Abstract
The “brain-gut” peptide ghrelin, which mediates food-seeking behaviors, is recognized as a very strong endogenous modulator of dopamine (DA) signaling. Ghrelin binds the G protein-coupled receptor GHSR1a, and administration of ghrelin increases the rewarding properties of psychostimulants while ghrelin receptor antagonists decrease them. Additionally, the GHSR1a signals through βarrestin-2 to regulate actin/stress fiber rearrangement, suggesting βarrestin-2 participation in the regulation of actin-mediated synaptic plasticity for addictive substances like cocaine. The effects of ghrelin receptor ligands on reward strongly suggest that modulation of ghrelin signaling could provide an effective strategy to ameliorate undesirable behaviors arising from addiction. To investigate this possibility, we tested the effects of ghrelin receptor antagonism in a cocaine behavioral sensitization paradigm using DA neuron-specific βarrestin-2 KO mice. Our results show that these mice sensitize to cocaine as well as wild-type littermates. The βarrestin-2 KO mice, however, no longer respond to the locomotor attenuating effects of the GHSR1a antagonist YIL781. The data presented here suggest that the separate stages of addictive behavior differ in their requirements for βarrestin-2 and show that pharmacological inhibition of βarrestin-2 function through GHSR1a antagonism is not equivalent to the loss of βarrestin-2 function achieved by genetic ablation. These data support targeting GHSR1a signaling in addiction therapy but indicate that using signaling biased compounds that modulate βarrestin-2 activity differentially from G protein activity may be required.
Keywords: Ghrelin, GHSR1a, Arrestin, addiction, cocaine
Graphical Abstract

Introduction
Areas in the brain that are responsible for mediating reward respond to a variety of natural and synthetic chemical reinforcers. Stereotypic behaviors associated with reinforcers like cocaine or food are consequences of cue-triggered anticipatory (wanting) phases and hedonic consumption (liking) phases (Berridge, Robinson, & Aldridge, 2009), and these behaviors may be associated with changes in the activity of restricted groups of dopaminergic neurons (DA). For example, it has recently been reported that food restriction increases the basal firing rate of neurons in the ventral tegmental area (VTA) associated with anticipating cue-induced reward (van der Plasse et al., 2015). Food restriction physiologically results in increased plasma levels of the hormone ghrelin, and presumably a corresponding increase in signaling of its VTA target, the growth hormone secretagogue receptor 1a (GHSR1a). Ghrelin concentration normally peaks in anticipation of and before a meal, and it falls quickly right after eating. This series of events indicates that ghrelin and its cognate receptor are capable of mediating an initiation of rewarding behavior (Williams & Cummings, 2005). Thus, and similar to meal initiation, ghrelin/GHSR1a signaling may also have pronounced effects on the anticipatory, compulsive, seeking phase of other reinforcers.
The ghrelin receptor GHSR1a is G protein-coupled receptor (GPCR) that regulates diverse physiological processes such as growth hormone secretion, food intake, mood, and memory formation (for review see (Muller et al., 2015). It is expressed directly on DA neurons in in the VTA and has a pivotal role in mediating dopamine-dependent reward (Abizaid et al., 2006; Zigman, Jones, Lee, Saper, & Elmquist, 2006). GHSR1a activation in the VTA increases DA release in the nucleus accumbens (NAc) and stimulates locomotor activity in response to the natural ligand ghrelin, (Abizaid et al., 2006; Jerlhag et al., 2007; Quarta et al., 2009). Most significantly, the pharmacological antagonism of the GHSR1a reverses cocaine, amphetamine, morphine and alcohol-induced hyperactivity and the rewarding properties of these drugs in tests of conditioned place preference and alcohol intake (Engel, Nylander, & Jerlhag, 2015; Jerlhag, Egecioglu, Dickson, & Engel, 2010; Jerlhag et al., 2009).
The GHSR1a signals through both heterotrimeric G proteins and separately through a βarrestin pathway (Evron et al., 2014), βarrestin-1 and-2 are also known as arrestins 2 and 3. Using a model cell system, we identified measurable GHSR1a /βarrestin signaling selectivity in which GHSR1a βarrestin recruitment led to RhoA activated, actin/stress fiber rearrangement (Evron et al., 2014). Potentially important to behavior and reward, actin regulation is associated with the cocaine-induced expansion of NAc dendritic spines (Dietz et al., 2012). Finding biased ligands that selectively engage either G-protein or /βarrestin signaling is an ever-growing strategy for dissecting the signaling of GPCRs regulating reward-associated behaviors. Examples of biased ligands have already been identified for neurotensin and dopamine receptors (Allen et al., 2011; Peddibhotla et al., 2013). Alternatively, genetic strategies remain useful to study signaling bias in the context of the rewarding properties of drugs of abuse. Employing a genetic approach with a global βarrestin-2 KO mouse model, our laboratory has shown that βarrestin-2 regulates psychostimulant-induced DA-dependent behaviors (Beaulieu et al., 2005; Bohn et al., 2003; Urs, Daigle, & Caron, 2011).
The phenomenon that repeated exposure to drugs of abuse results in a progressive and long-lasting enhancement of the locomotor response is termed psychomotor (or locomotor) sensitization. Locomotor sensitization is a commonly used assay in the modeling of psychostimulant abuse in animals. Although locomotor sensitization is not a direct correlate of reward, this response is thought to be due to alterations in neuronal plasticity that occur over the course of multiple exposures to psychostimulants. Furthermore, locomotor sensitization has been proposed to correspond to certain aspects of drug addiction such as compulsive drug-seeking behavior (Robinson & Berridge, 1993; Vanderschuren & Kalivas, 2000; Vezina & Leyton, 2009).
We now report a role for βarrestin-2 in early phases of drug addiction. We observed in a cocaine-sensitized mouse model that genetically ablating βarrestin-2 in pre-synaptic DA neurons prevents the GHSR1a antagonist YIL781 from inhibiting cocaine-induced hyperlocomotion. In contrast, deleting βarrestin-2 does not affect the preceding phase of cocaine sensitization. This suggests that βarrestin-2 function differentially regulates distinct stages of drug addiction. Thus, our findings have important pharmacological implications for matching stage with therapy.
Material and Methods
Animals and Drugs
Adult age-matched male and female (8–12 week old and 25–35 g body weight) C57BL/6J (The Jackson Laboratory Bar Harbor, ME), global βarrestin-2 KO and two distinct lines of dopamine neuron-specific βarrestin-2 KO mice (defined as DAβarr2KO-1 and DAβarr2KO-2) and WT littermate mice were used. To obtain DAβarr2KO mice, a βarrestin-2 flox/flox mouse line was crossed to two independent dopamine transporter Cre mouse lines, respectively (for DAβarr2KO-1: DAT-Cre, SG62 GENSAT, for DAβarr2KO-2: DAT-Cre #006660 The Jackson Laboratory). All mouse lines used in the experiments had or were backcrossed to the same C57BL/6J genetic background. All mice were group housed and maintained at a 12/12 hour light/dark cycle. Experiments were carried out at the beginning of the light cycle. Tap water and standard laboratory chow were supplied ad libitum, except for the time of testing. Mice were randomly assigned into treatment groups and the individuals performing injections were blind to the genotype of the injected animal. GHSR1a antagonist YIL781 (5, 10 or 20 mg/kg, Tocris 3959) and cocaine HCl (5 or 20 mg/kg, Sigma C5776) were dissolved in saline and were injected intraperitoneally (i.p.) according to the experimental schedule. Saline was used as vehicle controls. All drugs were injected at a volume of 10 ml/kg body weight. All mouse studies were conducted in accordance with the National Institutes of Health Guidelines for Animal Care and Use and with an approved animal protocol from the Duke University Animal Care and Use Committee.
Cultured cell-based assays
Ca2+ mobilization
The calcium response of GHSR1a receptor was measured using HEK293 cells that permanently expressed both the human GHSR1a and mitochondrial apoaequorin (Evron et al., 2014; Rizzuto, Simpson, Brini, & Pozzan, 1992). On day 0 the cells were plated at 7×106 cell on a 10 cm diameter culture plate. The following day (test day) growth media was replaced with cMEM+HEPES+GlutaMAX (OptiMEM, Gibco, 51985034) for 2–4 hours before adding 2.5µl/ml coelenterazine-h (1 µM stock solution,(Promega, 2011) to the media. Following incubation for 1–2 hours, the cells were washed from the plate and dispensed (30µl, 15000 cells/well) into increasing doses of 2× concentration YIL781 pre-dispensed into the wells of a 96-well plate (Corning Costar 3903, Corning). Luminescence was recorded using a Mithras LB 940 plate reader (Berthold Technologies, Oak Ridge, TN) for 10s/well immediately after dispensing the cells. For an antagonist assay using YIL781, 50nM (final concentration) of the GHSR1a agonist L-692,585 (L585) was added to each well and the luminescence was recorded for another 10s/well. Subsequently, 80µl of calcium lysis buffer (100mM CaCl2 + 0.2% Triton-X) was added to each well and the luminescence was recorded for 5 s to determine a total remaining signal (TRS) per well. To control for variations in cell numbers, a test compound-stimulated response (net Aeq) was determined by dividing the test compound-induced response (L) by a total signal (response) per cell (net Aeq = L/(L+TRS). Each experiment comprised two or three technical replicates for each test compound concentration. A minimum of three independent experiments were performed.
βarrestin-2 recruitment
The activation of βarrestin2 by the GHSR1a was assessed using a U2OS cell line permanently expressing the GHSR1a-(vasopressin receptor2-tail) chimera (GHSR1a-V2T) and green fluorescent protein (GFP) tagged βarrestin2. On day 1, stable cells were split into MGB101-1-2-LG glass-bottom 384-well plates (MatriCal, Spokane, WA). Each well contained 30µL aliquots of 8000 cells in Minimum Eagle’s medium (MEM) containing 10% fetal bovine serum (FBS) and 100 U/mL penicillin/ streptomycin (Life Technologies, Grand Island, NY). The plates were incubated overnight at 37 °C in 5% CO2, and on the following day the media was changed to 30 µL clear MEM without serum. Increasing doses of YIL781 in 5% DMSO were added to the wells and diluted 10-fold to reach final concentration. To test the antagonist property of YIL781, 1µM of the GHSR1a agonist L585 was injected into each well, the plates were returned to the incubator for 40 min, and then the cells were fixed by adding 30 µL of 2% paraformaldehyde-phosphate buffered saline (PBS) to each well. Plates were stored at 4 °C until analysis at 488 nm on a robotic imager (ImageXpress Ultra, Molecular Devices, Sunnyvale, CA). Images were analyzed using a wavelet algorithm to measure formation of fluorescence aggregates (Evron et al., 2014). Image results were also visually confirmed
Cre Expression Verification
DAT-Cre mice (SG62 GENSAT and Jackson Laboratory #006660) were bred to floxed βarrestin-2 mice (Urs et al., 2015) for homozygosity at the floxed allele and were used for behavioral experiments. To check for Cre activity in dopamine neurons, the SG62 GENSAT DAT-Cre line was also bred to a Cre reporter mouse line consisting of a Cre-dependent GFP-tagged ribosomal L10a subunit. For the DAT-Cre line from Jackson Laboratory, Adeno-associated virus serotype 10 containing a Cre-inducible hM3D-mCherry was stereotactically injected bilaterally into the VTA/SNc (AP: − 3.1mm ML: ±0.6mm DV: −4.5mm). Cre activity for both DAT-Cre mouse lines was verified histologically. Briefly, the mice were transcardially perfused with 2% formaldehyde with heparin in PBS. Brains were removed and post-fixed overnight. On the next day, 200 micron thick sections were cut with a vibratome (VT1000S, Leica) and were blocked in Fish Gelatin Extract in PBS and then incubated with primary rabbit antibodies against tyrosine hydroxylase (Invitrogen, OPA-04050, 1:1000) overnight at 4°C. After incubation with primary antibody, the sections were washed in PBS and incubated for 1 hour at RT with Goat anti-rabbit alexa fluor 488 or 568 (1:1000). Sections were then washed and mounted on slides with vectashield and coverslipped. Images were taken on an Axiozoom fluorescent dissecting scope with the appropriate filter cubes (Zeiss).
Locomotor Activity
Locomotor activity was measured with an Omnitech Digiscan activity monitor (20 × 20 cm; Accuscan Instruments, Columbus, OH) at 5 min intervals, and data were analyzed for the total distance traveled per interval over 120 min (Bohn et al., 2003). The total distance traveled over a 30 min period post-cocaine injection was also calculated to analyze the outcome of pre-treatments on the peak effect of cocaine. Mice were allowed to acclimatize to the activity monitor for 30 min before any drug treatments. Drugs were administered at various time points depending on the experiment as shown on the figures.
Blood Pressure Measurement
To assess the possible effect of YIL781 on blood pressure, hemodynamic measurements were performed on WT mice anesthetized with a mixture of Ketamine (100 mg/kg) and Xylazine (10 mg/kg). A 1.4-Fr pressure-conductance catheter (Millar Instruments, Houston, TX) was inserted retroaortically into the LV to record hemodynamics. Subsequently, parallel conductance (Vp) was determined by 10µl injection of 15% saline into the right jugular vein to establish the parallel conductance of the blood pool. The derived Vp was used to correct the P-V loop data. Data were recorded digitally at 1,000 Hz and analyzed with pressure volume analysis software (PVAN data analysis software version 3.3; Millar Instruments). Each mouse received the following injection scheme, vehicle i.p followed after 10 minutes with either 5, 10 or 20 mg/kg i.p. YIL781.
Locomotor Sensitization to Cocaine
Mice were sensitized as described (Bohn et al., 2003). They were placed in Omnitech activity chambers on day 1 and allowed to acclimatize for 30 minutes, removed, injected with 20 mg/kg cocaine (i.p.), and immediately returned to the open field for 90 min. Subsequently and for 3 consecutive days, the mice received daily injections of 20 mg/kg cocaine in their home cages. On day 5 of sensitization, the mice were again tested in the locomotor chamber. The mice were then given a hiatus of 4 days, and on test day 10 were placed into the open field for 30 min, removed and (i.p.) administered either the vehicle or 10 mg/kg YIL781 and returned immediately to the open field for 15 min. The mice were removed again after this period, injected (i.p.) with 5 mg/kg cocaine and immediately returned to the open field for another 90 min. Locomotor activity was assessed for total distance traveled (cm) at 5 min intervals as well as the total distance traveled over 30 min post-cocaine injection.
Statistical Analyses
For dose-response curves data were analyzed by nonlinear regression to obtain ECmax, EC50, and IC50 values. Locomotor data were analyzed by a standard one-way or two-way ANOVA test for comparison between genotypes, treatments, or doses (GraphPad Prism 7.02 software). Individual genotypes, treatments, or doses were compared using Tukey`s post hoc test whenever ANOVA showed significance to either treatment or a treatment × time interaction. For the cocaine sensitization data, areas under the curve was also calculated in 15 min consecutive segments. A probability value of p<0.05 was considered as statistically significant. Statistical results are described in the figure legends. All data are presented as mean ± SEM.
Results
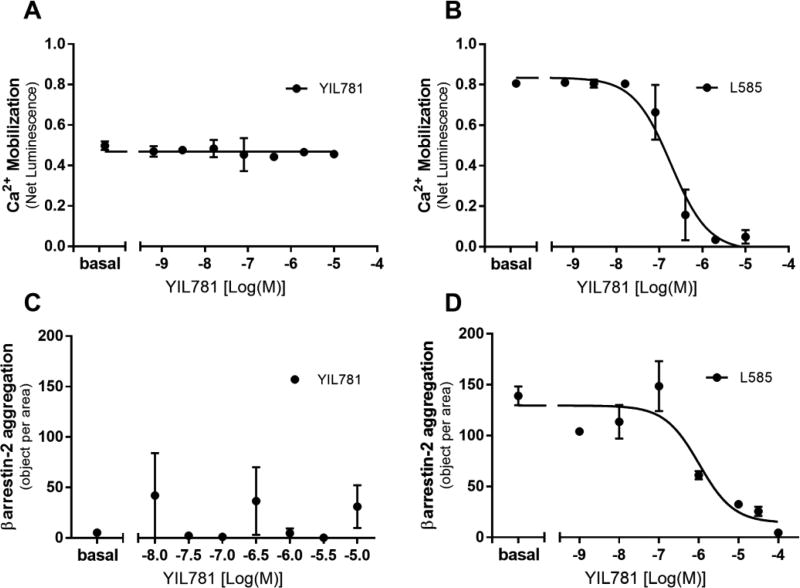
YIL781 is reported to competitively block ghrelin peptide binding to GHSR1a as well as G protein binding and βarrestin-2 activation (Esler et al., 2007; Evron et al., 2014). We verified these properties suggesting that YIL781 is a full GHSR1a antagonist using a combination of in cellulo assays for Gq mediated Ca2+ response (Figure 1A–B) and βarrestin-2 translocation (Figure 1C–D). The data show that YIL781 has no agonist activity of its own (Figure.1A and 1C) and dose dependently and with low nanomolar affinity blocks GHSR1a calcium (Figure 1B) and βarrestin-2 (Figure 1D) responses of the potent small molecule GHSR1a agonist L-692,585. Since YIL781 does not activate the receptor when applied alone, it is also not a weak partial agonist. These results suggest that YIL781 should be able to antagonize GHSR1a-based regulation of locomotion occurring through both the receptor’s G-protein and βarrestin-2 signaling pathways.
Figure 1. Effects of YIL781 on in cellulo signaling of the GHSR1a determined by G-protein mediated Ca2+ responsiveness and βarrestin-2 translocation.
(A–B) Ca2+ response of the GHSR1a in HEK-293 cells was assessed by bioluminescence in the presence of an aequorin reporter. (A) YIL781 treatment alone over the range of concentrations was modeled by a line with slope = 0 ± 0.0078 and intercept = 0.47 ± 0.0077 (B) The ability of the GHSR1a agonist L-692,585 to activate calcium was inhibited by increasing doses of YIL781, IC50= (1.85 ± 0.093) × 10−7 M). (C–D) βarrestin-2 translocation to the activated GHSR1a in U2OS cells was measured by assessing the formation of receptor/ βarrestin-2 aggregates using a robotic microscope plate reader. (C) Linear regression of YIL781 treatment over the dose range, Intercept = 18 ± 43, slope = 0.35 ± 6.2. (D) The ability of L-692,585 to induce βarrestin-2 translocation was inhibited by YIL781 with an IC50= (1 ± 0.26) × 10−6 M. Data were analyzed by GraphPad Prism, version 7.02 and are presented as mean ± sem, N = 3 independent experiments.
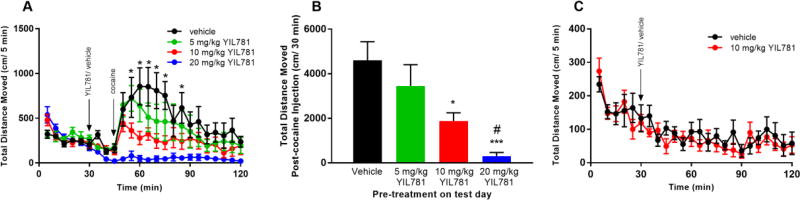
We began in vivo locomotor testing in WT C57BL/J6 mice exposed to graded i.p. doses of 5, 10, and 20 mg/kg YIL781 followed 15 min later by an i.p. dose of either vehicle or cocaine, Figure 2A). Each tested concentration of YIL781 significantly reduced cocaine-induced hyperlocomotion. However, post-cocaine, the total distance traveled over 30 min was significantly reduced only in the 10 and 20 mg/kg YIL781 treated mice, Figure 2B. The 10mg/kg dose of YIL781 when injected alone also did not affect basal locomotion, Figure 2C, suggesting this dose would be appropriate for subsequent behavioral testing.
Figure 2. Acute cocaine-induced locomotion after GHSR1a antagonist treatment.
(A) Cocaine-induced locomotion was measured in C57BL/6J YIL781 treated WT mice (n=5–6 mice/group; ANOVA: interaction P < 0.0001; treatment P < 0.01). Tukey`s post hoc comparisons of different doses of YIL781 and vehicle revealed significant differences at multiple time points (*p<0.05). (B) Effect of pre-treatment with 10 and 20 mg/kg YIL781 on cocaine-mediated locomotion evaluated by total distanced moved over the 30 min post-cocaine injection period (ANOVA: treatment P < 0.0006) Tukey`s post hoc test: 10mg/kg YIL781 vs saline, *p< 0.05; 20mg/kg YIL781 vs saline, ***p < 0.001; 20mg/kg YIL781 vs 5 or 10 mg/kg YIL781, #p<0.05). (C) ANOVA analysis of 10 mg/kg YIL781 treatment on locomotor activity compared to vehicle (treatment P=0.66), interaction P=0.29).
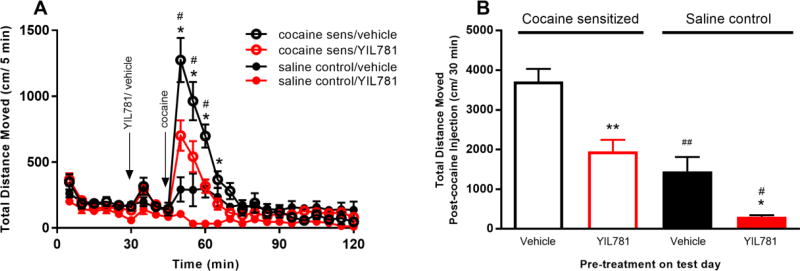
We next examined the effect of YIL781 on locomotor sensitization induced by chronic cocaine administration (see methods). A subset of the C57BL/6J WT mice that received daily saline injections instead of cocaine over the sensitization period served as controls. YIL781 administration reduced cocaine-induced locomotion at multiple time points in both sensitized and control mice (Figure 3A), but the control mice demonstrated less horizontal locomotion compared to cocaine-sensitized mice at 5, 10, 15 and 20 min following the administration of 5 mg/kg cocaine (Figure 3A, symbol #). Additionally, over the 30 min interval post 5 mg/kg i.p. cocaine, the 10 mg/kg YIL781 pre-treatment decreased locomotion compared to vehicle pre-treated controls (Figure 3B, symbol *).
Figure 3. Locomotor sensitization to cocaine in C57BL/6J mice.
(A) On the test day, the GHSR1a antagonist pre-treatment reduced cocaine locomotion in both saline sensitized and cocaine sensitized groups at multiple time points (n= 6 mice/group, ANOVA: interaction P < 0.0001; treatment P< 0.0001; Tukey`s post hoc test: cocaine-sensitized group compared to saline sensitized group, #p<0.05; YIL781 compared to saline pre-treatment, *p<0.05). (B) Data showing the sum of distance traveled during 30 min after cocaine administration (ANOVA: treatment P < 0.0001; Tukey`s post hoc test: cocaine-sensitized group compared to saline sensitized group, #p<0.05, ##p<0.01; YIL781 compared to saline pre-treatment, *p<0.05, **p<0.01).
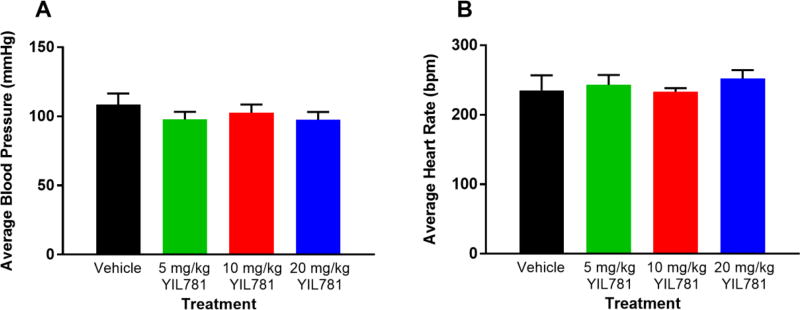
Rodent open field activity can be affected by either high or low blood pressure. High blood pressure delays the habituation to the arena (Sestakova, Puzserova, Kluknavsky, & Bernatova, 2013) while low blood pressure may impair motor performance and environment exploration. It has been reported that peripheral injection of ghrelin and other GHSR1a agonists decrease blood pressure, and this occurs with or without a decrease in sympathetic nerve discharge (Callaghan et al., 2012). Therefore, we tested the effect of i.p. YIL781 on blood pressure and heart rate under conditions similar to those used in the locomotor sensitization experiments. We found no significant difference between vehicle and i.p. administered YIL781 at 5, 10 and 20 mg/kg (Figure 4), suggesting that YIL781’s locomotor effect is not due to blood pressure fluctuations.
Figure 4. Blood pressure and heart rate in WT mice treated with YIL781.
(A, B) Treatment with different doses of the GHSR1a antagonist YIL781 did not change either blood pressure (n=3, ANOVA for treatment P=0.60) or heart rate (n=3, ANOVA for treatment P=0.80) compared to vehicle treatment in WT mice.
To examine in presynaptic DA neurons whether the inhibitory effect of YIL781 on locomotor sensitization to cocaine is mediated by βarrestin-2, we generated KO mice by crossing dopamine transporter (DAT)-Cre positive mice with βarrestin2-flox/flox mice. This deletes βarrestin-2 selectively from the DAT expressing DA positive neurons. In comparison to global βarrestin-2 KOs, these limited KO mice (DAβarr2KO) better isolate the CNS circuits involved in rewarding behaviors.
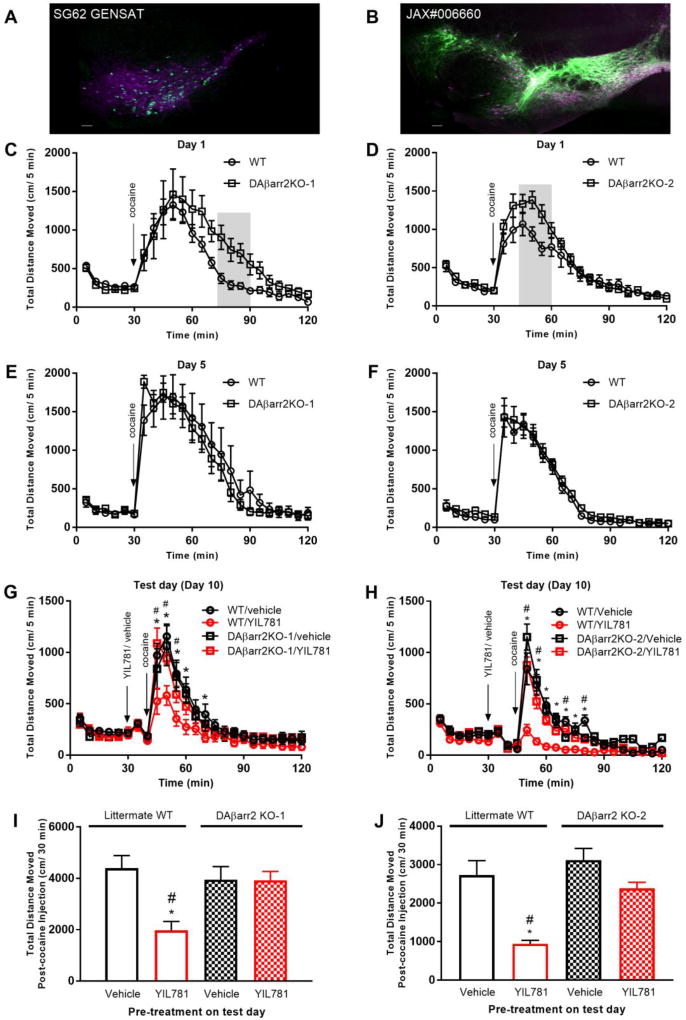
It has been reported that even among littermates, mouse strains expressing Cre may have quite variable expression patterns (Heffner et al., 2012). Consequently, they may also have unexpected and variable phenotypes (Heffner et al., 2012; Vuong, Perez de Sevilla Muller, Hardi, McMahon, & Brecha, 2015). Our first attempt at DAβarr2KO mice using SG62 GENSAT DAT-Cre resulted in apparently limited Cre expression. Most likely, speed congenic breeding to a C57BL/J6 background produced a DAT-Cre mosaic in DA+ neurons (Figure 5A). We therefore generated a new line of DA neuron selective βarrestin-2 KO by crossing βarrestin2-flox/flox mice with an independent line of C57BL/6J background DAT-Cre mice (Figure 5B; DAT-Cre #006660) to control for variable Cre expression. The new KO mice had much more extensive Cre expression, implying relatively greater βarrestin-2 depletion in the target neurons. In fact, the second DAT-Cre mouse line we used has been shown to have the highest fidelity for Cre expression in DA neurons, which has been a problem in the field (Lammel et al., 2015). Importantly, together the two KO lines offered an opportunity to determine upper limits on the distribution of ghrelin receptor containing cells involved in dopamine regulation.
Figure 5. Locomotor sensitization to cocaine in DAβarrestin-2 KO mice.
(A, B) Representative images showing Cre-expression patterns (green) in midbrain dopamine neurons (DA) (magenta) in the two different DAT-Cre mouse lines. Scale bars = 100 µm. (C, D) Plots of 20 mg/kg cocaine induced locomotion for DAβarr2KO-1 and DAβarr2KO-2 mice on sensitization Day 1. Differences in the areas under the respective curves were observed for the grey shaded regions, (grey shaded area; n= 8–9 mice/group, for DAβarr2KO-1: p <0.0001; for DAβarr2KO-2: p <0.0001). (E, F) Cocaine-mediated locomotion on Day 5 of sensitization. (G, H) On the test day (Day 10), 10 mg/kg YIL781 pre-treatment reduced 5 mg/kg cocaine-induced locomotion in sensitized WT mice only (ANOVA: DAβarr2KO-1 interaction P < 0.0001; treatment P = 0.099; DAβarr2KO-2 interaction P < 0.0001; treatment P < 0.0001; Post hoc comparison: WT/YIL781 compared to WT/saline, *p<0.05; WT/YIL781 compared to DAβarr2KO-1 or DAβarr2KO--2/YIL781, #p<0.05). (I, J) Total distance traveled 30 min after cocaine administration. (ANOVA: DAβarr2KO-1 treatment P < 0.004; DAβarr2KO-2 treatment P < 0.005; post hoc test: WT/YIL781 compared to WT/saline, *p<0.05; WT/YIL781 compared to DAβarr2KO-1 or DAβarr2KO-2/YIL781, #p<0.05).
As shown in Figure 5, both DAβarr2KO-1 and DAβarr2KO-2 mice showed more sensitivity to cocaine on the first day of injections (Day 1, Figure 5 C, D). However, both KO lines were sensitized similarly to their littermate WT controls (Day 5, Figure 5 E, F). Four days after the sensitization paradigm and on the test day 10 (Figure 5 G through J), a 10mg/kg YIL781 pre-treatment reduced 5 mg/kg cocaine-induced locomotion in the WT mice but not in either of the DAβarr2KO-1 and DAβarr2KO-2 lines. These data suggest that intact βarrestin-2 signaling in DA neurons is not necessary for cocaine sensitization but surprisingly βarrestin-2 is required for the inhibitory effect of YIL781 on cocaine-mediated hyperlocomotion. Furthermore, our data suggest that a subset of cells may be sufficient to cause the behavioral phenotype since we did not observe a significant difference between the two KO mouse lines in their response to YIL781.
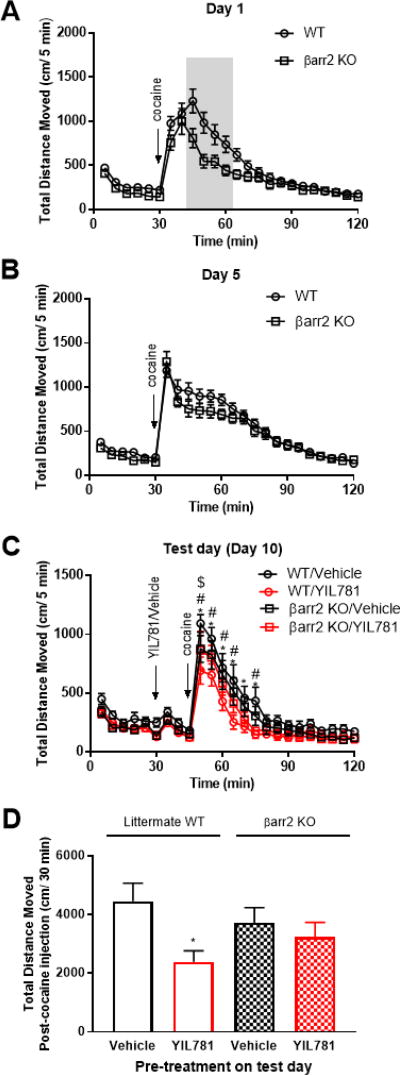
We employed two alternative approaches to confirm that βarrestin-2 depletion underlies the observations described in figure 5 above. The first approach attempted to use βarrestin-2 specific antibodies to directly assess the extent of Cre-dependent βarrestin-2 knockdown in brain slices. However, the antibodies available to us for in situ immunochemical staining were unable to discriminate local changes in βarrestin-2 expression versus βarrestin-1 and background. We therefore employed a second approach to assess the effects of βarrestin-2 depletion that utilized a validated whole-body βarrestin-2 knockout mouse (Bohn et al., 1999). These KO mice and their littermate controls were sensitized (Figure 6 A–B) and treated with YIL781 (Figure 6 C–D) as above. The whole-body KO mice behaved like the DAβarr2KO-1 and DAβarr2KO-2 animals. YIL781 effectively reduced expression of cocaine sensitization in the wild-type littermate controls, but in contrast no such effect was evident in the whole-body βarrestin-2 KO animals (Figure 6 C–D). These data confirm that βarrestin-2 is required for YIL781 to attenuate expression of cocaine sensitization.
Figure 6. Locomotor sensitization to cocaine in whole body βarrestin-2 KO mice.
(A) Plot of 20 mg/kg cocaine-induced locomotion for whole body βarr2KO mice and littermate WT control mice on sensitization Day 1. Differences in the areas under the respective curves were observed for the grey shaded regions (n = 24 mice/genotype, t = 2.623, p <0.05). (B) Cocaine-mediated locomotion on Day 5 of sensitization. (C) On the test day (Day 10), 10 mg/kg YIL781 pre-treatment reduced 5 mg/kg cocaine-induced locomotion in sensitized WT mice only. A two-way ANOVA revealed significant main effects for time (n = 11–12 mice/group, F (23, 989) = 64.5, p<0.0001) and treatment/genotype group (F (3, 43) = 3.499, p<0.05). Tukey’s post hoc comparison: WT/Vehicle compared to WT/YIL781, *p<0.05; βarr2KO/Vehicle compared to WT/YIL781, #p<0.05; WT/ YIL781 compared to βarr2KO/YIL781, $p<0.05. (D) Total distance traveled 30 min after cocaine administration. A one-way ANOVA identified a treatment group effect, F (3, 42) = 2.958, p < 0.05. Tukey’s post hoc comparison: WT/Vehicle compared to WT/YIL781, *p<0.05.
Discussion
Over the past three decades our laboratory has investigated addiction in the context of DA signaling. Drug addiction can be viewed as a composite neurological disorder composed of a pre-addictive phase followed by three alternating stages: (1) preoccupation/anticipation (craving), (2) binge/intoxication, and (3) withdrawal/negative affect (Koob & Volkow, 2010; Volkow, Koob, & McLellan, 2016). An ideal addiction therapy would seek to reestablish normal brain function by disrupting this ongoing cycle at one or more of its stages, with the ultimate goal of preventing relapse. To date, there are limited pharmacological tools to achieve these overall goals let alone specifically target and interdict any one particular stage. Important factors governing this limitation are the functional heterogeneity of participating receptors, the large spectrum of available signaling pathways, and the complex circuitry that participates in regulating the formation and loss of addictive behaviors. A minimally invasive approach to blocking the addictive cycle is desirable and potentially pharmacologically obtainable. Here we showed that both WT mice and DA-neuron specific βarrestin-2 KO mice can be sensitized to cocaine. In contrast, the GHSR1a antagonist YIL781 cannot attenuate cocaine-induced locomotion in the KO mice. This indicates that βarrestin-2 activity at GHSR1a plays a distinct role in regulating an established cocaine induced behavior. Thus, this drug seeking stage should be amenable to pharmacological therapy aimed at GHSR1a/ βarrestin-2 activity whereas the pre-addictive drug sensitization phase would not. This provides the first evidence for a selective requirement of βarrestin-2 activity in the distinct stages of addictive behavior.
Our observation that βarrestin-2 KO mice displayed reduced sensitivity to cocaine on Day 1 but WT-like expression of cocaine sensitization on Day 10 (test day), extend the findings of Bohn et al., 2003. Bohn et al. reported a small, non-significant reduction in cocaine responsivity in βarrestin-2 KOs prior to sensitization and both genotypes are shown to sensitize to the same extent. Our ability to identify a significant genotype effect in unsensitized mice here may stem from the larger cohort (n = 24 vs. 12 mice/genotype). The cocaine sensitization results with DA neuron-selective KO of βarrestin-2 are also in agreement with those reported with global βarrestin-2 KO, where the mice in the latter case have basal and cocaine-induced extracellular DA levels similar to their WT littermates (Bohn et al., 2003). A possible mechanism to explain our sensitization observations lies in the partial redundancy exhibited by the two βarrestin isoforms in cell models. Alternatively, because locomotor sensitization may predominantly involve enhanced postsynaptic DA receptor sensitivity on NAc medium spiny neurons (Koob & Nestler, 1997), knocking out βarrestin-2 pre-synaptically in VTA DA neurons may only have a minor effect on behavioral sensitization.
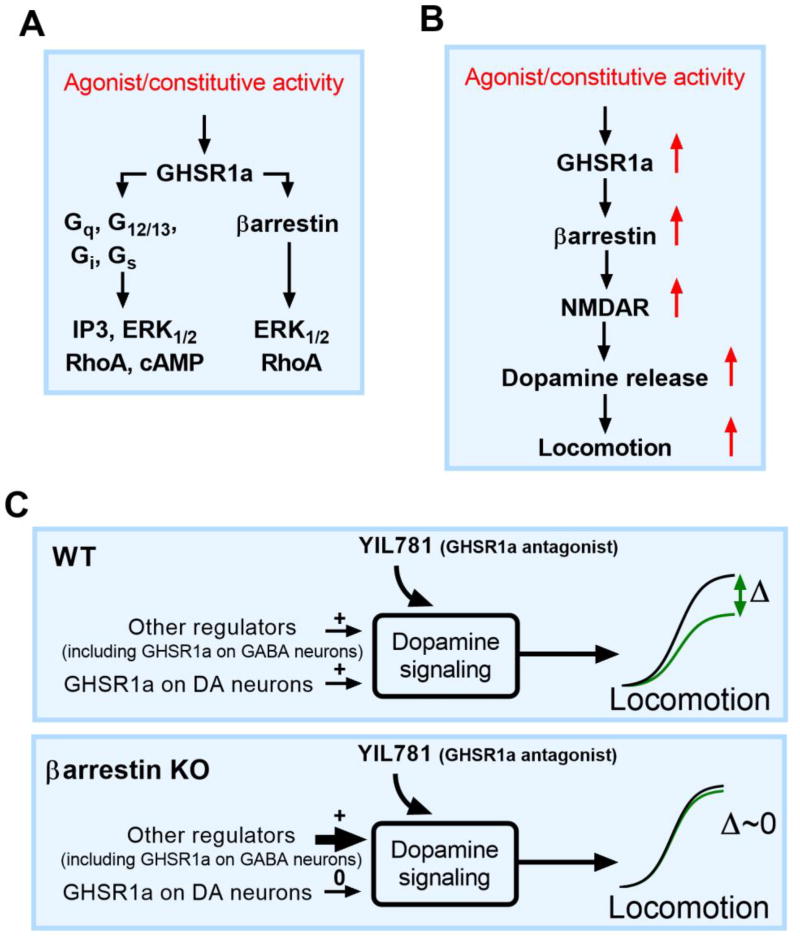
Changes in extracellular DA in the striatum regulate locomotion, and a functionally significant fraction of DA release is controlled by GHSR1a signaling (Jerlhag et al., 2007; Quarta et al., 2009). We believe that antagonists like YIL781 may reduce DA release by inhibiting excitatory glutamatergic neurotransmission because an injection of ghrelin increases it (Abizaid et al., 2006 (Figure 7B). Characterizing the role of βarrestin-2 in YIL781/GHSR1a modulation of hyperlocomotion in cocaine-sensitized mice requires that we reconcile two seemingly contradictory results. First, pharmacological blockade of the ghrelin receptor in sensitized WT mice prevents both G protein and βarrestin-2 activity and reverses cocaine-induced hyperlocomotion. Thus, reducing βarrestin-2 activity by genetic ablation should also reduce hyperlocomotion. However, cocaine-sensitized mice lacking βarrestin-2 globally or selectively in presynaptic DA neurons do not respond to YIL781, and hyperlocomotion persists with cocaine exposure. This is exactly opposite to what is expected and appears to rule out both G protein and βarrestin regulation of hyperlocomotion at the GHSR1a. To explain these observations, we need to address how genetic depletion of βarrestin-2 activity in the KO differs from its pharmacological loss/inhibition of the receptor. To explain the role of βarrestin-2 in YIL781/GHSR1a modulation of hyperlocomotion in cocaine-sensitized mice, we first invoke a basic axiom of signaling theory; most all biological processes can be modeled by logistic (sigmoid) curves (Black & Leff, 1983). Sigmoid functions are defined by three characteristic parameters, an affinity (which we need not consider for purposes of this discussion), a maximal response (top) and a baseline (bottom). A simple hypothesis that provides an explanation for the YIL781 results in the KO mice is that the signaling dynamic range Δ (top minus bottom) is constrained near zero in absence of βarrestin-2 and compensatory mechanisms by other modulatory systems regulate the observed DA mediated locomotion (Figure 7C). Therefore, not only is the attenuation of hyperlocomotion by ghrelin receptor βarrestin-2-dependent, it apparently is also G protein independent and not rescued by redundancy of the remaining cellular βarrestin-1.
Figure 7. Schematic model for G protein and βarrestin-mediated GHSR1a signaling.
(A) Individual G protein- and βarrestin-mediated signaling pathways of activated GHSR1a. (B) Conceptual model for GHSR1a regulation of VTA dopamine neurons underlying locomotion. Activation of βarrestin (red arrows) leads to N-methyl-D-aspartic acid receptor (NMDAR) enhanced excitability of VTA dopamine neurons, dopamine release, and locomotion. Inhibition of this pathway with a GHSR1a antagonist reduces dopamine neuron firing, dopamine release and locomotion, blocking the hyper-locomotor effect of psychostimulants like cocaine. (C) Model of the effect of a genetic ablation of βarrestin-2 in DAT neurons. DAT neurons in WT mice are still responsive to GHSR1a antagonists (upper panel, green sigmoid curve) and the negative change in locomotion, Δ locomotion = Δ locomotion (βarrestin, other factors) is a significant fraction of the total locomotion. In the YIL781 unresponsive βarrestin-2 KO mouse, (lower panel), Δ locomotion at the GHSR1a is fixed at zero because of compensation by other remaining regulatory mechanisms (lower panel, green sigmoid curve).
In conclusion, the findings presented here identify separable behavioral changes in the addiction cycle that differ in their requirements for βarrestin-2 and demonstrate that the acute pharmacological ablation of its activity at the GHSR1a is not equivalent to its permanent genetic ablation. Additionally, the notion that chronically targeting the GHSR1a with antagonists to alleviate adverse aspects on dopamine regulation occurring with drug addiction must be balanced by our data in KO mice suggesting that the modulatory ghrelin system may readjust to counter objectives in treatment. To be able to better achieve a long term goal of using a drug that simultaneously reduce GHSR1a signaling and abnormal dopamine activity, we should first identify which of the G-protein or βarrestin pathways provides the superior target and then tailor the pharmacological treatment to the more relevant pathway using functionally selective compounds that also maintain chronic βarrestin activity.
Acknowledgments
Funding Information
This work was supported by National Institutes on Drug Abuse grant R21-DA35421 (KT, LSB) and 5P30DA029925 (KT, MGC, LSB) and National Institutes of Mental Health grant 5R37-MH-073853 (MGC, LSB).
Footnotes
Author`s Roles
Krisztian Toth, Lauren Slosky and Lawrence Barak were responsible for experimental design, data analysis, composing the manuscript. Krisztian Toth, Lauren Slosky and Peter Boone performed the behavioral studies. Thomas Pack was responsible for the characterization of Cre expression pattern. Dennis Abraham and Lan Mao designed, performed and analyzed blood pressure and heart rate measurements. Marc Caron and Nikhil Urs aided with experimental design. All authors contributed to and approved the final manuscript.
Conflict of Interest
The authors declare no competing financial interests.
References
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116(12):3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M, Jin J. Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci U S A. 2011;108(45):18488–18493. doi: 10.1073/pnas.1104807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122(2):261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: 'liking', 'wanting', and learning. Curr Opin Pharmacol. 2009;9(1):65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JW, Leff P. Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci. 1983;220(1219):141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Sotnikova TD, Medvedev IO, Lefkowitz RJ, Dykstra LA, Caron MG. Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. J Neurosci. 2003;23(32):10265–10273. doi: 10.1523/JNEUROSCI.23-32-10265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286(5449):2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Callaghan B, Hunne B, Hirayama H, Sartor DM, Nguyen TV, Abogadie FC, Brock JA. Sites of action of ghrelin receptor ligands in cardiovascular control. Am J Physiol Heart Circ Physiol. 2012;303(8):H1011–1021. doi: 10.1152/ajpheart.00418.2012. [DOI] [PubMed] [Google Scholar]
- Dietz DM, Sun H, Lobo MK, Cahill ME, Chadwick B, Gao V, Nestler EJ. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat Neurosci. 2012;15(6):891–896. doi: 10.1038/nn.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JA, Nylander I, Jerlhag E. A ghrelin receptor (GHS-R1A) antagonist attenuates the rewarding properties of morphine and increases opioid peptide levels in reward areas in mice. Eur Neuropsychopharmacol. 2015 doi: 10.1016/j.euroneuro.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Esler WP, Rudolph J, Claus TH, Tang W, Barucci N, Brown SE, Sweet LJ. Small-molecule ghrelin receptor antagonists improve glucose tolerance, suppress appetite, and promote weight loss. Endocrinology. 2007;148(11):5175–5185. doi: 10.1210/en.2007-0239. [DOI] [PubMed] [Google Scholar]
- Evron T, Peterson SM, Urs NM, Bai Y, Rochelle LK, Caron MG, Barak LS. G Protein and beta-arrestin signaling bias at the ghrelin receptor. J Biol Chem. 2014;289(48):33442–33455. doi: 10.1074/jbc.M114.581397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner CS, Herbert Pratt C, Babiuk RP, Sharma Y, Rockwood SF, Donahue LR, Murray SA. Supporting conditional mouse mutagenesis with a comprehensive cre characterization resource. Nat Commun. 2012;3:1218. doi: 10.1038/ncomms2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12(1):6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Engel JA. Ghrelin receptor antagonism attenuates cocaine- and amphetamine-induced locomotor stimulation, accumbal dopamine release, and conditioned place preference. Psychopharmacology (Berl) 2010;211(4):415–422. doi: 10.1007/s00213-010-1907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, Engel JA. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A. 2009;106(27):11318–11323. doi: 10.1073/pnas.0812809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9(3):482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Steinberg EE, Foldy C, Wall NR, Beier K, Luo L, Malenka RC. Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron. 2015;85(2):429–438. doi: 10.1016/j.neuron.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Tschop MH. Ghrelin. Mol Metab. 2015;4(6):437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peddibhotla S, Hedrick MP, Hershberger P, Maloney PR, Li Y, Milewski M, Pinkerton AB. Discovery of ML314, a Brain Penetrant Non-Peptidic beta-Arrestin Biased Agonist of the Neurotensin NTR1 Receptor. ACS Med Chem Lett. 2013;4(9):846–851. doi: 10.1021/ml400176n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta D, Di Francesco C, Melotto S, Mangiarini L, Heidbreder C, Hedou G. Systemic administration of ghrelin increases extracellular dopamine in the shell but not the core subdivision of the nucleus accumbens. Neurochem Int. 2009;54(2):89–94. doi: 10.1016/j.neuint.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Simpson AW, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358(6384):325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Sestakova N, Puzserova A, Kluknavsky M, Bernatova I. Determination of motor activity and anxiety-related behaviour in rodents: methodological aspects and role of nitric oxide. Interdiscip Toxicol. 2013;6(3):126–135. doi: 10.2478/intox-2013-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urs NM, Bido S, Peterson SM, Daigle TL, Bass CE, Gainetdinov RR, Caron MG. Targeting beta-arrestin2 in the treatment of L-DOPA-induced dyskinesia in Parkinson's disease. Proc Natl Acad Sci U S A. 2015;112(19):E2517–2526. doi: 10.1073/pnas.1502740112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urs NM, Daigle TL, Caron MG. A dopamine D1 receptor-dependent beta-arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice. Neuropsychopharmacology. 2011;36(3):551–558. doi: 10.1038/npp.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plasse G, van Zessen R, Luijendijk MC, Erkan H, Stuber GD, Ramakers GM, Adan RA. Modulation of cue-induced firing of ventral tegmental area dopamine neurons by leptin and ghrelin. Int J Obes (Lond) 2015;39(12):1742–1749. doi: 10.1038/ijo.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151(2–3):99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology. 2009;56(Suppl 1):160–168. doi: 10.1016/j.neuropharm.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med. 2016;374(4):363–371. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong HE, Perez de Sevilla Muller L, Hardi CN, McMahon DG, Brecha NC. Heterogeneous transgene expression in the retinas of the TH-RFP, TH-Cre, TH-BAC-Cre and DAT-Cre mouse lines. Neuroscience. 2015;307:319–337. doi: 10.1016/j.neuroscience.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Cummings DE. Regulation of ghrelin in physiologic and pathophysiologic states. J Nutr. 2005;135(5):1320–1325. doi: 10.1093/jn/135.5.1320. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494(3):528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]