Abstract
Children who are iron deficient (ID) or iron-deficient anemic (IDA) have been shown to seek and receive less stimulation from their caregivers, contributing to functional isolation. Over time, the reduced interactions between child and caregiver are thought to interfere with the acquisition of normative social competencies and adversely affect the child’s development. The current study examined functional isolation in children who were ID or IDA in infancy in relation to social difficulties in middle childhood and problem behaviors in adolescence. Using a sample of 873 Chilean children, 45% of whom were ID or IDA in infancy, structural equation modeling results indicated that infant IDA was associated with children’s dull affect and social reticence at age 5, which were related to mothers’ unresponsiveness and under-stimulation. Mothers’ limited responsiveness and stimulation were, in turn, related to children’s peer rejection at age 10, which further linked to problem behaviors and associating with deviant peers at adolescence. Findings support the functional isolation hypothesis and suggest that early limited caregiver responsiveness and stimulation contribute to long-term social difficulties in adolescents who were iron-deficient anemic in infancy.
Keywords: adolescent problem behaviors, anemia, child affect, functional isolation, iron deficiency, maternal responsiveness
It is well documented that malnourished children have flat affect, are less active and show less exploration of their environments (Brown & Pollitt, 1996). These altered behavioral patterns make such children less able to seek and receive developmentally facilitating caregiving, resulting in functional isolation (Levitsky & Strupp, 1995). Over time, the diminished child-caregiver interactions interfere with input from the physical and social environment and the child’s acquisition of social, motor and cognitive skills, which can adversely affect the child’s long-term development. Like severely malnourished children, iron-deficient anemic infants also have been observed to be emotionally dull and disengaged (Lozoff et al., 1998), and the mothers of iron-deficient anemic infants less stimulating and responsive than the mothers of non-anemic infants (Armony-Sivan, Kaplan-Estrin, Jacobson, & Lozoff, 2010). However, the long-term effects of these reduced interactive patterns are not known. Using longitudinal data, the current study tested whether iron deficiency in infancy relates to children’s dull affect and social reticence at age 5, which, in turn, contribute to mothers’ unresponsiveness and under-stimulation. We further examined whether mothers’ limited responsiveness and low social stimulation lead to peer rejection in middle childhood, and whether peer rejection contributes to problem behaviors and affiliation with deviant peers in adolescence (see conceptual model in Figure 1). Our conceptual model derives from a developmental cascade model, wherein an early health insult (infant iron deficiency) affects “downstream” development, with effects spreading across time and domains (Masten & Cicchetti, 2010). The connections between iron deficiency in infancy and later problem behaviors has widespread significance given that the estimated global prevalence of anemia in children younger than 5 years is 43%, or approximately 273 million children worldwide, mostly due to iron deficiency (Stevens et al., 2013). In the U.S., from 2007 to 2010, 7.1% of children 1–5 years were iron deficient, or approximately 1.5 million children (Gupta, Perrine, Mei, & Scanlon, 2016). Latino children have the highest prevalence (15–20% iron deficiency sustained over several decades) due in part to rapid postnatal growth and a greater likelihood of being overweight (Brotanek, Gosz, Weitzman, & Flores, 2007). Low-income children also have a high prevalence of iron deficiency due to nutritional deficiencies resulting from food insecurity (Park et al., 2009).
Figure 1.
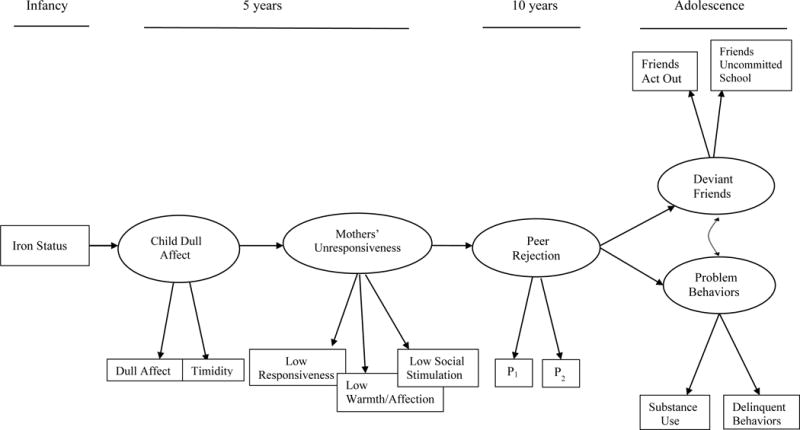
Conceptual model of the associations among infant iron status, child’s dull affect, mothers’ unresponsiveness, peer rejection, and outcomes at adolescence. P1 = parcel 1, etc.
Iron-Deficiency Anemia (IDA) and Affective Dullness and Unresponsive Parenting
Iron deficiency is characterized by depleted iron stores and reduced iron dependent protein production (Baker, Greer, & The Committee on Nutrition, 2010). Iron deficiency in infancy may be particularly harmful due to rapid brain growth, during which critical iron-containing enzymes and hemoproteins are needed for the brain’s hippocampal and cortical regional development (Georgieff, 2011; Lozoff, 2011). In animal studies, IDA also adversely affects the brain’s striatal dopaminergic systems (Beard, 2007). Because dopamine plays a major role in positive affect and the degree to which individuals experience inherent reward, alterations in the striatal-frontal and mesolimbic dopaminergic system connections associated with IDA would be consistent with affective dullness (Lozoff, 2011). Functionally, alterations of processes associated with the striatum and hippocampus in humans would be evidenced by a general disruption of systems regulating emotion, possibly resulting in delayed emotional responding or flat affect (Georgieff, 2011; Lozoff, 2011).
There is much evidence that early IDA in humans is associated with aberrant social behaviors that can interfere with developmentally facilitating caregiving. For example, infants who are iron deficient have been observed to be more solemn, listless, and easily fatigued than iron sufficient infants (Lozoff, 2007). Iron-deficient anemic preschool children in New Delhi displayed less social looking toward their mothers and were slower to show positive affect than non-anemic children when observed in a semi-structured in-home play setting (Lozoff et al., 2007). Five-year-olds from Costa Rica who had chronic, severe iron deficiency in infancy displayed lower levels of smiling, verbalization, and physical activity compared to children who had good infant iron status (Corapci, Radan, & Lozoff, 2006). Four-year-old Chinese children who had chronic IDA in infancy showed less positive affect and were more passive and unengaged than children who were non-anemic throughout infancy (Chang et al., 2011). In addition, children within the current study sample who were IDA in infancy were less socially engaging and less active in infancy and less attentive in childhood (East, et al., 2017; Lozoff et al., 2003, 2010, 2014).
The mothers of iron-deficient children also show impaired interactive behaviors. Compared to mothers of non-anemic infants, the mothers of iron-deficient anemic infants in Costa Rica showed less pleasure and affection in interaction with their child and were less likely to offer encouragement on a motor task (Lozoff et al., 1998). In a videotaped feeding interaction task, mothers of iron-deficient anemic infants were less sensitive to infant cues and showed less cognitive and social-emotional growth fostering behavior than mothers of non-anemic infants (Armony-Sivan et al., 2010). Other studies also have detected less mother-child reciprocity and maternal responsivity between iron-deficient infants or young children and their mothers (Corapci et al., 2006; Lozoff, Klein, & Prabucki, 1986; Lozoff et al., 1998).
Consistent with what one would expect from functional isolation, persistent limited social exchanges between iron-deficient children and their caregivers would provide fewer opportunities for the acquisition of critical social skills, thereby creating a lasting impairment in the child’s social development (Wachs, 2009). Although mothers’ responsiveness was not directly considered, children with chronic iron deficiency in infancy have higher levels of social problems at ages 10 and 14 than their counterparts with good iron status in infancy (Corapci et al., 2010; Lozoff, Jimenez, Hagen, Mollen, & Wolf, 2000). Parental unresponsiveness and low parental warmth and affection have been widely associated with children’s poor peer relations and peer rejection (Elicker, Englund, & Sroufe, 2016; Parke & Ladd, 2016). Studies also show that children who are actively disliked by their classmates are more likely to engage in later delinquency and substance use (Dodge et al., 2009; Kupersmidt & Coie, 1990). Youth who are socially rejected in the school setting tend to affiliate with deviant peers who are more tolerant of inept social behaviors (Snyder et al., 2005). Such affiliations, though, often foster “deviancy training,” whereby socially rejected youth are encouraged to also use substances and engage in delinquent-like behavior to solidify social bonding (Monahan, Steinberg, & Cauffman, 2009; Snyder et al., 2005). Given evidence of low maternal stimulation and responsiveness to iron deficient children, such children may be at risk for later social difficulties, such as peer exclusion, association with deviant peers, and delinquency.
The Current Study
The current study examined the process of functional isolation in children who were iron deficient or iron-deficient anemic in infancy and its relation to children’s social difficulties in middle childhood and problem behaviors in adolescence. To test these relations, we analyzed longitudinal data on over 800 Chilean children who were enrolled as infants when there was no national program for iron fortification in infant formula, resulting in a sample with relatively high rates of iron deficiency and iron-deficiency anemia in infancy. To best understand how iron deficiency relates to children’s affect, we analyzed iron status in two ways. First, we considered iron status as a continuous variable based on severity (coded [0] iron sufficiency, [1] iron deficiency, and [2] iron-deficiency anemia). Second, we considered iron status as a 3-level categorical (ordinal trichotomous) variable to assess group differences (i.e., comparing children who were iron sufficient [IS] as infants to those who were iron deficient only [ID-only], comparing children who were IS to those who were iron-deficient anemic [IDA], and comparing children who were ID to those who were IDA).
Regarding tests of severity effects, although some studies have found that iron deficiency severity is associated with more socioemotional impairment (Lozoff et al. 2008; Wachs, Pollitt, Cueto, Jacoby, & Creed-Kanashiro, 2005) and poorer cognitive functioning (Carter et al., 2010; Doom et al. 2014), several studies have not found severity effects (reviewed in Grantham-McGregor & Ani, 2001; McCann & Ames, 2007). Given that a dose-response relationship is one criterion used to establish causal relations (worse outcomes associated with higher levels of the exposure; Hill, 1965), it would be important to establish that an association exists between more severe iron deficiency and poorer socioemotional functioning.
The analyses involving iron status as an ordinal trichotomous variable will reveal whether differences exist in children’s affective behavior across groups, including the direct comparison of children who were iron deficient alone (no anemia) and those who were iron sufficient. Although some animal studies have demonstrated that iron deficiency without anemia is associated with poorer functioning, few studies of human infants have examined this question (McCann & Ames, 2007) (exceptions: Akman et al., 2004; Doom et al., 2014; Lozoff, Jimenez, & Wolf, 1991). Iron deficiency (alone, without anemia) is present when there is insufficient iron to maintain normal physiologic function, which may have harmful effects on brain and behavioral development (Baker et al., 2010). Given that iron deficiency without anemia is asymptomatic and often goes undetected, and is roughly 2.5 times more common than iron-deficiency with anemia (Centers for Disease Control and Prevention, 2002), understanding the effects of iron deficiency without anemia on children’s socioemotional behavior is important.
In the analyses comparing children who were iron deficient only to those who were iron-deficient anemic in infancy, we test whether iron-deficiency anemia is associated with deficits above and beyond those associated with iron deficiency alone. Anemia is a more severe and end-stage form of iron deficiency, characterized not only by depleted iron stores and functional alterations in iron-dependent compounds, but also by reduced hemoglobin concentrations (Zimmerman & Hurrell, 2007). Anemia in adults has been found in some studies to be associated with more severe symptoms of weakness, fatigue, and listlessness than iron deficiency without anemia (Zimmerman & Hurrell, 2007). We test whether children show more affective dullness and listlessness as a function of whether they were iron-deficient anemic in infancy compared to iron deficient only.
Tests of our conceptual model (Fig. 1) included all direct paths between distal variables, such as between children’s dull affect and problem behavior in adolescence. We operationalized mothers’ unresponsiveness using home observations of mothers’ low responsiveness, low warmth/affection, and low social stimulation. We operationalized peer rejection as active exclusion from the peer group. We did not focus on peer neglect (preferring solitary activities, uninvolved with peers) because peer rejection reflects more problematic social adjustment, whereas peer neglect is thought to derive from shyness and introversion and does not necessarily reflect social inadequacies (Parker, Rubin, Price, & de Rosier, 1995). Children’s problem behaviors at adolescence were operationalized as delinquent-like behaviors (cheating, stealing, truancy) and substance use, based on problem behavior theory, which asserts that such behaviors comprise a single multidimensional behavioral syndrome (Donovan & Jessor, 1985). We specifically analyzed early, regular, and problematic use of cigarettes, alcohol, and marijuana as our index of substance use, given that moderate use of these substances within this Chilean cohort of youth might be considered relatively normative. For example, data from the global school-based student health survey show that, in 2013 [the most recent Chile survey], 28% of 13- to 17-year-old Chilean students smoked cigarettes in the past 30 days and 25% had used marijuana (World Health Organization, 2013).
In addition, we tested an alternate ordering of study variables, specifically testing whether infant iron status relates directly to mothers’ unresponsiveness which, in turn, is associated with children’s dull affect. This analysis was conducted to potentially rule out this alternate ordering of effects and to derive evidence that children’s dull affect is best understood as a mediator linking infant iron deficiency and mothers’ unresponsiveness. Further analyses tested whether the pathways outlined in our analytic model differ for males and females.
Analyses controlled for several child, family, and socioeconomic factors that are related to the study variables. Because iron deficiency is more likely to occur in disadvantaged family circumstances (Lozoff, 2007) and among males (due in part to greater postnatal weight gain and lower neonatal iron stores in boys; Domellof et al., 2002), we control for family socioeconomic status (SES) and child sex. We also control for whether infants were iron-supplemented as part of the original infancy study, as this is negatively associated with poor iron status (Lozoff et al., 2003), and whether infants were breastfed as the sole source of milk at 6 months, given that breast feeding into the second half year of life is associated with increased risk of iron deficiency and iron deficiency anemia (Baker et al., 2010; Clark et al., 2017). Dull temperament at 6 months of age was also included as a covariate to strengthen interpretation that dull affect at 5 years results from iron deficiency or iron-deficiency anemia independent of temperamental dullness. We also control for mothers’ depressive symptoms, IQ, and educational level, as well as number of children in the family and family stressors on mothers’ unresponsiveness. Lastly, we control for family stress, SES, mothers’ education, child sex, and age at the adolescent follow-up on deviant friends and problem behaviors at adolescence.
Method
Participants and Study Design
Participants were 873 children (53% male) who have been studied since infancy as part of an iron-deficiency anemia randomized controlled preventive trial and follow-up study in Santiago, Chile (Lozoff et al., 2003). Table 1 displays descriptive information about the analytic sample and the study variables. The study originally involved 1,657 infants recruited from community clinics in Santiago, Chile between 1991 and 1996, a period during which iron deficiency in infancy was widespread and there was no national program for iron fortification in infant formula. All infants were born healthy at term (birth weight ≥ 3.0 kg) and had no perinatal complications or acute or chronic illnesses. Participants were generally from lower middle-class families, for whom most heads of households held stable skilled jobs (22%, e.g., carpenter), stable semi-skilled jobs (37%, e.g., taxi driver), or sporadic unskilled jobs (29%, e.g., house painter). Most mothers had a 9th-grade education (only 9 years of schooling was compulsory in Chile at the time of the study), with the mean and median of mothers’ education the 9th grade. (Adults with a 6th-through 10th-grade education comprise most of the lower middle class in Chile; Barozet & Fierro, 2011).
Table 1.
Descriptive Statistics of Sample and Study Measures
| N | Min | Max | Mean or % | Standard deviation | |
|---|---|---|---|---|---|
| Infant assessment | |||||
| Iron sufficient (IS) | 483 | 0 | 1 | 55.3% | |
| Iron deficient without anemia (ID) | 248 | 0 | 1 | 28.4% | |
| Iron deficient with anemia (IDA) | 142 | 0 | 1 | 16.3% | |
| † Infant sex (1=male) | 873 | 0 | 1 | 53% | |
| † Breastfed solely at 6 months | 864 | 0 | 1 | 62% | |
| † Family socioeconomic statusa | 814 | 13 | 43 | 27.06 | 6.19 |
| † Mothers’ education (years) | 814 | 0 | 19 | 9.79 | 2.66 |
| † Family stressors | 814 | 0 | 30 | 4.66 | 2.66 |
| † Number of children < 15 yrs | 814 | 1 | 8 | 2.14 | 1.20 |
| † Dull temperament | 678 | 3 | 21 | 7.81 | 2.81 |
| † Iron supplementationb | 814 | 0 | 1 | 55% | |
| † Mothers’ IQ | 873 | 51 | 110 | 83.56 | 9.41 |
| 5 Year Assessment | |||||
| † Child age (years) | 873 | 5.42 | 5.83 | 5.51 | 0.40 |
| † Mothers’ depressive symptoms | 873 | 0 | 60 | 19.69 | 13.40 |
| Child’s Dull Affect and Timidity | |||||
| Child dull affect | 873 | 6 | 24 | 9.64 | 2.70 |
| Child timidity | 873 | 7 | 28 | 14.57 | 4.58 |
| Mothers’ Unresponsivenessc | |||||
| Mothers’ low responsiveness | 873 | 0 | 2 | 0.85 | 0.41 |
| Mothers’ low warmth/affection | 873 | 0 | 6 | 0.77 | 0.89 |
| Mothers’ low social stimulation | 873 | 0 | 16 | 2.68 | 1.48 |
| 10 Year Assessment | |||||
| † Child age | 790 | 9.90 | 11.00 | 10.02 | 0.09 |
| Peer Rejection | 790 | 0 | 14 | 4.45 | 2.41 |
| Adolescent Assessment | |||||
| †Adolescent age (years) | 813 | 11.92 | 17.83 | 14.63 | 1.54 |
| Deviant Friends | |||||
| Friends act out | 812 | 0 | 32 | 9.91 | 5.21 |
| Friends uncommitted school | 812 | 0 | 16 | 6.80 | 2.73 |
| Problem Behaviors | |||||
| Delinquent-like behaviors | 806 | 0 | 24 | 4.58 | 3.30 |
| Total substance used | 806 | 0 | 12 | 1.76 | 2.46 |
| Cigarette use | 806 | 0 | 4 | 0.68 | 1.13 |
| Alcohol use | 806 | 0 | 4 | 0.76 | 1.02 |
| Marijuana use | 806 | 0 | 4 | 0.33 | 0.93 |
Note. Bolded variables are latent variables.
A control variable.
Higher scores indicate greater poverty.
0 = did not receive iron supplementation in infancy, 1 = received some form of iron supplementation.
Higher scores indicate less responsiveness.
Higher scores indicate ever, earlier, current, and more problematic use.
At 5½ years of age, 888 children were assessed on various behaviors and caregiver interactions. The entire original sample could not be studied due to funding constraints. The focus was on children in the high-iron supplement and no-added iron groups but not the low-iron supplement group, since the low-iron supplement and high-iron supplement groups did not differ in IDA prevalence and were similar in behavior in infancy (Walter, Pino, Pizarro, & Lozoff, 1998). Because infants were randomly assigned to iron-supplementation or no supplementation, these groups were similar on most background characteristics.
At subsequent follow-ups (age 10 and adolescence), funding allowed us to reach out to the entire infancy sample. At 10 years, 1,127 of the study children participated, and at adolescence, 1,119 youth (M = 14.63 years; range 11.92–17.83) completed assessments of their friendships, behaviors, and substance use. The 873 youth who had complete data at most study time points form the core sample for this analysis. These 873 participants had good coverage (<10% missing) on all model variables (see Table 1), except for the variable dull infant temperament, which was deemed important to retain despite relatively high numbers of missing participants. There were no differences between the 873 children in the current analytic sample and the original sample of 1,657 regarding: infant iron deficiency or iron-deficiency anemia status, number of children in the household, mothers’ education, mothers’ IQ, age, or depression, or any of the model variables (child’s dull affect, mothers’ unresponsiveness at 5 years, etc.). However, compared to children who were not part of the current analytic sample, those who were studied here were less likely to have received iron supplementation in infancy, were from lower socioeconomic families, and slightly younger at the adolescent follow-up (p < .05). These factors were controlled in all analyses. A detailed description of the study is published elsewhere (Lozoff et al., 2003).
Procedure
The infant study and the 5-year, 10-year, and adolescent follow-ups were approved by the relevant institutional review boards in the U.S. and Chile. Signed informed consent was obtained from parents at each time point; assent was obtained from children at 10 years and at adolescence. At the adolescent follow-up, youth completed a two-hour, interviewer-administered questionnaire in a private room. The questionnaires were administered by Chilean psychologists who were trained in the administration of standardized questionnaires.
Measures
At all study time points, Spanish versions of the study measures were used. They were extensively pilot-tested with the population under investigation prior to conducting the study and shown to have good reliability and high equivalence to the English versions. Measures administered at the adolescent follow-up were back-translated to verify comparability with the English version. All Cronbach’s coefficient alphas (α) are reported using the current sample.
Infant iron deficiency (ID) and iron-deficiency anemia (IDA)
At 6 months, children underwent a finger stick to determine hemoglobin levels. Infants with finger stick hemoglobin values ≤ 103 g/L had a venipuncture performed to determine iron status. Anemia at 6 months was defined as a venous hemoglobin concentration ≤ 100 g/L and two of three iron measures in the iron deficient range (mean corpuscular volume < 70 fL, erythrocyte protoporphyrin > 100 μg/L red blood cells, and serum ferritin < 12 mg/L). At 12 months, venipuncture blood specimens (7–10 ml) were drawn on all infants; at 18 months, infants in the low- and no-added iron groups received another venipuncture. Anemia at 12 and 18 months was defined as venous hemoglobin < 110 g/L. Iron deficiency at 12 and 18 months was defined as two of three iron measures in the iron-deficient range (described above). These criteria were the standard in the field (Dallman, Reeves, Driggers, & Lo, 1981). Infants with iron-deficiency anemia at 6, 12 or 18 months of age were treated with therapeutic doses of oral iron and followed up for improvement. With testing and treatment for IDA every 6 months, the longest a child could have been iron-deficient anemic was typically 6 months. Since iron status fluctuated across infancy, we categorized infants’ iron status as the most severe diagnosis at any of the three time points, or as: ever IDA, ever ID, or IS (iron sufficient, or not iron deficient or iron-deficient anemic at any time point in infancy). The various iron groups were mutually exclusive, such that no child was coded as both ever ID or ever IDA. The proportions of the iron status groups are shown in Table 1. Venous blood samples were also obtained at 5, 10, and 16 years. We defined ID as 2 or more abnormal iron measures and anemia using age-appropriate cutoffs. Iron status was generally good after infancy. No child had IDA at age 5 and only 5% had ID at 10 years or adolescence.
Child dull affect 5 years
Mothers completed the Children’s Adaptive Behavior Inventory (CABI; Cowan & Cowan, 1990), which includes scales of dull affect (6 items: e.g., my child shows no smiles, fixed expression, sits idly, not interested in things: α = .64) and timidity/social reticence (7 items: e.g., my child is shy with adults, is shy with children, is inhibited; α = .76). Response options were: never (coded as 1), rarely (2), repeatedly (3), and most of the time (4). Scores were summed to yield a possible score range of 6–24 for dull affect, and 7–28 for timidity/social reticence.
Mothers’ low responsiveness, low warmth/affection, and low social stimulation to child at 5 years
Researcher observation ratings on the Home Observation for Measurement of the Environment Inventory-Early Childhood version (HOME-EC) were used to assess mothers’ low responsiveness, low warmth/affection, and low social stimulation toward her child (Bradley, Corwyn, & Whiteside-Mansell, 1996). The HOME-EC is a well-established measure that is sensitive to variations in family life, including in Latin American countries (Bradley et al., 1996). There have been several factor analytic studies of the HOME-EC, some of which show different factor structures for families of different race and ethnicity (Bradley Mundfrom, Whiteside, Casey, & Barrett, 1994). To clarify the factor structure within the current sample, we conducted a principal components analysis on 13 items that appeared to capture the maternal behaviors of interest while excluding items that would be correlated with socioeconomic status (e.g., has toys for learning). Three factors were identified using the Kaiser (K1) criteria (all factor loadings ≥ .42): responsivity (2 items: e.g., mother responds to child, mother responds to child’s verbalization), warmth/affection (3 items: mother kisses, holds, praises child), and social stimulation (8 items: e.g., mother talks to child, mother encourages child to talk). The Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy was .722, and the Bartlett’s test of sphericity was Chi-square [78] = 1292.15, p < .000. These procedures and criterion cutoff values are stated in Meyers, Gamst and Guarino (2006). Ratings were scored as binary-choice options of yes = 1 or no = 0. Scores were reversed so that high scores indicate low responsiveness, low warmth/affection, and low social stimulation.
Peer rejection at age 10
Mothers’ ratings on seven items on the social problems scale of the Child Behavior Checklist (CBCL; Achenbach & Ruffle, 2000) were used to assess children’s peer rejection (e.g., not liked, not get along with others, has no friends, is teased; α = .69). Response options were not true (coded as 0), somewhat or sometimes true (1), and very true or often true (2). Scores were summed to yield a possible score range of 0–14.
Deviant friends in adolescence
Adolescents answered eight questions about the extent to which “the kids you spend time with” engage in acting-out behaviors (e.g., they get into a lot of trouble at school, they cheat on tests; α = .74) and four items about how committed their friends are to school (e.g., they do well in school, they like school; α = .69). Response options ranged from 0 (none of them) to 4 (all of them). These scores were reversed, such that higher scores indicate having many friends who act out (range: 0–32) and having many friends who are uncommitted to school (range: 0–16).
Problem behavior at adolescence
Items from the self-report youth version of the CBCL rule-breaking scale (Achenbach, 1991) were used to assess delinquent-like behavior at adolescence (12 items: e.g., lies, cheats, steals, cuts class; α = .75). Response options ranged from not true (coded as 0) to very true or often true (2). Scores were summed to yield a possible score range of 0–24. Youth also completed an extensive substance use inventory in which they responded to questions about their use of cigarettes, alcohol, and marijuana. All youth were assured of the confidentiality of their responses. Separate scores were derived for each substance. A score of 1 was coded for each of the following: ever used, early age of onset (≤ 14 years), and regular use (cigarettes: smoked ≥ 10 days within the last month; alcohol: drank ≥ 3 days within the last month; marijuana: used/smoked ≥ 2 occasions within the last month). These cutoff criteria were based on a median split per each substance. Youth also responded to 14 questions per each substance about problems resulting from their use of cigarettes, alcohol, and marijuana (e.g., “Your use of marijuana [cigarettes, alcohol] has … hurt your relationship with your parents; hurt your school or job performance; caused physical health problems?”). Participants who reported at least 3 problems associated with their substance use received a score of 1 for problem use. All scores were summed, resulting in a possible score range of 0 to 12 for total substance use (or 0 to 4 for each substance, with one point for each of the following: ever used, early use, regular use, and problem use; Table 1).
Background Variables
Family socioeconomic status was assessed by parent responses on the Graffar instrument, which is an often-used measure of poverty in developing countries and asks about the family’s living and housing conditions, material possessions, and parents’ occupation (Graffar, 1956). Mothers’ IQ was assessed at the infant assessment by an abbreviated Wechsler Adult Intelligence Scale (WAIS-III; Wechsler, 1955), which yields a full-scale IQ score (Mean = 100, SD = 15). Children’s temperamental dullness at infancy was measured by mothers’ ratings at 6 months on the Infant Characteristics Questionnaire (Bates, Freeland, & Lounsbury, 1979), which asks three questions about the infant’s smiling, happy excitement, and activity level. Response options ranged from not at all (coded as 1) to all the time (7). Scores were reversed coded, with high scores indicating temperamental dullness (range: 3–21). Children’s mothers were interviewed by research staff when the infant was 8 months and asked to indicate their highest level of schooling, the number of children living in the household who were younger than 15 years, and the presence of 30 possible stressors (e.g., chronic illness of a family member; α = .91) on a modified Social Readjustment Rating Scale (Holmes & Rahe, 1967). Iron supplementation as part of the preventive trial was also included as a covariate, coded as receiving some form of iron supplementation (1) or not receiving iron supplementation (0). Additionally, mothers’ depressive symptoms at the 5-year assessment were assessed by their responses on the 20-item Center for Epidemiologic Studies-Depression Scale (CES-D; Radloff, 1977), which asks about the frequency of depressed mood within the last week (e.g., I could not get going) using response options of rarely or none of the time (0) to most or all the time (3).
Analytic Strategy
Structural equation modeling using Mplus 6.0 (Muthén & Muthén, 2010) was used to evaluate the model shown in Figure 1. We assessed this model twice: first, we considered iron status as a continuous variable reflecting severity of iron deficiency, and second, we treated iron status as an ordinal trichotomous variable and assessed pairwise comparisons among the three iron status classifications. For this second model, iron status was dummy coded into three categories: IDA, ID, and IS. IS was the omitted category because it served as the reference group. Thus, the two exogenous variables in the second analysis were IS vs. ID and IS vs. IDA. To compare ID and IDA groups directly, we specified new parameters derived from parameter estimates for the two dummy variables in the full model. The standard errors for these new parameters were produced using the delta method (Muthén, 2011). We also analyzed an alternate ordering of study variables to rule out a different ordering of effects (i.e., that increasing levels of iron deficiency relate directly to mothers’ unresponsiveness, which relates directly to child’s dull affect). Finally, we tested whether the strength of relations within our model differed by child sex by conducting an omnibus test using multiple-group analyses in the model analyzing iron status as a continuous variable reflecting increasing severity. Model fit was examined by reviewing indices of good model fit (Kline, 2011), including a nonsignificant chi-square, comparative fit index (CFI) > .90, root mean square error of approximation (RMSEA) < .06, and standardized root mean square residual (SRMR) < .08 Mediation was tested using the INDIRECT command within Mplus, which estimates indirect effects with the delta method standard errors (Muthén, 2011). Analyses were conducted using maximum likelihood estimators, which are robust to non-normality. Missing data were treated within Mplus with the full information maximum likelihood method (FIML). Given their expected association, deviant friends and problem behaviors in adolescence were correlated a priori.
Inclusion of covariates
Based on correlational results (available upon request and described below) as well as the relations found in the literature (described in the Introduction under The Current Study), several covariates were included. For infant iron status, covariates included child sex, family socioeconomic status (SES), mothers’ educational level, breastfeeding as the sole source of milk at 6 months, and iron supplementation. For child’s dull affect, covariates included child age at 5 years, child sex, temperamental dullness at infancy, and family SES. For maternal unresponsiveness, covariates included child age at 5 years, child sex, family stress, mothers’ depressive symptoms, educational level, and IQ, and number of children in the household < 15 years. For peer rejection, covariates included child sex, age at the 10-year assessment, family SES, family stress, and number of children in household < 15 years. For deviant friends and problem behaviors in adolescence, child sex, age at the adolescent follow-up, family SES, mothers’ education, depression, and IQ, and iron supplementation were covariates. Modification indices within Mplus did not indicate additional covariates for any of the models, and all covariates remained in the model regardless of significance level.
Prior to conducting this study’s main analyses, we computed the main and interaction effects of iron status and iron supplementation on the model’s 10 (observed) endogenous variables (in Fig. 1: child dull affect, child timidity, mothers’ low responsiveness, low warmth/affection, low stimulation, child peer rejection, friends who act out, friends uncommitted to school, substance use, and delinquent behaviors). This was done to rule out the possibility that the effects of iron status are moderated by iron supplementation. There were no statistically significant interactions for any of the endogenous variables. Thus, given that the effects of iron status do not vary across iron supplementation group, we model only the main effects of IDA and ID. We keep iron supplementation in the model as a covariate to control for confounding, as it relates to our predictor, iron status.
Results
Formation of Latent Variables and Measurement Model
We constructed latent factors within Mplus for the model’s endogenous variables. A latent variable for peer rejection was estimated using the parceling procedure of randomly selecting items as outlined by Bandalos and Finney (2001). The individual factor loadings for all latent variables were significant in all models (.45–.87, p < .001). The measurement model had good fit: χ2 (34) = 74.54, CFI = .962, RMSEA = .037, SRMR = .037. When tested separately by child sex in a model where all paths were allowed to vary freely, the pattern of loadings was nearly identical for males and females. Table 2 displays the bivariate correlations among the study variables.
Table 2.
Intercorrelations among Model Variables
| Model variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Infant iron status (IS-ID-IDA)a | – | ||||||||||||
| 2. Iron deficient only (ID)b- infancy | – | – | |||||||||||
| 3. Iron-deficient anemic (IDA)c-infancy | – | – | – | ||||||||||
| 4. ID v IDAd - infancy | – | – | – | – | |||||||||
| 5. Child dull affect - 5 yrs | .07* | .01 | .06 | .07 | – | ||||||||
| 6. Child timidity – 5 yrs | .07* | .04 | .07 | .05 | .40*** | – | |||||||
| 7. Mother unresponsiveness −5 yrs | .07* | .01 | .07 | .09 | .18*** | .07* | – | ||||||
| 8. Mother low warmth/affection-5 yrs | .08* | .02 | .10* | .13** | .12*** | .05 | .32*** | – | |||||
| 9. Mother lows stimulation – 5 yrs | .00 | .03 | .02 | .04 | .19*** | .12** | .50*** | .33*** | – | ||||
| 10. Peer rejection – 10 yrs | .03 | .01 | .04 | .09 | .16*** | .04 | .17*** | .27*** | .20*** | – | |||
| 11. Deviant friends – adolescence | .09* | .07* | .05 | .02 | .06 | .02 | .07 | .06 | .07 | .14*** | – | ||
| 12. Friends uncommitted school –adolescence | .08* | .04 | .06 | .04 | .04 | −.05 | .09* | .04 | .08* | .05 | .32*** | – | |
| 13. Substance use - adolescence | .05 | .00 | −.06 | .02 | −.07 | −12** | .01 | .10** | .06 | .12** | .23*** | .26*** | – |
| 14. Delinquent behaviors - adolescence | .06 | .07* | .02 | .06 | .01 | −.07 | .02 | .09* | .02 | .22*** | .48*** | .33*** | .41*** |
Note. Coded as a continuous variable, reflecting increasing severity: 0 = iron sufficient (IS), 1 = iron deficient (ID), 2 = iron-deficient anemic (IDA). N = 873.
0 = iron sufficient, 1 = iron deficient; N = 731.
0 = iron sufficient, 1 = iron-deficient anemic; N = 625.
0 = iron deficient only, 1 = iron-deficient anemic; N = 390.
p < .05.
p < .01.
p < .001.
Modeling Results
Figure 2 shows the model results for infant iron status analyzed as a continuous variable representing severity. The fit statistics are listed in Figure 2 and indicate good model fit. Path coefficients indicate that more severe iron deficiency in infancy was associated with higher levels of dull affect at 5 years (β = .09, B = .25, SE = .12, p < .05), and child’s dull affect was strongly associated with mothers’ unresponsiveness (β = .26, B = .04, SE = .01, p < .001). Mothers’ unresponsiveness at 5 years was related to children’s peer rejection at 10 years (β = .38, B = 1.03, SE = .21, p < .001), and peer rejection was related to both deviant friends and problem behaviors in adolescence (β = .19, B = .67, SE = .29, p < .05 and β = .31, B = .51, SE = .14, p < .001, respectively). Direct effects between distal variables were also tested; none were significant.
Figure 2.
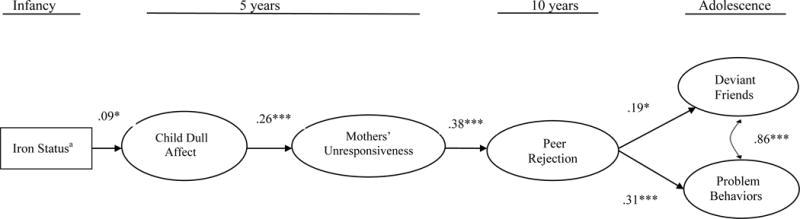
aIron status coded as a continuous variable reflecting increasing severity: 0 = iron sufficient, 1 = iron deficient, 2 = iron-deficient anemic (N = 873). Standardized coefficients are shown. Direct effects between distal variables (e.g., child dull affect → problem behaviors) were tested; no significant direct distal effects emerged. The model had good fit: χ2 (182) = 416.47, CFI = .881, RMSEA = .038, SRMR = .038. *p < .05. **p < .001. ***p < .001.
Figure 3 shows the path coefficients and fit statistics for the model testing the various iron category comparisons. Model fit was adequate and modification indices indicated that no additional covariates would improve model fit. Results indicated that, compared to IS in infancy, ID in infancy was not related to child’s dull affect at age 5 (β = .06, B = .21, SE = .23), but IDA in infancy was significantly related to dull affect at 5 years (β = .09, B = .50, SE = .26, p < .05). The path coefficient comparing IDA to ID-only was not significant (β = .001, B = .25, SE = .30, ns). The remainder of the paths from child’s dull affect to adolescent outcomes were highly similar to the model testing iron deficiency severity (Fig. 2).
Figure 3.
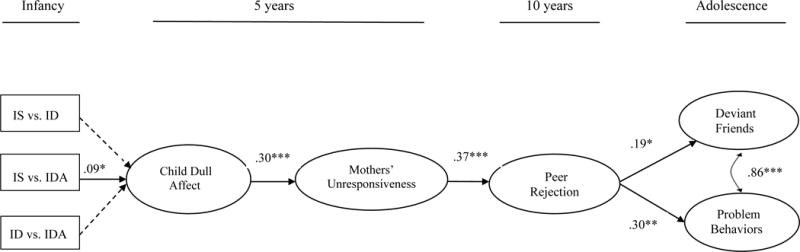
IS = iron sufficient. ID = iron deficient. IDA = iron-deficient anemic (N = 873). Standardized coefficients are shown. Direct effects between distal variables (e.g., child dull affect → problem behaviors) were tested; no significant direct distal effects emerged. The model had good fit: χ2 (195) = 523.90, CFI = .801, RMSEA = .044, SRMR = .040. *p < .05. **p < .001. ***p < .001.
Tests of indirect effects
Tests of the indirect effects indicated that the full mediated model from iron status severity in infancy to problem behavior in adolescence approached significance (top, Table 3). The 3- and 4-variable pathways (starting from child’s dull affect) that comprise this indirect effect were significant. Importantly, and as a test of functional isolation, children’s dull affect significantly mediated iron status severity in infancy to mothers’ unresponsiveness at child’s age 5. When analyzing iron status as an ordinal trichotomous variable (bottom, Table 3), the indirect effect from IDA in infancy to peer rejection at age 10 approached significance. In addition, the two mediated pathways from child’s dull affect to deviant friends and problem behavior in adolescence were significant.
Table 3.
Summary of Significant Indirect Effects
| Severity Model (Figure 2) | B | (SE) |
| Iron statusa → dull affect → mothers’ unresponsiveness → peer rejection → problem behavior | .005+ | (.003) |
| Iron statusa → dull affect → mothers’ unresponsiveness → peer rejection | .008+ | (.004) |
| Iron statusa → dull affect → mothers’ unresponsiveness | .010* | (.005) |
| Child dull affect → mothers’ unresponsiveness → peer rejection → problem behavior | .022** | (.008) |
| Child dull affect → mothers’ unresponsiveness → peer rejection → deviant friends | .028* | (.014) |
| Child dull affect → mothers’ unresponsiveness → peer rejection | 042*** | (.011) |
| Mothers’ unresponsiveness → peer rejection → problem behavior | .528** | (170) |
| Mothers’ unresponsiveness → peer rejection → deviant friends | .694* | (312) |
| Multiple Comparison Model (Figure 3) | ||
| IDAb → dull affect → mothers’ unresponsiveness → peer rejection | .010+ | (.006) |
| IDAb → dull affect → mothers’ unresponsiveness | .028+ | (015) |
| Child dull affect → mothers’ unresponsiveness → peer rejection → problem behavior | .034** | (.013) |
| Child dull affect → mothers’ unresponsiveness → peer rejection → deviant friends | .021* | (010) |
| Child dull affect → mothers’ unresponsiveness → peer rejection | .111*** | (.028) |
| Mothers’ unresponsiveness → peer rejection → problem behavior | .508** | (165) |
| Mothers’ unresponsiveness → peer rejection → deviant friends | .666* | (.305) |
Note. Iron status coded as a continuous variable reflecting increasing severity: 0 = iron sufficient, 1 = iron deficient, 2 = iron-deficient anemic.
Iron-deficiency anemia compared to iron sufficiency. +p < .09.
p < .05.
p < .01.
p <.001.
Tests of alternate ordering of effects
Given the possibility that infant iron deficiency might relate directly to mothers’ unresponsiveness, we computed a model where infant iron status (analyzed as a continuous variable reflecting severity) leads directly to mothers’ unresponsiveness, and mothers’ unresponsiveness leads to child’s dull affect, which in turn, leads to peer rejection and the two exogenous outcomes at adolescence. All covariates controlled in previous models were included, and all paths between distal variables were included. The model had good fit (χ2 [182] = 410.77, CFI = .884, RMSEA = .038, SRMR = .037, N=873). Results indicated that iron status was not directly related to mothers’ unresponsiveness (β = .05, B = .03, SE = .02, ns), but iron status was directly related to children’s dull affect at age 5 (β = .08, B = .25, SE = .12, p < .05). As in the previous two models (Figures 2 and 3), mothers’ unresponsiveness was positively related to child’s dull affect (β = .29, B = 1.88, SE = .47, p < .001) and to peer rejection (β = .38, B = 1.03, SE = .21, p < .001). Child’s dull affect at age 5 was not related to peer rejection, however, peer rejection at age 10 was positively related to adolescent problem behavior (β = .31, B = .52, SE = .14, p < .001) and having deviant friends (β = .20, B = .68, SE = .29, p < .05). Thus, the significant paths that emerged in this alternate model are the significant paths shown in Figure 2.
Sex differences in model paths
The omnibus chi-square difference test was nonsignificant for the multiple group analyses comparing model paths for males and females (χ2 [36] = 44.43, p > .10), indicating that the relations in the model do not differ by child sex.
Discussion
The findings of this study provide evidence of functional isolation among children who were iron-deficient anemic in infancy. Specifically, infant iron-deficiency anemia was found to be associated with children’s dull socioemotional expressiveness and social reticence at age 5, which were related to mothers’ lower nurturance and stimulation. Mothers’ low nurturance and stimulation, in turn, predicted children’s subsequent peer rejection, which linked to children’s problem behaviors and association with deviant peers in adolescence.
These findings extend earlier research in several ways. First, results confirm that functional isolation processes occur among children who were iron-deficient anemic in infancy. Functional isolation has been largely studied among critically malnourished infants (Brown & Pollitt, 1996; Levitsky & Strupp, 1995; Wachs, 2009). Yet, both malnourished infants and iron-deficient anemic infants show similar aberrant behaviors of infrequent vocalizations and less engagement and exploration of their environments (Black et al., 2004; Lozoff et al., 1998, 2007). The current findings confirm that functional isolation can occur among children who, other than their iron deficiency, were healthy as infants and not malnourished.
Second, although earlier studies have noted low responsiveness in mothers of iron-deficient infants (Armony-Sivan et al., 2010; Corapci et al., 2006; Lozoff et al., 1986, 1998), the current results highlight the key role of children’s affective dullness and social reticence as important behavioral mediators linking infant iron-deficiency anemia to later maternal unresponsiveness. Results also showed linear relations between increasing severity of iron deficiency and higher levels of children’s dull affect and social reticence, and between higher levels of children’s dull affect-social reticence and mothers’ greater unresponsiveness. Thus, these findings confirm the process outlined in functional isolation and illustrate a developmental sequence by which infant iron deficiency-anemia is associated with behaviors that reduce children’s engagement with, and receipt of input from, the environment.
Third, the current findings indicate that the limited interactions observed between mothers and their iron-deficient infants (Armony-Sivan et al., 2010; Lozoff et al., 1998) continue into early childhood and are associated with long-lasting adverse effects. The current findings add to the literature, then, by showing that maternal unresponsiveness may be rooted in their child’s early-life nutritional deficiency, can last into early childhood, and can adversely affect their child’s future social functioning and integration.
The findings related to the comparisons among the three iron status groups indicated that children who were iron-deficient anemic as infants had deficits in affective expressiveness at 5 years compared to those who had been iron sufficient (Fig. 3). This corroborates the results from several previous studies (Chang et al.,2011; Corapci et al., 2006; reviewed in Lozoff, 2011). There were, however, no differences between those who were iron sufficient in infancy and those who were iron deficient-only (without anemia) in terms of their affective dullness at 5 years. Only a few studies have found effects associated with iron deficiency without anemia. A recent review of studies concluded that the evidence is suggestive but limited with regard to impaired social-affective functioning associated with iron deficiency alone (McCann & Ames, 2007). It may be that more subtle effects occur with iron deficiency only, which could explain the weak and inconclusive findings (McCann & Ames, 2007).
Finally, study findings confirm earlier research that shows that childhood peer rejection is associated with later deviant friends, delinquent behaviors, and substance use (Dodge et al., 2009; Kupersmidt & Coie, 1990; Snyder et al., 2005). Previous studies show that children who lack critical social skills and are largely rejected from normative social cliques are more likely to associate with deviant peers, with substance use and delinquent acts a means of strengthening ties with such peers (Monahan et al., 2009). Because deviant friendships and problem behaviors were measured concurrently, we cannot establish the direction of effects. Nevertheless, having deviant friends and engaging in problem behaviors were highly correlated and, thus, could possibly be reinforcing each other (Dodge et al., 2009; Snyder et al., 2005).
Limitations and Strengths
Certain study limitations are important for interpreting the findings. For instance, iron deficiency and iron-deficiency anemia may adversely affect children’s cognitive and motor development (Georgieff, 2011; Lozoff, 2007). Indirect effects through these mechanisms could be at work in producing poor child and adolescent adjustment and should be considered in future work. In addition, while our sample was lower middle income rather than abject poverty, it is possible that early iron deficiency was associated with unmeasured disadvantaged circumstances (Lozoff, 2007). Although we statistically controlled for many family, home, and child and adolescent characteristics in attempts to adjust for these factors, unmeasured features in the environments of formerly anemic children could contribute to their poorer outcomes. For example, an inadequate diet, food insecurity, and not attending preschool are risk factors know to be associated with iron deficiency and could contribute to the poor outcomes of formerly iron deficient children (Brotanek et al., 2007; Park et al., 2009). Another limitation is that iron status was not determined for most mothers. It is possible that maternal ID or IDA contributed to their unresponsiveness. This possibility should be considered in future studies.
Important strengths of the current study are its use of multiple-wave longitudinal data, the specificity with which iron deficiency and iron-deficiency anemia were assessed, the use of observer-, parent-, and youth-reports in the assessment of study variables, the large sample, and good retention rates. It is also noteworthy that all study children were healthy as newborns. Thus, there were no neonatal health problems confounding infants’ health status. Almost all child participants also had good iron status levels in childhood, making it unlikely that chronic iron deficiency and/or anemia contributed to adolescent outcomes.
Another strength is our consideration of an alternate ordering of effects. The results did not support an alternate ordering, strengthening interpretation about the direction of effects. Specifically, it appears that infant iron deficiency severity does not lead directly to mothers’ low responsiveness but, rather, predicts mothers’ unresponsiveness by its association with children’s affective dullness. Similarly, children’s dull affect at age 5 was not directly related to peer rejection in middle childhood but, rather, children’s dull affect predicted subsequent peer rejection through its association with mothers’ low responsiveness and stimulation.
Modeling analyses also incorporated key covariates on all variables. Earlier studies that found that the mothers of iron-deficient anemic infants were less expressive did not rule out the possibility that these mothers were also depressed (Lozoff et al., 1986, 1998, 2007). Statistically controlling for mothers’ depressive symptoms in the current study (as well as controlling for numerous other family and home characteristics) increases confidence in the relations found
Conclusion and Future Directions
The findings of this study provide an understanding of the processes by which iron-deficiency anemia in infancy constitutes an early health insult, which has cascading effects that give rise to “downstream” problems, notably social difficulties, substance use, and delinquent-like behavior. Iron-deficiency anemia has been consistently linked to altered child affect, energy and activity, but little is known about how these aberrations affect later development or adjustment in other contexts. For example, it would be informative to study formerly iron-deficient children’s interactions with peers and teachers in the school setting. Do these individuals also provide less stimulation and responsivity? Educators who serve children who were anemic during infancy might benefit from knowing about their reticent behavioral tendencies and might provide social skill interventions to maximize these children’s integration in the school-peer context. In addition, home-based interventions aimed at fostering responsive and stimulating parenting throughout early childhood would also likely be beneficial (Landry, Smith, Swank, & Guttentag, 2008; Lozoff et al., 2010). On a broad scale, it is important to continue primary prevention efforts to reduce the high worldwide prevalence of iron deficiency. The persistence of negative outcomes derived from infant iron-deficiency anemia found here highlights the need for prevention, screening, and treatment to lessen the long-term effects of this pervasive nutrient disorder.
Contributor Information
Patricia East, University of California, San Diego.
Betsy Lozoff, University of Michigan.
Estela Blanco, University of California, San Diego.
Erin Delker, University of California, San Diego.
Jorge Delva, University of Michigan.
Pamela Encina, University of Chile.
Sheila Gahagan, University of California, San Diego.
References
- Achenbach TM. Manual for the youth self-report and 1991 profile. Burlington, VT: University of Vermont; 1991. [Google Scholar]
- Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatrics in Review. 2000;21:265–271. doi: 10.1542/pir.21-8-265. [DOI] [PubMed] [Google Scholar]
- Akman M, Cebeci D, Okur V, Angin H, Abali O, Akman AC. The effects of iron deficiency on infants’ developmental test performance. Acta Paediatr. 2004;93:1391–96. doi: 10.1080/08035250410030946. [DOI] [PubMed] [Google Scholar]
- Armony-Sivan R, Kaplan-Estrin M, Jacobson SW, Lozoff B. Iron-deficiency anemia (IDA) in infancy and mother-infant interaction during feeding. Journal of Developmental and Behavioral Pediatrics. 2010;31:326–32. doi: 10.1097/DBP.-0b013e3181dc525d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RD, Greer FR, The Committee on Nutrition Clinical report: Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age) Pediatrics. 2010;126:1040–1050. doi: 10.1542/peds.2010-2576. [DOI] [PubMed] [Google Scholar]
- Bandalos DL, Finney SJ. Item parceling issues in structural equation modeling. In: Marcoulides GA, Schumacker RE, editors. Advanced structural equation modeling: New developments and techniques. Mahwah, NJ: Erlbaum; 2001. pp. 269–296. [Google Scholar]
- Barozet E, Fierro J. The middle class in Chile: The characteristics and evolution 1990–2011. KAS International Reports. 2011:25–41. [Google Scholar]
- Bates JE, Freeland CB, Lounsbury ML. Measurement of infant difficultness. Child Development. 1979;50:794–803. doi: 10.2307/1128946. [DOI] [PubMed] [Google Scholar]
- Beard J. Recent evidence from human and animal studies regarding iron status and infant development. Journal of Nutrition. 2007;137:524S–530S. doi: 10.1093/jn/137.2.524S. [DOI] [PubMed] [Google Scholar]
- Black MM, Baqui AH, Zaman K, Persson LA, Arifeen SE, Le K, …Black RE. Iron and zinc supplementation promotes motor development and exploratory behavior among Bangladeshi infants. American Journal of Clinical Nutrition. 2004;80:903–10. doi: 10.1093/ajcn/80.4.903. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF, Whiteside-Mansell L. Life at home: Same time, different places – An examination of the Home Inventory in different cultures. Early Development and Parenting. 1996;5:251–269. doi:10.1002/(SICI)1099-0917(199612)5:4<251-::AID-EDP137> 3.0.CO;2. [Google Scholar]
- Bradley RH, Mundfrom DJ, Whiteside L, Casey PH, Barrett K. A factor analytic study of the infant-toddler and early childhood versions of the HOME Inventory administered to White, Black, and Hispanic American parents of children born preterm. Child Development. 1994;65:880–888. doi: 10.2307/1131425. [DOI] [PubMed] [Google Scholar]
- Brotanek JM, Gosz J, Weitzman M, Flores G. Iron deficiency in early childhood in the United States: Risk factors and racial/ethnic disparities. Pediatrics. 2007;120:568–575. doi: 10.1542/peds.2007-0572. [DOI] [PubMed] [Google Scholar]
- Brown JL, Pollitt E. Malnutrition, poverty and intellectual development. Scientific American. 1996;274:38–43. doi: 10.1038/scientificamerican0296-38. [DOI] [PubMed] [Google Scholar]
- Carter RC, Jacobson JL, Burden MJ, Armony-Sivan R, Dodge NC, Angelilli ML, Lozoff B, Jacobson SW. Iron deficiency anemia and cognitive function in infancy. Pediatrics. 2010;126:e427–e434. doi: 10.1542/peds.2009-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Iron deficiency: United States, 1999–2000. Morbidity and Mortality Weekly Reports. 2002;51:897–899. [PubMed] [Google Scholar]
- Chang S, Wang L, Wang Y, Brouwer ID, Kok FJ, Lozoff B, Chen C. Iron-deficiency anemia in infancy and social emotional development in preschool-aged Chinese children. Pediatrics. 2011;127:e927–e933. doi: 10.1542/peds.2010-1659. [DOI] [PubMed] [Google Scholar]
- Clark KM, Li M, Zhu B, Liang F, Shao J, Zhang Y, Lozoff B. Breastfeeding, mixed, or formula feeding at 9 months of age and the prevalence of iron deficiency and iron deficiency anemia in two cohorts of infants in China. Journal of Pediatrics. 2017;181:56–61. doi: 10.1016/j.jpeds.2016.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corapci F, Calatroni A, Kaciroti N, Jimenez E, Lozoff B. Longitudinal evaluation of externalizing and internalizing behavior problems following iron deficiency in infancy. Journal of Pediatric Psychology. 2010;35:296–305. doi: 10.1093/jpepsy/jsp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corapci F, Radan AE, Lozoff B. Iron deficiency in infancy and mother-child interaction at 5 years. Journal of Developmental and Behavioral Pediatrics. 2006;27:371–8. doi: 10.1093/jpepsy/jsp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan PA, Cowan CP. Becoming a family: Research and intervention. In: Sigel I, Brody A, editors. Family research. Hillsdale, NJ: Erlbaum; 1990. pp. 1–51. [Google Scholar]
- Dallman PR, Reeves JD, Driggers DA, Lo YTE. Diagnosis of iron deficiency: The limitations of laboratory tests in predicting response to iron treatment in 1-year-old infants. The Journal of Pediatrics. 1981;99:376–381. doi: 10.1016/S0022-3476(81)80321-6. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Malone PS, Lansford JE, Miller S, Pettit GS, Bates JE. A dynamic cascade model of the development of substance-use onset: Early peer relations problem factors. Monographs of the Society for Research in Child Development. 2009;74:66–70. doi: 10.1111/j.1540-5834.2009.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domellof M, Lonnerdal B, Dewey KG, Cohen RJ, Rivera LL, Hernell O. Sex differences in iron status during infancy. Pediatrics. 2002;110:545–552. doi: 10.1542/peds.110.3.545. [DOI] [PubMed] [Google Scholar]
- Donovan JE, Jessor R. Structure of problem behavior in adolescence and young adulthood. Journal of Consulting and Clinical Psychology. 1985;53:890–904. doi: 10.1037/0022-006X.53.6.890. [DOI] [PubMed] [Google Scholar]
- Doom JR, Gunnar MR, Georgieff MK, Kroupina MC, Frenn K, Fuglestad AJ, Carlson SM. Beyond stimulation deprivation: Iron deficiency and cognitive deficits in post institutionalized children. Child Development. 2014;85:1805–1812. doi: 10.1111/cdev.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East P, Delker E, Lozoff B, Delva J, Castillo M, Gahagan S. Associations among infant iron deficiency, childhood emotion and attention regulation, and adolescent problem behaviors. Child Development. 2017 doi: 10.1111/cdev.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elicker J, Englund M, Sroufe LA. Predicting peer competence and peer relationships in childhood from early parent-child relationships. In: Parke R, Ladd G, editors. Family-peer relationships: Modes of linkage. New York: Routledge; 2016. pp. 77–106. [Google Scholar]
- Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutritional Review. 2011;69:S43–48. doi: 10.1111/j.1753-4887.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graffar M. A method for social classification of samples of population. Courier. 1956;6:455–459. [Google Scholar]
- Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. Journal of Nutrition. 2001;131:649S–668S. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- Gupta PM, Perrine CG, Mei Z, Scanlon KS. Iron, anemia, and iron deficiency anemia among young children in the United States. Nutrients. 2016;8(6):330–333. doi: 10.-3390/nu8060330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AB. The environment and disease: Association or causation? Proceedings of the Royal Society of Medicine. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes TH, Rahe RH. The Social Readjustment Rating Scale. Journal of Psychosomatic Research. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 3rd. New York: Guilford Press; 2011. [Google Scholar]
- Kupersmidt JB, Coie JD. Preadolescent peer status, aggression, and school adjustment as predictors of externalizing problems in adolescence. Child Development. 1990;61:1350–62. doi: 10.2307/1130747. [DOI] [PubMed] [Google Scholar]
- Landry SH, Smith KE, Swank PR, Guttentag C. A responsive parenting intervention: The optimal timing across early childhood for impacting maternal behaviors and child outcomes. Developmental Psychology. 2008;44:1335–53. doi: 10.1037/a0013030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitsky DA, Strupp BJ. Malnutrition and the brain: Changing concepts, changing concerns. Journal of Nutrition. 1995;125:2212S–20S. doi: 10.1093/jn/125.suppl_8.2212S. [DOI] [PubMed] [Google Scholar]
- Lozoff B. Iron deficiency and child development. Food Nutrition Bulletin. 2007;28:S560–71. doi: 10.1177/15648265070284S409. [DOI] [PubMed] [Google Scholar]
- Lozoff B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. Journal of Nutrition. 2011;141:740S–46S. doi: 10.3945/jn.110.131169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Castillo M, Clark KM, Smith JB, Sturza J. Iron supplementation in infancy contributes to more adaptive behavior at 10 years of age. Journal of Nutrition. 2014;144:838–845. doi: 10.3945/jn.113.182048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Clark KM, Jing Y, Armony-Sivon R, Angelilli ML, Jacobson SW. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. Journal of Pediatrics. 2008;152:696–702. doi: 10.1016/j.jpeds.-2007.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Corapci F, Burden MJ, Kaciroti N, Angulo-Barroso R, Sazawa S, Black M. Preschool-aged children with iron deficiency anemia show altered affect and behavior. Journal of Nutrition. 2007;137:683–689. doi: 10.1093/jn/137.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, De Andraca I, Castillo M, Smith J, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112:846–854. [PubMed] [Google Scholar]
- Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf A. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51–E61. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Jimenez E, Wolf A. Long-term developmental outcome of infants with iron deficiency. New England Journal of Medicine. 1991;325:687–94. doi: 10.1056/-NEJM199109053251004. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME. Behavior of infants with iron-deficiency anemia. Child Development. 1998;69:24–36. doi: 10.2307/1132067. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Klein NK, Prabucki KM. Iron-deficient anemic infants at play. Journal of Developmental and Behavioral Pediatrics. 1986;7:152–158. doi: 10.1097/00004703-198606000-00004. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Smith JB, Clark KM, Perales CG, Rivera F, Castillo M. Home intervention improves cognitive and social-emotional scores in iron-deficient anemic infants. Pediatrics. 2010;126:e884–e894. doi: 10.1542/peds.2009-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, Cicchetti D. Developmental cascades. Development and Psychopathology. 2010;22:491–495. doi: 10.1017/S0954579410000_222. [DOI] [PubMed] [Google Scholar]
- McCann JC, Ames BN. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. American Journal of Clinical Nutrition. 2007;85:931–45. doi: 10.1093/ajcn/85.4.931. [DOI] [PubMed] [Google Scholar]
- Meyers LS, Gamst G, Guarino AJ. Applied multivariate research: Design and interpretation. Thousand Oaks, CA: Sage Publications; 2006. [Google Scholar]
- Monahan KC, Steinberg L, Cauffman E. Affiliation with antisocial peers, susceptibility to peer influence, and antisocial behavior during the transition to adulthood. Developmental Psychology. 2009;45(6):1520–1530. doi: 10.1037/a0017417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén BO. Applications of causally defined direct and indirect effects in mediation analysis using SEM in MPlus. Los Angeles, CA: Author; 2011. [Google Scholar]
- Muthén LK, Muthén BO. 6th. Los Angeles: Authors; 2010. Mplus user’s guide. [Google Scholar]
- Park K, Kersey M, Geppert J, Story M, Cutts D, Himes JH. Household food insecurity is a risk factor for iron-deficiency anaemia in a multi-ethnic, low-income sample of infants and toddlers. Public Health Nutrition. 2009;12:2120–28. doi: 10.1017/-S1368980009005540. [DOI] [PubMed] [Google Scholar]
- Parke RD, Ladd GW, editors. Family-peer relationships: Modes of linkage. New York: Routledge; 2016. [Google Scholar]
- Parker J, Rubin K, Price J, de Rosier M. Peer relationships, child development, and adjustment. In: Cicchetti D, Cohen D, editors. Developmental psychopathology. Vol. 2. New York: Wiley; 1995. pp. 96–161. Risk, disorder, and adaptation. [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. doi: 10.1177/-014662167700100306. [DOI] [Google Scholar]
- Snyder J, Schrepferman L, Oeser J, Patterson G, Stoolmiller M, Johnson K, Snyder A. Deviancy training and association with deviant peers in young children: Occurrence and contribution to early-onset conduct problems. Development and Psychopathology. 2005;17:397–413. doi: 10.1017/s0954579405050194. doi: 10.10170S0954579405050194. [DOI] [PubMed] [Google Scholar]
- Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, Ezzati M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet: Global Health. 2013;1:e16–e25. doi: 10.1016/S2214-109X(13)70001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachs TD. Models linking nutritional deficiencies to maternal and child mental health. American Journal of Clinical Nutrition. 2009;89:935S–39S. doi: 10.3945/ajcn.2008.26692B. [DOI] [PubMed] [Google Scholar]
- Wachs TD, Pollitt E, Cueto S, Jacoby E, Creed-Kanashiro H. Relation of neonatal iron status to individual variability in neonatal temperament. Developmental Psychobiology. 2005;46:141–53. doi: 10.1002/dev.20049. [DOI] [PubMed] [Google Scholar]
- Walter T, Pino P, Pizarro F, Lozoff B. Prevention of iron-deficiency anemia: Comparison of high- and low-iron formulas in term healthy infants after six months of life. Journal of Pediatrics. 1998;132:635–640. doi: 10.1016/S0022-3476(98)70352-X. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Manual for the Wechsler Adult Intelligence Scale. Oxford, England: Psychological Corp; 1955. [Google Scholar]
- World Health Organization. Global School-based Student Health Survey Chile: 2013 Fact Sheet. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:511–20. doi: 10.1016/s01406736(07)61235-5. [DOI] [PubMed] [Google Scholar]


