Abstract
The pace of therapeutic drug development in multiple myeloma has reached unprecedented levels, with five regulatory approvals for relapsed and/or refractory disease of either new drugs or new regimens in 2015, and one already in 2016, while still others are anticipated. This has provided a wide array of options to be considered by patients and their healthcare providers in the event of relapse after, or progression on front-line therapy. Most of these agents are currently being evaluated in earlier patient populations, including as parts of induction, consolidation, and maintenance therapy approaches, where their benefits may be even greater. Moreover, additional randomized studies have been completed with our previous stable of novel agents that inform their use in these settings as well. In the current contribution to this CCR Focus on multiple myeloma, we will present an overview of some of the key recent data that have supported the addition of these new therapeutics to our armamentarium against multiple myeloma. Also, we will provide some guidelines about possible best practices in applying these regimens, and attempt to extrapolate how they will be used as parts of our future standards of care.
Introduction
Statistics from the Surveillance, Epidemiology, and End Results Program indicate that over 30,000 new myeloma cases will be diagnosed in the United States in 2016 (1). Meanwhile, the International Agency for Research on Cancer predicted the worldwide myeloma incidence in 2012 would be roughly double that figure (2). Due in part to an aging populace, and possibly to an increased disease incidence in at least some areas (3), myeloma cases are predicted to grow almost 60% between 2010 and 2030, ranking it third among all cancers in the rate of increase during this period (4). Also of note, the cost of myeloma care on a per-patient basis has been among the highest of any malignancy (5). This has been in part due to the sub-optimal ability of our previously available therapies to completely eradicate neoplastic cell clones and achieve minimal residual disease (MRD)-negativity (6). Together, these and other data strongly support the need for development of new myeloma therapeutics despite the impressive gains made possible by the first generation of novel agents, including bortezomib, thalidomide, and lenalidomide. Fortunately, recent registration-enabling studies have substantially expanded our toolkit, and led to approval of new drugs in old classes, such as the proteasome inhibitors (PIs) carfilzomib and ixazomib (Table 1). In addition, new drugs in new classes have been developed, including the deacetylase inhibitor panobinostat, and the monoclonal antibodies daratumumab and elotuzumab. We will here review the current state of knowledge about their clinical use as the myeloma community strives to optimally incorporate them to maximize patient outcomes.
Table 1.
Recent and Possible Future Food and Drug Administration (FDA) Approvals of Novel Agents for Patients with Relapsed and/or Refractory Multiple Myeloma1
| Novel Agent or Regimen |
FDA Approval Date |
Regimen Used in Registration Study Leading to FDA Approval |
Patient Population |
|---|---|---|---|
|
| |||
| Panobinostat + Bortezomib/ dexamethasone | February 23, 2015 | Panobinostatat (20 mg p.o. on days 1, 3, 5, 8, 10, 12) q 3 weeks in cycles 1–8 + bortezomib (1.3 mg/m2 i.v. on days 1, 4, 8, 11) + dexamethasone (20 mg p.o. on days 1, 2, 4, 5, 8, 9, 11, 12) | Patients with ≥2 prior standard therapies, including bortezomib and an immunomodulatory agent |
| For cycles 9–12, panobinostat (20 mg p.o. on days 1, 3, 5, 8, 10, 12, 22, 24, 26, 29, 31, 33) q 6 weeks + bortezomib (1.3 mg/m2 i.v. on days 1, 8, 22, 29) + dexamethasone (20 mg p.o. on days 1, 2, 8, 9, 22, 23, 29, 30) | |||
|
| |||
| Carfilzomib + Lenalidomide/dexamethasone | July 27, 2015 | Carfilzomib (20 mg/m2 i.v. on days 1, 2 of cycle 1, then 27 mg/m2 i.v. on days 8, 9, 15, 16 of cycle 1, and days 1, 2, 8, 9, 15, and 16 for cycles 2–12) q 28 days + lenalidomide (25 mg p.o. on days 1–21) + dexamethasone (40 mg p.o. on days 1, 8, 15, 22) | Patients with relapsed disease who had received 1–3 prior lines of therapy |
| For cycles 12–18, carfilzomib (27 mg/m2 i.v. on days 1, 2, 15, 16) q 28 days + lenalidomide/ dexamethasone as above | |||
| For cycles 19 and beyond, only lenalidomide and dexamethasone were continued | |||
|
| |||
| Daratumumab | November 16, 2015 | Daratumumab (16 mg/kg i.v. on days 1, 8, 15, 22) q 28 days of cycles 1–2, on days 1, 15 of cycles 3–6, and on day 1 of all subsequent cycles | Patients with at least 3 prior treatments |
|
| |||
| Ixazomib + Lenalidomide/ dexamethasone | November 20, 2015 | Ixazomib (4 mg p.o. on days 1, 8, 15) q 28 days + lenalidomide (25 mg p.o. on days 1–21) + dexamethasone (40 mg p.o. on days 1, 8, 15, 22) | Patients who had received at least 1 prior therapy |
|
| |||
| Elotuzumab + Lenalidomide/dexamethasone | November 30, 2015 | Elotuzumab (10 mg/kg i.v. on days 1, 8, 15, 22) q 28 days of cycles 1–2 + lenalidomide (25 mg p.o. on days 1–21) + dexamethasone (40 mg p.o. on days 1, 8, 15, 22) | Patients with 1-3 prior therapies |
| For cycles 3 and beyond, elotuzumab (10 mg/kg i.v. on days 1, 15) q 28 days + lenalidomide/ dexamethasone as above | |||
|
| |||
| Carfilzomib + Dexamethasone | January 21, 2016 | Carfilzomib (20 mg/m2 i.v. on days 1, 2 of cycle 1, then 56 mg/m2 i.v. on days 8, 9, 15, 16 of cycle 1, and 56 mg/m2 i.v. on days 1, 2, 8, 9, 15, and 16 for cycles 2 and onward) q 28 days + dexamethasone (20 mg p.o. or i.v. on days 1, 2, 8, 9, 15, 16, 22, 23) | Patients with relapsed disease and 1–3 prior therapies |
|
| |||
| Daratumumab + Bortezomib/ dexamethasone | FDA review pending | Daratumumab (16 mg/kg i.v. on days 1, 8, 15) q 21 days of cycles 1–3, on day 1 of cycles 4–8 q 21 days, and on day 1 of cycles 9 and onward q 28 days + for cycles 1–8, bortezomib (1.3 mg/m2 s.c. on days 1, 4, 8, 11) + dexamethasone (20 mg p.o. or i.v. on days 1, 2, 4, 5, 8, 9, 11, 12) | Patients who had received at least 1 prior therapy |
|
| |||
| Daratumumab + Lenalidomide/ dexamethasone | FDA review pending | Daratumumab dosing as detailed above for single–agent daratumumab + lenalidomide + dexamethasone as described above for elotuzumab | Patients who had received at least 1 prior therapy |
Abbreviations: FDA, Food and Drug Administration; i.v., intravenous; p.o., oral; s.c., subcutaneous
New Drug Approvals in Relapsed and/or Refractory Multiple Myeloma
Panobinostat
Histone deacetylases (HDACs) are involved predominantly in post-translational modification of both histone and non-histone proteins, and the multiple HDAC isoforms are divided into four broad classes in man. HDAC inhibitors are active against myeloma through multiple mechanisms, including induction of cell cycle arrest and apoptosis, inhibition of cytokine-mediated survival signaling, generation of reactive oxygen species, and blockade of alternative proteolytic pathways needed for cell survival in the face of proteasome inhibition (recently reviewed in (7)). The last of these provided a strong rationale for the combination of the pan-HDAC inhibitor panobinostat with bortezomib and dexamethasone (FVD), which showed an overall response rate (ORR) of 51.6% in an initial phase Ib study (8). These findings prompted the phase III PANORAMA1 trial (Table 2), which evaluated FVD in comparison with bortezomib and dexamethasone (VD)(9). Progression-free survival (PFS) was the primary endpoint and was significantly improved by FVD, as was the ORR and the duration of response (DOR)(Table 3). Trends that did not quite reach significance were also seen for an improvement in the complete response (CR) rate and overall survival (OS). Subgroup analyses indicated that lower hazard ratios (HRs) were seen in younger patients and men, and those who had received prior bortezomib but not an immunomodulatory drug (IMiD). Also, patients with higher risk cytogenetic features had attractive HRs, indicating a substantial benefit from FVD. Addition of panobinostat was associated with an at least 10% absolute increase in a number of toxicities (Table 2). Patients in particular should be monitored for diarrhea, which needs early and aggressive management, while QT segment prolongations can rarely be seen. On-treatment deaths were noted in 18 patients treated with VD, and 30 with FVD.
Table 2.
Patient Populations Targeted in Recent Registration Studies of Novel Agents in Multiple Myeloma and Associated Adverse Events1
| Trial | Agent or Regimen |
Target Population |
Enrolled Population (% with 1, 2, 3 prior lines) |
Key Exclusion Criteria |
Adverse Events Increased in Experimental Arm* |
|---|---|---|---|---|---|
| PANORAMA1 | Panobinostat/Bortezomib/dex | Relapsed or refractory with 1–3 prior therapies | 51% / 32% / 17% | Primary refractory or bortezomib-refractory myeloma | Diarrhea, fatigue, nausea, peripheral edema, anorexia, pyrexia, vomiting, thrombo-cytopenia, leukopenia, neutropenia, and anemia |
| Placebo/Bortezomib/dex | 52% / 28% / 20% | ||||
| ASPIRE | Carfilzomib/Lenalidomide/dex | Relapsed disease after 1–3 prior lines of therapy | 46.5% / 53.3% with 2 or 3 lines | Bortezomib-refractory disease excluded. RD allowed if not most recent tx, and no progression during first three RD months. | Cough and hypokalemia |
| Lenalidomide/dex | 39.6% / 60.1% with 2 or 3 lines | ||||
| SIRIUS | Daratumumab | Refractory disease with ≥3 lines including PI & IMiD, or refractory to both. | Median of 5 (range 2–14) | None | Not applicable (non-randomized study) |
| TOURMALINE 1 | Ixazomib/Lenalidomide/dex | Relapsed or refractory with 1–3 prior therapies | 62% / 27% / 11% | Patients with disease refractory to lenalidomide or a PI | Thrombocytopenia, rash, and vomiting |
| Placebo/Lenalidomide/dex | 60% / 31% / 9% | ||||
| ELOQUENT-2 | Elotuzumab/Lenalidomide/dex | Relapsed after 1–3 prior therapies | 47% with 1 regimen, 37% with 2, 16% with ≥3 | Prior len permitted (in ≤10%) if achieved ≥PR, did not progress on or within 9 months of therapy, did not discontinue due to ≥grade 3 AE, had <9 cycles, had ≥9 months between last dose & PD. | Pyrexia, diarrhea, cough |
| Lenalidomide/dex | 49% with 1 regimen, 35% with 2, 16% with ≥3 | ||||
| ENDEAVOR | Carfilzomib/dex | Patients with relapsed or refractory myeloma after 1–3 prior lines of therapy. | 49.8% / 50.2% with 2 or 3 lines | Bortezomib or carfilzomib allowed if these were tolerated, induced a ≥PR before progression, and if patients had a six-month PI-free period | Anemia, dyspnea, pyrexia, cough, hypertension, and muscle spasms |
| Bortezomib/dex | 49.2% / 50.8% with 2 or 3 lines | ||||
| CASTOR | Daratumumab/Bortezomib/dex | Relapsed or refractory myeloma after ≥1 prior line(s) of therapy. | 48.6% / 27.9% / 14.7% / 8.8% >3 prior lines | Primary refractory disease. Patients refractory or intolerant to bortezomib, or another proteasome inhibitor. | Thrombocytopenia, cough |
| Bortezomib/dex | 45.7% / 30.0% / 13.0% / 11.3% >3 prior lines | ||||
| POLLUX | Daratumumab/Lenalidomide/dex | Relapsed or refractory myeloma after ≥1 prior line of therapy | Median of 1 (range 1–11) | Lenalidomide-refractory or intolerant disease | Neutropenia, diarrhea, upper respiratory infection, cough |
| Lenalidomide/dex | Median of 1 (range 1–8) |
Abbreviations: AE, adverse event; dex, dexamethasone, IMiD, immunomodulatory drug; len, lenalidomide; PD, disease progression; PI, proteasome inhibitor; PR, partial remission; RD, lenalidomide a›nd dexamethasone; tx, therapy
Adverse events of all grades are listed whose overall incidence was increased in the experimental arm by at least an absolute value of 10% compared with the control arm, where applicable
Table 3.
Outcomes Data from Key Trials Supporting Recent and Possible Future Food and Drug Administration Approvals1
| Trial | Agent or Regimen |
PFS | HR for PFS |
ORR | DOR | CR Rate |
Median OS |
|---|---|---|---|---|---|---|---|
| PANORAMA1 | Panobinostat/Bortezomib/dex | 11.99 months* | 0.63 | 235/387 (60.7%)* | 13.14 months* | 42/387 (11%) | 33.64 months |
| Placebo/Bortezomib/dex | 8.08 months | 208/381 (54.6%) | 10.87 months | 22/381 (6%) | 30.39 months | ||
| ASPIRE | Carfilzomib/ Lenalidomide/dex | 26.3 months* | 0.69 | 345/396 (87.1%)* | 28.6 months* | 126/396 (31.8%)* | 73.3% at 24 months* |
| Lenalidomide/dex | 17.6 months | 264/396 (66.7%) | 21.2 months | 37/396 (9.3%) | 65% at 24 months | ||
| SIRIUS | Daratumumab | 3.7 months | N.A. | 31/106 (29.2%) | 7.4 months | 3/106 (2.8%) | 64.8% at 12 months |
| TOURMALINE 1 | Ixazomib/Lenalidomide/dex | 20.6 months* | 0.74 | 282/360 (78%)* | 20.5 months | 42/360 (12%)* | 81 deaths at 23-months |
| Placebo/Lenalidomide/dex | 14.7 months | 259/362 (72%) | 15.0 months | 24/362 (7%) | 90 deaths at 23-months | ||
| ELOQUENT 2 | Elotuzumab/Lenalidomide/dex | 19.4 months* | 0.70 | 252/321 (79%)* | 20.73 months | 14/321 (4%) | 14 deaths |
| Lenalidomide/dex | 14.9 months | 213/325 (66%) | 16.62 months | 24/325 (7%) | 22 deaths | ||
| ENDEAVOR | Carfilzomib/dex | 18.7 months* | 0.53 | 365/464 (76.7%)* | 21.3 months* | 58/464 (13%)* | 75 deaths |
| Bortezomib/dex | 9.4 months | 290/465 (62.3%) | 10.4 months | 29/465 (6%) | 88 deaths | ||
| CASTOR | Daratumumab/Bortezomib/dex | Not reached* | 0.39 | 199/240 (82.9%)* | Not reached* | 46/240 (19.2%) | 29 deaths |
| Bortezomib/dex | 7.2 months | 148/234 (63.2%) | 7.9 months | 21/234 (9.0%) | 36 deaths | ||
| POLLUX | Daratumumab/Lenalidomide/dex | Not reached* | 0.37 | 261/281 (92.9%)* | Not reached* | 121/281 (43.1%)* | 86.1% at 18 months |
| Lenalidomide/dex | 18.4 months | 211/276 (76.4%) | 17.4 months | 53/276 (19.2%) | 75.6% at 18 months* |
Abbreviations: CR, complete response; dex, dexamethasone; DOR, duration of response; HR, hazard ratio; N.A.; not applicable; OS, overall survival; ORR, overall response rate, defined as partial response or better; PFS, progression-free survival;
Indicates that the value in question is statistically significantly better than the relevant control, where significance is defined by a p value of <0.05
Additional support for FVD comes from PANORAMA2, which enrolled 55 patients with bortezomib-refractory disease and ≥2 prior lines of therapy, including an IMiD (10). The ORR was 34.5%, while the PFS and DOR were 5.4 and 6.0 months, respectively, in this heavily pre-treated population. Panobinostat may also be safely and efficaciously combined with carfilzomib and dexamethasone (11). Finally, novel HDAC inhibitors with greater specificity, such as for HDAC6, are being investigated, and could provide comparable or greater benefits with enhanced safety (7).
Carfilzomib
This irreversible epoxyketone PI, which showed encouraging phase I data (12, 13), and was then initially approved as a single-agent for refractory myeloma (14), has recently garnered additional approvals (Table 1) in two different combinations for earlier populations. PIs in general have anti-myeloma efficacy by suppressing plasma cell survival signaling, including through nuclear factor-kappa B, activating extrinsic and intrinsic apoptotic pathways, interfering with permissive stromal and microenvironmental influences, and enhancing proteotoxic stress (reviewed in (15)). Pre-clinical studies supported development of carfilzomib in combinations, such as with lenalidomide and/or dexamethasone (16). The three-drug regimen looked especially active in phase I and II studies targeting patients with relapsed/refractory (17, 18) or newly diagnosed myeloma (19), prompting its further evaluation. In the ASPIRE study (Table 2), patients received lenalidomide and dexamethasone with (KRD) or without (RD) carfilzomib (20). Outcomes data showed a significant improvement for KRD in the primary endpoint, PFS, and a superior ORR and CR rate, DOR, and OS (Table 3). Hazard ratios favored KRD in virtually all analyzed subgroups, although smaller improvements occurred in patients with baseline neuropathy, and with disease that was bortezomib non-responsive and IMiD-refractory. Few adverse events (AEs) were increased by ≥10% (Table 2), while diarrhea, pyrexia, upper respiratory infection, and hypertension approached this threshold. A similar combination with very nice activity is carfilzomib with pomalidomide and dexamethasone (21), though phase III trial data are not yet available.
A second expanded indication for carfilzomib is in combination with dexamethasone from the ENDEAVOR study (Table 2)(22), which compared higher-dose carfilzomib (Table 1), given over 30-minutes, with dexamethasone (KD) to VD. KD essentially doubled the PFS of VD (Table 3), and was significantly superior in other outcome measures in all of the subgroup analyses, though the least relative benefit was seen in patients with lenalidomide-refractory disease, and in those with a decreased creatinine clearance. Some AEs were increased in the KD group (Table 2), while peripheral neuropathy and neuralgia were lower. One other toxicity of interest has been cardiac failure, which on the ASPIRE study (20) was seen in 6.4% and 4.1% overall with KRD or RD, respectively, and 3.8% and 1.8% at ≥grade 3. In the context of the ENDEAVOR study, cardiac failure was seen in a total of 38 (8.2%) and 13 (2.9%) patients who received KD or VD, respectively, but reached ≥grade 3 in only 5 (1.1%) and 3 (0.7%). These studies suggest that the higher-dose carfilzomib regimen may be less attractive in some patients, such as perhaps those who are over 75 years of age, or have a prior history of thoracic radiation and anthracycline exposure (23). Additional data from randomized studies are needed to help identify firm risk factors, and could be forthcoming from the S1304 study (NCT01903811) comparing high-dose to standard-dose KD.
Ixazomib
Bortezomib and carfilzomib are available only through parenteral routes, but ixazomib is an orally bioavailable PI. Ixazomib was studied initially as a single-agent on two different schedules, and both showed activity against relapsed and/or refractory myeloma, with ORRs of 15–18%, and very manageable toxicity profiles (24, 25). Moreover, a phase I combining ixazomib with lenalidomide/dexamethasone (IRD) induced a PR or better in 88% of newly-diagnosed patients, including 60% with a very good partial remission (VGPR) or better (26). This supported the design of the TOURMALINE-MM1 study, which led to regulatory approval of IRD (Table 2)(27). PFS was significantly prolonged by IRD (Table 3), which also improved the ORR and CR rates, and DOR, though OS data were not mature. Clinically relevant subgroups almost uniformly benefitted more from IRD, including a larger benefit in patients with three prior therapy lines. Especially attractive data were reported for high-risk subgroups, such as those with deletion (del) 17p, where the median PFS was 21.4 months with IRD but only 9.7 months with RD (HR 0.60), and in patients with the t(4;14) translocation, who saw an absolute 6.5 month PFS improvement (HR 0.65). Remarkably little increased toxicity was noted (Table 2), with the majority reaching only grade 1–2 severity, and many, including gastrointestinal effects, were seen after only the first ixazomib dose. Such enhanced efficacy and convenience, along with a minimally enhanced risk profile, would seem to make the therapeutic index of IRD among the most attractive of these new standards of care.
Daratumumab
Daratumumab is an IgG kappa monoclonal antibody targeting human CD38, which is highly expressed on plasma cells, and to some extent on other hematopoietic cells. Of interest, in vitro and in vivo data suggest that higher CD38 expression is associated with higher response rates, and has led investigators to evaluate strageties to increase CD38 expression (28). The first trial to evaluate daratumumab was the GEN501 phase I/II study, on which 4/13 patients with refractory disease achieved ≥PR with doses >4 mg/kg. In a dose expansion phase, patients received 8 or 16 mg/kg, and the ORR was 10% and 36%, respectively. Among the latter, two patients each achieved a CR and a VGPR, and the 1-year OS was 77% (29). This led to a larger, phase 2 study that initially retested the 8 and 16 mg/kg doses, but the former was found to be inadequate, leading to a subsequent 106 patient expansion using 16 mg/kg (Table 2). Among these, the median number of prior lines was 5, all had PI- and IMiD-resistant disease, and some were refractory to other recently approved agents. Included in the 29% ORR, 3% achieved a strigent-CR, another 9% achieved a VGPR, and the 1-year OS and median DOR were impressive (Table 3)(30). Adverse events included fatgue, nausea, anemia, cough, and upper respiratory infection, most of which were likely related to the severity of the underlying myeloma. Infusion reactions were seen in 48%, with >90% occurring during the first dose, nearly all of grade 1–2. Together, these encouraging findings prompted an accelerated approval in the US (Table 1).
A multi-arm phase II study was performed next adding daratumumab to commonly used regimens including VD, bortezomib/thalidomide/dexamethasone, melphalan/bortezomib/dexamethasone, and pomalidomide/dexamethasone (PD). The bortezomib-based combinations were tested in untreated patients, and all responded to each regimen, while in the PD cohort, 4/6 patients achieved ≥PR (31). This small study set the groundwork for the phase III CASTOR trial, which compared VD with daratumumab/VD (Table 1)(32). A higher ORR was seen in the daratumumab group (Table 3), and the VGPR or higher rate (59% vs. 29%, respectively) was superior, as was the response durability. Preclinical data evaluating daratumumab with lenalidomide suggested that the combinations resulted in synergistic effects (33), and a phase I/II trial reported an ORR of 81% (34). These findings supported the phase III POLLUX study, which evaluated 569 patients who received RD or daratumumab/RD (Table 2). Similar to the CASTOR trial, the 18-month PFS (78% vs. 52%) and ≥VGPR rate (76% vs. 44%) was superior with daratumumab, as was the ORR and DOR (Table 3). Notably, the magnitude of benefit from daratumumab was similar among patients who were previously exposed to, or lenalidomide-naïve (35). Both the POLLUX and CASTOR trials demonstrated that daratumumab combined safely and effectively with PIs or IMiDs, and may therefore represent a new ‘backbone’ agent for myeloma.
Elotuzumab
Elotuzumab is a humanized IgG kappa monoclonal protein that targets Signaling lymphocyte activating molecule family member 7 (SLAMF7, previously CS1), which is highly expressed on myeloma and natural killer (NK) cells (36). Functionally, plasma cell-associated SLAMF7 is not linked to intracellular signaling, while NK cell-associated SLAMF7 is linked to the EWS/FLI1-activated transcript 2 signaling cascade, resulting in NK activation following binding (37). While single-agent elotuzumab showed no clinical responses (38), pre-clinical data suggested synergy with lenalidomide and dexamethasone (39), and a phase Ib trial of elotuzumab/RD showed an 82% ORR (40). A randomized phase II extension was developed comparing 10 to 20 mg/kg of elotuzumab with RD, and showed an ORR of 92% and 76%, respectively, with a better PFS at 10 mg/kg (41). Based upon these encouraging data, the phase III ELOQUENT2 trial (Table 2) randomized 646 patients to RD or elotuzumab/RD. PFS favored the three-drug arm, as did the ORR and DOR, and elotuzumab-treated patients in the high-risk cohorts of del 17p and t(4;14) had a longer PFS (42).
Preclinical data supported the use of bortezomib in combination with elotuzumab and dexamethasone (43), and a phase I study demonstrated an ORR of 48% with a median PFS of 9.5 months (44). In a larger phase II trial, elotuzumab/VD induced a median PFS of 9.7 months compared to 6.6 with VD (HR 0.72, p=0.09), with no statistical difference in ORR or OS. A subset analysis among 13 patients treated with elotuzumab who had the FcγRIIIa high-affinity allele achieved a median PFS of 22.3 months, compared to 9.8 months among patients with the low-affinity allele (45).
Developments that Influence Initial Myeloma Therapy
Induction Therapy
Lenalidomide with bortezomib and dexamethasone (RVD) has been considered a standard of care for newly diagnosed symptomatic myeloma based on promising phase I/II trial data (46). This regimen has now been validated by the S0777 study performed through the National Clinical Trials Network, which compared RD to RVD in patients who were not eligible for, or willing to delay stem cell transplant (ASCT). RVD induced a superior median PFS (43 vs. 30 months; HR=0.712; confidence interval (CI) 0.560–0.906; p-value=0.0018), and had a significantly better ORR and CR rate, and median OS (75 vs. 64 months; HR=0.709; CI 0.516–0.973; p-value=0.025). These findings prompted the National Comprehensive Cancer Network (NCCN) to accord it category 1 designation as a recommended induction for both transplant-eligible and –ineligible patients (47). In contrast, bortezomib with cyclophosphamide and dexamethasone (CyBorD), which had also been popular based on an initial phase I/II study (48), has not recently fared well. The Intergroupe Francophone du Myélome (IFM) reported data from a study comparing bortezomib with thalidomide and dexamethasone (VTD) to CyBorD (49). After four cycles, VTD had a superior VGPR or better rate (66.3% vs. 56.2%; P=0.05), as well as a higher ORR (92.3% vs. 83.4%; P=0.01).
Role of Transplant
The IFM also reported data from the randomized IFM/DFCI 2009 trial (50) comparing RVD induction for three cycles followed by high-dose melphalan with ASCT, two consolidation RVD cycles, and then maintenance lenalidomide for two years, with RVD for eight cycles and then the same maintenance lenalidomide but without early ASCT. Incorporating transplantation into front-line therapy improved the CR rate (59% vs. 49%; P=0.02) and MRD-negativity by flow cytometry (80% vs. 65%; P=0.001). Median PFS, which was the primary endpoint, was improved as well (43 vs. 34 months; HR 0.69; CI 0.56–0.84; P<0.001), supporting early high-dose therapy. However, four-year survival was similar (83% vs. 81%; HR 1.2; CI 0.7–1.8), and there were more deaths (54 vs. 48) and second primary malignancies (6 vs.1) in the early transplant group. Correlative studies showed that MRD-negativity by flow cytometry or next-generation sequencing (51), as well as by magnetic resonance imaging or positron emission tomography (52), was generally associated with a better PFS, and in some cases with a better OS.
Optimal Current Use of Novel Agents in Myeloma
Given the plethora of available drugs and combination regimens for multiple myeloma, their use should ideally be guided by data from large, well-designed, randomized phase III studies, including those described earlier. Patients with newly diagnosed symptomatic disease who are transplant-eligible have both two-drug regimens, such as VD and RD as options, as well as three-drug combinations, including VTD and RVD, as well as bortezomib with doxorubicin and dexamethasone as category one NCCN recommendations. While data comparing all of these regimens are not available, it is very reasonable to utilize a PI/IMiD combination, such as either VTD or RVD, as the current standard of care for patients who are sufficiently robust to be able to tolerate a triplet regimen, and who have access to these agents. Several phase III studies are evaluating KRD as an induction based on encouraging earlier pilot trials (19, 53), and the ability of this regimen to induce high rates of MRD-negativity make it a very attractive option as well. Also of note, monoclonal antibodies have already been added to RVD as part of induction (54), and it is likely that such four-drug regimens will be in use in the future, especially for patients with a good performance status.
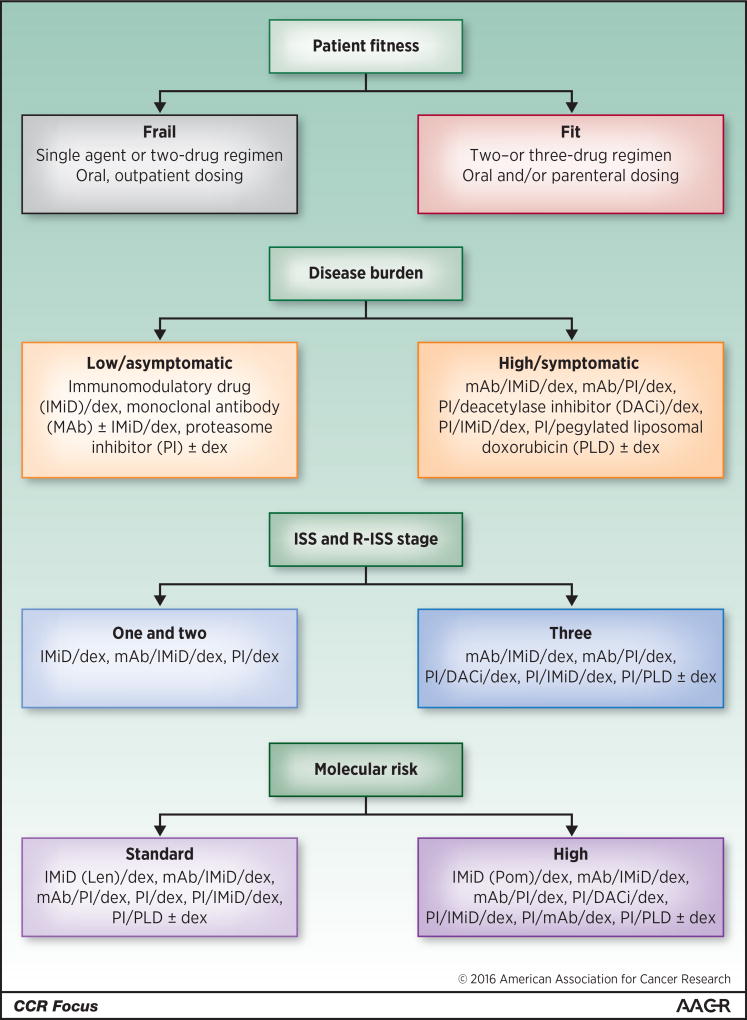
For transplant-ineligible patients, RD and RVD are also very reasonable, as are triplets based on melphalan and prednisone (MP), including MP with thalidomide, bortezomib, or lenalidomide. Once again, the available comparative data support the possibility that RVD, with some dose modifications to enhance tolerability, is an excellent option for fit patients, with KRD as a worthy contender (55). Selected patients may need to be considered for other therapies based on clinical grounds, such as with severe neuropathy, where RD or possibly KD could be used. Other clinical considerations should include the patient’s fitness based on validated scoring systems (56–58), disease burden, as in part measured by classical International Staging System (ISS) stage (59) or, preferably, Revised-ISS stage (60), and molecular risk (61). Indeed, given the higher comorbidity in this population, less robust patients should be considered for RD while we await the results of phase III studies with RD and either elotuzumab, daratumumab, or ixazomib. The relative lack of added toxicity due to incorporation of monoclonal antibodies, with the notable exception of infusion reactions with the first drug dose, and with ixazomib in general, and data from the relapsed/refractory setting reviewed earlier, suggest that these should be both highly efficacious and tolerable. Figure 1 provides some general guidelines about the use of various clinical criteria in selecting appropriate therapies for newly diagnosed and relapsed and/or refractory patients. However, it should be noted that randomized controlled trials (RCTs) addressing the use of these factors have largely not been performed. These suggestions are therefore made from the clinical experience of the authors, and are not as evidence-based as we would ideally like. In general, the authors feel that a three-drug, novel agent-based regimen should be given in as many situations as it is fesible to do so.
Figure 1. Factors to be wighed in deciding on therapy for patients with multiple myeloma.
Patient fitness, disease burden, International Staging System (ISS) and Revised-ISS stage, and molecular risk should be considered in making treatment decisions for myeloma patients in either the newly-diagnosed, relapsed, or refractory settings. However, randomized controlled trials (RCTs) trials addressing the use of these factors have largely not been performed. These suggestions are therefore grounded in the clinical experience of the authors, and are not as evidence-based as we would ideally like. Also, practitioners will need to keep in mind the settings for which these drugs have or have not been approved. In particular, many of the options, including some of the immunomodulatory drugs (IMiDs), pegylated liposomal doxorubicin (PLD), proteasome inhibitors (PIs), monoclonal antibodies (MAbs), and the deacetylase inhibitor (DACi) are not approved for front-line therapy. Finally, the options are presented alphabetically, and their order should not be construed as a suggestion of their appropriate sequencing, since such studies have not been performed. Additional abbreviation: dex, dexamethasone.
Single-agent lenalidomide or thalidomide (62–64), or bortezomib (65) remain the standards of care for maintenance of myeloma patients who have received ASCT. For transplant-ineligible patients after induction therapy, continued treatment with lenalidomide alone, or with RD should be considered (66, 67), while bortezomib alone (68) or with other agents (69) are additional options, though they have not been validated in placebo-controlled trials. Oral ixazomib could be an attractive option for both of these populations, and results from phase III studies in this setting (NCT02181413 and NCT02312258) are eagerly awaited. Bortezomib or bortezomib with RD should be especially considered for maintenance of patients with molecularly defined (61) high risk disease (70).
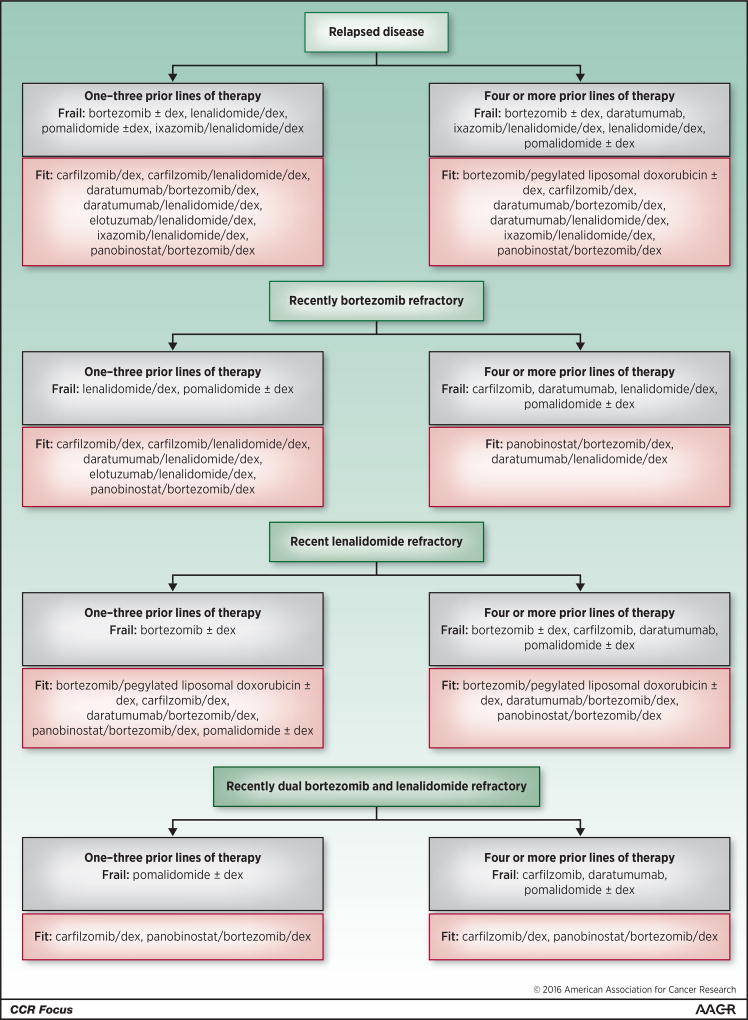
In the second line or later setting, no single regimen will be optimal for everyone since more options are available, and because choices will be influenced by the number and types of prior regimens, whether the patient’s disease is relapsed or refractory, and other considerations. Figure 2 provides one possible algorithm that can be used as a rough map based in part on the NCCN guidelines, and taking into account recent regulatory drug approvals (Table 1) and the design and study populations included and excluded in their respective trials (Table 2). Many options are available after 1–3 prior therapies in relapsed disease, where progression occurs off treatment, and myeloma is usually more drug-sensitive, while the number is smaller, but still robust for those with 4 or more prior therapies. Refractory disease, defined as progression on the prior treatment, or within sixty days of completing therapy, is more challenging, but several options remain for patients with bortezomib-, lenalidomide-, and dual-refractory-disease, and can provide substantial response quality and durability. It is worth noting that some patients, especially if they have progressed on a low-dose lenalidomide or bortezomib maintenance regimen, may not have disease that is fully refractory to these drug classes. In such cases, it may be valuable to consider, for example, increasing the dose of lenalidomide and adding dexamethasone along with another agent, such as bortezomib, carfilzomib, daratumumab, elotuzumab, or ixazomib. Molecular risk should be considered as well (Figure 1), and is increasingly being studied in the context of randomized trials. Deletion of 17p, for example, which is one of the higher risk myeloma features, seems to respond well based on recent studies to elotuzumab- (42) and ixazomib-based (27) therapies. Thus, high-risk patients in both the up-front and relapsed and/or refractory settings should likely especially be targeted for PI- and antibody-based therapies, while panobinostat should be considered in later lines. Another agent of interest in the latter setting is pomalidomide, which was approved in 2013 based on phase II (71) and III studies (72), and may have enhanced activity against del 17p myeloma (73). Importantly, opportunities should be sought at all times to enroll patients onto well-designed clinical trials of other, even more novel combinations and agents.
Figure 2. Evidence-based algorithm for treatment of patients with relapsed and/or refractory myeloma using our currently approved novel agents.
This diagram provides an overview of just some of the considerations and options that are involved in management of patients with relapsed and/or refractory disease. Please consult the text for further details about the use of this approach, which will need to be individualized to the molecular characteristics of each patient’s disease (Figure 1). The patient’s prior exposure to, and tolerance of these drug classes needs consideration, as do their comorbidities. With regard to the latter, some suggestions are provided about appropriate options in the fit (pink background) and frail patient (gray background). Also, please note that this list includes only recently approved novel single agents and combination regimens, as well as some combinations that will likely be approved based on already available phase III data. For the sake of brevity, we have not incorporated other drug classes, such as conventional alkylating agents, which may be of substantial use in this setting either at standard doses, or in the context of high-dose therapy with autologous stem cell rescue. Moreover, other combinations that have been reported to show encouraging outcomes, such as carfilzomib with pomalidomide and dexamethasone, are not included due to the lack of phase III data. Thus, this algorithm should not be taken as a representation of the full array of available therapies in this setting. “Carfilzomib” refers to the use of this drug as a single-agent at the standard dose (20 mg/m2 on days 1, 2, 8, 9, 15, and 15 in cycle 1, and then 27 mg/m2 on the same days starting cycle 2), while “Carfilzomib/dex” refers to its use at a high dose, which is detailed in Table 1. As in Figure 1, the options are presented alphabetically, and their order should not be construed as a suggestion of their appropriate sequencing, since such studies have not been performed.
Conclusions
Outcomes for patients with multiple myeloma have improved substantially over the past decade, and the impact of the new generation of novel(er) agents, including panobinostat, carfilzomib, ixazomib, daratumumab, elotuzumab, and pomalidomide, and combinations based on them, will enhance outcomes further. Challenges remain, however, as little is known about optimal drug and combination regimen sequencing. Some agents that were previously effective can probably be reused for relapsed disease (74, 75), but cross-resistance between drugs with similar mechanisms of action may limit this in the refractory setting. To achieve further progress against multiple myeloma, we still need additional new drugs and new drug classes to be validated. Promising candidates include selective inhibitors of nuclear export, selective HDAC inhibitors, and inhibitors of anti-apoptotic plasma cell proteins such as B-cell Lymphoma (BCL)-2 and Myeloid cell leukemia (MCL)-1, as well as immunotherapies such as checkpoint inhibitors, bi-specific T-cell engagers, and chimeric antigen receptor-directed T- and natural killer cells. There remains an unmet medical need as patients continue to develop relapsed and refractory myeloma which requires novel approaches, and many of these novel agents and approaches will probably show efficacy in this setting. However, the path towards approvals in the relapsed, and especially the front-line setting, will become more difficult given our current treatment landscape. For example, since several three-drug regimens are now more effective than either VD or RD, one could reasonably argue that these may no longer be appropriate general control arms for RCTs. Exceptions could be trials that target specific populations, such as frail patients, where two-drug regimens may still be standards of care. Studies comparing a triplet to a quadruplet will likely happen, but with the expected expense of the latter, a higher bar for regulatory approval may need to be set than the commonly used 30% improvement in PFS, to insure that the incremental cost provides high value in return.
Another challenge is that we still tend to deploy our options in a somewhat empiric fashion, and rely on clinical disease characteristics, rather than on the molecular features of each patient’s disease, or based on biomarkers. Fortunately, gene expression profiling and sequencing are increasingly being used, as in part detailed in the CCR Focus article by Szalat and colleagues (76), to drive treatment decisions. Moreover, biomarkers such as Cereblon, which may help predict IMiD sensitivity (77), and the recently described Tight junction protein 1, which influences PI sensitivity and resistance (78), are being validated. Also, expression of cell surface receptors such as CD38 and Complement inhibitory proteins may influence responses to monoclonal antibodies (79), suggesting that immunophenotyping and receptor density studies could be of value in deciding on drugs from this class. Future therapy will also likely be driven by MRD status, since MRD-negativity is associated with a superior outcome. This holds the exciting possibility that MRD-negativity could be used as an early endpoint for new drug approvals, and that, especially with molecular MRD-negativity, therapy could be stopped, thereby reducing cumulative patient clinical toxicity and financial burden. These last two parameters, along with patient-reported outcomes, need also to become a greater focus, since we hope to soon have several regimens to choose from which induce MRD-negative remissions in 100% of patients, at which point selections will be made using these factors. Together, these approaches will hopefully soon usher in the true era of precision myeloma therapy, and bring us to the verge of a cure for this disease.
Acknowledgments
R.Z. Orlowski reports receiving commercial research grants from Amgen, Bristol-Myers Squibb, Celgene, Spectrum Pharmaceuticals, and Takeda Oncology and is a consultant/advisory board member for Amgen, Bristol-Myers Squibb, Celgene, Forma Therapeutics, Incyte, Janssen, Novartis, Rigel Pharmaceuticals, and Takeda Oncology. S. Lonial reports receiving commercial research support from Bristol-Myers Squibb, Celgene, Janssen, Novartis, and Takeda Oncology and is a consultant/advisory board member for Amgen, Bristol-Myers Squibb, Celgene, Janssen, Merck, Novartis, and Takeda Oncology.
Grant Support
R.Z. Orlowski, the Florence Maude Thomas Cancer Research Professor, was supported by The MD Anderson Cancer Center SPORE in Multiple Myeloma (P50 CA142509), The MD Anderson Cancer Center Support Grant (P30 CA016672), the NCI (U10 CA032102, R01 CA184464 and CA194264), the Brock Family Myeloma Research Fund, and the MD Anderson Cancer Center High Risk Multiple Myeloma Moon Shot. S. Lonial would like to acknowledge support from the Levine Family Foundation and the Richard and Annelly Deets Fund for Multiple Myeloma.
Footnotes
Disclosure of Potential Conflicts of Interest:
No other potential conflicts of interest were disclosed.
References
- 1.Surveillance, Epidemiology, and End Results Program [database on the Internet] Bethesda (MD): National Cancer Institute; 2016. [[cited 2016 Sep 20]]. Available from: http://seer.cancer.gov. [Google Scholar]
- 2.GLOBOCAN 2012: Estimated Cancer incidence, Mortality and Prevalence Worldwide in 2012 [database on the Internet] Lyon (France): International Agency for Research on Cancer; 2016. [[cited 2016 Sep 20]]. Available from: http://globocan.iarc.fr/Default.aspx. [Google Scholar]
- 3.Mistry M, Parkin DM, Ahmad AS, Sasieni P. Cancer incidence in the United Kingdom: projections to the year 2030. British journal of cancer. 2011;105:1795–803. doi: 10.1038/bjc.2011.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. Journal of clinical oncology. 2009;27:2758–65. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 5.Cook R. Economic and clinical impact of multiple myeloma to managed care. Journal of managed care pharmacy. 2008;14:19–25. doi: 10.18553/jmcp.2008.14.S7-A.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paiva B, van Dongen JJ, Orfao A. New criteria for response assessment: role of minimal residual disease in multiple myeloma. Blood. 2015;125:3059–68. doi: 10.1182/blood-2014-11-568907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harada T, Hideshima T, Anderson KC. Histone deacetylase inhibitors in multiple myeloma: from bench to bedside. International journal of hematology. 2016;104:300–9. doi: 10.1007/s12185-016-2008-0. [DOI] [PubMed] [Google Scholar]
- 8.San-Miguel JF, Richardson PG, Gunther A, Sezer O, Siegel D, Blade J, et al. Phase Ib study of panobinostat and bortezomib in relapsed or relapsed and refractory multiple myeloma. Journal of clinical oncology. 2013;31:3696–703. doi: 10.1200/JCO.2012.46.7068. [DOI] [PubMed] [Google Scholar]
- 9.San-Miguel JF, Hungria VT, Yoon SS, Beksac M, Dimopoulos MA, Elghandour A, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. The Lancet Oncology. 2014;15:1195–206. doi: 10.1016/S1470-2045(14)70440-1. [DOI] [PubMed] [Google Scholar]
- 10.Richardson PG, Schlossman RL, Alsina M, Weber DM, Coutre SE, Gasparetto C, et al. PANORAMA 2: panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma. Blood. 2013;122:2331–7. doi: 10.1182/blood-2013-01-481325. [DOI] [PubMed] [Google Scholar]
- 11.Berdeja JG, Hart LL, Mace JR, Arrowsmith ER, Essell JH, Owera RS, et al. Phase I/II study of the combination of panobinostat and carfilzomib in patients with relapsed/refractory multiple myeloma. Haematologica. 2015;100:670–6. doi: 10.3324/haematol.2014.119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Connor OA, Stewart AK, Vallone M, Molineaux CJ, Kunkel LA, Gerecitano JF, et al. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clinical cancer research. 2009;15:7085–91. doi: 10.1158/1078-0432.CCR-09-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsina M, Trudel S, Furman RR, Rosen PJ, O'Connor OA, Comenzo RL, et al. A phase I single-agent study of twice-weekly consecutive-day dosing of the proteasome inhibitor carfilzomib in patients with relapsed or refractory multiple myeloma or lymphoma. Clinical cancer research. 2012;18:4830–40. doi: 10.1158/1078-0432.CCR-11-3007. [DOI] [PubMed] [Google Scholar]
- 14.Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–25. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreau P, Richardson PG, Cavo M, Orlowski RZ, San Miguel JF, Palumbo A, et al. Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120:947–59. doi: 10.1182/blood-2012-04-403733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–90. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niesvizky R, Martin TG, 3rd, Bensinger WI, Alsina M, Siegel DS, Kunkel LA, et al. Phase Ib dose-escalation study (PX-171-006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Clinical cancer research. 2013;19:2248–56. doi: 10.1158/1078-0432.CCR-12-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M, Martin T, Bensinger W, Alsina M, Siegel DS, Kavalerchik E, et al. Phase 2 dose-expansion study (PX-171-006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Blood. 2013;122:3122–8. doi: 10.1182/blood-2013-07-511170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakubowiak AJ, Dytfeld D, Griffith KA, Lebovic D, Vesole DH, Jagannath S, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120:1801–9. doi: 10.1182/blood-2012-04-422683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Spicka I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. The New England journal of medicine. 2015;372:142–52. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 21.Shah JJ, Stadtmauer EA, Abonour R, Cohen AD, Bensinger WI, Gasparetto C, et al. Carfilzomib, pomalidomide, and dexamethasone for relapsed or refractory myeloma. Blood. 2015;126:2284–90. doi: 10.1182/blood-2015-05-643320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hajek R, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. The Lancet Oncology. 2016;17:27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 23.Danhof S, Schreder M, Rasche L, Strifler S, Einsele H, Knop S. 'Real-life' experience of preapproval carfilzomib-based therapy in myeloma - analysis of cardiac toxicity and predisposing factors. European journal of haematology. 2016;97:25–32. doi: 10.1111/ejh.12677. [DOI] [PubMed] [Google Scholar]
- 24.Kumar SK, Bensinger WI, Zimmerman TM, Reeder CB, Berenson JR, Berg D, et al. Phase 1 study of weekly dosing with the investigational oral proteasome inhibitor ixazomib in relapsed/refractory multiple myeloma. Blood. 2014;124:1047–55. doi: 10.1182/blood-2014-01-548941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson PG, Baz R, Wang M, Jakubowiak AJ, Laubach JP, Harvey RD, et al. Phase 1 study of twice-weekly ixazomib, an oral proteasome inhibitor, in relapsed/refractory multiple myeloma patients. Blood. 2014;124:1038–46. doi: 10.1182/blood-2014-01-548826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar SK, Berdeja JG, Niesvizky R, Lonial S, Laubach JP, Hamadani M, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. The Lancet Oncology. 2014;15:1503–12. doi: 10.1016/S1470-2045(14)71125-8. [DOI] [PubMed] [Google Scholar]
- 27.Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. The New England journal of medicine. 2016;374:1621–34. doi: 10.1056/NEJMoa1516282. [DOI] [PubMed] [Google Scholar]
- 28.Nijhof IS, Groen RW, Lokhorst HM, van Kessel B, Bloem AC, van Velzen J, et al. Upregulation of CD38 expression on multiple myeloma cells by all-trans retinoic acid improves the efficacy of daratumumab. Leukemia. 2015;29:2039–49. doi: 10.1038/leu.2015.123. [DOI] [PubMed] [Google Scholar]
- 29.Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. The New England journal of medicine. 2015;373:1207–19. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 30.Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387:1551–60. doi: 10.1016/S0140-6736(15)01120-4. [DOI] [PubMed] [Google Scholar]
- 31.Moreau P, Mateos M-V, Blade J, Benboubker L, de la Rubia J, Facon T, et al. An open-label, multicenter, phase 1b study of daratumumab in combination with backbone regimens in patients with multiple myeloma. Blood. 2014;124:176. [Google Scholar]
- 32.Palumbo A, Chanan-Khan AAA, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Phase III randomized controlled study of daratumumab, bortezomib, and dexamethasone (DVd) versus bortezomib and dexamethasone (Vd) in patients (pts) with relapsed or refractory multiple myeloma (RRMM): CASTOR study. Journal of clinical oncology. 2016;34(suppl) abstr LBA4. [Google Scholar]
- 33.Nijhof IS, Groen RW, Noort WA, van Kessel B, de Jong-Korlaar R, Bakker J, et al. Preclinical evidence for the therapeutic potential of CD38-targeted immuno-chemotherapy in multiple myeloma patients refractory to lenalidomide and bortezomib. Clinical cancer research. 2015;21:2802–10. doi: 10.1158/1078-0432.CCR-14-1813. [DOI] [PubMed] [Google Scholar]
- 34.Plesner T, Arkenau H-K, Gimsing P, Krejcik J, Lemech C, Minnema MC, et al. Daratumumab in combination with lenalidomide and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: Updated results of a phase 1/2 study (GEN503) Blood. 2015;126:507. [Google Scholar]
- 35.Dimopoulos MA, Oriol A, Nahi H, San Miguel J, Bahlis NJ, Rabin N, et al. An open-label, randomised phase 3 study of daratumumab, lenalidomide, and dexamethasone (DRD) versus lenalidomide and dexamethasone (RD) in relapsed or refractory multiple myeloma (RRMM): POLLUX [abstract]. Proceedings of the 21st Congress of the European Hematology Association; Copenhagen, Denmark. The Hague (The Netherlands): EHA; 2016; 2016. Jun 9–12, Abstract nr LB2238. [Google Scholar]
- 36.Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clinical cancer research. 2008;14:2775–84. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palumbo A, Sonneveld P. Preclinical and clinical evaluation of elotuzumab, a SLAMF7-targeted humanized monoclonal antibody in development for multiple myeloma. Expert review of hematology. 2015;8:481–91. doi: 10.1586/17474086.2015.1053866. [DOI] [PubMed] [Google Scholar]
- 38.Zonder JA, Mohrbacher AF, Singhal S, van Rhee F, Bensinger WI, Ding H, et al. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood. 2012;120:552–9. doi: 10.1182/blood-2011-06-360552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008;112:1329–37. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lonial S, Vij R, Facon T, Moreau P, Leleu X, Mazumder A, et al. Phase I trial of elotuzumab, lenalidomide, and low-dose dexamethasone in patients with relapsed or refractory multiple myeloma. Journal of clinical oncology. 2011;29 doi: 10.1200/JCO.2011.37.2649. Abstract 8076. [DOI] [PubMed] [Google Scholar]
- 41.Richardson PG, Jagannath S, Moreau P, Jakubowiak AJ, Raab MS, Facon T, et al. Elotuzumab in combination with lenalidomide and dexamethasone in patients with relapsed multiple myeloma: final phase 2 results from the randomised, open-label, phase 1b-2 dose-escalation study. The Lancet Haematology. 2015;2:e516–27. doi: 10.1016/S2352-3026(15)00197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. The New England journal of medicine. 2015;373:621–31. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 43.van Rhee F, Szmania SM, Dillon M, van Abbema AM, Li X, Stone MK, et al. Combinatorial efficacy of anti-CS1 monoclonal antibody elotuzumab (HuLuc63) and bortezomib against multiple myeloma. Molecular cancer therapeutics. 2009;8:2616–24. doi: 10.1158/1535-7163.MCT-09-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jakubowiak AJ, Benson DM, Bensinger W, Siegel DS, Zimmerman TM, Mohrbacher A, et al. Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma. Journal of clinical oncology. 2012;30:1960–5. doi: 10.1200/JCO.2011.37.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jakubowiak A, Offidani M, Pegourie B, De La Rubia J, Garderet L, Laribi K, et al. Randomized phase 2 study: elotuzumab plus bortezomib/dexamethasone vs bortezomib/dexamethasone for relapsed/refractory MM. Blood. 2016;127:2833–40. doi: 10.1182/blood-2016-01-694604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–86. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Comprehensive Cancer Network [database on the Internet] Fort Washington (PA): National Comprehensive Cancer Network; 2016. [[cited 2016 Sep 20]]. NCCN guidelines for treatment of cancer by site. Multiple myeloma. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines_nojava.asp. [Google Scholar]
- 48.Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Hentz J, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23:1337–41. doi: 10.1038/leu.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moreau P, Hulin C, Macro M, Caillot D, Chaleteix C, Roussel M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 2016;127:2569–74. doi: 10.1182/blood-2016-01-693580. [DOI] [PubMed] [Google Scholar]
- 50.Attal M, Lauwers-Cances V, Hulin C, Facon T, Caillot D, Escoffre M, et al. Autologous transplantation for multiple myeloma in the era of new drugs: a phase III study of the Intergroupe Francophone du Myelome (IFM/DFCI 2009 trial) Blood. 2015;126:391. [Google Scholar]
- 51.Avet-Loiseau H, Corre J, Lauwers-Cances V, Chretien M-L, Robillard N, Leleu X, et al. Evaluation of minimal residual disease (MRD) by next generation sequencing (NGS) Is highly predictive of progression free survival in the IFM/DFCI 2009 trial. Blood. 2015;126:191. [Google Scholar]
- 52.Moreau P, Attal M, Karlin L, Garderet L, Facon T, Macro M, et al. Prospective evaluation of MRI and PET-CT at diagnosis and before maintenance therapy in symptomatic patients with multiple myeloma included in the IFM/DFCI 2009 trial. Blood. 2015;126:295. [Google Scholar]
- 53.Korde N, Roschewski M, Zingone A, Kwok M, Manasanch EE, Bhutani M, et al. Treatment with carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA oncology. 2015;1:746–54. doi: 10.1001/jamaoncol.2015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Usmani SZ, Sexton R, Ailawadhi S, Shah JJ, Valent J, Rosenzweig M, et al. Phase I safety data of lenalidomide, bortezomib, dexamethasone, and elotuzumab as induction therapy for newly diagnosed symptomatic multiple myeloma: SWOG S1211. Blood cancer journal. 2015;5:e334. doi: 10.1038/bcj.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dytfeld D, Jasielec J, Griffith KA, Lebovic D, Vesole DH, Jagannath S, et al. Carfilzomib, lenalidomide, and low-dose dexamethasone in elderly patients with newly diagnosed multiple myeloma. Haematologica. 2014;99:e162–4. doi: 10.3324/haematol.2014.110395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palumbo A, Bringhen S, Mateos MV, Larocca A, Facon T, Kumar SK, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125:2068–74. doi: 10.1182/blood-2014-12-615187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palumbo A, Rajkumar SV, San Miguel JF, Larocca A, Niesvizky R, Morgan G, et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. Journal of clinical oncology. 2014;32:587–600. doi: 10.1200/JCO.2013.48.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larocca A, Palumbo A. How I treat fragile myeloma patients. Blood. 2015;126:2179–85. doi: 10.1182/blood-2015-05-612960. [DOI] [PubMed] [Google Scholar]
- 59.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. Journal of clinical oncology. 2005;23:3412–20. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 60.Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised International Staging System for multiple myeloma: a report from International Myeloma Working Group. Journal of clinical oncology. 2015;33:2863–9. doi: 10.1200/JCO.2015.61.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127:2955–62. doi: 10.1182/blood-2016-01-631200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. The New England journal of medicine. 2012;366:1770–81. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. The New England journal of medicine. 2012;366:1782–91. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 64.Attal M, Harousseau JL, Leyvraz S, Doyen C, Hulin C, Benboubker L, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289–94. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 65.Sonneveld P, Schmidt-Wolf IG, van der Holt B, El Jarari L, Bertsch U, Salwender H, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. Journal of clinical oncology. 2012;30:2946–55. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]
- 66.Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. The New England journal of medicine. 2012;366:1759–69. doi: 10.1056/NEJMoa1112704. [DOI] [PubMed] [Google Scholar]
- 67.Benboubker L, Dimopoulos MA, Dispenzieri A, Catalano J, Belch AR, Cavo M, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. The New England journal of medicine. 2014;371:906–17. doi: 10.1056/NEJMoa1402551. [DOI] [PubMed] [Google Scholar]
- 68.Niesvizky R, Flinn IW, Rifkin R, Gabrail N, Charu V, Clowney B, et al. Community-Based Phase IIIB Trial of Three UPFRONT Bortezomib-Based Myeloma Regimens. Journal of clinical oncology. 2015;33:3921–9. doi: 10.1200/JCO.2014.58.7618. [DOI] [PubMed] [Google Scholar]
- 69.Mateos MV, Oriol A, Martinez-Lopez J, Gutierrez N, Teruel AI, de Paz R, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. The Lancet Oncology. 2010;11:934–41. doi: 10.1016/S1470-2045(10)70187-X. [DOI] [PubMed] [Google Scholar]
- 70.Nooka AK, Kaufman JL, Muppidi S, Langston A, Heffner LT, Gleason C, et al. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia. 2014;28:690–3. doi: 10.1038/leu.2013.335. [DOI] [PubMed] [Google Scholar]
- 71.Richardson PG, Siegel DS, Vij R, Hofmeister CC, Baz R, Jagannath S, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123:1826–32. doi: 10.1182/blood-2013-11-538835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.San Miguel J, Weisel K, Moreau P, Lacy M, Song K, Delforge M, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. The Lancet Oncology. 2013;14:1055–66. doi: 10.1016/S1470-2045(13)70380-2. [DOI] [PubMed] [Google Scholar]
- 73.Leleu X, Karlin L, Macro M, Hulin C, Garderet L, Roussel M, et al. Pomalidomide plus low-dose dexamethasone in multiple myeloma with deletion 17p and/or translocation (4;14): IFM 2010-02 trial results. Blood. 2015;125:1411–7. doi: 10.1182/blood-2014-11-612069. [DOI] [PubMed] [Google Scholar]
- 74.Petrucci MT, Giraldo P, Corradini P, Teixeira A, Dimopoulos MA, Blau IW, et al. A prospective, international phase 2 study of bortezomib retreatment in patients with relapsed multiple myeloma. British journal of haematology. 2013;160:649–59. doi: 10.1111/bjh.12198. [DOI] [PubMed] [Google Scholar]
- 75.Alici E, Chrobok M, Lund J, Ahmadi T, Khan I, Duru AD, et al. Re-challenging with anti-CD38 monotherapy in triple-refractory multiple myeloma patients is a feasible and safe approach. British journal of haematology. 2016;174:473–7. doi: 10.1111/bjh.13776. [DOI] [PubMed] [Google Scholar]
- 76.Szalat R, Avet-Loiseau H, Munshi NC. Gene expression profile in clinical practice. Clin Cancer Res. 2016;22:xxxx–xxxx. doi: 10.1158/1078-0432.CCR-16-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2012;118:4771–9. doi: 10.1182/blood-2011-05-356063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang XD, Baladandayuthapani V, Lin H, Mulligan G, Li B, Esseltine DL, et al. Tight Junction Protein 1 Modulates Proteasome Capacity and Proteasome Inhibitor Sensitivity in Multiple Myeloma via EGFR/JAK1/STAT3 Signaling. Cancer cell. 2016;29:639–52. doi: 10.1016/j.ccell.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nijhof IS, Casneuf T, van Velzen J, van Kessel B, Axel AE, Syed K, et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128:959–70. doi: 10.1182/blood-2016-03-703439. [DOI] [PubMed] [Google Scholar]