Summary
N6-methyladenosine (m6A) is the most abundant internal modification of mRNAs and is implicated in all aspects of post-transcriptional RNA metabolism. However, little is known about m6A modifications to circular (circ) RNAs. We developed a computational pipeline (AutoCirc) that together with depletion of ribosomal RNA and m6A immunoprecipitation defined thousands of m6A-circRNAs, with cell-type-specific expression. The presence of m6A-circRNAs is corroborated by interaction between circRNAs and YTHDF1/YTHDF2, proteins that read m6A sites in mRNAs, and by reduced m6A levels upon depletion of METTL3, the m6A writer. Despite sharing m6A readers and writers, m6A-circRNAs are frequently derived from exons that are not methylated in mRNAs, while mRNAs that are methylated on the same exons that compose m6A-circRNAs exhibit less stability, in a process regulated by YTHDF2. These results expand our understanding of the breadth of m6A modifications and uncover regulation of circRNAs through m6A modification.
Keywords: circular RNAs, m6A modification, noncoding RNAs, embryonic stem cells, METTL3, YTHDF2
Introduction
N6-methyladenosine (m6A) was the first identified mammalian internal messenger (m) RNA modification and remains the most abundant modification known on mRNAs and long noncoding (lnc) RNAs (Gilbert and Bell, 2016). A renewed interest in RNA modifications catalyzed by technological advances has revealed the widespread nature of m6A in eukaryotic cells from yeast to humans as well as its reversibility in mammalian cells (Dominissini et al., 2012; Jia et al., 2013; Meyer et al., 2012; Schwartz et al., 2013). The identification of proteins that act as “writers,” “readers,” and “erasers” of m6A, as well as recognition of other internal modifications such as 5-methylcytosine (Squires et al., 2012), N1-methyladenosine (Ozanick et al., 2005), and pseudouridine (Staehelin, 1971) have led to the field coined ‘epitranscriptomics’. m6A has been implicated in all aspects of post-transcriptional RNA metabolism including half-life, splicing, translational efficiency, nuclear export, and RNA structure (Lichinchi et al., 2006; Spitale et al., 2015; Wang et al., 2014, 2015).
The development of m6A location analyses utilizing anti-m6A antibodies coupled to RNA-sequencing after RNA fragmentation has revealed sites of m6A modifications located on thousands of mRNAs and hundreds of lncRNAs in numerous primary and transformed cells (Chen et al., 2015; Linder et al., 2015). Along with site and cell/tissue specificity, m6A modifications exhibit global enrichment in the 3′UTR near mRNA stop-codons and long internal exons, leading to unique m6A-derived transcriptome topology (Batista et al., 2014; Dominissini et al., 2012; Ke et al., 2015; Meyer et al., 2012). The writing of m6A is accomplished via an m6A methyltransferase complex composed of a core METTL3 and METTL14 heterodimer (Liu et al., 2014; Wang et al., 2016a, 2016b), and depletion of either METTL3 or METTL14 decreases m6A levels in mRNAs to a similar degree (Liu et al., 2014; Ping et al., 2014). Proteins containing the YTH domain directly bind m6A sites and act as readers of the m6A signal (Wang et al., 2014, 2015; Xiao et al., 2016). YTHDF2 proteins recruit m6A-modified mRNAs to nuclear p-bodies, promoting RNA degradation (Wang et al., 2014), while YTHDF1 promotes the translation of m6A-modified mRNAs through interaction with translation initiation machinery (Wang et al., 2015).
We asked how broadly the concept of an epitranscriptome extends from linear RNAs to circRNAs, which are created by the covalent linkage of the 3′ and 5′ ends of spliced RNA transcripts resulting in circularization (Salzman et al., 2012). These back splice events were initially described in mammalian cells as a source of scrambled exons (Nigro et al., 1991) before they were linked to circRNAs (Capel et al., 1993). Nearly two decades later, high-throughput sequencing of total RNAs depleted of ribosomal (r) RNAs revealed the abundance of circRNAs (Salzman et al., 2012). Subsequent studies suggested that circRNAs can interact with transcriptional machinery, cyclin-dependent kinases, and microRNAs (Du et al., 2016; Hansen et al., 2013; Memczak et al., 2013; Zhang et al., 2013) and are potential biomarkers for disease (Shang et al., 2016; Xuan et al., 2016). However, the extent to which circRNAs are marked by the same m6A modification found in mRNAs and lncRNAs is unclear.
Here we identify more than one thousand m6A-circRNAs in human embryonic stem cells (hESCs) and show that m6A-circRNAs are also abundant in HeLa cells. Comparison of m6A-circRNA maps between hESCs and HeLa cells reveals both common and cell-type-specific m6A-circRNA expression patterns. Surprisingly, a large percentage of m6A-circRNAs do not overlap with exons containing m6A peaks in mRNAs in both cell types. The m6A readers YTHDF1 and YTHDF2 interact with m6A-circRNAs, and the m6A writer METTL3 regulates m6A levels, suggesting that much of the same cellular machinery is shared between m6A modified circRNAs and mRNAs. Our analyses also uncovered an unexpected connection between m6A-circRNAs and mRNA half-life regulated by YTHDF2. These results expand our understanding of the breadth and regulatory aspects of m6A modifications through identification of the circRNA epitranscriptome.
Results
RNase R Resistant RNA Species are Modified by m6A
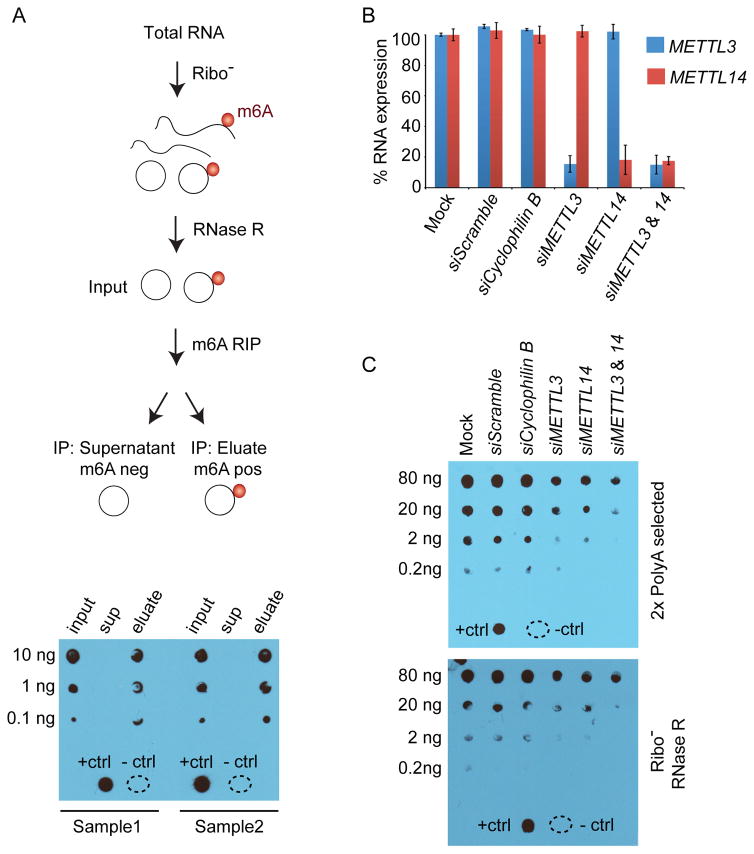
m6A modifications have been described in mRNAs and lncRNAs, and we wanted to determine if circRNAs may also be modified by m6A. We analyzed three fractions of hESC RNA for the presence of m6A modifications by anti-m6A dot blot after rRNA depletion and RNAse R digestion to degrade linear RNAs (Suzuki et al., 2006) (Figure 1). Bioanalyzer analysis was also performed to evaluate the RNA at each step in this process (Figure S1A). The input RNA (rRNA-depleted, RNAse R-treated) and eluate fraction (m6A-positive fraction following m6A RNA immunoprecipitation (RIP)) both contain m6A modifications, while the supernatant (m6A-negative fraction) shows no evidence of m6A (Figure 1A bottom). It is unlikely that the positive signal in the eluate fraction is due to tRNAs, which are also resistant to RNase R, because tRNAs are not modified by m6A in mammalian cells (Mishima et al., 2015). This conclusion is supported by the loss of the RNA peak around 75 nucleotides (nt) following m6A RIP (Figure S1A, eluate), which contains tRNAs (Holley et al., 1965). These results show that RNase R-resistant (nonlinear) RNAs contain a strong m6A signal, and suggest that circRNAs contained in this pool may be modified by m6A.
Figure 1. RNase R resistant RNA species are m6A-modified.
(A) The diagram describes how total RNA from hESCs was processed. Dot blots for m6A were performed for the indicated amount of RNA. RNA from input (after rRNA-depletion and RNase R treatment), supernatant (sup) and eluate were probed to detect the m6A modification for two replicates (Sample 1 and Sample 2). A positive control (+ctrl) and negative control (−ctrl) containing water are at the bottom of the blot. (B) 293T cells were transfected with siRNAs to deplete METTL3 and METTL14 as well as negative controls without siRNA (mock), with scrambled siRNA, and with siRNAs that deplete Cyclophilin B. Expression of METTL3 (blue) and METTL14 (red) was normalized to mock transfection. Error bars represent standard deviation. (C) Two rounds of polyadenylated RNA selection (top) were performed for each condition in (B). Decreasing amounts of RNA from each condition were probed to detect the m6A. Controls were performed as in (A). Total RNA was isolated from each condition in (B). RNA was rRNA-depleted and treated with RNase R to digest linear RNA (Ribo- RNase R). Decreasing amounts of RNA from each condition were probed to detect m6A in the fraction of RNAs enriched for circRNAs (bottom).
METTL3 and METTL14 are Required for m6A Modifications of Non-linear RNAs
METTL3 and METTL14 physically interact in a synergistic complex, which is required for m6A modification of polyadenylated (polyA) RNAs (Liu et al., 2014), and we asked if METTL3 and/or METTL14 are also required for m6A modification of non-linear RNAs. Depletion of METTL3 and/or METTL14 by small interfering (si) RNAs in HEK-293T cells (Figure 1B and S1B) resulted in reduced m6A methylation of polyA RNAs as expected (Figure 1C, top and S1C, top) (Batista et al., 2014; Liu et al., 2014). Analysis of rRNA-depleted and RNase R-digested RNA after depletion of METTL3 or METTL14 also demonstrated a reduction in m6A modification of non-linear (circRNA-enriched) RNA compared to negative controls, and this reduction was more pronounced upon combined METTL3/14 depletion. (Figure 1C, bottom and S1C, bottom). Together, these data show that there is an RNase R-resistant fraction of RNA that is dependent on METTL3/14 for m6A modification and suggest that circRNAs may be modified by the METTL3/14 complex.
Computational Pipeline to Identify CircRNAs
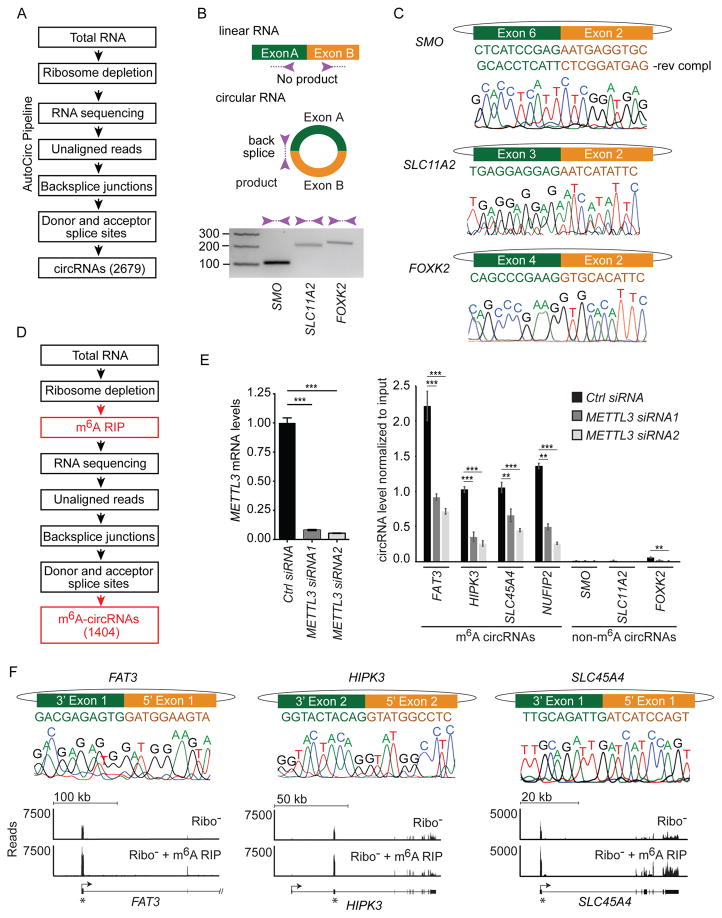
To test for the existence of m6A-modified circRNAs, we developed a computational pipeline (AutoCirc) to identify back splice junctions characteristic of circRNAs (Figure 2A, see Materials and Methods). AutoCirc identified 2,679 total circRNAs by the presence of at least two unique reads spanning a back splice in the union of biological replicates. We validated the presence of back splice junctions identified by AutoCirc with reverse transcription (RT)-PCR (Figure 2B) and Sanger sequencing (Figure 2C). The analyzed circRNAs were chosen to include examples from the most abundant (encoded by SMO), moderately abundant (encoded by SLC11A2), and least abundant (encoded by FO XK2) groups of circRNAs based on back splice counts (Supplemental Table S1).
Figure 2. A customized pipeline to identify circRNAs and m6A-modifiied circRNAs.
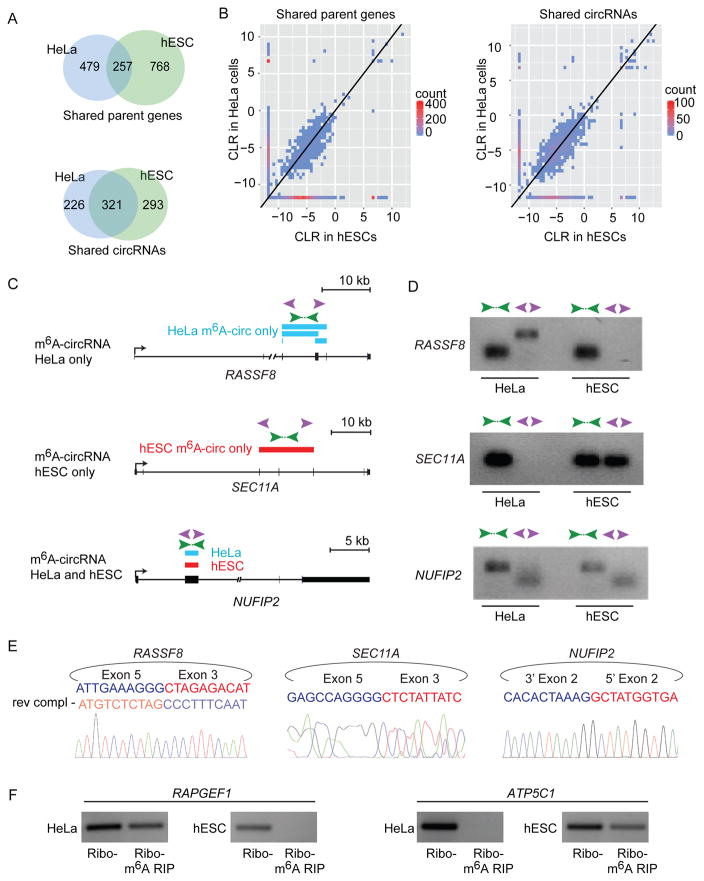
(A) AutoCirc pipeline to detect circRNAs from RNA-seq data. (B) RT-PCR validation of identified circRNAs. Divergent primers (purple arrows) only amplify across back splice junctions created in circRNAs. PCR was performed on total RNA after rRNA-depletion. Amplicon size is indicated on the left of the gel. (C) Sanger sequencing validation of circRNAs. The exons involved in the back splice junction and the predicted sequence are shown at the top for each transcript. Sanger sequencing results across each back splice junction is shown below each gene. (D) Application of AutoCirc to define m6A-circRNAs with new steps in red. (E) METTL3 expression was quantified by qRT-PCR in hESCs transfected with Control (Ctrl) siRNA and two siRNAs depleting METTL3 (left). Error bars represent standard deviation. *** indicates p<0.001. Detection of back splice junctions for circRNAs encoded by the indicated genes after m6A RIP is shown in the right. Black boxes represent hESCs transfected with Ctrl siRNA and the two shades of gray indicate hESCs transfected with siRNAs targeting METTL3 (right). m6A-circRNAs and non-m6A-circRNAs are indicated. Error bars represent standard deviation. *** indicates p< 0.0001 and ** indicates p< 0.001. Amplification of each back splice was tested for each sample prior to m6A-RIP (Figure S2G). (F) Sanger sequencing across the back splice forming m6A-circRNAs. The exons involved and predicted sequence is show at the top for each transcript. Sanger sequencing results across each back splice junction are shown in the middle. RNA-seq tracks are shown at the bottom for total RNA following rRNA depletion (Ribo-) and after rRNA depletion followed by m6A RIP. The gene structure is shown below the tracks. Arrows indicate the direction of transcription and asterisks indicate the exons that are circularized.
We then compared AutoCirc to CIRCexplorer and MapSplice, which have performed well in other studies (Hansen et al., 2015; Zhang et al., 2014). All three pipelines identify similar populations of circRNAs (2,679 by AutoCirc, 2,425 by CIRCexplorer and 2,704 by MapSplice) using a threshold of two unique reads spanning a back splice junction in our rRNA-depleted hESC RNA samples. Approximately 80% of the back splice junctions identified by CIRCexplorer were also identified by AutoCirc. The circRNAs identified by AutoCirc or CIRCexplorer each have a slightly smaller degree of overlap with the circRNAs identified by MapSplice than they do with each other (Figure S2A). 887 back splice junctions identified by MapSplice are unique compared to 254 for CIRCexplorer and 479 for AutoCirc. The increased number of unique back splice junctions identified by MapSplice is likely due to the use of the ENCODE gene annotation by MapSplice compared to RefSeq for CIRCexplorer and AutoCirc, as the ENCODE annotation contains a larger number of genes. As a negative control for circRNA detection, we analyzed polyA RNA from hESCs (Sigova et al., 2013), and all three pipelines identified a similar low frequency of back splice junctions (Figure S2B). AutoCirc is about 10 fold faster than CIRCexplorer and about 250 fold faster than MapSplice and consumes fewer computing resources (memory threads and number of processes) (Supplemental Table S2).
Identification of m6A-circRNAs in hESCs
To test for the existence of m6A-modified circRNAs in hESCs, we prepared libraries for RNA sequencing after rRNA depletion (input) followed by m6A RIP (eluate) (Figure S2C). To confirm the specificity of the m6A RIP, we added exogenous RNA spike-ins with and without m6A modifications to each RNA sample after rRNA-depletion. Only spike-ins containing m6A-modified RNAs were detected after m6A RIP (Figure S2D), consistent with our previous experience (Batista et al., 2014; Molinie et al., 2016).
We applied AutoCirc after m6A RIP and identified 1,404 m6A-circRNAs (Figure 2D). Fifty-four percent of m6A-circRNAs were contained in the pool of total circRNAs (Figure S2E), and this increased to 83% when we expanded the pool of total circRNAs by including additional hESC data sets (Figure S2F). We quantified expression of m6A-circRNAs verses those predicted not to be methylated by their presence in the input and absence in m6A RIP samples. We performed these experiments before and after depletion of METTL3 to assess for the requirement of METTL3 for m6A modifications in circRNAs (Figure 2E). m6A-circRNAs encoded by FAT3, HIPK3, and SLC45A4 were chosen due to their high abundance, and the m6A-circRNA encoded by NUFIP2 was chosen as an example of an m6A-circRNA with lower abundance (Supplemental Table S1). Black bars represent samples without depletion of METTL3 and show enrichment of m6A-circRNAs (encoded by FAT3, HI PK3, SLC45A4, and NUFIP2) after m6A RIP compared to non-m6A-circRNAs (encoded by SMO, SLC11A2, and FOXK2) (Figure 2E, right). Dark and light gray shading represent two independent siRNAs used to deplete METTL3 (Figure 2E, left). Depletion of METTL3 did not affect the total expression levels of circRNAs regardless of whether they were identified as m6A-circRNAs or non-m6A-circRNAs (Figure S2G). However, depletion of METTL3, was associated with a decrease in the level of m6A-circRNAs (Figure 2E, right), suggesting that METTL3 regulates m6A modification in circRNAs. The back splices that define each m6A-circRNA were confirmed by Sanger sequencing, and RNA-seq tracks show enrichment of the exons encoding circRNAs following m6A RIP (Figure 2F, S2H). These results establish a transcriptome-wide map of m6A-circRNAs in hESCs.
m6A Methylation is Enriched in CircRNAs Composed of Long Single Exons
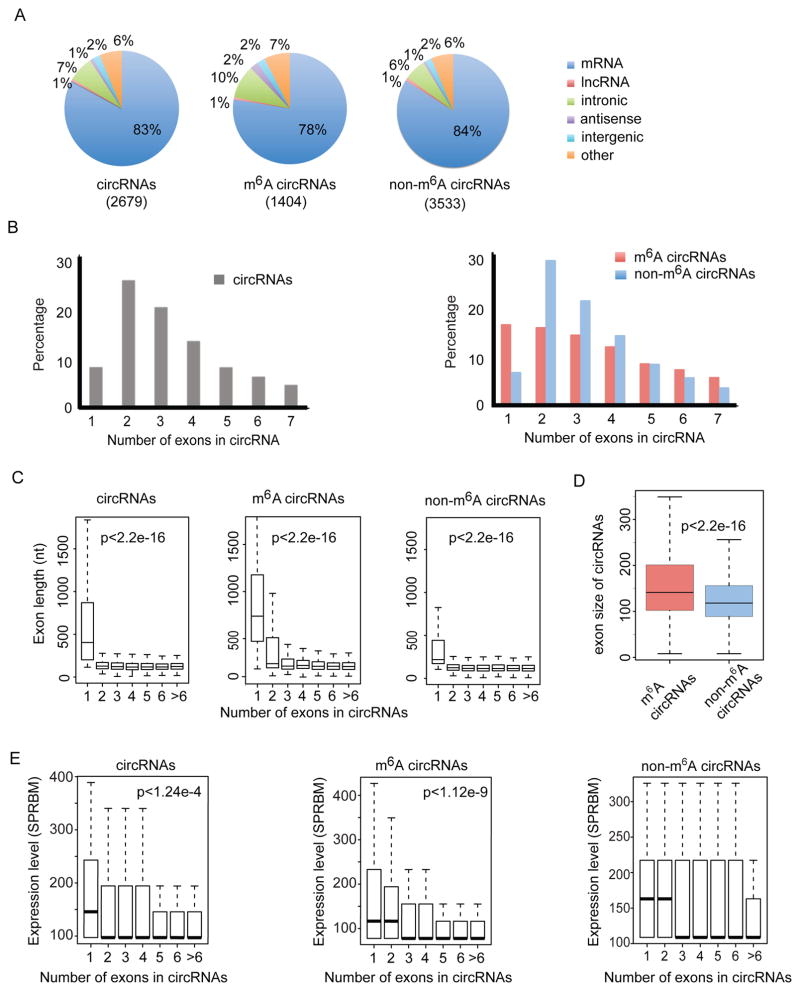
We next evaluated the features of m6A-circRNAs and non-m6A-circRNAs. We sequenced the m6A-depleted RNA (supernatant from m6A RIP) and applied AutoCirc (Figure 2A) to provide more stringent criteria to define the population of circRNAs enriched in non-m6A-circRNAs. We examined the distributions of genomic origins of total circRNAs identified from input, m6A-circRNAs identified from eluate, and non-m6A-circRNAs from supernatant. m6A-circRNAs and non-m6A-circRNAs show a similar genomic distribution; approximately 80% of circRNAs from both categories are derived from exons of protein-coding genes (Figure 3A). Parent genes for both m6A-circRNAs and non-m6A-circRNAs are also similarly enriched in the gene ontogeny (GO) categories of enzyme binding and transcription factor activities (Figure S3A). The majority of total circRNAs that originate from protein-coding genes span two or three exons (Figure 3B, left), whereas m6A-circRNAs are more commonly encoded by single exons compared to non-m6A-circRNAs (Figure 3B, right). The exons of single exon circRNAs tend to be longer than the exons of multi-exon circRNAs for all groups of circRNAs (Figure 3C). Furthermore, the lengths of all exons in m6A-circRNAs tend to be longer than those in non-m6A-circRNAs (Figure 3D). Single exon circRNAs are also more abundant than multiple-exon circRNAs in m6A-circRNAs compared to non-m6A-circRNAs (p<1.2e-9 in m6A-circRNAs vs p=0.52 in non-m6A-circRNAs, Figure 3E). Thus, m6A methylation is enriched in circRNAs composed of long single exons, which are more abundant than multi-exon m6A-circRNAs.
Figure 3. CircRNAs are frequently methylated in human embryonic stem cells.
(A) Genomic distribution of total circRNAs (input, left), m6A-circRNAs (eluate, center), and non-m6A-circRNAs (supernatant, right). The total number of circRNAs identified in each condition is shown in parenthesis. (B) The percentage of circRNAs (y-axis) was calculated based on the number of exons each circRNA spans (x-axis) for input circRNAs (left), m6A-circRNAs (red, right panel) and non-m6A-circRNAs (blue, right panel). The number of exons up to seven is displayed. (C) The distributions of exon length (y-axis) for input circRNAs (left), m6A-circRNAs (middle) and non-m6A-circRNAs (right) are plotted based on the number of exons spanned by each circRNA (x-axis). (D) Comparison of exon size of m6A-circRNAs and non-m6A-circRNAs. (E) Expression levels (y-axis) for input circRNAs, m6A-circRNAs, and non-m6A-circRNAs are plotted based on the number of exons spanned by each circRNA (x-axis). P values indicate that single exon circRNAs are more abundant than circRNAs composed of more than one exon.
m6A-circRNAs Exhibit Distinct Patterns of m6A Modifications Compared to mRNAs
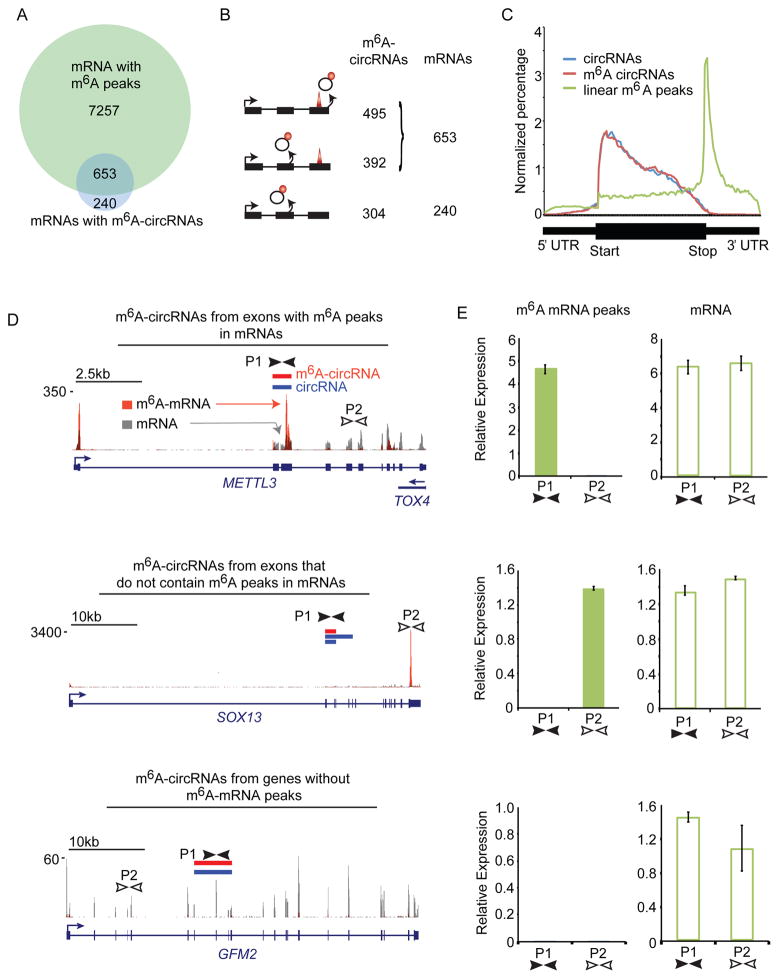
m6A sites in mRNAs are most common in the last exon (Meyer et al., 2012); however, circularization of the last exon of genes is uncommon (Zhang et al., 2014). We found that 73% of parent genes of m6A-circRNAs also encode m6A-mRNAs in hESCs (Figure 4A). We then examined if exons methylated in mRNAs are the same exons that form m6A-circRNAs. Surprisingly, the majority (59%) of m6A-circRNAs were produced from exons without m6A peaks in mRNAs (Figure 4B). Thirty-three percent of m6A-circRNAs were produced from genes that encode m6A-mRNAs methylated on different exons, and 26% of m6A-circRNAs were produced from genes that encode mRNAs without detectable m6A modification. This observation is also reflected in the different distributions of m6A-circRNAs and m6A peaks in mRNAs across genes (Figure 4C). These results suggest a different set of rules may govern m6A biogenesis in circRNAs.
Figure 4. m6A-circRNAs are often methylated in regions where m6A-mRNAs are not methylated.
(A) Venn diagram showing the overlap between genes encoding m6A-circRNAs and genes encoding m6A-mRNAs. The total number of genes in each category is shown. (B) Distribution of m6A-modified exons between circRNAs and mRNAs: m6A sites in the same exons (top); m6A sites are on different exons from the same parent gene (middle); m6A sites are only present on circRNAs (bottom). The total number of m6A-circRNAs and mRNAs in each category are shown. (C) Distribution of exons encoding m6A-circRNAs (red) and total circRNAs (blue) across genes compared to the distribution of m6A peaks in mRNAs across genes (green). The region from the transcription start site (TSS) to the start of the coding sequence (start) represents the 5′ UTR and the region from the end of the coding sequence (Stop) to the 3′ end represents the 3′ UTR. (D) Examples of genes described in (B). Red bars indicate m6A-circRNAs detected by sequencing. Blue bars represent circRNAs identified by sequencing. Tracks below the circRNAs represent m6A peaks from polyadenylated mRNAs before and after m6A RIP (Batista et al., 2014). Gray peaks represent mRNA background levels, and orange peaks represent sites of m6A modification in mRNAs. The locations of primer sets for each transcript are indicated by black and white arrows (P1 and P2). (P1, P2) (E) qRT-PCR was performed on cDNA following polyA selection and m6A RIP (m6A mRNA peaks) and following polyA selection alone (mRNA) for the genes in (D).
We performed qRT-PCR on fragmented, polyA-selected RNA before and after m6A RIP to confirm the m6A status of linear transcripts that also encode circRNAs. The first category represents genes including METTL3 and ZNF398 that encode m6A-circRNAs from exons that also have an m6A peak in mRNAs. We confirmed the presence of m6A-circRNAs encoded by METTL3 and ZNF398 by RT-PCR (Figure S4A). Primers were designed to detect the m6A mRNA peak within the exons encoding the m6A-circRNA (primers P1) and to detect a region of the mRNA without m6A enrichment (primers P2) (Figures 4D, top and S4B, top). After m6A RIP, we detected amplification with P1 but not P2 primers (Figures 4E and S4C, top-left). P1 and P2 primers both amplified fragmented mRNA confirming the presence of both exons in polyA-selected RNA (Figures 4E and S4C, top-right). The second category of genes, including SO X13 and ARHG EF19, encode m6A-circRNAs from exons different from those containing m6A peaks in mRNAs. Primers P1 amplify RNA from exons encoding the m6A-circRNA, and primers P2 amplify the m6A-mRNA peak defined by sequencing (Figures 4D and S4B, middle). After m6A RIP, we detected amplification of with P2 but not P1 primers, consistent with different sites of m6A modifications between m6A circRNAs and m6A mRNAs encoded by the same genes. (Figures 4E and S4C, middle). Both primer sets amplified mRNA before m6A RIP (Figures 4E and S4C, middle-right). The third category of genes, including G FM2 and MAPKAP1, encode m6A-circRNAs from genes that do not encode m6A mRNAs. We did not detect m6A modifications in mRNAs within the exons encoding m6A-circRNAs (P1) or other regions (P2), (Figures 4D and S4B, bottom), but both primer sets amplified mRNA (Figures 4E and S4C, bottom). These results validate the finding that numerous m6A-circRNAs are generated from exons that do not contain m6A peaks in mRNAs.
CircRNAs Exhibit Unique Patterns of m6A Methylation in Different Cell Types
To determine if m6A modifications in hESCs were representative of mammalian cells in general, we repeated the sequencing analysis in HeLa cells. We identified 854 circRNAs from input (rRNA-depletion), 987 m6A-circRNAs from eluate (rRNA-depletion, m6A RIP), and 899 non-m6A-circRNAs from supernatant after m6A RIP (Figure S5A). The genomic distribution, exon length, and number of exons in m6A-circRNAs and non-m6A-circRNAs are similar between hESCs and HeLa cells (Figures S5A–C). Similar to m6A-circRNAs in hESCs, half of the m6A-circRNAs identified in HeLa cells originate from exons that do not contain the m6A modification in mRNAs (Figure S5D).
More than half of the m6A-circRNAs detected in HeLa cells were not detected in hESCs (Figure S5E), suggesting that many m6A-circRNAs are uniquely expressed in the two cell types. HeLa cells and hESCs do not express all of the same genes or circRNAs, so we asked if the differences in m6A-circRNAs between HeLa cells and hESCs could be explained by differences in gene or circRNA expression. Sixty-five percent of m6A-circRNAs detected in HeLa cells were not detected in hESCs even when the parent genes of these circRNAs are expressed in both cell types (Figure 5A, top). Furthermore, 41% of m6A-circRNAs detected in HeLa cells were not detected in hESCs even when circRNAs are expressed in both cell types (Figure 5A, bottom). HeLa cells and hESCs produce circRNAs from a small number of parent genes that do not express detectable mRNAs, which explains why there are more common m6A-circRNAs between HeLa cells and hESCs among the shared circRNAs group (Figure 5A, bottom) compared to the shared parent genes group (Figure 5A, top). When m6A-circRNAs are expressed in both cell types, they tend to be expressed at similar levels (Figure 5B and S5F).
Figure 5. m6A-circRNAs show cell-type-specific patterns of expression.
(A) Venn diagram shows the overlap of m6A-circRNAs encoded by genes expressed in HeLa cells and hESCs with RPKM > 1 in both cell types (Shared parent genes, top). The overlap between m6A-circRNAs among circRNAs that are detected in both HeLa cells and hESCs is shown below (Shared circRNAs). (B) Two-dimensional histograms comparing the expression levels of m6A-circRNAs in HeLa cells and hESCs. m6A-circRNAs in the left panel are encoded by genes that are expressed in both HeLa cells and hESCs (Shared parent genes). m6A-circRNAs in the right panel show m6A-circRNA levels for all circRNAs common in both HeLa cells and hESCs (Shared circRNAs). CircRNA counts are indicated on the scale to the right of each plot. m6A-circRNA expression levels are calculated by the circular-to-linear ratio (CLR). (C) Examples of m6A-circRNAs that that are unique to HeLa cells (top), unique to hESCs (middle), and common between HeLa cells and hESCs (bottom). Blue rectangles indicated m6A-circRNAs identified in HeLa cells, and red rectangles indicate m6A-circRNAs identified in hESCs. Green arrows indicate the location of primers that amplify across forward splice junctions and purple arrows indicate primers that amplify across back splices. (D) RT-PCR was performed on RNA prepared following rRNA depletion in HeLa cells and hESC cells, respectively. (E) Sanger sequencing was performed on PCR products generated by amplifying across back splice junctions for the indicated genes. Sequencing across the junction is shown in sense for SEC11A and NUFIP2 and antisense (rev compl) for RASSF8. (F) RT-PCR was performed on rRNA-depleted RNA (Ribo-) and after m6A RIP (Ribo-, m6A RIP) in HeLa cells and hESCs, respectively.
RT-PCR confirmed that unique m6A-circRNAs could be detected in the two different cell types. RASSF8 and KANK1 are expressed in both HeLa cells and hESCs, yet circRNAs from these two genes are detected only in HeLa cells, where they are m6A-modified (Figure 5C, 5D, top and S5G, S5H, top). In contrast, SEC11A and TMEFF1 are expressed in both HeLa cells and hESCs, but circRNAs are only detected in hESCs, where they are m6A-modified (Figure 5C, 5D, center and S5G, S5H, center). CircRNAs encoded by KIF20B and NUFIP2 are detected in both HeLa cells and hESCs and are both modified by m6A based on m6A RIP seq (Figure 5C, 5D, bottom and S5G, S5H bottom). Sanger sequencing confirmed the predicted back splice junctions for circRNAs encoded by RASSF8, SEC11A, and NUFIP2 (Figure 5E). Furthermore, circRNAs encoded by RAPGEF1 and ATP5C1 are detected in both HeLa cells and hESCs, but are only detected after m6A RIP in HeLa cells (Figure 5F, left) and hESCs (Figure 5F, right), respectively. These results show that many m6A-circRNAs are expressed in a cell-type-specific manner.
m6A-circRNA Levels
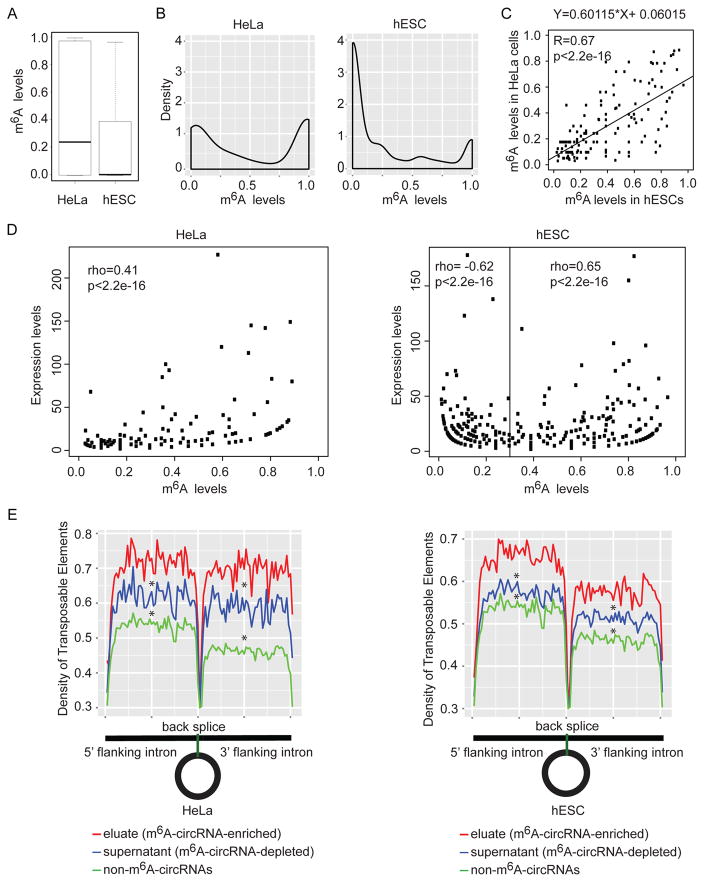
We next evaluated m6A levels in circRNAs in HeLa cells and hESCs. We quantified the m6A level for each circRNA by RNA abundance, defined as eluate/(eluate+supernatant) where eluate and supernatant samples were normalized using synthetic ERCC control RNAs (Molinie et al., 2016) (Figure S6A). The median methylation levels of circRNAs are reduced in hESCs compared to HeLa cells (Figure 6A) due to an enrichment of unmethylated or lowly methylated circRNAs in hESCs compared to HeLa cells (Figure 6B). Because the majority of unmethylated or lowly methylated circRNAs are only detected in supernatant samples, we also measured the distribution of m6A levels for circRNAs detected in both eluate and supernatant. These circRNAs follow an almost bimodal distribution where most circRNAs exhibit less than 50% methylation (Figure S6B), similar to the distribution observed in mRNAs (Molinie et al., 2016). The overall m6A-levels in circRNAs are higher in hESCs than in HeLa cells when restricting analysis to circRNAs detectable in both eluate and supernatant (Figure 6C). These results show that there are variable levels of m6A-modification across circRNA species in a distribution similar to mRNAs.
Figure 6. m6A levels.
(A) Comparison of m6A levels in circRNAs in HeLa cells and hESCs with at least 2 reads supporting the back splice junctions in eluate and supernatant data. (B) Density distributions of m6A levels in circRNAs using the same criteria as in (B). (C) Scatter plot showing the linear correlations of m6A levels among the m6A-circRNAs identified in both HeLa cells and hESCs. The linear regression fit equation is indicated in the top. The Pearson correlation coefficient and p-value are indicated at the top left corner. (D) The relationship between expression levels of circRNAs and m6A levels of circRNAs in HeLA cells (left) and hESCs (right). Expression levels are represented by back splice read counts of circRNAs in eluate and supernatant data. (E) Density distribution of transposable elements (TEs) flanking circRNAs. Eluate (red) contains circRNAs identified by m6A RIP (m6A-circRNA-enriched). Supernatant (blue) contains circRNAs not precipitated by m6A RIP (m6A-circRNA-depleted). Non-m6A-circRNAs (green) contains circRNAs identified in either HeLa or hESC supernatant that were not detected in either HeLa or hESC eluates. * indicates p<2.2e-16 compared to eluate.
m6A Levels and CircRNA Expression
m6A promotes mRNA degradation (Wang et al., 2014), and m6A levels are inversely correlated with steady-state mRNA expression levels (Molinie et al., 2016). We asked how m6A-levels are related to expression levels of circRNAs. In HeLa cells, m6A-levels in circRNAs are positively correlated with the expression levels of circRNAs (Spearman correlation rho=0.41, p<2.2e-16), and in hESCs, m6A-levels in circRNAs are positively correlated with the expression levels of circRNAs with m6A levels > 0.3 (Spearman correlation rho=0.65, p<2.2e-16, Figure 6D). We did observe a negative correlation with circRNA expression for low m6A levels (<0.3) in hESCs (Spearman correlation rho= - 0.62, p<2.2e-16). These results suggest that at low levels of m6A-modification, an increase in m6A level can be associated with reduced circRNA expression in some conditions, but at m6A levels over 0.3, increasing levels of m6A-modification is not linked to decreased circRNA expression.
Transposable Elements are Enriched in the Flanking Regions of m6A-circRNAs
Reverse complementary sequence in transposable elements (TEs) in the flanking regions of circRNAs promote the formation of circRNAs (Ashwal-Fluss et al., 2014; Chen et al., 2017; Liang and Wilusz, 2014; Zhang et al., 2014). We asked if there were characteristics of TEs in the flanking regions of circRNAs that are associated with m6A modification. We found that TEs are significantly enriched in both the 5′ and 3′ flanking regions of m6A-circRNAs (eluate) compared to circRNA pools depleted of m6A (supernatant). The population of m6A-circRNAs changes between cell types (Figure 5A and B), so we also analyzed the density of flanking TEs in the group of circRNAs that do not have evidence of m6A modification in either HeLa cells or hESCs. These circRNAs have the lowest density of flanking TEs (Figure 6E). These results suggest that the density of TEs flanking circRNAs may be linked to formation of m6A-circRNAs.
m6A-circRNAs are recognized by YTHDF1 and YTHDF2
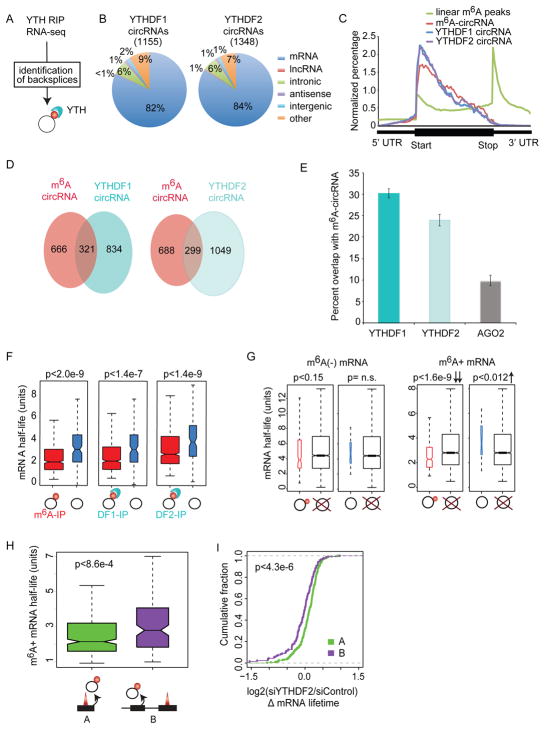
YTH-domain family member 1 (YTHDF1) recognizes m6A-mRNAs and promotes translation (Wang et al., 2015), while YTHDF2 forms a complex with m6A-mRNAs to target RNAs to decay sites (Wang et al., 2014). We asked if the YTH domain that recognizes m6A-mRNAs also recognizes m6A-circRNAs. We re-analyzed YTHDF1 and YTHDF2 RIP-seq data (Wang et al., 2014, 2015) using AutoCirc (Figure 2A) to identify circRNAs bound by YTHDF1 and YTHDF2 (Figure 7A). We identified 1,155 circRNAs interacting with YTHDF1 and 1,348 circRNAs interacting with YTHDF2 (Figure 7B). These circRNAs show a similar distribution across the genome to that of m6A-circRNAs (Figure 7B and Figure S5A) and are generated from the same categories of genes by GO analysis (Figure S7A). In addition, the circRNAs that interact with YTHDF1 and YTHDF2 are formed primarily from exons immediately downstream of the start of coding regions (Figure 7C). Twenty-eight percent and 22% of the YTHDF1 and YTHDF2 bound circRNAs, respectively, were also identified as m6A-circRNAs in HeLa cells (Figure 7D), and 51% of circRNAs interacting with YTHDF1 also interact with YTHDF2 (Figure S7B). To evaluate the possibility that circRNAs may interact with proteins independent of m6A modifications, we analyzed RIP-seq data for AGO2 (Polioudakis et al., 2015), a protein which is not known to bind m6A. Analyses are normalized to sequencing depth, and show that YTHDF1 and YTHDF2-bound circRNAs are significantly enriched in m6A-circRNAs compared to AGO2-bound circRNAs (p<0.0041) (Figure 7E).
Figure 7. m6A-circRNAs bind YTHDF1 and YTHDF2 and identify transcripts with shorter half-lives.
(A) Data from YTHDF1 and YTHDF2 RIP-seq (Wang et al., 2014, 2015) were used to identify m6A-circRNAs in HeLa cells. (B) The genomic distribution of circRNAs bound by YTHDF1 and YTHDF2 are shown. The number of circRNAs associated with each protein is indicated in parenthesis. (C) The distribution of exons encoding circRNAs associated with YTHDF1 (blue) and YTHDF2 (purple) compared to exons encoding m6A-circRNAs (red) and the distribution of m6A peaks across mRNAs (green) is shown. (D) Venn diagram shows the overlap between m6A-circRNAs identified in HeLa cells and m6A-circRNAs bound by YTHDF1 (left) and YTHDF2 (right). (E) The percentage of m6A-circRNAs identified by m6A RIP in HeLa cells and bound by YTHDF1, YTHDF2, or AGO2 are shown. Error bars represent standard deviation. (F) mRNA half-life for parent genes that encode m6A-circRNAs (left), YTHDF1-bound (DF1-IP) circRNAs (middle), or YTHDF2-bound (DF2-IP) circRNAs (right). Black rings represent circRNAs, red circles indicate m6A modification, and light blue structures represent YTH proteins. (G) The mRNA half-life for m6A negative (m6A (−) mRNA) (left) and m6A positive mRNAs (m6A (+) mRNA) (right) whose parent genes encode m6A-circRNAs (black ring with red circle attached), circRNAs without m6A (black ring), or no circRNAs (black ring with red “X”). p values are indicated above each plot. (H) The half-life of m6A-mRNAs whose parent genes also encode m6A-circRNAs from the same exon(s) where an m6A peak is found in mRNA (labeled A) is compared to the half-life of m6A-mRNA whose parent gene encodes an m6A circRNA at exon(s) where no m6A peak is found in mRNAs (labeled B). (I) The change in half-life (log2(siYTHDF2/siControl)) was calculated after depletion of YTHDF2 as described (Wang et al., 2014) for condition A (green) and condition B (purple). The p values were calculated using Wilcoxon-Mann-Whitney test. Figure S6 shows the second replicate.
m6A-circRNAs are Linked to mRNA Stability by YTHDF2
YTHDF2 interacts with m6A-mRNAs to regulate mRNA stability (Wang et al., 2014), and mRNAs encoded by the parent genes of m6A-circRNAs also have a shorter half-life than mRNAs encoded by the parent genes of non-m6A-circRNAs (Figures 7F and S7C). This finding is observed for m6A-circRNAs identified by m6A RIP or interaction with YTHDF1/YTHDF2, regardless of whether the mRNA contains an m6A modification. We then performed the same analysis but separated m6A negative (m6A(−)) from m6A positive (m6A(+)) mRNAs (Figure 7G and S7D). This analysis showed that m6A-mRNAs encoded by the parent genes of m6A-circRNAs are the only group with significantly reduced half-lives compared to genes not encoding any circRNAs. These results suggest that in addition to the m6A modification of mRNAs being associated with a shorter mRNA half-life (Wang et al., 2014), those m6A-mRNAs encoded by the parent genes of m6A-circRNAs have shorter half-lives among all m6A-mRNAs. Genes encoding both m6A-mRNAs and m6A-circRNAs can produce these transcripts from the same (Figure 7H, diagram A) or different exons (diagram B). The m6A-mRNAs with the shortest half-lives are those in which m6A-circRNAs are produced from the same exons that have m6A modifications in mRNAs (Figure 7H and S7E). m6A is most commonly enriched in the 3′ UTR of mRNAs (Batista et al., 2014; Dominissini et al., 2012; Ke et al., 2015; Meyer et al., 2012), while circRNAs are enriched in the gene body (Figure 4C and 7C). We examined if differences in the location of the m6A modification in mRNAs could explain the difference in half-lives and found no significant difference in the half-lives of m6A-mRNAs that are modified in the gene body or 3′ UTR (Figure S7F). Finally, the half-lives of m6A-mRNAs that are methylated in the same exons that are methylated in circRNAs increase with depletion of YTHDF2 (Figures 7I and S7G), suggesting that stability of this subset of RNAs is controlled by YTHDF2 in a process that may involve recognition of m6A-circRNAs.
Discussion
This study brings together two rapidly expanding fields: RNA modifications and circRNAs. Here, we present transcriptome-wide identification of m6A-circRNAs, extending the concept of the RNA epitranscriptome to circRNAs. We provide evidence that m6A modifications in circRNAs are written and read by the same machinery (METTL3/14, YTH proteins) used for mRNAs, but often at different locations. We implicate m6A-circRNAs in mRNA stability mediated by YTHDF2, but m6A does not appear to promote degradation of circRNAs as it does for mRNAs. Furthermore, we identify many m6A-circRNAs expressed in a cell-type-specific pattern even when their parental genes (or circRNAs) are expressed in both cell types, suggesting that the m6A modification of circRNAs may regulate different biological processes in different cell types. Our results establish a fertile area of investigation to define the breadth and function of covalent modifications in circRNAs.
The discovery of m6A-circRNAs raises many questions that will need to be addressed in future studies, including the significance of m6A-modifications on exons that compose circRNAs but are not modified in mRNAs. Nevertheless, our results show that identification of m6A-circRNA patterns may be useful to identify different cell types/states even in the absence of significant changes in baseline mRNA expression, leading to methodologies to fingerprint cells. Furthermore, it will be of interest to address whether m6A-circRNAs are found in extracellular vesicles, which have been proposed as a mechanism to clear circRNAs from cells (Lasda and Parker, 2016).
In terms of m6A-circRNA functionality, we provide evidence of cross-talk between m6A-modified mRNAs and circRNAs that affects mRNA half-life in a YTHDF2-dependent manner. However, it is unclear if recognition of m6A-circRNAs by YTHDF2 plays a direct role. One potential model is that m6A-circRNAs and m6A-mRNAs encoded by the same exons are bundled as part of a chromatin-associated liquid phase transition leading to a nuclear “liquid droplet” (Caudron-Herger et al., 2016; Marzahn et al., 2016) and continue to be a topologically and organizationally distinct information packets. Whereas m6A-circRNAs arising from non-methylated exons of mRNAs may not be bundled with mRNAs or are contained in other bundles. It is possible that these information packet(s) are transmitted to the cytosol leading to differential mRNA processing via interaction with cytosolic liquid droplets, that may include m6A binding to YTH domain proteins, which harbor poly Q unstructured domains (Guo and Shorter, 2015; Wang et al., 2014; Zhang et al., 2015). We postulate that circRNAs in general may exhibit unique tuning qualities on liquid droplets, affecting surface tension, stability, size and/or longevity. m6A-circRNAs may further modify these characteristics given their ability to interact with YTH proteins as well as other RNA binding proteins (Guo and Shorter, 2015; Lin et al., 2015).
Controlling the state of m6A modifications in circRNAs may act as switches to control circRNA functionality. For example, the presence of m6A modifications can promote the translation of m6A-circRNAs (Yang et al., 2017). This study focused on m6A modifications located near back splice junctions, which were identified by sequencing circRNAs that underwent m6A RIP after fragmentation. This approach yielded fewer circRNAs compared to our analysis because we performed m6A RIP on un-fragmented circRNAs to identify the abundance of m6A-circRNAs. CircRNAs can also be engineered to be translatable with internal ribosome entry sites (Chen and Sarnow, 1995; Wang and Wang, 2015), and a few circRNAs are found to be translated into peptides in a splice-dependent/cap-independent manner (Legnini et al., 2017; Pamudurti et al., 2017). m6A modification is also implicated in the splicing of mRNAs (Liu et al., 2015), and m6A modification could be involved in alternative splicing of some circRNAs.
Many m6A-circRNAs originate from exons where m6A modifications are absent on mRNAs, indicating that while the writing and reading machinery of m6A modifications are similar in both mRNAs and circRNAs, different patterns of m6A modifications are produced in different types of RNA. How do we reconcile the presence of m6A-circRNAs arising from exons that are non-methylated in polyA-selected mRNA species? The locations of m6A modifications in nascent pre-mRNAs and mRNAs are highly conserved, suggesting that m6A modifications in mRNAs occur by the time nascent pre-mRNA is formed (Ke et al., 2017). These conclusions, together with our findings that m6A-circRNAs are frequently modified in exons that are not m6A-modified in mRNAs suggest that some m6A modifications to circRNAs may occur during or after circRNA formation. TEs are present in the flanking regions of circRNAs to facilitate the formation of stem loops during back splicing events (Ashwal-Fluss et al., 2014; Liang and Wilusz, 2014; Zhang et al., 2014), METTL3/14 have been shown to bind TEs (Kelley et al., 2014), and TEs are enriched in the flanking regions of m6A-circRNAs compared to non-m6A-circRNAs (Figure 6E). These findings support a model in which methylation of some circRNAs is linked to circRNA formation.
Experimental Procedures
RNA isolation, m6A immunoprecipitation, and library preparation
Total RNA was obtained by TRIzol extraction followed by DNAse I treatment prior to rRNA depletion. RNase R treatment (5 units per μg RNA) was performed in duplicate with 5 μg of rRNA-depleted RNA input. m6A RIP was performed using an anti-m6A antibody (Synaptic Systems # 202 003). PolyA RNA selection was performed twice using the Dynabeads mRNA Purification Kit with 7.5 μg of total RNA input. 100 ng of RNA was used for library construction.
Computational pipeline for detecting circRNAs
We performed directional, 100 × 100 paired-end sequencing to define circRNAs. Paired reads were treated independently and mapped to the human reference genome (hg19). We used Bowtie2 (Langmead and Salzberg, 2012) to identify and discard all sequences that mapped to a contiguous region of genomic DNA. We developed AutoCirc to scan 20 nucleotides at both ends of each 100 nt sequence of unmapped reads to identify sequences that contain back splice junctions of circRNAs (see Supplemental Materials and Methods for details). AutoCirc is available at https://github.com/chanzhou/AutoCirc.
Identification of circRNAs interacting with YTHDF1, YTHDF2, and AGO2
We applied the AutoCirc pipeline to identify circRNAs from YTHDF1 (GSE63591), YTHDF2 (GSE49339) and AGO2 RIP (GSE64615) in HeLa cells. We counted the circRNAs with a single read or greater as present in a replicate as long as there were at least two reads supporting a specific back splice in the union of all replicates.
Evaluation of mRNA half-life
We obtained mRNA half-life data from siControl and siYTHDF2 in HeLa cells (Wang et al., 2014). We separated the mRNAs into groups as described. mRNAs produced by genes that did not produce circRNAs were used as a control group. To examine how the interaction between YTHDF2 and m6A-circRNAs affects m6A-mRNA half-life, we plotted the accumulation fraction curve of the log2-transformed changed half-life between siYTHDF2 and siControl cells for m6A-mRNAs methylated in the same exons as m6A-circRNAs and m6A-mRNAs methylated in different exons from m6A-circRNAs encoded by the same gene.
Data access
The accession number for RNA-seq data produced for this study is GEO: GSE85324.
Supplementary Material
Figure S1, related to Figure 1: RNase R resistant RNA species are m6A modified.
(A) Bioanalyzer analysis of RNA samples processed for dot blot (Figure 1A). RIN scores are indicated for total RNA for each replicate. Eluate refers to RNA precipitated by m6A RIP and supernatant is the RNA pool that was not precipitated by m6A RIP.
(B) 293T cells were transfected with siRNAs that target METTL3 and METTL14 as well as negative controls without siRNA (mock), with scrambled siRNA and siRNAs that target Cyclophilin B. RNA expression of METTL3 (blue) and METTL14 (red) was normalized to mock transfection. Error bars represent standard deviation. Data shown are biological replicates of Figure 1C.
(C) Two rounds of polyadenylated RNA selection (top) were performed for each siRNA condition in (C). Decreasing amounts of RNA from each condition was probed to detect the m6A modification. Total RNA was isolated from each siRNA condition (bottom). The RNA was depleted of rRNA and treated with RNase R to digest linear RNAs. Decreasing amounts of RNA from each condition was probed to detect the m6A modification (bottom). Controls were performed as described in Figure 1C.
Figure S2, Related to Figure 2: A customized pipeline to identify m6A-modified circRNAs.
(A) Venn diagram showing the circRNAs identified by our AutoCirc pipeline, CIRCexplorer (CIRCe) pipeline, and MapSplice pipeline. About 80% of circRNAs found by CIRCexplorer are also identified by our AutoCirc pipeline. The pools of circRNAs identified by AutoCirc and CIRCexplorer are very similar (p<1.6e-295 by hypergeometric test).
(B) The expression levels of circRNAs in SPRBM (back-spliced reads per billion mapping metric see Materials and Methods for details) as identified by our AutoCirc computational pipeline, CIRCexplorer pipeline, and MapSplice pipeline from our hESC rRNA-depleted (Ribo-) RNA-seq data and the polyadenlyated (polyA) RNA-seq data (GEO: GSE41009). Each computational pipeline identified more than 2000 circRNAs from Ribo-RNA-seq data containing 109 million reads. Poly-A selected RNA served a negative control, and each pipeline only identified several hundred back splice junctions from polyA-selected RNA-seq data which contained 557 million reads. The mean SPRBM counts for poly-A RNA were much lower than the counts for Ribo- RNA for each pipeline.
(C) Bioanalyzer analysis of RNA in each step of sample preparation prior to library construction.
(D) Non-human RNAs were added to RNA samples before m6A RIP at the indicated amounts. Plus signs indicate detection of the spike-ins before and after m6A RIP. Minus signs indicate conditions where the spike-ins are not detected.
(E) Venn diagram showing the total circRNAs identified from the input sample (Ribo- RNA) and m6A-circRNAs identified after m6A IP.
(F) Venn diagram showing the total circRNAs identified from all hESC samples (including our input and ENCODE non-polyA RNA) and m6A-circRNAs identified after m6A IP.
(G) qRT-PCR was performed following rRNA depletion using the primers described in Figure 2E to detect circRNAs with Control (Ctrl siRNA) and two different siRNAs targeting METTL3 (METTL3 siRNA1 and 2). Amplification is quantified as 1/(average Ct of input cDNA). Error bars represent standard deviation.
(H) Sanger sequencing validation of the m6A-circRNA NUFIP2. The exons involved in the back splice junction and predicted sequence is show at the top. Sanger sequencing results across the back splice junction is shown in the middle. RNA-seq tracks are shown at the bottom for total RNA following rRNA depletion (Ribo-) and after rRNA depletion followed by m6A RIP. The gene structure is shown below the tracks. The arrow indicates the direction of transcription and the asterisk indicates the exons that are circularized in each example.
Figure S3, Related to Figure 3: CircRNAs are frequently methylated in human embryonic stem cells.
(A) The most enriched GO molecular functional categories of parent genes of m6A-circRNAs (left) and non-m6A-circRNAs (right).
Figure S4, related to Figure 4: m6A-circRNAs are often methylated in regions where m6A-mRNAs are not methylated.
(A) PCR was performed to detect circRNAs that were methylated (METTL3 and ZNF398) and unmethylated (FOXK2 and SLC11A2). Convergent primers (green) were used to amplify forward splice junctions that were predicted to be contained in the indicated circRNAs, and divergent primers (purple) were used to amplify back splice junctions produced by the indicated circRNAs. Additional convergent primers (light blue) were used to amplify splice junctions not contained in circRNAs as a control for RNase R digestion of linear RNAs. PCR was performed on total RNA after rRNA-depletion and RNase R digestion (Ribo- + RNase R, top), RNA that subsequently underwent m6A RIP (m6A RIP, center) and genomic DNA (bottom). PCR products from convergent primers that amplify across splice junctions outside of circRNAs (light blue) were not detected after RNase R digestion. Forward (green) and back splice primers (purple) that amplified products contained in m6A-circRNAs produced PCR products before and after m6A RIP for m6A-circRNAs. Forward (green) and back splice primers (purple) that amplified products contained in non-m6A-circRNAs produced PCR products only before m6A RIP. GAPDH primers were designed to amplify across forward splice junctions and across an artificial back splice junction as negative controls. Amplification of primers in genomic DNA was used as a positive control to show that convergent primers that can amplify forward splice junctions outside of circRNAs (light blue) can amplify the intended product. Exons (Ex) are shown as colored rectangles, and intronic regions are indicated by a black line. The genomic distance between Ex A and Ex B for each forward splice junction (amplified by green primers) was too long to be amplified under the PCR conditions that were used.
(B) Gene tracks showing the location of m6A-circRNAs and m6A mRNA peaks in three example genes. ZNF398 (top) produces an m6A-circRNA (red box) from the same exon that contains an m6A mRNA peak. ARHGEF19 (middle) produces an m6A-circRNA from an exon that does not contain an m6A peak in the mRNA, and the mRNA contains an m6A peak in a different exon. MAPKAP1 (bottom) produces an m6A-circRNA, and there is no m6A peak in the mRNA produced by the same gene. Red bars indicate m6A-circRNAs detected by sequencing. Blue bars represent total circRNAs identified by sequencing. Orange peaks represent m6A peaks from identified in mRNAs (Batista et al., 2014) and gray peaks represent mRNA background levels. The structure of each gene is indicated below each track and arrows indicate the direction of transcription. The location of primer sets for each transcript is indicated by black and white arrows (P1 and P2).
(C) qRT-PCR was performed on cDNA prepared following polyA selection and m6A RIP (m6A mRNA peaks) and following polyA selection alone (mRNA) for the genes in (B). P1 primers amplify m6A circRNAs, but only in the top example do the same primers amplify mRNA that contains an m6A peak. Amplification of the P2 primers for ARHGEF19 (middle) indicates that the mRNA contains an m6A peak at a different exon. No m6A peaks are detected in MAPKAP1 mRNA (bottom). Each primer is amplified in polyA-selected RNA (right), indicating that all primers can amplify mRNAs before m6A RIP. These data represent additional examples of what is shown in Figures 4D and 4E.
Figure S5, related to Figure 5: m6A-circRNAs show cell-type-specific patterns of expression.
(A) Genomic distribution of input circRNAs (left), m6A-circRNAs (middle) and non-m6A-circRNAs (right) in HeLa cells. The total number of circRNAs identified in each condition is shown in parenthesis.
(B) The distribution of exon length (y-axis) for input circRNAs (left), m6A-circRNAs (middle) and non-m6A-circRNAs (right) is plotted based on the number of exons spanned by each circRNA (x-axis).
(C) The number of exons spanned by each m6A-circRNA (red) and each non-m6A-circRNA (blue) was calculated. The percentage of circRNAs (y-axis) spanning up to seven exons (x-axis) is shown.
(D) The distribution of m6A-modified exons between circRNAs and mRNAs in HeLa cells.
(E) Venn diagram showing the overlap between all m6A-circRNAs identified in HeLa cells and all identified in hESCs.
(F) Two-dimensional histogram comparing the expression levels of all m6A circRNAs in HeLa cells and these in hESCs.
(G) Tracks showing additional examples of m6A circRNAs that are unique to HeLa cells (top), that are unique to hESCs (middle), and are common between hESC and HeLa cells (bottom). Blue rectangles indicated m6A-circRNAs identified in HeLa cells, and red rectangles indicate m6A-circRNAs identified in hESCs. Green arrows indicate the location of primers that amplify across forward splice junctions and purple arrows indicate primers that amplify across back splices.
(H) RT-PCR was performed on RNA prepared following rRNA depletion in HeLa cells and hESCs, respectively.
Figure S6, related to Figure 6: Multiple properties of m6A levels in HeLa cells and hESCs.
(A) Linear regression plot of log2 ERCC read counts in eluate and supernatant are shown for Hela cells (left) and hESCs cells (right). Red circles represent ERCC RNAs. Solid lines indicate the regression line and the two parallel dashed lines indicate ± 0.5. Vertical and horizontal dashed lines indicate the cutoff of read counts > 100 used for filtering out low-concentration RNAs before modeling (Molinie et al., 2016). Regression function is indicated at the top of each panel.
(B) The density of m6A levels for circRNAs identified in both eluate and supernatant data with at least 3 reads supporting back splice junctions in HeLa cells (left) and hESCs (right).
Figure S7, related to Figure 7: m6A-circRNAs bind YTHDF1 and YTHDF2 and identify transcripts with shorter half-lives.
(A) The most enriched GO molecular functional categories of the parent genes of m6A-circRNAs (left), YTHDF1-bound circRNAs (middle) and YTHDF2-bound circRNAs (right).
(B) Venn diagram showing the overlap between circRNAs interacting with YTDHF1 (left) and YTHDF2 (right) in Hela cells.
(C) The half-life of mRNAs for parent genes that encode m6A circRNAs (left), YTHDF1-bound (DF1-IP) circRNAs (middle) or YTHDF2-bound (DF2-IP) circRNAs (right). Black rings represent circRNAs, red circles indicate m6A modification, and light blue structures represent YTH proteins.
(D) The mRNA half-life for m6A negative (−) (left) and m6A positive (+) (right) mRNAs whose parent genes encode m6A-circRNAs (black ring with red circle attached), circRNAs without m6A (black ring) or no circRNAs (black ring with red “X”). The p-values are indicated above each comparison and downward arrows indicate a statistically significant change in half-life.
(E) The half-life of m6A-mRNAs whose parent genes also encode m6A-circRNAs from the same exon(s) where an m6A peak is found in mRNA (labeled A) is compared to the half-life of m6A-mRN whose parent gene encodes an m6A circRNA at exon(s) where no m6A peak is found in mRNAs (labeled B). The p-value is indicated.
(F) The half-life was calculated for m6A-mRNAs containing m6A modifications in the 3′ UTR and m6A-mRNAs with modifications outside the 3′UTR for both replicates.
(G) The change in half-life (log2(siYTHDF2/siControl)) was calculated as described (Wang et al., 2014) for condition A (green) and condition B (purple). The p-values were calculated using Wilcox Mann-Whitney test. Data shown in Figure S7C–S7E and S7G are the results of a second biological replicate of the data shown in Figures 7F–I.
Acknowledgments
We would like to thank P. Dedon and K. Jeffrey for helpful discussions, X. Zhao for suggestions on pipeline implementation, and the MGH Sequencing core and Tufts University Genomics core for RNA sequencing. This work was supported National Institutes of Health (NIH) grant R01GM088342, an Eli & Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA and Rose Hills Foundation Research Award, and an Alfred Sloan Research Fellowship (Y.X.). This work was also supported by Massachusetts General Hospital start-up funds (C.C.G. and A.C.M.).
Footnotes
Supplemental information includes Extended Experimental Procedures, seven supplemental figures and four supplemental tables.
Author Contributions
C.C.G. and A.C.M. conceived the study. C.C.G. and A.C.M. designed experiments with C.Z., B.M. and K.D. Computational analyses were performed by C.Z. with support from J.V.P., J.W. and Y.X. Bench experiments were performed by B.M. and K.D. with assistance from J.V.P and N.O.V.W. The manuscript was written by C.Z., C.C.G. and A.C.M. with input from B.M.
References
- Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. circRNA Biogenesis Competes with Pre-mRNA Splicing. Mol Cell. 2014:1–12. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Batista PJ, Molinie B, Wang J, Qu K, Zhang J, Li L, Bouley DM, Lujan E, Haddad B, Daneshvar K, et al. m6A RNA Modification Controls Cell Fate Transition in Mammalian Embryonic Stem Cells. Cell Stem Cell. 2014;15:707–719. doi: 10.1016/j.stem.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel B, Swain a, Nicolis S, Hacker a, Walter M, Koopman P, Goodfellow P, Lovell-Badge R. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell. 1993;73:1019–1030. doi: 10.1016/0092-8674(93)90279-y. [DOI] [PubMed] [Google Scholar]
- Caudron-Herger M, Pankert T, Rippe K. Regulation of nucleolus assembly by non-coding RNA polymerase II transcripts. Nucleus. 2016;7:308–318. doi: 10.1080/19491034.2016.1190890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science. 1995;268:415–417. doi: 10.1126/science.7536344. [DOI] [PubMed] [Google Scholar]
- Chen K, Lu Z, Wang X, Fu Y, Luo GZ, Liu N, Han D, Dominissini D, Dai Q, Pan T, et al. High-resolution N6-methyladenosine (m6A) map using photo-crosslinking-assisted m6A sequencing. Angew Chemie - Int Ed. 2015;54:1587–1590. doi: 10.1002/anie.201410647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhang P, Fan Y, Huang J, Lu Q, Li Q, Yan J. Transposons modulate transcriptomic and phenotypic variation via the formation of circular RNAs in maize 2017 [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44:2846–2858. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert WV, Bell TA. Messenger RNA modifications: Form, distribution, and function. Science. 2016;352 doi: 10.1126/science.aad8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Shorter J. It’s Raining Liquids: RNA Tunes Viscoelasticity and Dynamics of Membraneless Organelles. Mol Cell. 2015;60:189–192. doi: 10.1016/j.molcel.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- Hansen TB, Venø MT, Damgaard CK, Kjems J. Comparison of circular RNA prediction tools. Nucleic Acids Res. 2015;44:1–8. doi: 10.1093/nar/gkv1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley RW, Apgar J, Everett GA, Madison JT, Marquisee M, Merrill SH, Penswick JR, Zamir A. Structure of a Ribonucleic Acid. Science. 1965;147:1462–1465. doi: 10.1126/science.147.3664.1462. [DOI] [PubMed] [Google Scholar]
- Jia G, Fu Y, He C. Reversible RNA adenosine methylation in biological regulation. Trends Genet. 2013;29:108–115. doi: 10.1016/j.tig.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, Haripal B, Zucker-Scharff I, Moore MJ, Park CY, et al. A majority of m6A residues are in the last exons, allowing the potential for 3′ UTR regulation. Genes Dev. 2015;29:2037–2053. doi: 10.1101/gad.269415.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke S, Pandya-jones A, Saito Y, Fak JJ, Vågbø CB, Geula S, Hanna JH, Black DL, Jr, JED, Darnell RB. m 6 A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. 2017:990–1006. doi: 10.1101/gad.301036.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DR, Hendrickson DG, Tenen D, Rinn JL. Transposable elements modulate human RNA abundance and splicing via specific RNA-protein interactions. Genome Biol. 2014;15:537. doi: 10.1186/s13059-014-0537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasda E, Parker R. Circular RNAs Co-Precipitate with Extracellular Vesicles: A Possible Mechanism for circRNA Clearance. PLoS One. 2016;11:1–11. doi: 10.1371/journal.pone.0148407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade M, et al. Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Mol Cell. 2017;66:22–37. e9. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Wilusz JE. Short intronic repeat sequences facilitate circular RNA production. Genes Dev. 2014 doi: 10.1101/gad.251926.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichinchi G, Gao S, Saletore Y, Gonzalez GM, Bansal V, Wang Yinsheng, Mason CE, Rana TM. Dynamics of the human and viral m6A RNA methylomes during HIV-1 infection of T cells. Nat Microbiol. 2006;1:2016. doi: 10.1038/nmicrobiol.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Protter DSW, Rosen MK, Parker R. Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol Cell. 2015;60:208–219. doi: 10.1016/j.molcel.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods. 2015;12:767–772. doi: 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzahn MR, Marada S, Lee J, Nourse A, Kenrick S, Zhao H, Ben-Nissan G, Kolaitis RM, Peters JL, Pounds S, et al. Higher-order oligomerization promotes localization of SPOP to liquid nuclear speckles. EMBO J. 2016;35:1–22. doi: 10.15252/embj.201593169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive Analysis of mRNA Methylation Reveals Enrichment in 3′ UTRs and near Stop Codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima E, Jinno D, Akiyama Y, Itoh K, Nankumo S, Shima H, Kikuchi K, Takeuchi Y, Elkordy A, Suzuki T, et al. Immuno-northern blotting: Detection of RNA modifications by using antibodies against modified nucleosides. PLoS One. 2015;10:1–17. doi: 10.1371/journal.pone.0143756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinie B, Wang J, Lim KS, Hillebrand R, Lu Z, Van Wittenberghe N, Howard BD, Daneshvar K, Mullen AC, Dedon P, et al. m6A-LAIC-seq reveals the census and complexity of the m6A epitranscriptome. Nat Methods. 2016;13:692–698. doi: 10.1038/nmeth.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro JM, Cho KR, Fearon ER, Kern SE, Ruppert JM, Oliner JD, Kinzler KW, Vogelstein B. Scrambled exons. Cell. 1991;64:607–613. doi: 10.1016/0092-8674(91)90244-s. [DOI] [PubMed] [Google Scholar]
- Ozanick S, Krecic A, Andersland J, Anderson JT. The bipartite structure of the tRNA m1A58 methyltransferase from S. cerevisiae is conserved in humans. RNA. 2005;11:1281–1290. doi: 10.1261/rna.5040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E, Perez-Hernandez D, Ramberger E, et al. Translation of CircRNAs. Mol Cell. 2017;66:9–21. e7. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polioudakis D, Abell NS, Iyer VR. miR-503 represses human cell proliferation and directly targets the oncogene DDHD2 by non-canonical target pairing. BMC Genomics. 2015;16:40. doi: 10.1186/s12864-015-1279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Agarwala SD, Mumbach MR, Jovanovic M, Mertins P, Shishkin A, Tabach Y, Mikkelsen TS, Satija R, Ruvkun G, et al. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell. 2013;155:1409–1421. doi: 10.1016/j.cell.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang X, Li G, Liu H, Li T, Liu J, Zhao Q, Wang C. Comprehensive Circular RNA Profiling Reveals That hsa_circ_0005075, a New Circular RNA Biomarker, Is Involved in Hepatocellular Crcinoma Development. Medicine (Baltimore) 2016;95:e3811. doi: 10.1097/MD.0000000000003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigova AA, Mullen AC, Molinie B, Gupta S, Orlando DA, Guenther MG, Almada AE, Lin C, Sharp PA, Giallourakis CC, et al. Divergent transcription of long noncoding RNA/mRNA gene pairs in embryonic stem cells. Proc Natl Acad Sci U S A. 2013;110:2876–2881. doi: 10.1073/pnas.1221904110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitale RC, Flynn Ra, Zhang QC, Crisalli P, Lee B, Jung JW, Kuchelmeister HY, Batista PJ, Torre Ea, Kool ET, et al. Structural imprints in vivo decode RNA regulatory mechanisms. Nature. 2015;519:486–490. doi: 10.1038/nature14263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, Suter CM, Preiss T. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023–5033. doi: 10.1093/nar/gks144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin M. The primary structure of transfer ribonucleic acid. Experientia. 1971;27:1–11. doi: 10.1007/BF02137708. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Zuo Y, Wang J, Zhang MQ, Malhotra A, Mayeda A. Characterization of RNase R-digested cellular RNA source that consists of lariat and circular RNAs from pre-mRNA splicing. Nucleic Acids Res. 2006;34:e63. doi: 10.1093/nar/gkl151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang Z. Efficient backsplicing produces translatable circular mRNAs. RNA. 2015;21:172–179. doi: 10.1261/rna.048272.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Doxtader KA, Nam Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol Cell. 2016a;63:306–317. doi: 10.1016/j.molcel.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N6-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, Gong Z, Wang Q, Huang J, Tang C, et al. Structural basis of N6-adenosine methylation by the METTL3–METTL14 complex. Nature. 2016b;534:1–15. doi: 10.1038/nature18298. [DOI] [PubMed] [Google Scholar]
- Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY, et al. Nuclear m6A Reader YTHDC1 Regulates mRNA Splicing. Mol Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Xuan L, Qu L, Zhou H, Wang P, Yu H, Wu T, Wang X, Li Q, Tian L, Liu M, et al. Circular RNA: a novel biomarker for progressive laryngeal cancer. Am J Transl Res. 2016;8:932–939. [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, Chen L, Wang Y, et al. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 2017:1–16. doi: 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Elbaum-Garfinkle S, Langdon EM, Taylor N, Occhipinti P, Bridges AA, Brangwynne CP, Gladfelter AS. RNA Controls PolyQ Protein Phase Transitions. Mol Cell. 2015;60:220–230. doi: 10.1016/j.molcel.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL, Yang L. Complementary Sequence-Mediated Exon Circularization. Cell. 2014;159:1–14. doi: 10.1016/j.cell.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang XO, Chen T, Xiang JF, Yin QF, Xing YH, Zhu S, Yang L, Chen LL. Circular Intronic Long Noncoding RNAs. Mol Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, related to Figure 1: RNase R resistant RNA species are m6A modified.
(A) Bioanalyzer analysis of RNA samples processed for dot blot (Figure 1A). RIN scores are indicated for total RNA for each replicate. Eluate refers to RNA precipitated by m6A RIP and supernatant is the RNA pool that was not precipitated by m6A RIP.
(B) 293T cells were transfected with siRNAs that target METTL3 and METTL14 as well as negative controls without siRNA (mock), with scrambled siRNA and siRNAs that target Cyclophilin B. RNA expression of METTL3 (blue) and METTL14 (red) was normalized to mock transfection. Error bars represent standard deviation. Data shown are biological replicates of Figure 1C.
(C) Two rounds of polyadenylated RNA selection (top) were performed for each siRNA condition in (C). Decreasing amounts of RNA from each condition was probed to detect the m6A modification. Total RNA was isolated from each siRNA condition (bottom). The RNA was depleted of rRNA and treated with RNase R to digest linear RNAs. Decreasing amounts of RNA from each condition was probed to detect the m6A modification (bottom). Controls were performed as described in Figure 1C.
Figure S2, Related to Figure 2: A customized pipeline to identify m6A-modified circRNAs.
(A) Venn diagram showing the circRNAs identified by our AutoCirc pipeline, CIRCexplorer (CIRCe) pipeline, and MapSplice pipeline. About 80% of circRNAs found by CIRCexplorer are also identified by our AutoCirc pipeline. The pools of circRNAs identified by AutoCirc and CIRCexplorer are very similar (p<1.6e-295 by hypergeometric test).
(B) The expression levels of circRNAs in SPRBM (back-spliced reads per billion mapping metric see Materials and Methods for details) as identified by our AutoCirc computational pipeline, CIRCexplorer pipeline, and MapSplice pipeline from our hESC rRNA-depleted (Ribo-) RNA-seq data and the polyadenlyated (polyA) RNA-seq data (GEO: GSE41009). Each computational pipeline identified more than 2000 circRNAs from Ribo-RNA-seq data containing 109 million reads. Poly-A selected RNA served a negative control, and each pipeline only identified several hundred back splice junctions from polyA-selected RNA-seq data which contained 557 million reads. The mean SPRBM counts for poly-A RNA were much lower than the counts for Ribo- RNA for each pipeline.
(C) Bioanalyzer analysis of RNA in each step of sample preparation prior to library construction.
(D) Non-human RNAs were added to RNA samples before m6A RIP at the indicated amounts. Plus signs indicate detection of the spike-ins before and after m6A RIP. Minus signs indicate conditions where the spike-ins are not detected.
(E) Venn diagram showing the total circRNAs identified from the input sample (Ribo- RNA) and m6A-circRNAs identified after m6A IP.
(F) Venn diagram showing the total circRNAs identified from all hESC samples (including our input and ENCODE non-polyA RNA) and m6A-circRNAs identified after m6A IP.
(G) qRT-PCR was performed following rRNA depletion using the primers described in Figure 2E to detect circRNAs with Control (Ctrl siRNA) and two different siRNAs targeting METTL3 (METTL3 siRNA1 and 2). Amplification is quantified as 1/(average Ct of input cDNA). Error bars represent standard deviation.
(H) Sanger sequencing validation of the m6A-circRNA NUFIP2. The exons involved in the back splice junction and predicted sequence is show at the top. Sanger sequencing results across the back splice junction is shown in the middle. RNA-seq tracks are shown at the bottom for total RNA following rRNA depletion (Ribo-) and after rRNA depletion followed by m6A RIP. The gene structure is shown below the tracks. The arrow indicates the direction of transcription and the asterisk indicates the exons that are circularized in each example.
Figure S3, Related to Figure 3: CircRNAs are frequently methylated in human embryonic stem cells.
(A) The most enriched GO molecular functional categories of parent genes of m6A-circRNAs (left) and non-m6A-circRNAs (right).
Figure S4, related to Figure 4: m6A-circRNAs are often methylated in regions where m6A-mRNAs are not methylated.
(A) PCR was performed to detect circRNAs that were methylated (METTL3 and ZNF398) and unmethylated (FOXK2 and SLC11A2). Convergent primers (green) were used to amplify forward splice junctions that were predicted to be contained in the indicated circRNAs, and divergent primers (purple) were used to amplify back splice junctions produced by the indicated circRNAs. Additional convergent primers (light blue) were used to amplify splice junctions not contained in circRNAs as a control for RNase R digestion of linear RNAs. PCR was performed on total RNA after rRNA-depletion and RNase R digestion (Ribo- + RNase R, top), RNA that subsequently underwent m6A RIP (m6A RIP, center) and genomic DNA (bottom). PCR products from convergent primers that amplify across splice junctions outside of circRNAs (light blue) were not detected after RNase R digestion. Forward (green) and back splice primers (purple) that amplified products contained in m6A-circRNAs produced PCR products before and after m6A RIP for m6A-circRNAs. Forward (green) and back splice primers (purple) that amplified products contained in non-m6A-circRNAs produced PCR products only before m6A RIP. GAPDH primers were designed to amplify across forward splice junctions and across an artificial back splice junction as negative controls. Amplification of primers in genomic DNA was used as a positive control to show that convergent primers that can amplify forward splice junctions outside of circRNAs (light blue) can amplify the intended product. Exons (Ex) are shown as colored rectangles, and intronic regions are indicated by a black line. The genomic distance between Ex A and Ex B for each forward splice junction (amplified by green primers) was too long to be amplified under the PCR conditions that were used.
(B) Gene tracks showing the location of m6A-circRNAs and m6A mRNA peaks in three example genes. ZNF398 (top) produces an m6A-circRNA (red box) from the same exon that contains an m6A mRNA peak. ARHGEF19 (middle) produces an m6A-circRNA from an exon that does not contain an m6A peak in the mRNA, and the mRNA contains an m6A peak in a different exon. MAPKAP1 (bottom) produces an m6A-circRNA, and there is no m6A peak in the mRNA produced by the same gene. Red bars indicate m6A-circRNAs detected by sequencing. Blue bars represent total circRNAs identified by sequencing. Orange peaks represent m6A peaks from identified in mRNAs (Batista et al., 2014) and gray peaks represent mRNA background levels. The structure of each gene is indicated below each track and arrows indicate the direction of transcription. The location of primer sets for each transcript is indicated by black and white arrows (P1 and P2).
(C) qRT-PCR was performed on cDNA prepared following polyA selection and m6A RIP (m6A mRNA peaks) and following polyA selection alone (mRNA) for the genes in (B). P1 primers amplify m6A circRNAs, but only in the top example do the same primers amplify mRNA that contains an m6A peak. Amplification of the P2 primers for ARHGEF19 (middle) indicates that the mRNA contains an m6A peak at a different exon. No m6A peaks are detected in MAPKAP1 mRNA (bottom). Each primer is amplified in polyA-selected RNA (right), indicating that all primers can amplify mRNAs before m6A RIP. These data represent additional examples of what is shown in Figures 4D and 4E.
Figure S5, related to Figure 5: m6A-circRNAs show cell-type-specific patterns of expression.
(A) Genomic distribution of input circRNAs (left), m6A-circRNAs (middle) and non-m6A-circRNAs (right) in HeLa cells. The total number of circRNAs identified in each condition is shown in parenthesis.
(B) The distribution of exon length (y-axis) for input circRNAs (left), m6A-circRNAs (middle) and non-m6A-circRNAs (right) is plotted based on the number of exons spanned by each circRNA (x-axis).
(C) The number of exons spanned by each m6A-circRNA (red) and each non-m6A-circRNA (blue) was calculated. The percentage of circRNAs (y-axis) spanning up to seven exons (x-axis) is shown.
(D) The distribution of m6A-modified exons between circRNAs and mRNAs in HeLa cells.
(E) Venn diagram showing the overlap between all m6A-circRNAs identified in HeLa cells and all identified in hESCs.
(F) Two-dimensional histogram comparing the expression levels of all m6A circRNAs in HeLa cells and these in hESCs.
(G) Tracks showing additional examples of m6A circRNAs that are unique to HeLa cells (top), that are unique to hESCs (middle), and are common between hESC and HeLa cells (bottom). Blue rectangles indicated m6A-circRNAs identified in HeLa cells, and red rectangles indicate m6A-circRNAs identified in hESCs. Green arrows indicate the location of primers that amplify across forward splice junctions and purple arrows indicate primers that amplify across back splices.
(H) RT-PCR was performed on RNA prepared following rRNA depletion in HeLa cells and hESCs, respectively.
Figure S6, related to Figure 6: Multiple properties of m6A levels in HeLa cells and hESCs.
(A) Linear regression plot of log2 ERCC read counts in eluate and supernatant are shown for Hela cells (left) and hESCs cells (right). Red circles represent ERCC RNAs. Solid lines indicate the regression line and the two parallel dashed lines indicate ± 0.5. Vertical and horizontal dashed lines indicate the cutoff of read counts > 100 used for filtering out low-concentration RNAs before modeling (Molinie et al., 2016). Regression function is indicated at the top of each panel.
(B) The density of m6A levels for circRNAs identified in both eluate and supernatant data with at least 3 reads supporting back splice junctions in HeLa cells (left) and hESCs (right).
Figure S7, related to Figure 7: m6A-circRNAs bind YTHDF1 and YTHDF2 and identify transcripts with shorter half-lives.
(A) The most enriched GO molecular functional categories of the parent genes of m6A-circRNAs (left), YTHDF1-bound circRNAs (middle) and YTHDF2-bound circRNAs (right).
(B) Venn diagram showing the overlap between circRNAs interacting with YTDHF1 (left) and YTHDF2 (right) in Hela cells.
(C) The half-life of mRNAs for parent genes that encode m6A circRNAs (left), YTHDF1-bound (DF1-IP) circRNAs (middle) or YTHDF2-bound (DF2-IP) circRNAs (right). Black rings represent circRNAs, red circles indicate m6A modification, and light blue structures represent YTH proteins.
(D) The mRNA half-life for m6A negative (−) (left) and m6A positive (+) (right) mRNAs whose parent genes encode m6A-circRNAs (black ring with red circle attached), circRNAs without m6A (black ring) or no circRNAs (black ring with red “X”). The p-values are indicated above each comparison and downward arrows indicate a statistically significant change in half-life.
(E) The half-life of m6A-mRNAs whose parent genes also encode m6A-circRNAs from the same exon(s) where an m6A peak is found in mRNA (labeled A) is compared to the half-life of m6A-mRN whose parent gene encodes an m6A circRNA at exon(s) where no m6A peak is found in mRNAs (labeled B). The p-value is indicated.
(F) The half-life was calculated for m6A-mRNAs containing m6A modifications in the 3′ UTR and m6A-mRNAs with modifications outside the 3′UTR for both replicates.
(G) The change in half-life (log2(siYTHDF2/siControl)) was calculated as described (Wang et al., 2014) for condition A (green) and condition B (purple). The p-values were calculated using Wilcox Mann-Whitney test. Data shown in Figure S7C–S7E and S7G are the results of a second biological replicate of the data shown in Figures 7F–I.