Abstract
Adenovirus 36 (Ad36) is the only adenovirus to date that has been linked with obesity in humans. Our previous studies in late-adolescent females suggest that excess weight in the form of fat mass is associated with lower cortical bone strength. The purpose of this study was to assess the relationship between Ad36-specific antibodies, adiposity, and bone strength in our sample of late-adolescent females. A cross-sectional study of 115 females aged 18 to 19 years was performed. Participants were classified according to adiposity by dual-energy X-ray absorptiometry (body fat percentage as normal-fat [<32% body fat; n=93] or high-fat [≥ 32% body fat; n=22]), and according to the presence of Ad36-specific neutralizing antibodies. Peripheral quantitative computed tomography measured bone parameters at the 4% (trabecular bone) and 20% (cortical bone) site, and muscle cross-sectional area (MCSA) at the 66% site, from the distal metaphyses of the radius and the tibia. Bone strength was determined from volumetric bone mineral density and bone geometry to calculate bone strength index (BSI; trabecular site) and polar strength–strain index (SSI; cortical site). After adjustment for MCSA and limb length, radial SSI was lower in Ad36+ versus Ad36− subjects from the high-fat group (p<0.03), but not the normal-fat group. No significant differences were observed between groups in tibial SSI or BSI. These data support an association of adiposity and cortical bone strength at the radius with the presence of neutralizing antibodies to Ad36 in late-adolescent females.
Keywords: ADENOVIRUS, OBESITY, PERIPHERAL QUANTITATIVE COMPUTED TOMOGRAPHY, BONE STRENGTH, BONE GEOMETRY
Introduction
Environmental and genetic influences have been identified as contributing factors to the escalating obesity problem(1,2) and recently, the idea that obesity arises from viral infections has received increased attention.(3) Several viruses have been reported to cause obesity in animal models,(4) but adenoviruses are the only adipogenic viruses linked with human obesity.(1,5) To date, the human adenovirus 36 (Ad36) is the only adenovirus that has been linked with obesity.(6) The Ad36–obesity relationship has been investigated in children and adults, where between 22%7 and 30%(2,8) of obese children were found to be positive for Ad36 antibodies, and Ad36 positive versus negative subjects had higher body mass index (BMI; in kg/m2) and waist circumference. In a recent review of existing Ad36 studies,(9) pooled data (n=559) showed an overall prevalence of 28% Ad36 positive status in obese children and 10% in non-obese children.
The significance of greater Ad36 infection rates in obese individuals is unknown, but it is possible that infection with Ad36 may partially explain various adverse health consequences associated with obesity, such as an increased risk for osteoporosis and related fractures, particularly at the forearm.(10) The idea that viral infections negatively affect bone has been reported in studies of human immunodeficiency virus (HIV) patients, with osteoporosis prevalence estimates in cross-sectional studies varying from 55%(11) to 89%.(12) Given the relationship between Ad36 and adipogenesis, it is plausible that being Ad36 positive could be a contributing factor in the bone-fat relationship. However, this proposition warrants investigation.
The purpose of this study, in a sample of late-adolescent females, was to test the hypothesis that bone strength would be lower in participants with high levels of body fat and Ad36 positive as compared to those with normal levels of body fat and Ad36 negative.
Subjects and Methods
Study participants
Data were obtained from late-adolescent (ie, postpubertal) females (n=115), aged 18 to 19 years who participated in a study published previously on adiposity and bone.(13) The age group was selected to minimize any influence of pubertal maturation on the bone outcome variables. The 115 subjects included in this work were chosen from the 120 originally recruited because these participants had successful pQCT scans that could be used in the analysis. Participants were divided into two groups on the basis of their percentage body fat (%body fat): normal-fat (<32% body fat; n=93) and high-fat (≥ 32% body fat; n=22). The cutoff of 32% was selected based on levels of body fat associated with cardiovascular disease risk factors.(14–16) Cut points used to denote Ad36+ (≥ 0.4 optical density [OD] 450 nm; n=62) and Ad36− (<0.4 OD 450 nm; n=53) groups were determined based on control OD values. Participant ethnicity and race were classified with the use of the National Institutes of Health Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research.(17) In this study, 108 participants were white, 10 were Asian, 5 were Hispanic, and 2 were black. When race was included as a covariate in the analyses of bone, %body fat and adenovirus outcomes, it did not have a statistically significant effect; therefore, participants from all ethnic and racial groups were included in the analyses. The main inclusion criteria in this study were being female, between 18 to 19 years of age and having normal menstrual cycles (ie,≥4 menstrual periods in the past 6 months). Exclusion criteria were determined by self-report of premenarcheal status (as determined by menstrual history), history of weight loss or gain in the past 6 months (ie,±10% initial body weight), present illness or chronic disease, known bone disease or disease known to influence bone metabolism (eg, cerebral palsy, juvenile rheumatoid arthritis), growth disorders, eating disorders, parity, participation in Division I collegiate athletics, and the use of supplements or medications that may influence body composition or bone metabolism (eg, corticosteroids). Procedures were approved by the Institutional Review Board for Human Subjects at The University of Georgia, and all participants provided written consent.
Anthropometry
Measures of height and weight were completed after the removal of shoes and outdoor clothing. Height was measured to the nearest 0.1 cm by wall-mounted stadiometer (Novel Products Inc, Rockton, IL, USA). Body weight was measured to the nearest 0.1 kg by electronic scale (Seca Bella 840; Seca, Columbia, MD, USA). The scale was checked weekly for accuracy with the use of known weights. One-way random-effects model, single-measure intraclass coefficients (ICCs) were calculated in females aged 6 to 10 years (n=10) measured twice in a 2-week period by the same individual. The height and weight ICC (R value) and test-retest coefficient of variation (CV) (percentage) values were 0.99 and 0.4% and 0.99 and 1.4%, for height and weight, respectively. Limb length was measured with anthropometric tape (Rosscraft, Inc, Surrey, Canada) to the nearest 0.10mmat the tibia (the distal edge of the medial malleolus to the tibial plateau) and forearm (distance between the ulnar styloid process and olecranon).
Body composition
Body composition variables (fat mass [kg], fat-free soft tissue [FFST] mass [kg], and %body fat) were measured by dual-energy X-ray absorptiometry (DXA) (Delphi A, S/N 70467; Hologic Inc., Bedford, MA, USA). All scans were measured by the same technician using Hologic Whole Body Analysis software (version 11.2). Quality assurance for fat mass, FFST mass, and %body fat was performed by calibration against a three-step soft tissue wedge (Hologic anthropomorphic spine phantom, Model DPA/QDR-1;SN9374) composed of varying thicknesses of aluminum and Lucite, calibrated against stearic acid (100% fat) and water (8.6% fat). In our laboratory, a CV of 0.36% was observed from 648 scans of the spine phantom during a 3-year period. On the basis of a one-factor random effects model, single measure ICCs were calculated in 5 females, aged 18 to 30 years, scanned twice in our laboratory during a 7-day period for fat mass, FFST mass, and %body fat, and all values were R ≥ 0.87.
Dietary intake and energy expenditure
Dietary intake and physical activity data were collected to account for nutrient and loading activity that may confound the bone results. Three-day diet records, including 2 weekdays and 1 weekend day, were used to estimate average daily intakes of energy, macronutrients, calcium, and vitamin D. The 3-day diet records were analyzed by Food Processor for Windows version 8.0 (ESHA Research, Salem, OR, USA). In our laboratory, the reliability of diet records was investigated in a previous study of females 6 to 10 years of age (n=10) who completed 3-day diet records twice over a 2-week period. In that investigation, one-way random effects model ICCs were computed for 3-day energy intake and 3-day calcium intake and found to be R=0.47 and calcium R=0.71, respectively. Information on physical activity for the past week was collected using the intervieweradministered 7-day recall questionnaire,(18) which has been validated in females within this age group.(19) From this questionnaire, participants’ average daily energy expenditure (kcal/d) was estimated.
Determination of Ad36 seropositivity
Seropositivity to Ad36 was determined by ELISA. For the indirect ELISA assay, 10 µg virus antigen in 100µL PBS was added to each well and incubated at 37°C for 1 hour. Cells were washed and blotted, then primary antibody test sera (1:10 dilution) or control (PBS) was added to wells and incubated at 37°C for 1 hour. Secondary antibody (goat anti-human immunoglobulin G [IgG] (H+L) whole molecule-alkaline phosphatase conjugated) diluted 1:500 was added to wells (100 µL/well) and incubated at 37°C for 1 hour. Wells were washed and 100 µL para-nitrophenyl phosphate substrate/well was added and incubated at 37°C for 10 to 15 minutes. Absorbance was measured at OD 405/495 nm.
Peripheral quantitative computed tomography
Peripheral quantitative computed tomography (pQCT) (Stratec XCT-2000; Stratec Medizintechnic GmbH, Pforzheim, Germany) measures were taken at the 4% and 20% site of the nondominant tibia and radius from the distal metaphysis, and represent areas high in trabecular and cortical bone, respectively. Image processing and calculation of the bone indices were determined using Stratec software (version 5.50d).(13) Each scan was obtained with a 0.4-mm voxel, at a slice thickness of 2.4mm and a scan speed of 20 mm/s. The positioning of the scans were determined in a scout view using the medial endplate as an anatomic marker and automatically set by the software at 4% or 20% sites.
The following variables were assessed at the tibia and radius for the 4% site: Total and trabecular volumetric bone mineral density (vBMD; mg/cm3), total bone mineral content (BMC; mg/mm), and total bone cross-sectional area (mm2) were analyzed using contour mode 2 to analyze the outer bone edge and peel mode 2 to separate the cortical and trabecular compartments. The following variables were assessed at the tibia and radius for the 20% site: cortical vBMD (mg/cm3), cortical bone area (mm2), total bone cross-sectional area (mm2), cortical BMC (mg/mm), and cortical thickness (mm). Cortical bone variables for the 20% sites were assessed using cort mode 1 and a threshold of 710 mg/cm3. Estimated bone strength was obtained from vBMD and bone geometry by calculating the bone strength index (BSI; trabecular site) and polar strength-strain index (SSI; cortical site), which represent the strength of bone against compression and the density-weighted section modulus, respectively, and are valid measures of bone strength.(20,21) The SSI was analyzed with cort mode 1 and a threshold of 280 mg/cm3, and was calculated as the section modulus multiplied by the ratio of cortical vBMD and normal physiologic density (1,200mg/mm3):
| (1) |
| (2) |
Section modulus (mm3) is calculated as (a × d2)/dmax, where a is the cross-sectional area of a voxel (mm2), d is the distance of the voxel from the center of gravity (mm), and dmax is the maximum distance (eccentricity) of one voxel to the center of gravity (mm).
A third measurement was taken at the 66% site to assess muscle cross-sectional area (MCSA; mm2), an estimate of muscle strength,(22,23) and muscle density (mm3), an estimate of fat content in muscle.(24) The MCSA was determined by placing a region of interest within the subcutaneous fat tissue. Contour mode 3 with a threshold of 34 mg/cm3 and peel mode 1 was used to locate the area of muscle plus bone. Contour mode 1, threshold of 280 mg/cm3 and peel mode 1 determined the area of bone. MCSA is calculated by subtracting the area of bone from the area of muscle plus bone. To assess muscle density, muscle and bone area were separated from fat using a threshold of 40 mg/cm3. Bone area and density were found using a threshold of 150 mg/cm3. Muscle density was calculated using the bone area and density and muscle area results:
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
All pQCT measurements were performed and analyzed by one trained operator who scanned a cone phantom at the beginning of each scan day to confirm machine calibration. Test-retest measurements were performed in 5 females, aged 18 to 24 years, to determine reliability of the pQCT in our laboratory. The one-factor random effects model ICCs for all pQCT measurements were calculated as R ≥ 0.97.
Statistical analyses
Data were analyzed with the use of SPSS version 20.0.0 (SPSS Inc., Chicago, IL, USA). Normal distribution and homogeneity of variances were confirmed by Shapiro-Wilks W and Levene’s tests, respectively. Group differences for age, anthropometric, body composition, dietary intake, physical activity, Ad36, and unadjusted bone response variables were determined with the use of independent samples two-tailed t tests. Descriptive statistics for raw variables are presented as mean ± SD. Bone measures were adjusted for MCSA, an acceptable surrogate of muscle strength,(22,23) and limb length, because the rate of bone formation during growth is highly influenced by mechanical loading generated by muscle forces.(25) Because muscle density has been linked to fat infiltration, we subsequently tested whether muscle density could explain the differences in the groups. An F test was performed to test the assumption of homogeneity of regression slopes for the interaction between the independent variables (ie, adiposity groups and Ad36 groups) and the covariates (eg, MCSA and limb length). Because there was no interaction, a 2 × 2 analysis of covariance (Ad36 seropositivity as positive or negative) × (adiposity as high-fat versus normal-fat) was used to determine effects of Ad36 seropositivity and %body fat on bone strength at the tibia and radius. Estimated means of bone variables in the adjusted analyses are reported as mean ± SE. Statistically significant differences are reported if p<0.05.
Results
Participant characteristics
Baseline characteristics of the participants are presented in Table 1 as unadjusted values. Results in Table 1 are grouped first by Ad36 seropositivity and second by adiposity. Approximately 52% and 64% in normal-fat and high-fat groups, respectively, had antibodies for Ad36 based on cut points used: normal-fat (n=93): Ad36+ (n=48), Ad36− (n=45); high-fat (n=22): Ad36+ (n=14), Ad36− (n=8). Data for the total study sample have been published elsewhere,(13) with the exception of Ad36 (0.372 ± 0.107 OD 450 nm) and muscle density of the radius (78.7 ± 1.5 mg/cm3) and the tibia (76.3 ± 1.1 mg/cm3).
Table 1.
Participant Characteristics
| Ad36 +a | Ad36−a | |||
|---|---|---|---|---|
|
|
|
|||
| Characteristic | Normal-fat | High-fat | Normal-fat | High-fat |
| Age (years) | 18.1 ± 0.36 | 18.4 ± 0.51 | 18.3 ± 0.45 | 18.3 ± 0.46 |
| Weight (kg) | 58.0 ± 6.1 | 68.8 ± 7.7* | 58.6 ± 6.4 | 65.8 ± 10.0* |
| Height (cm) | 164 ± 6.8 | 165 ± 5.6 | 164 ± 5.6 | 164 ± 4.9 |
| Percent body fat | 27.1 ± 2.9 | 35.8 ± 2.9* | 27.2 ± 3.1 | 34.5 ± 3.2* |
| BMI-for-age (kg/m2) | 21.7 ± 1.8 | 25.3 ± 2.9* | 21.7 ± 2.0 | 24.4 ± 2.5* |
| BMI-for-age (%) | 51.3 ± 19.3 | 77.3 ± 16.0* | 51.3 ± 22.3 | 73.4 ± 16.7* |
| Tibia muscle cross-sectional area (mm2) | 6841 ± 1046 | 7735 ± 1571* | 6940 ± 1103 | 7851 ± 1777 |
| Radius muscle cross-sectional area (mm2) | 2511 ± 320 | 2685 ± 386 | 2429 ± 312 | 2305 ± 598 |
| Tibia muscle density (mg/cm3) | 76.3 ± 1.0 | 76.0 ± 1.8 | 76.3 ± 1.0 | 75.7 ± 1.2 |
| Radius muscle density (mg/cm3) | 79.0 ± 1.8 | 78.1 ± 1.8 | 78.5 ± 1.0 | 78.5 ± 1.9 |
| Tibia length (mm) | 370 ± 21.1 | 374 ± 17.8 | 376 ± 21.2 | 369 ± 21.8 |
| Radius length (mm) | 256 ± 14.5 | 260 ± 12.4 | 259 ± 14.4 | 254 ± 14.5 |
| Adenovirus-36 (OD 450 nm) | 0.442 ± 0.077 | 0.457 ± 0.110 | 0.291 ± 0.044 | 0.258 ± 0.072 |
Values are means ± SD.
Ad36 = adenovirus 36; BMI = body mass index; OD = optical density.
Cut points used to denote normal-fat and high-fat were determined using cardiovascular risk factors.(14,15) Cut points used to denote Ad36+ and Ad36− were determined by ELISA and were based on control OD values; normal-fat (n=93): Ad36+ (n=48), Ad36− (n=45); high-fat (n=22): Ad36+ (n=14), Ad36− (n=8).
Tests of significance between groups are based on one-way ANOVA; significantly different from normal-fat, p ≤ 0.05.
Correlations between body composition, adenovirus, and bone measurements
Both bivariate and partial correlations, controlling for limb length and MCSA, between adiposity and bone variables showed significant inverse associations between %body fat and bone variables have been reported.(13) Bivariate and partial correlations, controlling for limb length and MCSA, between Ad36 and bone variables did not show significant results when the entire sample was included in the analyses. However, when partial correlations were calculated in the high-fat group only, Ad36 was positively associated with %body fat (r=0.528; p=0.017) and negatively associated with SSI of the radius (r=−0.451; p=0.046).
Comparisons between adiposity and adenovirus groups
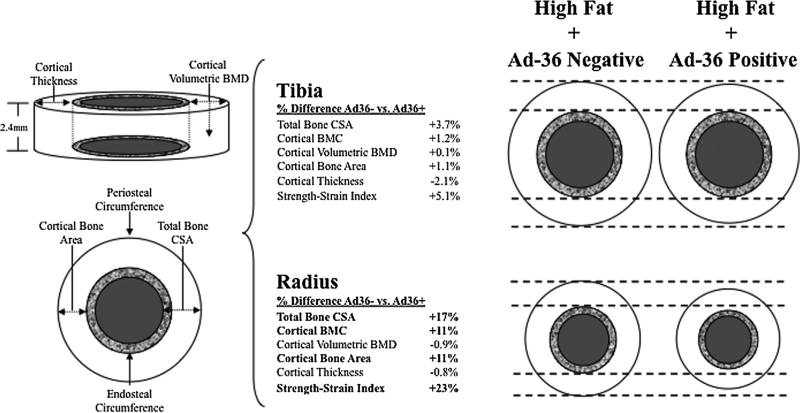
Table 2 summarizes group-specific means for each bone variable based on analysis of covariance. After controlling for MCSA and limb length, statistically significant adiposity × virus interactions were observed, such that total area (at both the 4% and 20% radial sites), total BMC at the 4% site, and measures of cortical BMC, cortical area, and SSI of the 20% radius were all significantly lower in Ad36+ versus Ad36− subjects from the high-fat group, but not the normal-fat group (p<0.03). When correcting for muscle density in addition to MCSA and limb length, the statistically significant results shown in Table 2 were not altered. No significant obesity × Ad36 interactions were observed at the 4% or 20% sites of the tibia. Figure 1 shows a visual representation in the high-fat group of the overall effect by which smaller cortical bone dimensions at the 20% site, as was observed in the Ad36+ compared with the Ad36− group, had on estimated bone strength (SSI), an estimate of torsional bone strength,(20) relative to %body fat, and radial and tibial MCSA and length.
Table 2.
Radial and Tibial Bone Variables at the Trabecular and Cortical Sites
| Ad36+a | Ad36−a | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Bone variable | Normal-fat | High-fat | Normal-fat | High-fat | Virus × adiposity (p)b |
| Radius | |||||
| Trabecular (4%) site | |||||
| Total area (mm2) | 280 ± 6.02 | 246 ± 11.4c | 267 ± 6.25 | 296 ± 14.9 | 0.003 |
| Total BMC (mg/mm) | 95.9 ± 1.85 | 86.8 ± 3.48 | 92.6 ± 1.91 | 97.3 ± 4.56 | 0.032 |
| Total vBMD (mg/cm3) | 348 ± 8.00 | 358 ± 15.1 | 350 ± 8.29 | 332 ± 19.7 | 0.306 |
| Trabecular vBMD (mg/cm3) | 214 ± 4.46 | 207 ± 8.41 | 214 ± 4.62 | 206 ± 11.0 | 0.987 |
| BSI (mg2/mm4) | 3397 ± 120 | 3125 ± 226 | 3267 ± 124 | 3291 ± 296 | 0.475 |
| Cortical (20%) site | |||||
| Total area (mm2) | 97.4 ± 1.65 | 83.7 ± 3.11c | 93.2 ± 1.71 | 99.4 ± 4.07 | 0.001 |
| Cortical BMC (mg/mm) | 83.4 ± 1.07 | 76.1 ± 2.01c | 81.8 ± 1.11 | 84.7 ± 2.64 | 0.006 |
| Cortical vBMD (mg/cm3) | 1191 ± 2.99 | 1196 ± 5.63 | 1196 ± 3.09 | 1185 ± 7.37 | 0.107 |
| Cortical area (mm2) | 70.1 ± .880 | 63.6 ± 1.66c | 68.4 ± .913 | 71.3 ± 2.17 | 0.003 |
| Cortical thickness (mm) | 2.64 ± .031 | 2.67 ± .059 | 2.66 ± .032 | 2.64 ± .077 | 0.707 |
| SSI (mm3) | 225 ± 4.97 | 186 ± 9.37c | 217 ± 5.15 | 235 ± 12.3 | 0.001 |
| Tibia | |||||
| Trabecular (4%) site | |||||
| Total area (mm2) | 938 ± 13.6 | 922 ± 25.4 | 912 ± 14.0 | 939 ± 33.6 | 0.343 |
| Total BMC (mg/mm) | 291 ± 5.26 | 283 ± 9.85 | 290 ± 5.42 | 277 ± 13.0 | 0.743 |
| Total vBMD (mg/cm3) | 311 ± 5.49 | 312 ± 10.3 | 320 ± 5.66 | 293 ± 13.6 | 0.138 |
| Trabecular vBMD (mg/cm3) | 252 ± 3.70 | 250 ± 6.93 | 260 ± 3.82 | 245 ± 9.16 | 0.309 |
| BSI (mg2/mm4) | 9165 ± 303 | 8973 ± 567 | 9358 ± 312 | 8174 ± 749 | 0.334 |
| Cortical (20%) site | |||||
| Total area (mm2) | 342 ± 5.69 | 314 ± 10.7 | 330 ± 5.86 | 326 ± 14.1 | 0.218 |
| Cortical BMC (mg/mm) | 228 ± 3.28 | 213 ± 6.15 | 224 ± 3.38 | 216 ± 8.12 | 0.589 |
| Cortical vBMD (mg/cm3) | 1173 ± 2.34 | 1177 ± 4.41 | 1172 ± 2.43 | 1179 ± 5.83 | 0.699 |
| Cortical area (mm2) | 194 ± 2.72 | 181 ± 5.09 | 191 ± 2.80 | 183 ± 6.72 | 0.615 |
| Cortical thickness (mm) | 3.61 ± .065 | 3.54 ± .121 | 3.61 ± .067 | 3.46 ± .160 | 0.750 |
| SSI (mm3) | 1315 ± 28.4 | 1166 ± 53.2 | 1257 ± 29.3 | 1227 ± 70.3 | 0.221 |
Values are adjusted means ± SEM for muscle cross sectional area (mm2) and limb (radial or tibial) length (mm).
Ad36 = adenovirus 36; Area = cross-sectional area of bone; BMC = bone mineral content; vBMD = volumetric bone mineral density; BSI = bone strength index; BMC = cortical bone mineral content; SSI = strength strain index; OD = optical density.
Cut points used to denote normal-fat and high-fat were determined using cardiovascular risk factors.(14,15) Cut points used to denote Ad36+ and Ad36− were determined by ELISA and were based on control OD values; normal-fat (n=93): Ad36+ (n=48), Ad36− (n=45); high-fat (n=22): Ad36+ (n=14), Ad36− (n=8).
Two × two (virus presence × adiposity) analysis of covariance results for radial and tibial bone variables, adjusting for muscle cross-sectional area and limb length.
Post hoc comparisons show significantly lower bone strength values in Ad36+ versus Ad36− subjects in the high-fat group, but not the normal-fat group.
Fig. 1.
Schematic representation of the average magnitude of difference A–B/[(A+B)/2]× 100 at the cortical site of the tibia and radius in the High-Fat+Ad36 Negative group (n=8) versus High-Fat+Ad36 Positive group (n=14) adjusted for muscle cross-sectional area (mm2) and limb-specific length (mm). The outer white circles represent cortical bone, the textured circles represent trabecular bone, and the gray circles represent medullary cavity. Significant associations are in bold. Ad36+=Ad36 positive; Ad36−=Ad36 negative.
Discussion
Obesity is a multifaceted condition whose causes and consequences are strongly interrelated. In humans, a higher prevalence of Ad36 antibodies is present in obese individuals(1,2) and Ad36 has been associated with increased adiposity in animals. Our data using DXA-derived %body fat agree with the BMI data from previous Ad36 studies.(2,7,9,26–28) In the present study, the prevalence of Ad36 seropositivity was found in one-half of our subject group as a whole (53.9%) and was higher in subjects with high %body fat than those with a normal %body fat (66% versus 46%). This study also found that being Ad36+ in the presence of high levels of body fat is associated with lower trabecular and cortical bone structure and strength parameters at the radius, a non–weight-bearing skeletal site. These relationships were independent of potentially confounding factors such as muscle size, muscle density, and limb length.(24,29–31) Our data therefore suggest that when infection for Ad36 is present alongside excess adiposity, it could have a negative effect on bone. To our knowledge, there are no studies reporting the effects of Ad36 or any adipogenic virus on osteogenesis, in spite of the fact that adipogenesis and osteogenesis are interrelated processes.(32–34)
Adult obesity has traditionally been thought to protect against bone loss at various skeletal sites(35) and body weight has previously been used as a positive predictor of areal bone mineral density (aBMD). However, recent reports, including those from our group, have shown that obesity is not protective against decreases in bone mass; instead, increased fat mass is associated with low aBMD, BMC,(36,37) and bone strength,(13,38) and an increased risk for skeletal fractures.(37,39) In studies of children and adolescents,(13,38,40) overweight or obesity was inversely related to bone strength parameters at a predominantly cortical site of the radius, suggesting that bone strength of higher-weight individuals might not be adequate for bone health at this site. The lower radial SSI in high-fat subjects from the Ad36+ group in the present study is important because SSI is a valid estimate of torsional bone strength.(20) It may be that obesity increases risk for fractures at non–weight-bearing skeletal sites, such as the forearm, given that the mechanical force during a fall is proportional to body weight. This may also explain why studies report the radius as the site with the highest percentage of child fractures, and higher fracture rates in overweight compared to normal weight children.(39,41,42)
Why our data showed lower bone strength with Ad36+ in the high-fat, but not the normal-fat group, is unclear. The effects on obesity may be mediated by epigenetic events, events linked to Ad36 itself, or both, and we do not yet understand the tempo of events that trigger these effects. It is unlikely there are sustained effects linked to past Ad36 infection or repeated infections, because adenoviruses should be neutralized due to antibody responses. However, Ad36 exposure could lead to health effects that are secondary to the infection and may result in permanent tissue changes. Though the timing of initial Ad36 exposure in our study is unknown, if past Ad36 infection in the obese subjects led to secondary effects on bone, this sequence of events would be consistent with other health hazards associated with Ad36 and obesity, such as cardiovascular diseases, type 2 diabetes,(43) and hypertension.(26) In this regard, some outcomes linked with early infection with Ad36 may be permanent, but it may also be possible to vaccinate to prevent or reduce these features. Because both osteoblasts and adipocytes share a common progenitor cell, the inverse relationship between adipogenesis and osteogenesis in bone marrow may lead to decreased bone mass when adipogenesis is stimulated.(44) Among the investigated mechanisms explaining Ad36 infection, obesity, and bone, Ad36 infection has been linked to inflammation via increased production of interleukin-6 (IL-6) and to greater lipid accumulation in preadipocytes,(45) leading to increased bone mobilization and reduced bone formation. Specifically, Ad36 infection may affect preadipocyte differentiation and/or mesenchymal stem cell differentiation, an effect that could contribute to bone loss through a virus-associated preponderance of osteoclasts over osteoblasts. As Ad36 seropositive status could be a hallmark of a clinical-metabolic profile preceding obesity, cardiovascular disease, diabetes, and osteoporosis, it would be prudent to evaluate in future studies if Ad36 infections early in life drive changes that are hallmarks of obesity later in life.
Limitations of this study included its cross-sectional nature, which prevented ascertaining historical or prospective data on the length of time since infection and/or becoming obese. Because obesity is associated with metabolic disturbances, such as insulin resistance and increased inflammation that have been shown to negatively affect bone,(46–48) a wider array of biochemical and endocrine factors, such as peroxisome proliferator-activated receptor gamma (PPARγ), NF-κB, and serum 25(OH)D, may have helped explain our findings and should be included as a focus for future studies. Moreover, we did not collect information on the types and frequencies of physical activities performed during puberty, which are influential for bone mineral accrual particularly during growth.(49,50) Though, participants who may have been more physically active would have likely had greater MCSA, which was controlled for in our analyses. Strengths of this study include the use of contemporary DXA and pQCT technologies that allowed us to explore the novel hypothesis that seropositivity for Ad36 is not only associated with increased adiposity, but also lower bone strength. By grouping participants by %body fat versus BMI, we excluded the possibility of misclassification of those with high amounts of body fat that may have otherwise been classified as normal weight if BMI had been used in the grouping process. Last, an advantage of this sample was that key factors known to influence bone, such as sex, age, and maturational status, were controlled by study design.
In summary, our results suggest that excess %body fat and Ad36 seropositivity adversely influence forearm bone strength in late-adolescent females entering adulthood. Though our study suggests a concern for bone health in a population experiencing the highest global rates of obesity, additional research should validate a cause and effect relationship that considers physical activity, metabolic abnormalities, and environmental influences, which can be extended to other race and age groups. If these relationships are confirmed, the information gained will advance our understanding of bone development with potentially long-lasting benefits, including the development of vaccines or other treatment modalities to counteract the effects of the virus.
Acknowledgments
This work was supported by The University of Georgia Research Foundation and College of Family and Consumer Sciences. We are grateful to the study subjects for their participation. We also thank Ruth Taylor, Ashley Ferira, and Maria Breen for coordinating the project, Jackelyn Crabtree for facilitating the adenovirus studies, and Daniel Schiferl for assistance with attaining the muscle density data.
Footnotes
Disclosures
All authors state that they have no conflicts of interest.
Authors’ roles: Study concept and design: NKP, RDL, EML, CAB, RAT, SMT, MADF, and SR. Acquisition of the data: NKP, EML, RDL, and RAT. Statistical analysis: EML, DAK, and NKP. Interpretation of the data and drafting the manuscript: EML, RDL, RAT, CAB, MADF, and SR. All authors contributed to the revision of the manuscript.
References
- 1.Atkinson RL, Dhurandhar NV, Allison DB, Bowen RL, Israel BA, Albu JB, Augustus AS. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int J Obes (Lond) 2005;29:281–6. doi: 10.1038/sj.ijo.0802830. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson RL, Lee I, Shin HJ, He J. Human adenovirus-36 antibody status is associated with obesity in children. Int J Pediatr Obes. 2010;5:157–60. doi: 10.3109/17477160903111789. [DOI] [PubMed] [Google Scholar]
- 3.Rossner S. Can obesity be an infectious disease? Lakartidningen. 2005;102:1896–8. [PubMed] [Google Scholar]
- 4.van Ginneken V, Sitnyakowsky L, Jeffery JE. Infectobesity: viral infections (especially with human adenovirus-36: Ad-36) may be a cause of obesity. Med Hypotheses. 2009;72:383–8. doi: 10.1016/j.mehy.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Dhurandhar NV, Kulkarni PR, Ajinkya SM, Sherikar AA, Atkinson RL. Association of adenovirus infection with human obesity. Obes Res. 1997;5:464–9. doi: 10.1002/j.1550-8528.1997.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson RL. Viruses as an etiology of obesity. Mayo Clin Proc. 2007;82:1192–8. doi: 10.4065/82.10.1192. [DOI] [PubMed] [Google Scholar]
- 7.Gabbert C, Donohue M, Arnold J, Schwimmer JB. Adenovirus 36 and obesity in children and adolescents. Pediatrics. 2010;126:721–6. doi: 10.1542/peds.2009-3362. [DOI] [PubMed] [Google Scholar]
- 8.Na HN, Hong YM, Kim J, Kim HK, Jo I, Nam JH. Association between human adenovirus-36 and lipid disorders in Korean schoolchildren. Int J Obes (Lond) 2010;34:89–93. doi: 10.1038/ijo.2009.207. [DOI] [PubMed] [Google Scholar]
- 9.Atkinson RL. Human adenovirus-36 and childhood obesity. Int J Pediatr Obes. 2011;6(Suppl 1):2–6. doi: 10.3109/17477166.2011.590200. [DOI] [PubMed] [Google Scholar]
- 10.Kalkwarf HJ, Laor T, Bean JA. Fracture risk in children with a forearm injury is associated with volumetric bone density and cortical area (by peripheral QCT) and areal bone density (by DXA) Osteoporos Int. 2011;22:607–16. doi: 10.1007/s00198-010-1333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold J, Pocock N, Li Y. Bone mineral density abnormalities in patients with HIV infection. J Acquir Immune Defic Syndr. 2002;30:131–2. doi: 10.1097/00042560-200205010-00020. [DOI] [PubMed] [Google Scholar]
- 12.Knobel H, Guelar A, Vallecillo G, Nogues X, Diez A. Osteopenia in patients HIV-infected is it the disease or is it the treatment? AIDS. 2001;15:807–8. doi: 10.1097/00002030-200104130-00022. [DOI] [PubMed] [Google Scholar]
- 13.Pollock NK, Laing EM, Baile CA, Hamrick MW, Hall DB, Lewis RD. Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am J Clin Nutr. 2007;86:1530–8. doi: 10.1093/ajcn/86.5.1530. [DOI] [PubMed] [Google Scholar]
- 14.Williams DP, Going SB, Lohman TG, Harsha DW, Srinivasan SR, Webber LS, Berenson GS. Body fatness and risk for elevated blood pressure, total cholesterol, and serum lipoprotein ratios in children and adolescents. Am J Public Health. 1992;82:358–63. doi: 10.2105/ajph.82.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Going SB, Lohman TG, Falls HB. FitnessGram-ActivityGram reference guide 2008. [Internet] Dallas, TX: The Cooper Institute; 2008. Body composition assessments. [cited 2012 Oct 2]. Available from; http://www.cooperinstitute.org/ourkidshealth/fitnessgram/references.cfm. [Google Scholar]
- 16.Pollock NK, Bernard PJ, Gutin B, Davis CL, Zhu H, Dong Y. Adolescent obesity, bone mass, and cardiometabolic risk factors. J Pediatr. 2011;158:727–34. doi: 10.1016/j.jpeds.2010.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institutes of Health. Office of Extramural Research. NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research, Amended, October, 2001. Bethesda, MD: Office of Extramural Research, National Institutes of Health; 2001. [cited 2012 Oct 2]. Available from; http://grants.nih.gov/grants/funding/women_min/guidelines_amended_10_2001.htm. [Google Scholar]
- 18.Blair SN, Haskell WL, Ho P, Paffenbarger RSJ, Jr, Vranizan KM, Farquhar JW, Wood PD. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 19.Washburn RA, Jacobsen DJ, Sonko BJ, Hill JO, Donnelly JE. The validity of the Stanford Seven-Day Physical Activity Recall in young adults. Med Sci Sports Exerc. 2003;35:1374–80. doi: 10.1249/01.MSS.0000079081.08476.EA. [DOI] [PubMed] [Google Scholar]
- 20.Schiessl H, Ferretti J, Tysarczyk-Neimeyer G, Willnecker J. Paediatric osteology: new developments in diagnostics and therapy. Amsterdam: Elsevier; 1996. [Google Scholar]
- 21.Kontulainen SA, Johnston JD, Liu D, Leung C, Oxland TR, McKay HA. Strength indices from pQCT imaging predict up to 85% of variance in bone failure properties at tibial epiphysis and diaphysis. J Musculoskelet Neuronal Interact. 2008;8:401–9. [PubMed] [Google Scholar]
- 22.Schoenau E. From mechanostat theory to development of the “Functional Muscle-Bone-Unit”. J Musculoskelet Neuronal Interact. 2005;5:232–8. [PubMed] [Google Scholar]
- 23.Maughan RJ, Watson JS, Weir J. Strength and cross-sectional area of human skeletal muscle. J Physiol. 1983;338:37–49. doi: 10.1113/jphysiol.1983.sp014658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farr JN, Funk JL, Chen Z, Lisse JR, Blew RM, Lee VR, Laudermilk M, Lohman TG, Going SB. Skeletal muscle fat content is inversely associated with bone strength in young girls. J Bone Miner Res. 2011;26:2217–25. doi: 10.1002/jbmr.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rauch F, Bailey DA, Baxter-Jones A, Mirwald R, Faulkner R. The ‘muscle-bone unit’ during the pubertal growth spurt. Bone. 2004;34:771–5. doi: 10.1016/j.bone.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Trovato GM, Castro A, Tonzuso A, Garozzo A, Martines GF, Pirri C, Trovato F, Catalano D. Human obesity relationship with Ad36 adenovirus and insulin resistance. Int J Obes (Lond) 2009;33:1402–9. doi: 10.1038/ijo.2009.196. [DOI] [PubMed] [Google Scholar]
- 27.Trovato GM, Martines GF, Garozzo A, Tonzuso A, Timpanaro R, Pirri C, Trovato FM, Catalano D. Ad36 adipogenic adenovirus in human nonalcoholic fatty liver disease. Liver Int. 2010;30:184–90. doi: 10.1111/j.1478-3231.2009.02127.x. [DOI] [PubMed] [Google Scholar]
- 28.Trovato GM, Martines GF, Trovato FM, Pirri C, Pace P, Garozzo A, Castro A, Catalano D. Adenovirus-36 seropositivity enhances effects of nutritional intervention on obesity, bright liver, and insulin resistance. Dig Dis Sci. 2012;57:535–44. doi: 10.1007/s10620-011-1903-8. [DOI] [PubMed] [Google Scholar]
- 29.Rauch F, Schoenau E. The developing bone: slave or master of its cells and molecules? Pediatr Res. 2001;50:309–14. doi: 10.1203/00006450-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 31.Frost HM, Schonau E. The “muscle-bone unit” in children and adolescents: a. 2000 overview. J Pediatr Endocrinol Metab. 2000;13:571–90. doi: 10.1515/jpem.2000.13.6.571. [DOI] [PubMed] [Google Scholar]
- 32.Musri MM, Gomis R, Parrizas M. A chromatin perspective of adipogenesis. Organogenesis. 2010;6:15–23. doi: 10.4161/org.6.1.10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahn CR. Medicine Can we nip obesity in its vascular bud? Science. 2008;322:542–3. doi: 10.1126/science.1165667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–6. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liel Y, Edwards J, Shary J, Spicer KM, Gordon L, Bell NH. The effects of race and body habits on bone mineral density of the radius, hip and spine in premenopausal women. J Clin Endocrinol Metab. 1988;78:1247–50. doi: 10.1210/jcem-66-6-1247. [DOI] [PubMed] [Google Scholar]
- 36.Goulding A, Taylor RW, Jones IE, McAuley KA, Manning PJ, Williams SM. Overweight and obese children have low bone mass and area for their weight. Int J Obes Relat Metab Disord. 2000;24:627–32. doi: 10.1038/sj.ijo.0801207. [DOI] [PubMed] [Google Scholar]
- 37.Hsu YH, Venners SA, Terwedow HA, Feng Y, Niu T, Li Z, Laird N, Brain JD, Cummings SR, Bouxsein ML, Rosen CJ, Xu X. Relation of body composition fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr. 2006;83:146–54. doi: 10.1093/ajcn/83.1.146. [DOI] [PubMed] [Google Scholar]
- 38.Pollock NK, Laing EM, Hamrick MW, Baile CA, Hall DB, Lewis RD. Bone and fat relationships in postadolescent black females: a pQCT study. Osteoporos Int. 2011;22:655–65. doi: 10.1007/s00198-010-1266-6. [DOI] [PubMed] [Google Scholar]
- 39.Goulding A, Jones IE, Taylor RW, Williams SM, Manning PJ. Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy X-ray absorptiometry study. J Pediatr. 2001;139:509–15. doi: 10.1067/mpd.2001.116297. [DOI] [PubMed] [Google Scholar]
- 40.Ducher G, Bass SL, Naughton GA, Eser P, Telford RD, Daly RM. Overweight children have a greater proportion of fat mass relative to muscle mass in the upper limbs than in the lower limbs: implications for bone strength at the distal forearm. Am J Clin Nutr. 2009;90:1104–11. doi: 10.3945/ajcn.2009.28025. [DOI] [PubMed] [Google Scholar]
- 41.Goulding A, Cannan R, Williams SM, Gold EJ, Taylor RW, Lewis-Barned NJ. Bone mineral density in girls with forearm fractures. J Bone Miner Res. 1998;13:143–8. doi: 10.1359/jbmr.1998.13.1.143. [DOI] [PubMed] [Google Scholar]
- 42.Skaggs DL, Loro ML, Pitukcheewanont P, Tolo V, Gilsanz V. Increased body weight and decreased radial cross-sectional dimensions in girls with forearm fractures. J Bone Miner Res. 2001;16:1337–42. doi: 10.1359/jbmr.2001.16.7.1337. [DOI] [PubMed] [Google Scholar]
- 43.Bouwman JJ, Visseren FL, Bouter KP, Diepersloot RJ. Infection-induced inflammatory response of adipocytes in vitro. Int J Obes (Lond) 2008;32:892–01. doi: 10.1038/ijo.2008.36. [DOI] [PubMed] [Google Scholar]
- 44.Pasarica M, Mashtalir N, McAllister EJ, Kilroy GE, Koska J, Permana P, de Courten B, Yu M, Ravussin E, Gimble JM, Dhurandhar NV. Adipogenic human adenovirus Ad-36 induces commitment, differentiation, and lipid accumulation in human adipose-derived stem cells. Stem Cells. 2008;26:969–78. doi: 10.1634/stemcells.2007-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vangipuram SD, Yu M, Tian J, Stanhope KL, Pasarica M, Havel PJ, Heydari AR, Dhurandhar NV. Adipogenic human adenovirus-36 reduces leptin expression and secretion and increases glucose uptake by fat cells. Int J Obes (Lond) 2007;31:87–96. doi: 10.1038/sj.ijo.0803366. [DOI] [PubMed] [Google Scholar]
- 46.Khosla S, Riggs BL, Robb RA, Camp JJ, Achenbach SJ, Oberg AL, Rouleau PA, Melton LJ., 3rd Relationship of volumetric bone density and structural parameters at different skeletal sites to sex steroid levels in women. J Clin Endocrinol Metab. 2005;90:5096–103. doi: 10.1210/jc.2005-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schiessl H, Frost HM, Jee WS. Estrogen and bone-muscle strength and mass relationships. Bone. 1998;22:1–6. doi: 10.1016/s8756-3282(97)00223-8. [DOI] [PubMed] [Google Scholar]
- 48.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–7. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 49.Laing EM, Massoni JA, Nickols-Richardson SM, Modlesky CM, O’Connor PJ, Lewis RD. A prospective study of bone mass and body composition in female adolescent gymnasts. J Pediatr. 2002;141:211–6. doi: 10.1067/mpd.2002.126599. [DOI] [PubMed] [Google Scholar]
- 50.Laing EM, Wilson AR, Modlesky CM, O’Connor PJ, Hall DB, Lewis RD. Initial years of recreational artistic gymnastics training improves lumbar spine bone mineral accrual in 4- to 8-year-old females. J Bone Miner Res. 2005;20:509–19. doi: 10.1359/JBMR.041127. [DOI] [PubMed] [Google Scholar]