Summary
Background
The anti-HER2 monoclonal antibody trastuzumab and the tyrosine kinase inhibitor lapatinib have complementary mechanisms of action and synergistic antitumour activity in models of HER2-overexpressing breast cancer. We argue that the two anti-HER2 agents given together would be better than single-agent therapy.
Methods
In this parallel groups, randomised, open-label, phase 3 study undertaken between Jan 5, 2008, and May 27, 2010, women from 23 countries with HER2-positive primary breast cancer with tumours greater than 2 cm in diameter were randomly assigned to oral lapatinib (1500 mg), intravenous trastuzumab (loading dose 4 mg/kg, subsequent doses 2 mg/kg), or lapatinib (1000 mg) plus trastuzumab. Treatment allocation was by stratified, permuted blocks randomisation, with four stratification factors. Anti-HER2 therapy alone was given for the first 6 weeks; weekly paclitaxel (80 mg/m2) was then added to the regimen for a further 12 weeks, before definitive surgery was undertaken. After surgery, patients received adjuvant chemotherapy followed by the same targeted therapy as in the neoadjuvant phase to 52 weeks. The primary endpoint was the rate of pathological complete response (pCR), analysed by intention to treat. This trial is registered with ClinicalTrials.gov, NCT00553358.
Findings
154 patients received lapatinib, 149 trastuzumab, and 152 the combination. pCR rate was significantly higher in the group given lapatinib and trastuzumab (78 of 152 patients [51·3%; 95% CI 43·1–59·5]) than in the group given trastuzumab alone (44 of 149 patients [29·5%; 22·4–37·5]; difference 21·1%, 9·1–34·2, p=0·0001). We recorded no significant difference in pCR between the lapatinib (38 of 154 patients [24·7%, 18·1–32·3]) and the trastuzumab (difference −4·8%, −17·6 to 8·2, p=0·34) groups. No major cardiac dysfunctions occurred. Frequency of grade 3 diarrhoea was higher with lapatinib (36 patients [23·4%]) and lapatinib plus trastuzumab (32 [21·1%]) than with trastuzumab (three [2·0%]). Similarly, grade 3 liver-enzyme alterations were more frequent with lapatinib (27 [17·5%]) and lapatinib plus trastuzumab (15 [9·9%]) than with trastuzumab (11 [7·4%]).
Interpretation
Dual inhibition of HER2 might be a valid approach to treatment of HER2-positive breast cancer in the neoadjuvant setting.
Funding
GlaxoSmithKline.
Introduction
The human epidermal growth factor receptor 2 (HER2) is a potent mediator of cellular growth and proliferation.1 Amplification of the HER2 gene, and the corresponding overexpression of the HER2 receptor, occurs in roughly 20% of breast tumours and is associated with a poor outcome.2 Molecular targeting of the HER2 receptor with the humanised monoclonal antibody trastuzumab (herceptin, Genentech, San Francisco, CA, USA) has improved disease-free and overall survival in patients with both metastatic and early HER2-positive breast cancer.3–5 Another anti-HER2 agent, the tyrosine kinase inhibitor lapatinib (tykerb, GlaxoSmithKline, Brentford, UK), given in combination with capecitabine, improves progression-free survival in patients who have progressed on trastuzumab and is approved for treatment of patients with advanced HER2-positive breast cancer.6
Dual targeting of HER2-positive tumours with trastuzumab and lapatinib is undertaken because of primary and acquired resistance to both agents, their partly non-overlapping mechanisms of action, and the well characterised synergistic interaction between them in HER2 breast-cancer models.7–9 Trastuzumab inhibits ligand-independent HER2 and HER3 signalling10 and triggers antibody-dependent cellular cytotoxicity.11 By contrast, lapatinib blocks ligand-induced heterodimer signalling and prevents signalling via a frequently expressed truncated version of the HER2 receptor that could render cells resistant to trastuzumab. Additionally, lapatinib leads to an accumulation of HER2 at the cell surface, enhancing trastuzumab-dependent antibody-dependent cellular cytotoxicity.9 In a clinic setting, trastuzumab induces mostly a pro apoptotic effect, but lapatinib inhibits proliferation.12,13 Evidence from clinic settings shows indirect evidence in support of dual HER2 blockade. In patients with trastuzumab-refractory breast cancer, lapatinib plus trastuzumab improves progression-free survival when compared with lapatinib alone.14
Preoperative systemic (neoadjuvant) treatment of breast cancer yields disease-free and overall survival results similar to adjuvant systemic therapy of breast cancer and improves breast conservation rates because of tumour response to therapy. The preoperative setting also allows monitoring of response to therapy in previously untreated patients. In HER2-positive breast tumours, pathological complete response (pCR) at time of surgery has been shown to correlate with improved disease outcomes in randomised studies containing trastuzumab and chemotherapy suggesting that it may serve as a surrogate marker of clinical benefit.15,16
In the NeoAdjuvant Lapatinib and/or Trastuzumab Treatment Optimisation (NeoALTTO) study, we assessed lapatinib, trastuzumab, and their combination as neoadjuvant therapy for women with HER2-positive early breast cancer.
Methods
Study design and patients
The NeoALTTO trial (Breast International Group 01–06) is a randomised, multicentre, open-label, phase 3 study. From Jan 5, 2008, to May 27, 2010, 455 patients entered the study from 86 sites in 23 countries in Europe, Asia, North and South America, and South Africa. The trial included three parallel treatment groups: oral lapatinib, intravenous trastuzumab, or lapatinib plus trastuzumab given for 6 weeks (the biological window), followed by an additional 12 weeks of the assigned anti-HER2 therapy plus paclitaxel given every week. Within 4 weeks after the last dose of paclitaxel, patients underwent definitive breast surgery. The webappendix provides detailed study design and postoperative treatment.
Eligible patients had histologically confirmed invasive breast cancer with HER2 overexpression or amplification as per guidelines.17 HER2 status was assessed locally at participating institutions that had been accredited by our certified laboratory (Vall d’Hebron Institute of Oncology). Participating patients had primary breast tumours greater than 2 cm in diameter measured by either mammography or echography. Patients had to have adequate baseline hepatic, renal, cardiac, and bone marrow function for inclusion. Adequate cardiac function was defined as a baseline left ventricular ejection fraction of 50% or more measured by echo cardiography or multiple gate acquisition scan. Patients were not eligible if they had bilateral breast cancer, inflammatory breast cancer, or distant metastases.
The ethics committee and relevant health authorities at each participating institution approved the study protocol. All women gave written informed consent before study entry.
Randomisation and masking
Treatment allocation was by stratified, permuted blocks randomisation. Block size was six (three groups randomly assigned in a 2:2:2 ratio) and was masked from any individuals actively participating in the trial. We used four stratification factors: hormone-receptor status (oestrogen-receptor or progesterone-receptor positive, or both, vs both oestrogen-receptor and progesterone-receptor negative); clinical involvement of axillary lymph nodes (N0–1 vs ≥N2); clinical tumour size (T2 [2–5 cm diameter]) vs ≥T3 [>5 cm diameter]); and suitability for breast-conserving surgery (yes vs no). We did the randomisation centrally at the Frontier Science and Technology Research Foundation Randomisation Center, which was accessed by participating sites using a web-based system so that patients were enrolled before the treatment assignment was revealed. This trial was open label, but investigators assessing outcome and those analysing data were masked.
Procedures
Lapatinib was given daily at a dose of 1500 mg (250 mg tablets) or of 1000 mg when given concomitantly with trastuzumab. To reduce the occurrence of diarrhoea, the lapatinib dose was reduced from 1000 mg to 750 mg when paclitaxel was added to the combination (protocol amendment 2, approved Oct 10, 2008). However, because of rapid accrual, only 54 of 152 patients in the group given all three drugs received treatment according to amendment 2. Trastuzumab was given before surgery with a loading dose of 4 mg/kg; subsequent doses were 2 mg/kg every week. Paclitaxel was given at a dose of 80 mg/m2 every week for 12 weeks. In case of non-haematological grade 3 or 4 toxic effects related to study agents such as diarrhoea and abnormal liver function, therapy was interrupted and dose reductions were implemented (webappendix).
During the biological window, patients were assessed every 2 weeks, and then every 3 weeks during the 12 weeks of concomitant paclitaxel. Objective measurements to assess the disease response were obtained by physical examination (calliper), mammography, and echography or MRI at 6 weeks and at the time of surgery. For each patient, the same imaging techniques were used throughout the study treatment period. Objective response reported was based on calliper measurements.
Left ventricular ejection fraction was measured by either echocardiography or multiple gate acquisition scan. Measurements were repeated 6 weeks after randomisation, and before surgery. An absolute difference in incidence of severe congestive heart failure or cardiac death of more than 4% between any of the treatment groups would have triggered a recommendation by the independent data monitoring committee to stop or modify the trial.
The primary endpoint was rate of pCR (according to National Surgical Adjuvant Breast and Bowel Project guidelines,18 with absence of invasive tumour cells in the breast) at time of surgery. Secondary endpoints from the neoadjuvant phase included: locoregional total pCR, as defined by no invasive cancer in the breast and no pathological involvement of axillary lymph nodes; objective tumour response rate (complete plus partial) at the end of the biological window and at the time of definitive surgery on the basis of physical examination with WHO criteria; patients with node-negative disease at surgery; patients having breast-conserving surgery; rate of conversion to breast-conserving surgery; and safety and tolerability. The secondary endpoints disease-free and overall survival, molecular characteristics of responding tumours, and biomarker expression will be reported elsewhere.
Statistical analysis
We planned to enrol 450 patients to detect a difference in pCR rate from 25% in the trastuzumab group to 42% in either of the experimental groups, with 80% power and 0·025 two-sided significance level. Comparison between the two experimental groups given lapatinib was not protocol specified. One formal efficacy interim analysis, reviewed exclusively by the independent data monitoring committee, was done when pCR results were available for the first 210 assessable patients. The boundary was p≤0·001 with a Lan-DeMets implemen tation of an O’Brien-Fleming plan,19 and a Bonferroni adjustment for the two pairwise comparisons was applied. The committee recommended that the trial continue as planned.
For the final analysis of the primary endpoint, the two null hypotheses were tested by unstratified binomial tests using Hochberg’s20 modification of the Bonferroni adjustment. A supplementary analysis was done with logistic regression21 with the stratification factors as covariates. The efficacy analyses were done according to the intention-to-treat principle, with all patients included according to their randomised assignment for the primary endpoint. Any patient without a recorded pCR was regarded as a non-responder. Analyses were done with SAS (version 9·1·2) and R (version 12·2).
The independent data monitoring committee received safety data roughly every 6 months throughout the trial, and reviewed one predefined interim efficacy analysis and the final efficacy analysis. This trial is registered with ClinicalTrials.gov, NCT00553358.
Role of the funding source
GlaxoSmithKline, the manufacturer of lapatinib, distributed the study drugs and provided financial support, but imposed no restrictions on the investigators with respect to study design, data collection, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all data and had final responsibility for the decision to submit for publication.
Results
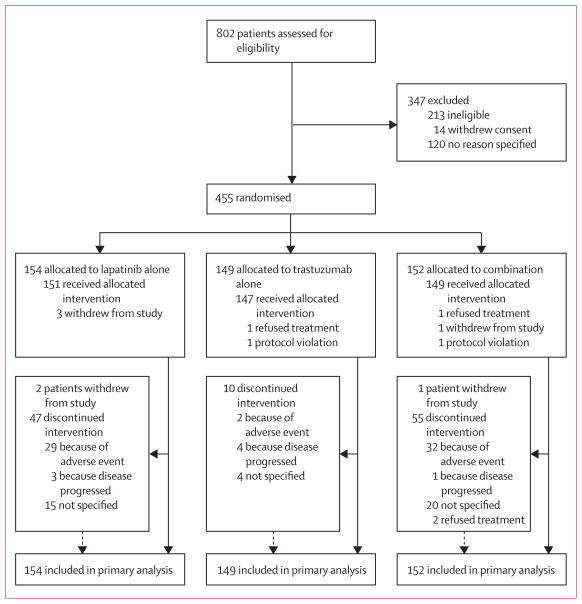
Figure 1 shows the trial profile. Of 455 patients who were enrolled, 154 women were assigned to the lapatinib group, 149 to the trastuzumab group, and 152 to the group given a combination of lapatinib and trastuzumab (figure 1). The groups were well balanced for hormone-receptor and clinical lymph-node status (table 1). More patients had tumours larger than 5 cm and fewer were candidates for breast-conserving surgery in the trastuzumab group than in the other groups (table 1).
Figure 1.
Trial profile
Table 1.
Baseline patient and tumour characteristics
| Lapatinib (n=154) | Trastuzumab (n=149) | Lapatinib and trastuzumab (n=152) | |
|---|---|---|---|
| Age (years) | 50 (42–56) | 49 (44–57) | 50 (43–59) |
| Status of steroid hormone receptors | |||
| Positive | 80 (51·9%) | 75 (50·3%) | 77 (50·7%) |
| Negative | 74 (48·1%) | 74 (49·7%) | 75 (49·3%) |
| Clinical tumour size (cm) | |||
| Missing | 0 (0·0%) | 1 (0·7%) | 2 (1·3%) |
| ≤2 | 4 (2·6%) | 3 (2·0%) | 2 (1·3%) |
| >2–≤5 | 91 (59·1%) | 66 (44·3%) | 94 (61·8%) |
| >5 | 59 (38·3%) | 79 (53·0%) | 54 (35·5%) |
| Clinical status of lymph nodes | |||
| N0/1 | 129 (83·8%) | 126 (84·6%) | 128 (84·2%) |
| N2+, Nx, or missing | 25 (16·2%) | 23 (15·4%) | 24 (15·8%) |
| Planned conservative breast surgery | |||
| Not a candidate | 107 (69·5%) | 112 (75·2%) | 106 (69·7%) |
| Candidate | 47 (30·5%) | 37 (24·8%) | 46 (30·3%) |
Data are median (IQR) or n (%) unless otherwise stated.
Overall, we recorded more grade 3 and 4 adverse events (graded according to National Cancer Institute Common Terminology Criteria for Adverse Events) in both groups given lapatinib than in the group given trastuzumab alone (table 2). The incidence of neutropenia and diarrhoea and concentration of liver enzymes increased when paclitaxel was started at week 6 (data not shown). Specifically, all grade 3 or worse neutropenia events occurred after paclitaxel treatment began.
Table 2.
Frequency of grade 3 or 4 adverse events
| Lapatinib (n=154) | Trastuzumab (n=149) | Lapatinib and trastuzumab (n=152) | |
|---|---|---|---|
| Grade 3 | |||
| Diarrhoea | 36 (23·4%) | 3 (2·0%) | 32 (21·1%) |
| Hepatic* | 27 (17·5%) | 11 (7·4%) | 15 (9·9%) |
| Neutropenia | 22 (14·3%) | 2 (1·3%) | 11 (7·2%) |
| Skin disorder | 10 (6·5%) | 4 (2·7%) | 10 (6·6%) |
| Grade 4 | |||
| Diarrhoea | 0 (0·0%) | 0 (0·0%) | 0 (0·0%) |
| Hepatic | 1 (0·6%) | 0 (0·0%) | 1 (0·7%) |
| Neutropenia | 2 (1·3%) | 2 (1·3%) | 2 (1·3%) |
| Skin disorder | 0 (0·0%) | 0 (0·0%) | 0 (0·0%) |
Data are n (%). No major cardiac dysfunctions were recorded. One death occurred in the lapatinib and trastuzumab group immediately after end of treatment.
Includes two patients with Hy’s law criteria22 (drug-related concomitant elevation of alanine transaminase or aspartate transaminase > three times upper limit of normal and total bilirubin > two times upper limit of normal) in the trastuzumab group and one in the lapatinib group.
In the combination group, one patient with a history of diabetes mellitus died immediately after the end of treatment, related to hypoglycaemia. We recorded no major cardiac dysfunction, with only one patient in each group having a left ventricular ejection fraction of less than 50% and a decrease of more than 10% from baseline. One patient in the combination group developed class III coronary heart failure and left ventricular ejection fraction fell from 66% to 55% after start of paclitaxel, but recovered after therapy was stopped (webappendix). Generally, events in the group given both lapatinib and trastuzumab were not more frequent than in the group given lapatinib alone (table 2).
Patients assigned to trastuzumab alone were able to complete HER2 therapy as planned more often than were those assigned to the groups given lapatinib (table 3). Treatment was discontinued in 30 patients because of hepatic adverse events (table 3). Strict protocol-defined discontinuation criteria for liver events applied only to the groups given lapatinib, and caused permanent discontinuation of lapatinib and reporting of serious adverse events. The webappendix shows the numbers of patients that had dose reductions.
Table 3.
Reasons for discontinuation of anti-HER2 therapy
| Lapatinib (n=154) | Trastuzumab (n=149) | Lapatinib and trastuzumab (n=152) | |
|---|---|---|---|
| Completed as planned | 102 (66·2%) | 137 (91·9%) | 92 (60·5%) |
| Not completed as planned | 52 (33·8%) | 12 (8·1%) | 60 (39·5%) |
| Adverse event | 29 (18·8%) | 2 (1·3%) | 33 (21·7%) |
| Hepatic* | 18 (11·7%) | 0 (0·0%) | 12 (7·9%) |
| Diarrhoea | 2 (1·3%) | 0 (0·0%) | 13 (8·6%) |
| Other† | 8 (5·2%) | 2 (1·3%) | 8 (5·3%) |
| No recorded AE | 1 (0·6%) | 0 (0·0%) | 0 (0·0%) |
| Protocol violation | 0 (0·0%) | 1 (0·7%) | 1 (0·7%) |
| Disease progression‡ | 3 (1·9%) | 4 (2·7%) | 1 (0·7%) |
| Subject decision | 5 (3·2%) | 0 (0·0%) | 4 (2·6%) |
| Other§ | 15 (9·7%) | 5 (3·4%) | 21 (13·8%) |
Data are n (%).
Moderate rises in liver enzymes caused discontinuation of lapatinib, per protocol.
In the lapatinib group, other adverse events included skin rash and other well known toxicities, such as asthenia.
Two patients (both given trastuzumab alone) had early surgery (before 17 weeks) for disease progression, two other patients with disease progression did not have surgery, and four had surgery as scheduled.
Mistakes by site or patient, or patient non-compliance.
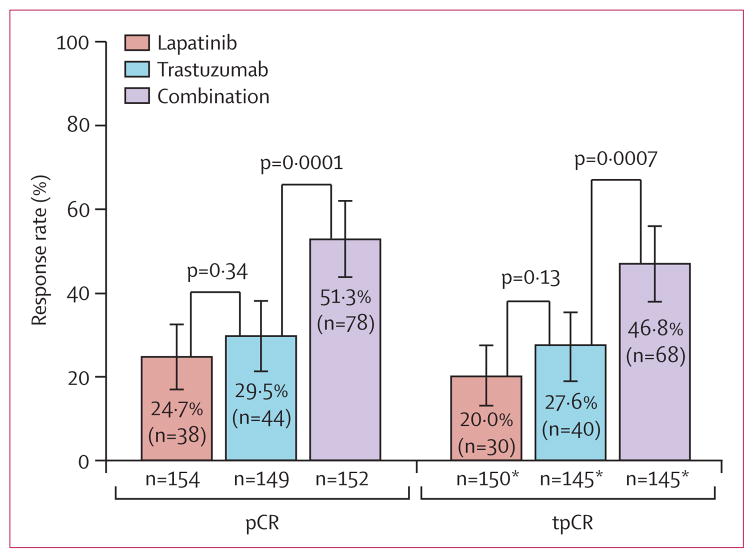
pCR rate was significantly higher in the combination group (51·3% [95% CI 43·1–59·5]) than in the trastuzumab group (29·5% [22·4–37·5]; absolute difference 21·1%, 95% CI 9·1–34·2; figure 2). pCR did not differ between the lapatinib group (24·7% [18·1–32·3] and the trastuzumab group (difference −4·8%, −17·6 to −8·2; figure 2). The odds ratio for pCR, adjusted for stratification factors, in the combination group relative to the trastuzumab-alone group was 2·6 (97·5% CI 1·50–4·58; p=0·0001).
Figure 2. Rates of pCR and of locoregional total pCR in the three treatment groups.
Error bars show 95% CIs. pCR=pathological complete response.
tPCR=locoregional total pCR. *Excludes 15 patients because their nodal status could not be assessed.
For the locoregional total pCR analysis, 15 patients with non-evaluated nodal status at time of surgery were excluded. Similar to pCR, the locoregional total pCR in the combination group was significantly higher than in the trastuzumab group (figure 2; odds ratio 2·39, 97·5% CI 1·36–4·26, p=0·0007; absolute difference 19·3%, 97·5% CI 5·9–32·3). We noted no significant difference in locoregional total pCR between the lapatinib and the trastuzumab groups (20·0% [97·5% CI 13·9–27·3] vs 27·6% [20·5–36·2]; absolute difference −7·6%, 97·5% CI −20·9 to −5·6; figure 2). The proportion of patients with pathologically negative nodes at surgery was higher in the combination (100 of 137 assessed, 73·0%) than in the trastuzumab group (82 of 140 assessed, 58·6%; p=0·0115). Node negativity at time of surgery did not differ between the lapatinib (72 of 139 assessed, 51·8%) and the trastuzumab groups (p=0·14).
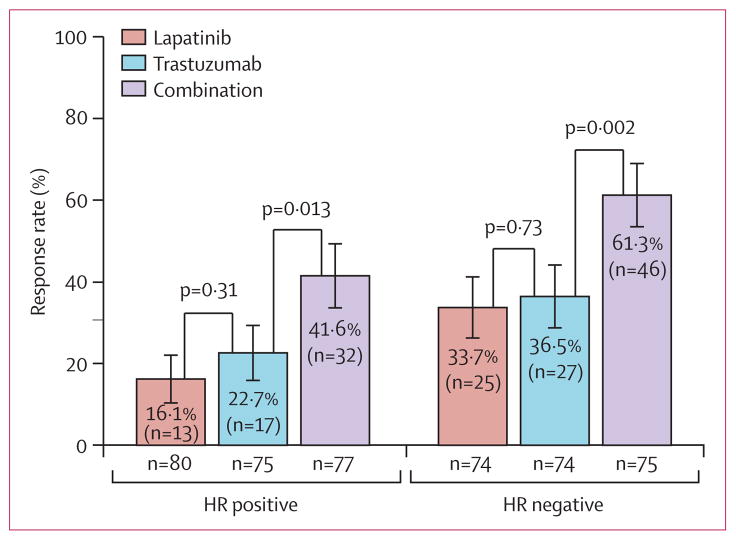
Overall, the pCR rate was higher in patients with hormone-receptor (HR) negative tumours than in those with HR positive tumours in all groups (figure 3, table 4). Additionally, pCR rate for the combination group was significantly higher than for the trastuzumab group for both HR-positive and HR-negative tumours (table 4, figure 3). The ordering of treatment groups with respect to pCR rates for the overall analysis was the same for subgroup analyses defined by the other stratification factors (data not shown). Table 5 shows pCR rates in the different groups by tumour size at baseline.
Figure 3. Rate of pathological complete response by hormone-receptor status of the primary tumour.
Error bars show 95% CIs. HR=hormone receptor.
Table 4.
Proportion of patients with pCR at time of surgery, split by status of hormone receptors
| Lapatinib | Trastuzumab | Lapatinib and trastuzumab | |
|---|---|---|---|
| Positive receptor status | |||
| Number of patients | 80 | 75 | 77 |
| Number of patients with pCR | 13 | 17 | 32 |
| Rate of pCR (95% CI) | 16·25% (8·95 to 26·18) | 22·67% (13·79 to 33·79) | 41·56% (30·43 to 53·36) |
| Difference (97·5% CI)* | −6·42% (−24·2% to 11·66) | ·· | 18·89% (0·90 to 36·34) |
| Binomial p value* | 0·3123 | ·· | 0·0127 |
| Negative receptor status | |||
| Number of patients | 74 | 74 | 75 |
| Number of patients with pCR | 25 | 27 | 46 |
| Rate of pCR (95% CI) | 33·78% (23·19 to 45·72) | 36·49% (25·60 to 48·49) | 61·33% (49·38 to 72·36) |
| Difference (97·5% CI)* | −2·70% (−21·5 to 16·26) | ·· | 24·85% (6·24 to 42·29) |
| Binomial p value* | 0·7306 | ·· | 0·0024 |
pCR=pathological complete response.
Comparisons with trastuzumab group.
Table 5.
Proportion of patients with pCR at time of surgery, by baseline calliper measure of tumour size at baseline
| Lapatinib | Trastuzumab | Lapatinib and trastuzumab | |
|---|---|---|---|
| Tumour ≤ 5 cm | |||
| Number of patients | 95 | 69 | 96 |
| Number of patients with pCR | 27 | 18 | 53 |
| Rate of pCR (95% CI) | 28·42% (19·64 to 38·60) | 26·09% (16·25 to 38·06) | 55·21% (44·71 to 65·37) |
| Difference (97·5% CI)* | 2·33% (−15·3 to 19·91) | ·· | 29·12% (11·62 to 45·28) |
| Binomial p value* | 0·7409 | ·· | 0·0002 |
| Tumour > 5 cm | |||
| Number of patients | 59 | 79 | 54 |
| Number of patients with pCR | 11 | 26 | 23 |
| Rate of pCR (95% CI) | 18·64% (9·69 to 30·91) | 32·91% (22·75 to 44·40) | 42·59% (29·23 to 56·79) |
| Difference (97·5% CI)* | −14·3% (−32·9 to 4·90) | ·· | 9·68% (−10·2 to 29·03) |
| Binomial p value* | 0·0612 | ·· | 0·2557 |
pCR=pathological complete response.
Comparisons with trastuzumab group.
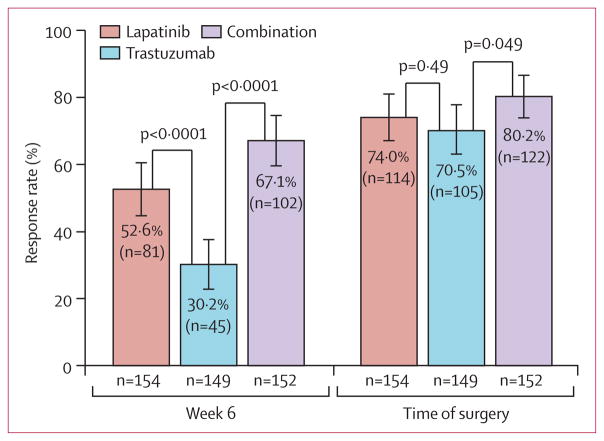
At completion of the 6-week biological window before chemotherapy, a higher objective clinical response rate was recorded for the lapatinib (52·6% [95% CI 44·4–60·7]) and combination (67·1% [59·0–74·5]) groups than for the trastuzumab group (30·2% [23·0–38·3]; figure 4). We recorded no significant differences in objective response rate between the lapatinib (74·0% [66·3–80·7]), trastuzumab (70·5% [62·5–77·7]), and combination groups (80·2% [73·0–86·3]) at time of surgery (figure 4).
Figure 4. Objective clinical tumour response rate at completion of 6 weeks of anti-HER2 therapy and at time of surgery.
Error bars show 95% CIs.
66 (42·9%) patients given lapatinib alone, 58 (38·9%) given trastuzumab alone, and 63 (41·4%) given the combination had conservative surgery. Of patients who were not candidates for conservative surgery at time of randomisation, 33 (30·8%) of 107 patients in the lapatinib group, 31 (27·7%) of 112 in the trastuzumab group, and 28 (26·4%) of 106 in the combination group subsequently received conservative surgery.
Discussion
Our study provides proof of concept that dual HER2 blockade is better than single agent anti-HER2 therapy, as predicted by findings from laboratory studies.7–9 The combination of lapatinib and trastuzumab resulted in a significantly higher pCR rate than did trastuzumab or lapatinib alone. The higher pCR rate for the combination was clear across all subgroups tested and was consistent with other studies of anti-HER2 therapies in HER2-positive tumours.23 The pCR rate was higher in patients with oestrogen-receptor-negative tumours in all treatment groups, as has been recorded in other neoadjuvant trials with anti-HER2 agents in HER2-positive disease.23 Although the absence of masking might have affected the treatment completion rates, pCR rates—an objective measure of outcome—are unlikely to be biased.
The clinical response rate at the end of 6 weeks of anti-HER2 therapy alone (before chemotherapy) with the combination was also superior to trastuzumab alone. Notably, we noted a high clinical response rate for the lapatinib group at the end of 6 weeks compared with that for trastuzumab alone. This finding, which should be cautiously interpreted because the assessment method is imprecise, might be related to the profound anti-proliferative effects of lapatinib in the neoadjuvant setting, as shown in a pilot study.13 The reduced clinical response rate noted at 6 weeks with trastuzumab alone might be explained by the time necessary for this agent to reach fully therapeutic steady-state serum concentration. We recorded similar clinical response rates for the three treatment groups at the time of surgery, which could explain the similar numbers of patients undergoing breast-conserving surgery.
As expected, toxicity rates were higher in the groups given lapatinib than in the group given trastuzumab. The increase in diarrhoea, and in excess neutropenia, could be because combined therapy with lapatinib and paclitaxel causes a 20% increase in systemic exposure to both drugs.24 After enrolment had started, the results of a pilot study showing excess diarrhoea with all three drugs at a dose of 1000 mg were reported.25 We then made a protocol amendment with a dose reduction to 750 mg of lapatinib in the combination group and inclusion of a diarrhoea management algorithm for all patients. The addition of trastuzumab did not increase the rate of diarrhoea secondary to lapatinib. Patients given lapatinib also had more hepatic adverse events, which caused treatment discontinuation in 30 cases. These adverse events consisted of transient and reversible rise in transaminases in 29 patients, which fulfilled Hy’s law criteria (table 2).
We noted two cases of Hy’s law in the trastuzumab group. The criteria for discontinuation of lapatinib and reporting of serious adverse events due to liver dysfunction were not applied to trastuzumab, which could partly explain the reported differences. Additionally, the lapatinib-discontinuation criteria were more stringent in our study than in others and in the agent label itself. A pooled analysis of more than 3000 patients with metastatic cancer treated with lapatinib in 16 trials showed a toxicity rate of 0·3%, fulfilling Hy’s law.22 The higher incidence of diarrhoea and the stringent criteria for treatment discontinuation due to rises in liver enzymes in the lapatinib-containing regimens contributed to the increased rate of treatment discontinuation noted in these groups (table 2). Therefore, that the combination regimen was still able to produce the highest pCR rate is remarkable.
In HER2-positive breast cancer, neoadjuvant studies with anti-HER2 agents15,16 have shown that pCR correlates with disease-free survival. The NOAH study,15 in which patients with HER2-positive locally advanced or inflammatory breast cancer were randomly allocated to chemotherapy or chemotherapy and trastuzumab, showed a doubling in pCR rate in the trastuzumab group and a strong correlation between pCR and improved event-free survival. Investigators of the TECHNO study16 also reported a correlation between pCR and improved disease-free and overall survival. If our study progresses similarly to these previous studies, neoadjuvant dual HER2 blockade will probably improve disease-free survival after further follow-up. Disease-free survival is the primary endpoint for the large ALTTO adjuvant study (ClinicalTrials.gov, NCT00490139), which includes more than 8300 patients and is assessing treatments similar to those used in our trial.
Our findings are similar to the recently reported NeoSphere neoadjuvant study,23 investigating dual HER2 blockade with a combination of two anti-HER2 antibodies, trastuzumab and pertuzumab. Pertuzumab, as for lapatinib, has a complementary mechanism of action with trastuzumab.26 NeoSphere also showed a doubling of pCR rate when the two antibodies were given in combination. Three other smaller neoadjuvant trials27–29 also showed improved pCR rates for the lapatinib plus trastuzumab combination. The high pCR rates with combination treatment recorded in NeoALTTO and NeoSphere are similar to those obtained in similar populations receiving single-agent anti-HER2 therapy (trastuzumab or lapatinib) together with longer duration and more aggressive anthracycline-based and taxane-based regimens in 24 weeks,30 and in 36 weeks.15 Thus, dual HER2 blockade could lead to use of short chemotherapy-containing regimens with few toxic effects for early HER2-positive breast cancer.
Panel: Research in context.
Systematic review
We searched Medline from Jan 1, 2001, to Oct 30, 2011, for full reports of randomised clinical trials in patients with HER2-positive breast cancer with the term “trastuzumab and lapatinib”. We identified one randomised study of patients with HER2-positive metastatic breast cancer that had progressed on trastuzumab.14 We did not find any report of the combination of lapatinib and trastuzumab in the early disease setting, either primary or adjuvant, or in patients with metastatic disease not previously exposed to trastuzumab. However, preliminary results from three small neoadjuvant trials showing improved responses with the combination were presented in June, 2011.27–29
Interpretation
Overall, dual HER2 blockade could be an improved approach to treatment of patients with HER2-positive tumours. Our study shows that dual inhibition of HER2 by lapatinib and trastuzumab in combination with paclitaxel is better than single-agent targeting of HER2 in the neoadjuvant setting. Dual HER2 blockade might be a valid approach in patients with early HER2-positive disease.
The design of this trial is unique because patients received the same anti-HER2 therapy after surgery as was assigned during the neoadjuvant phase (panel, webappendix). Disease-free and overall survival endpoints will be reported after further follow-up. Additionally, the 12 193 biospecimens collected at predetermined timepoints during the trial will enable us to do correlative studies in the future.
In conclusion, this open-label, multicentre, phase 3 study showed that dual inhibition of HER2 with lapatinib and trastuzumab in combination with paclitaxel is better than single-agent HER2 targeting. Our study also supports investigation of novel targeted agents for breast cancer in the neoadjuvant setting, when tumours have not yet acquired resistance to therapy and when chances of clinical benefit are highest.
Supplementary Material
Acknowledgments
This trial was funded by GlaxoSmithKline. We thank Lorena de la Peña (SOLTI Breast Cancer Research group) for her assistance in data collection, analysis, and report writing. The Breast International Group, the Breast European Adjuvant Study Team, and SOLTI Breast Cancer Study Group were responsible for the design and coordination of the study, the collection and management of data, the medical review, analysis of the data, and reporting of the results. The members of the trial steering committee reviewed the report and were responsible for the decision to submit it for publication. The report was prepared by the members of the writing committee, who had unrestricted access to study data and made the final decisions about content. All authors and members of the Executive Committee (including a minority representation from GlaxoSmithKline) reviewed the article and suggested changes. The data were analysed by statisticians at Frontier Science Scotland.
Footnotes
Contributors
JB, IB, SDC, EdA, GA, AG, RDG, and MP-G were involved in the conception and design of the study. JB, IB, HE, SDC, EdA, CA, HG, KF, VVD, GA, AG, T-WC, ZH, LP, MU, RDG, and MP-G supervised the study as part of the steering committee. JB, HE, SDC, HG, MC-P, JD, L-MT, GK, JHS, VS, GL, MP, VP, and MP-G were involved in the provision of patients or data acquisition, or both. JB, IB, EdA, RDG, and MP-G analysed and interpreted data. All authors were involved in the writing and reviewing of the report and approved the final version.
Study investigators per country
Argentina Guillermo Lerzo, Luis Fein, Mirta Varela, Juan Zarba, Cesar Blajman. Belgium Martine Piccart, Hans Wildiers, Peter Vuylsteke, Claire Nouwynck. Brazil Helio Pinczowski, Jeferson Jose Vinholes, Célia Oliveira. Canada: Josee-Anne Roy. Czech Republic Marketa Palacova, Barbara Donocikova, Maria Bendova. France Julien Domont, Thierry Petit, Hervé Cure, Mihaela Achille. Germany Holger Eidtmann, Elke Keil, Oliver Tomé, Sherko Kuemmel, John Hackmann, Georg Kunz, Elisabeth Kloepper, Toralf Reimer, Christoph Thomssen, Kathrin Schwedler. Hong Kong Wai Man Josephin Ng, Ava Kwong, Louis Chow. Hungary István Láng. India Lokanatha Dasappa, Rakesh Chopra, Ajay Mehta, Sudeep Gupta. Italy Antonella Ferro, Marilena Visini, Lucia Del Mastro, Marco Colleoni, Loredana Miglietta, Paolo Bidoli. South Korea Sung-Bae Kim, Joo Hyuk Sohn, Young-Hyuck Im. Lithuania Audrone Ciceniene. Norway Bjorn Naume, Erik Wist. Peru Henry Gomez, Fernando Hurtado de Mendoza. Romania Alexandru Eniu. Russia Vera Gorbunova, Vladimir Semiglazov, Marina Shomova, Alexey Manikhas. South Africa Maria Coccia-Portugal, Anca Pirjol, Lydia Dreosti, Shun Moodley. Spain José Baselga, Eva Ciruelos, Serena Di Cosimo, Antonio Llombart, Miguel Angel de la Cruz Mora, Juan Carlos Toral Peña, Manuel Ruiz Borrego, Antonio González Martín, Rafael López. Sweden Stig Holmberg. Taiwan Ling-Min Tseng, Tsai-Wang Chang, Chiun-Sheng Huang, Ruey-Kuen Hsieh, Dar-Ren Chen. UK Tamas Hickish, Ashraf Patel, Stephen Chan, Rebecca Roylance.
Conflicts of interest
JB has received honoraria from Roche. IB’s institution has received funding from GlaxoSmithKline and Roche. HE has been a speaker and received travel grants from GlaxoSmithKline. SDC has been a speaker for GlaxoSmithKline. EdA has served on an advisory board and received a travelling grant from GlaxoSmithKline, and has been a speaker for Roche. KF’s institution has received travelling grants from GlaxoSmithKline. VVD’s and T-WC’s institutions have received research funding from GlaxoSmithKline. AG has received honoraria from GlaxoSmithKline and Roche. T-WC has been a speaker for GlaxoSmithKline, Roche, Novartis, and Amgen; and has received consultancy funding from GlaxoSmithKline, Roche, Abbott, AstraZeneca, Novartis, and Amgen. LP has received consultancy fees from Pfizer, Sanofi, and Bristol-Myers Squibb; and research grants from Bristol-Myers Squibb and AstraZeneca. RDG’s institution has received research funding from GlaxoSmithKline and Roche. MP-G has received honoraria from GlaxoSmithKline and Roche, and her institution has received research funding from GlaxoSmithKline. GA is a salaried employee of GlaxoSmithKline and retains stock and stock options at the company. The other authors declare that they have no conflicts of interest.
References
- 1.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 4.Smith I, Procter M, Gelber RD, et al. for the HERA study team. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 5.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. the FinHer Study Investigators. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–20. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 6.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 7.Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–39. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 8.Xia W, Gerard CM, Liu L, Baudson NM, Ory TL, Spector NL. Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene. 2005;24:6213–21. doi: 10.1038/sj.onc.1208774. [DOI] [PubMed] [Google Scholar]
- 9.Scaltriti M, Verma C, Guzman M, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28:803–14. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 10.Junttila TT, Akita RW, Parsons K, et al. Ligand-independent HER2/ HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–40. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 12.Mohsin SK, Weiss HL, Gutierrez MC, et al. Neoadjuvant trastuzumab induces apoptosis in primary breast cancers. J Clin Oncol. 2005;23:2460–68. doi: 10.1200/JCO.2005.00.661. [DOI] [PubMed] [Google Scholar]
- 13.Dave B, Migliaccio I, Gutierrez MC, et al. Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J Clin Oncol. 2011;29:166–73. doi: 10.1200/JCO.2009.27.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–30. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 15.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–84. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 16.Untch M, Fasching P, Konecny G, et al. Pathological complete response after neoadjuvant chemotherapy + trastuzumab treatment predicts survival and detects a patient subgroup at high need for improvement of anti-HER2 therapy: three year median follow-up data of the TECHNO Trial. Cancer Res. 2011;70(suppl 2) P1-11-03. [Google Scholar]
- 17.Wolff AC, Hammond ME, Schwartz JN, et al. for the American Society of Clinical Oncology, and the College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 18.Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002;95:681–95. doi: 10.1002/cncr.10741. [DOI] [PubMed] [Google Scholar]
- 19.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–63. [Google Scholar]
- 20.Hochberg Y. A sharper bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–02. [Google Scholar]
- 21.Cox DR. Analysis of binary data. London: Methuen and Company; 1970. [Google Scholar]
- 22.Moy B, Rappold E, Williams L, et al. Hepatobiliary abnormalities in patients with metastatic cancer treated with lapatinib. J Clin Oncol. 2009;27(suppl):1043. [Google Scholar]
- 23.Gianni L, Pienkowski T, Im Y-H, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 24.Crown JP, Burris HA, 3rd, Jones S, et al. Safety and tolerability of lapatinib in combination with taxanes (T) in patients with breast cancer (BC) J Clin Oncol. 2007;25(suppl):1027. [Google Scholar]
- 25.Dang C, Lin N, Moy B, et al. Dose-dense doxorubicin and cyclophosphamide followed by weekly paclitaxel with trastuzumab and lapatinib in HER2/neu-overexpressed/amplified breast cancer is not feasible because of excessive diarrhea. J Clin Oncol. 2010;28:2982–88. doi: 10.1200/JCO.2009.26.5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–75. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 27.Chang JC, Mayer IA, Forero-Torres A, et al. TBCRC 006: A multicenter phase II study of neoadjuvant lapatinib and trastuzumab in patients with HER2-overexpressing breast cancer. J Clin Oncol. 2011;29(suppl):505. doi: 10.1200/JCO.2012.44.8027. (abstr) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmes FA, Nagarwala YM, Espina VA, et al. Correlation of molecular effects and pathologic complete response to preoperative lapatinib and trastuzumab, separately and combined prior to neoadjuvant breast cancer chemotherapy. J Clin Oncol. 2011;29(suppl):506. (abstr) [Google Scholar]
- 29.Guarneri V, Frassoldati A, Bottini A, et al. Final results of a phase II randomized trial of neoadjuvant anthracycline-taxane chemotherapy plus lapatinib, trastuzumab, or both in HER2-positive breast cancer (CHER-LOB trial) J Clin Oncol. 2011;29(suppl):507. (abstr) [Google Scholar]
- 30.Untch M, Loibl S, Bischoff J, et al. for the German Breast Group (GBG) and the Arbeitsgemeinschaft Gynäkologische Onkologie-Breast (AGO-B) study group. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 2012 doi: 10.1016/S1470-2045(11)70397-7. published online Jan 17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.