl Infect Dis. 2013; 15: 104–9. doi: 10.1111/j.1399-3062.2012.00765.x [DOI] [PubMed] [Google Scholar]
- 169.Papay P, Eser A, Winkler S, Frantal S, Primas C, Miehsler W, et al. Factors impacting the results of interferon-gamma release assay and tuberculin skin test in routine screening for latent tuberculosis in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2011; 17: 84–90. doi: 10.1002/ibd.21427 [DOI] [PubMed] [Google Scholar]
- 170.Ramos JM, Masiá M, Rodríguez JC, López C, Padilla S, Robledano C, et al. Negative effect of immunosuppressive therapy in the performance of the QuantiFERON gold in-tube test in patients with immune-mediated inflammatory diseases. Clin Exp Med. 2013; 13: 177–86. doi: 10.1007/s10238-012-0192-7 [DOI] [PubMed] [Google Scholar]
- 171.Ramos JM, Robledano C, Masiá M, Belda S, Padilla S, Rodríguez JC, et al. Contribution of interferon gamma release assays testing to the diagnosis of latent tuberculosis infection in HIV-infected patients: a comparison of QuantiFERON-TB Gold In Tube, T-SPOT.TB and tuberculin skin test. BMC Infect Dis. 2012; 12: 169 doi: 10.1186/1471-2334-12-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sauzullo I, Mengoni F, Scrivo R, Valesini G, Potenza C, Skroza N, Marocco R, et al. Evaluation of QuantiFERON-TB Gold In-Tube in human immunodeficiency virus infection and in patient candidates for anti-tumour necrosis factor-alpha treatment. Int J Tuberc Lung Dis. 2010; 14: 834–40. [PubMed] [Google Scholar]
- 173.Talati NJ, Seybold U, Humphrey B, Aina A, Tapia J, Weinfurter P, et al. Poor concordance between interferon-gamma release assays and tuberculin skin tests in diagnosis of latent tuberculosis infection among HIV-infected individuals. BMC Infect Dis. 2009; 9: 15 doi: 10.1186/1471-2334-9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vassilopoulos D, Tsikrika S, Hatzara C, Podia V, Kandili A, Stamoulis N, et al. Comparison of two gamma interferon release assays and tuberculin skin testing for tuberculosis screening in a cohort of patients with rheumatic diseases starting anti-tumor necrosis factor therapy. Clin Vaccine Immunol. 2011; 18: 2102–8. doi: 10.1128/CVI.05299-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hoffmann M, Tsinalis D, Vernazza P, Fierz W, Binet I, et al. Assessment of an Interferon-gamma release assay for the diagnosis of latent tuberculosis infection in haemodialysis patient. Swiss Med Wkly. 2010; 140: 286–92. [DOI] [PubMed] [Google Scholar]
- 176.Mariette X, Baron G, Tubach F, Lioté F, Combe B, Miceli-Richard C, et al. Influence of replacing tuberculin skin test with ex vivo interferon gamma release assays on decision to administer prophylactic antituberculosis antibiotics before anti-TNF therapy. Ann Rheum Dis. 2012; 71: 1783–90. doi: 10.1136/annrheumdis-2011-200408 [DOI] [PubMed] [Google Scholar]
- 177.Ponce de Leon D, Acevedo-Vasquez E, Alvizuri S, Gutierrez C, Cucho M, Alfaro J, et al. Comparison of an interferon-gamma assay with tuberculin skin testing for detection of tuberculosis (TB) infection in patients with rheumatoid arthritis in a TB-endemic population. J Rheumatol. 2008; 35: 776–81. [PubMed] [Google Scholar]
- 178.Scrivo R, Sauzullo I, Mengoni F, Iaiani G, Vestri AR, Priori R, et al. Serial interferon-gamma release assays for screening and monitoring of tuberculosis infection during treatment with biologic agents. Clin Rheumatol. 2012; 31: 1567–75. doi: 10.1007/s10067-012-2049-6 [DOI] [PubMed] [Google Scholar]
- 179.Cho H, Kim YW, Suh CH, Jung JY, Um YJ, Jung JH, et al. Concordance between the tuberculin skin test and interferon gamma release assay (IGRA) for diagnosing latent tuberculosis infection in patients with systemic lupus erythematosus and patient characteristics associated with an indeterminate IGRA. Lupus. 2016; 25: 1341–8. doi: 10.1177/0961203316639381 [DOI] [PubMed] [Google Scholar]
- 180.Kurti Z, Lovasz BD, Gecse KB, Balint A, Farkas K, Morocza-Szabo A, et al. Tuberculin skin test and Quantiferon in BCG vaccinated, immunosuppressed patients with moderate-to-severe inflammatory bowel disease. J Gastrointestin Liver Dis. 2015; 24: 467–72. doi: 10.15403/jgld.2014.1121.244.bcg [DOI] [PubMed] [Google Scholar]
- 181.Kussen GM, Dalla-Costa LM, Rossoni A, Raboni SM. Interferon-gamma release assay versus tuberculin skin test for latent tuberculosis infection among HIV patients in Brazil. Braz J Infect Dis. 2016; 20: 69–75. doi: 10.1016/j.bjid.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Palomar R, Arias Guillén M, Robledo C, Agüero R, Agüero J, Rodríguez C, et al. Detection of latent tuberculosis infection in peritoneal dialysis patients: new methods. Nefrologia. 2011; 31: 169–73. doi: 10.3265/Nefrologia.pre2011.Jan.10765 [DOI] [PubMed] [Google Scholar]
- 183.Ferguson TW, Tangri N, Macdonald K, Hiebert B, Rigatto C, Sood MM, et al. The diagnostic accuracy of tests for latent tuberculosis infection in hemodialysis patients: a systematic review and meta-analysis. Transplantation. 2015; 99: 1084–91. doi: 10.1097/TP.0000000000000451 [DOI] [PubMed] [Google Scholar]
- 184.Irwig L, Macaskill P, Glasziou P, Fahey M. Meta-analytic methods for diagnostic test accuracy. J Clin Epidemiol. 1995; 48: 119–30. [DOI] [PubMed] [Google Scholar]
- 185.Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K, et al. Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am J Respir Crit Care Med. 2004; 170: 59–64. doi: 10.1164/rccm.200402-179OC [DOI] [PubMed] [Google Scholar]
- 186.Song GG, Bae SC, Lee YH. Interferon-gamma release assays versus tuberculin skin testing in patients with rheumatoid arthritis. Int J Rheum Dis. 2013; 16: 279–83. doi: 10.1111/1756-185X.12098 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
IGRA, interferon gamma release assay; N, No; Q, Question; TST, tuberculin skin test; U, Unclear; Y, Yes.
(PDF)
*Results are for immune-competent adults. BCG, Bacillus Calmette-Guérin; CrI, credible interval; QFT-GIT, QuantiFERON-TB Gold In Tube; TB, tuberculosis; TST, tuberculin skin test.
(PDF)
*Results are for immune-competent adults. BCG, Bacillus Calmette-Guérin; CrI, credible interval; QFT-GIT, QuantiFERON-TB Gold In Tube; TB, tuberculosis; TST, tuberculin skin test.
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.