Abstract
HIV infection often causes neurological symptoms including cognitive and motor dysfunction, which have been collectively termed HIV/neuroAIDS. Neuropsychological assessment and clinical symptoms have been the primary diagnostic criteria for HIV/neuroAIDS, even for the mild cognitive and motor disorder, the most prevalent form of HIV/neuroAIDS in the era of combination antiretroviral therapy. Those performance-based assessments and symptoms are generally descriptive and do not have the sensitivity and specificity to monitor the diagnosis, progression, and treatment response of the disease when compared to objective and quantitative laboratory-based biological markers, or biomarkers. In addition, effects of demographics and comorbidities such as substance abuse, psychiatric disease, nutritional deficiencies, and co-infection on HIV/neuroAIDS could be more readily determined using biomarkers than using neuropsychological assessment and clinical symptoms. Thus, there have been great efforts in identification of HIV/neuroAIDS biomarkers over the past two decades. The need for reliable biomarkers of HIV/neuroAIDS is expected to increase as the HIV-infected population ages and their vulnerability to neurodegenerative diseases, particularly Alzheimer’s disease increases. Currently, three classes of HIV/neuroAIDS biomarkers are being pursued to establish objective laboratory-based definitions of HIV-associated neurologic injury: cerebrospinal fluid biomarkers, blood biomarkers, and neuroimaging biomarkers. In this review, we will focus on the current knowledge in the field of HIV/neuroAIDS biomarker discovery.
Keywords: HIV/neuroAIDS, Biomarkers, CSF, Blood, Neuroimaging
Graphical abstract
1. Introduction
Thirty-three million people are estimated to be currently living with HIV worldwide, with over 2.7 million newly infected individuals annually. Within the United States, over one million adults and adolescents may be living with diagnosed or undiagnosed HIV infection, with an estimated 56,000 new HIV infections occurring annually (Hall et al., 2008; UNAIDS, 2015). The introduction of combination antiretroviral therapy (cART) in the mid-1990’s led to reduced viral replication, improved immune function and increased life expectancy among HIV-infected individuals (Egger et al., 2002; May et al., 2007; May et al., 2006; Munoz et al., 1997; Pedersen et al., 2015).
HIV-1 infects the central nervous system (CNS) and often causes neurological symptoms that include motor and cognitive dysfunction (Etherton et al., 2015; Price et al., 1988), which are collectively called HIV/neuroAIDS. In the era of cART, a more discrete form of CNS dysfunction so-called minor cognitive motor disorder (MCMD) has become more common (Brew, 2009; Cohen and Gongvatana, 2009; Sacktor, 2002; Simoes and Justino, 2015). HIV-associated neuropathologies mainly include widespread reactive astrocytes so-called astrocytosis and other changes in the brain depending on the severity of the diseases (Bell et al., 2006; Brew, 2009; Cohen and Gongvatana, 2009; Del Valle and Pina-Oviedo, 2006; Ellis et al., 2007; Gelman, 2015; Sacktor, 2002). At the cellular level, the primary cell targets for HIV infection are macrophages/microglia and, to a lesser extent, astrocytes (Gorry et al., 1998; Lee et al., 1993; Liu et al., 2004; Luo and He, 2015; Saito et al., 1994; Schweighardt and Atwood, 2001; Tornatore et al., 1994). However, neurons that are mostly affected in the brain of HIV-infected individuals are rarely infected. Therefore, indirect mechanisms have been proposed for HIV/neuroAIDS pathogenesis; they can generally be grouped into three categories: improper immune activation of macrophages/microglia, soluble factors of both viral and host origins from macrophages/microglia and astrocytes, and astrocyte dysfunction.
Since its recognition more than two decades ago, clinical symptoms, neuropsychological testing, and post-mortem neuropathology have been used for HIV/neuroAIDS diagnosis. Meanwhile, more objective laboratory-based biological markers, so called biomarkers have also been pursued to predict and define HIV/neuroAIDS as well as to monitor treatment response and help therapeutic development for HIV/neuroAIDS. Those biomarkers can be derived from cerebrospinal fluid (CSF) such as beta-2-microglobulin, neopterin, quinolinic acid, and monocyte chemo-attractant protein-1 (MCP-1) (Brew et al., 1990; Brew et al., 1992; Cinque et al., 2005; Heyes et al., 1991; Letendre et al., 1999; Thames et al., 2015), or from peripheral blood such as CD16+ monocytes (Kusdra et al., 2002; Luo et al., 2003; Pulliam et al., 1997; Shiramizu et al., 2005); Neuroimaging markers have also been proposed as HIV/neuroAIDS biomarkers to document changes in the size and structure of brain and metabolites in the context of HIV infection (Cao et al., 2015; Cloak et al., 2004; Cysique et al., 2004; Dousset et al., 1997; Filippi et al., 2001; Ge et al., 2003; Hall et al., 1996; Jakobsen et al., 1989; Lee et al., 2003; Paul et al., 2002; Pomara et al., 2001a; Ragin et al., 2004a; Ragin et al., 2004b; Ragin et al., 2005; Tarasow et al., 2003; Thompson et al., 2006; Thompson et al., 2005; Wu et al., 2006). While not eliminating the need for clinical recognition and perhaps neuropsychological testing to measure severity and given that neuroimaging is expensive and not readily accessible, identification of objective laboratory-based quantitative CSF/blood biomarkers for HIV/neuroAIDS represents a major advance in all aspects of HIV/neuroAIDS.
Most of our knowledge about HIV/neuroAIDS is derived from clade B HIV. However, it should be pointed out that clade C HIV have been shown to be less neurotoxic compared to clade B HIV (Rao et al., 2008). Several factors may have attributed to this difference, and they include mutations in the dicysteine motif of clade C Tat protein (Mishra et al., 2008), weaker chemotactic activity of clade C Tat protein (Gandhi et al., 2009), and lower replication efficiency of clade C HIV in macrophages (Constantino et al., 2011). However, a more recent study has found no significant differences in the incidence of cognitive impairment between clade B and C HIV (de Almeida et al., 2013). This study has attributed lower prevalence of HIV/neuroAIDS complications in regions with higher clade C infection (India and Sub-Saharan Africa) to less intensive study of impairments, presence of a myriad of comorbid disorders of CNS, and application of routine neurological exam instead of standardized neuropsychological assessments. In the review, we will aim to summarize all studies on HIV/neuroAIDS biomarkers.
2. CSF biomarkers for HIV/neuroAIDS
CSF is the optimal medium for monitoring the alterations in the CNS environment due to its proximity to brain parenchyma (Mattsson, 2011). The enrichment of CNS proteins in this biofluid makes it superior to blood in studies of neurodegenerative disorders. The relatively simple, cost-effective, and routine sampling in clinics is considered the great advantage of CSF over neuroimaging markers which require highly specialized instruments (Blennow and Zetterberg, 2015).
Alterations in CSF frequently occur throughout the course of systemic HIV infection (McArthur et al., 1988). In the pre-cART era, detection of viral RNA in the CSF was an indicator of presence and severity of HIV-associated neurocognitive disorders (HAND) (Fox, 2013). This association however no longer exists in the cART era since both neurocognitively unimpaired and HIV/neuroAIDS patients have been reported to have a low level of CSF viral RNA while replication of the virus has been undetectable in blood. Although there is debate on the nature of this CSF viral escape, a plausible explanation is ongoing replication in the CNS (Dahl et al., 2014). In the cART era, the quest for the identification of objective markers has shifted towards the CSF-secreted products by the CNS cells that are specifically damaged by HIV and its toxic products.
Most of the CSF biomarkers that have been studied to date can be grouped into one of the three categories: viral, immune-related, and neural markers. Studies of CSF HIV can be quantitative measurement of virus, most commonly assessing viral RNA and the CSF viral load, and qualitative characterization of CSF virus, including genotypic and functional properties. Despite the fact that HIV infection is the pre-requisite for HIV/neuroAIDS (Spudich et al., 2005) and the fact that HIV exhibit CNS compartmentalization with respect to blood virus (Harrington et al., 2005; Power et al., 1995; Strain et al., 2005), none of these has so far proven to be predictive of CNS injury. On the other hand, the relationship of intrathecal immunoactivation to CNS injury was recognized early in the epidemic, several CSF immunologic markers were found at elevated levels during CNS infection of HIV before introduction of assays for HIV RNA (Brew et al., 1990; Brew et al., 1992). Among the biomarkers of immune activation that have been studied most extensively are beta-2-microglobulin, neopterin, quinolinic acid, and MCP-1 (Brew et al., 1990; Brew et al., 1992; Cinque et al., 2005; Heyes et al., 1991; Letendre et al., 1999). None of these has entered mainstream clinical practice related to the diagnosis of HIV/neuroAIDS. Use of brain-specific biomarkers to document neurodegeneration has been pursued in a variety of neurologic diseases, but until more recently, not with the same enthusiasm in HIV/neuroAIDS. Candidate biomarkers in this category include molecular products of neurons, astrocytes, oligodendrocytes, and microglia. There are two general issues with these biomarkers. The first relates to their individual sensitivity to detect ongoing brain injury in the setting of HIV/neuroAIDS. This needs to be established for each biomarker. The second relates to the intrinsic non-specificity of these biomarkers as a group. Thus, virtually by definition, many types of injury can result in release of neural biomarkers into CSF (Norgren et al., 2003). However, this limitation becomes less critical if one uses this class of biomarkers as indicators of active injury rather than to define the type of injury. In that context, sensitive neural biomarkers should have a critical and useful role. To date, several CSF biomarkers of cellular injury have been studied in the context of HIV/neuroAIDS; we will discuss them based on the type of the cell involved and the significance and correlation of those biomarkers with HIV-induced injury.
2.1 Cellular biomarkers
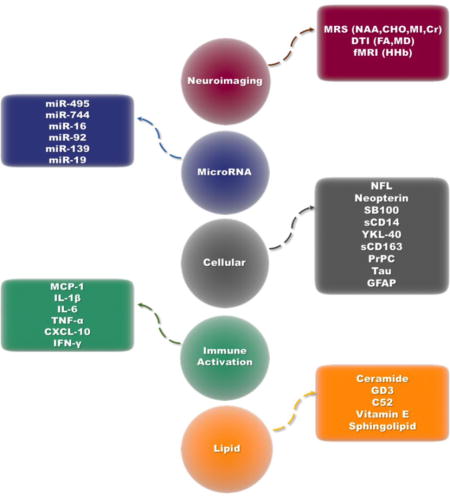
The consequence of HIV invasion of the CNS is neuronal damage in the form of synaptodendritic loss and neuron death (Rao et al., 2014). Identifying sensitive surrogate biomarkers of HIV-induced neuronal damage in the CSF is of great importance in staging of HIV/neuroAIDS, response to therapy, and uncovering other mechanisms of injury to neurons. We have summarized the available cellular HIV/neuroAIDS biomarkers in the order of their significance along with their detection methods, strengths, and limitation (Table 1).
Table 1.
Cellular biomarkers for HIV/neuroAIDS
| Biomarker | Detection Method | Strengths | Limitations | References |
|---|---|---|---|---|
| NFL | ELISA | -Levels are reliable reflection of disease progression or therapy -Differentiates primary, chronic, and neuroasymptomatic HIV -Correlates positively with plasma viral load and negatively with CD4 count |
-Also elevated in other CNS disorders -Detectable only in mid-late phase of disease |
Abdulle et al. 2007 Jessen Krut et al. 2014 McGuire et al. 2015 |
| Neopterin | HPLC,ELISA | -Levels are reliable reflection of disease progression or therapy -Correlates positively with NFL levels -Reliable biomarker of macrophage and microglial activation |
-Also elevated in other CNS Disorders -Detectable only in mid-late phase of disease |
Griffin et al. 1991 Abdulle et al. 2007 Fuchs et al. 1988 |
| SB100 | ELISA | -Levels are reliable indicator of early damage to BBB and astrocyte activation -Correlates with severity of neurocognitive Impairment |
-Also elevated in other CNS disorders |
Rohlwink et al. 2014 Pemberton et al. 2001 |
| sCD14 | ELISA | -Correlates with severity of neurocognitive impairment -Correlates with neuroimaging biomarkers -Levels predict risk of death |
-Also elevated in other CNS disorders |
Lyons et al. 2011 Ryan et al. 2001 Sandler et al. 2011 |
| YKL-40 | ELISA | -Correlates with productive HIV infection of microglia and with acute neuroinflammation | - Also elevated in other CNS Disorders -Few human studies conducted |
Kolson et al. 2008 Bonneh-Barkay et al. 2012 |
| sCD163 | ELISA | -Correlates with viral load in plasma and with monocyte expansion -Levels are reliable reflection of disease progression or therapy |
-Detectable only in plasma - Also elevated in other CNS disorders |
Burdo et al. 2011 Burdo et al. 2013 |
| PrPC | ELISA | -Levels correlate with CNS compromise and with monocyte influx into CNS | - Also elevated in other CNS -Does not correlate with viral load or CD4 count |
Roberts et al. 2010 Megra et al. 2013 |
| Tau | ELISA | -Correlates with severity of neurocognitive impairment | -Also elevated in other CNS disorders -Results not consistent between groups |
Peterson et al. 2014 Gisslen et al. 2009 |
| GFAP | ELISA | -Correlates with astrocytosis | -Also elevated in other CNS disorders -Few studies conducted |
Norenberg 1998 Andersson et al. 2006 |
2.1.1 Neurofilament proteins (NF)
Subunits of the NF have been proposed as suitable biomarkers of neuronal damage in multiple neurodegenerative disorders (Skillbäck et al., 2014). These are neuron-specific intermediate filaments with roles in structural support for axons and are classified based upon the molecular weight of their core chains as light, medium or heavy NF. Elevated levels of neurofilament proteins, especially light NF, have been found in the CSF following damage to the axons in Alzheimer’s (Zetterberg et al., 2015), multiple sclerosis (MS) (Gresle et al., 2011), and amyotrophic lateral sclerosis (ALS) (Norgren et al., 2003).
In the context of HIV infection, elevation in CSF light NF has been reported by several groups. Highest levels of CSF NFL are observed in HIV-associated dementia (HAD), decreasing with cART and resurging following the interruption of treatment (Abdulle et al., 2007). In primary HIV infection, chronic neuro-asymptomatic infection, and HAD, CSF light NF seems to be the most sensitive CSF biomarker when compared to other CSF biomarkers such as soluble amyloid precursor protein (sAPP) and total tau protein (t-Tau) (Jessen Krut et al., 2014). CSF light NF has been shown to be positively correlated to plasma viral load, and negatively with CD4 cell count, suggesting a link between neuronal injury and systemic HIV infection (McGuire et al., 2015). Direct association of monocyte activation with neuronal injury at all stages of HAND has also been noted because of the significant correlation of CSF light NF with monocyte activation markers, soluble CD163 (sCD163) and soluble CD14 (sCD14) (McGuire et al., 2015). A recent study indicates lack of elevation in CSF light NF in acute HIV infection, confirming light NF as a suitable marker of neuronal injury supported by the fact that this injury occurs later in the disease (Peluso et al., 2015).
2.1.2 Amyloid precursor protein (APP)
APP is a membrane spanning protein with neuroprotective roles; it is ubiquitously expressed in neuronal cells. Secretase cleavage products of this precursor protein however have been linked to a variety of neurodegenerative disorders. β-amyloid 42 (Aβ42) is the product of APP cleavage by β-secretase, and its aggregation is considered a major contributor to Alzheimer’s pathology (Nhan et al., 2015).
The inflammatory cascade initiated by HIV invasion of the CNS and some of the viral products are capable of altering amyloid metabolism. Tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) secreted by HIV-infected microglia have been shown to induce elevated APP transcription and cleavage. MCP-1 secreted by HIV-infected and activated astrocytes increases extracellular Aβ42 levels. HIV Tat protein, a neurotoxic protein of HIV-1, has been shown to inhibit neprilysin, the proteolytic enzyme that prevents Aβ42 aggregation, leading to the elevation of Aβ42 and soluble forms of APP and altered metabolism of Aβ42 and APP (Liu et al., 2000; Ortega and Ances, 2014).
The effect of HIV infection of the CNS on amyloid levels has been studied for both soluble APP forms (sAPPα/β), and Aβ42 deposits in the brain. The consistent finding seems to be a decrease in soluble forms of CSF APP with HIV/neuroAIDS, but there are discrepancies in reports regarding Aβ42 deposition. Among these studies, one reports reduced sAPPα/β in HAD but no change in primary HIV infection (Peterson et al., 2014). Another study reports a decrease only in CSF Aβ42 of HIV/neuroAIDS patients compared to HIV-infected individuals without cognitive impairment (Clifford et al., 2009); and the third study finds reductions in both CSF sAPPα/β and Aβ42 of HAD patients (Krut et al., 2013). Reductions in soluble forms of CSF APP could be explained by an increase in deposition of Aβ42 in neurons; and one study confirms this relationship in early infection with HIV (Peluso et al., 2013), and another suggests that increase in Aβ42 deposition is concomitant with increased frequency of detection in plasma of Aβ42 in HIV-related cognitive impairments (Mothapo et al., 2015). Two other studies however report no change in Aβ42 deposition in the context of HIV/neuroAIDS (Ances et al., 2012a; Steinbrink et al., 2013). An explanation for the discrepancies among studies of Aβ42 deposition in HIV/neuroAIDS patients would be the age difference of the cohorts studied. The reports with increased amyloid deposition within cognitively impaired HIV patients happen to have more middle-aged and older cohorts, while studies that have reported no change in deposition seem to have recruited younger cognitively impaired HIV patients. It is conceivable to conclude that sAPP reduction in CSF serves as a suitable biomarker for HAND while aging has to be considered when amyloid deposition is being studied.
2.1.3 Protease resistant protein, cellular isoform (PrPC)
PrPC is a GPI-anchored protein found in the lipid raft domains of plasma membrane in neurons and astrocytes (Coleman and Hill, 2015). PrPC is the non-pathological isoform of the prion protein and is involved in adhesion and signaling, transmigration of leukocytes across the endothelium, and neuroprotection. In prion diseases however, this normal host protein is converted to PrPSc, the transmissible agent of prion diseases which causes neurotoxicity (Bujdoso et al., 2015). Prion-like, misfolded protein transmission is a common feature of neurodegenerative disorders such as Alzheimer’s disease (AD), Parkinson, and Huntington.
PrPC has been shown to be significantly increased in the CNS of HIV patients with neurocognitive impairments and also in SIV-infected macaques with encephalitis. The soluble form of this protein (sPrPC) is released into CSF and its levels have been shown to be correlated with the compromised CNS (Roberts et al., 2010). Increased monocyte influx into the CNS due to HIV infection seems to be involved in PrPC elevation and release in the CSF, since PrPC levels are correlated with increased MCP-1 in CSF, but are independent of viral load and CD4 count (Megra et al., 2013). Anti-HIV properties of PrPC are another reason for making this protein a valuable biomarker of CNS infection. PrPC has been shown to bind viral RNA, inhibiting its translation, and depleting PrPC favors HIV-1 replication (Alais et al., 2012).
2.1.4 Tau protein
Tau is a microtubule-associated protein involved in the formation and stabilization of microtubules and movement of organelles along the axons and dendrites. Phosphorylation of tau occurs following inflammation, and its hyper-phosphorylated form (p-Tau) detaches from microtubules, destabilizing axons and leading to self-assembly of neurofibrillary tangles (Calcagno et al., 2015). Hyper-phosphorylated Tau detected in tangles is a hallmark of Alzheimer’s disease and detection of elevated total tau (t-Tau) in CSF is considered a valuable biomarker in the diagnosis of AD (Peterson et al., 2014). Epidemiological studies on Tau levels in HIV/neuroAIDS patients however have been controversial; some reports find no correlations while others show elevated total Tau (t-Tau), p-Tau or both in cognitively impaired HIV patients. Of the 12 total studies in the literature that investigated Tau levels in CSF of HIV/neuroAIDS patients, four recent studies show elevations of t-Tau (Gisslén et al., 2009; Peterson et al., 2014; Smith et al., 2014; Steinbrink et al., 2013). Three early studies also show increases in CSF levels of p-Tau in HIV/neuroAIDS (Anthony et al., 2006; Cohen et al., 2015; Patrick et al., 2011), and two other studies show increase in both t-Tau and p-Tau (Brew et al., 2005; Calcagno et al., 2015). The other three studies report no change in the levels of t-Tau or p-Tau in HIV/neuroAIDS patients (Clifford et al., 2009; Green et al., 2000; McNamara et al., 2015). Since Tau, unlike amyloid plaques, is detected in brains of the majority of normally aged older adults, it is necessary to consider the age of the patient population while using this protein as a biomarker for HIV/neuroAIDS. Total Tau seems to be more closely correlated with the severity of cognitive impairment and therefore a more accurate biomarker for staging of HIV/neuroAIDS in younger patients.
2.1.5 Human cartilage glycoprotein 39 (YKL-40)
YKL-40 is a secreted glycoprotein expressed in several cell types with functions in growth stimulation, adhesion, and migration. Up-regulated levels of YKL-40 have been reported in several inflammatory diseases such as ulcerative colitis, Crohn’s disease, rheumatoid arthritis, as well as in cancer (Craig-Schapiro et al., 2010). YKL-40 in both plasma and CSF has been shown to increase in AD and both YKL-40 and Aβ-42 have been proposed as a potential prognostic biomarker for pre-clinical AD (Craig-Schapiro et al., 2010). Previous studies have only detected YKL-40 expression in macrophages/microglia, but not in neurons and astrocytes in the context of HIV infection (Bonneh-Barkay et al., 2008). Nevertheless, differential up-regulation of CSF YKL-40 has been reported in SIV-infected pigtailed macaques that had developed encephalitis, and have been correlated with increases in the CSF viral load (Beck et al., 2015). These studies have attributed the elevated YKL-40 expression to productive HIV and SIV infections of microglia (Kolson, 2008). More recent reports however have found increased detection of YKL-40 in activated astrocytes and perivascular macrophages in the context of multiple sclerosis and lentiviral encephalitis (Bonneh-Barkay et al., 2010). The induction of YKL-40 in astrocytes begins immediately following activation and continues for approximately 12 days. CSF levels of YKL-40 have also been positively correlated with IL-1β and TNF-α (Bonneh-Barkay et al., 2012). These properties make YKL-40 a valuable biomarker of acute neuroinflammation. But it is important to note that most of the studies have focused on SIV models; therefore, further investigation of the usefulness of this biomarker for HIV/neuroAIDS is needed.
2.1.6 Calcium binding protein B (S100B)
S100B is a small acidic protein mainly expressed in astrocytes in the CNS and has important roles in cellular energy metabolism, cytoskeletal rearrangement, proliferation, and differentiation (Rothermundt et al., 2003). S100B secretion is elevated in the CSF in various neurodegenerative, inflammatory, and psychiatric disorders including AD, dementia, stroke, subarachnoid hemorrhage, ischemia, and brain trauma. The elevation is indicative of tissue damage or blood brain barrier disruption (Rohlwink and Figaji, 2014). In HIV infection, patients with advanced neurocognitive problems have been shown to have higher levels of CSF S100B (Pemberton and Brew, 2001). In normal physiological concentrations, S100B has been shown to have trophic effects on neurons, but following injury and rise in concentration, this protein could exacerbate the damage. Increased S100B secretion also induces interleukin-6 (IL-6) secretion in CSF, which has been shown to contribute to neuroinflammation and further damage (Piazza et al., 2013). S100B also functions as an inhibitor of glial fibrillary acidic protein (GFAP), the structural intermediate filament (IF) exclusive to astrocytes, by inhibiting the phosphorylation and assembly of GFAP (Sorci et al., 1998). Elevated concentrations of S100B along with IL-1β have been shown to induce gliosis, disrupt neurite growth, and cause calcium mediated neuronal loss in HAD (Stanley et al., 1994). Considering the specificity of this protein to astrocyte dysfunction and blood brain barrier disruption, S100B could be a useful biomarker of early CNS damage and BBB penetration in the context of HIV/neuroAIDS.
2.1.7 Neopterin
Neopterin is a pteridine and a metabolite of guanosine triphosphate catabolism; it is synthesized in macrophages and microglia following stimulation with interferon γ (IFN-γ). Increased neopterin production and secretion in CSF have been noted in viral, bacterial, and parasitic infections, autoimmune disorders, cancer, and neurocognitive impairments. For this reason, neopterin is considered an excellent intrathecal biomarker of macrophage and microglial activation in the CNS (Fuchs et al., 1988). Neopterin is elevated in CSF of untreated HIV patients (Fuchs et al., 1989). Its CSF concentrations initially drop with cART, but following a short plateau, bounce back and persist at higher levels throughout the course of infection despite continued treatment. Highest levels of CSF neopterin have been associated with advanced neurological impairment in HAD, while neuro-asymptomatic HIV patients do not demonstrate such elevation (Griffin et al., 1991). Positive correlation between concentrations of CSF neopterin and NFL has been reported in HAND, indicating association of axonal degeneration with macrophage activation (Abdulle et al., 2007). Neopterin is a well-studied biomarker of immune activation; it can be helpful in staging and therapeutic monitoring of HIV/neuroAIDS, because of its microglial specificity, initial drop following cART, and continued high levels through cART therapy.
2.1.8 Soluble CD163 (sCD163)
sCD163 is the secreted form of the scavenger receptor for hemoglobin; it is expressed on monocytes and macrophages and has roles in endothelial cell adhesion and dampening of systemic inflammation. Several pro- and anti-inflammatory mediators regulate the expression of sCD163; for instance, TNF-α and IFN-γ inhibit its expression while IL-6 and interleukin-10 (IL-10) induce it. sCD163 has been used as a biomarker for systemic monocyte activation in coronary artery disease, rheumatoid arthritis, Gaucher lysosomal storage disease, and cancer (Onofre et al., 2009). In SIV-infected pigtailed macaques, plasma sCD163 is significantly higher in individuals with encephalitis compared to those without encephalitis (Beck et al., 2015). In humans, plasma levels of sCD163 but not CSF levels are shown to be elevated in both early and chronic infection with HIV. Plasma levels have also been correlated with viral load and associated with monocyte expansion following infection (Burdo et al., 2011). Higher levels of sCD163 in plasma have been reported in HIV patients with more severe neurological impairments. These levels decreased with effective cART, but did not return to the pre-infection baseline levels, indicating persistent immune activation (Burdo et al., 2013). Tracking studies on CD163+ monocytes and macrophages have shown that these cells accumulated in the perivascular regions of brain in HIV encephalitis, and that their numbers are correlated with plasma HIV load (Fischer-Smith et al., 2008).
Despite its solubility and release in plasma, sCD163 levels have not been found to be significantly increased in the CSF of HIV/neuroAIDS patients. This might be due to phenotypic change in monocytes following transmigration and establishment in the perivascular regions. Plasma levels however have been useful in staging, as they indicate an initial drop following cART but present at higher levels during the course of infection. Overall, sCD163 can be useful in staging and therapeutic monitoring of HIV/neuroAIDS in a panel along with other CSF and blood biomarkers.
2.1.9 Soluble CD14 (sCD14)
sCD14 is the soluble form of the monocyte lipopolysaccharide (LPS) receptor which is cleaved and released from the membrane following the activation of monocytes (McGuire et al., 2015). Low concentrations of sCD14 are sufficient to confer LPS responsiveness to non-CD14-expressing cells. Soluble CD14 has also been shown to be present in human milk and linked to the regulation of microbial growth in infant gut (Funda et al., 2001).
Both plasma and CSF levels of sCD14 have been shown to be elevated in HIV infection regardless of cART status and correlated with the severity of neurocognitive impairment; HIV patients with lower test scores in attention and learning had higher sCD14 (Lyons et al., 2011). HIV patients with cognitive impairments showed elevated plasma sCD14, which was correlated with neuropsychological test scores and neuroimaging biomarkers of HIV/neuroAIDS. These levels have also been found to be elevated in HIV patients with cerebral atrophy compared to those without atrophy (Ryan et al., 2001). Plasma levels of sCD14 have been shown to independently predict mortality in HIV infection; patients with highest plasma levels of sCD14 have six times higher risk of death. This is due to the association of chronic HIV infection with intestinal permeability which increases the risk for bacterial sepsis (Sandler et al., 2011). Altogether, soluble CD14 is a useful plasma and CSF biomarker in HIV/neuroAIDS, as it reflects the severity of cognitive impairments, effectiveness of therapy, and risk of death.
2.1.10. Glial fibrillary acidic protein (GFAP)
GFAP is a type III intermediate filament protein that has a characteristic structure composed of a highly conserved central-helical rod domain flanked by nonhelical head and tail domains (Fuchs, 1996; Fuchs and Weber, 1994). It is expressed in all astroglial cells but predominantly in the fibrillary astrocytes and has been widely used as an astrocyte marker (Eng et al., 2000). GFAP expression is developmentally and pathophysiologically regulated. It begins to express as astrocytes mature (Chiu and Goldman, 1985), and its expression is always increased in reactive astrocytes, or astrocytosis, which is one of the main characteristics of the astrocytic reaction commonly observed in CNS injury, either as a result of physical, pathological, or chemical insults (Norenberg, 1998). GFAP is mainly located in cytoskeletal compartments, while little is found in the cytosolic (Bertelli et al., 2000). In normal and pathological conditions GFAP leaks out from the central nervous system to the CSF (Rosengren et al., 1995)
The serum and CSF GFAP levels have been shown to be a sensitive and specific indicator for a number of subacute or chronic CNS diseases including AD (Schmued and Hopkins, 2000), normal pressure hydrocephalus (Lord and Papoian, 2004; Ridet et al., 1997), cerebral vasculitis (Lord and Papoian, 2004; Ridet et al., 1997), multiple sclerosis (Kreutzberg, 1996), Lyme neuroborreliosis (de Olmos et al., 1994) and trypanosomiasis (Norton et al., 1992; O’Callaghan and Sriram, 2005). In HIV infection, one study shows only slight to moderate increase of the CSF GFAP level in some HIV-infected patients without detectable CNS complications (Andersson et al., 2006), while the other two studies show no differences in the CSF GFAP levels and the frequency of increased GFAP levels between the three groups of HIV-infected patients with different degrees of neuroAIDS (Andersson et al., 1998; Sporer et al., 2000). The difference may be likely due to the small size of the samples in these later two studies. Thus, to further evaluate the exact sensitivity and specificity of the CSF GFAP as a HIV/neuroAIDS biomarker is clearly warranted.
2.2 Immune activation biomarkers
Infiltration of HIV-infected macrophages, monocytes and T cells into the CNS establishes a cascade of inflammation that leads to the activation of microglia, astrocytes, and perivascular macrophages. The combination of cytokines and chemokines produced by these infiltrated cells induces more inflammation and invites more immune cells from the periphery, contributing to neuronal injury. TNF-α, IL-1-β, and IFN-γ produced by infected monocytes and T cells activate microglia and astrocytes. These cells in turn produce IL-6, MCP-1, and macrophage inflammatory protein 1 (MIP-1), which bring in more immune cells into the CNS and contribute to neuroinflammation and injury (Hong and Banks, 2015). We have summarized the most commonly used immune activation biomarkers for HIV/neuroAIDS in the order of their significance along with their detection methods, strengths, and limitation (Table 2).
Table 2.
Immune activation biomarkers for HIV/neuroAIDS
| Biomarker | Detection Method | Strengths | Limitations | References |
|---|---|---|---|---|
| MCP-1 | ELISA | -Correlates with Monocyte infiltration and with severity of cognitive impairment -Correlates with Neopterin levels and CSF viral load -Can be used for cART monitoring |
-Also elevated in other CNS disorders |
Thames et al. 2015 Price et al. 2007 Tiraboschi et al. 2015 |
| IL-1β, IL-6, TNF-α CXCL10 | ELISA | -Can be used to monitor infiltration of HIV infected cells into the CNS -Correlate with early stage of disease prior to astrocyte activation |
-Also elevated in other CNS disorders -Do not correlate with cognitive status |
Hong et al. 2015 Kamat et al. 2012 |
| IFN-γ | ELISA | -Correlates with CD8 T cell transmigration into CNS -Associated with increased risk of cognitive impairment |
-Also elevated in other CNS disorders | Schrier et al. 2015 |
Several soluble factors in both plasma and CSF of HIV patients have been studied as biomarkers for immune activation before and after the advent of cART. Early studies show that CSF and serum IL-1β, IL-6, and TNF-α are more frequently detected in HIV patients presenting with HAD (Perrella et al., 1992). Elevation of plasma and CSF HIV RNA, TNF-α, MCP-1, matrix metalloprotease-2 and macrophage colony stimulating factor have also been shown to be predictive of HAD (Sevigny et al., 2004). Higher levels of chemokines such as MCP-1 and interferon gamma-induced protein 10 (CXCL-10) are also reported in the CSF of HIV patients with dementia (Mehla et al., 2012). Astrocytes are the major producers of MCP-1 following exposure to or expression of HIV-1 Tat protein (Conant et al., 1998). CSF MCP-1 has been reported to be particularly higher in CSF of HIV patients with dementia, suggesting that monocyte infiltration of CNS is closely correlated with severe cognitive impairment. Other studies have confirmed the significant correlation of CSF MCP-1 with cognitive impairment and found that other immune activation biomarkers of CSF such as IL-6, interleukin-8 (IL-8), MIP-1α, CXCL10, and IFN-γ are elevated in HIV independent of cognitive status (Kamat et al., 2012). A recent study has shown that HIV-positive individuals with a specific genotype of MCP-1 have significantly higher CSF levels of this chemokine and suffer more severe cognitive decline (Thames et al., 2015). CSF MCP-1 has been shown to be correlated with neopterin (Price et al., 2007), and both are elevated in HAD, dropping with cART regimen with more CNS penetration (Tiraboschi et al., 2015). Elevated MCP-1 in CNS induces leukocytosis (Eugenin et al., 2006) and is associated with increased CSF viral loads following the interruption of cART (de Almeida et al., 2006). Transmigration of CD8 T cells into the CNS resulting in elevated CSF IFN-γ has specifically been correlated with increased risk of HIV-associated cognitive impairments (Schrier et al., 2015). These findings have encouraged combinational analysis of cytokines and chemokines for the purpose of monitoring severity and progression of neurological impairments in HIV infection. In general, most of the immune activation markers with the exception of monocyte/macrophage markers have shown normalization within one year after the initiation of cART (Wada et al., 2015). This observation maintains combinational monitoring of soluble immune activation biomarkers for HIV/neuroAIDS.
2.3 Lipid biomarkers
Disruption in the lipid metabolism in the CNS is a common feature of CNS disorders with acute or chronic inflammation. As the second fattiest tissue in the body, the CNS, the high concentration of lipid components in the CNS, is susceptible to the insults of cytokines, chemokines, and reactive oxygen species. Other than HIV, the presence of activated microglia in the CNS capable of producing copious amounts of inflammatory factors has been observed in neurodegenerative disorders such as AD, Parkinson, Huntington, and MS (Takeuchi et al., 2005). There is elevation of lipid metabolites in the brain and CSF for several above-mentioned disorders; for example, ceramide, a product of sphingomyelinase activation, is elevated in astroglia of frontal cortex and CSF of AD and ALS patients (Satoi et al., 2005), contributing to neuronal apoptosis. Accumulation of ganglioside GD3 has been shown in the CSF of MS (Miyatani et al., 1990) and prion disease (Ohtani et al., 1996). Free fatty acids and their carrier, carnitine, have been documented to increase in meningitis, seizures, neuropathies (Shinawi et al., 1998), traumatic brain injury, and stroke (Pilitsis et al., 2003).
Cytokines and chemokines produced by glial cells are capable of inducing enzymes involved in lipid metabolism such as phospholipase A2 (PLA2), phospholipase C (PLC), and cyclooxygenase (Farooqui et al., 2007). This induction of enzymes occurs through transcription factors that control cytokine and chemokine gene expression. For example, nuclear factor kappa light-chain enhancer of activated B cells directly stimulates PLA2, resulting in phospholipid membrane disintegration and release of arachidonic acid along with the generation of reactive oxygen species (Shmelzer et al., 2003). Phospholipid oxidation of neural membranes also occurs in the process of ongoing inflammation in the CNS, leading to the accumulation of oxidized lipid products, which can influence the inflammatory process, themselves. Peroxisome proliferator-activated receptors (PPAR) are a family of nuclear hormone receptors that exist in different isoforms in neural tissue (α, γ, δ). PPAR have anti-inflammatory properties once activated by their ligands most of which are composed of long-chain poly-unsaturated fatty acids, eicosanoid derivatives, and phospholipids. Deficiency in PPARs or modifications in their ligands, including oxidation of phospholipids, have been associated with delay in anti-inflammatory responses (Drew et al., 2006).
Aside from their indirect effects on lipid metabolism, cytokine and chemokines produced in the CNS directly bind to receptors that are coupled with PLA2 and PLC and induce their enzymatic activity against lipids. TNF-α and IL-1β, for instance, have been shown to stimulate PLC and sphingomyelinase, leading to increased ceramide generation (Machleidt et al., 1996). Ceramide is also produced as a result of oxidative assault on lipid membranes in the CNS; its accumulation has been reported in CSF of HIV/neuroAIDS patients. Inhibition of inducible nitric oxide synthase through minocycline treatment resulted in normalization of this metabolite in CSF (Sacktor et al., 2014). We have summarized the lipid HIV/neuroAIDS biomarkers in the order of their significance along with their detection methods, strengths, and limitation (Table 3).
Table 3.
Lipid biomarkers for HIV/neuroAIDS
| Biomarker | Detection Method | Strengths | Limitations | References |
|---|---|---|---|---|
| Ceramide, GD3 2004 | Immunoblotting Immunostaining |
-Correlate with more severe cognitive impairment in late stages of HAND -Associated with activation of microglia and astrocytes -Accumulation of multiple species indicator of worsening cognitive status |
-Also elevated in other CNS disorders -Only detectable in late stages |
Haughey et al. Andersson et al. 1998 Bandaru et al. 2013 |
| C52, Vitamin E 2007 Sphingolipid |
Mass Spectrometry | -Correlate with stage of cognitive impairment -Correlates with earlier stage of disease and with moderate cognitive dysfunction |
-Also elevated in other CNS disorders | Bandaru et al. |
Initial investigations of lipid accumulation in CSF of HIV patients began with cell-specific lipid species. Ganglioside GD3, for example, is considered a microglial/macrophage- and astrocyte-enriched lipid component and has been found to be elevated in CSF of HIV patients. This elevation has been associated with the activation of microglia and astrocytes in HIV infection, resulting in a higher cell turnover (Andersson et al., 1998). Then a global, mass spectrometric analysis of CNS lipid components was introduced and has revealed the accumulation of sphingolipids and ceramide in frontal, parietal, and temporal cortices of brain with HIV infection (Haughey et al., 2004). It has also shown that the degree of sphingolipid and ceramide accumulation is correlates with severity of cognitive impairments of HIV (Haughey et al., 2004). Using the same approach, additional elevated components such as triglyceride C52 and vitamin E have also been detected in the CSF and correlated with the stage of cognitive impairment progression (Bandaru et al., 2007). Sphingolipid accumulation is correlated with moderate cognitive dysfunction in earlier stages of HAD when anti-oxidant defense barriers of the brain fails to protect CNS cells against acute oxidative and inflammatory storm. Ceramide is produced from sphingolipid in later stages and are associated with declining and more severe cognitive impairments. A mass-based metabolomics study shows elevation in phospholipids, and free fatty acids associated with the activation of phospholipases in SIV encephalitis (Wikoff et al., 2008). Recent efforts in characterizing the relationship between lipidoses of the CNS and HIV infection indicate similarities of HIV/neuroAIDS to lysosomal storage disorders, suggesting disruptive effects of HIV proteins on the endolysosomal system (Bandaru et al., 2013). Worsening of cognitive impairment is associated with a shift in lipid accumulation in the CNS from single lipid species to multiple species. These findings together support the utility of lipids as prognostic and staging biomarkers for HIV/neuroAIDS.
2.4 microRNA (miRNA) biomarkers
Circulating, small, non-coding RNA have become attractive surrogates for evaluating disease progression, pathogenesis, therapeutic effect, and prognosis. Potential use of miRNA as biomarkers is supported by the accumulating evidence from numerous studies, which show that conditions of disease change the physiological expression patterns of these transcription-regulating molecules. Moreover, the resistance to degradation, the tissue specificity, the fast response to cellular environment, and the predictable effects on biological pathways also make miRNA ideal candidates for monitoring processes and aberrations in biological systems (Rao et al., 2013). Extracellular miRNA can be detected in all biofluids in association with lipoproteins, proteins of the argonaute family, or exosomes, which adds to their value as resistant molecules capable of tolerating common problems of sample handling without degradation.
Studies on tractable miRNA signatures in the context of CNS disorders are particularly attractive because these brain-specific small RNA can be identified in the CSF or plasma and provide a rare snapshot of the status of the inaccessible brain tissues. Although several studies have successfully demonstrated differential expression patterns of brain-specific miRNA in plasma, CSF remains the biofluid of choice. The main reasons are the less heterogeneity of sources of miRNA in CSF compared to blood, the small volume of CSF imposing less diluting effects on miRNA concentrations, and the proximity to the source tissue. All of those unique properties allow use of miRNA to precisely predict the pathological process. miRNA profiling has been performed in the context of various CNS disorders including AD, Parkinson, Schizophrenia, ALS, and MS and have led to identification of specific miRNA expression patterns associated with each of the diseases (Jin et al., 2013).
Investigating the global expression pattern of miRNA in response to HIV infection has also revealed disease-specific miRNA signatures that could potentially be used as biomarkers of staging, progression, and therapeutic monitoring. The initial study compared miRNA expression in peripheral blood mononuclear cells (PBMC) between HIV patients and healthy controls (Houzet et al., 2008). Expression of 63 miRNA is altered in PBMC of HIV infected patients. Several mostly down-regulated miRNA are T- cell specific and have the anti-HIV property. In a follow-up study, HIV patients are divided into four groups: asymptomatic, cART naïve, cART treated, and cART resistant, significant correlation is found in miRNA expression between plasma and PBMC. In addition, two differentially expressed miRNA in both plasma and PBMC could potentially predict progression of disease and response to therapy (Munshi et al., 2014; Witwer et al., 2012). miRNA profile in PBMC of viremic subjects and elite controllers show the same pattern of down-regulation of anti-HIV miRNA in both groups, but also differentially expressed miRNA specific to patients with HIV viremia (Witwer et al., 2012). Down-regulation of the let-7 miRNA family has been noted in HIV-infected T cells and correlated with increase in IL-10 expression and inhibition of T cell responses against HIV infection (Swaminathan et al., 2012). Besides those studies, the other study also shows a general trend in down-regulation of anti-viral defenses in T cells and PBMC following infection with HIV (Duskova et al., 2013). Overall down-regulation in miRNA expression in HIV infection has also been associated with RNA silencing suppressor (RSS) activity of HIV Tat (Bennasser and Jeang, 2006; Hayes et al., 2011) and Vpr (Coley et al., 2010).
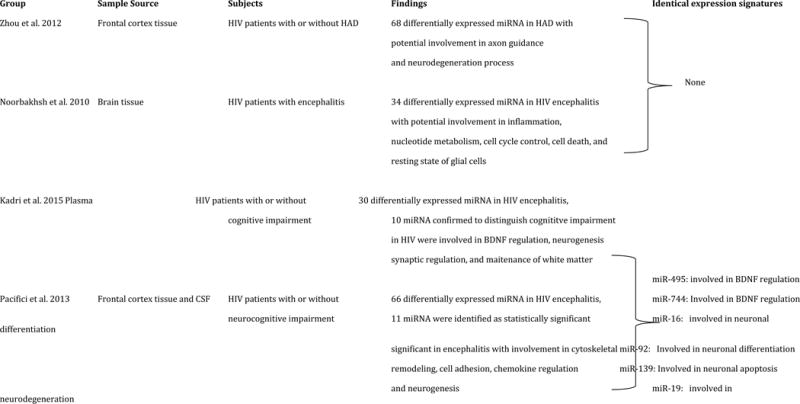
To date, only four comprehensive miRNA profiling studies have been conducted to identify suitable biomarkers for HIV/neuroAIDS. Two of these studies have been performed on archived and post-mortem brain tissue, one on plasma, and one on CSF. Parallel analysis of mRNA and miRNA expression profiles in the frontal cortex of HIV patients with or without HAD have shown 68 differentially expressed miRNA in HAD, 49 of which are down-regulated and 19 are up-regulated (Zhou et al., 2012). Furthermore, gene ontology and clustering analysis reveal involvement of significantly altered miRNA in axon guidance and down-stream signaling. Five miRNA are identified as potential biomarkers of neurodegeneration based on the fact that they are all enriched in neurons and have previously been shown to be involved in processes related to pathology in AD, Huntington, MS, and Schizophrenia. miRNA expression profiling in the brains of four HIV patients with encephalitis shows that the majority of up-regulated miRNA are involved in the transcriptional control of gene products important in immune response and inflammation, nucleotide metabolism, and cell cycle control (Noorbakhsh et al., 2010). These findings are consistent with infiltration and proliferation of immune cells in the CNS. Down-regulated miRNA are shown to be involved in cell death and resting state of glial cells (Noorbakhsh et al., 2010). Paired miRNA profiling of the plasma in HIV patients with or without cognitive impairment shows that 30 pairs of miRNA are differentially expressed but only 10 of them can be used to distinguish cognitively impaired HIV patients from unimpaired (Kadri et al., 2015). Interestingly, three of these identified miRNA targeted brain-derived neurotrophic factor (Kadri et al., 2015).
The first and only CSF miRNA profiling study compared frontal cortex tissue and CSF of HIV patients with or without neurocognitive impairment to healthy controls (Pacifici et al., 2013). The study found 66 differentially expressed miRNA in CSF samples and 35 of those miRNA were also detected in frontal cortex. There were 4 miRNA with identical expression patterns between both sample sources and one miRNA with more than 20 fold higher expression in CSF compared to brain tissue. Computer-assisted target prediction of significantly altered miRNA indicated involvement in cytoskeletal remodeling, cell adhesion, chemokine expression, neurogenesis, axonal guidance, notch signaling, synaptogenesis, and nerve impulses.
Comparing the reported miRNA signatures of these four studies, we have found 6 brain-enriched miRNA with shared differential expression patterns. Interestingly, each of the identified miRNA have been reported as significant in various processes in the brain; miR-495 and miR-744 in BDNF regulation (Mellios et al., 2008; Wu et al., 2010), miR-19 in the pathogenesis of neurodegenerative disorders (Lee et al., 2008), miR-16 in neurotransmitter transport regulation (Baudry et al., 2010), miR-92 in neuronal differentiation (Bian et al., 2013), and miR-139 in regulation of neuronal apoptosis (Qu et al., 2014). We have summarized the miRNA studies on brain tissue and CSF of HIV patients along with shared signatures of expression with potential use as HIV/neuroAIDS biomarkers (Table 4).
Table 4.
MicroRNA biomarkers for HIV/neuroAIDS
|
More studies of miRNA profiling in both brain tissue and CSF of HIV patients need to be performed in order to define HIV/neuroAIDS-specific miRNA signatures. Overall, relevant disease-specific changes in miRNA have been noted in the above-mentioned studies, those miRNA could potentially enlighten the path to accurate biomarker discovery. This is particularly important with the changing dynamics of HIV/neuroAIDS and potential effects of the aging co-morbidity. Considering the close relationship of circulating miRNA with exosomes and the fact that these extracellular vesicles serve as significant intercellular communication vehicles between cells in the CNS, profiling of the exosome-associated miRNA in the CSF in the context of HIV infection is necessary for identification of potentially more specific miRNA for HIV/neuroAIDS.
3. Blood biomarkers for HIV/neuroAIDS
Compared to CSF, blood has the advantage of being easily sampled and is a desirable source for identification of biomarkers for diagnosis and management. However, this source has proved less useful than CSF in most of the CNS diseases including HIV/neuroAIDS. A number of studies have examined blood concentrations of the same type of biomarkers as discussed for CSF, without suggesting any clinical utility. One exception is that circulating proviral HIV DNA within peripheral blood CD16+ monocytes has recently been suggested as a prognostic marker for HIV/neuroAIDS (Shiramizu et al., 2005). In the pre-cART era, monocytes from individuals with HAD exhibit increased expression of CD69, CD16, and TNF-α (Pulliam et al., 1997). Subsequently, in cART-treated patients, circulating CD69 and CD16 monocytes are decreased compared to earlier observations, suggesting either inactive disease or that treatment might down-regulate monocyte activation even in patients with HAD/HIV encephalitis (HIVE) (Kusdra et al., 2002). Proteomics has been used to analyze CSF and monocyte/macrophage products derived from HIV-infected individuals with varying cognitive impairments (Luo et al., 2003). This methodology holds the great promise in discovering truly novel molecules associated with HIV/neuroAIDS.
4. Neuroimaging biomarkers for HIV/neuroAIDS
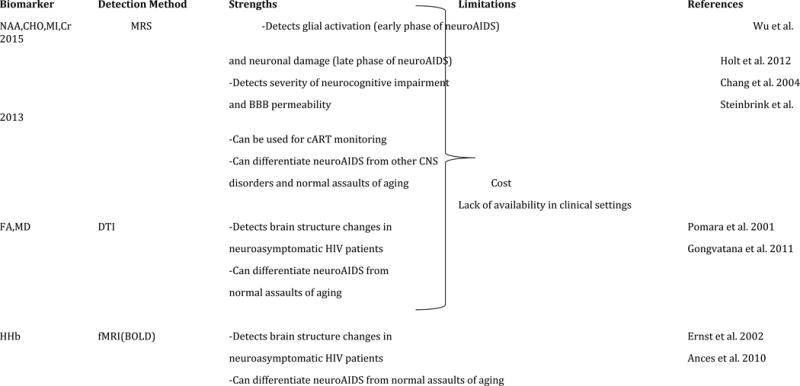
The rationale for applying neuroimaging modalities to HAD/HIVE parallels those for CSF biomarkers. In fact, neuroimaging has contributed significantly to defining the neurologic manifestations of HIV-1 infection since the onset of the AIDS pandemic; neuroimaging remains invaluable in distinguishing lesions due to opportunistic CNS disease. Computerized tomography (CT) and magnetic resonance imaging (MRI) were utilized early in the HIV epidemic to detect encephalitis and damage to white matter (WM) (Thompson and Jahanshad, 2015). These earlier studies found that WM and caudate loss was accompanied with a larger CNS space in HIV patients (Stout et al., 1998). We have summarized the most commonly utilized neuroimaging methodologies in HIV/neuroAIDS (Table 5).
Table 5.
Neuroimaging biomarkers for HIV/neuroAIDS
|
In the context of HIV/neuroAIDS, neuroimaging methods have the obvious advantage of anatomic definition that can be combined with various functional assessments. Ideally, a neuroimaging marker should be (1) specific for the diagnosis of CNS injury that underlies HIV associated neurocognitive impairment; (2) sensitive in detecting these processes during the presymptomatic stages; and (3) able to define disease stage and track the effects of treatment. Morphometric MRI studies focus on quantitative volumetric changes of the brain or its subanatomic regions and have shown that the reduced size of various brain regions including basal ganglia, caudate nucleus, corpus callosum, cortex, hippocampus, and lateral ventricles in HIV/neuroAIDS patients is correlated with impaired neuropsychological test performance of these individuals (Cysique et al., 2004; Hall et al., 1996; Jakobsen et al., 1989; Paul et al., 2002; Thompson et al., 2006; Thompson et al., 2005). Smaller thalamic volume and larger frontal sulcal volume has been reported in MRI studies of HIV compared to controls (Pfefferbaum et al., 2012). Cortical atrophy has also been reported from MRI studies of HIV-affected brain and is interpreted as a sign of neuronal and myelin loss (Archibald et al., 2004).
HIV infection of the CNS is associated with accelerated and/or accentuated aging. Particularly in the post-cART era, an increasing number of HIV patients are presenting with more severe symptoms and co-morbidities of aging compared to their seronegative counterparts. Signs of accelerated aging are identifiable even through the MRI screening in the pre-cART era (Holt et al., 2012). Later, voxel-based morphometry has been used to characterize age-related changes in the HIV-affected brain. Based on this method, change in the gray matter (GM) due to ageing becomes identifiable from HIV-related atrophy in the GM (Towgood et al., 2012). HIV is still causing volume loss in WM and GM in the post-cART era and these reductions in volume are independent of ageing (Becker et al., 2011). Contribution of HIV and aging to volume reduction in several brain regions are shown to be independent and distinct. Aging has been shown to be associated with atrophy in amygdala, while in HIV, a reduction in corpus callosum is observed (Ances et al., 2012b). Also, HIV-associated atrophy in frontal, temporal, parietal, and cerebellar regions, as well as basal ganglia are found to be greater than age-related atrophy (Chang et al., 2011). These studies confirm the advantage of imaging studies in differentiating respective assaults of HIV and aging on the HIV population that is experiencing dramatically improved life expectancy.
Both diffusion tensor imaging (DTI) and magnetization transfer imaging (MTR) are quantitative MRI modalities that provide information on brain microstructures. DTI is to measure diffusion parameters of water in the brain, while MTR is based on the study of interactions between protons in free and restricted (macromolecular) environments. DTI is a useful method in studying WM changes since neuroinflammation is associated with increase in fluid volume in the brain. Two main values are measured by DTI techniques: fractional anisotropy (FA), which reflects integrity and organization of WM fibers, and mean diffusion (MD), which indicates the average of diffusion along the axons (axial diffusion) and diffusion perpendicular to axon fibers (radial diffusion). This technique has enabled the detection of WM abnormalities in seemingly normal MRI screenings of the HIV brain (Pomara et al., 2001b). Studies have shown using both strategies that structural changes in white matter, subcortex, and corpus callosum correlate with cognitive status measures, including the severity of dementia and the degree of impairment in specific cognitive domains (Cloak et al., 2004; Filippi et al., 2001; Pomara et al., 2001a; Ragin et al., 2004a). Also, higher diffusion and lower FA in several brain regions have been documented in HIV. In a one-year follow up study of neuroasymptomatic HIV patients, MD values are recorded as highly significant with lower FA in parietal WM at baseline. Follow-up evaluation however shows increased MD in frontal and parietal WM, putamen and genu regions (Chang et al., 2008). DTI has enabled the differentiation of age-related changes in WM, as well as changes associated with co-infections, from HIV-related abnormalities in brain regions. Reductions in FA of frontal regions has been shown to be an age-specific change in HIV, while a lower WM FA with higher diffusion in parietal and occipital regions indicates co-infection with the hepatitis C virus (Gongvatana et al., 2011). These findings confirm the utility of DTI in differentiating the assaults of aging and other co-morbidities from HIV specific effects in an aging HIV affected population.
Magnetic resonance spectroscopy (MRS) is a widely available MRI modality that measures regional changes of the metabolites in the brain. These neurometabolites include N-acetylaspartate (NAA) which is a marker for neuronal and axonal damage, choline (CHO) and myoinositol (MI) which are indicators of membrane turnover and glial activation, and creatine (Cr) which is a marker of high energy metabolism associated with glial activation. MRS studies have shown that a reduced level of N-acetylaspartate and an increased level of myoinositol in frontal white matter are markers for neuronal or axonal loss or damage in basal ganglia and glial activation, respectively and have been correlated with the severity of cognitive impairment and the permeability of the blood-brain barrier (Dousset et al., 1997; Ge et al., 2003; Lee et al., 2003; Ragin et al., 2004b; Ragin et al., 2005; Tarasow et al., 2003; Wu et al., 2006). Reduced NAA along with increased CHO has been noted in the frontal WM and lenticular nuclei of cognitively unimpaired HIV patients (Chong et al., 1993; McCONNELL et al., 1994). A recent proton MRS study on SIV model of rhesus macaques has shown that in acute infection, MRS glial markers MI, Cr, and CHO increase by 28%, 15%, and 10% respectively while NAA remains unchanged. These results indicate that activation of glia occurs prior to neuronal injury (Wu et al., 2015). Another study on the basis for creatine alterations in a rapid SIV progression model in macaques shows that reduction of NAA and elevation in creatine in this model have been correlated with reduced synaptophysin and MAP-2 levels, indicating neuronal injury, while GFAP and Iba-I are significantly upregulated. These findings establish a relationship between astrocytosis and enhanced high-energy phosphate turnover as indicated by creatine levels (Ratai et al., 2011).
MRS studies have proven to be useful in monitoring cART treatment. Initiation of cART has been shown to normalize NAA/Cr and Cho/Cr ratios (Chang et al., 2004b; Stankoff et al., 2001; Tarasów et al., 1999), while lactate/Cr has been reported to be significantly higher in HIV patients receiving cART who have moderate to severe cognitive impairment (Roc et al., 2007). Increase in lactate is an indicator of anaerobic glycolysis in macrophages and glia due to the ongoing inflammation despite HAART implementation. Decrease in NAA has been associated with more severe dementia in HIV (Holt et al., 2012). MRS studies of cART-naïve HIV patients have shown that elevation of MI in frontal WM is linked to poor performance on executive function test while increase in CHO in the same region of the brain is correlated with slower performance in working memory tasks (Chang et al., 2002).
Several groups have shown significant correlations between magnetic resonance data and other CSF biomarkers of HIV/neuroAIDS. A cross-sectional study of HIV patients investigating cognitive status, CSF biomarkers of HIV/neuroAIDS, and MRI structural evaluation has found that the severity of brain atrophy, signal changes in basal ganglia, and degree of cognitive impairment are positively correlated with the amount of Tau protein in CSF (Steinbrink et al., 2013). This study demonstrates that HIV/neuroAIDS can be differentiated from AD using this combinational approach to biomarker detection since p-Tau is unchanged by HIV infection. The recent study has looked at global biomarkers of chronic inflammation in plasma and CSF of HIV patients along with proton MRS of the brain (Anderson et al., 2015). The study shows an association between increased MCP-1 in plasma and CSF with reductions in NAA/Cr of midfrontal cortex and basal ganglia. The study further shows that sCD14 in plasma and CSF is exclusively correlated with Glucose/Cr ratio. The inverse relationship between MCP-1 levels and NAA/Cr has been described previously as the link between transmigration of monocytes and neuronal injury in HIV/neuroAIDS (Chang et al., 2004a). These results emphasize again the presence of persistent immune activation in chronic HIV and its role in neuronal injury.
MRS can be used to reliably differentiate age-related changes in neurometabolites in HAND from other cognitive impairments. Applying this technique in aging research has shown that in the process of normal aging, metabolites such as creatine, myoinositol, and choline increase in a linear fashion (Chang et al., 1996). Discrimination of healthy aging individuals from subjects with mild cognitive impairment has also been reported by comparing MI/Cr ratios through MRS (Catani et al., 2001). In HIV, changes in MRS metabolites due to accelerated aging of the brain have been documented in both cART-naïve and cART-treated subjects. A study on young, untreated HIV patients shows the usual reduction in NAA along with substantial increases in MI and CHO which are significantly higher than age-associated changes in these markers (Ernst and Chang, 2004). In cART-treated HIV patients, NAA/Cr ratios are lower even in young individuals compared to seronegatives; this ratio decreases with age. These data indicate that abnormalities in MRS metabolites resulting from pre-mature aging can be detected in cART-treated HIV patients. Evaluation of glutamate levels using MRS has shown that the levels of this metabolite are decreased in parietal region of HIV/neuroAIDS patients while patients without cognitive impairment have higher glutamate in basal ganglia (Ernst et al., 2010). In addition, glutamate levels have been found to be lower in young HIV patients, which are comparable to the levels normally seen in seronegatives who are 10 years older. These MRS studies of aging HIV population suggest the great utility of this technique in the aged HIV population.
Functional MRI (fMRI) measures cerebral blood flow by detecting changes in the concentration of paramagnetic deoxyhemoglobin. This technology has allowed documentation of compensatory brain hyperactivation in mildly impaired patients, prior to the appearance of abnormalities on neuropsychological testing (Ernst et al., 2002). The blood oxygen level-dependent contrast imaging (BOLD) method of fMRI can be used as an indirect measure of neuronal function and activity (Arthurs and Boniface, 2002). In HIV infection, fMRI studies have consistently shown reduced activity of normal attention networks along with increased usage of reserve networks of the brain (Chang et al., 2004c). This increased involvement of reserve networks that acts as a compensatory mechanism to maintain normal cognitive performance is also observed in neuroasymptomatic HIV subjects. Greater need for the activation of brain reserve networks has also been shown in studies that employ both MRS and fMRI. Elevated glial metabolites such as MI, CHO, and Cr lead to stronger BOLD signals, associating neuroinflammation with greater need for reserve network activation (Ernst et al., 2003). A longitudinal fMRI study of cognitively unimpaired HIV patients and seronegative controls has shown greater baseline BOLD signals in occipital, cerebellar, and right prefrontal regions of HIV brain during the completion of visual attention tasks. Followup measurements one year after the baseline reading have shown significantly increased BOLD activity in prefrontal and parietal regions of HIV brain and decreased BOLD activity in seronegatives (Ernst et al., 2009). These results are consistent with signs of HIV-associated premature aging, as increased activation of reserve networks have also been observed in normal aging brains following visual attention tasks. Evaluating resting cerebral blood flow (CBF) and its alterations in response to visual stimulation has shown that HIV patients have reduced CBF when compared to seronegatives who are 15 years older. Assessing activation of brain regions during visual tasks, HIV patients show a reduced activity that is equivalent to the brain activity of seronegatives who are 21 years older (Ances et al., 2010). The data from fMRI studies of HIV brain suggest that although this technique can identify changes in the activity of brain networks and cerebral blood flow in the context of HIV infection, it cannot discriminate the similar effects from the aging brain. However, this technique along with MRS is useful in demonstrating increased need for brain reserves in HIV infection. Moreover, despite the technical complexity of fMRI that limits its general utility in clinical settings, advances in areas associated with language lateralization (e.g., prior to temporal lobe surgery for intractable epilepsy) suggest that this technique should soon be more readily available in clinical settings.
5. Conclusion
Clinical symptoms, neuropsychological testing and post-mortem pathology have been the main approaches for diagnosis of HIV/neuroAIDS. A great deal of studies have been attempted to identify more objective laboratory-based quantitative CSF/blood biomarkers for HIV/neuroAIDS. Neuroimaging for HIV/neuroAIDS still remains an attractive option, despite the nature of being expensive and not readily accessible. It is conceivable that proteomics and metabolomics will expedite the HIV/neuroAIDS biomarker discovery and validation and will surely facilitate development of anti-HIV/neuroAIDS therapeutics and clinical management of HIV/neuroAIDS. Considering the variable specificity of each biomarker, a combinational approach in clinical implementation of multiple HIV/neuroAIDS biomarkers is expected to improve the detection, staging, and therapeutic monitoring of HIV/neuroAIDS.
Acknowledgments
This work was supported by the grants NIH/NINDS R01NS065785, NIH/NINDS R01094108 and NIH/NIMH R01MH092673 (to JJH) from National Institutes of Health. PJ was supported in part by the Neurobiology of Ageing Training Grant T32 AG020494 (PI: Dr. Meharvan Singh).
List of Abbreviations
- Aβ42
β-amyloid of 42 kiloDaltons
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- ALS
(amyotrophic lateral sclerosis
- BOLD
blood oxygen level-dependent contrast imaging
- cART
combination anti-retroviral therapy
- CBF
cerebral blood flow
- CHO
choline
- CNS
central nervous system
- Cr
creatine
- CSF
cerebrospinal fluid
- CT
computerized tomography
- CXCL-10
interferon gamma-induced protein 10
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- fMRI
functional magnetic resonance imaging
- GFAP
glial fibrillary acidic protein
- GM
grey matter
- HAD
HIV-associated dementia
- HAND
HIV-associated neurocognitive disorders
- HIV
human immune deficiency virus
- HIVE
HIV encephalitis
- IF
intermediate filament
- IFN-γ
interferon γ
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- IL-8
interleukin-8
- IL-10
interleukin-10
- LPS
lipopolysaccharide
- MCMD
mild cognitive and motor disorder
- MCP-1
monocyte chemo-attractant protein-1
- MD
mean diffusion
- MI
myoinositol
- MIP-1
macrophage inflammatory protein
- miRNA
microRNA
- MRI
magnetic resonance imaging
- MS
multiple sclerosis
- MRS
magnetic resonance spectroscopy
- MTR
magnetization transfer imaging
- NAA
N-acetylaspartate
- NF
neurofilament protein
- PLA2
phospholipase A2
- PLC
phospholipase C
- PMBC
peripheral blood mononuclear cells
- PPAR
peroxisome proliferator-activated receptors
- PrPC
protease resistant protein, cellular isoform
- p-Tau
hyperphosphorylated Tau protein
- S100B
calcium binding protein B
- sAPP
soluble amyloid precursor protein
- sCD14
soluble CD14
- sCD163
soluble CD163
- SIV
simian immune deficiency virus
- sPrPC
soluble protease resistant protein, cellular isoform
- TNF-α
Tumor necrosis factor α
- t-Tau
total Tau protein
- WM
white matter
- YKL-40
human cartilage glycoprotein 39
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdulle S, Mellgren Å, Brew BJ, Cinque P, Hagberg L, Price RW, Rosengren L, Gisslén M. CSF neurofilament protein (NFL)—a marker of active HIV-related neurodegeneration. Journal of neurology. 2007;254:1026–1032. doi: 10.1007/s00415-006-0481-8. [DOI] [PubMed] [Google Scholar]
- Alais S, Soto-Rifo R, Balter V, Gruffat H, Manet E, Schaeffer L, Darlix JL, Cimarelli A, Raposo G, Ohlmann T. Functional mechanisms of the cellular prion protein (PrPC) associated anti-HIV-1 properties. Cellular and Molecular Life Sciences. 2012;69:1331–1352. doi: 10.1007/s00018-011-0879-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Benzinger TL, Christensen JJ, Thomas J, Venkat R, Teshome M, Aldea P, Fagan AM, Holtzman DM, Morris JC. HIV associated neurocognitive disorder (HAND) is not associated with increased fibrillar amyloid deposits using 11C-PiB in middle-aged HIV+ participants. Archives of neurology. 2012a;69:72. doi: 10.1001/archneurol.2011.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. Journal of acquired immune deficiency syndromes (1999) 2012b;59:469. doi: 10.1097/QAI.0b013e318249db17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Vaida F, Yeh MJ, Liang CL, Buxton RB, Letendre S, McCutchan JA, Ellis RJ. HIV infection and aging independently affect brain function as measured by functional magnetic resonance imaging. Journal of Infectious Diseases. 2010;201:336–340. doi: 10.1086/649899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AM, Harezlak J, Bharti A, Mi D, Taylor MJ, Daar ES, Schifitto G, Zhong J, Alger JR, Brown MS. Plasma and cerebrospinal fluid biomarkers predict cerebral injury in HIV-infected individuals on stable combination antiretroviral therapy. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2015;69:29–35. doi: 10.1097/QAI.0000000000000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson LM, Fredman P, Lekman A, Rosengren L, Gisslen M. Increased cerebrospinal fluid ganglioside GD3 concentrations as a marker of microglial activation in HIV type 1 infection. AIDS Res Hum Retroviruses. 1998;14:1065–1069. doi: 10.1089/aid.1998.14.1065. [DOI] [PubMed] [Google Scholar]
- Andersson LM, Hagberg L, Rosengren L, Fuchs D, Blennow K, Gisslen M. Normalisation of cerebrospinal fluid biomarkers parallels improvement of neurological symptoms following HAART in HIV dementia–case report. BMC Infect Dis. 2006;6:141. doi: 10.1186/1471-2334-6-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol. 2006;111:529–538. doi: 10.1007/s00401-006-0037-0. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, Heaton RK, Grant I, Mallory M, Miller A. Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Archives of Neurology. 2004;61:369–376. doi: 10.1001/archneur.61.3.369. [DOI] [PubMed] [Google Scholar]
- Arthurs OJ, Boniface S. How well do we understand the neural origins of the fMRI BOLD signal? TRENDS in Neurosciences. 2002;25:27–31. doi: 10.1016/s0166-2236(00)01995-0. [DOI] [PubMed] [Google Scholar]
- Bandaru VV, McArthur JC, Sacktor N, Cutler RG, Knapp EL, Mattson MP, Haughey NJ. Associative and predictive biomarkers of dementia in HIV-1–infected patients. Neurology. 2007;68:1481–1487. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru VVR, Mielke MM, Sacktor N, McArthur JC, Grant I, Letendre S, Chang L, Wojna V, Pardo C, Calabresi P. A lipid storage–like disorder contributes to cognitive decline in HIV-infected subjects. Neurology. 2013;81:1492–1499. doi: 10.1212/WNL.0b013e3182a9565e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- Beck SE, Queen SE, Witwer KW, Pate KAM, Mangus LM, Gama L, Adams RJ, Clements JE, Zink MC, Mankowski JL. Paving the path to HIV neurotherapy: Predicting SIV CNS disease. European journal of pharmacology. 2015;759:303–312. doi: 10.1016/j.ejphar.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, Cohen B, Goodkin K, Martin E, Miller EN. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain imaging and behavior. 2011;5:77–85. doi: 10.1007/s11682-011-9113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JE, Anthony IC, Simmonds P. Impact of HIV on regional & cellular organisation of the brain. Curr HIV Res. 2006;4:249–257. doi: 10.2174/157016206777709401. [DOI] [PubMed] [Google Scholar]
- Bennasser Y, Jeang KT. HIV-1 Tat interaction with Dicer: requirement for RNA. Retrovirology. 2006;3:95. doi: 10.1186/1742-4690-3-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelli E, Regoli M, Gambelli F, Lucattelli M, Lungarella G, Bastianini A. GFAP is expressed as a major soluble pool associated with glucagon secretory granules in A-cells of mouse pancreas. J Histochem Cytochem. 2000;48:1233–1242. doi: 10.1177/002215540004800907. [DOI] [PubMed] [Google Scholar]
- Bian S, Hong J, Li Q, Schebelle L, Pollock A, Knauss JL, Garg V, Sun T. MicroRNA cluster miR-17-92 regulates neural stem cell expansion and transition to intermediate progenitors in the developing mouse neocortex. Cell reports. 2013;3:1398–1406. doi: 10.1016/j.celrep.2013.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Zetterberg H. Understanding Biomarkers of Neurodegeneration: Ultrasensitive detection techniques pave the way for mechanistic understanding. Nature medicine. 2015;21:217–219. doi: 10.1038/nm.3810. [DOI] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Bissel SJ, Wang G, Fish KN, Nicholl GC, Darko SW, Medina-Flores R, Murphey-Corb M, Rajakumar PA, Nyaundi J. YKL-40, a marker of simian immunodeficiency virus encephalitis, modulates the biological activity of basic fibroblast growth factor. The American journal of pathology. 2008;173:130–143. doi: 10.2353/ajpath.2008.080045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Wang G, Starkey A, Hamilton RL, Wiley CA. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. Journal of neuroinflammation. 2010;7:34. doi: 10.1186/1742-2094-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Bissel SJ, Kofler J, Starkey A, Wang G, Wiley CA. Astrocyte and Macrophage Regulation of YKL-40 Expression and Cellular Response in Neuroinflammation. Brain pathology. 2012;22:530–546. doi: 10.1111/j.1750-3639.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew BJ. HIV, the brain, children, HAART and ‘neuro-HAART’: a complex mix. AIDS. 2009;23:1909–1910. doi: 10.1097/QAD.0b013e32832ec4c6. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Bhalla RB, Paul M, Gallardo H, McArthur JC, Schwartz MK, Price RW. Cerebrospinal fluid neopterin in human immunodeficiency virus type 1 infection. Ann Neurol. 1990;28:556–560. doi: 10.1002/ana.410280413. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Bhalla RB, Paul M, Sidtis JJ, Keilp JJ, Sadler AE, Gallardo H, McArthur JC, Schwartz MK, Price RW. Cerebrospinal fluid beta 2-microglobulin in patients with AIDS dementia complex: an expanded series including response to zidovudine treatment. AIDS. 1992;6:461–465. [PubMed] [Google Scholar]
- Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology. 2005;65:1490–1492. doi: 10.1212/01.wnl.0000183293.95787.b7. [DOI] [PubMed] [Google Scholar]
- Bujdoso R, Landgraf M, Jackson WS, Thackray AM. Prion-induced neurotoxicity: Possible role for cell cycle activity and DNA damage response. World journal of virology. 2015;4:188–197. doi: 10.5501/wjv.v4.i3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, Rosenberg ES, Williams KC, Grinspoon S. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. Journal of Infectious Diseases. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS (London, England) 2013;27 doi: 10.1097/QAD.0b013e32836010bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagno A, Atzori C, Romito A, Vai D, Audagnotto S, Stella M, Montrucchio C, Imperiale D, Di Perri G, Bonora S. Blood brain barrier impairment is associated with cerebrospinal fluid markers of neuronal damage in HIV-positive patients. Journal of neurovirology. 2015:1–5. doi: 10.1007/s13365-015-0371-x. [DOI] [PubMed] [Google Scholar]
- Cao B, Kong X, Kettering C, Yu P, Ragin A. Determinants of HIV-induced brain changes in three different periods of the early clinical course: A data mining analysis. NeuroImage Clinical. 2015;9:75–82. doi: 10.1016/j.nicl.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Cherubini A, Howard R, Tarducci R, Pelliccioli G, Piccirilli M, Gobbi G, Senin U, Mecocci P. 1H-MR spectroscopy differentiates mild cognitive impairment from normal brain aging. Neuroreport. 2001;12:2315–2317. doi: 10.1097/00001756-200108080-00007. [DOI] [PubMed] [Google Scholar]
- Chang L, Andres M, Sadino J, Jiang C, Nakama H, Miller E, Ernst T. Impact of apolipoprotein E ε4 and HIV on cognition and brain atrophy: antagonistic pleiotropy and premature brain aging. Neuroimage. 2011;58:1017–1027. doi: 10.1016/j.neuroimage.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, Poland RE, Jenden DJ. In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life sciences. 1996;58:2049–2056. doi: 10.1016/0024-3205(96)00197-x. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, St Hillaire C, Conant K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antiviral therapy. 2004a;9:431–440. doi: 10.1177/135965350400900302. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naıve HIV patients. Neuroimage. 2002;17:1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- Chang L, Lee P, Yiannoutsos C, Ernst T, Marra C, Richards T, Kolson D, Schifitto G, Jarvik J, Miller E. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004b;23:1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- Chang L, Tomasi D, Yakupov R, Lozar C, Arnold S, Caparelli E, Ernst T. Adaptation of the attention network in human immunodeficiency virus brain injury. Annals of neurology. 2004c;56:259–272. doi: 10.1002/ana.20190. [DOI] [PubMed] [Google Scholar]
- Chang L, Wong V, Nakama H, Watters M, Ramones D, Miller EN, Cloak C, Ernst T. Greater than age-related changes in brain diffusion of HIV patients after 1 year. Journal of Neuroimmune Pharmacology. 2008;3:265–274. doi: 10.1007/s11481-008-9120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu FC, Goldman JE. Regulation of glial fibrillary acidic protein (GFAP) expression in CNS development and in pathological states. J Neuroimmunol. 1985;8:283–292. doi: 10.1016/s0165-5728(85)80067-9. [DOI] [PubMed] [Google Scholar]
- Chong W, Sweeney B, Wilkinson I, Paley M, Hall-Craggs M, Kendall B, Shepard J, Beecham M, Miller R, Weller I. Proton spectroscopy of the brain in HIV infection: correlation with clinical, immunologic, and MR imaging findings. Radiology. 1993;188:119–124. doi: 10.1148/radiology.188.1.8099750. [DOI] [PubMed] [Google Scholar]
- Cinque P, Bestetti A, Marenzi R, Sala S, Gisslen M, Hagberg L, Price RW. Cerebrospinal fluid interferon-gamma-inducible protein 10 (IP-10, CXCL10) in HIV-1 infection. J Neuroimmunol. 2005;168:154–163. doi: 10.1016/j.jneuroim.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Clifford D, Fagan A, Holtzman D, Morris J, Teshome M, Shah A, Kauwe J. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73:1982–1987. doi: 10.1212/WNL.0b013e3181c5b445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloak CC, Chang L, Ernst T. Increased frontal white matter diffusion is associated with glial metabolites and psychomotor slowing in HIV. J Neuroimmunol. 2004;157:147–152. doi: 10.1016/j.jneuroim.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Gongvatana A. HIV-associated brain dysfunction in the era of HAART: reasons for hope, but continued concern. Neurology. 2009;73:338–339. doi: 10.1212/WNL.0b013e3181b1220d. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Seider TR, Navia B. HIV effects on age-associated neurocognitive dysfunction: premature cognitive aging or neurodegenerative disease? Alzheimer’s research & therapy. 2015;7:37. doi: 10.1186/s13195-015-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman BM, Hill AF. Extracellular vesicles–Their role in the packaging and spread of misfolded proteins associated with neurodegenerative diseases. Seminars in cell & developmental biology. 2015;40:89–96. doi: 10.1016/j.semcdb.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Coley W, Van Duyne R, Carpio L, Guendel I, Kehn-Hall K, Chevalier S, Narayanan A, Luu T, Lee N, Klase Z. Absence of DICER in monocytes and its regulation by HIV-1. Journal of Biological Chemistry. 2010;285:31930–31943. doi: 10.1074/jbc.M110.101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proceedings of the National Academy of Sciences. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino AA, Huang Y, Zhang H, Wood C, Zheng JC. HIV-1 clade B and C isolates exhibit differential replication: relevance to macrophage-mediated neurotoxicity. Neurotoxicity research. 2011;20:277–288. doi: 10.1007/s12640-011-9241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ, Mintun MA, Peskind ER, Li G, Galasko DR, Clark CM, Quinn JF, D’Angelo G, Malone JP, Townsend RR, Morris JC, Fagan AM, Holtzman DM. YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biological psychiatry. 2010;68:903–912. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10:350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- Dahl V, Peterson J, Fuchs D, Gisslen M, Palmer S, Price RW. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. Aids. 2014;28:2251–2258. doi: 10.1097/QAD.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida SM, Letendre S, Zimmerman J, Kolakowski S, Lazzaretto D, McCutchan JA, Ellis R. Relationship of CSF leukocytosis to compartmentalized changes in MCP-1/CCL2 in the CSF of HIV-infected patients undergoing interruption of antiretroviral therapy. Journal of neuroimmunology. 2006;179:180–185. doi: 10.1016/j.jneuroim.2006.06.018. [DOI] [PubMed] [Google Scholar]
- de Almeida SM, Ribeiro CE, de Pereira AP, Badiee J, Cherner M, Smith D, Maich I, Raboni SM, Rotta I, Barbosa FJ, Heaton RK, Umlauf A, Ellis RJ. Neurocognitive impairment in HIV-1 clade C- versus B-infected individuals in Southern Brazil. Journal of neurovirology. 2013;19:550–556. doi: 10.1007/s13365-013-0215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Olmos JS, Beltramino CA, de Olmos de Lorenzo S. Use of an amino-cupric-silver technique for the detection of early and semiacute neuronal degeneration caused by neurotoxicants, hypoxia, and physical trauma. Neurotoxicol Teratol. 1994;16:545–561. doi: 10.1016/0892-0362(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Del Valle L, Pina-Oviedo S. HIV disorders of the brain: pathology and pathogenesis. Front Biosci. 2006;11:718–732. doi: 10.2741/1830. [DOI] [PubMed] [Google Scholar]
- Dousset V, Armand JP, Huot P, Viaud B, Caille JM. Magnetization transfer imaging in AIDS-related brain diseases. Neuroimaging Clin N Am. 1997;7:447–460. [PubMed] [Google Scholar]
- Drew PD, Xu J, Storer PD, Chavis JA, Racke MK. Peroxisome proliferator-activated receptor agonist regulation of glial activation: relevance to CNS inflammatory disorders. Neurochemistry international. 2006;49:183–189. doi: 10.1016/j.neuint.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Duskova K, Nagilla P, Le HS, Iyer P, Thalamuthu A, Martinson J, Bar-Joseph Z, Buchanan W, Rinaldo C, Ayyavoo V. MicroRNA regulation and its effects on cellular transcriptome in human immunodeficiency virus-1 (HIV-1) infected individuals with distinct viral load and CD4 cell counts. BMC infectious diseases. 2013;13:250. doi: 10.1186/1471-2334-13-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, Costagliola D, D’Arminio Monforte A, de Wolf F, Reiss P, Lundgren JD, Justice AC, Staszewski S, Leport C, Hogg RS, Sabin CA, Gill MJ, Salzberger B, Sterne JA. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L. Effect of aging on brain metabolism in antiretroviral-naive HIV patients. Aids. 2004;18:61–67. [PubMed] [Google Scholar]
- Ernst T, Chang L, Arnold S. Increased glial metabolites predict increased working memory network activation in HIV brain injury. Neuroimage. 2003;19:1686–1693. doi: 10.1016/s1053-8119(03)00232-5. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Jovicich J, Ames N, Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59:1343–1349. doi: 10.1212/01.wnl.0000031811.45569.b0. [DOI] [PubMed] [Google Scholar]
- Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: A new mechanism for HIV-associated neurocognitive disorder. Journal of Magnetic Resonance Imaging. 2010;32:1045–1053. doi: 10.1002/jmri.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Yakupov R, Nakama H, Crocket G, Cole M, Watters M, Ricardo-Dukelow ML, Chang L. Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Annals of neurology. 2009;65:316–325. doi: 10.1002/ana.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton MR, Lyons JL, Ard KL. HIV-associated Neurocognitive Disorders and Antiretroviral Therapy: Current Concepts and Controversies. Current infectious disease reports. 2015;17:485. doi: 10.1007/s11908-015-0485-6. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood–brain barrier: a potential mechanism of HIV–CNS invasion and NeuroAIDS. The Journal of neuroscience. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. Journal of neurochemistry. 2007;101:577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- Filippi CG, Ulug AM, Ryan E, Ferrando SJ, van Gorp W. Diffusion tensor imaging of patients with HIV and normal-appearing white matter on MR images of the brain. AJNR Am J Neuroradiol. 2001;22:277–283. [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Tedaldi EM, Rappaport J. CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS research and human retroviruses. 2008;24:417–421. doi: 10.1089/aid.2007.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HS. Biomarkers for NeuroAIDS: recent progress in the field. Journal of Neuroimmune Pharmacology. 2013;8:1055–1058. doi: 10.1007/s11481-013-9515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs D, Chiodi F, Albert J, Asjö B, Hagberg L, Hausen A, Norkrans G, Reibnegger G, Werner ER, Wachter H. Neopterin concentrations in cerebrospinal fluid and serum of individuals infected with HIV-1. Aids. 1989;3:285–288. doi: 10.1097/00002030-198905000-00006. [DOI] [PubMed] [Google Scholar]
- Fuchs D, Hausen A, Reibnegger G, Werner ER, Dierich MP, Wachter H. Neopterin as a marker for activated cell-mediated immunity: application in HIV infection. Immunology today. 1988;9:150–155. doi: 10.1016/0167-5699(88)91203-0. [DOI] [PubMed] [Google Scholar]
- Fuchs E. The cytoskeleton and disease: genetic disorders of intermediate filaments. Annu Rev Genet. 1996;30:197–231. doi: 10.1146/annurev.genet.30.1.197. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Funda DP, Tuckova L, Farre MA, Iwase T, Moro I, Tlaskalova-Hogenova H. CD14 is expressed and released as soluble CD14 by human intestinal epithelial cells in vitro: lipopolysaccharide activation of epithelial cells revisited. Infection and immunity. 2001;69:3772–3781. doi: 10.1128/IAI.69.6.3772-3781.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi N, Saiyed Z, Thangavel S, Rodriguez J, Rao KV, Nair MP. Differential effects of HIV type 1 clade B and clade C Tat protein on expression of proinflammatory and antiinflammatory cytokines by primary monocytes. AIDS Res Hum Retroviruses. 2009;25:691–699. doi: 10.1089/aid.2008.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Kolson DL, Babb JS, Mannon LJ, Grossman RI. Whole brain imaging of HIV-infected patients: quantitative analysis of magnetization transfer ratio histogram and fractional brain volume. AJNR Am J Neuroradiol. 2003;24:82–87. [PMC free article] [PubMed] [Google Scholar]
- Gelman BB. Neuropathology of HAND With Suppressive Antiretroviral Therapy: Encephalitis and Neurodegeneration Reconsidered. Current HIV/AIDS reports. 2015;12:272–279. doi: 10.1007/s11904-015-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisslén M, Krut J, Andreasson U, Blennow K, Cinque P, Brew BJ, Spudich S, Hagberg L, Rosengren L, Price RW. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC neurology. 2009;9:63. doi: 10.1186/1471-2377-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongvatana A, Cohen RA, Correia S, Devlin KN, Miles J, Kang H, Ombao H, Navia B, Laidlaw DH, Tashima KT. Clinical contributors to cerebral white matter integrity in HIV-infected individuals. Journal of neurovirology. 2011;17:477–486. doi: 10.1007/s13365-011-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorry P, Purcell D, Howard J, McPhee D. Restricted HIV-1 infection of human astrocytes: potential role of nef in the regulation of virus replication. J Neurovirol. 1998;4:377–386. doi: 10.3109/13550289809114536. [DOI] [PubMed] [Google Scholar]
- Green AJ, Giovannoni G, Hall-Craggs MA, Thompson EJ, Miller RF. Cerebrospinal fluid tau concentrations in HIV infected patients with suspected neurological disease. Sexually transmitted infections. 2000;76:443–446. doi: 10.1136/sti.76.6.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresle MM, Butzkueven H, Shaw G. Neurofilament proteins as body fluid biomarkers of neurodegeneration in multiple sclerosis. Multiple sclerosis international. 2011 doi: 10.1155/2011/315406. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DE, McArthur JC, Cornblath DR. Neopterin and interferon-gamma in serum and cerebrospinal fluid of patients with HIV-associated neurologic disease. Neurology. 1991;41:69–69. doi: 10.1212/wnl.41.1.69. [DOI] [PubMed] [Google Scholar]
- Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, Karon J, Brookmeyer R, Kaplan EH, McKenna MT, Janssen RS. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M, Whaley R, Robertson K, Hamby S, Wilkins J, Hall C. The correlation between neuropsychological and neuroanatomic changes over time in asymptomatic and symptomatic HIV-1-infected individuals. Neurology. 1996;46:1697–1702. doi: 10.1212/wnl.46.6.1697. [DOI] [PubMed] [Google Scholar]
- Harrington PR, Haas DW, Ritola K, Swanstrom R. Compartmentalized human immunodeficiency virus type 1 present in cerebrospinal fluid is produced by short-lived cells. J Virol. 2005;79:7959–7966. doi: 10.1128/JVI.79.13.7959-7966.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Annals of neurology. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- Hayes AM, Qian S, Yu L, Boris-Lawrie K. Tat RNA silencing suppressor activity contributes to perturbation of lymphocyte miRNA by HIV-1. Retrovirology. 2011;8:36. doi: 10.1186/1742-4690-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes MP, Brew BJ, Martin A, Price RW, Salazar AM, Sidtis JJ, Yergey JA, Mouradian MM, Sadler AE, Keilp J, et al. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann Neurol. 1991;29:202–209. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- Holt JL, Kraft-Terry SD, Chang L. Neuroimaging studies of the aging HIV-1-infected brain. Journal of neurovirology. 2012;18:291–302. doi: 10.1007/s13365-012-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain, behavior, and immunity. 2015;45:1–12. doi: 10.1016/j.bbi.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houzet L, Yeung ML, de Lame V, Desai D, Smith SM, Jeang KT. MicroRNA profile changes in human immunodeficiency virus type 1 (HIV-1) seropositive individuals. Retrovirology. 2008;5:118. doi: 10.1186/1742-4690-5-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen J, Gyldensted C, Brun B, Bruhn P, Helweg-Larsen S, Arlien-Soborg P. Cerebral ventricular enlargement relates to neuropsychological measures in unselected AIDS patients. Acta Neurol Scand. 1989;79:59–62. doi: 10.1111/j.1600-0404.1989.tb03710.x. [DOI] [PubMed] [Google Scholar]
- Jessen Krut J, Mellberg T, Price RW, Hagberg L, Fuchs D, Rosengren L, Nilsson S, Zetterberg H, Gisslén M. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PloS one. 2014;9:e88591. doi: 10.1371/journal.pone.0088591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin XF, Wu N, Wang L, Li J. Circulating microRNAs: a novel class of potential biomarkers for diagnosing and prognosing central nervous system diseases. Cellular and molecular neurobiology. 2013;33:601–613. doi: 10.1007/s10571-013-9940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadri F, LaPlante A, De Luca M, Doyle L, Velasco-Gonzalez C, Patterson JR, Molina PE, Nelson S, Zea A, Parsons CH. Defining Plasma MicroRNAs Associated With Cognitive Impairment in Hiv-Infected Patients. Journal of cellular physiology. 2015 doi: 10.1002/jcp.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. Journal of acquired immune deficiency syndromes (1999) 2012;60:234. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolson DL. YKL-40: a candidate biomarker for simian immunodeficiency virus and human immunodeficiency virus encephalitis? The American journal of pathology. 2008;173:25–29. doi: 10.2353/ajpath.2008.080389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Krut JJ, Zetterberg H, Blennow K, Cinque P, Hagberg L, Price RW, Studahl M, Gisslén M. Cerebrospinal fluid Alzheimer’s biomarker profiles in CNS infections. Journal of neurology. 2013;260:620–626. doi: 10.1007/s00415-012-6688-y. [DOI] [PubMed] [Google Scholar]
- Kusdra L, McGuire D, Pulliam L. Changes in monocyte/macrophage neurotoxicity in the era of HAART: implications for HIV-associated dementia. AIDS. 2002;16:31–38. doi: 10.1097/00002030-200201040-00005. [DOI] [PubMed] [Google Scholar]
- Lee PL, Yiannoutsos CT, Ernst T, Chang L, Marra CM, Jarvik JG, Richards TL, Kwok EW, Kolson DL, Simpson D, Tang CY, Schifitto G, Ketonen LM, Meyerhoff DJ, Lenkinski RE, Gonzalez RG, Navia BA. A multi-center 1H MRS study of the AIDS dementia complex: validation and preliminary analysis. J Magn Reson Imaging. 2003;17:625–633. doi: 10.1002/jmri.10295. [DOI] [PubMed] [Google Scholar]
- Lee SC, Hatch WC, Liu W, Brosnan CF, Dickson DW. Productive infection of human fetal microglia in vitro by HIV-1. Ann N Y Acad Sci. 1993;693:314–316. doi: 10.1111/j.1749-6632.1993.tb26295.x. [DOI] [PubMed] [Google Scholar]
- Lee Y, Samaco RC, Gatchel JR, Thaller C, Orr HT, Zoghbi HY. miR-19, miR-101 and miR-130 co-regulate ATXN1 levels to potentially modulate SCA1 pathogenesis. Nature neuroscience. 2008;11:1137–1139. doi: 10.1038/nn.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre SL, Lanier ER, McCutchan JA. Cerebrospinal fluid beta chemokine concentrations in neurocognitively impaired individuals infected with human immunodeficiency virus type 1. J Infect Dis. 1999;180:310–319. doi: 10.1086/314866. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nature medicine. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu H, Kim BO, Gattone VH, Li J, Nath A, Blum J, He JJ. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J Virol. 2004;78:4120–4133. doi: 10.1128/JVI.78.8.4120-4133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord PG, Papoian T. Genomics and drug toxicity. Science. 2004;306:575. doi: 10.1126/science.1105854. [DOI] [PubMed] [Google Scholar]
- Luo X, Carlson KA, Wojna V, Mayo R, Biskup TM, Stoner J, Anderson J, Gendelman HE, Melendez LM. Macrophage proteomic fingerprinting predicts HIV-1-associated cognitive impairment. Neurology. 2003;60:1931–1937. doi: 10.1212/01.wnl.0000064396.54554.26. [DOI] [PubMed] [Google Scholar]
- Luo X, He JJ. Cell-cell contact viral transfer contributes to HIV infection and persistence in astrocytes. Journal of neurovirology. 2015;21:66–80. doi: 10.1007/s13365-014-0304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons JL, Uno H, Ancuta P, Kamat A, Moore DJ, Singer EJ, Morgello S, Gabuzda D. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. Journal of acquired immune deficiency syndromes (1999) 2011;57:371. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machleidt T, Krämer B, Adam D, Neumann B, Schütze S, Wiegmann K, Krönke M. Function of the p55 tumor necrosis factor receptor “death domain” mediated by phosphatidylcholine-specific phospholipase C. The Journal of experimental medicine. 1996;184:725–733. doi: 10.1084/jem.184.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N. CSF biomarkers in neurodegenerative diseases. Clinical chemistry and laboratory medicine : CCLM/FESCC. 2011;49:345–352. doi: 10.1515/CCLM.2011.082. [DOI] [PubMed] [Google Scholar]
- May M, Sterne JA, Sabin C, Costagliola D, Justice AC, Thiebaut R, Gill J, Phillips A, Reiss P, Hogg R, Ledergerber B, D’Arminio Monforte A, Schmeisser N, Staszewski S, Egger M. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. AIDS. 2007;21:1185–1197. doi: 10.1097/QAD.0b013e328133f285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MT, Sterne JA, Costagliola D, Sabin CA, Phillips AN, Justice AC, Dabis F, Gill J, Lundgren J, Hogg RS, de Wolf F, Fatkenheuer G, Staszewski S, d’Arminio Monforte A, Egger M. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006;368:451–458. doi: 10.1016/S0140-6736(06)69152-6. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Cohen BA, Farzedegan H, Cornblath DR, Selnes OA, Ostrow D, Johnson RT, Phair J, Polk BF. Cerebrospinal fluid abnormalities in homosexual men with and without neuropsychiatric findings. Ann Neurol. 1988;23(Suppl):S34–37. doi: 10.1002/ana.410230712. [DOI] [PubMed] [Google Scholar]
- McCONNELL JR, SWINDELLS S, ONG CS, GMEINER WH, CHU WK, BROWN DK, GENDELMAN HE. Prospective utility of cerebral proton magnetic resonance spectroscopy in monitoring HIV infection and its associated neurological impairment. AIDS research and human retroviruses. 1994;10:977–982. doi: 10.1089/aid.1994.10.977. [DOI] [PubMed] [Google Scholar]
- McGuire JL, Gill AJ, Douglas SD, Kolson DL. Central and peripheral markers of neurodegeneration and monocyte activation in HIV-associated neurocognitive disorders. Journal of neurovirology. 2015:1–10. doi: 10.1007/s13365-015-0333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara P, Dunne P, Lawlor B, Redmond J, Doherty C, Doherty D, Bergin C. Cerebrospinal Fluid (CSF) Findings of Patients with HIV Associated Neurocognitive Disorders (HAND)(P2.316) Neurology. 2015;84 [Google Scholar]
- Megra B, Eugenin E, Roberts T, Morgello S, Berman J. Protease Resistant Protein Cellular Isoform (PrPc) as a Biomarker: Clues into the Pathogenesis of HAND. Journal of Neuroimmune Pharmacology. 2013;8:1159–1166. doi: 10.1007/s11481-013-9458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehla R, Bivalkar-Mehla S, Nagarkatti M, Chauhan A. Programming of neurotoxic cofactor CXCL-10 in HIV-1-associated dementia: abrogation of CXCL-10-induced neuro-glial toxicity in vitro by PKC activator. J Neuroinflammation. 2012;9:2094–2099. doi: 10.1186/1742-2094-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellios N, Huang HS, Grigorenko A, Rogaev E, Akbarian S. A set of differentially expressed miRNAs, including miR-30a-5p, act as post-transcriptional inhibitors of BDNF in prefrontal cortex. Hum Mol Genet. 2008;17:3030–3042. doi: 10.1093/hmg/ddn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M, Vetrivel S, Siddappa NB, Ranga U, Seth P. Clade-specific differences in neurotoxicity of human immunodeficiency virus-1 B and C Tat of human neurons: significance of dicysteine C30C31 motif. Annals of neurology. 2008;63:366–376. doi: 10.1002/ana.21292. [DOI] [PubMed] [Google Scholar]
- Miyatani N, Saito M, Ariga T, Yoshino H, Robert KY. Glycosphingolipids in the cerebrospinal fluid of patients with multiple sclerosis. Molecular and chemical neuropathology. 1990;13:205–216. doi: 10.1007/BF03159923. [DOI] [PubMed] [Google Scholar]
- Mothapo K, Stelma F, Janssen M, Kessels R, Miners S, Verbeek M, Koopmans P, van der Ven A. Amyloid beta-42 (Aβ-42), neprilysin and cytokine levels. A pilot study in patients with HIV related cognitive impairments. Journal of neuroimmunology. 2015;282:73–79. doi: 10.1016/j.jneuroim.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Munoz A, Sabin CA, Phillips AN. The incubation period of AIDS. Aids. 1997;11(Suppl A):S69–76. [PubMed] [Google Scholar]
- Munshi SU, Panda H, Holla P, Rewari BB, Jameel S. MicroRNA-150 is a potential biomarker of HIV/AIDS disease progression and therapy. PloS one. 2014;9:e95920. doi: 10.1371/journal.pone.0095920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nhan H, Chiang K, Koo E. The multifaceted nature of amyloid precursor protein and its proteolytic fragments: friends and foes. Acta Neuropathol. 2015;129:1–19. doi: 10.1007/s00401-014-1347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorbakhsh F, Ramachandran R, Barsby N, Ellestad KK, LeBlanc A, Dickie P, Baker G, Hollenberg MD, Cohen ÉA, Power C. MicroRNA profiling reveals new aspects of HIV neurodegeneration: caspase-6 regulates astrocyte survival. The FASEB Journal. 2010;24:1799–1812. doi: 10.1096/fj.09-147819. [DOI] [PubMed] [Google Scholar]
- Norenberg MD. Active and passive roles of astrocytes in neurologic disease: commentary on forum position paper. Neurotoxicology. 1998;19:23–26. discussion 37-28. [PubMed] [Google Scholar]
- Norgren N, Rosengren L, Stigbrand T. Elevated neurofilament levels in neurological diseases. Brain Res. 2003;987:25–31. doi: 10.1016/s0006-8993(03)03219-0. [DOI] [PubMed] [Google Scholar]
- Norton WT, Aquino DA, Hozumi I, Chiu FC, Brosnan CF. Quantitative aspects of reactive gliosis: a review. Neurochem Res. 1992;17:877–885. doi: 10.1007/BF00993263. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Sriram K. Glial fibrillary acidic protein and related glial proteins as biomarkers of neurotoxicity. Expert Opin Drug Saf. 2005;4:433–442. doi: 10.1517/14740338.4.3.433. [DOI] [PubMed] [Google Scholar]
- Ohtani Y, Tamai Y, Ohnuki Y, Miura S. Ganglioside alterations in the central and peripheral nervous systems of patients with Creutzfeldt-Jakob disease. Neurodegeneration. 1996;5:331–338. doi: 10.1006/neur.1996.0045. [DOI] [PubMed] [Google Scholar]
- Onofre G, Kolackova M, Jankovicova K, Krejsek J. Scavenger receptor CD163 and its biological functions. Acta medica. 2009;52:57–61. [PubMed] [Google Scholar]
- Ortega M, Ances B. Role of HIV in Amyloid Metabolism. Journal of Neuroimmune Pharmacology. 2014;9:483–491. doi: 10.1007/s11481-014-9546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici M, Delbue S, Ferrante P, Jeansonne D, Kadri F, Nelson S, Velasco-Gonzalez C, Zabaleta J, Peruzzi F. Cerebrospinal fluid miRNA profile in HIV-encephalitis. Journal of cellular physiology. 2013;228:1070–1075. doi: 10.1002/jcp.24254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick C, Crews L, Desplats P, Dumaop W, Rockenstein E, Achim CL, Everall IP, Masliah E. Increased CDK5 expression in HIV encephalitis contributes to neurodegeneration via tau phosphorylation and is reversed with Roscovitine. The American journal of pathology. 2011;178:1646–1661. doi: 10.1016/j.ajpath.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neurosci Biobehav Rev. 2002;26:353–359. doi: 10.1016/s0149-7634(02)00006-4. [DOI] [PubMed] [Google Scholar]
- Pedersen KK, Eiersted MR, Gaardbo JC, Pedersen M, Gerstoft J, Troseid M, Nielsen SD. Lower Self-Reported Quality of Life in HIV-Infected Patients on cART and With Low Comorbidity Compared With Healthy Controls. Journal of acquired immune deficiency syndromes. 2015;70:16–22. doi: 10.1097/QAI.0000000000000697. [DOI] [PubMed] [Google Scholar]
- Peluso MJ, Meyerhoff DJ, Price RW, Peterson J, Lee E, Young AC, Walter R, Fuchs D, Brew BJ, Cinque P. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. Journal of Infectious Diseases. 2013:jit088. doi: 10.1093/infdis/jit088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso MJ, Valcour V, Ananworanich J, Sithinamsuwan P, Chalermchai T, Fletcher JL, Lerdlum S, Chomchey N, Slike B, Sailasuta N, Gisslen M, Zetterberg H, Spudich S, Rv254/Search, Teams, S.S. Absence of Cerebrospinal Fluid Signs of Neuronal Injury Before and After Immediate Antiretroviral Therapy in Acute HIV Infection. The Journal of infectious diseases. 2015 doi: 10.1093/infdis/jiv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton LA, Brew BJ. Cerebrospinal fluid S-100β and its relationship with AIDS dementia complex. Journal of clinical virology. 2001;22:249–253. doi: 10.1016/s1386-6532(01)00196-2. [DOI] [PubMed] [Google Scholar]
- Perrella O, Carrieri PB, Guarnaccia D, Soscia M. Cerebrospinal fluid cytokines in AIDS dementia complex. Journal of neurology. 1992;239:387–388. doi: 10.1007/BF00812156. [DOI] [PubMed] [Google Scholar]
- Peterson J, Gisslen M, Zetterberg H, Fuchs D, Shacklett BL, Hagberg L, Yiannoutsos CT, Spudich SS, Price RW. Cerebrospinal Fluid (CSF) Neuronal Biomarkers across the Spectrum of HIV Infection: Hierarchy of Injury and Detection. PloS one. 2014;9:e116081. doi: 10.1371/journal.pone.0116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Sassoon SA, Kemper CA, Deresinski S, Rohlfing T, Sullivan EV. Regional brain structural dysmorphology in human immunodeficiency virus infection: effects of acquired immune deficiency syndrome, alcoholism, and age. Biological psychiatry. 2012;72:361–370. doi: 10.1016/j.biopsych.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza O, Leggiero E, De Benedictis G, Pastore L, Salvatore F, Tufano R, De Robertis E. S100B induces the release of pro-inflammatory cytokines in alveolar type I-like cells. International journal of immunopathology and pharmacology. 2013;26:383–391. doi: 10.1177/039463201302600211. [DOI] [PubMed] [Google Scholar]
- Pilitsis JG, Coplin WM, O’Regan MH, Wellwood JM, Diaz FG, Fairfax MR, Michael DB, Phillis JW. Free fatty acids in cerebrospinal fluids from patients with traumatic brain injury. Neuroscience letters. 2003;349:136–138. doi: 10.1016/s0304-3940(03)00803-6. [DOI] [PubMed] [Google Scholar]
- Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO. White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Res. 2001a;106:15–24. doi: 10.1016/s0925-4927(00)00082-2. [DOI] [PubMed] [Google Scholar]
- Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO. White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Research: Neuroimaging. 2001b;106:15–24. doi: 10.1016/s0925-4927(00)00082-2. [DOI] [PubMed] [Google Scholar]
- Power C, McArthur JC, Johnson RT, Griffin DE, Glass JD, Dewey R, Chesebro B. Distinct HIV-1 env sequences are associated with neurotropism and neurovirulence. Curr Top Microbiol Immunol. 1995;202:89–104. doi: 10.1007/978-3-642-79657-9_7. [DOI] [PubMed] [Google Scholar]
- Price R, Epstein L, Becker J, Cinque P, Gisslén M, Pulliam L, McArthur J. Biomarkers of HIV-1 CNS infection and injury. Neurology. 2007;69:1781–1788. doi: 10.1212/01.wnl.0000278457.55877.eb. [DOI] [PubMed] [Google Scholar]
- Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- Qu Y, Wu J, Chen D, Zhao F, Liu J, Yang C, Wei D, Ferriero DM, Mu D. MiR-139-5p inhibits HGTD-P and regulates neuronal apoptosis induced by hypoxia-ischemia in neonatal rats. Neurobiology of disease. 2014;63:184–193. doi: 10.1016/j.nbd.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Ragin AB, Storey P, Cohen BA, Edelman RR, Epstein LG. Disease burden in HIV-associated cognitive impairment: a study of whole-brain imaging measures. Neurology. 2004a;63:2293–2297. doi: 10.1212/01.wnl.0000147477.44791.bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragin AB, Storey P, Cohen BA, Epstein LG, Edelman RR. Whole brain diffusion tensor imaging in HIV-associated cognitive impairment. AJNR Am J Neuroradiol. 2004b;25:195–200. [PMC free article] [PubMed] [Google Scholar]
- Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG. Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. J Neurovirol. 2005;11:292–298. doi: 10.1080/13550280590953799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P, Benito E, Fischer A. MicroRNAs as biomarkers for CNS disease. Frontiers in molecular neuroscience. 2013;6:39. doi: 10.3389/fnmol.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VR, Ruiz AP, Prasad VR. Viral and cellular factors underlying neuropathogenesis in HIV associated neurocognitive disorders (HAND) AIDS research and therapy. 2014;11:13. doi: 10.1186/1742-6405-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VR, Sas AR, Eugenin EA, Siddappa NB, Bimonte-Nelson H, Berman JW, Ranga U, Tyor WR, Prasad VR. HIV-1 clade-specific differences in the induction of neuropathogenesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:10010–10016. doi: 10.1523/JNEUROSCI.2955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratai EM, Annamalai L, Burdo T, Joo CG, Bombardier JP, Fell R, Hakimelahi R, He J, Lentz MR, Campbell J. Brain creatine elevation and N-acetylaspartate reduction indicates neuronal dysfunction in the setting of enhanced glial energy metabolism in a macaque model of NeuroAIDS. Magnetic Resonance in Medicine. 2011;66:625–634. doi: 10.1002/mrm.22821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Roberts TK, Eugenin EA, Morgello S, Clements JE, Zink MC, Berman JW. PrP C, the cellular isoform of the human prion protein, is a novel biomarker of HIV-associated neurocognitive impairment and mediates neuroinflammation. The American journal of pathology. 2010;177:1848–1860. doi: 10.2353/ajpath.2010.091006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roc AC, Ances BM, Chawla S, Korczykowski M, Wolf RL, Kolson DL, Detre JA, Poptani H. Detection of human immunodeficiency virus–induced inflammation and oxidative stress in lenticular nuclei with magnetic resonance spectroscopy despite antiretroviral therapy. Archives of neurology. 2007;64:1249–1257. doi: 10.1001/archneur.64.9.noc60125. [DOI] [PubMed] [Google Scholar]
- Rohlwink UK, Figaji AA. Biomarkers of brain injury in cerebral infections. Clinical chemistry. 2014;60:823–834. doi: 10.1373/clinchem.2013.212472. [DOI] [PubMed] [Google Scholar]
- Rosengren LE, Lycke J, Andersen O. Glial fibrillary acidic protein in CSF of multiple sclerosis patients: relation to neurological deficit. J Neurol Sci. 1995;133:61–65. doi: 10.1016/0022-510x(95)00152-r. [DOI] [PubMed] [Google Scholar]
- Rothermundt M, Peters M, Prehn JH, Arolt V. S100B in brain damage and neurodegeneration. Microscopy research and technique. 2003;60:614–632. doi: 10.1002/jemt.10303. [DOI] [PubMed] [Google Scholar]
- Ryan LA, Zheng J, Brester M, Bohac D, Hahn F, Anderson J, Ratanasuwan W, Gendelman HE, Swindells S. Plasma levels of soluble CD14 and tumor necrosis factor-α type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. Journal of Infectious Diseases. 2001;184:699–706. doi: 10.1086/323036. [DOI] [PubMed] [Google Scholar]
- Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;8(Suppl 2):115–121. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Miyahara S, Evans S, Schifitto G, Cohen B, Haughey N, Drewes JL, Graham D, Zink MC, Anderson C. Impact of minocycline on cerebrospinal fluid markers of oxidative stress, neuronal injury, and inflammation in HIV-seropositive individuals with cognitive impairment. Journal of neurovirology. 2014;20:620–626. doi: 10.1007/s13365-014-0292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Sharer LR, Epstein LG, Michaels J, Mintz M, Louder M, Golding K, Cvetkovich TA, Blumberg BM. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994;44:474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Ruxrungtham K, Lewin SR, Emery S, Neaton JD. Plasma levels of soluble CD14 independently predict mortality in HIV infection. Journal of Infectious Diseases. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoi H, Tomimoto H, Ohtani R, Kitano T, Kondo T, Watanabe M, Oka N, Akiguchi I, Furuya S, Hirabayashi Y. Astroglial expression of ceramide in Alzheimer’s disease brains: a role during neuronal apoptosis. Neuroscience. 2005;130:657–666. doi: 10.1016/j.neuroscience.2004.08.056. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade: novel fluorochromes for detecting toxicant-induced neuronal degeneration. Toxicol Pathol. 2000;28:91–99. doi: 10.1177/019262330002800111. [DOI] [PubMed] [Google Scholar]
- Schrier RD, Hong S, Crescini M, Ellis R, Pérez-Santiago J, Spina C, Letendre S, Group, H. Cerebrospinal Fluid (CSF) CD8+ T-Cells That Express Interferon-Gamma Contribute to HIV Associated Neurocognitive Disorders (HAND) PloS one. 2015;10:e0116526. doi: 10.1371/journal.pone.0116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighardt B, Atwood WJ. HIV type 1 infection of human astrocytes is restricted by inefficient viral entry. AIDS Res Hum Retroviruses. 2001;17:1133–1142. doi: 10.1089/088922201316912745. [DOI] [PubMed] [Google Scholar]
- Sevigny J, Albert S, McDermott M, McArthur J, Sacktor N, Conant K, Schifitto G, Selnes O, Stern Y, McClernon D. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63:2084–2090. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- Shinawi M, Gruener N, Lerner A. CSF levels of carnitine in children with meningitis, neurologic disorders, acute gastroenteritis, and seizure. Neurology. 1998;50:1869–1871. doi: 10.1212/wnl.50.6.1869. [DOI] [PubMed] [Google Scholar]
- Shiramizu B, Gartner S, Williams A, Shikuma C, Ratto-Kim S, Watters M, Aguon J, Valcour V. Circulating proviral HIV DNA and HIV-associated dementia. AIDS. 2005;19:45–52. doi: 10.1097/00002030-200501030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmelzer Z, Haddad N, Admon E, Pessach I, Leto TL, Eitan-Hazan Z, Hershfinkel M, Levy R. Unique targeting of cytosolic phospholipase A2 to plasma membranes mediated by the NADPH oxidase in phagocytes. The Journal of cell biology. 2003;162:683–692. doi: 10.1083/jcb.200211056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes E, Justino JD. HIV-associated neurocognitive disorders: A review for NPs. The Nurse practitioner. 2015;40:1–7. doi: 10.1097/01.NPR.0000466501.42049.99. [DOI] [PubMed] [Google Scholar]
- Skillbäck T, Farahmand B, Bartlett JW, Rosén C, Mattsson N, Nägga K, Kilander L, Religa D, Wimo A, Winblad B. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology. 2014;83:1945–1953. doi: 10.1212/WNL.0000000000001015. [DOI] [PubMed] [Google Scholar]
- Smith DB, Simmonds P, Bell JE. Brain viral burden, neuroinflammation and neurodegeneration in HAART-treated HIV positive injecting drug users. Journal of neurovirology. 2014;20:28–38. doi: 10.1007/s13365-013-0225-3. [DOI] [PubMed] [Google Scholar]
- Sorci G, Agneletti AL, Bianchi R, Donato R. Association of S100B with intermediate filaments and microtubules in glial cells. Biochimica et biophysica acta. 1998;1448:277–289. doi: 10.1016/s0167-4889(98)00134-7. [DOI] [PubMed] [Google Scholar]
- Sporer B, Koedel U, Paul R, Kohleisen B, Erfle V, Fontana A, Pfister HW. Human immunodeficiency virus type-1 Nef protein induces blood-brain barrier disruption in the rat: role of matrix metalloproteinase-9. J Neuroimmunol. 2000;102:125–130. doi: 10.1016/s0165-5728(99)00170-8. [DOI] [PubMed] [Google Scholar]
- Spudich SS, Nilsson AC, Lollo ND, Liegler TJ, Petropoulos CJ, Deeks SG, Paxinos EE, Price RW. Cerebrospinal fluid HIV infection and pleocytosis: relation to systemic infection and antiretroviral treatment. BMC Infect Dis. 2005;5:98. doi: 10.1186/1471-2334-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankoff B, Tourbah A, Suarez S, Turell E, Stievenart J, Payan C, Coutellier A, Herson S, Baril L, Bricaire F. Clinical and spectroscopic improvement in HIV-associated cognitive impairment. Neurology. 2001;56:112–115. doi: 10.1212/wnl.56.1.112. [DOI] [PubMed] [Google Scholar]
- Stanley LC, Mrak RE, Woody RC, Perrot LJ, Zhang S, Marshak DR, Nelson SJ, Griffin WST. Glial cytokines as neuropathogenic factors in HIV infection: pathogenic similarities to Alzheimer’s disease. Journal of Neuropathology & Experimental Neurology. 1994;53:231–238. doi: 10.1097/00005072-199405000-00003. [DOI] [PubMed] [Google Scholar]
- Steinbrink F, Evers S, Buerke B, Young P, Arendt G, Koutsilieri E, Reichelt D, Lohmann H, Husstedt IW. Cognitive impairment in HIV infection is associated with MRI and CSF pattern of neurodegeneration. European Journal of Neurology. 2013;20:420–428. doi: 10.1111/ene.12006. [DOI] [PubMed] [Google Scholar]
- Stout JC, Ellis RJ, Jernigan TL, Archibald SL, Abramson I, Wolfson T, McCutchan JA, Wallace MR, Atkinson JH, Grant I. Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. Archives of neurology. 1998;55:161–168. doi: 10.1001/archneur.55.2.161. [DOI] [PubMed] [Google Scholar]
- Strain MC, Letendre S, Pillai SK, Russell T, Ignacio CC, Gunthard HF, Good B, Smith DM, Wolinsky SM, Furtado M, Marquie-Beck J, Durelle J, Grant I, Richman DD, Marcotte T, McCutchan JA, Ellis RJ, Wong JK. Genetic composition of human immunodeficiency virus type 1 in cerebrospinal fluid and blood without treatment and during failing antiretroviral therapy. J Virol. 2005;79:1772–1788. doi: 10.1128/JVI.79.3.1772-1788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S, Suzuki K, Seddiki N, Kaplan W, Cowley MJ, Hood CL, Clancy JL, Murray DD, Méndez C, Gelgor L. Differential regulation of the Let-7 family of microRNAs in CD4+ T cells alters IL-10 expression. The Journal of Immunology. 2012;188:6238–6246. doi: 10.4049/jimmunol.1101196. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Mizuno T, Zhang G, Wang J, Kawanokuchi J, Kuno R, Suzumura A. Neuritic beading induced by activated microglia is an early feature of neuronal dysfunction toward neuronal death by inhibition of mitochondrial respiration and axonal transport. Journal of Biological Chemistry. 2005;280:10444–10454. doi: 10.1074/jbc.M413863200. [DOI] [PubMed] [Google Scholar]
- Tarasów E, Wiercińska-Drapało A, Jaroszewicz J, Orzechowska-Bobkiewicz A, Dzienis W, Prokopowicz D, Walecki J. Antiretroviral therapy and its influence on the stage of brain damage in patients with HIV–1H MRS evaluation. Medical Science Monitor. 1999;10:101–106. [PubMed] [Google Scholar]
- Tarasow E, Wiercinska-Drapalo A, Kubas B, Dzienis W, Orzechowska-Bobkiewicz A, Prokopowicz D, Walecki J. Cerebral MR spectroscopy in neurologically asymptomatic HIV-infected patients. Acta Radiol. 2003;44:206–212. doi: 10.1080/j.1600-0455.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- Thames AD, Briones MS, Magpantay LI, Martinez-Maza O, Singer EJ, Hinkin CH, Morgello S, Gelman BB, Moore DJ, Heizerling K. The role of chemokine CC motif ligand 2 genotype and cerebrospinal fluid chemokine CC motif ligand 2 in neurocognition among HIV-infected patients. AIDS. 2015;29:1483–1491. doi: 10.1097/QAD.0000000000000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Lu A, Lee SE, Lee JY, Lopez OL, Aizenstein HJ, Toga AW, Becker JT. 3D mapping of ventricular and corpus callosum abnormalities in HIV/AIDS. Neuroimage. 2006;31:12–23. doi: 10.1016/j.neuroimage.2005.11.043. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A. 2005;102:15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Jahanshad N. Novel Neuroimaging Methods to Understand How HIV Affects the Brain. Current HIV/AIDS reports. 2015;12:289–298. doi: 10.1007/s11904-015-0268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraboschi JM, Munoz-Moreno JA, Puertas MC, Alonso-Villaverde C, Prats A, Ferrer E, Rozas N, Maso M, Ouchi D, Martinez-Picado J, Podzamczer D. Viral and inflammatory markers in cerebrospinal fluid of patients with HIV-1-associated neurocognitive impairment during antiretroviral treatment switch. HIV medicine. 2015;16:388–392. doi: 10.1111/hiv.12243. [DOI] [PubMed] [Google Scholar]
- Tornatore C, Chandra R, Berger JR, Major EO. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994;44:481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- Towgood KJ, Pitkanen M, Kulasegaram R, Fradera A, Kumar A, Soni S, Sibtain NA, Reed L, Bradbeer C, Barker GJ. Mapping the brain in younger and older asymptomatic HIV-1 men: Frontal volume changes in the absence of other cortical or diffusion tensor abnormalities. Cortex. 2012;48:230–241. doi: 10.1016/j.cortex.2011.03.006. [DOI] [PubMed] [Google Scholar]
- UNAIDS. UNAIDS Fact Sheet 2015. 2015 http://www.unaids.org/en/resources/campaigns/HowAIDSchangedeverything/factsheet.
- Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, Martínez-Maza O, Bream JH. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS (London, England) 2015;29:463. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikoff WR, Pendyala G, Siuzdak G, Fox HS. Metabolomic analysis of the cerebrospinal fluid reveals changes in phospholipase expression in the CNS of SIV-infected macaques. The Journal of clinical investigation. 2008;118:2661. doi: 10.1172/JCI34138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witwer KW, Watson AK, Blankson JN, Clements JE. Relationships of PBMC microRNA expression, plasma viral load, and CD4+ T-cell count in HIV-1-infected elite suppressors and viremic patients. Retrovirology. 2012;9:1–15. doi: 10.1186/1742-4690-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Tao J, Chen PJ, Shahab A, Ge W, Hart RP, Ruan X, Ruan Y, Sun YE. Genome-wide analysis reveals methyl-CpG-binding protein 2-dependent regulation of microRNAs in a mouse model of Rett syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18161–18166. doi: 10.1073/pnas.1005595107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WE, Babb JS, Tal A, Kirov II, George AE, Ratai EM, Gonzalez RG, Gonen O. Early glial activation precedes neurodegeneration in the cerebral cortex after SIV infection: a 3D, multivoxel proton magnetic resonance spectroscopy study. HIV medicine. 2015;16:381–387. doi: 10.1111/hiv.12222. [DOI] [PubMed] [Google Scholar]
- Wu Y, Storey P, Cohen BA, Epstein LG, Edelman RR, Ragin AB. Diffusion alterations in corpus callosum of patients with HIV. AJNR Am J Neuroradiol. 2006;27:656–660. [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H, Skillback T, Mattsson N, Trojanowski JQ, Portelius E, Shaw LM, Weiner MW, Blennow K, Alzheimer’s Disease Neuroimaging, I. Association of Cerebrospinal Fluid Neurofilament Light Concentration With Alzheimer Disease Progression. JAMA neurology. 2015:1–8. [Google Scholar]
- Zhou L, Pupo GM, Gupta P, Liu B, Tran SL, Rahme R, Wang B, Rua R, Rizos H, Carroll A, Cairns MJ, Saksena NK. A parallel genome-wide mRNA and microRNA profiling of the frontal cortex of HIV patients with and without HIV-associated dementia shows the role of axon guidance and downstream pathways in HIV-mediated neurodegeneration. BMC genomics. 2012;13:677. doi: 10.1186/1471-2164-13-677. [DOI] [PMC free article] [PubMed] [Google Scholar]


