Abstract
BACKGROUND
The anti–human epidermal growth factor receptor 2 (HER2) humanized monoclonal antibody trastuzumab improves the outcome in patients with HER2-positive metastatic breast cancer. However, most cases of advanced disease eventually progress. Pertuzumab, an anti-HER2 humanized monoclonal antibody that inhibits receptor dimerization, has a mechanism of action that is complementary to that of trastuzumab, and combination therapy with the two antibodies has shown promising activity and an acceptable safety profile in phase 2 studies involving patients with HER2-positive breast cancer.
METHODS
We randomly assigned 808 patients with HER2-positive metastatic breast cancer to receive placebo plus trastuzumab plus docetaxel (control group) or pertuzumab plus trastuzumab plus docetaxel (pertuzumab group) as first-line treatment until the time of disease progression or the development of toxic effects that could not be effectively managed. The primary end point was independently assessed progression-free survival. Secondary end points included overall survival, progression-free survival as assessed by the investigator, the objective response rate, and safety.
RESULTS
The median progression-free survival was 12.4 months in the control group, as compared with 18.5 months in the pertuzumab group (hazard ratio for progression or death, 0.62; 95% confidence interval, 0.51 to 0.75; P<0.001). The interim analysis of overall survival showed a strong trend in favor of pertuzumab plus trastuzumab plus docetaxel. The safety profile was generally similar in the two groups, with no increase in left ventricular systolic dysfunction; the rates of febrile neutropenia and diarrhea of grade 3 or above were higher in the pertuzumab group than in the control group.
CONCLUSIONS
The combination of pertuzumab plus trastuzumab plus docetaxel, as compared with placebo plus trastuzumab plus docetaxel, when used as first-line treatment for HER2-positive metastatic breast cancer, significantly prolonged progression-free survival, with no increase in cardiac toxic effects.
Approximately 20% of all breast cancers have gene amplification or overexpression (or both) of human epidermal growth factor receptor 2 (HER2),1 a tyrosine kinase trans-membrane receptor, resulting in a more aggressive phenotype and a poor prognosis.2 Treatment with the anti-HER2 humanized monoclonal antibody trastuzumab in addition to chemotherapy, as compared with chemotherapy alone, significantly improves progression-free and overall survival among patients with HER2-positive metastatic breast cancer.3,4 Trastuzumab binds to subdomain IV of the HER2 extracellular domain and exerts its antitumor effects by blocking HER2 cleavage,5 stimulating antibody-dependent, cell-mediated cytotoxicity6 and inhibiting ligand-independent, HER2-mediated mitogenic signaling.7 However, in most patients with HER2-positive metastatic breast cancer, the disease progresses,8 highlighting the need for new targeted therapies for advanced disease.
New therapies directed at HER2 are being developed,9–13 among them pertuzumab, a humanized monoclonal antibody that binds HER2 at a different epitope of the HER2 extracellular domain (subdomain II) than that at which trastuzumab binds.14 Pertuzumab prevents HER2 from dimerizing with other ligand-activated HER receptors, most notably HER3.9,15 Like trastuzumab, pertuzumab stimulates antibody-dependent, cell-mediated cytotoxicity.6 Because pertuzumab and trastuzumab bind to different HER2 epitopes and have complementary mechanisms of action, these two agents, when given together, provide a more comprehensive blockade of HER2 signaling and result in greater antitumor activity than either agent alone in HER2-positive tumor models.6,16 In phase 2 studies, a pertuzumab–trastuzumab regimen has shown activity in patients with HER2-positive metastatic breast cancer17,18 and in patients with early breast cancer.19
The Clinical Evaluation of Pertuzumab and Trastuzumab (CLEOPATRA) study assessed the efficacy and safety of pertuzumab plus trastuzumab plus docetaxel, as compared with placebo plus trastuzumab plus docetaxel, as first-line treatment for patients with HER2-positive metastatic breast cancer.
METHODS
STUDY DESIGN
We conducted a randomized, double-blind, placebo-controlled, phase 3 trial involving patients with HER2-positive metastatic breast cancer who had not received chemotherapy or biologic therapy for their metastatic disease. The study was conducted in full accordance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. Written informed consent was obtained from each participant. Approval for the protocol and for any modifications was obtained from an independent ethics committee for each participating site.
Patients were randomly assigned, in a 1:1 ratio, to receive placebo plus trastuzumab (Her-ceptin, F. Hoffmann–La Roche/Genentech) plus docetaxel or pertuzumab (F. Hoffmann–La Roche/Genentech) plus trastuzumab plus docetaxel. Randomization was performed with the use of an interactive voice-response system, with stratification according to geographic region (Asia, Europe, North America, or South America) and prior treatment status (prior adjuvant or neoadjuvant chemotherapy vs. none).
The primary end point was progression-free survival, as determined on the basis of the assessment of tumors at an independent review facility (independently assessed progression-free survival). Progression-free survival was defined as the time from randomization to the first documented radiographic evidence of progressive disease according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0,20 or death from any cause within 18 weeks after the last independent assessment of tumors. Data for patients who did not have evidence of independently assessed progressive disease or who had not died within 18 weeks after the last tumor assessment were censored at the time of the last independent tumor assessment that provided results that could be evaluated. Secondary end points included overall survival, progression-free survival as assessed by the investigator, the objective response rate, and safety. Safety was monitored by an independent data and safety monitoring committee and an independent cardiac review committee.
STUDY OVERSIGHT
The study was designed by the senior academic authors and representatives of the sponsor (F. Hoffmann–La Roche/Genentech). The data were collected by the sponsor and were analyzed by the sponsor in collaboration with the senior academic authors, who vouch for the completeness and accuracy of the data and analyses and for the fidelity of the study to the protocol. The corresponding author prepared the initial draft of the manuscript with support from a medical writer who was paid by F. Hoffmann–La Roche. All the authors contributed to subsequent drafts and made the decision to submit the manuscript for publication. The protocol, including the statistical analysis plan, is available with the full text of this article at NEJM.org.
PATIENTS
Eligible patients had locally recurrent, unresectable, or metastatic HER2-positive breast cancer. HER2-positive status was confirmed centrally, by means of immunohistochemistry (with 3+ indicating positive status) or fluorescence in situ hybridization (with an amplification ratio ≥2.0 indicating positive status).21 Patients were eligible whether they had measurable disease or non-measurable disease. Tumor hormone-receptor status was determined locally. Additional eligibility criteria were an age of 18 years or older, a left ventricular ejection fraction of 50% or more at baseline (determined by echocardiography or multiple-gated acquisition scanning), and an Eastern Cooperative Oncology Group (ECOG) performance status22 of 0 or 1 (with 0 indicating that the patient is fully active and able to carry on all predisease activities without restriction and 1 indicating that the patient is restricted in physically strenuous activity but is ambulatory and able to carry out work of a light or sedentary nature). Patients may have received one hormonal treatment for metastatic breast cancer before randomization. Patients may have received adjuvant or neoadjuvant chemotherapy with or without trastuzumab before randomization, with an interval of at least 12 months between completion of the adjuvant or neoadjuvant therapy and the diagnosis of meta-static breast cancer.
Exclusion criteria were therapy for metastatic breast cancer (other than that described above), central nervous system metastases, prior exposure to a cumulative dose of doxorubicin that exceeded 360 mg per square meter of body-surface area or its equivalent, a previous decline in the left ventricular ejection fraction to less than 50% during or after prior trastuzumab therapy, and current uncontrolled medical conditions that could limit a patient’s ability to undertake study therapy.
PROCEDURES
Patients received a loading dose of 8 mg of trastuzumab per kilogram of body weight, followed by a maintenance dose of 6 mg per kilogram every 3 weeks until disease progression, as assessed by the investigator on the basis of radiographic, cytologic, or photographic evidence, or the development of toxic effects that could not be effectively managed. Docetaxel was administered every 3 weeks at a starting dose of 75 mg per square meter; at the discretion of the investigator, the dose could be increased to 100 mg per square meter if the side-effect profile was acceptable. Per protocol, the investigator could reduce the dose by 25%, from 75 mg per square meter to 55 mg per square meter or from 100 mg per square meter to 75 mg per square meter, if the drug had toxic effects. It was recommended that patients receive at least six cycles of docetaxel. Pertuzumab or placebo was given at a fixed loading dose of 840 mg, followed by 420 mg every 3 weeks until disease progression or the development of toxic effects that could not be effectively managed. In the case of discontinuation of chemotherapy owing to toxic effects, antibody therapy was continued until disease progression, the development of unacceptable toxic effects, or withdrawal of consent. All drugs were administered intravenously.
ASSESSMENTS
Routine tumor assessments, based on RECIST, were performed every 9 weeks by the investigator and by personnel at the independent review facility; these assessments were performed until the time of independently assessed disease progression or death. Decisions regarding treatment were made by the investigator, solely on the basis of the investigator’s assessment of disease progression. Assessments of left ventricular ejection fraction were performed at baseline, every 9 weeks during the treatment period, at the time of discontinuation of treatment, every 6 months in the first year after discontinuation, and annually thereafter for up to 3 years. Laboratory tests were performed and ECOG performance status was assessed every cycle (i.e., every 3 weeks). Adverse events were monitored continuously and were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf).
Patients with cardiac events or serious adverse events that were ongoing at the time of discontinuation of treatment were followed until resolution of the event or stabilization of the patient’s condition, up to 1 year after administration of the final dose of the study drug. Patients with cardiac events or treatment-related serious adverse events that occurred after discontinuation of treatment were followed for 1 year after the onset of the event.
STATISTICAL ANALYSIS
We planned to enroll 800 patients in the study and to perform the primary analysis of progression-free survival after the occurrence of approximately 381 events of independently assessed disease progression or death from any cause within 18 weeks after the last independent assessment of tumors; with that number of events, it was estimated that the study would have 80% power to detect a 33% improvement in median progression-free survival in the pertuzumab group (hazard ratio, 0.75) at a two-sided significance level of 5%. A prespecified interim analysis of overall survival was performed at the time of the primary analysis of independently assessed progression-free survival. A Lan–DeMets alpha spending function with the O’Brien–Fleming stopping boundary was applied to the interim analysis of overall survival. If the stopping boundary was not crossed, patients were to continue to receive the study therapy (with group assignments remaining concealed) until the final analysis of overall survival, which is to be performed after 385 deaths have occurred. With this number of deaths, we estimate that the study will have 80% power to detect a 33% improvement in overall survival in the pertuzumab group. Analyses of progression-free survival, overall survival, and objective response rate were performed in the intention-to-treat population (all patients who underwent randomization). The log-rank test, with stratification according to prior treatment status and region, was used to compare independently assessed progression-free survival between the two groups. The Kaplan–Meier approach was used to estimate the median independently assessed progression-free survival in each group. A Cox proportional-hazards model, with stratification according to prior treatment status and region, was used to estimate the hazard ratio and 95% confidence intervals.
Prespecified subgroup analyses of independently assessed progression-free survival were performed to determine the consistency of the treatment effect according to key baseline characteristics. Progression-free survival as assessed by the investigator and overall survival were analyzed with the same methods as those used for the analysis of independently assessed progression-free survival. The objective response rate was analyzed on data from patients who had independently assessed measurable disease at baseline and was compared between the groups with the use of the Mantel–Haenszel test, with stratification according to prior treatment status and region. Adverse events were evaluated descriptively in the safety population (all patients who received at least one dose of a study drug).
RESULTS
STUDY POPULATION
During the period from February 2008 through July 2010, a total of 808 patients were enrolled at 204 centers in 25 countries; 406 were randomly assigned to placebo plus trastuzumab plus docetaxel (control group), and 402 to pertuzumab plus trastuzumab plus docetaxel (pertuzumab group) (Fig. 1 in the Supplementary Appendix, available at NEJM.org). The cutoff date for collection of data was May 13, 2011. The baseline characteristics of the patients were similar in the two groups (Table 1).
Table 1.
Baseline Characteristics of the Intention-to-Treat Population.*
| Characteristic | Placebo plus Trastuzumab plus Docetaxel (N = 406) | Pertuzumab plus Trastuzumab plus Docetaxel (N = 402) |
|---|---|---|
| Female sex — no. (%) | 404 (99.5) | 402 (100.0) |
| Age — yr | ||
| Median | 54.0 | 54.0 |
| Range | 27–89 | 22–82 |
| Race or ethnic group — no. (%)† | ||
| Asian | 133 (32.8) | 128 (31.8) |
| Black | 20 (4.9) | 10 (2.5) |
| White | 235 (57.9) | 245 (60.9) |
| Other | 18 (4.4) | 19 (4.7) |
| Region — no. (%) | ||
| Asia | 128 (31.5) | 125 (31.1) |
| Europe | 152 (37.4) | 154 (38.3) |
| North America | 68 (16.7) | 67 (16.7) |
| South America | 58 (14.3) | 56 (13.9) |
| ECOG performance status — no. (%)‡ | ||
| 0 | 248 (61.1) | 274 (68.2) |
| 1 | 157 (38.7) | 125 (31.1) |
| ≥2 | 1 (0.2) | 3 (0.7) |
| Disease type at screening — no. (%) | ||
| Nonvisceral | 90 (22.2) | 88 (21.9) |
| Visceral | 316 (77.8) | 314 (78.1) |
| Hormone-receptor status — no. (%) | ||
| ER-positive, PgR-positive, or both | 199 (49.0) | 189 (47.0) |
| ER-negative and PgR-negative | 196 (48.3) | 212 (52.7) |
| Unknown | 11 (2.7) | 1 (0.2) |
| HER2 status, assessed by immunohistochemistry — no. (%) | ||
| 0 or 1+ | 2 (0.5) | 4 (1.0) |
| 2+ | 32 (7.9) | 47 (11.7) |
| 3+ | 371 (91.4) | 350 (87.1) |
| Data not available | 1 (0.2) | 1 (0.2) |
| HER2 status, assessed by FISH — no. (%) | ||
| Positive | 383 (94.3) | 384 (95.5) |
| Negative | 4 (1.0) | 1 (0.2) |
| Data not available | 19 (4.7) | 17 (4.2) |
| Prior adjuvant or neoadjuvant chemotherapy — no. (%) | ||
| No | 214 (52.7) | 218 (54.2) |
| Yes§ | 192 (47.3) | 184 (45.8) |
| Anthracycline | 164 (40.4) | 150 (37.3) |
| Hormone | 97 (23.9) | 106 (26.4) |
| Taxane | 94 (23.2) | 91 (22.6) |
| Trastuzumab | 41 (10.1) | 47 (11.7) |
Baseline characteristics did not differ significantly between the two groups. ER denotes estrogen receptor, FISH fluorescence in situ hybridization, HER2 human epidermal growth factor receptor 2, and PgR progesterone receptor.
Race or ethnic group was determined by the investigator. The category of “Other” includes American Indian and Alaska Native.
The Eastern Cooperative Oncology Group (ECOG) performance status reflects the daily-living abilities of the patient, on a scale of 0 (fully active without symptoms) to 5 (dead).
Patients may have received more than one form of adjuvant or neoadjuvant chemotherapy.
PROGRESSION-FREE SURVIVAL
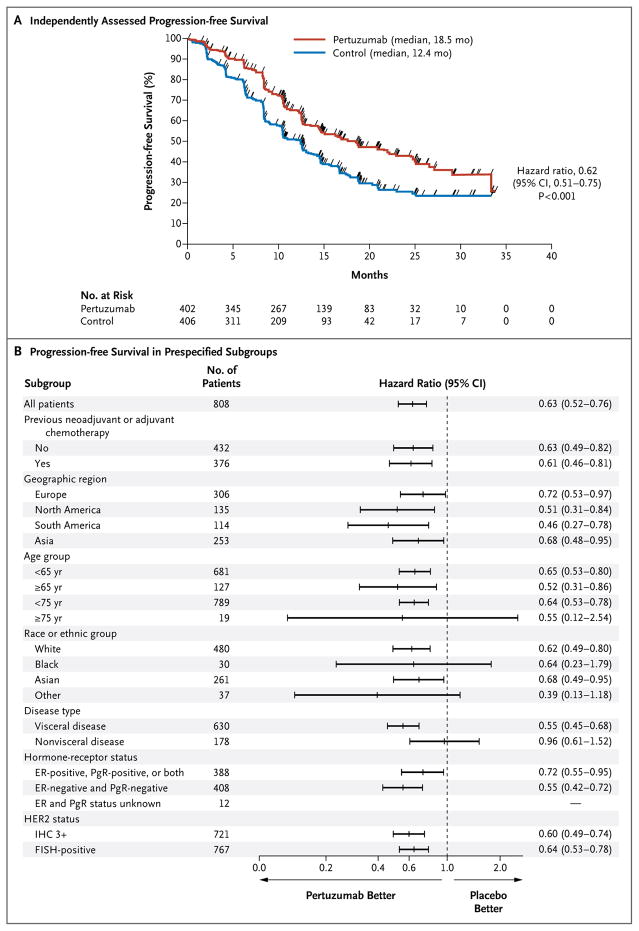
Treatment with pertuzumab plus trastuzumab plus docetaxel, as compared with placebo plus trastuzumab plus docetaxel, significantly improved independently assessed progression-free survival, with patients stratified according to prior treatment status and region. The median independently assessed progression-free survival was prolonged by 6.1 months, from 12.4 months in the control group to 18.5 months in the pertuzumab group (hazard ratio for progression or death, 0.62; 95% confidence interval [CI], 0.51 to 0.75; P<0.001) (Fig. 1A). The benefit of pertuzumab–trastuzumab–docetaxel therapy with respect to progression-free survival was observed across all predefined subgroups (Fig. 1B). Among the 88 patients who had received adjuvant or neoadjuvant chemotherapy with trastuzumab, the median independently assessed progression-free survival was 10.4 months in the control group, as compared with 16.9 months in the pertuzumab group (hazard ratio, 0.62; 95% CI, 0.35 to 1.07). Among the 288 patients who had received adjuvant or neoadjuvant chemotherapy without trastuzumab, the median independently assessed progression-free survival was 12.6 months in the control group, as compared with 21.6 months in the pertuzumab group (hazard ratio, 0.60; 95% CI, 0.43 to 0.83). The median investigator-assessed progression-free survival was 12.4 months in the control group, as compared with 18.5 months in the pertuzumab group (hazard ratio, 0.65; 95% CI, 0.54 to 0.78; P<0.001).
Figure 1. Progression-free Survival, as Assessed at an Independent Review Facility.
Panel A shows Kaplan–Meier estimates of progression-free survival in the intention-to-treat population, stratified according to prior treatment and region. The median progression-free survival was longer by 6.1 months in the pertuzumab group (pertuzumab plus trastuzumab plus docetaxel) than in the control group (placebo plus trastuzumab plus docetaxel). The tick marks indicate the times at which events were recorded. Panel B shows hazard ratios and 95% confidence intervals for progression-free survival in all prespecified subgroups according to baseline characteristics, without stratification. The hazard ratio for the category of unknown status of estrogen receptor (ER) and progesterone receptor (PgR) was not quantifiable, owing to the small number of patients in the group. FISH denotes fluorescence in situ hybridization, and IHC immunohistochemistry.
KEY SECONDARY EFFICACY END POINTS
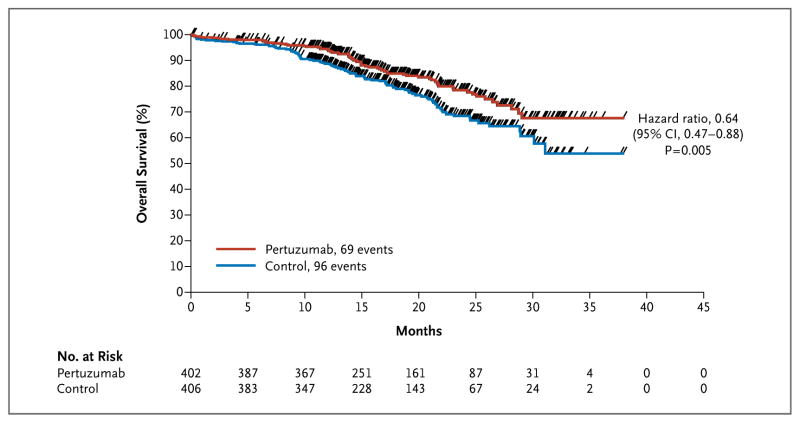
The interim analysis of overall survival was performed after 165 events (43% of the prespecified total number for the final analysis) had occurred. More deaths occurred in the control group than in the pertuzumab group (96 [23.6%] vs. 69 [17.2%]) (Fig. 2). The hazard ratio was 0.64 (95% CI, 0.47 to 0.88; P = 0.005), which did not meet the O’Brien–Fleming stopping boundary of the Lan–DeMets alpha spending function for this interim analysis of overall survival (hazard ratio, ≤0.603; P≤0.0012) and was therefore not significant. However, the data showed a strong trend toward a survival benefit with pertuzumab–trastuzumab–docetaxel therapy. The median follow-up period in both groups was 19.3 months (Kaplan–Meier estimate).
Figure 2. Overall Survival.
Kaplan–Meier estimates of overall survival in patients in the intention-to-treat population are shown. The tick marks indicate the times at which events were recorded. The interim overall survival analysis was performed after 165 events (43% of the prespecified total number for the final analysis) had occurred: 96 events in the control group (placebo plus trastuzumab plus docetaxel) and 69 events in the pertuzumab group (pertuzumab plus trastuzumab plus docetaxel). The interim analysis of overall survival did not cross the O’Brien–Fleming stopping boundary threshold; therefore, the interim result is not statistically significant and is deemed exploratory.
The objective response rate was 69.3% in the control group, as compared with 80.2% in the pertuzumab group. The difference in response rates was 10.8 percentage points (95% CI, 4.2 to 17.5; P = 0.001) (Table 2). A fixed-sequence testing hierarchy was prespecified: independently assessed progression-free survival was to be tested first, followed by the secondary end point of overall survival and then by the secondary end point of objective response rate. Since overall survival at the interim analysis did not cross the stopping boundary for significance, the statistical test result for objective response rate is deemed to be exploratory.
Table 2.
Overall Response, as Assessed at an Independent Review Facility.*
| Response | Placebo plus Trastuzumab plus Docetaxel (N = 336) | Pertuzumab plus Trastuzumab plus Docetaxel (N = 343) |
|---|---|---|
| number (percent) | ||
| Objective response | 233 (69.3) | 275 (80.2) |
|
| ||
| Complete response | 14 (4.2) | 19 (5.5) |
|
| ||
| Partial response | 219 (65.2) | 256 (74.6) |
|
| ||
| Stable disease | 70 (20.8) | 50 (14.6) |
|
| ||
| Progressive disease | 28 (8.3) | 13 (3.8) |
|
| ||
| Not assessable | 2 (0.6) | 2 (0.6) |
|
| ||
| No assessment performed | 3 (0.9) | 3 (0.9) |
Total numbers in the two groups represent the number of patients with measurable disease at baseline, as assessed at an independent review facility.
TREATMENT EXPOSURE
The median number of study-treatment cycles per patient was 15 (range, 1 to 50) in the control group and 18 (range, 1 to 56) in the pertuzumab group, and the median duration of study treatment was estimated to be 11.8 months and 18.1 months in the two groups, respectively. Dose reductions were not permitted for placebo, pertuzumab, or trastuzumab. Patients in each group received docetaxel for a median of 8 cycles, with a range of 1 to 41 cycles in the control group and 1 to 35 in the pertuzumab group. In the safety population, the dose of docetaxel was increased to 100 mg per square meter for one or more cycles in 61 patients in the control group (15.4%) as compared with 48 patients in the pertuzumab group (11.8%). The median dose intensity of docetaxel was 24.8 mg per square meter per week in the control group and 24.6 mg per square meter per week in the pertuzumab group. The reasons for permanent discontinuation of all study treatment are shown in Figure 1 in the Supplementary Appendix.
SIDE-EFFECT PROFILE AND CARDIAC SAFETY
The adverse-event profile during the treatment period was generally balanced between the two groups (Table 3). The incidences of the adverse events (any grade) of diarrhea, rash, mucosal inflammation, febrile neutropenia, and dry skin were higher by at least 5 percentage points in the pertuzumab group than in the control group. The incidences of grade 3 or higher febrile neutropenia and diarrhea were higher by at least 2 percentage points in the pertuzumab group than in the control group (Table 3). The incidence of febrile neutropenia of grade 3 or higher among patients from Asia was 12% in the control group and 26% in the pertuzumab group; in all other geographic regions, the incidence was 10% or less in both groups.
Table 3.
Adverse Events in the Safety Population.*
| Adverse Event | Placebo plus Trastuzumab plus Docetaxel (N = 397) | Pertuzumab plus Trastuzumab plus Docetaxel (N = 407) |
|---|---|---|
| number (percent) | ||
| Most common events, all grades† | ||
|
| ||
| Diarrhea | 184 (46.3) | 272 (66.8) |
|
| ||
| Alopecia | 240 (60.5) | 248 (60.9) |
|
| ||
| Neutropenia | 197 (49.6) | 215 (52.8) |
|
| ||
| Nausea | 165 (41.6) | 172 (42.3) |
|
| ||
| Fatigue | 146 (36.8) | 153 (37.6) |
|
| ||
| Rash | 96 (24.2) | 137 (33.7) |
|
| ||
| Decreased appetite | 105 (26.4) | 119 (29.2) |
|
| ||
| Mucosal inflammation | 79 (19.9) | 113 (27.8) |
|
| ||
| Asthenia | 120 (30.2) | 106 (26.0) |
|
| ||
| Peripheral edema | 119 (30.0) | 94 (23.1) |
|
| ||
| Constipation | 99 (24.9) | 61 (15.0) |
|
| ||
| Febrile neutropenia | 30 (7.6) | 56 (13.8) |
|
| ||
| Dry skin | 17 (4.3) | 43 (10.6) |
|
| ||
| Grade 3 or higher events‡ | ||
|
| ||
| Neutropenia | 182 (45.8) | 199 (48.9) |
|
| ||
| Febrile neutropenia | 30 (7.6) | 56 (13.8) |
|
| ||
| Leukopenia | 58 (14.6) | 50 (12.3) |
|
| ||
| Diarrhea | 20 (5.0) | 32 (7.9) |
|
| ||
| Peripheral neuropathy | 7 (1.8) | 11 (2.7) |
|
| ||
| Anemia | 14 (3.5) | 10 (2.5) |
|
| ||
| Asthenia | 6 (1.5) | 10 (2.5) |
|
| ||
| Fatigue | 13 (3.3) | 9 (2.2) |
|
| ||
| Granulocytopenia | 9 (2.3) | 6 (1.5) |
|
| ||
| Left ventricular systolic dysfunction | 11 (2.8) | 5 (1.2) |
|
| ||
| Dyspnea | 8 (2.0) | 4 (1.0) |
The safety population includes all patients who received at least one dose of the study drug.
Included are all grades of adverse events with an incidence of 25% or more in either group or at least a 5% difference in incidence between groups.
Included are adverse events of grade 3 or higher with an incidence of at least 2%.
Left ventricular systolic dysfunction (any grade) was reported more frequently in the control group than in the pertuzumab group (8.3% vs. 4.4%). Left ventricular systolic dysfunction of grade 3 or higher was reported in 2.8% of the patients in the control group and in 1.2% of the patients in the pertuzumab group. Among patients in whom the left ventricular ejection fraction was assessed after the baseline assessment, 6.6% in the control group and 3.8% in the pertuzumab group had declines of 10 percentage points or more from baseline that resulted in a left ventricular ejection fraction of less than 50%.
In the safety population, most of the deaths that occurred were attributed to disease progression: 81 of the deaths in the control group (20.4% of the patients in the control-group safety population) and 57 of the deaths in the pertuzumab group (14.0% of the patients in the pertuzumab-group safety population). The number and causes of deaths other than deaths from disease progression were generally balanced between the two groups, and a similar number of patients died as a result of adverse events (10 [2.5%] in the control group and 8 [2.0%] in the pertuzumab group); infections were the most common cause of death due to an adverse event.
DISCUSSION
We found that the combination of the anti-HER2 monoclonal antibodies pertuzumab and trastuzumab with docetaxel as first-line therapy prolonged progression-free survival in patients with HER2-positive metastatic breast cancer. Treatment with pertuzumab plus trastuzumab plus docetaxel, as compared with placebo plus trastuzumab plus docetaxel, resulted in a significant reduction in the risk of progression or death (hazard ratio, 0.62) and an increase of 6.1 months in median progression-free survival. The survival data are not yet mature, since the interim analysis of overall survival was performed after 165 events had occurred (43% of the prespecified total number of events for the final analysis). Although there is a strong trend toward prolonged survival with pertuzumab plus trastuzumab plus docetaxel, the result is exploratory, since it did not cross the O’Brien–Fleming stopping boundary. The final analysis of overall survival is event-driven and is estimated to be performed in late 2013. To place our results in perspective, the median progression-free survival in the control group in our study (12.4 months) was similar to that among HER2-positive patients with metastatic breast cancer in two other randomized studies who were treated with the combination of trastuzumab and docetaxel (11.7 months4 and 11.1 months23).
The combination therapy with pertuzumab did not increase the rates of symptomatic or asymptomatic cardiac dysfunction. Adverse events (any grade) of diarrhea, rash, mucosal inflammation, febrile neutropenia, and dry skin were reported more frequently in the pertuzumab group than in the control group. The events were mostly grade 1 or 2 and occurred during the period of concomitant docetaxel administration. Grade 3 or higher febrile neutropenia and diarrhea were also increased in the pertuzumab group.
The underlying basis for the observed clinical benefit of pertuzumab is likely to be related to the biologic characteristics of the HER family of transmembrane-spanning tyrosine kinase receptors, which consists of four closely related members: epidermal growth factor receptor (EGFR; HER1), HER2, HER3, and HER4. Each receptor is composed of an extracellular domain, where ligand binding occurs, and an intracellular domain with tyrosine kinase activity. HER2 has no ligand, and HER3 does not have tyrosine kinase activity.16 Receptor dimerization is an essential requirement for HER function and can occur between two different HER members (heterodimerization) or between two molecules of the same receptor (homodimerization). On receptor dimerization, transactivation of the tyrosine kinase domains of the dimer moiety occurs as each receptor phosphorylates its partner. Although there are multiple homomeric and heteromeric complexes, the HER2–HER3 heterodimer is considered to be the most potent signaling pair,15 driving cell proliferation in HER2-positive cancer.16,24 On binding of the ligand to HER3, dimerization of HER2 with HER3 occurs, and HER2 transphosphorylates HER3, resulting in activation of the critically important phosphoinositide 3-kinase pathway.25 Pertuzumab prevents HER2 dimerization and, as a consequence, inhibits HER2–HER3 signaling.26
Our findings suggest that targeting HER2-positive tumors with two anti-HER2 monoclonal antibodies that have complementary mechanisms of action results in a more comprehensive blockade of HER2 and highlights the clinical importance of preventing the ligand-dependent formation of HER2 dimers in order to silence HER2 signaling to the greatest extent possible.9,27 Although the number of patients who had received prior adjuvant or neoadjuvant chemotherapy with trastuzumab was small, the progression-free survival benefit of pertuzumab plus trastuzumab plus docetaxel therapy in those patients (hazard ratio, 0.62) was similar to the benefit observed in patients who had received prior adjuvant or neoadjuvant chemotherapy without trastuzumab (hazard ratio, 0.60). Finally, our findings suggest that a study of pertuzumab and trastuzumab in earlier stages of the disease is warranted. A study of adjuvant therapy in patients with newly diagnosed HER2-positive breast cancer has been initiated (Adjuvant Pertuzumab and Herceptin in Initial Therapy in Breast Cancer [APHINITY], NCT01358877).
Supplementary Material
Acknowledgments
Supported by F. Hoffmann–La Roche/Genentech, a member of the Roche Group. Support for writing assistance by a third party (Vilma Graupner, Ph.D.) was provided by F. Hoffmann–La Roche.
(Funded by F. Hoffmann–La Roche/Genentech; ClinicalTrials.gov number, NCT00567190.)
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 2.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–68. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a mono-clonal antibody against HER2 for meta-static breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 4.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–74. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 5.Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–9. [PubMed] [Google Scholar]
- 6.Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–6. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 7.Junttila TT, Akita RW, Parsons K, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–40. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Nahta R, Esteva FJ. Trastuzumab: triumphs and tribulations. Oncogene. 2007;26:3637–43. doi: 10.1038/sj.onc.1210379. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–75. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 10.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. doi: 10.1056/NEJMoa064320. [Erratum, N Engl J Med 2007;356:1487.] [DOI] [PubMed] [Google Scholar]
- 11.Rabindran SK, Discafani CM, Rosfjord EC, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–65. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- 12.Burris HA, III, Rugo HS, Vukelja SJ, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29:398–405. doi: 10.1200/JCO.2010.29.5865. [DOI] [PubMed] [Google Scholar]
- 13.Burstein HJ. Novel agents and future directions for refractory breast cancer. Semin Oncol. 2011;38(Suppl 2):S17–S24. doi: 10.1053/j.seminoncol.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–28. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 15.Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–37. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 16.Lee-Hoeflich ST, Crocker L, Yao E, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–87. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 17.Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive meta-static breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–44. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Portera CC, Walshe JM, Rosing DR, et al. Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. Clin Cancer Res. 2008;14:2710–6. doi: 10.1158/1078-0432.CCR-07-4636. [Erratum, Clin Cancer Res 2008; 14:3641.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianni L, Pienkowski T, Im Y-H, et al. Neoadjuvant pertuzumab (P) and trastuzumab (H): antitumor and safety analysis of a randomized phase II study (“NeoSphere”). Presented at the 33rd annual San Antonio Breast Cancer Symposium; San Antonio, TX. December 8–12, 2010; abstract. [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Carlson RW, Moench SJ, Hammond ME, et al. HER2 testing in breast cancer: NCCN Task Force report and recommendations. J Natl Compr Canc Netw. 2006;4(Suppl 3):S1–S22. [PubMed] [Google Scholar]
- 22.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 23.Valero V, Forbes J, Pegram MD, et al. Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified meta-static breast cancer (BCIRG 007 study): two highly active therapeutic regimens. J Clin Oncol. 2011;29:149–56. doi: 10.1200/JCO.2010.28.6450. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh AC, Moasser MM. Targeting HER proteins in cancer therapy and the role of the non-target HER3. Br J Cancer. 2007;97:453–7. doi: 10.1038/sj.bjc.6603910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soltoff SP, Carraway KL, III, Prigent SA, Gullick WG, Cantley LC. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol Cell Biol. 1994;14:3550–8. doi: 10.1128/mcb.14.6.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sliwkowski MX, Schaefer G, Akita RW, et al. Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J Biol Chem. 1994;269:14661–5. [PubMed] [Google Scholar]
- 27.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.