Abstract
Objective:
To demonstrate single abobotulinumtoxinA injection efficacy in lower limb vs placebo for adults with chronic hemiparesis and assess long-term safety and efficacy of repeated injections.
Methods:
In a multicenter, double-blind, randomized, placebo-controlled, single-cycle study followed by a 1-year open-label, multiple-cycle extension, adults ≥6 months after stroke/brain injury received one lower limb injection (abobotulinumtoxinA 1,000 U, abobotulinumtoxinA 1,500 U, placebo) followed by ≤4 open-label cycles (1,000, 1,500 U) at ≥12-week intervals. Efficacy measures included Modified Ashworth Scale (MAS) in gastrocnemius–soleus complex (GSC; double-blind primary endpoint), physician global assessment (PGA), and comfortable barefoot walking speed. Safety was the open-label primary endpoint.
Results:
After a single injection, mean (95% confidence interval) MAS GSC changes from baseline at week 4 (double-blind, n = 381) were as follows: −0.5 (−0.7 to −0.4) (placebo, n = 128), −0.6 (−0.8 to −0.5) (abobotulinumtoxinA 1,000 U, n = 125; p = 0.28 vs placebo), and −0.8 (−0.9 to −0.7) (abobotulinumtoxinA 1,500 U, n = 128; p = 0.009 vs placebo). Mean week 4 PGA scores were as follows: 0.7 (0.5, 0.9) (placebo), 0.9 (0.7, 1.1) (1,000 U; p = 0.067 vs placebo), and 0.9 (0.7, 1.1) (1,500 U; p = 0.067); walking speed was not significantly improved vs placebo. At cycle 4, week 4 (open-label), mean MAS GSC change reached −1.0. Incremental improvements in PGA and walking speed occurred across open-label cycles; by cycle 4, week 4, mean PGA was 1.9, and walking speed increased +25.3% (17.5, 33.2), with 16% of participants walking >0.8 m/s (associated with community mobility; 0% at baseline). Tolerability was good and consistent with the known abobotulinumtoxinA safety profile.
Conclusions:
In chronic hemiparesis, single abobotulinumtoxinA (Dysport Ipsen) administration reduced muscle tone. Repeated administration over a year was well-tolerated and improved walking speed and likelihood of achieving community ambulation.
Clinicaltrial.gov identifiers:
Classification of evidence:
The double-blind phase of this study provides Class I evidence that for adults with chronic spastic hemiparesis, a single abobotulinumtoxinA injection reduces lower extremity muscle tone.
Hemiparesis from acquired brain injury can impair mobility, related to abnormal passive and active antagonist muscle resistance.1,2 In chronic hemiparesis, walking speed stabilizes at a low plateau level, inadequate for sustainable community ambulation.3,4 Slow walking speed and inability to ambulate in the community represents a significant health issue, associated with accelerated health decline.5,6
Intramuscular injection of botulinum toxin type A (BoNT-A), a neurotoxin produced by Clostridium botulinum bacteria,7 produces muscle relaxation for 12–16 weeks or longer and is an effective treatment for muscle overactivity in spastic paresis.8–13 While abobotulinumtoxinA (Dysport; IpsenPharma, Wrexham, UK) reduces muscle tone and improves spasticity-related features in upper and lower limb muscles, long-term safety of repeated lower limb injections and concomitant effect on walking speed in hemiparesis have not been explored.8–13
We evaluated efficacy and safety of abobotulinumtoxinA in participants with chronic hemiparesis causing walking impairment, from a double-blind, single-cycle study followed by an open-label, repeated-cycle extension.
METHODS
Primary research question.
The double-blind study asked whether abobotulinumtoxinA is effective at reducing lower extremity muscle tone, measured using the Modified Ashworth Scale (MAS) score. This study provides Class I evidence that for adults with chronic spastic hemiparesis, a single abobotulinumtoxinA injection reduces lower extremity muscle tone.
Standard protocol approvals, registrations, and participant consents.
The research protocol and all study documents were approved by an independent ethics committee. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Consolidated Guidelines on Good Clinical Practice. Written informed consent was obtained prior to participants entering the study (Clinical Trial identifiers, double-blind NCT01249404; open-label NCT01251367).
Study population.
Inclusion criteria were ambulatory participants aged 18–80 years with spastic hemiparesis causing gait dysfunction; comfortable barefoot walking speed 0.1–0.8 m/s, measured on a 10-m walking speed test without walking aids; 1 clinically defined stroke episode or brain trauma ≥6 months prior to enrollment; MAS score ≥2 in gastrocnemius–soleus complex (GSC; measured with knee extended) in toxin-naive participants (no previous BoNT-A injection in affected lower limb) or ≥3 in toxin non-naive participants (≥4 months after last BoNT-A injection); and GSC spasticity angle ≥5° (Tardieu Scale).14 Exclusion criteria were major limitation in passive range of motion at hip, knee, or ankle; known sensitivity to BoNT or abobotulinumtoxinA excipients; pregnancy; and severe cognitive impairment that interfered with consent provision. No standardized physiotherapy regimen was associated with this protocol, but community physiotherapy initiated before study had to remain unchanged to week 4 and whenever possible until study end. No physiotherapy was initiated <4 weeks prior to study or during the first 4 study weeks.
Study design.
This was a phase III, multicenter, prospective, double-blind, randomized, placebo-controlled, single-treatment-cycle study in adults with chronic hemiparesis, followed by a phase III, multicenter, prospective, open-label, multiple-cycle extension. Fifty-two centers across Australia, Belgium, Czech Republic, France, Hungary, Italy, Poland, Portugal, Russia, Slovakia, and the United States participated.
The double-blind study consisted of a single injection of abobotulinumtoxinA 1,000 U, 1,500 U, or placebo into both soleus and gastrocnemius muscles and ≥1 other (investigator-selected) lower limb muscle (e-Methods at Neurology.org). For the open-label extension, participants were offered abobotulinumtoxinA for ≤4 treatment cycles at ≥12-week intervals, over ≤18 months. In both studies, selected muscles were targeted using electrical stimulation. At open-label cycle 1, all participants received 1,500 U except participants who experienced treatment-emergent adverse events (TEAEs) during the double-blind phase, which the investigator considered posed unacceptable risk, who received 1,000 U. For subsequent cycles, abobotulinumtoxinA 1,000 or 1,500 U was administered at weeks 12, 16, 20, or 24, based on the investigator's judgment. Retreatment was possible at intervals ≥12 weeks (e-Methods). For cycles 3/4, concomitant 500 U injection into the upper limb was allowed at the investigator’s discretion, while keeping the total body dose ≤1,500 U.
Study objectives.
The double-blind study primary objective was to demonstrate single abobotulinumtoxinA injection efficacy vs placebo in the lower extremity; safety was a secondary objective. The open-label study primary objective was to assess long-term safety of repeated abobotulinumtoxinA injections; long-term efficacy was a secondary objective.
Outcome measures.
The double-blind primary endpoint was GSC muscle tone (MAS knee extended) change from baseline after 4 weeks. Secondary endpoints, also measured after 4 weeks, were physician global assessment (PGA) score (9-point scale; −4 [markedly worse] to 4 [markedly improved]) assessed by an investigator different from the one assessing MAS; and 10-m comfortable barefoot walking speed without walking aids change from baseline. Exploratory endpoints included soleus muscle tone (MAS knee flexed) mean change from baseline, spasticity (Tardieu Scale; angle of arrest XV1, angle of catch XV3, spasticity grade Y), range of active ankle dorsiflexion (XA), measured knee flexed and extended,14 and Short-Form Health Survey (SF-36) and European Quality of Life (EQ-5D) questionnaires. Safety assessment included TEAEs elicited by direct, nonleading questioning and spontaneous reports; laboratory measures; vital signs; ECG analysis; and neutralizing antibodies analysis (e-Methods). Identical safety and efficacy endpoints were assessed throughout the open-label study. This report focuses on key efficacy endpoints of muscle tone, PGA, comfortable barefoot walking speed, spasticity, and active range of motion.3,14
Double-blind study randomization.
Participants were randomized (1:1:1) using a block design to receive abobotulinumtoxinA 1,000 U, 1,500 U, or placebo and stratified by toxin baseline status (naive and non-naive; e-Methods).
Statistical analysis.
Sample size calculation.
For the double-blind study, 156 randomized participants (n = 52 per treatment group) were necessary to demonstrate significance on MAS change from baseline to week 4 in GSC (primary endpoint) with a 2-sided comparison-wise type I error rate at 0.025% and 90% power, assuming mean MAS changes of −0.9 with active treatments and −0.4 with placebo, common SD of 0.7, and 3% dropout rate. To meet long-term safety objectives, a sample size of 348 randomized participants was considered necessary (assuming 97% double-blind participants would enter the open-label study and a 5% dropout rate at each subsequent cycle). Centers that recruited fewer than 6 participants were considered small centers and were pooled with one another (e-Methods).
Double-blind phase.
The primary population for efficacy analyses was the intent-to-treat (ITT) population, defined as all randomized participants who received ≥1 study medication injection, with baseline and week 4 MAS scores.
MAS consists of 6 grades: 0, 1, 1+, 2, 3, or 4. For quantitative analyses, 1+ was considered as 2 and higher numeric scores were incremented by 1, giving a 6-point MAS range (0–5). For the primary endpoint, mean data were tested using 2 contrast analyses within a single mixed-effect analysis of covariance (ANCOVA) model, controlled for baseline MAS score, baseline BoNT treatment status, and center, all as fixed factors (post hoc analysis was conducted using rank transform and proportional odds models to address possible lack of data normality [e-Results]). To control family-wise type I error rate, a 2-step Hochberg method (e-Methods) was applied to demonstrate superiority of either abobotulinumtoxinA dose to placebo. For PGA, identical analyses were performed on mean week 4 values. Post hoc analysis using ranked PGA scores and proportional odds analysis (e-Results) was undertaken to restore power. For other endpoints, a single, mixed-effect ANCOVA model was performed.
Open-label phase.
Descriptive statistics were performed for all efficacy and safety endpoints. Post hoc analyses by subgroup tested whether walking speed changes differed depending on time since lesion and concomitant physiotherapy, and whether participants changed gait speed range categories, reported according to functions of household ambulation (<0.4 m/s), limited community mobility (0.4–0.8 m/s), and full community mobility (>0.8 m/s).15 Additional post hoc analyses were performed using Pearson correlation coefficients estimated by treatment group (baseline–study end) exploring linear relationships between (1) time since lesion and walking speed change; and (2) composite XA (XA against GSC + XA against soleus) and walking speed. Additional post hoc analysis compared 10-m walking speed change at week 4 vs 12 across cycles using a model for repeated measures.
Clinical trial data disclosure
Results of the double-blind study (NCT01249404) were posted on the EudraCT website on August 1, 2015. Results of the open-label extension (NCT01251367) were posted on the EudraCT website on March 31, 2017.
RESULTS
Study population characteristics.
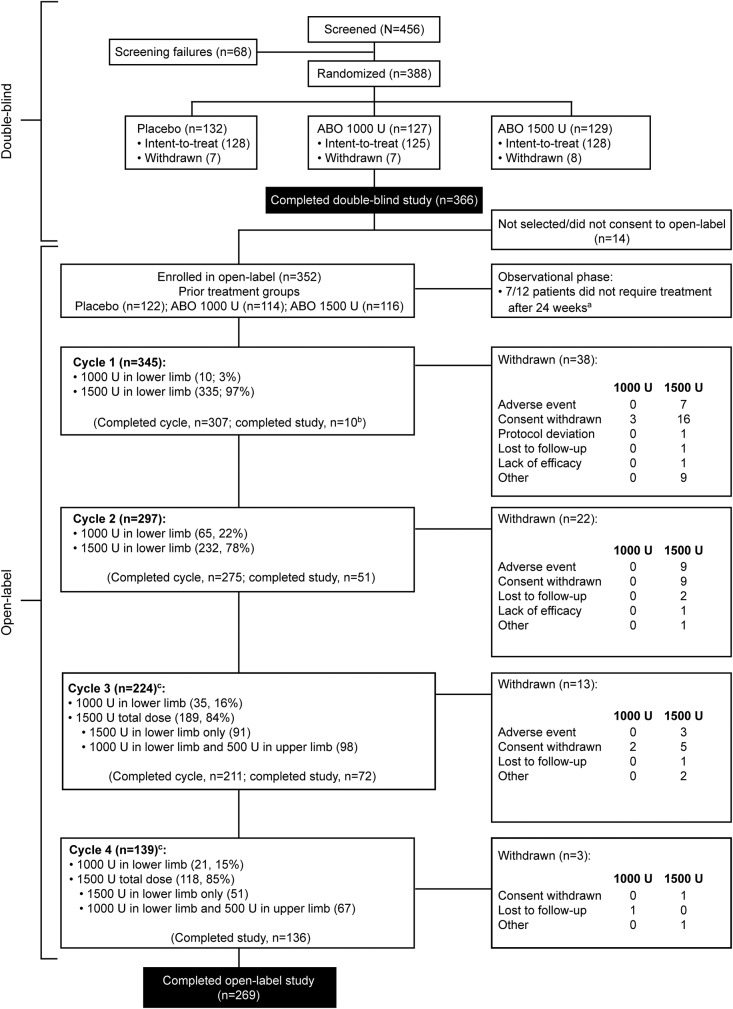
Of 381 double-blind phase participants (128 placebo, 125 abobotulinumtoxinA 1,000 U, 128 abobotulinumtoxinA 1,500 U; ITT population; study initiation March 2011; completion May 2014), 352 continued in the open-label phase (figure 1; study initiation June 2011; completion April 2015). Participant characteristics are presented in table 1. Approximately 60% of participants underwent concomitant physiotherapy, including during the open-label phase. To appreciate loss of gastrocnemius and soleus extensibility, baseline coefficients of muscle shortening (CS) were retrospectively calculated (CS = [XN − XV1]/XN, where XN is the normal expected amplitude [115° GSC; 120° soleus]).16 Mean (SD) baseline CS were 23.7% (8.5) in GSC and 20.6% (7.9) in soleus. Mean treatment exposure duration (2 studies combined) was 54 weeks; table e-1 lists doses injected per muscle.
Figure 1. Participant disposition.
aOf the 12 patients in the observational phase, 5 required retreatment and entered cycle 1 and 7 did not require retreatment. Of these 7 patients, 3 withdrew early and 4 completed the study without retreatment. bIncluding 2 participants who entered an observational phase and received no further abobotulinumtoxinA (ABO) injections during the study. cIn cycles 3 and 4, concomitant treatment of the affected upper limb muscles was allowed.
Table 1.
Baseline demographics and participant characteristics (intent-to-treat population)
Efficacy of a single injection (double-blind study).
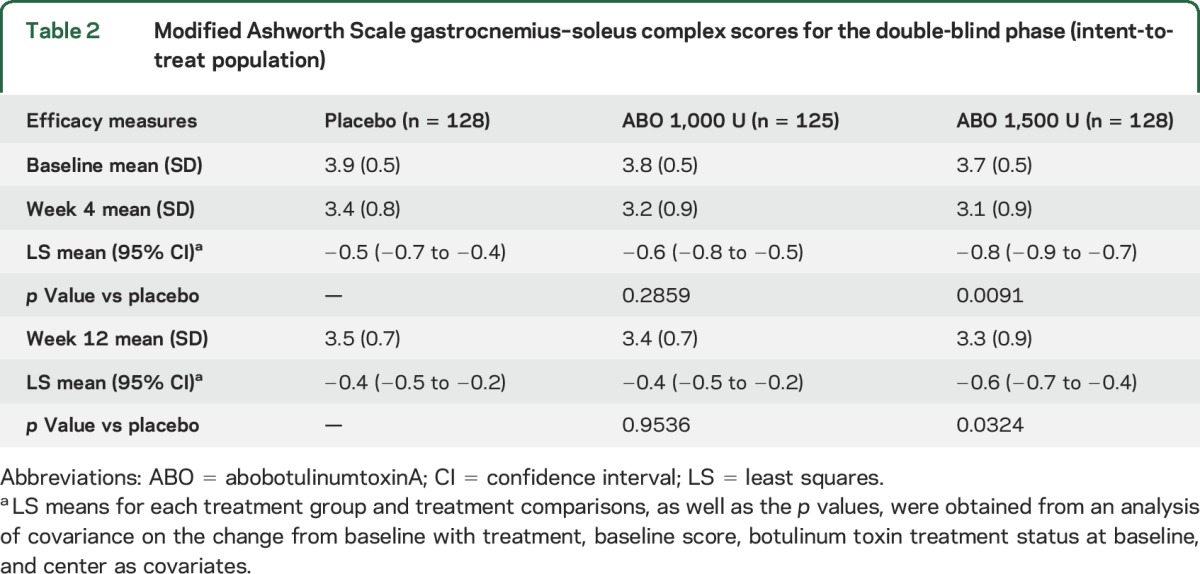
The main efficacy results after a single injection are presented in table 2. At week 4, a single injection of abobotulinumtoxinA 1,500 U reduced MAS GSC to a greater extent than placebo (p = 0.009), with no significant difference seen between abobotulinumtoxinA 1,000 U and placebo (p = 0.28). GSC tone reduction vs placebo at week 4 in the 1,500 U group was maintained at week 12 (1,000 U not statistically different). Tone reduction occurred in soleus with both doses at weeks 4 and 12 (table e-2). Exploratory analysis of treatment-by-center interaction is presented in e-Results.
Table 2.
Modified Ashworth Scale gastrocnemius–soleus complex scores for the double-blind phase (intent-to-treat population)
According to the planned analysis, neither dose was significantly more effective than placebo at week 4 for PGA (table e-2). However, week 4 PGA distribution was heavily skewed, undermining the assumption of normality necessary to support a statistically valid and powerful ANCOVA. Exploratory analysis using ranked PGA scores showed both doses were superior to placebo (table e-2). There was no difference in comfortable barefoot walking speed change from baseline among the 3 groups at weeks 4 or 12 (table e-2), nor for quality of life (QoL) scales. For changes in active range of ankle dorsiflexion and spasticity (XV1, XV3, Y), see e-Results (figure e-1, table e-3).
Baseline characteristics were similar in BoNT-A-naive and non-naive participants, and BoNT-A status at baseline (naive vs non-naive) did not affect efficacy outcomes (table e-4). AbobotulinumtoxinA had similar efficacy in poststroke and traumatic brain injury (TBI) participants (table e-5).
Effects of repeated injections (open-label study).
From here, results are presented with doses combined as participants could alternate abobotulinumtoxinA doses between cycles based on clinical need, and because open-label results were similar across doses. Muscle tone improvements observed in the double-blind study (table 2) remained stable from cycle 2 week 4 onwards, with −0.9 from baseline in GSC (figure 2A) and −1.1 in soleus. PGA continuously improved from the double-blind study, reaching 1.9 at cycle 4 week 4 (figure 2B). Comfortable barefoot walking speed progressively increased across treatment cycles, reaching +25.35% (95% confidence interval 17.48–33.21) at cycle 4 week 4 from double-blind study baseline (figure 2C). Greater improvement was observed at week 12 than week 4 across cycles (figure 2C; p < 0.001, table e-6). Correlation between time since event and walking speed improvement suggested the more recent the event (stroke or TBI), the greater the improvement (e-Results, tables e-7 and e-8). For changes in passive, active range of ankle dorsiflexion and spasticity (XV1, XV3, XA, Y), see e-Results (figure e-1; table e-3). For QoL, by cycle 4 week 4, there were mean increases from baseline in SF-36 physical component (+2.80, SD 6.65) and EQ-5D visual analogue scale (+5.5, SD 21.0).
Figure 2. Main efficacy results (abobotulinumtoxinA [ABO] doses combined).
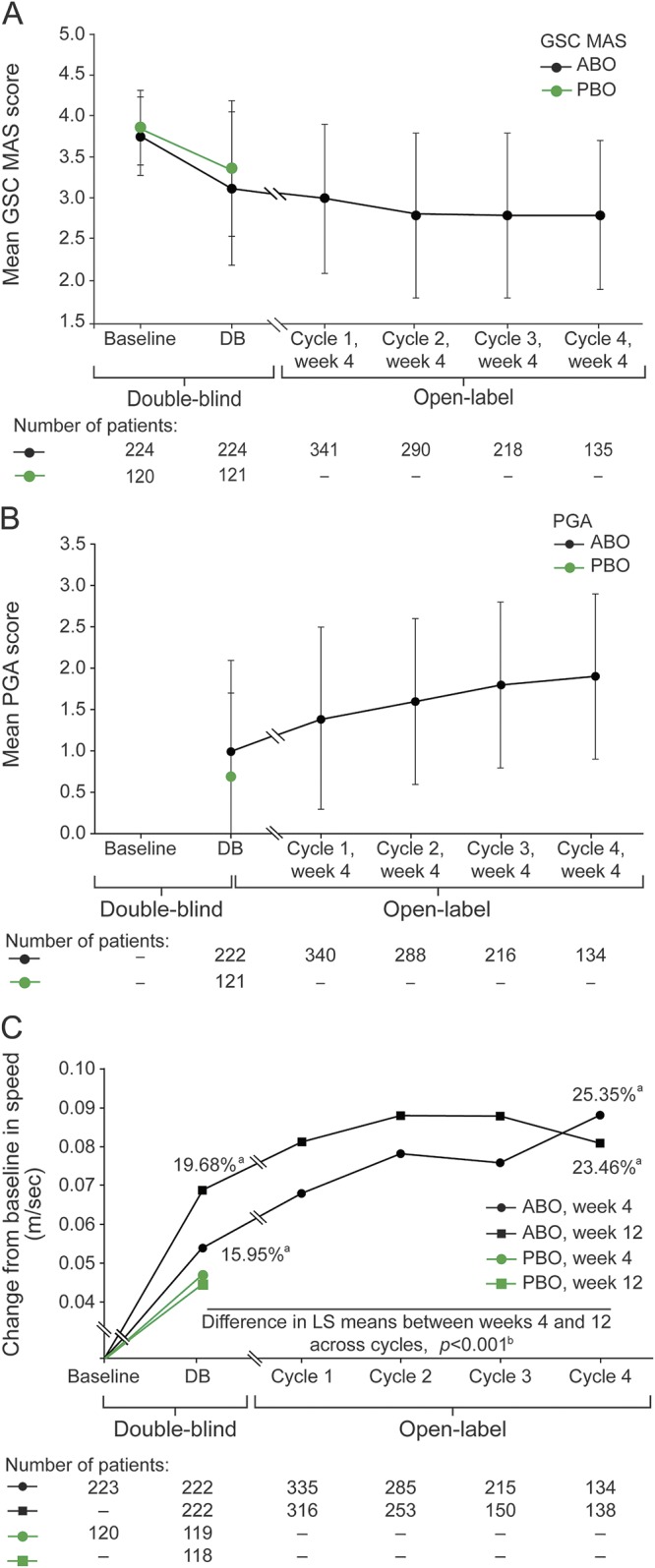
(A) Gastrocnemius–soleus complex (GSC) Modified Ashworth scale (MAS) and (B) physician global assessment (PGA) mean scores at baseline or week 4 and across treatment cycles and (C) mean change in comfortable barefoot walking speed from baseline and week 4 and week 12 of each cycle. Baseline refers to baseline of double-blind study, prior to first injection. Error bars in A and B show SD. aPercentage improvement from baseline. bA post hoc analysis compared change from baseline in 10-m walking speed at week 4 vs week 12 across cycles using a model for repeated measures; greater improvement was observed at week 12 compared with week 4 across cycles. DB = double-blind study; LS = least-squared; PBO = placebo.
Time to retreatment.
Among participants who received abobotulinumtoxinA in the double-blind study and continued in open-label cycle 1, 20.1% were not considered by the investigator to need reinjection at week 12 (9.8% were reinjected at week 16, 4.9% at week 20, 5.4% at week 24 or later). For open-label cycle 2, 32% were not reinjected at week 12 (16.5% retreated at week 16, 8.8% at week 20, 6.7% at week 24 or later). For open-label cycle 3, 15.2% were not reinjected at week 12.
Safety.
After a single injection (double-blind study), TEAE incidence was slightly higher with abobotulinumtoxinA than placebo (table 3). Most TEAEs were mild to moderate and considered unrelated to study treatment. Overall, TEAEs were most frequently falls, pain in extremity, and muscle weakness (see e-Results and table e-9). Six participants (2 per group) withdrew due to TEAEs: pulmonary embolism, loss of consciousness (placebo); arthralgia, pancreatic carcinoma (abobotulinumtoxinA 1,000 U); and generalized muscle weakness (abobotulinumtoxinA 1,500 U). Twenty serious adverse events (SAEs) occurred in 17 participants, equally distributed between groups. There were 2 deaths (1 pulmonary embolism, 1 “natural causes”; both placebo). One SAE was suggestive of remote toxin spread (generalized muscular weakness, abobotulinumtoxinA 1,500 U).
Table 3.
Summary of treatment-emergent adverse events (TEAEs) following abobotulinumtoxinA injections at any cycle (safety population)
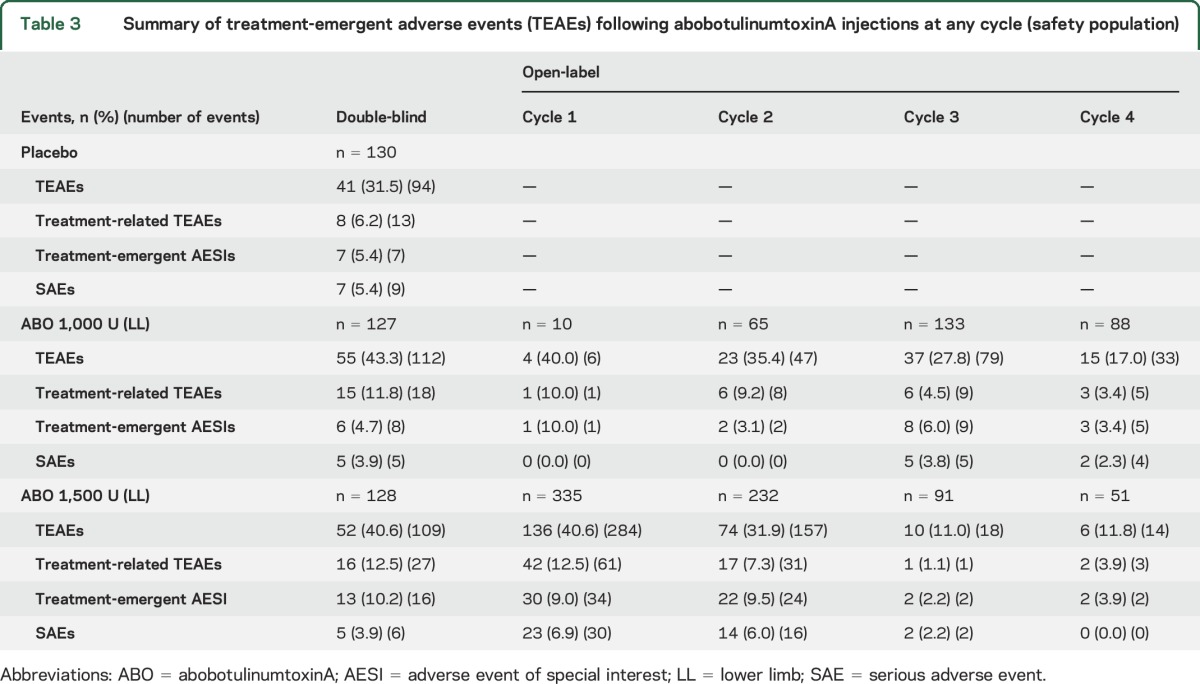
During the open-label extension, TEAE incidence decreased across cycles with both doses (table 3), considering that most participants leaving between cycles had reached maximal study duration (figure 1). Most TEAEs were mild to moderate. Falls and muscular weakness were reported (table e-9) with 9/345 participants (2.6%) presenting fractures following falls across all open-label cycles. Nineteen participants withdrew due to TEAEs; 11 were considered treatment-related. SAEs were experienced by 11% of participants across all cycles. There were 2 deaths (suicide and respiratory failure; both abobotulinumtoxinA 1,500 U), neither considered treatment-related. Four participants treated with abobotulinumtoxinA 1,500 U reported 5 SAEs suggestive of remote toxin spread (3 generalized muscular weaknesses, 2 dysphagia; e-Results); 1 participant withdrew as a result, 1 (2 SAEs) was reinjected at 2 consecutive treatment cycles, and 2 withdrew for independent reasons.
There were no significant hematology or clinical biochemistry changes and no clinically significant changes in vital signs or ECG measures. No participant presented with seroconversion for neutralizing antibodies (baseline antibodies reported in e-Results).
DISCUSSION
In this large, international, multicenter, dose-ranging, placebo-controlled study of abobotulinumtoxinA in chronic hemiparesis, a single injection of abobotulinumtoxinA 1,500 U significantly reduced muscle tone (MAS GSC) at weeks 4 (p = 0.009) and 12 (p = 0.03). No significant effect on PGA was seen in preplanned analyses, nor was a significant difference seen between abobotulinumtoxinA and placebo on walking speed improvement after a single treatment cycle.
In the double-blind phase, a 0.05 m/s walking speed increase was observed with placebo, consistent with previous placebo-controlled studies of botulinum toxin in hemiparesis (+0.04 m/s and +0.03 m/s with placebo at week 4).17,18 Subsequent walking speed changes observed here should be interpreted with caution; no study has evaluated repeated placebo injections. Yet, while the degree of significance for PGA difference between abobotulinumtoxinA and placebo was p = 0.06 in the planned analysis, significant PGA improvement (p = 0.04) was shown with both abobotulinumtoxinA doses in nonparametric post hoc analyses.
In the open-label extension, repeated abobotulinumtoxinA injections (both doses) were well-tolerated over 1 year and associated with progressive improvements in PGA, spasticity (angle of catch), active range of dorsiflexion, walking speed, and QoL. These continuing functional improvements occurred while muscle tone and passive range of motion stabilized beyond the third injection. Across cycles, 15%–32% of participants did not need reinjection at week 12.
In the open-label study, mean decrease from baseline in MAS score reached +1 by cycle 2 week 4 with both abobotulinumtoxinA doses. MAS and XV1 stabilization beyond this time point may reflect residual structural changes in calf muscles insensitive to BoNT, as suggested by ≥20% CS.16,19 In contrast, continuing improvements in angle of catch and active dorsiflexion may represent increasing extinction of motor units recruited in response to fast stretch (XV3) and co-contraction (XA) with repeated BoNT injections.
Walking speed normally plateaus at ∼0.7 m/s in chronic (>9 months) poststroke hemiparesis.4,20,21 Of 136 participants treated for 4 cycles, 16% achieved walking speed >0.8 m/s (vs 0% at baseline), a threshold associated with community mobility.15 This walking speed improvement paralleled gradual increases in PGA (physician-rated) and QoL (participant-rated). Walking speed improvement was consistently greater at week 12 than week 4, contrasting with other outcome measures and prior placebo-controlled BoNT studies,8,10,13 probably linked to the functional nature of this outcome. Twenty-five percent walking speed increase and 16% of participants reaching full community mobility are meaningful achievements in chronic hemiparesis, improving QoL.15,22,23 While modest effects on walking speed/active range of dorsiflexion occurred 4 weeks after a single injection (double-blind study), the correlated improvements in walking speed and active dorsiflexion, knee extended (table e-10) after 1 year of repeated injections may correspond to cumulative effect over time and the need for accommodation periods while participants adapt their walking pattern with reduced co-contraction and increased range of motion produced by abobotulinumtoxinA.
Lower limb multilevel injections in distal (mainly gastrocnemius, soleus, tibialis posterior) or proximal muscles (e.g., hamstrings, adductors) with both abobotulinumtoxinA doses were well-tolerated over repeated, open-label cycles, with an expected safety profile based on previous experience.10,11
Open-label extension efficacy data should be considered in the context of no placebo comparator and increasingly smaller participant numbers because of study completion or withdrawals. Yet the discrepancy between some outcome measures that reach a plateau of improvement (mostly passive outcomes) long before others (mostly active or functional outcomes) remains a compelling observation in chronic spastic paresis. Finally, participant-centered interviews on ambulation performance in daily life could have optimized evaluation.23
Regarding optimal dosing to treat adult lower limb spastic paresis, although only the 1,500 U dose produced a significant effect on the primary outcome, both doses showed significant differences vs placebo in soleus alone. In addition, results after repeated injections were similar across both doses. Taking these data together, doses up to 1,500 U should probably be considered for adult patients with lower limb spastic paresis, tailored to individual patient impairment and treatment objectives. Considering maximal approved abobotulinumtoxinA dosing by different countries for adult upper and lower extremities, the remaining dose not used in the lower limb could be used for the upper limb, depending on patient priorities and needs.13
A single injection of abobotulinumtoxinA reduced muscle tone (MAS GSC) in participants with chronic hemiparesis. Passive outcome measure improvements (muscle tone), demonstrated after a single abobotulinumtoxinA injection, stabilized over time. While PGA and walking speed were not significantly improved compared with placebo in preplanned double-blind analysis (PGA was significantly improved in post hoc nonparametric analysis), continuous walking speed, PGA, and QoL improvements were observed over 1 year of open-label, repeated abobotulinumtoxinA administration, with 16% reaching a walking speed range associated with capacity for achieving community ambulation. This is the first report of such functional improvements in gait and QoL in this chronic population with repeated abobotulinumtoxinA injections (open-label study), and moreover, in the absence of a protocol-defined, standardized, physiotherapy regimen. Repeated abobotulinumtoxinA injections combined with a tailored rehabilitation program should be studied to determine whether even greater improvements over time could occur in chronic hemiparesis.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the participants and their families and Sylvie Guillory (Ipsen) for study coordination.
GLOSSARY
- ANCOVA
analysis of covariance
- BoNT-A
botulinum toxin type A
- CI
confidence interval
- CS
coefficients of muscle shortening
- EQ-5D
European Quality of Life
- GSC
gastrocnemius–soleus complex
- ITT
intent-to-treat
- MAS
Modified Ashworth Scale
- PGA
physician global assessment
- QoL
quality of life
- SAE
serious adverse event
- SF-36
Short-Form Health Survey
- TBI
traumatic brain injury
- TEAE
treatment-emergent adverse event
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: International AbobotulinumtoxinA Adult Lower Limb Spasticity Study Group, Steven Faux, John Olver, Senen Gonzalez, Ian Baguley, Katya Kotschet, John Estell, Thierry Deltombe, Thierry Lejeune, Robert Jech, Jean-Michel Gracies, Alexis Schnitzler, Pierre Decavel, Philippe Marque, François Boyer, Marie-Eve Isner-Horobeti, Claude Desnuelles, Zoltan Denes, Attila Csányi, Gábor Fazekas, Katalin Guba, Michele Vecchio, Ugo Dimanico, Giancarlo Comi, Marta Banach, Monika Rudzinska, Anna Kaminska, Jan Ilkowski, Stanisław Ochudlo, Małgorzata Krawczyk, Luis Jorge Jacinto, Fernando Parada, Svetlana Khatkova, Alexander Skoromets, Sofia Timerbaeva, Jan Benetin, Peter Valkovic, Serdar Kocer, Allison Brashear, Alberto Esquenazi, Steven Edgley, David Simpson, Michael O’Dell, Gerard Francisco, Erika Driver Dunckley, Peter McAllister, Jeffrey Gross, Peter Hedera, Heather Walker, Fatma Gul, Bruce Rubin, Ziyad Ayyoub, Stuart Isaacson, Michael Wimmer, and James Sutton
AUTHOR AFFILIATIONS
From EA 7377 BIOTN (J.-M.G.), Université Paris-Est, Hospital Albert Chenevier-Henri Mondor, Service de Rééducation Neurolocomotrice, Créteil, France; Gait and Motion Analysis Laboratory (A.E.), Moss Rehab, Elkins Park, PA; Department of Neurology (A.B.), Wake Forest School of Medicine, Winston-Salem, NC; Department of Neurology (M.B.), Jagiellonian University Medical College, Krakow, Poland; Centre de Rééducation de l'Hôpital du Jura (S. Kocer), Porrentruy, Switzerland; Department of Neurology (R.J.), First Faculty of Medicine, Charles University and General Faculty Hospital, Prague, Czech Republic; Neurology Department (S. Khatkova), Federal State Hospital, Treatments and Rehabilitation Center of the Ministry of Health and Social Development of the Russian Federation, Moscow, Russia; Department of Neurology (J.B.), Faculty of Medicine, Slovak Medical University in Bratislava and University Hospital Bratislava, Slovak Republic; Department of Medical Sciences (M.V.), UOC Physical Medicine and Rehabilitation, AOU “OVE-Policlinico,” Catania, Italy; New England Institute for Neurology and Headache (P.M.), Stamford, CT; Nzoz Neuro-Card (J.I.), Ilkowski Partner, Wierzbowa, Poznan; Stroke Department and Department of Neurology (S.O.), Central Clinical Hospital, Katowice, Poland; Assesoria Croissance (F.C.), Santiago, Chile; and Ipsen Innovation (A.S.G., C.V., P.P.), Les Ulis, France.
AUTHOR CONTRIBUTIONS
J.-M.G. was involved in concept and design of the study; participant enrollment into the study; analysis, collection, and interpretation of the data; and drafting the manuscript. A.E. and A.B. were involved in concept and design of the study; participant enrollment into the study; analysis, collection, and interpretation of the data; and critical review and revision of the manuscript. M.B., S. Kocer, R.J., S. Khatkova, J.B., M.V., P.M., J.I., and S.O. were involved in participant enrollment into the study; collection and interpretation of the data; and critical review and revision of the paper. A.S.G. was the statistician for this study and was involved in the analysis and interpretation of the data and drafting and critical review of the manuscript for data accuracy. F.C. was involved in concept and design of the study, analysis and interpretation of the data, and critical review and revision of the manuscript. P.P. was involved in concept and design of the study, analysis and interpretation of the data, and critical review and revision of the manuscript. C.V. was involved in analysis, interpretation of the data, and drafting the manuscript. All authors approved the final submitted manuscript.
STUDY FUNDING
This study was sponsored by Ipsen. Jean-Michel Gracies wrote the first draft of the manuscript. Medical writing assistance (technical editing, preparation of figures and tables) was provided by Catherine Risebro of Mudskipper Business Ltd., funded by Ipsen. The clinical research organization responsible for the study was INC Research. The Article Processing Charge was funded by Ipsen.
DISCLOSURE
J. Gracies served as a consultant for and received research grant support from Allergan, Ipsen, and Merz and has received compensation from Ipsen for conducting this clinical trial. A. Esquenazi has received research funding from Ipsen and Allergan and has received compensation from Ipsen for conducting this clinical trial. A. Brashear served as a consultant for Ipsen and for Worldmeds and Revance for protocol development; has received research and salary support from the National Institute of Neurologic Disorders and Stroke; her conflicts of interest are managed, and she is paid, by the Wake Forest School of Medicine; all research funds for this and other clinical trials are paid to the Wake Forest School of Medicine; and she has received compensation from Ipsen for conducting this clinical trial. M. Banach has received training fees and meeting sponsorship from Ipsen and Merz and has received compensation from Ipsen for conducting this clinical trial. S. Kocer served as a consultant for Ipsen, received training fees from Ipsen and Merz, and has received compensation from Ipsen for conducting this clinical trial. R. Jech has received grants from the Czech Science Foundation, Czech Ministry of Health, Czech Ministry of Education, and Charles University, Prague, and honoraria from Ipsen for consultations and lectures; and has received compensation from Ipsen for conducting this clinical trial. S. Khatkova received training fees and meeting sponsorship from Ipsen, Merz, and Allergan and has received compensation from Ipsen for conducting this clinical trial. J. Benetin has received compensation from Ipsen for conducting this clinical trial. M. Vecchio has received compensation from Ipsen for conducting this clinical trial. P. McAllister has received compensation for consulting, speakers' bureaus, and conduct of clinical trials for Allergan, Ipsen, and Merz and has received compensation from Ipsen for conducting this clinical trial. J. Ilkowski has received compensation for conducting clinical trials for Ipsen, Merz, and Allergan and has received compensation from Ipsen for conducting this clinical trial. S. Ochudlo has received compensation from Ipsen for conducting this clinical trial. F. Catus is a consultant for Ipsen. A. Grandoulier is a consultant for Ipsen. C. Vilain is an employee of Ipsen. P. Picaut is an employee of Ipsen. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Gracies JM. Pathophysiology of spastic paresis: I: paresis and soft tissue changes. Muscle Nerve 2005;31:535–551. [DOI] [PubMed] [Google Scholar]
- 2.Martin A, Abogunrin S, Kurth H, Dinet J. Epidemiological, humanistic, and economic burden of illness of lower limb spasticity in adults: a systematic review. Neuropsychiatr Dis Treat 2014;10:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moseley AM, Lanzarone S, Bosman JM, et al. Ecological validity of walking speed assessment after traumatic brain injury: a pilot study. J Head Trauma Rehabil 2004;19:341–348. [DOI] [PubMed] [Google Scholar]
- 4.Hutin E, Pradon D, Barbier F, Bussel B, Gracies JM, Roche N. Walking velocity and lower limb coordination in hemiparesis. Gait Posture 2012;36:205–211. [DOI] [PubMed] [Google Scholar]
- 5.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc 2003;51:314–322. [DOI] [PubMed] [Google Scholar]
- 6.Perera S, Patel KV, Rosano C, et al. Gait speed predicts incident disability: a pooled analysis. J Gerontol A Biol Sci Med Sci 2016;71:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson DM, Gracies JM, Graham HK, et al. Assessment: botulinum neurotoxin for the treatment of spasticity (an evidence-based review): report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology 2008;70:1691–1698. [DOI] [PubMed] [Google Scholar]
- 8.Bakheit AM, Thilmann AF, Ward AB, et al. A randomized, double-blind, placebo-controlled, dose-ranging study to compare the efficacy and safety of three doses of botulinum toxin type A (Dysport) with placebo in upper limb spasticity after stroke. Stroke 2000;31:2402–2406. [DOI] [PubMed] [Google Scholar]
- 9.Bakheit AM, Fedorova NV, Skoromets AA, Timerbaeva SL, Bhakta BB, Coxon L. The beneficial antispasticity effect of botulinum toxin type A is maintained after repeated treatment cycles. J Neurol Neurosurg Psychiatry 2004;75:1558–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burbaud P, Wiart L, Dubos JL, et al. A randomised, double blind, placebo controlled trial of botulinum toxin in the treatment of spastic foot in hemiparetic patients. J Neurol Neurosurg Psychiatry 1996;61:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hesse S, Jahnke MT, Luecke D, Mauritz KH. Short-term electrical stimulation enhances the effectiveness of botulinum toxin in the treatment of lower limb spasticity in hemiparetic patients. Neurosci Lett 1995;201:37–40. [DOI] [PubMed] [Google Scholar]
- 12.Suputtitada A, Suwanwela NC. The lowest effective dose of botulinum A toxin in adult patients with upper limb spasticity. Disabil Rehabil 2005;27:176–184. [DOI] [PubMed] [Google Scholar]
- 13.Gracies JM, Brashear A, Jech R, et al. Safety and efficacy of abobotulinumtoxinA for hemiparesis in adults with upper limb spasticity after stroke or traumatic brain injury: a double-blind randomised controlled trial. Lancet Neurol 2015;14:992–1001. [DOI] [PubMed] [Google Scholar]
- 14.Gracies JM, Bayle N, Vinti M, et al. Five-step clinical assessment in spastic paresis. Eur J Phys Rehabil Med 2010;46:411–421. [PubMed] [Google Scholar]
- 15.Schmid A, Duncan PW, Studenski S, et al. Improvements in speed-based gait classifications are meaningful. Stroke 2007;38:2096–2100. [DOI] [PubMed] [Google Scholar]
- 16.Gracies JM. Coefficients of impairment in deforming spastic paresis. Ann Phys Rehabil Med 2015;58:173–178. [DOI] [PubMed] [Google Scholar]
- 17.Pittock SJ, Moore AP, Hardiman O, et al. A double-blind randomised placebo-controlled evaluation of three doses of botulinum toxin type A (Dysport) in the treatment of spastic equinovarus deformity after stroke. Cerebrovasc Dis 2003;15:289–300. [DOI] [PubMed] [Google Scholar]
- 18.Kaji R, Osako Y, Suyama K, Maeda T, Uechi Y, Iwasaki M. Botulinum toxin type A in post-stroke lower limb spasticity: a multicenter, double-blind, placebo-controlled trial. J Neurol 2010;257:1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Bruin M, Smeulders MJ, Kreulen M, Huijing PA, Jaspers RT. Intramuscular connective tissue differences in spastic and control muscle: a mechanical and histological study. PLoS One 2014;9:e101038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esquenazi A, Sale P, Moon D, Wikoff A. Spatiotemporal changes in gait performance due to onabotulinumtoxinA injection to lower limb muscles in patients with upper motor neuron syndrome. Toxicon 2015;93(suppl):S24–S25. [Google Scholar]
- 21.Ochi F, Esquenazi A, Hirai B, Talaty M. Temporal-spatial feature of gait after traumatic brain injury. J Head Trauma Rehabil 1999;14:105–115. [DOI] [PubMed] [Google Scholar]
- 22.Dobkin BH, Plummer-D'Amato P, Elashoff R, Lee J. International randomized clinical trial, Stroke Inpatient Rehabilitation with Reinforcement of Walking Speed (SIRROWS), improves outcomes. Neurorehabil Neural Repair 2010;24:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kollen B, Kwakkel G, Lindeman E. Time dependency of walking classification in stroke. Phys Ther 2006;86:618–625. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.