Abstract
Background
The B2 receptor antagonist icatibant is approved for treatment of attacks of hereditary angioedema. Icatibant has been reported to decrease time-to-resolution of angiotensin-converting enzyme (ACE) inhibitor-associated angioedema in one study of European patients.
Methods
Patients with ACE inhibitor-associated angioedema (defined as swelling of lips, tongue, pharynx or face during ACE inhibitor use and no swelling in the absence of ACE inhibitor use) were enrolled at Vanderbilt University Medical Center from October 2007 through September 2015 and at Massachusetts General Hospital in 2012. C1 inhibitor deficiency and patients with bowel edema only were excluded. Patients were randomized within six hours of presentation to subcutaneous icatibant 30 mg or placebo at zero and six hours later. Patients assessed severity of swelling using a visual analog scale serially following study drug administration or until discharge.
Results
Thirty-three patients were randomized and 31 received treatment, with 13 receiving icatibant and 18 receiving placebo. One patient randomized to icatibant did not complete the visual analog scale and was excluded from analyses. Two-thirds of patients were African American and two-thirds were women. Time-to-resolution of symptoms was similar in placebo and icatibant treatment groups (p=0.19 for the primary symptom and p>0.16 for individual symptoms of face, lip, tongue, or eyelid swelling). Frequency of administration of H1 and H2 blockers, corticosteroids, and epinephrine was similar in the two treatment groups. Time-to-resolution of symptoms was similar in black and white patients.
Conclusions
This study does not support clinical efficacy of a bradykinin B2 receptor antagonist in ACE inhibitor-associated angioedema.
Keywords: Angiotensin-converting enzyme, Angioedema, Bradykinin, Icativant, Substance P
Introduction
Angioedema is a potentially life-threatening side effect sometimes seen in patients taking angiotensin-converting enzyme (ACE) inhibitors, and is characterized by well-demarcated edema of the lips, tongue, mucous membranes of the throat, nose, or other parts of the face, and occasionally the hands or gastrointestinal mucosa.(1) The incidence of angioedema among ACE inhibitor users ranges from 0.7 to 1.3 episodes per thousand person-years of exposure in whites and 3.3 to 4.6 person-years of exposure in blacks.(2)
The mechanism of angioedema is not fully understood but is presumed to involve bradykinin. Activation of the kallikrein-kinin system contributes to the pathogenesis of hereditary angioedema.(3) ACE or kininase II catalyzes the formation of angiotensin II from angiotensin I but is also involved in the breakdown of bradykinin to inactive metabolites. ACE inhibitors potentiate the effects of bradykinin through its action at the B2 receptor.(4;5) Stimulation of B2 receptors induces vasodilation and vascular permeability and also stimulates the release of substance P from sensory fibers.(6) Substance P increases vascular permeability and contributes to ACE inhibitor-associated angioedema in rodent models.(7;8)
The standard-of-care for ACE inhibitor-associated angioedema has been non-specific and consists of discontinuation of the ACE inhibitor, observation, and airway management. Antihistamines, corticosteroids, and epinephrine are often administered but ineffective in this non-allergic form of angioedema.(1) Icatibant or HOE 140 (D-Arg-[Hyp3, Thi5, D-Tic7, Oic8]) bradykinin is a selective bradykinin B2 receptor antagonist, which has been used in human studies to delineate the contribution of bradykinin to the beneficial effects of ACE inhibitors.(4;5;9) Icatibant reduces the time-to-resolution of symptoms in patients with hereditary angioedema(10;11) and has been approved by the FDA for this indication. Recently Bas and colleagues reported that icatibant significantly decreases time-to-complete resolution of ACE inhibitor-associated angioedema in patients of European descent.(12) Whether these findings can be applied to a more heterogeneous patient population remains to be determined. The purpose of this study was to test the hypothesis that administration of icatibant decreases the duration and severity of ACE inhibitor-associated angioedema.
Material and Methods
Subjects
Patients age 18 to 65 years with ACE inhibitor-associated angioedema were enrolled at Vanderbilt University Medical Center between October 2007 and August 2011 (NCT00517582) and at Massachusetts General Hospital (a single case enrolled in 2012) and VUMC between August 2011 and September 2015 (NCT01574248). Two other sites initiated the study but did not consent any patients. Patients were defined as having ACE inhibitor-associated angioedema if they had swelling of the lips, pharynx or face while taking an ACE inhibitor and had never had swelling in the absence of ACE inhibitor use. Patients with hereditary angioedema were excluded. Cases of ACE inhibitor-associated bowel edema were excluded because it would be difficult to define time of onset and time-to-resolution accurately. Patients who presented with features of hypersensitivity, such as pruritus or urticaria, were not considered as having ACE inhibitor-associated angioedema. Patients who had presented to medical care more than six hours prior to screening and randomization were excluded. Pregnant women were excluded by measurement of urine beta-human chorionic gonadotropin (HCG) on enrollment; initiation of oral contraceptive therapy within six months of presentation was also a criterion for exclusion.
Study Protocol
The double-blind, placebo-controlled study protocol was approved by the Institutional Review Boards of the participating institutions in accordance with the Declaration of Helsinki. All patients provided written informed consent prior to enrollment.
After consent was obtained patients underwent a baseline history and assessment of their angioedema. Patients were randomized in a one-to-one ratio to receive icatibant 30 mg (Firazyr, Jerini AG/Shire Human Genetic Therapies, Inc) or matching placebo (normal saline) at zero and six hours using a permuted-block randomization algorithm. Randomization was stratified by race. The Vanderbilt Investigational Drug Service was responsible for the storage, preparation and labeling of study drug. Study medication was delivered in two 1.5 mL syringes and was administered subcutaneously in the abdominal region by the nurse caring for the patient, so that investigators would remain blinded if there was any local irritation. Patients were not questioned about irritation at the injection site and nurses were instructed not to tell research personal if a patient commented on irritation so as to maintain blinding. Blood pressure and heart rate were measured prior to and every 15 minutes following study drug administration for one hour and then with each assessment of angioedema severity.
In addition to study drug, patients received standard-of-care medical therapy at the discretion of the treatment team and administration of anti-histamines, steroids, or epinephrine was recorded as a secondary endpoint. In all cases, the patient’s ACE inhibitor was stopped and the patient was given a list of ACE inhibitors so that he or she could avoid re-exposure. Disposition was also determined by the treatment team. Patients who were discharged home were offered the option of being admitted to the Vanderbilt Clinical Research Center (CRC) to complete study procedures up to 48 hours after study drug administration. Patient’s electronic medical records were also reviewed for recurrence of angioedema after discontinuation of ACE inhibitor use and study medication until completion of the study; one patient in the placebo group and one patient in the icatibant group were lost to follow-up.
Angioedema severity assessment
Patients were given a 10-cm non-hatched visual analog scale (VAS) that had been developed for trials of icatibant in hereditary angioedema to assess severity of face, lip, tongue and eyelid swelling, as well as shortness of breath, difficulty speaking, difficulty swallowing, and voice change.(10-12) Patients completed the VAS at baseline and 1, 2, 4, 6, 8, 16, 24, 48 hours following study drug administration or until discharge. Patients were provided with a mirror to view their swelling if desired prior to using the VAS and graded symptom score. At the same times, patients were also asked to contrast the current severity of their signs and symptoms to the prior time point with a graded symptom score using the following verbal descriptors: “none,” “much less,” “a little less,” “about the same,” “a little more,” and “much more.”
The investigator or research nurse independently assessed the severity of angioedema using a graded sign and symptom score, assigning a score equal to zero for “absence of symptoms,” 1 for “mild,” 2 for “moderate,” and 4 for “severe” symptom or signs. The study personnel completed this scale at baseline and 1, 2, 4, 6, 8, 16, 24, 48 hours after study drug or at discharge.
Laboratory Analysis
Blood for complete blood count, metabolic panel, prothrombin and partial thromboplastin time were collected at enrollment and at discharge and measured in the clinical laboratory. Blood for measurement of C1 esterase inhibitor and complement levels was collected at baseline. C1 esterase inhibitor protein was determined by quantitative nephelometry at a third party laboratory (ARUP Laboratories, Salt Lake City, UT), and C1 esterase inhibitor activity was determined using a commercially available chromogenic assay (Technoclone GmbH, Vienna, Austria). C3 and C4 levels were assessed by immunoturbidimetric method at the Vanderbilt Pathology Laboratory. Blood samples for future research assays were collected at baseline and 6, 24, 48 hours (or at discharge) after study drug administration and immediately centrifuged at 5°C for 20 minutes. Serum and plasma were then separated and stored at -80°C until the time of assay.
Statistical Analysis
The calculated sample size for the study was 16 patients per study arm. This sample size had a 0.8 power to detect a difference in the median times to resolution of 8.5 hours versus 23.3 hours between treatment based on a log-rank test with two-sided type I error rate of 0.05. The expected median times were based on time-to-resolution following treatment with icatibant versus placebo in hereditary angioedema.(10)
In February 2015, the principal investigator convened the Data and Safety Committee (DSMC) of the study to seek input regarding continuation of the study after Bas et al published the report of a randomized trial comparing the effect of icatibant to prednisolone/clemastine comparator on time-to-resolution of ACE inhibitor-induced angioedema.(12) The DSMC concluded that equipoise still existed and that the study should continue, based on important differences in outcomes and participant demographics between the published study and the current study. After the publication of the Bas trial, however, enrollment declined and, in September 2015, it was decided to discontinue the study after consultation with the DSMC.
An intention-to-treat analysis was completed in all patients who received any study drug. The primary analyses were 1) survival analysis using a log-rank test to assess the time-to-resolution of symptoms, and 2) generalized least squares (GLS) linear regression analysis of the VAS measurement of the primary (most severe) sign or symptom over time. In the log-rank test, signs or symptoms with a VAS measurement below or equal to one cm were defined as resolved. Data were also analyzed using a Cox proportional hazards model which controlled for severity at baseline (VAS score at time 0) and race.
Secondary outcomes included the frequency of concomitant medications, the incidence of admission to the intensive care unit, the incidence of intubation, the effect of treatment on blood pressure, and the frequency of adverse events.
A two-sided p-value less than 0.05 was considered statistically significant. All the analyses were performed using R 3.1.2.
Results
Among thirty-three eligible participants, 33 were randomized to study drug (15 to icatibant and 18 to placebo) (supplemental Figure 1). The imbalance resulted from incompletely filled randomization blocks within strata during both the Vanderbilt single-center study stage and the multi-center study stage. Two patients randomized to icatibant withdrew consent prior to study drug administration. All but one patient were enrolled at Vanderbilt. The single patient enrolled at the Massachusetts General Hospital was intubated and sedated and not able to complete the VAS; this patient, randomized to icatibant, was excluded from the final analysis. Two patients in the placebo group did not receive a second dose of study drug at six hours. (In one case, the patients symptoms had improved and he elected not to stay at the CRC. In the other, the patient’s plasma troponin I was elevated.) These patients are included in the analysis.
Baseline characteristics were similar in the two treatment groups (Table 1). Two-thirds of patients presenting with ACE inhibitor-associated angioedema were women and two-thirds were African American. All but one patient had hypertension and 47% were diabetic. One diabetic patient in the placebo group was taking a dipeptidyl peptidase 4 inhibitor and had taken this for five years. Fifty-three percent of patients presenting with ACE inhibitor-associated angioedema were current or former smokers; forty-seven percent had a history of seasonal allergies. No patient presented with pruritus; one patient in the placebo group has a small area of urticaria on his right thigh, which was observed after he had been randomized and treated. Six patients (20%) were taking an immunosuppressant or carried a diagnosis of immunodeficiency. In the placebo group this included two heart transplant patients, one renal transplant patient, a patient taking prednisone for chronic lung disease, and a patient with rheumatoid arthritis; one patient in the icatibant group carried the human immunodeficiency virus. Two patients had a history of prior angioedema while taking an ACE inhibitor. One patient with multiple myeloma in the placebo group had a low C4 level and C1 inhibitor activity with a normal C3 level and C1 inhibitor concentration. This patient had no prior history of angioedema or any episodes of angioedema during two years of follow-up following discontinuation of ACE inhibition. This patient was included in the final analysis, because exclusion did not change the findings. No patient had recurrent angioedema after discontinuation of his or her ACE inhibitor with a mean follow-up of 4.36±2.19 years.
Table 1.
Baseline Characteristics by Treatment Group
| Placebo N=18 | Icatibant N=12 | P-value | |
|---|---|---|---|
| Age | 60.7±10.8 | 56.3±13.4 | 0.36 |
| Gender | 0.64 | ||
| Female | 12 (67%) | 7 (58%) | |
| Male | 6 (33%) | 5 (42%) | |
| Race | 0.43 | ||
| White | 7 (39%) | 3 (25%) | |
| Black | 11 (61%) | 9 (75%) | |
| Sites with swelling at presentation* | |||
| Lip | 12 (67%) | 10 (83%) | 0.31 |
| Tongue | 11 (61%) | 5 (45%) | 0.41 |
| Face | 9 (50%) | 7 (58%) | 0.65 |
| Eyelid | 3 (17%) | 3 (25%) | 0.58 |
| Smoker | 0.43 | ||
| Never | 10 (56%) | 4 (33%) | |
| Past | 4 (22%) | 5 (42%) | |
| Current | 4 (22%) | 3 (25%) | |
| Diabetes | 0.54 | ||
| No | 10 (56%) | 8 (67%) | |
| Yes | 8 (44%) | 4 (33%) | |
| Hypertension | 0.41 | ||
| No | 1 (6%) | 0 | |
| Yes | 17 (94%) | 12 (100%) | |
| Seasonal allergy | 0.77 | ||
| No | 10 (56%) | 6 (50%) | |
| Yes | 8 (44%) | 6 (50%) | |
| Immunosuppressed† | 0.19 | ||
| No | 13 (72%) | 11 (92%) | |
| Yes | 5 (28%) | 1 (8%) | |
| Prior angioedema while taking an ACE inhibitor | 0.07 | ||
| No | 18 (100%) | 10 (83%) | |
| Yes | 0 | 2 (17%) | |
| Duration ACE inhibitor use (days) | 1903±1912 | 791±852 | 0.19 |
| ACE inhibitor | 0.51 | ||
| Lisinopril | 12 (67%) | 11 (92%) | |
| Enalapril | 2 (11%) | 1 (8%) | |
| Benazapril | 2 (11%) | ||
| Fosinopril | 1 (6%) | ||
| Quinapril | 1 (6%) |
Site was defined as involved with swelling if the patient marked the zero to ten cm visual analog scale for swelling at least one cm. More than one site may have been involved with swelling.
Immunosuppressants in the placebo group were prednisone (N=4), methotrexate (N=1), tacrolimus (N=1). One patient in the icatibant group was infected with the human immunodeficiency virus.
Although patients were treated with study drug within six hours of presentation to the hospital, the mean time from onset of symptoms to administration of study drug was 10.3 hours in the icatibant group. Figure 1 shows the Kaplan-Meier curves for time from treatment to complete resolution (symptoms less than one cm on the VAS) by treatment group. The time-to-resolution was similar in the placebo and icatibant treatment groups. There was also no significant effect of treatment on time-to-resolution in a Cox Proportional Hazards model in which we controlled for race and baseline severity (p=0.20 for the primary symptom and p>0.09 for all individual symptoms). Figure 2 shows mean symptom scores using the VAS over time following initiation of study drug. In a GLS model analysis, there was no significant effect of treatment on the amount of swelling over time (p=0.95 for the primary site of swelling). There were also no significant differences between treatment arms in the time course of patient-assessed changes in symptoms or physician-assessed severity of symptoms.
Figure 1.
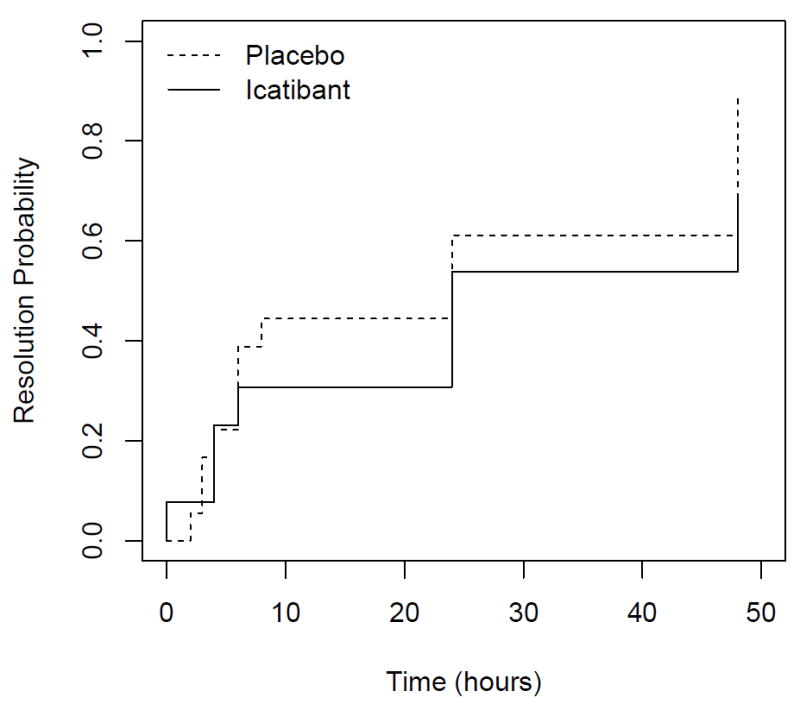
Kaplan-Meier Curve showing time-to-resolution of primary symptom, as assessed by patients using visual analog scale in patients treated with placebo (dotted line) or icatibant (solid line). Symptoms were deemed resolved when the patient marked the severity as less than one on a ten centimeter visual analog scale. P=0.192 for effect of treatment.
Figure 2.
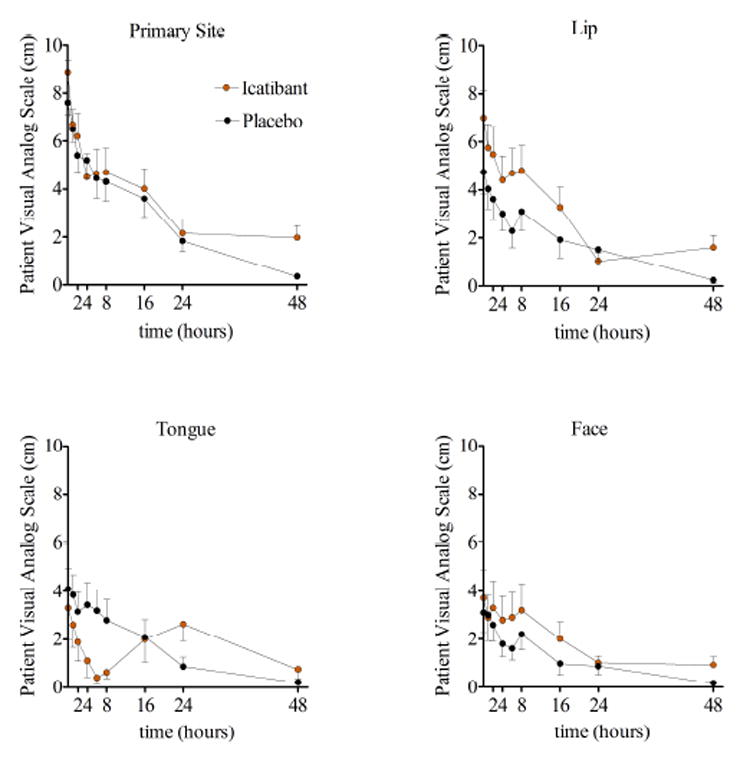
Time-course of symptom severity after treatment with placebo (grey line) or icatibant (red line) as indicated by patient on a visual analog scale. Data are presented as means ± standard error of the mean.
The frequency of administration of H1 and H2 blockers, corticosteroids, and epinephrine was similar in the two treatment groups (Table 2).
Table 2.
Concurrent Treatment by Treatment Group
| Placebo N=18 | Icatibant N=12 | P-value | |
|---|---|---|---|
| Symptom onset to study drug (hours) | 12.7 ± 8.1 | 10.3 ± 5.6 | 0.55 |
| Other Treatment | |||
| H1 blocker, N (%) | 16 (88.9%) | 11 (92%) | 0.80 |
| H2 blocker, N (%) | 14 (78%) | 11 (92%) | 0.32 |
| Corticosteroids, N (%) | 16 (88.9%) | 11 (92%) | 0.80 |
| Epinephrine, N (%) | 3 (17%) | 0 | 0.14 |
| ICU admission, N (%) | 6 (33%) | 6 (50%) | 0.36 |
| Intubation, N (%) | 1 (6%) | 2 (17%) | 0.32 |
Blood pressure trended down slightly over time after treatment and was similar in the two treatment groups (supplemental Table 1).
Relationship between race and presenting symptoms or time-to-resolution of ACE inhibitor-associated angioedema
The demographic characteristics of blacks and whites presenting with ACE inhibitor-associated angioedema were similar with one exception. Seventy-five percent of blacks presenting with ACE inhibitor-associated angioedema were women, whereas forty percent of whites presenting with angioedema were women (Table 3); this difference did not reach statistical significance.
Table 3.
Baseline Characteristics and Concurrent Treatment by Racial Group
| Black (N=20) | White (N=10) | P-value | |
|---|---|---|---|
| Age | 57.7±12.0 | 61.6±11.9 | 0.4 |
| Gender | |||
| Female | 15 (75%) | 4 (40%) | 0.06 |
| Male | 5 (25%) | 6 (60%) | |
| Smoker | 0.56 | ||
| Never | 8 (40%) | 6 (60%) | |
| Past | 7 (35%) | 2 (20%) | |
| Current | 5 (25%) | 2 (20%) | |
| Diabetes | 1.00 | ||
| No | 12 (60%) | 6 (60%) | |
| Yes | 8 (40%) | 4 (40%) | |
| Hypertension | 0.15 | ||
| No | 0 | 1 (10%) | |
| Yes | 20 (100%) | 9 (90%) | |
| Seasonal allergy | 0.80 | ||
| No | 11 (55%) | 5 (50%) | |
| Yes | 9 (45%) | 5 (50%) | |
| Immunosuppression | 0.33 | ||
| No | 15 (75%) | 9 (90%) | |
| Yes | 5 (25%) | 1 (10%) | |
| Prior angioedema | 0.60 | ||
| No | 19 (95%) | 9 (90%) | |
| Yes | 1 (5%) | 1 (10%) | |
| Duration ACE inhibitor use (days) | 1521±1829 | 1143±955 | 0.82 |
| Symptom onset to study drug (hours) | 11.6±6.6 | 12.0±8.7 | 0.82 |
| Primary site of swelling | 0.22 | ||
| Lip, N (%) | 10 (50%) | 2 (20%) | |
| Tongue, N (%) | 6 (30%) | 6 (60%) | |
| Face, N (%) | 4 (20%) | 2 (20%) | |
| Baseline patient ranking of swelling | |||
| Lip, cm on VAS | 6.6±3.8 | 3.4±3.7 | 0.03 |
| Tongue, cm | 2.7±3.5 | 6.4±3.9 | 0.02 |
| Face, cm | 2.96±3.85 | 4.08±3.07 | 0.32 |
| Eyelid, cm | 1.34±2.80 | 0.42±0.92 | 0.65 |
| Other treatment | |||
| H1 blocker, N (%) | 17 (85%) | 10 (100%) | 0.20 |
| H2 blocker, N (%) | 16 (80%) | 9 (90%) | 0.49 |
| Corticosteroids, N (%) | 18 (90%) | 9 (90%) | 1.00 |
| Epinephrine, N (%) | 0 | 3 (30%) | 0.01 |
| ICU admission, N (%) | 7 (35%) | 5 (50%) | 0.43 |
| Intubation, N (%) | 1 (5%) | 2 (20%) | 0.20 |
At presentation, lip swelling was more severe among black patients while angioedema of the tongue was more prominent in white patients (Table 3). White patients were significantly more likely to receive epinephrine. There were no other differences in the frequencies of treatments administered. In addition, the time-to-resolution of symptoms following administration of study drug was similar in the black and white patients presenting with ACE inhibitor-associated angioedema (p=0.53 for effect of race on resolution of the primary symptom in the Cox Proportional Hazards model, p=0.45 in the longitudinal analysis). There was also no effect of icatibant on time-to-resolution when analyzed separately in blacks or whites or in women or men (p>0.20 for time to resolution of the primary symptom in all subgroups).
There were no serious adverse events. One patient in the placebo arm, a transplant patient taking immunosuppressive drugs, reported having a low sperm count measured one month after completion of the study. Unbeknownst to the investigators, the patient and his wife had been undergoing an evaluation for infertility prior to the study; the research team consulted with the patient’s nephrologist who did not request unblinding of study medication. One patient in the placebo group had elevated troponin levels. One patient in the icatibant arm developed leukocytosis; this was attributed to concurrent administration of systemic steroids.
Discussion
This study compared the effect of placebo versus the bradykinin B2 receptor antagonist icatibant on symptoms of ACE inhibitor-associated angioedema in a mixed race population of patients. The study does not support the clinical efficacy of B2 receptor blockade in this form of angioedema.
The findings contrast those of Bas et al, who reported that administration of icatibant reduced the duration of symptoms of ACE inhibitor-associated angioedema in Europeans of Caucasian descent.(12) The present study differs from that study in several important aspects. Bas et al utilized active comparator treatment and it is not possible to exclude the interpretation that treatment worsens ACE inhibitor-associated angioedema. In contrast, the current study was placebo-controlled and administration of other therapies was considered a secondary outcome. In the earlier study, all of the patients were white and sixty-two percent were male. Patients enrolled in the present study were more representative of previously published risk factors for ACE inhibitor-induced angioedema in that the majority or patients were of African descent and female.(13;14) It is possible that the effect of icatibant differs in whites and blacks, or in men and women, but we did not find this in post-hoc analyses. Whereas in the Bas trial 57% of patients in the standard therapy group were taking ramipril and the majority in the icatibant group were taking ramipril (38%) or enalapril (38%),(12) the majority of patients in the present study were taking lisinopril. Bas et al reported that investigator-assessed injection-site reactions were more common in the icatibant group than in the standard therapy group.(12) Patients who were asked to assess injection site inflammation may have become biased or inadvertently conveyed information to investigators resulting in unblinding. In the present study, study drug was administered by a nurse who was not part of the study so that investigators could remain masked to therapy.
In contrast, our findings are similar to those of Sinert et al, who recently presented the results of a randomized, double-blind, placebo-controlled study in 121 patients at 31 centers.(15) In that study, the mean time from onset of angioedema to treatment with icatibant was 7.9 hours. The primary outcome was time to meeting discharge criteria and the median was 4.0 hours in both icatibant and placebo treatment groups. As in our study, the frequency of administration of antihistamine, steroids, and epinephrine was similar in the treatment groups. Our findings are also consistent with the observation that ecallantide, a kallikrein inhibitor which decreases the production of endogenous bradykinin, does not have significant effect on ACE inhibitor-associated angioedema.(16;17) The present study is smaller in size compared to these prior negative studies, a limitation, but similar in size to the study of Bas et al.(12)
The lack of effect of bradykinin receptor antagonism on symptoms in patients with ACE inhibitor-associated angioedema also contrasts the efficacy of the drug in hereditary angioedema.(10;11) There are several possible explanations for this divergence. First, hereditary angioedema results from excess formation of bradykinin whereas ACE inhibitor-associated angioedema results from decreased degradation of bradykinin as well as other vasoactive substrates of ACE such as substance P.(1;3) Unopposed effects of substance P or other peptide substrates of ACE may contribute to angioedema even when the B2 receptor is blocked.(8;18) Second, patients with hereditary angioedema often recognize their symptoms and can be given icatibant early in the course of angioedema, whereas patients who take ACE inhibitors may not present for medical attention immediately. In the present study, for example, patients did not receive icatibant until a mean of 10.3 hours after the onset of symptoms. In the Bas study, the median time from the onset of angioedema to treatment with icatibant was 6.1 hours.(12)
We compared the presentation and natural history of ACE inhibitor-associated angioedema in blacks and white patients enrolled in our study. Interestingly, the two groups differed in the site-specific severity of swelling involving the lips and tongue. White patients presenting with angioedema were more likely to receive epinephrine, perhaps reflecting concern regarding airway obstruction due to more severe tongue involvement. The tongue was also the most common site of angioedema involvement among European patients studied by Bas et al.(12) Importantly, even though ACE inhibitor-associated angioedema is more common in individuals of African descent than in those of Caucasian or Asian descent,(13) we found no relationship between race and the duration of symptoms.
Forty-seven percent of patients presenting with ACE inhibitor-associated angioedema in the present study had a history of seasonal allergies and six of 33 patients were immunosuppressed. Previous studies have reported an association between seasonal allergies and increased risk of ACE inhibitor associated angioedema. For example in the Omapatrilat Cardiovascular Treatment Assessment vs Enalapril (OCTAVE) Trial, seasonal allergies were associated with a significantly increased risk of angioedema during treatment with enalapril (OR, 1.79; 95% CI, 1.06-3.00).(14) Likewise, several groups have reported an increased incidence of ACE inhibitor-associated angioedema among transplant patients or patients taking an immunosuppressant.(19;20)
Of note, the average duration of ACE inhibitor use among patients presenting with angioedema in this study exceeded two years. This is consistent with prior studies indicating that while the risk of angioedema is highest shortly after initiation of therapy, angioedema often occurs after prolonged therapy,(2;13;21) likely due to an event that increases production of vasoactive substrates of ACE. This delay in onset can confound recognition of ACE inhibitor-associated angioedema by patients and their physicians.(22;23) Indeed, two patients had had prior episodes of angioedema while taking an ACE inhibitor and had been continued or restarted on an ACE inhibitor prior to enrollment in this study.
Supplementary Material
Key Messages.
The study does not support the clinical efficacy of bradykinin B2 receptor blockade in angiotensin-converting enzyme inhibitor-associated angioedema.
Black and white patients differed in the site-specific severity of swelling.
Acknowledgments
The investigators wish to thank the many physicians and nurses who contacted us when patients presented with ACE inhibitor-associated angioedema, as well as the patients who generously gave of their time.
Funding Sources
This study was partially supported by UL1 RR024975 and R01HL079184 from the National Institutes of Health, and the Investigator-Initiated Research Program from Jerini AG/Shire Pharmaceuticals, Inc. Drs. Straka, Byrd, and Woodard-Grice were funded by T32GM007569.
Abbreviations
- ACE
angiotensin-converting enzyme
- CRC
Clinical Research Center
- DSMC
Data and Safety Monitoring Committee
- GLS
generalized least squares
- OCTAVE
Omapatrilat Cardiovascular Treatment Assessment vs Enalapril
- VAS
visual analog scale
Footnotes
ClinicalTrials.gov Identifiers: NCT00517582, NCT01574248
Disclosures
Dr. Brown is a consultant for Novartis Pharmaceuticals and serves on the Scientific Advisory Board of Alynylam Pharmaceuticals. Dr. Brown has recently served as a consultant to Shire Pharmaceuticals, Inc. Dr. Brown has received research funding from New Haven Pharmaceuticals as well as funding from Shire Pharmaceuticals, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am. 2006;26(4):725–37. doi: 10.1016/j.iac.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Burkhart DG, Brown NJ, Griffin MR, Ray WA, Hammerstrom T, Weiss S. Angiotensin converting enzyme inhibitor-associated angioedema: higher risk in blacks than whites. Pharmacoepidemiol Drug Saf. 1996;5(3):149–54. doi: 10.1002/(SICI)1099-1557(199605)5:3<149::AID-PDS222>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 3.Zuraw BL. Clinical practice. Hereditary angioedema. N Engl J Med. 2008;359(10):1027–36. doi: 10.1056/NEJMcp0803977. [DOI] [PubMed] [Google Scholar]
- 4.Gainer JV, Morrow JD, Loveland A, King DJ, Brown NJ. Effect of bradykinin-receptor blockade on the response to angiotensin-converting-enzyme inhibitor in normotensive and hypertensive subjects. N Engl J Med. 1998;339(18):1285–92. doi: 10.1056/NEJM199810293391804. [DOI] [PubMed] [Google Scholar]
- 5.Pretorius M, Rosenbaum D, Vaughan DE, Brown NJ. Angiotensin-converting enzyme inhibition increases human vascular tissue-type plasminogen activator release through endogenous bradykinin. Circulation. 2003;107(579):585. doi: 10.1161/01.cir.0000046268.59922.a4. [DOI] [PubMed] [Google Scholar]
- 6.Seyedi N, Maruyama R, Levi R. Bradykinin activates a cross-signaling pathway between sensory and adrenergic nerve endings in the heart: a novel mechanism of ischemic norepinephrine release? J Pharmacol Exp Ther. 1999;290(2):656–63. [PubMed] [Google Scholar]
- 7.Sulpizio AC, Pullen MA, Edwards RM, Brooks DP. The effect of acute angiotensin-converting enzyme and neutral endopeptidase 24.11 inhibition on plasma extravasation in the rat. J Pharmacol Exp Ther. 2004;309(3):1141–7. doi: 10.1124/jpet.103.064105. [DOI] [PubMed] [Google Scholar]
- 8.Byrd JB, Shreevatsa A, Putlur P, Foretia D, McAlexander L, Sinha T, et al. Dipeptidyl peptidase IV deficiency increases susceptibility to angiotensin-converting enzyme inhibitor-induced peritracheal edema. J Allergy Clin Immunol. 2007;120(2):403–8. doi: 10.1016/j.jaci.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Witherow FN, Helmy A, Webb DJ, Fox KA, Newby DE. Bradykinin contributes to the vasodilator effects of chronic angiotensin-converting enzyme inhibition in patients with heart failure. Circulation. 2001;104(18):2177–81. doi: 10.1161/hc4301.098252. [DOI] [PubMed] [Google Scholar]
- 10.Cicardi M, Banerji A, Bracho F, Malbran A, Rosenkranz B, Riedl M, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med. 2010;363(6):532–41. doi: 10.1056/NEJMoa0906393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lumry WR, Li HH, Levy RJ, Potter PC, Farkas H, Moldovan D, et al. Randomized placebo-controlled trial of the bradykinin B(2) receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: the FAST-3 trial. Ann Allergy Asthma Immunol. 2011;107(6):529–37. doi: 10.1016/j.anai.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Bas M, Greve J, Stelter K, Havel M, Strassen U, Rotter N, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372(5):418–25. doi: 10.1056/NEJMoa1312524. [DOI] [PubMed] [Google Scholar]
- 13.Brown NJ, Ray WA, Snowden M, Griffin MR. Black americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clin Pharmacol Ther. 1996;60(1):8–13. doi: 10.1016/S0009-9236(96)90161-7. [DOI] [PubMed] [Google Scholar]
- 14.Kostis JB, Kim HJ, Rusnak J, Casale T, Kaplan A, Corren J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005;165(14):1637–42. doi: 10.1001/archinte.165.14.1637. [DOI] [PubMed] [Google Scholar]
- 15.Sinert R, Levy P, Bernstein JA, Body R, Sivilotti MLA, Moellman J, et al. Phase 3, Randomized, Double-Blind, Placebo-Controlled, Multicenter Clinical Study Evaluating the Safety and Efficacy of Icatibant for Angiotensin-Converting Enzyme Inhibitor-Induced Angioedema in Adults. Academic Emergency Medicine. 2016;23(S1):S11. [Google Scholar]
- 16.Bernstein JA, Moellman JJ, Collins SP, Hart KW, Lindsell CJ. Effectiveness of ecallantide in treating angiotensin-converting enzyme inhibitor-induced angioedema in the emergency department. Ann Allergy Asthma Immunol. 2015;114(3):245–9. doi: 10.1016/j.anai.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Lewis LM, Graffeo C, Crosley P, Klausner HA, Clark CL, Frank A, et al. Ecallantide for the acute treatment of angiotensin-converting enzyme inhibitor-induced angioedema: a multicenter, randomized, controlled trial. Ann Emerg Med. 2015;65(2):204–13. doi: 10.1016/j.annemergmed.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Emanueli C, Grady EF, Madeddu P, Figini M, Bunnett NW, Parisi D, et al. Acute ACE inhibition causes plasma extravasation in mice that is mediated by bradykinin and substance P. Hypertension. 1998;31(6):1299–304. doi: 10.1161/01.hyp.31.6.1299. [DOI] [PubMed] [Google Scholar]
- 19.Duerr M, Glander P, Diekmann F, Dragun D, Neumayer HH, Budde K. Increased incidence of angioedema with ACE inhibitors in combination with mTOR inhibitors in kidney transplant recipients. Clin J Am Soc Nephrol. 2010;5(4):703–8. doi: 10.2215/CJN.07371009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrd JB, Woodard-Grice A, Stone E, Lucisano A, Schaefer H, Yu C, et al. Association of angiotensin-converting enzyme inhibitor-associated angioedema with transplant and immunosuppressant use. Allergy. 2010;65(11):1381–7. doi: 10.1111/j.1398-9995.2010.02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller DR, Oliveria SA, Berlowitz DR, Fincke BG, Stang P, Lillienfeld DE. Angioedema incidence in US veterans initiating angiotensin-converting enzyme inhibitors. Hypertension. 2008;51(6):1624–30. doi: 10.1161/HYPERTENSIONAHA.108.110270. [DOI] [PubMed] [Google Scholar]
- 22.Brown NJ, Snowden M, Griffin MR. Recurrent angiotensin-converting enzyme inhibitor-associated angioedema. JAMA. 1997;278(3):232–3. doi: 10.1001/jama.278.3.232. [DOI] [PubMed] [Google Scholar]
- 23.Loftus PA, Tan M, Patel G, Lin J, Helman S, Badhey A, et al. Risk factors associated with severe and recurrent angioedema: an epidemic linked to ACE-inhibitors. Laryngoscope. 2014;124(11):2502–7. doi: 10.1002/lary.24777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


