Abstract
Objective:
To study the effect of pregnancy on the frequency of neuromyelitis optica spectrum disorder (NMOSD) relapse and evaluate rates of pregnancy-related complications in an international multicenter setting.
Methods:
We administered a standardized survey to 217 women with NMOSD from 7 medical centers and reviewed their medical records. We compared the annualized relapse rate (ARR) during a baseline period 2 years prior to a participant's first pregnancy to that during pregnancy and to the 9 months postpartum. We also assessed pregnancy-related complications.
Results:
There were 46 informative pregnancies following symptom onset in 31 women with NMOSD. Compared to baseline (0.17), ARR was increased both during pregnancy (0.44; p = 0.035) and during the postpartum period (0.69; p = 0.009). The highest ARR occurred during the first 3 months postpartum (ARR 1.33). A total of 8 of 76 (10.5%) with onset of NMOSD prior to age 40 experienced their initial symptom during the 3 months postpartum, 2.9 times higher than expected.
Conclusions:
The postpartum period is a particularly high-risk time for initial presentation of NMOSD. In contrast to published observations in multiple sclerosis, in neuromyelitis optica, relapse rate during pregnancy was also increased, although to a lesser extent than after delivery.
Neuromyelitis optica (NMO) is an inflammatory neurologic disease, manifested by clinical relapses primarily related to optic nerve and spinal cord involvement. A pathogenic autoantibody directed against aquaporin-4 (AQP4–immunoglobulin G [IgG]), a water channel expressed on astrocytes in spinal cord and optic nerve, is involved in the disease pathogenesis.1,2 New diagnostic criteria for NMO spectrum disorders (NMOSD), the new preferred term for this condition, acknowledge limited forms of the disease and allow for diagnosis following one clinical attack in AQP4-IgG seropositive patients.3
It is well-established that pregnancy plays a modulatory role in the course of autoimmune diseases. Typically, this has been explained as an effect of placentally produced factors or relative estrogen levels (estradiol or the pregnancy hormone estriol) on subsets of T-helper cells (TH cells) 1 and 2, leading to stabilization of TH1-related diseases such as rheumatoid arthritis and exacerbation of TH2-related diseases such as systemic lupus erythematosus, although studies vary.4–6 In multiple sclerosis (MS), the prototypic immune-mediated disease of the CNS, relapses decrease in frequency during pregnancy and increase in the immediate postpartum period.7,8 Breastfeeding may extend the period of pregnancy-conferred protection in MS.9,10
There are limited data regarding pregnancies in NMOSD; the outcomes of about 200 pregnancies report an increased frequency and aggressive presentation of pregnancy-related attacks, especially in the first 6 months postpartum,11–14 and, unlike MS, a lack of reduction in relapses during pregnancy.13,14 In addition, there may be an increased risk of spontaneous abortion,15,16 and case reports suggest placental transfer of the AQP4 antibody may occur without a detrimental effect on the infant's neurologic health.16,17
In the current study, we examined the effect of pregnancy on NMOSD onset, relapse rate, and pregnancy outcomes in a large, multicenter international cohort of women with NMOSD.
METHODS
Standard protocol approvals, registrations, and patient consents.
Informed consent was obtained from all participants under institutional review board policies at each site.
Study design.
Pregnancy and clinical data in women with NMOSD were collected from 7 participating centers. Data were collected using a standardized questionnaire. The questionnaire was completed either in person or over the phone. A copy of the questionnaire is included as an e-Questionnaire at Neurology.org. Inclusion criteria included (1) female and (2a) positive serum AQP4-IgG, consistent with NMOSD or (2b) negative serum AQP4-IgG, meeting 2006 published diagnostic criteria18 for NMO. NMOSD participants, regardless of pregnancy history or timing of symptom onset, were enrolled consecutively between November 2011 and May 2013 and all participants completed at least one component of the questionnaire.
The questionnaire obtained information related to relapses in prespecified time intervals in reference to pregnancy. Relapses were defined as new or worsening neurologic symptoms lasting longer than 24 hours without other etiology. A baseline interval of 2 years prior to the first pregnancy was chosen to provide an adequate time to establish a baseline rate but also to limit recall bias. Relapse information was obtained during the 9 months of pregnancy and 9 months following pregnancy to keep these intervals equal. To determine the temporal relationship of relapses to pregnancy, NMOSD participants served as their own controls for analysis. Relapse rates were annualized and a baseline relapse rate (2 years prior to the participant's first pregnancy) was compared to the 9-month period during pregnancy and a 9-month postpartum period.
Additional information was collected regarding type of relapse (optic neuritis, transverse myelitis, brainstem), duration of symptoms, and relapse-related treatment received. Relapse timing, duration, and treatment documented by the questionnaire were confirmed by review of medical records in 21 of 40 relapses. Unconfirmed relapses were due to absent or insufficient medical records. Participants without a history of pregnancy were queried whether concern over NMO-related disability or stopping NMO-related medications factored into decision-making processes.
Information related to menarche, menopause, and hormonal exposure in this group of women with NMOSD was queried and previously reported.19
Chart review was utilized to estimate Expanded Disability Status Scale (EDSS)20 from the most recent examination recorded in the medical record.
Statistical analysis.
Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at Massachusetts General Hospital (MGH).21 Comparisons of annualized relapse rate during the prespecified time intervals in relationship to pregnancy were made using a paired t test. Cases in which a patient's initial relapse occurred during pregnancy or in the postpartum period were not used for this analysis due to the inability to accurately determine a baseline relapse rate. Mann-Whitney U test was used to compare baseline relapse rate in those with and without relapses during or after pregnancy. χ2 Test was used to examine association between breastfeeding and postpartum disease activity in NMOSD. Statistical analyses utilized Statistical Package for the Social Sciences version 21 (SPSS, Chicago, IL).
RESULTS
Demographics and disease characteristics.
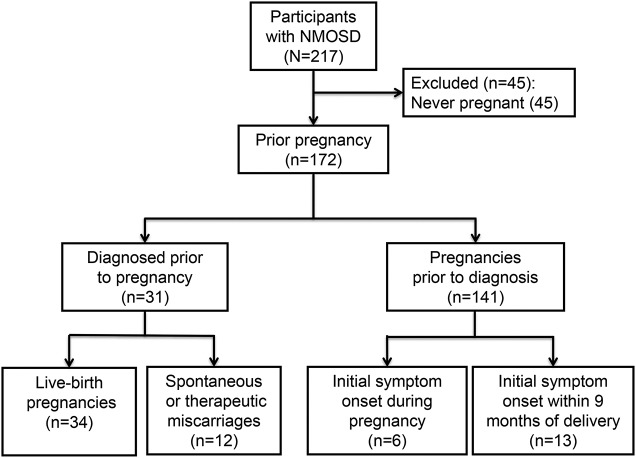
In total, 217 women with NMOSD were recruited from the Walton Centre, Liverpool, England (n = 56); Mayo Clinic, Rochester, Minnesota (n = 39); Washington University in St. Louis, Missouri (n = 36); Charité–Universitätsmedizin, Berlin, Germany (n = 26); Mayo Clinic, Scottsdale, Arizona (n = 25); Johns Hopkins University, Baltimore, Maryland (n = 19); and MGH/Brigham and Women's Hospital, Boston, Massachusetts (n = 16). A total of 81.6% (177/217) of participants with NMOSD were AQP4-seropositive. Mean age at symptom onset, race, disease comorbidities, disease duration, and EDSS are summarized in the table. The mean age at questionnaire administration was 49.4 ± 14.3 years. A total of 172 (79.3%) participants had been pregnant in the past, including those who did not carry to term, while 45 (20.7%) participants were nulliparous (figure 1). On average, the mean number of pregnancies was 2.1 ± 1.7 (SD); among those who reported pregnancy, the mean number of pregnancies was 2.7 ± 1.4 (SD).
Table.
Demographic and clinical information for participants with neuromyelitis optica spectrum disorders (NMOSD)
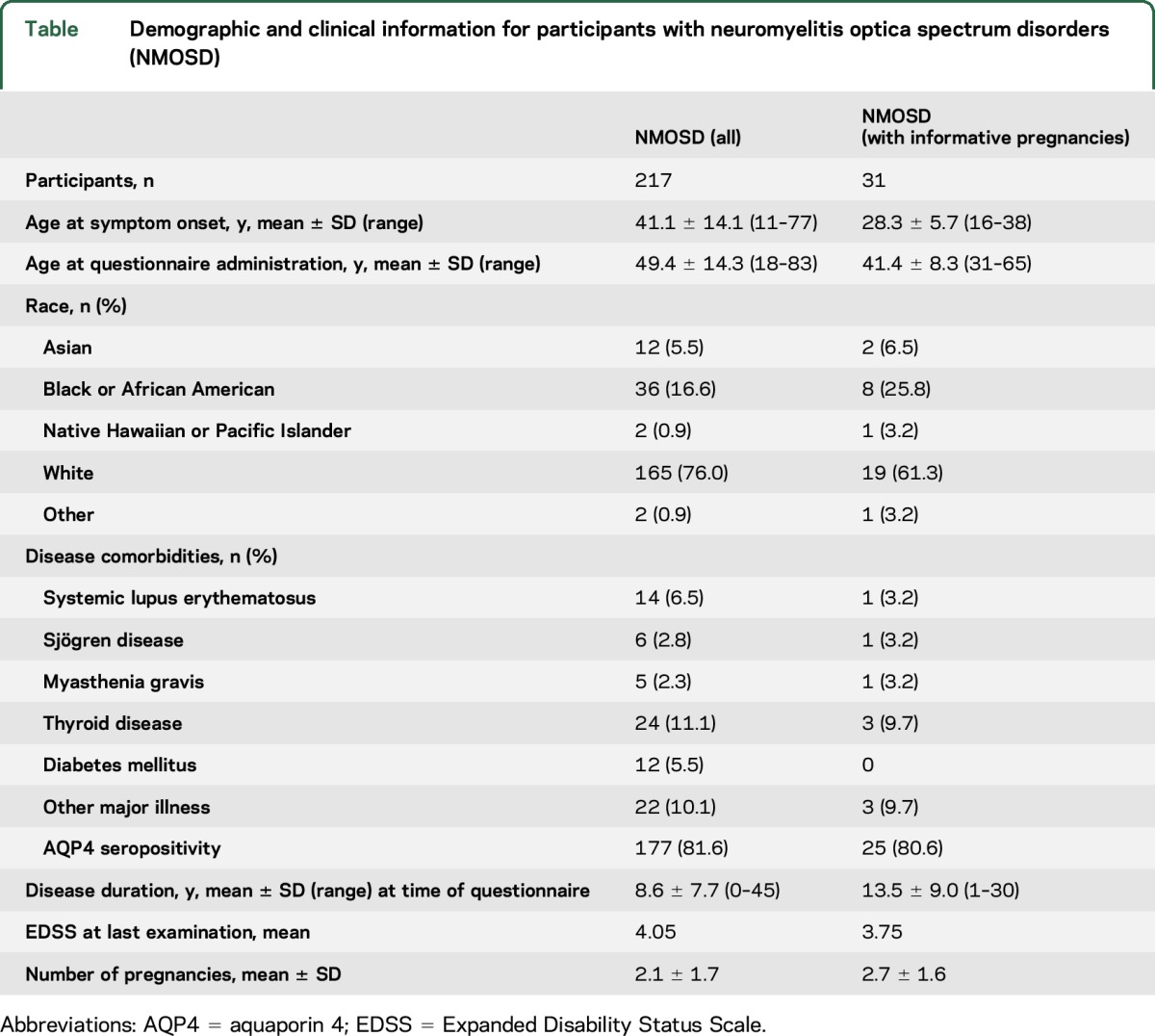
Figure 1. Flow chart of pregnancy history in participants with neuromyelitis optica.
Participant numbers are reported for pregnancy history, timing of pregnancy related to neurologic symptom onset, and pregnancy outcome. NMOSD = neuromyelitis optica spectrum disorders.
Risk of relapse related to pregnancy.
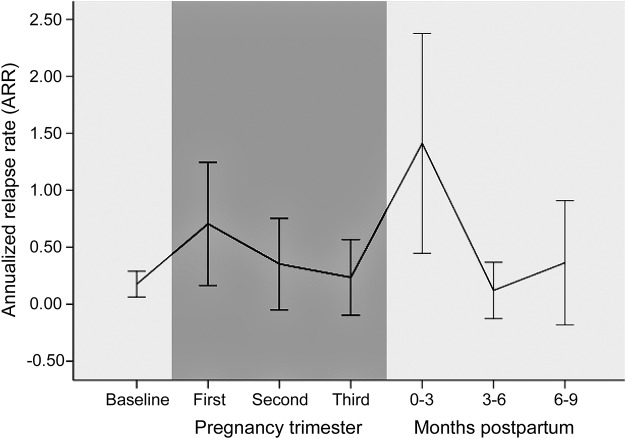
Of 172 participants who had been pregnant, 46 pregnancies in 31 participants occurred following NMOSD symptom onset, of which 34 pregnancies in 26 participants resulted in a live birth. Demographic and clinical information on this subgroup is summarized in the table. Compared to the 2 years before pregnancy, the annualized relapse rate (ARR) in the first trimester increased from 0.17 (±0.32) to 0.73 (±1.57; p = 0.037) (figure 2). In other trimesters of pregnancy, the ARR was unchanged compared to baseline. The relapse rate increased during the 0–3 months postpartum period (ARR 1.33 ± 2.77; p = 0.018) compared to the 2-year baseline before pregnancy but not during the 3–6 months or 6–9 months postpartum periods (figure 2). When analyzed in aggregate, both pregnancy and the 9-month postpartum periods (ARR 0.44 ± 0.72; p = 0.035 and ARR 0.69 ± 1.16; p = 0.009, respectively) were increased compared to baseline but not compared to one another (p = 0.228). Of the 9 pregnancies in which participants experienced a relapse during pregnancy and the 11 pregnancies in which participants experienced a relapse in the 9-month postpartum period, 3 participants experienced relapses both during pregnancy and within the 9 months following delivery. There was no statistical difference in baseline ARR in the 2 years prior to pregnancy between participants who experienced a relapse during pregnancy compared to those who remained relapse-free during pregnancy (0.26 vs 0.09; p = 0.113).
Figure 2. Annualized relapse rate (ARR) relative to pregnancy trimester and postpartum period in neuromyelitis optica.
ARR is reported for the 2 years prior to pregnancy (baseline), each trimester during pregnancy (dark area), and each 3-month period postpartum. Whiskers represent 95% confidence intervals.
Use of immunosuppressant and immunomodulatory therapy.
Of the 31 participants with pregnancies following NMOSD symptom onset, treatment with immunosuppressant or immunomodulatory therapy during baseline period included 4 on steroids, 4 on azathioprine, 1 on rituximab, 1 on cyclosporine, and 1 on glatiramer acetate. In 8 participants, these treatments were discontinued on average 11.9 months in advance of becoming pregnant. Two participants remained on azathioprine during pregnancy and another had therapeutic effect from rituximab during pregnancy. The majority of participants were untreated during the baseline periods and during pregnancy. Immunosuppressant and immunomodulatory treatment in the postpartum period was not adequately documented.
Relapse characteristics.
Relapses occurred during 9 of 34 (26.5%) pregnancies (8 had 1 relapse and 1 had 2 relapses). Eight relapses consisted of transverse myelitis and 2 of optic neuritis. Intractable vomiting thought unrelated to morning sickness/hyperemesis gravidarum or hiccups were associated with relapse in 2 cases. Symptoms resolved within 6 months in all but 2 cases (80%). Methylprednisolone was administered for 5 of the 10 relapses (50%); other relapses were untreated. Fifteen relapses in 11 individuals (32.4% of pregnancies) occurred in the postpartum period (8 had 1 relapse, 2 had 2 relapses, and 1 had 3 relapses). The relapses consisted of 12 transverse myelitis events and 3 optic neuritis events. Recovery was incomplete at 6 months in 8 of 15 postpartum relapses (53.3%). Compared to relapses during pregnancy, postpartum relapses were more likely to be treated with methylprednisolone, which was administered for all but 2 relapses (86.7%).
Risk of NMOSD disease onset postpartum.
Of the 76 participants with NMOSD with a history of pregnancy and initial disease onset during childbirth years (defined as ages 20–40 for the purposes of this study), 6 experienced their initial NMOSD presentation during pregnancy (7.9%), 8 presented within 3 months following delivery (10.5%), and an additional 5 presented within the next 6 months (6.6%). The observed presentation rate during pregnancy and postpartum months was compared to the expected presentation rate if the events of pregnancy and relapse were not associated. In the 147 participants with NMO with a history of pregnancy, 2,734 patient-years (32,808 months) were spent between the ages of 20 and 40 years prior to symptom onset. During that time, there were 399 pregnancies (1,197 postpartum months), suggesting the expected risk of disease onset to be 3.65% (1,197/32,808), assuming no association. The observed risk (10.5%) exceeds the expected risk (3.65%) of initial NMOSD symptom onset during the postpartum period by a factor of 2.9.
Attitudes toward pregnancy and disability.
Of the 45 participants with NMOSD who reported no history of pregnancy, 14 (31.1%) cited either concerns over disability accrual or stopping disease-modifying treatments as reasons for never becoming pregnant.
Pregnancy-related complications.
Of the 46 pregnancies following symptom onset, 34 resulted in a live birth (73.9%), 10 resulted in spontaneous abortion (21.7%), and 2 resulted in therapeutic abortion (4.3%). Of the 34 pregnancies following symptom onset that resulted in a live birth, there were 3 cases of preeclampsia or other hypertensive disorders (8.8%) and 4 cases of preterm labor (11.8%). The frequency of Cesarean section was 55.9% (19/34). Placental pathology included one case of placenta previa and one case of placental abruption. One child had congenital anomalies and 4 other children were recognized as having other health issues not otherwise specified. There are no reports of autoimmune disease or neurologic disease in these children. Participants with NMOSD breastfed postpartum in 27 of 34 pregnancies (79.4%) for an average of 6.7 months. Breastfeeding was considered exclusive in 21 of 27 instances. Breastfeeding (exclusive or otherwise) was not observed to be associated with risk of relapse (χ2 0.56; p = 0.51).
Of the 172 participants who had been pregnant either before or after disease onset, preeclampsia was reported in 12 (7.0%), preterm labor in 12 (7.0%), antenatal hospitalization in 6 (3.5%), intrauterine growth retardation in 3 (1.7%), and premature rupture of membranes in 1 (0.6%). The frequency of hypertensive disorders of pregnancy, including preeclampsia, in pregnancies following NMOSD symptom onset was 3 of 46 (6.5%). Fifty-seven of 172 (33.1%) participants reported a history of abortion (either therapeutic or spontaneous) with any of their pregnancies, although the spontaneous abortion frequency was not obtained in this group. Of those with pregnancies following symptom onset, 10 of 46 (21.7%) in 7 women ended in spontaneous abortion. Similar to the general population, the majority of spontaneous abortions were reported in the first trimester (8 of 10).
DISCUSSION
We examined the relationship of pregnancy and relapse rate in a large NMOSD dataset. With nearly 90% of patients with NMOSD being female,22 the effect of pregnancy on disease course is an important issue in the clinical care of patients with NMOSD and one that was reflected as a meaningful concern that modified family planning decisions in a substantial percentage of patients with NMOSD we studied. The results from this study support results from other retrospective studies from different geographic areas and different ethnicities describing an increase in relapse rate in the first 3 months postpartum in NMOSD.11–14 The high postpartum relapse rate relative to during pregnancy is also reported in MS7 but we observed a postpartum relapse rate increase relative to baseline rates in NMOSD that was higher than published in MS, consistent with other aggressive clinical features of NMOSD. This finding supports the concept of instituting a more aggressive treatment approach immediately after pregnancy.17 Although data here are limited for a potential conferred protection of breastfeeding, as is observed in MS,10 the observed high risk of postpartum relapses could tip the balance toward instituting disease-modifying treatments with fast onset of efficacy immediately postpartum and foregoing breastfeeding.
Our study suggests an increase in relapse rate during the first trimester of pregnancy compared to baseline, in contrast to patients with MS, who have a lower relapse rate during pregnancy, especially in the last trimester.23 It is possible this increased relapse rate could result from tapering off steroids and other immunosuppressants in the months leading up to pregnancy. Others have previously reported an increased risk of relapse during pregnancy in NMOSD. However, patients with NMOSD with relapses during pregnancy were more likely to have complete symptom resolution by 6 months and less likely to receive steroids or other treatments compared to postpartum relapses. These data could be interpreted as milder relapses occurring during pregnancy or a predilection for more aggressive relapses in the postpartum period, or both. One important unanswered question in NMOSD care surrounds what the safest approach to treating patients with NMOSD during pregnancy may be. In contrast to MS, where the vast majority of patients stop disease-modifying therapies, the increased relapse rate during pregnancy in NMOSD and potential for disability supports continuing an immunotherapy during pregnancy, especially in patients with a higher baseline relapse rate. A possible predilection for milder relapses during pregnancy bodes well for using milder treatment regimens during pregnancy in patients with well-controlled disease leading up to pregnancy. Patients with higher disease activity prior to pregnancy may require more aggressive treatment, such as continuing rituximab, where pregnancy safety data exist.24
The relatively low baseline ARR of 0.17 suggests a selection bias toward good disease activity control in those women with NMOSD electing to become pregnant. Recent large descriptive studies suggest an ARR of 0.8–1.3 in the general NMOSD population.22,25 Other retrospective studies involving pregnancy in NMOSD demonstrated prepregnancy baseline ARR ranging from ∼0.2 to 1.0, with all studies characterized by a higher prepregnancy relapse rate than our study. Results in our patient population with an observed low baseline relapse rate may not be applicable to patients with a history of more active disease when providing prepregnancy counseling.
We observed a high percentage of female patients with NMOSD with initial symptom onset in the 3 months following delivery. Initial symptom onset in the postpartum period is a well-recognized feature of MS. However, this suggests that while NMOSD is diagnosed at lower frequency than MS, it should be considered in women with new neurologic symptoms during the postpartum period, particularly relapses with clinical features suggestive of NMOSD.
Emerging data provide a plausible pathobiologic explanation for the apparent change in disease activity during pregnancy. AQP4 is upregulated during pregnancy and in the postpartum period.26 Furthermore, in addition to expression in astrocytes, AQP4 is expressed in the placenta and may play a role in ion homeostasis and water balance throughout pregnancy.27 Placental AQP4 expression is high during the second trimester and then progressively decreases through the end of pregnancy.28 Placental AQP4 expression theoretically could contribute to adverse pregnancy events. However, this study does not provide convincing evidence for an excess of pregnancy-related complications compared to what has been reported in the general population.29 The spontaneous abortion frequency in pregnancies after symptom onset in our study was lower than previously reported in a group of women with NMOSD from Japan, the United Kingdom, and Portugal.15 That study also reported a high risk of preeclampsia, in contrast to our study, which reports a frequency similar to the general population.30 Comprehensive evaluations of placental tissue from multiple pregnancies are needed in NMOSD to help address the role of AQP4 placental expression in pregnancy-related complications.
The retrospective design of this study is an important limitation, similar to other studies in the literature examining the effect of pregnancy on disease activity in NMOSD. These limitations extend to deriving disability scores, which were dependent upon the quality of the history and neurologic examination documented. Prospective pregnancy registries in NMOSD are needed. Prospective analyses of disease-modifying and acute relapse treatment courses during and following pregnancy, relapse history, pregnancy-related complications, and pregnancy outcomes would enlighten the medical community as to the most appropriate treatment regimens for pregnant and postpartum women with NMOSD and could inform consensus recommendations for pregnancy management of patients with NMOSD.
This large retrospective study corroborates the results of other studies demonstrating a high risk of relapse in the postpartum period in NMOSD, but also during pregnancy. In addition, the higher risk of initial disease onset in the months immediately following delivery is an important point of clinical and diagnostic recognition. These findings will facilitate counseling patients with NMOSD regarding family planning and appropriate management of disease-modifying therapy prior to, during, and after pregnancy.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Erin Longbrake, MD, PhD, for her contributions to data entry for the Washington University in St. Louis site.
GLOSSARY
- AQP4
aquaporin-4
- ARR
annualized relapse rate
- EDSS
Expanded Disability Status Scale
- IgG
immunoglobulin G
- MGH
Massachusetts General Hospital
- MS
multiple sclerosis
- NMO
neuromyelitis optica
- NMOSD
neuromyelitis optica spectrum disorders
Footnotes
Supplemental data at Neurology.org
Editorial, page 2220
AUTHOR CONTRIBUTIONS
E. Klawiter: design or conceptualization of the study, analysis or interpretation of the data, and drafting/revising the manuscript for intellectual content. R. Bove: design or conceptualization of the study, analysis or interpretation of the data, and drafting/revising the manuscript for intellectual content. L. Elsone: data collection and drafting/revising the manuscript for intellectual content. E. Alvarez: data collection and drafting/revising the manuscript for intellectual content. N. Borisow: data collection and drafting/revising the manuscript for intellectual content. M. Cortez: data collection and drafting/revising the manuscript for intellectual content. F. Mateen: data collection and drafting/revising the manuscript for intellectual content. M.A. Mealy: data collection and drafting/revising the manuscript for intellectual content. J. Sorum: data collection and drafting/revising the manuscript for intellectual content. K. Mutch: data collection and drafting/revising the manuscript for intellectual content. S. Tobyne: data collection and drafting/revising the manuscript for intellectual content. K. Ruprecht: data collection and drafting/revising the manuscript for intellectual content. G. Buckle: design or conceptualization of the study and drafting/revising the manuscript for intellectual content. M. Levy: design or conceptualization of the study drafting/revising the manuscript for intellectual content. D. Wingerchuk: design or conceptualization of the study and drafting/revising the manuscript for intellectual content. F. Paul: design or conceptualization of the study and drafting/revising the manuscript for intellectual content. A. Cross: design or conceptualization of the study and drafting/revising the manuscript for intellectual content. A. Jacob: design or conceptualization of the study and drafting/revising the manuscript for intellectual content. T. Chitnis: design or conceptualization of the study, analysis or interpretation of the data, drafting/revising the manuscript for intellectual content, and study supervision. B. Weinshenker: design or conceptualization of the study, analysis or interpretation of the data, drafting/revising the manuscript for intellectual content, and study supervision.
STUDY FUNDING
Study funding included the Guthy Jackson Charitable Foundation (Eureka Grant; E.C.K.). F.P. and K.R. were supported by the German Ministry for Education and Research (Competence Network Multiple Sclerosis). A.H.C. was supported the Manny & Rosalyn Rosenthal–Dr. John L. Trotter MS Center Chair.
DISCLOSURE
E. Klawiter has received consulting fees from Acorda Therapeutics, Atlas5d, Biogen Idec, EMD Serono, Genentech, and Shire and research support from Atlas5d, Biogen Idec, EMD Serono, and Roche. R. Bove and L. Elsone report no disclosures relevant to the manuscript. E. Alvarez has received consulting fees from Novartis, Biogen Idec, Teva Neuroscience, and Genzyme and support for research activities from Novartis, Biogen Idec, Acorda, and the Rocky Mountain Multiple Sclerosis Center. N. Borisow, M. Cortez, and F. Mateen report no disclosures relevant to the manuscript. M. Mealy has received honoraria from the Consortium of Multiple Sclerosis Centers. J. Sorum, K. Mutch, and S. Tobyne report no disclosures relevant to the manuscript. K. Ruprecht has received research support from Bundesministerium für Bildung und Forschung (Competence Network Multiple Sclerosis) and Novartis and honoraria from Bayer Healthcare, Biogen Idec, Merck Serono, Sanofi/Genzyme, Teva Neuroscience, Roche, and Novartis. G. Buckle has received personal compensation from Bayer, Biogen Idec, EMD-Serono, Genentech, Genzyme Corporation, Mallinckrodt, Novartis, and Teva Neuroscience as a consultant or speaker. Dr. Buckle has received research support from Biogen Idec. M. Levy has received research funding from Alexion, Genzyme, Acorda, Sanofi, TG Therapeutics, TerumoBCT, and Alnylam; consulted for Chugai/Roche, Sanofi, Alexion, and Acorda; and serves on the Scientific Advisory Board for Alexion and Acorda. D. Wingerchuk serves on the adjudication panel for MedImmune; is a consultant to Alexion, MedImmune, and Chugai Pharmaceuticals; is co-editor-in-chief for The Neurologist; and Alexion, TerumoBCT, and the Guthy Jackson Charitable Foundation. F. Paul has received honoraria and/or research support from Alexion, Bayer, Biogen, Chugai, MerckSerono, Novartis, Genyzme, MedImmune, Shire, and Teva Neuroscience; and has received funding from Deutsche Forschungsgemeinschaft (DFG Exc 257), Bundesministerium für Bildung und Forschung (Competence Network Multiple Sclerosis), Guthy Jackson Charitable Foundation, EU Framework Program 7, and National Multiple Sclerosis Society of the USA. A. Cross has received consulting fees from AbbVie, Biogen, EMD-Serono, Genentech, Genzyme/Sanofi, Roche, Teva Neuroscience, and Novartis. A. Jacob has received consulting and speaking fees and clinical trial grants from Biogen Idec, Alexion, Shire, and Chugai. T. Chitnis has received personal compensation for advisory board/consulting for Novartis and Biogen Idec and received financial support for research activities from Merck-Serono and Novartis Pharmaceuticals. B. Weinshenker has received consulting fees for participation on data safety monitoring boards for Novartis, Biogen Idec, and Mitsubishi Pharmaceuticals; consulting fees from MedImmune Pharmaceuticals for participation on an attack adjudication committee; and has received royalties from RSR Ltd. and Oxford University as a named coinventor of technology described in a patent held by Mayo Foundation for NMO-IgG for diagnosis of neuromyelitis optica. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 2005;202:473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matiello M, Schaefer-Klein J, Sun D, Weinshenker BG. Aquaporin 4 expression and tissue susceptibility to neuromyelitis optica. JAMA Neurol 2013;70:1118–1125. [DOI] [PubMed] [Google Scholar]
- 3.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marker-Hermann E, Fischer-Betz R. Rheumatic diseases and pregnancy. Curr Opin Obstet Gynecol 2010;22:458–465. [DOI] [PubMed] [Google Scholar]
- 5.Buyon JP, Kim MY, Guerra MM, et al. Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann Intern Med 2015;163:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyth A, Oliveira GH, Lahr BD, Bailey KR, Norby SM, Garovic VD. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol 2010;5:2060–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis: Pregnancy in Multiple Sclerosis Group. N Engl J Med 1998;339:285–291. [DOI] [PubMed] [Google Scholar]
- 8.D'Hooghe MB, D'Hooghe T, De Keyser J. Female gender and reproductive factors affecting risk, relapses and progression in multiple sclerosis. Gynecol Obstet Invest 2013;75:73–84. [DOI] [PubMed] [Google Scholar]
- 9.Pakpoor J, Disanto G, Lacey MV, Hellwig K, Giovannoni G, Ramagopalan SV. Breastfeeding and multiple sclerosis relapses: a meta-analysis. J Neurol 2012;259:2246–2248. [DOI] [PubMed] [Google Scholar]
- 10.Hellwig K, Rockhoff M, Herbstritt S, et al. Exclusive breastfeeding and the effect on postpartum multiple sclerosis relapses. JAMA Neurol 2015;72:1132–1138. [DOI] [PubMed] [Google Scholar]
- 11.Kim W, Kim SH, Nakashima I, et al. Influence of pregnancy on neuromyelitis optica spectrum disorder. Neurology 2012;78:1264–1267. [DOI] [PubMed] [Google Scholar]
- 12.Fragoso YD, Adoni T, Bichuetti DB, et al. Neuromyelitis optica and pregnancy. J Neurol 2013;260:2614–2619. [DOI] [PubMed] [Google Scholar]
- 13.Bourre B, Marignier R, Zephir H, et al. Neuromyelitis optica and pregnancy. Neurology 2012;78:875–879. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu Y, Fujihara K, Ohashi T, et al. Pregnancy-related relapse risk factors in women with anti-AQP4 antibody positivity and neuromyelitis optica spectrum disorder. Mult Scler 2016;22:1413–1420. [DOI] [PubMed] [Google Scholar]
- 15.Nour MM, Nakashima I, Coutinho E, et al. Pregnancy outcomes in aquaporin-4-positive neuromyelitis optica spectrum disorder. Neurology 2016;86:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asgari N, Henriksen TB, Petersen T, Lillevang ST, Weinshenker BG. Pregnancy outcomes in a woman with neuromyelitis optica. Neurology 2014;83:1576–1577. [DOI] [PubMed] [Google Scholar]
- 17.Ringelstein M, Harmel J, Distelmaier F, et al. Neuromyelitis optica and pregnancy during therapeutic B cell depletion: infant exposure to anti-AQP4 antibody and prevention of rebound relapses with low-dose rituximab postpartum. Mult Scler 2013;19:1544–1547. [DOI] [PubMed] [Google Scholar]
- 18.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489. [DOI] [PubMed] [Google Scholar]
- 19.Bove R, Elsone L, Alvarez E, et al. Female hormonal exposures and neuromyelitis optica symptom onset in a multicenter study. Neurol Neuroimmunol Neuroinflamm 2017;4:e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mealy MA, Wingerchuk DM, Greenberg BM, Levy M. Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Arch Neurol 2012;69:1176–1180. [DOI] [PubMed] [Google Scholar]
- 23.Cornelio DB, Braga RP, Rosa MW, Ayub AC. Devic's neuromyelitis optica and pregnancy: distinction from multiple sclerosis is essential. Arch Gynecol Obstet 2009;280:475–477. [DOI] [PubMed] [Google Scholar]
- 24.Chakravarty EF, Murray ER, Kelman A, Farmer P. Pregnancy outcomes after maternal exposure to rituximab. Blood 2011;117:1499–1506. [DOI] [PubMed] [Google Scholar]
- 25.Kitley J, Leite MI, Nakashima I, et al. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain 2012;135:1834–1849. [DOI] [PubMed] [Google Scholar]
- 26.Quick AM, Cipolla MJ. Pregnancy-induced up-regulation of aquaporin-4 protein in brain and its role in eclampsia. FASEB J 2005;19:170–175. [DOI] [PubMed] [Google Scholar]
- 27.De Falco M, Cobellis L, Torella M, et al. Down-regulation of aquaporin 4 in human placenta throughout pregnancy. In Vivo 2007;21:813–817. [PubMed] [Google Scholar]
- 28.Saadoun S, Waters P, Leite MI, Bennett JL, Vincent A, Papadopoulos MC. Neuromyelitis optica IgG causes placental inflammation and fetal death. J Immunol 2013;191:2999–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jurkovic D, Overton C, Bender-Atik R. Diagnosis and management of first trimester miscarriage. BMJ 2013;346:f3676. [DOI] [PubMed] [Google Scholar]
- 30.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011;25:391–403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.