Abstract
Myofibroblast-mediated fibrosis is important in the pathophysiology of diseases in most organs. The cornea, the transparent anterior wall of the eye that functions to focus light on the retina, is commonly affected by fibrosis and provides an optimal model due to its simplicity and accessibility. Severe injuries to the cornea, including infection, surgery, and trauma, may trigger the development of myofibroblasts and fibrosis in the normally transparent connective tissue stroma. Ultrastructural studies have demonstrated that defective epithelial basement membrane (EBM) regeneration after injury underlies the development of myofibroblasts from both bone marrow- and keratocyte-derived precursor cells in the cornea. Defective EBM permits epithelium-derived transforming growth factor beta, platelet-derived growth factor, and likely other modulators, to penetrate the stroma at sustained levels necessary to drive the development of vimentin+ alpha-smooth muscle actin+ desmin+ (V+A+D+) mature myofibroblasts and promote their persistence. Defective versus normal EBM regeneration likely relates to the severity of the stromal injury and a resulting decrease in fibroblasts (keratocytes) and their contribution of EBM components, including laminin alpha-3 and nidogen-2. Corneal fibrosis may resolve over a period of months to years if the inciting injury is eliminated through keratocyte-facilitated regeneration of normal EBM, ensuing apoptosis of myofibroblasts, and reorganization of disordered extracellular matrix by repopulating keratocytes. We hypothesize the corneal model of fibrosis associated with defective BM regeneration and myofibroblast development after epithelial or parenchymal injury may be a paradigm for the development of fibrosis in other organs where chronic injury or defective BM underlies the pathophysiology of disease.
Keywords: Basement membrane, Fibrosis, Cornea
Introduction
Fibrosis is important in the pathophysiology of diseases in most organs, including lung [1-5], heart [6], kidney [7-9], and skin [10,11], where epithelial or parenchymal cell injury is associated with disease. Important progress has been made over the past decade in understanding the pathophysiology of corneal fibrosis and these insights likely have relevance to fibrosis in other organs.
Fibrosis (clinically referred to as scarring) commonly occurs as a part of the overall wound healing response following more severe injuries involving the epithelium and stroma [12,13]. The epithelial basement membrane (BM) plays a critical role in determining the regenerative versus the fibrotic character of tissue repair after surgery or injury to the cornea (Fig. 1A, 1B, 1F, 1G, IH), the normally transparent anterior wall of the eye [13,14]. The structural and functional integrity of the epithelial BM—strategically placed between the epithelium and underlying connective tissue—is critical because of its function in regulating the bidirectional passage of cytokines, chemokines and growth factors that participate in epithelial-stromal- bone marrow-derived cell interactions, including processes that lead to the generation and persistence of fibrosis-associated myofibroblasts. The other major BM in the cornea, Descemet's membrane, that lies beneath the corneal endothelium on the posterior corneal surface, appears to have a similar role in regulating pro-fibrotic growth factors from the aqueous humor and corneal endothelium that could trigger posterior corneal myofibroblast development and fibrosis (Fig. 1D, 1E, 1G) [15].
Fig. 1.
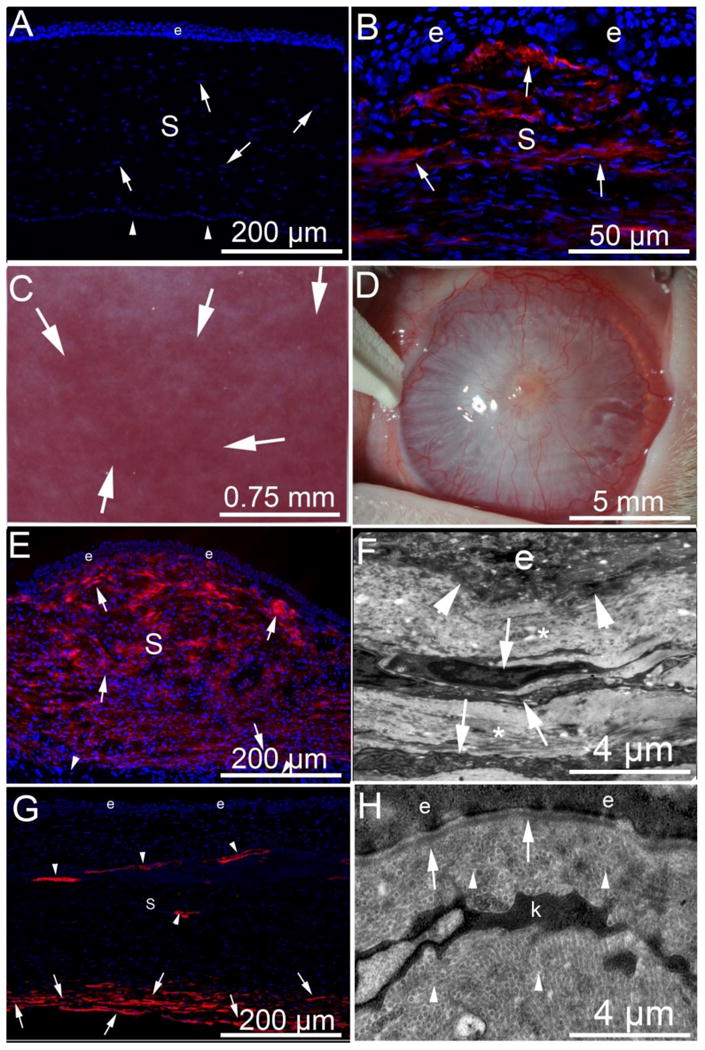
Fibrosis, myofibroblasts and the epithelial basement membrane after injury to the cornea. α-SMA (red) in immunohistochemistry (IHC). (A) α-SMA and DAPI IHC in a normal uninjured rabbit cornea with epithelium (e), the stroma (s) populated with stromal fibroblastic cells termed keratocytes (arrowheads, although a few of these nuclei could be Schwann cells associated with nerves) and corneal endothelium (arrows) that rests on Descemet's BM on the posterior surface of the cornea. Note there is no α-SMA in the unwounded normal cornea. Blue is DAPI staining of cell nuclei. Note, there are no α-SMA+ cells present. (B) α-SMA and DAPI IHC of a rabbit cornea at one month after excimer laser photorefractive keratectomy (PRK) surgery to correct high nearsightedness has alpha-smooth muscle actin (α-SMA) + myofibroblasts (arrows) in the anterior stroma beneath the epithelium. There are many DAPI+ nuclei in the stroma (S) and these are likely a heterogeneous population of cells including corneal fibroblasts, keratocytes, different bone marrow-derived cells such as fibrocytes and macrophages, and even Schwann cells associated with nerves. C. Slit lamp photo of a rabbit cornea at three months after PRK where clear areas (lacunae, arrows) have developed within the fibrosis. D. Slit lamp image of severe corneal fibrosis induced by pseudomonas aeruginosa keratitis in a rabbit cornea at one month after infection and sterilization with antibiotics. E. α-SMA and DAPI IHC shows myofibroblasts (arrows) nearly full thickness of the stroma (s) at one-month after pseudomonas keratitis. Note that the posterior corneal stroma is normal with α-SMA-negative keratocytes (arrowheads). e is epithelium. F. TEM of cornea at one-month after pseudomonas keratitis. Normal EBM lamina lucida and lamina densa cannot be recognized at the epithelio-stromal junction (arrowheads). e is epithelium. The anterior stroma has stacked myofibroblasts (arrows) with large amounts of rough endoplasmic reticulum that correspond to α-SMA+ cells in Fig. 1E and the surrounding extracellular matrix (*) is disorganized without detectible uniform fibrils. G. α-SMA and DAPI IHC at three months after pseudomonas keratitis that extended more posterior than in the cornea in Fig. 1E, and consequently also damaged the endothelium and Descemet's BM. Most anterior stromal α-SMA+ myofibroblasts have disappeared after regeneration of the epithelial BM noted with TEM (Fig. 1H), but α-SMA+ myofibroblasts persist in the posterior stroma (arrows) where Descemet's BM and the endothelium remain damaged or absent. Neovascular blood vessels persist in the stroma and have associated α-SMA+ pericytes (arrowheads). H. TEM of epithelial-stromal junction at three-months after pseudomonas keratitis. Normal EBM lamina lucida and lamina densa are present (arrows) and likely a keratocyte (k), but possibly a nerve-associated Schwann cell, is present, but myofibroblasts with large amounts of rough endoplasmic reticulum have disappeared [15]. Also note, normal regular collagen fibrils (arrowheads) are now present throughout the anterior stromal shown.
Myofibroblasts, when they are generated AND persist, alter the connective tissue (stroma) that underlies the epithelium or endothelium through the production of collagens and other matrix components not normally organized and/or present in the corneal stroma to produce fibrosis that interferes with organ functions, including the transparency of the cornea essential for normal vision. Survival of these cells is dependent on an ongoing exogenous source of TGFβ to suppress induction of apoptosis by paracrine interleukin-1 (IL-1) from adjacent cells or autocrine IL-1 [16-18].
Keratocytes, corneal fibroblasts, and myofibroblasts in the cornea
Keratocytes are neural crest-derived quiescent fibroblastic cells specific to the cornea that function to maintain the transparency of the normal corneal stroma through ongoing maintenance of the precise organization of the stromal extracellular matrix [19,20]. Keratocytes are distinguished fibroblasts (including corneal fibroblasts, as well as myofibroblasts) by the expression of high levels of keratocan and corneal crystallins such as transketolase and aldehyde dehydrogenase class 1 (ALDH1) [19,20]. After corneal injury, keratocytes in proximity to the injury are “activated” by growth factors to transform into “corneal fibroblasts” that express little keratocan or ALDH1, and develop an actin cytoskeleton and focal adhesions [19]. Conversion of keratocytes to corneal fibroblasts in vitro occurs with exposure to serum. Corneal fibroblasts secrete relatively small amounts of disordered extracellular matrix into the stroma and produce mild stromal opacity. Corneal fibroblasts may revert to the keratocyte phenotype as the wound healing response subsides in the stroma. Sustained exposure to TGFβ, either in vivo or in vitro, triggers corneal fibroblasts to differentiate into myofibroblasts that express alpha-smooth muscle actin, desmin, and biglycan, but little ALDH1 and no keratocan [19,21]. Myofibroblasts produce large amounts of disordered extracellular matrix that markedly reduces stromal transparency [13]. In the cornea, myofibroblasts that are indistinguishable from keratocyte-derived myofibroblasts also develop from bone marrow-derived cells, likely fibrocytes [22]. There is some evidence myofibroblasts can transdifferentiate back to corneal fibroblasts after resolution of the corneal wound healing response [23], but they have also been shown to undergo apoptosis in response to IL-1 alpha when TGFβ is withdrawn or during the resolution of severe stromal opacity [24,17].
Another possible progenitor for corneal myofibroblasts has been recently identified. Bargagna-Mohan and colleagues [25] showed that in transgenic mice with constitutive activation of ERK-1/2 in neural crest-derived non-myelinating Schwann cells in the corneal stroma, the normally quiescent Schwann cells begin to express alpha-smooth muscle actin and become myofibroblasts. Nothing is about the potential role of non-myelinating Schwann cell-derived myofibroblasts in corneal wound healing but further study is warranted.
Very little is known about possible epithelial-mesenchymal transition after corneal injury [26]. However, it could provide another source for corneal myofibroblasts in some situations.
Basement membrane structure and assembly
BMs are highly specialized extracellular matrices assembled primarily from laminins, collagens, heparin sulfate proteoglycans, and nidogens, although the precise composition is tissue-specific [27-29]. They are frequently recognized at the transmission electron microscope (TEM) level as two adjacent layers termed the lamina lucida and lamina densa (Fig. 1H, 2A). Although the appearance of these layers may be an artifact of TEM fixation [30], their presence is an indicator of normal BM composition in situ. Of critical relevance, studies in many organs have demonstrated that BM components are contributed by both epithelial/parenchymal cells and fibroblastic cells [31-34], but in most cases the importance of these contributions has not been fully appreciated.
Fig. 2.
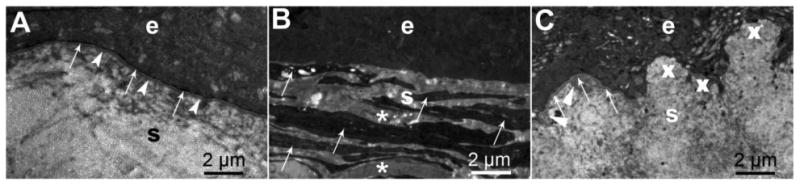
Transmission electron microscopy of the corneal epithelium and stroma after surgical injury in rabbits. A. A clear cornea at 1-month after photorefractive keratectomy (PRK) for low-nearsightedness showing the epithelium (e) and regenerated epithelial basement membrane with normal lamina lucida (arrows) and lamina densa (arrowheads). S is the stroma of the cornea. B. Fibrotic scarred cornea at 1-month after PRK for high-nearsightedness with no detectible lamina lucida or lamina densa. Stacked myofibroblasts (arrows), that correspond to α-SMA+ cells in Fig. 1B, and the disordered extracellular matrix they produced (*) fill the anterior stroma (s) beneath the epithelium (e). C. Cornea at three months after PRK for high-nearsightedness in which clear spaces (lacunae) developed in the fibrosis. These clear areas of stroma correlated with islands of fully-regenerated epithelial basement membrane (arrows) with apoptosis of underlying myofibroblasts, whereas adjacent areas (X) had no detectible epithelial basement membrane. Myofibroblasts were no longer present in the stroma (s) but high density extracellular matrix produced by keratocytes (arrowheads) in the subepithelial stroma is likely contributing to regeneration of the EBM.
BM regeneration after injury occurs by a self-assembly process mediated by surface adhesion, intercomponent binding, and polymerization in which laminins are essential initiators because of their capacity to bind to other laminin molecules, other BM components, and cell surface molecules [27]. Once initiated, the laminin scaffold enables recruitment and assembly of remaining BM components, including nidogen-1, nidogen-2, perlecan, and collagen type IV, to regenerate the structurally and functionally mature BM [27-29].
Corneal basement membranes and myofibroblasts
The cornea provides an excellent model to study BM because of its relatively simple organization with epithelium, epithelial BM, stroma (connective tissue with fibroblastic “keratocytes” and nerves), Descemet's BM, and corneal endothelium (Fig. 1A)—without blood vessels, hair follicles, sebaceous glands and other structures found in skin and other organs that make interpretations more difficult—and its accessibility for reproducible manipulations and observation [13,14]. Following many types of injuries, infections (Fig. 1D) diseases or surgeries of the cornea in which the BM is damaged, large numbers of myofibroblasts are generated and persist in the corneal stroma (Fig. 1B, 1F, 2B). These cells, and the disordered extracellular matrix components they secrete (Fig. 2B), produce fibrosis that alters the structure and function of the corneal stroma and a loss of normal transparency (corneal scarring or haze) (Fig. 1C and 1D). Studies using chimeric mice transplanted with bone marrow derived from green fluorescent protein (GFP)+ donors have demonstrated conclusively that corneal myofibroblasts originate from both bone marrow-derived cells (likely fibrocytes) and resident stromal fibroblastic cells (keratocytes that transition to corneal fibroblasts following activation triggered by cytokines released by corneal injury) [16,22]. The development of mature myofibroblasts from these precursor cells, and persistence in the stroma, is dependent on an adequate ongoing supply of transforming growth factor (TGF) β and platelet-derived growth factor (PDGF) [16]. In the normal transparent cornea, the epithelium produces TGFβ1 and PDGF, at least a low levels, but these growth factors cannot penetrate into the stroma to sufficient sustained levels to drive myofibroblast development due to the barrier function of the normal EBM. TGFβ also requires activation by modulators such as avb6 intergrin and thrombospondin -1 that are upregulated by injury [35,36]. After minor injuries to the cornea that do not result in scarring—such as an abrasion—the EBM is temporarily disrupted and epithelium-derived TGFβ and PDGF penetrate the stroma and initiate the development of myofibroblasts from precursor cells [16,21,22,37]. However, the EBM is soon fully-regenerated, cutting off the supply of epithelium-derived TGFβ and PDGF. Thus, the TGFβ- and PDGF-dependent immature myofibroblasts that have begun development undergo apoptosis before they produce sufficient disordered extracellular matrix to reduce corneal transparency [13,14]. With more severe injuries, such as bacterial infections or photorefractive keratectomy (PRK) surgery to correct high nearsightedness, normal regeneration of the EBM may be delayed (Fig 2) [13,15]. This defective EBM allows ongoing penetration of high levels of TGFβ1 and PDGF from the epithelium into the stroma to drive development and persistence of large numbers of myofibroblasts, resulting in fibrotic scarring of the cornea (Fig 1C). Studies of this pathophysiology [37,38], including laser capture quantitative RT-PCR studies [39], demonstrated that defective EBM regeneration likely results from inadequate keratocyte production and/or localization of EBM components, such as laminin alpha-3 and nitogen-2, due to extensive apoptotic death of stromal keratocytes at the time of the injury. Our working hypothesis is that once the epithelium regenerates over the injured stroma, it lays down a nascent EBM, that in the cornea consists of self-polymerizing laminin 332, but full regeneration of mature functional EBM requires EBM component contributions to the more posterior EBM that must be provided by contiguous keratocytes (Fig 3). If large numbers of mature myofibroblasts develop and secrete disordered extracellular matrix, a physical tissue barrier is produced (Fig. 2B and 3B) that blocks surviving keratocytes in the more peripheral and posterior corneal stroma from repopulating the anterior stroma. Thus, normal keratocytes are blocked from proximity to the nascent EBM and, therefore, the defective EBM and myofibroblasts, and the disordered extracellular matrix they produce, persist for many months to years. Eventually, in most scarred corneas, after the injury is eliminated for a period of months to years, small areas of clearing called “lacunae” appear in the stromal fibrosis (Fig. 1C). In these clear areas, normal keratocytes have repopulated the stroma, mature EBM has regenerated, and the underlying myofibroblasts that are deprived of TGFβ and PDGF have undergone apoptosis, whereas the EBM continues to be morphologically and functionally defective in adjacent scarred areas where myofibroblasts persist (Fig. 2C and 3C) [40]. Over time, these lacunae tend to enlarge and coalesce as more surrounding EBM regenerates and full transparency of the cornea can be restored.
Fig. 3.
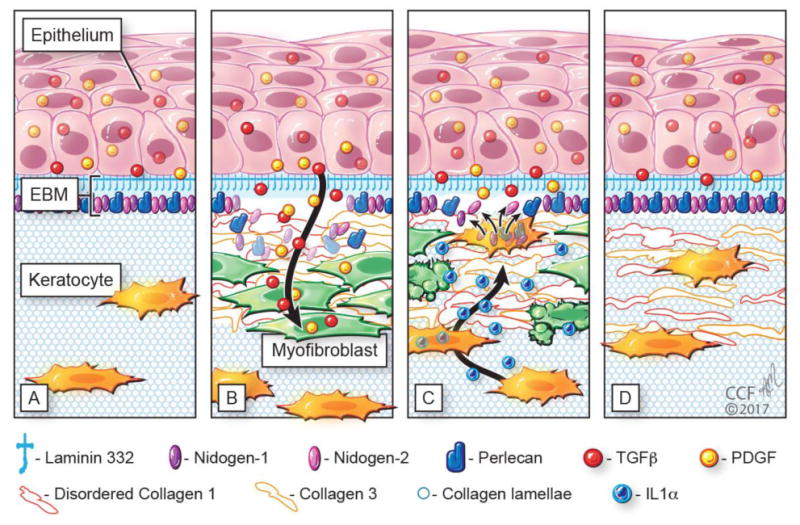
Schematic diagram illustrating injury to the corneal epithelial basement membrane (EBM) and defective regeneration leading to myofibroblast development and fibrosis, followed by hypothesized keratocyte contributions to regeneration of the EBM and resolution of fibrosis. A) Normal unwounded cornea with intact epithelial basement membrane (EBM) comprised of laminin 332, nidogen-1, nidogen-2, perlecan, and other components not depicted such as collagen type IV. The underlying stroma is populated with fibroblastic cells (keratocytes) that function to maintain the highly-organized stromal collagen lamellae that provide the cornea transparency. Epithelial transforming growth factor beta (TGFβ) and platelet-derived growth factor (PDGF) are blocked from penetration into the underlying stroma by the normal EBM. B) After severe epithelial-stromal injuries, such as infections, trauma and some surgeries, the epithelium and EBM are disrupted and TGFβ and PDGF are activated and penetrate the underlying stroma at sufficient concentrations to drive the development of myofibroblasts from keratocyte-derived and bone marrow-derived (fibrocyte) precursors [16]. Myofibroblasts are themselves opaque relative to keratocytes and secrete disordered collagen type 1, collagen type 3 and other matrix materials that disrupt the normal stromal lamellae to produce corneal opacity or scarring. C) Over months to years following the initial injury, keratocytes penetrate the anterior stromal myofibroblasts and facilitate EBM regeneration via the production of laminins, nidogens, and perlecan [38,39,43] in coordination with epithelial cell production of EBM components. We hypothesize that once the nascent laminin-332 layer is produced by the epithelium, more posterior EBM components must, at least in part, be derived from keratocytes to fully regenerate the EBM. The resulting decrease in TGFβ and PDGF penetration from the epithelium into the stroma triggers myofibroblast apoptosis via unopposed paracrine IL-1α from adjacent keratocytes and/or autocrine IL-1α produced by the myofibroblasts themselves (autocrine suicide) [17]. This process begins in a random spotty distribution within the stromal opacity to produce clear areas of stroma called “lacunae” (Fig. 1C) that enlarge and coalesce over weeks to months to fully restore transparency. D. In many corneas, depending on the severity of the injury, all myofibroblasts disappear and keratocytes fully-repopulate the stroma and reabsorb the remaining disorganized collagens and matrix materials secreted by the myofibroblasts to completely restore the normal morphology of the collagen lamellae and, thereby, stromal transparency.
With more extensive injuries to the cornea, such as severe microbial keratitis (Fig 1D and 1E), both the epithelial BM and Descemet's BM can be damaged, leading to extraordinary myofibroblast generation and fibrosis of the full-thickness cornea (Fig. 1E). In this situation, the epithelial BM can eventually be repaired, leading to apoptosis of anterior stromal myofibroblasts, while posterior stromal myofibroblasts survive due to persistent damage to Descemet's membrane BM that allows activation and penetration of TGFβ from the aqueous humor within the anterior chamber of the eye into the posterior stroma (Fig 1F) [15]. In some species, in which the corneal endothelium can regenerate, Descemet's BM may also eventually be repaired, leading to apoptosis of the posterior stromal myofibroblasts and restoration of full corneal transparency.
Bowman's layer and fibrosis in the cornea
Bowman's layer is an acellular layer located immediately beneath the EBM of the central, but not limbal, cornea that is composed primarily of collagen type 1 fibrils that are more tightly woven than those intermixed with other extracellular matrix materials in the remaining stroma [41]. Bowman's layers from 3 to 12 microns thick are present in most primates and some herbivores (chicken, reindeer, elk, deer, giraffe, zebu, and ox), but is absent in most other species, including all carnivores that have been studied [41]. The function, if any, of Bowman's layer remains uncertain, although it has been hypothesized to be a visible indicator of ongoing prominent stromal-epithelial interactions [42]. Millions of humans who have undergone photorefractive keratectomy have had it removed from the central cornea—some up to 30 years ago—with no apparent untoward effects, although a Bowman's-like layer may regenerate years later [42]. Bowman's layer is damaged or destroyed at the site of many pathologies that lead to corneal fibrosis such as photorefractive keratectomy, lacerations, microbial infections, and acid and alkali burns. In any case, if present, Bowman's layer doesn't appear to impede stromal contributions to EBM regeneration since after corneal epithelial scrape injury in humans, that does not injure Bowman's layer, EBM components such as perlecan are produced in large quantities by keratocytes in the subepithelial stroma and likely penetrate Bowman's layer to the site of EBM regeneration [43]. In the corneal ectatic disease keratoconus, many breaks are typically noted in Bowman's layer in advanced cases treated with corneal transplantation [44]. However, the EBM is intact in these corneas and few, if any, myofibroblasts are noted in the stroma, even when apical stromal scarring is present [44]. This supports the hypothesis that Bowman's layer has no role in modulating myofibroblast development in the cornea.
Fibrogenic factors
Several factors promote a fibrotic healing response in the cornea where the anterior corneal stroma is populated with mature myofibroblasts rather than normal regenerative healing that reestablishes normal morphology in which the stroma is repopulated with keratocytes. Injuries, surgeries or diseases that injure the epithelium trigger apoptosis of the underlying keratocytes mediated by the Fas-Fas ligand system [45-47]. The larger the initial injury to the epithelium and stroma, the greater the loss of keratocytes via apoptosis and necrosis [48]. This extensive loss of keratocytes delays EBM regeneration due to deficiency and/or inadequate localization of EBM components provided by keratocytes [13,14,39]. Repeated or chronic injury to the epithelium, EBM and stroma, or delayed healing of the epithelium, augments TGFβ and PDGF penetration into the stroma to drive mature myofibroblast generation and their persistence. Injuries that produce surface irregularity can mechanically impede normal EBM regeneration and thereby augment TGFβ and PDGF penetration from the epithelium into the stroma and promote myofibroblast development and persistence [49]. Age can be a factor in the development of corneal fibrosis since older individuals have less tendency to develop corneal fibrosis (haze) than younger individuals after the same surgical injury [50]. Finally, it is likely there are poorly characterized genetic factors [50], perhaps related to BM components, or their production or localization, that promote myofibroblast generation and persistence in the corneal stroma after mild injury. Thus, the eyes of some patients develop severe stromal myofibroblast-associated fibrosis after minor injuries or surgeries that heal without scarring in most individuals. More work is needed to delineate these genetic factors underlying fibrotic wound healing responses in the cornea and other organs, and to explore potential therapeutic measures to limit myofibroblast proliferation and development in the cornea such as injection of stromal stem cells [51] and application of Rho-associated kinase inhibitors that modulate TGF-beta signaling [52].
BM injury and regeneration, and fibrosis in other organs
We hypothesize that the corneal model of basement membrane injury and defective regeneration associated with mature myofibroblast development and persistence, and attributable, at least in some diseases, to insufficient fibroblastic/mesenchymal contributions of BM components, likely has a role in the development of fibrosis in other organs [1-11] where epithelial or parenchymal cell injury is linked to disease. More emphasis on BM injury and defective BM regeneration in other organs could provide important insights into the pathophysiology of fibrotic diseases, especially if studies are focused on early disease before tissue morphology is severely altered to the point that ultrastructure and histology are difficult to interpret. Importantly, in the cornea, if the injury is interrupted, then BM repair can occur and fibrosis resolve. It seems likely that the fibrosis process can be similarly ameliorated in other organs by reducing or eliminating the inciting injuries and promoting basement membrane repair as the specifics of injury and repair are better delineated. For example, liver fibrosis has been shown to be reversible in the early stages of disease caused by ethanol or hepatitis B virus infection [53-55], although this has not yet been linked to regeneration of hepatocyte basement membranes.
Highlights Steven E. Wilson.
Corneal epithelial basement membrane (EBM) injury and defective generation underlie fibrosis
Corneal fibroblasts (keratocytes) contribute EBM components during regeneration
The pathophysiology of corneal fibrosis is likely a paradigm for fibrosis in other organs
Acknowledgments
Supported in part by US Public Health Service grants RO1EY10056 (SEW) and EY025585 from the National Eye Institute, National Institutes of Health, Bethesda, MD and Research to Prevent Blindness, New York, NY.
Abbreviations used
- BM
basement membrane
- EBM
epithelial basement membrane
- TGFβ
transforming growth factor beta
- PDGF
platelet-derived growth factor
- α-SMA
alpha-smooth muscle actin
- DAPI
4′,6-diamidine-2′-phenylindole dihydrochloride
Footnotes
None of the authors has any commercial or proprietary interest in this topic.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Strieter RM. What differentiates normal lung repair and fibrosis? Proc Am Thor Soc. 2008;5:305–310. doi: 10.1513/pats.200710-160DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coward WR, Saini G, Jenkins G. The pathogenesis of idiopathic pulmonary fibrosis. Adv Respir Dis. 2010;4:367–88. doi: 10.1177/1753465810379801. [DOI] [PubMed] [Google Scholar]
- 3.Strieter RM, Mehrad B. New mechanisms of pulmonary fibrosis. Chest. 2009;136:1364–1370. doi: 10.1378/chest.09-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camelo A, Dunmore R, Sleenan MA, Clarke DL. The epithelium in idiopathic pulmonary fibrosis: breaking the barrier. Frontiers Pharmacol. 2014;4:1–11. doi: 10.3389/fphar.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, Dave A, Engelhardt JF, Liu X, White ES, Thannickal VJ, Moore BB, Christensen PJ, Simon RH. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:254–63. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, Lin SCJ, Aronow BJ, Tallquist MD, Molkentin JD. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nature Comm. 2016;7:12260. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mack M, Yanagita M. Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. 2015;87:297–307. doi: 10.1038/ki.2014.287. [DOI] [PubMed] [Google Scholar]
- 8.Sun YBY, Qu X, Caruana G, Li J. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation. 2016 doi: 10.1016/j.diff.2016.05.008. http://dx.doi.org/10.1016.05.008. [DOI] [PubMed]
- 9.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial. 1. Origin of myofibroblasts in kidney fibrosis. Am J Path. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellström M, Hellström S, Engström-Laurent A, Bertheim U. The structure of the basement membrane zone differs between keloids, hypertrophic scars and normal skin: a possible background to an impaired function. J Plast Reconstr Aesthet Surg. 2014;67:1564–72. doi: 10.1016/j.bjps.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Hashimoto K, Shirakata Y. Epidermogenesis in a skin wound deep through the basement membrane contributes to scar formation. J Dermatol Sci. 2012;65:224–6. doi: 10.1016/j.jdermsci.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res. 2015;49:17–45. doi: 10.1016/j.preteyeres.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torricelli AA, Santhanam A, Wu J, Singh V, Wilson SE. The corneal fibrosis response to epithelial-stromal injury. Exp Eye Res. 2016;142:110–8. doi: 10.1016/j.exer.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torricelli AA, Singh V, Santhiago MR, Wilson SE. The corneal epithelial basement membrane: Structure, function and disease. Invest Ophthalmol Vis Sci. 2013;54:6390–400. doi: 10.1167/iovs.13-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marino GK, Santhiago MR, Santhanam A, Dibbin LL, Thangavadivel S, Tam KP, Wilson SE. Epithelial basement membrane injury and regeneration modulates corneal fibrosis after pseudomonas corneal ulcers in rabbits. Exp Eye Res. May 12; doi: 10.1016/j.exer.2017.05.003. pii: S0014-4835(17)30107-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh V, Jaini R, Torricelli AA, Santhiago MR, Singh N, Ambati BK, Wilson SE. TGFβ and PDGF-B signaling blockade inhibits myofibroblast development from both bone marrow-derived and keratocyte-derived precursor cells in vivo. Exp Eye Res. 2014;121:35–40. doi: 10.1016/j.exer.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaur H, Chaurasia SS, Agrawal V, Wilson SE. Corneal myofibroblast viability: Opposing effects of IL-1 and TGF beta-1. Exp Eye Res. 2009;89:152–8. doi: 10.1016/j.exer.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbosa FL, Lin M, Santhiago MR, Singh V, Agrawal V, Wilson SE. Interleukin-1 receptor role in the viability of corneal myofibroblasts. Exp Eye Res. 2012;96:65–9. doi: 10.1016/j.exer.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte Phenotype Mediates Proteoglycan Structure. A role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278:45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J. The cellular basis of corneal transparency: evidence for ‘corneal crystallins’. J Cell Sci. 1999;112:613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- 21.Chaurasia SS, Kaur H, Medeiros FW, Smith SD, Wilson SE. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp Eye Res. 2009;89:133–9. doi: 10.1016/j.exer.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbosa FL, Chaurasia SS, Cutler A, Asosingh K, Kaur H, Medeiros FW, Agrawal V, Wilson SE. Corneal myofibroblast generation from bone marrow-derived cells. Exp Eye Res. 2010;91:92–6. doi: 10.1016/j.exer.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maltseva O, Folger P, Zekaria D, Petridou S, Masur SK. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest Ophthalmol Vis Sci. 2001;42:2490–5. [PubMed] [Google Scholar]
- 24.Wilson SE, Chaurasia SS, Medeiros FW. Apoptosis in the initiation, modulation and termination of the corneal wound healing response. Exp Eye Res. 2007;85:305–11. doi: 10.1016/j.exer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bargagna-Mohan P, Ishii A, Lei L, Sheehy D, Pandit S, Chan G, Bansal R, Mohan R. Sustained activation of ERK1/2 MAPK in Schwann cells causes corneal neurofibroma. J Neurosci Res. 2017 May 10; doi: 10.1002/jnr.24067. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai TH, Sun MH, Ho TC, Ma HI, Liu MY, Tsao YP. Notch prevents transforming growth factor-beta-assisted epithelial-mesenchymal transition in cultured limbal progenitor cells through the induction of Smad7. Mol Vis. 2014;20:522–34. [PMC free article] [PubMed] [Google Scholar]
- 27.Ponzi A, Yurchenco PD, Iozzo RV. The nature and biology of basement membranes. Matrix Biol. 2017;57-58:1–11. doi: 10.1016/j.matbio.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erickson AC, Couchman JR. Still more complexity in mammalian basement membranes. J Histochem Cytochem. 2000;48:1291–306. doi: 10.1177/002215540004801001. [DOI] [PubMed] [Google Scholar]
- 29.Gubbiotti MA, Neill T, Iozzo RV. A current view of perlecan in physiology and pathology: A mosaic of functions. Matrix Biol. 2017;57-58:285–298. doi: 10.1016/j.matbio.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miosge N. The ultrastructural composition of basement membranes in vivo. Histol Histopathol. 2001;16:1239–48. doi: 10.14670/HH-16.1239. [DOI] [PubMed] [Google Scholar]
- 31.Hassell JR, Schrecengosr PK, Rada JA, SundarRaj N, Sossi J, Thoft RA. Biosynthesis of stromal matrix proteoglycans and basement membrane components by human corneal fibroblasts. Invest Ophth Vis Sci. 1992;33:547–557. [PubMed] [Google Scholar]
- 32.Marnkovich MP, Keene DR, Rimberg CS, Burgeson RE. Cellular origin of the dermal-epidermal basement membrane. Developmental Dynam. 1993;197:255–267. doi: 10.1002/aja.1001970404. [DOI] [PubMed] [Google Scholar]
- 33.Breitkreutz D, Mirancea M, Schmidt C, Beck R, Werner U, Stark HJ, Gerl M, Fusenig N. Inhibition of basement membrane formation by a nidogen-binding laminin γ1- chain fragment in human skin-organotypic co-cultures. J Cell Sci. 2004;117:2611–622. doi: 10.1242/jcs.01127. [DOI] [PubMed] [Google Scholar]
- 34.Simon-Assmann P, Bouziges F, Arnold C, Haffen K, Kedinger M. Epithelial-mesenchymal interactions in the production of basement membrane components in the gut. Development. 1988;102:339–347. doi: 10.1242/dev.102.2.339. [DOI] [PubMed] [Google Scholar]
- 35.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 36.Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. J Cell Biol. 1993;122:923–932. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torricelli AAM, Singh V, Agrawal V, Santhiago MR, Wilson SE. Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze. Invest Ophth Vis Sci. 2013;54:4026–33. doi: 10.1167/iovs.13-12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santhanam A, Torricelli AAM, Wu J, Marino GK, Wilson SE. Differential expression of epithelial basement membrane components nidogens-1 and -2 and perlecan in corneal stromal cells in vitro. Mol Vision. 2015;21:1318–27. [PMC free article] [PubMed] [Google Scholar]
- 39.Santhanam A, Marino GK, Torricelli AAM, Wilson SE. Epithelial basement membrane (EBM) regeneration and changes in EBM component mRNA expression in the anterior stroma after corneal injury. Mol Vision. 2017;23:39–51. [PMC free article] [PubMed] [Google Scholar]
- 40.Marino GK, Santhiago MR, Santhanam A, Dibbin LL, Thangavadivel S, Medeiros CS, Torricelli AAM, Wilson SE. Regeneration of defective epithelial basement membrane and restoration of corneal transparency. J Ref Surg. 2017 doi: 10.3928/1081597X-20170126-02. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merindano MaD, Costa J, Canals M, Potau JM, Ruano D. A comparative study of Bowman's layer in some mammals: Relationships with other constituent corneal structures. Eur J Anat. 2002;6:133–139. [Google Scholar]
- 42.Wilson SE, Hong JW. Bowman's layer structure and function: critical or dispensable to corneal function? A hypothesis. Cornea. 2000;19:417–20. doi: 10.1097/00003226-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Torricelli AAM, Marino GK, Santhanam A, Wu J, Singh A, Wilson SE. Epithelial basement membrane proteins perlecan and nidogen-2 are up-regulated in stromal cells after epithelial injury in human corneas. Exp Eye Res. 2015;134:33–8. doi: 10.1016/j.exer.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kenney MC, Nesburn AB, Burgeson RE, Butkowski RJ, Ljubimov AV. Abnormalities of the extracellular matrix in keratoconus corneas. Cornea. 1997;16:345–51. [PubMed] [Google Scholar]
- 45.Wilson SE, He YG, Weng J, Li Q, McDowall AW, Vital M, Chwang EL. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996;62:325–8. doi: 10.1006/exer.1996.0038. [DOI] [PubMed] [Google Scholar]
- 46.Wilson SE, Li Q, Weng J, Barry-Lane PA, Jester JV, Liang Q, Wordinger RJ. The Fas/Fas ligand system and other modulators of apoptosis in the cornea. Invest Ophthalmol Vis Sci. 1996;37:1582–92. [PubMed] [Google Scholar]
- 47.Mohan RR, Liang Q, Kim WJ, Helena MC, Baerveldt F, Wilson SE. Apoptosis in the cornea: further characterization of Fas-Fas ligand system. Exp Eye Res. 1997;65:575–89. doi: 10.1006/exer.1997.0371. [DOI] [PubMed] [Google Scholar]
- 48.Mohan RR, Hutcheon AEK, Choi R, Hong JW, Lee JS, Mohan RR, Ambrósio R, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- 49.Netto MV, Mohan RR, Sinha S, Sharma A, Dupps WJ, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006;82:788–97. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ang BC, Foo RC, Lim EW, Tan MM, Nah GK, Thean LS, Tan CW, Zhao PS. Risk factors for early-onset corneal haze after photorefractive keratectomy in an Asian population: Outcomes from the Singapore Armed Forces Corneal Refractive Surgery Programme 2006 to 2013. J Cat Refract Surg. 2016;42:710–6. doi: 10.1016/j.jcrs.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 51.Du Y, Carlson EC, Funderburgh ML, Birk DE, Pearlman E, Guo N, Kao WW, Funderburgh JL. Stem cell therapy restores transparency to defective murine corneas. Stem Cells. 2009;27:1635–42. doi: 10.1002/stem.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sijnave D, Van Bergen T, Castermans K, Kindt N, Vandewalle E, Stassen JM, Moons L, Stalmans I. Inhibition of Rho-Associated Kinase Prevents Pathological Wound Healing and Neovascularization After Corneal Trauma. Cornea. 2015;34:1120–9. doi: 10.1097/ICO.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 53.Xu J, Liu X, Koyama Y, Wang P, Lan T, Kim IG, Kim IH, Ma HY, Kisseleva T. The types of hepatic myofibroblasts contributing to liver fibrosis of different etiologies. Front Pharmacol. 2014;5:167. doi: 10.3389/fphar.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mehal W, To U. New approaches for fibrosis regression in alcoholic cirrhosis. Hepatol Int. 2016;10:773–8. doi: 10.1007/s12072-016-9752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rockey DC. Liver fibrosis reversion after suppression of hepatitis B virus. Clin Liver Dis. 2016;20:667–679. doi: 10.1016/j.cld.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


