Dear Editor
ABC1Ks (activity of bc1 complex kinases) are atypical protein kinases that do not display many of the typical eukaryotic protein kinase features. Most ABC1K members have been demonstrated to localize in chloroplasts or mitochondria and regulation of quinone synthesis is the ancestral function of ABC1Ks (Lundquist et al., 2012a). The ABC1K gene family is evolutionarily conserved and has greatly expanded in photosynthetic organisms. In Arabidopsis, the ABC1K family consists of 17 members, eight of which are predicted to localize to chloroplasts. Six of these eight ABC1K members have been detected in the proteome of highly purified plastoglobules (PGs) in mass spectrometry analyses (Vidi et al., 2006; Ytterberg et al., 2006; Lundquist et al., 2012a, 2012b).
ABC1K1 and ABC1K3 are two PG-localized members of the ABC1K protein family. ABC1K1 (also named AtACDO1) is required for the stability of chlorophyll-binding proteins and prevents the accumulation of chlorophyll degradation products, which in turn serves adaptation to high-intensity light in plants (Yang et al., 2012; Lundquist et al., 2013). ABC1K1 may work in concert with its homolog ABC1K3 to phosphorylate and regulate tocopherol cyclase VTE1, a key enzyme in vitamin E synthesis and recycling in PG (Lundquist et al., 2013; Martinis et al., 2014). ABC1K1 and ABC1K3 may have non-redundant contributions to the light stress response since the abc1k1-1 abc1k3 double mutant displayed a synergistic effect under high light irradiance (Lundquist et al., 2013). However, the regulatory roles of ABC1K1 and ABC1K3 at the seedling stage are not fully understood; previous studies concerning ABC1Ks were undertaken only in adult rosette leaves. Here, we isolated abc1k1/bdr1 and abc1k3/rbd1 mutant alleles and described the seedling phenotype characteristics of ABC1K1 and ABC1K3 under continuous red light.
Plastids function not only as a biochemical factory for photosynthesis and other biochemical processes but also as a sensor for interaction between developmental and environmental cues (Xiao et al., 2012). To explore novel regulatory factors in the plastid signaling network, we screened an Arabidopsis T-DNA insertional mutagenized population (Zhang et al., 2010) for mutants that exhibited growth defects under continuous red light. One recessive mutant, designated as bleaching and dwarf in red light 1-1 (bdr1-1), was identified from this screening. The bdr1-1 mutant seedlings exhibited striking albino cotyledons (Supplemental Figure 1A) and paler mesophyll cells (Figure 1B). The content of chlorophylls and carotenoid was significantly reduced in the cotyledons of bdr1 mutant seedlings compared with the wild type (WT) (Supplemental Figure 2). However, more anthocyanin accumulated in bdr1 than in WT under red and blue light conditions (Supplemental Figure 2), although bdr1 displayed no visible phenotype under blue light. It should be noted that only the level of D1 protein, one of the four core subunits (D1, D2, CP43, and CP47) of photosystem II (PSII), was greatly reduced in bdr1, indicating that BDR1 might specifically affect protein stability or turnover of D1 protein (Figure 1C). Meanwhile, after exposure to red light for 5 days before being transferred to the soil, bdr1-1 mutant plants were dwarf and showed pale green rosette leaves (Supplemental Figure 1B), implying that the continuous red light irradiance at the seedling stage had prolonged effects on later adult morphogenesis of bdr1-1.
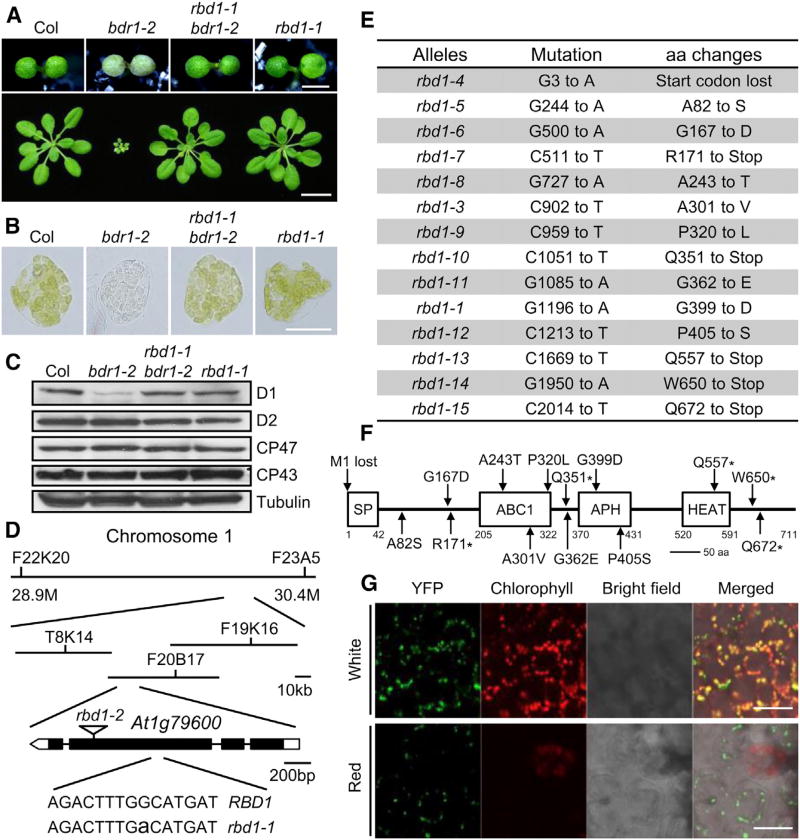
Figure 1. rbd1 is a Genetic Repressor of bdr1-2 and Subcellular Localization of RBD1.
(A) Upper panel. Phenotype of 7-day-old Col, bdr1-2, rbd1-1 bdr1-2, and rbd1-1 seedlings grown under continuous red light (50 µmol·m−2·s−1). Bars represent 1 mm. Lower panel. Morphology of Col, bdr1-2, rbd1-1 bdr1-2 and rbd1-1 plants on soil. The photograph shows soil-grown plants 2 weeks after planting. Bars represent 10 mm.
(B) Microscopic images representative of mesophyll cells separated from the first true leaves of Col, bdr1-2, rbd1-1 bdr1-2, and rbd1-1. Plants were grown under red light for 5 days and then transferred to continuous white light for 9 days. Bars represent 20 µm.
(C) Immunoblot analyses of the total proteins from the cotyledons of 7-day-old seedlings grown under continuous red light using anti-D1, -D2, -CP43, or -CP47 antibodies. An anti-tubulin immunoblot is shown below to indicate approximately equal loadings.
(D) Map-based cloning of RBD1 (At1g79600) and the mutation position of rbd1-1 and rbd1-2 (T-DNA insertion line, Salk_128696C).
(E) Mutations identified in the rbd1 alleles and the consequences of mutations to RBD1 protein. aa, amino acids.
(F) Protein structure of RBD1. SP, signal peptide; ABC1, Absence of bc1 complex; APH, amino-glycoside phosphotransferase; HEAT, Huntingtin, elongation factor 3 (EF3), protein phosphatase 2A (PP2A), and the yeast kinase TOR1; aa, amino acids. The positions of the amino acid affected in each rbd1 mutant are indicated.
(G) RBD1-YFP localization in cotyledons. From left panel to right panel: confocal YFP signals, chlorophyll autofluorescence signals, bright field image and merged image of bright field, chlorophyll autofluorescence, and YFP signals. Bars represent 20 µm.
We carried out thermal asymmetric interlaced PCR (TAIL-PCR) to amplify the genomic sequences flanking the T-DNA insertion site (Supplemental Figure 1C) and found that bdr1-1 contains a T-DNA inserted in the seventh exon of AT4G31390 (Supplemental Figure 1D). bdr1-2/abc1k1-1 (Salk_057147), a null mutant that carries a T-DNA in the 13th exon of AT4G31390, exhibited the same phenotypes as the bdr1-1 tested (Figure 1 and Supplemental Figure 1), indicating that a functional BDR1 gene is indeed necessary for proper red light growth. BDR1 encodes ABC1K1, a chloroplast member of the ABC1K protein family in Arabidopsis and comprises a putative chloroplast localization signal at the N terminus and an ABC1K kinase domain in the middle (Supplemental Figure 1E).
To identify additional components required for ABC1K1/BDR1 function, we performed a genetic screen to find mutations that suppress the bleaching and dwarf phenotype of bdr1-2 in red light. Mutants that have green cotyledons and can grow to normal plant size as WT were identified in the M2 population of ethyl methanesulfonate-mutagenized bdr1-2 mutant. A total of 60 mutants were identified as rbd (repressor of bdr1-2) mutants. rbd1-1 bdr1-2 is one of the most complete suppressor lines and the albino and dwarf phenotype observed in bdr1 mutants were suppressed in rbd1-1 bdr1-2 (Figure 1A). Meanwhile, the reduction of chlorophylls and carotenoid contents combined with the D1 protein level were all rescued to the WT levels (Figure 1C and Supplemental Figure 2). Furthermore, rbd1-1 could also rescue the phenotype of bdr1-1 to WT (Supplemental Figure 3A). Using map-based cloning, we found rbd1-1 bdr1-2 contained a single G to A nucleotide change in the coding region of At1g79600, resulting in an amino acid change from Gly399 to Asp (Figure 1D). At1g79600 also encodes an ABC1K atypical kinase, ABC1K3, one of the closest homologs of BDR1/ABC1K1 in Arabidopsis.
BDR1 and RBD1 share ~40% identity and 60% similarity at the amino acid level but RBD1 has an additional weak HEAT repeat domain (Huntingtin, elongation factor 3 [EF3], protein phosphatase 2A [PP2A], and the yeast kinase TOR1) at the C terminus, which forms rod-like helical structures that are involved in intracellular transport (Figure 1F). rbd1-2, a T-DNA insertion mutant (Salk_128696C) in the third exon of At1g79600, had no visible phenotype in red light and can suppress the albino phenotype of bdr1-1 and bdr1-2 to WT (Figure 1D and Supplemental Figure 3B).
To determine whether the other rbd mutants also contain mutations in At1g79600, we sequenced At1g79600 in the other 59 mutants and identified 13 additional alleles of rbd1 (Figure 1E). Most of these mutants were single lines, while rbd1-7 and rbd1-14 had two and four independent lines, respectively. Mutations resulting in amino acid changes happened frequently in the protein kinase domain. Five of these mutants (rbd1-7, rbd1-10, rbd1-13, rbd1-14, and rbd1-15 in the bdr1-2 mutant background) were introduced as early premature stop codons. Interestingly, protein short truncation in rbd1-14 and rbd1-15 (losing 61 and 40 amino acids at the C terminus, respectively) could disrupt the function of RBD1, implying this short C-terminal end had an important regulation effect (Figure 1E and 1F). There was also a G to A mutation in the start codon in rbd1-4 (ATG to ATA), which causes protein translation miss- or no starting. So far, no junction site mutations were found in all these rbd1 mutants. All rbd1 suppressor lines were backcrossed to Col to get rbd1 single mutants. All single mutations were recessive and could suppress the phenotype of bdr1-2. These data suggest that RBD1 is At1g79600.
As RBD1 has a signal peptide at the N terminus that is critical for its correct localization, we could only construct expression vectors of RBD1 tagged with fluorescence protein at the C-terminal end (YFP-HA-tag). Considering disrupting the C-terminal end of RBD1 may strongly affect its function; we also overexpressed native RBD1 in WT plants. But interestingly, all transgenic plants (both tagged and native RBD1) exhibited albino and dwarf phenotypes, suggesting that YFP-HA fused RBD1 could work as native RBD1. Two representative RBD1 overexpressors with RBD1-YFP-HA expressed at different levels were characterized in detail. As shown in Supplemental Figure 4, both line OX#1 and OX#2 seedlings had albino phenotype in red light, and line OX#1 was much smaller than the WT and line OX#2 at the adult stage (Supplemental Figure 4A and 4C). Line OX#1 also accumulated higher levels of RBD1-YFP content (Supplemental Figure 4D) compared with WT and line OX#2. In addition, the protein levels of D1 decreased in line OX#1 and line OX#2 compared with those in the WT (Supplemental Figure 4D). Accordingly, the pigment content and the diameter of adult plants also decreased in line OX#1 and line OX#2 (Supplemental Figure 4B and 4C). These data suggest that overexpressing RBD1 leads to photodamage of chloroplasts mimicking the bdr1-2 mutants under continuous red light and thus works oppositely with BDR1.
Transgenic plants expressing the RBD1-YFP-HA fusion protein were also used to determine the subcellular localization of RBD1. In the cotyledons of seedlings grown in white light, the fluorescence signal excited from RBD1-YFP merged exactly with the chlorophyll autofluorescence of mature chloroplasts (Figure 1G). Surprisingly, in the cotyledons of seedlings grown in continuous red light, YFP fluorescence was only observed on the chloroplasts losing chlorophyll autofluorescence (Figure 1G). Previous studies have demonstrated that ABC1K1/BDR1 localized in the chloroplasts using transient expression in adult Arabidopsis protoplasts (Yang et al., 2012). These data suggest that RBD1 localized in the chloroplasts but had unique actions in contrast to BDR1.
BDR1 and RBD1 are homologs of the ABC1K atypical kinase family but they work oppositely to regulate plant’s adapting to the light environments. This triggered us to examine the possible evolutionary relationship of these two genes. Based on the phylogenetic analysis, these 17 ABC1K proteins were divided into three clades: plastid clade, ancestral clade, and mitochondrial clade. Most members in the plastid clade were multiple introns containing genes except RBD1 (Supplemental Figure 5). Thus, RBD1 may be a young gene derived from a recent retrotransposition with novel function. In the typical eukaryotic protein kinases family, mitogen-activated protein kinase kinase kinase MEKK1 (At4g08500) and MEKK2 (At4g08480) are homologs but they also work oppositely in regulating the activation of plant innate immunity (Kong et al., 2012). This phenomenon happens accordingly in the atypical protein kinase family that predominates in prokaryotes. Future studies on other rbd mutants may help us to identify genes that function with ABC1K1/BDR1 and ABC1K3/RBD1, and lead to better understanding of how ABC1K1/BDR1 and ABC1K3/RBD1 regulate plant fitness to the environment.
Supplementary Material
Acknowledgments
We acknowledge the Arabidopsis Biological Resource Center for providing T-DNA insertion mutant seeds Salk_057147 and Salk_128696C.
FUNDING
This work was supported by grants from the National Basic Research Program of China (973 Program: 2012CB910900), the National Natural Science Foundation of China (31330048), and a grant from NIH (GM-47850), and Peking-Tsinghua Center for Life Sciences (to X.W.D), and State Key Laboratory of Protein and Plant Gene Research.
Footnotes
Supplemental Information is available at Molecular Plant Online.
No conflict of interest declared.
References
- Kong Q, Qu N, Gao M, Zhang Z, Ding X, Yang F, Li Y, Dong OX, Chen S, Li X, et al. The MEKK1-MKK1/MKK2-MPK4 kinase cascade negatively regulates immunity mediated by a mitogen-activated protein kinase kinase kinase in Arabidopsis. Plant Cell. 2012;24:2225–2236. doi: 10.1105/tpc.112.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist PK, Davis JI, van Wijk KJ. ABC1K atypical kinases in plants: filling the organellar kinase void. Trends Plant Sci. 2012a;17:546–555. doi: 10.1016/j.tplants.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist PK, Poliakov A, Bhuiyan NH, Zybailov B, Sun Q, van Wijk KJ. The functional network of the Arabidopsis plastoglobule proteome based on quantitative proteomics and genome-wide coexpression analysis. Plant Physiol. 2012b;158:1172–1192. doi: 10.1104/pp.111.193144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist PK, Poliakov A, Giacomelli L, Friso G, Appel M, McQuinn RP, Krasnoff SB, Rowland E, Ponnala L, Sun Q, et al. Loss of plastoglobule kinases ABC1K1 and ABC1K3 causes conditional degreening, modified prenyl-lipids, and recruitment of the jasmonic acid pathway. Plant Cell. 2013;25:1818–1839. doi: 10.1105/tpc.113.111120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinis J, Glauser G, Valimareanu S, Stettler M, Zeeman SC, Yamamoto H, Shikanai T, Kessler F. ABC1K1/PGR6 kinase: a regulatory link between photosynthetic activity and chloroplast metabolism. Plant J. 2014;77:269–283. doi: 10.1111/tpj.12385. [DOI] [PubMed] [Google Scholar]
- Vidi PA, Kanwischer M, Baginsky S, Austin JR, Csucs G, Dormann P, Kessler F, Brehelin C. Tocopherol cyclase (VTE1) localization and vitamin E accumulation in chloroplast plastoglobule lipoprotein particles. J. Biol. Chem. 2006;281:11225–11234. doi: 10.1074/jbc.M511939200. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Savchenko T, Baidoo EE, Chehab WE, Hayden DM, Tolstikov V, Corwin JA, Kliebenstein DJ, Keasling JD, Dehesh K. Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell. 2012;149:1525–1535. doi: 10.1016/j.cell.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Yang S, Zeng X, Li T, Liu M, Zhang S, Gao S, Wang Y, Peng C, Li L, Yang C. AtACDO1, an ABC1-like kinase gene, is involved in chlorophyll degradation and the response to photooxidative stress in Arabidopsis. J. Exp. Bot. 2012;63:3959–3973. doi: 10.1093/jxb/ers072. [DOI] [PubMed] [Google Scholar]
- Ytterberg AJ, Peltier JB, van Wijk KJ. Protein profiling of plastoglobules in chloroplasts and chromoplasts. A surprising site for differential accumulation of metabolic enzymes. Plant Physiol. 2006;140:984–997. doi: 10.1104/pp.105.076083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Guo H, Zhang J, Guo G, Schumaker KS, Guo Y. Arabidopsis cockayne syndrome A-like proteins 1A and 1B form a complex with CULLIN4 and damage DNA binding protein 1A and regulate the response to UV irradiation. Plant Cell. 2010;22:2353–2369. doi: 10.1105/tpc.110.073973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.