Abstract
Tissue composition impacts the interpretation of magnetic resonance spectroscopy metabolite quantification. The goal of applying tissue correction is to decrease the dependency of metabolite concentrations on the underlying voxel tissue composition. Tissue correction strategies have different underlying assumptions to account for different aspects of the voxel tissue fraction. The most common tissue correction is the CSF-correction that aims to account for the cerebrospinal fluid (CSF) fraction in the voxel, in which it is assumed there are no metabolites. More recently, the α-correction was introduced to account for the different concentrations of GABA+ in gray matter and white matter. In this paper, we show that the selected tissue correction strategy can alter the interpretation of results using data from a healthy aging cohort with GABA+ measurements in a frontal and posterior voxel. In a frontal voxel, we show an age-related decline in GABA+ when either no tissue correction (R2= 0.25, p < 0.001) or the CSF-correction is applied (R2= 0.08, p < 0.01). When applying the α-correction to the frontal voxel data, we find no relationship between age and GABA+ (R2= 0.02, p = 0.15). However, with the α-correction we still find that cognitive performance is correlated with GABA+ (R2= 0.11, p < 0.01). These data suggest that in healthy aging, while there is normal atrophy in the frontal voxel, GABA+ in the remaining tissue is not decreasing on average. This indicates that the selection of tissue correction can significantly impact the interpretation of MRS results.
Keywords: α-correction, aging, atrophy, CSF-correction, GABA, GABA-edited MRS, gray matter, tissue-correction, white matter
1. Introduction
As the primary inhibitory neurotransmitter in the human brain, γ-Aminobutyric acid (GABA) has a fundamental role in shaping cortical activity as well as in plasticity and in cognitive function. Measurement of GABA in vivo, in humans is possible using specialized magnetic resonance spectroscopy (MRS) techniques; in this study we apply GABA-edited MEGA-PRESS (1). Due to limitations with the method, measurements of GABA are contaminated with macromolecules. Consistent with conventions in the literature, in the current manuscript, when referring to MRS measures of GABA, we will use GABA+ to represent GABA+macromolecules. Previous MRS studies have shown that GABA+ levels in the brain decline with age. Specifically, GABA+/Cr levels in frontal and parietal brain regions have been shown to decrease with age (2). This finding was subsequently replicated in comparable voxel locations (3) and was associated with age-related changes in cognitive performance. Cognitive performance was strongly correlated with frontal GABA+ concentrations, even after accounting for age, the fraction of cerebral spinal fluid (CSF) in the MRS voxel (a coarse measure of brain atrophy) and behavioral factors such as education or age related normative performance. This is consistent with prior animal work showing age-related declines in GABA+ (4) and a cross-sectional study observing lower GABA+ concentrations in the right hippocampus in healthy elderly controls compared to young controls, though no differences were observed in the anterior cingulate (5).
While the majority of age-related atrophy research has been in the context of specific pathologies (e.g. Alzheimer’s dementia), age-related atrophy will affect many people as part of the normal aging process. It is well established that even with healthy aging, brain volume declines with age (6–9). Age-related brain volume loss is not equal between tissue types (i.e., white or gray matter) and the age at which tissue volume begins to decrease also varies (10–13). Furthermore, the rate of tissue atrophy during healthy aging varies regionally, with frontal and medial temporal atrophy occurring at a more rapid rate than parietal and occipital regions (10–12). How the heterogeneity of white and gray matter atrophy impacts cognitive decline is unclear (7). The impact of this dynamic, regionally variant atrophy necessitates a sophisticated consideration of voxel tissue composition to ensure that the age-related GABA+ changes are not unduly impacted by the tissue composition of the measurement voxel (14). The current study implements and compares different tissue corrections for GABA+ in the context of healthy aging. Specifically, the most commonly used approach, the CSF-correction, a more recently proposed tissue correction, here referred to as the α-correction (14), and no tissue correction are compared.
Tissue composition of MRS voxels has a significant impact on metabolite quantification (14–16), as metabolite levels (and reference signals) differ between tissue and CSF, but also between gray and white matter. Tissue correction is applied to account for the differences in signal between different tissues within a voxel; however both the use of the term and implementation of the method are inconsistently applied. In the simplest form, tissue correction refers to accounting for the CSF fraction of the voxel (CSF-correction) and is applied by normalizing the voxel by the non-CSF voxel fraction (1-fCSF), with the implicit assumption that the metabolites in question are not contained in CSF or, if present, do not contribute to the MRS signal. More sophisticated tissue corrections include accounting for the differential relaxation constants and water visibility between white matter, gray matter and CSF (14–16). In addition to the differences in water signal between white matter, gray matter and CSF, GABA+-MRS measurements are further complicated by the differences in GABA+ between tissue types. It is well established that the concentration of GABA+ in gray matter is greater than in white matter (14, 17–21). Recently, Harris et al. (14) proposed a tissue correction strategy to account for the different concentrations of GABA+ in gray matter and white matter in addition to accounting for differential tissue relaxations and signal constants to better address the underlying dependency of GABA+ on the tissue composition of the voxel.
The primary goal of tissue-correction is to remove the dependency of metabolite measurements, in this case GABA+, on the voxel tissue composition. To most fully address the issues associated with the dependency of measures on underlying tissue composition, the correction needs to accounts for differences in GABA+ concentration between white matter and gray matter (accomplished by applying the constant α) and normalize GABA+ values to a standard voxel composition (in this case, we chose the group-average voxel composition of white matter and gray matter). In the current manuscript, we refer to this entire procedure (correction and normalization) as the α-correction. As shown by Harris et al (14), equation 3, the correction is:
where ccorr is the tissue-corrected GABA+ level, cmeas is the measured GABA+ level that accounts for individual tissue T1, T2 and water visibility constants, μGM and μwm are the group average voxel fractions for gray and white matter, fGM and fWM are the individual voxel fractions of gray matter and white matter and α is the ratio of GABA+ in white matter to gray matter, which is assumed to be 0.5. This correction accounts for the for the fact that GABA+ in gray matter is twice that of white matter and normalizes the GABA+ measurement to a standardized voxel, in this case it is the group average voxel fractions of white matter and gray matter. The normalization of the voxel fractions to a standard voxel is similar to registration or normalization of T1-weighted anatomical images in imaging studies. It is common to register and warp an individual’s T1-weighted anatomical image to a group template (as per the FSL-VBM tools) or standard space (e.g., MNI or Talairach space) for a structural imaging analysis. The process of this registration impacts the absolute volume of measurements of each anatomical structure for each individual; however, it enables direct comparisons of structural volumes because there is a normalized baseline. Similarly, selecting a normalized voxel composition of white matter and gray matter enables direct comparison GABA+ measures between individuals. The selection of the group-average voxel is based on the desire to normalize the GABA+ measurements to a voxel that is representative of the group and accurately reflects these data. This approach and selection of normalization voxel has been used previously (14, 22). As discussed by Harris et al (14), the selection of the voxel fractions for normalization may vary depending on the study; for example, if two groups with different structural changes are being compared, it may be appropriate to use the control group to determine the voxel for normalization. For the present study, the group-average voxel fractions was selected as the normalized voxel that best reflects the population. In absence of normalization to a group-average voxel, but applying the α-ratio in the tissue correction (i.e., applying the first correction term but not the last in the above equation), GABA is corrected to a voxel of pure gray matter. While this may be useful in some cases, the reported GABA values will be inflated and do not represent the tissue examined. For simplicity and to maintain consistency with the literature, we have used the term α-correction to refer to the procedure including: the correction accounting for the CSF-fraction, the difference in GABA concentration between gray matter and white matter (α), and inclusion of all tissue specific relaxation constants; and the normalization procedure. It is important to note that the normalization of GABA levels to the group-average voxel is a normalization procedure in addition what is more conventionally thought of as tissue correction (i.e., the CSF-correction or the GM-correction) that accounts for the gross tissue composition of the voxel used across the sample.
In this paper, we investigate the impact of applying the different tissue corrections to GABA+-MRS data in a large, healthy-aging cohort. Given the expectation that tissue volume declines with age, this cohort provides a unique opportunity to examine the impact of different tissue corrections on the interpretation of GABA+-MRS data. We compare the most commonly applied CSF-based tissue correction with a more sophisticated tissue correction, the α-correction, that accounts for differences in signal and GABA+ concentration between white and gray matter. Two voxel locations are included in this study, a frontal voxel and a posterior voxel. To investigate the relationship between GABA+ levels and functional processes, the Montreal Cognitive Assessment (MoCA) was used as an easily administered test of cognitive performance that is appropriate for this cohort (23). This assessment evaluates cognitive performance across multiple cognitive domains including: visuospatial ability, executive function, attention and concentration, memory, language, and time and space orientation (23). The total MoCA score is widely used and has strong psychometric properties including consistency and reliability. In particular, MoCA includes items that are sensitive to dysfunction in frontal brain regions, and performance on these tasks often declines with age. We show that the selection of tissue correction strategy can impact the results; specifically, we show differences in the age-GABA+ and MoCA-GABA+ relationships depending on the voxel of interest and the tissue-correction strategy. These results provide support for the application of the α-correction and evidence that previous reports, including our own previous results, may underestimate the influence of age-related atrophy when reporting age-related GABA+ decreases.
2. Methods
2.1 Participants
Ethical approval was obtained from the University of Florida Institutional Review Board and all participants provided signed, informed consent. Some of these data presented here were previously reported addressing a different research question and objective (3).
Ninety-three participants aged 40 and above were recruited from the local community; subjects were free from neurological and psychiatric disease as established through self-reports on extensive medical questionnaires and forms. Through these questionnaires, participants exhibiting potential dementia and cognitive impairments symptoms were identified and excluded. Participants were asked to abstain from alcohol on the day of data collection.
2.2 Cognitive Assessment
A cognitive assessment was performed using the standardized administration of the MoCA. Participants with a MoCA score <20 were not enrolled in this study. This assessment reports on cognitive performance by investigating multiple cognitive domains (23). The total MoCA score is widely used and has strong psychometric properties (e.g., consistency, reliability). Employing a threshold of ≤25 the MoCA (24–26) been shown to be more sensitive to mild cognitive impairment as well and other varieties of cognitive decline, including Korsakoff’s syndrome, than the mini-mental state exam (MMSE) (27).
2.3 MRS
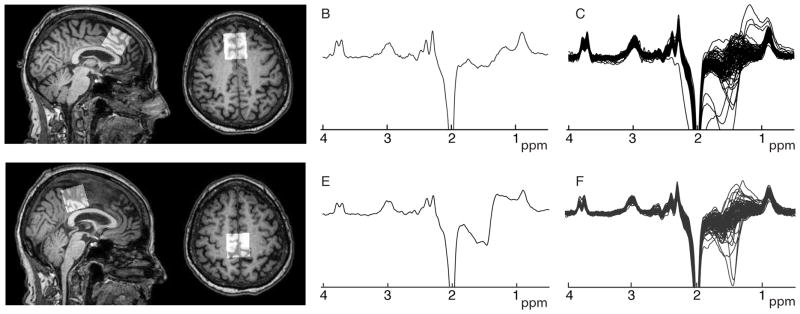
MR data were collected using a 3T Philips Achieva Scanner with a 32-channel head coil. Anatomical images were collected for MRS voxel placement and segmentation using a 3D, T1-weighted, magnetization-prepared rapid gradient-echo (MP-RAGE) acquisition, prescribed in the sagittal plane, repetition time = 8 ms, echo time = 3.7 ms, 1-mm3 isotropic voxels. A standard GABA+-edited MEGA-PRESS acquisition (1) was used (TR/TE = 2s/68 ms, 14 ms editing pulses placed at 1.9 ppm in the “ON” condition and at 7.46 ppm in the “OFF” condition, 320 averages, 2048 data points at 2 kHz sampling rate, 3×3×3 cm3 voxels, VAPOR water suppression and 8 transients of unsuppressed water data for water-referenced GABA+ quantification. Measurements were acquired in mid-sagittal frontal and posterior voxels (locations shown in Figure 1). The frontal voxel was placed superior to the genu of the corpus callosum in the frontal lobe and the posterior voxel was placed in the parietal lobe, aligned with the parieto-occiptal sulcus. Both voxels were centered on the midline.
Figure 1.
Frontal and posterior voxel placement (A and D), a single sample spectrum (B and E) and all spectra (C and F) for each region.
Data were processed using Gannet2 (14, 28) with integrated voxel registration to the T1-weighted image and voxel segmentation, which was performed using SPM 12 (29). While other segmentation tools are available, SPM is widely used and accepted. Furthermore, SPM is Matlab-based and has been integrated with the Gannet Toolkit, which is also Matlab-based. GABA+ data were water-referenced for quantification. In this manuscript, we compare GABA+ levels with no tissue correction, the conventional CSF-correction and the more sophisticated tissue correction in which application of tissue-specific T1, T2 and water-visibility constants, α-correction (α = 0.5; i.e., corresponding to the concentration of GABA+ in gray matter being twice that of white matter as per (14)) and tissue normalization were applied. Tissue constants were: T1 of white matter = 832 ms, T2 of white matter = 79.2 ms, T1 of gray matter = 1331 ms and T2 of gray matter = 110 ms (30), T1 of CSF = 3817 (31) and T2 of CSF = 503 ms (32). Applying the α-correction factor accounts for the differences in GABA+ concentration between white matter and gray matter. Therefore, reported GABA+ levels are less dependent on the underlying voxel tissue composition compared to uncorrected data or CSF− or GM− correction (normalizing by the voxel fraction of gray matter alone (33)) approaches (14).
2.4 Statistical Analysis
All analyses were performed for both the frontal and posterior voxels. In order to understand the changes in voxel content as measured across the aging cohort, a regression analysis of the tissue fractions (gray matter, white matter and CSF) and age was performed first. Multiple linear regression analyses were then performed to investigate the impact of the tissue correction strategy – either uncorrected, CSF-corrected or α-corrected – as applied to (1) the relationship of GABA+ and age and (2) the relationship between GABA+ and cognitive performance on the MoCA. A follow-up, more complex model was applied to include age and education in addition to GABA+ as factors impacting MoCA performance.
3. Results
Demographics of the 93 recruited participants are shown in Table 1. Six frontal voxel data sets and 4 posterior voxel data sets were excluded due to poor spectral data quality resulting from gross movement or missing MRS data (could not be collected due to scanner time constraints).
Table 1.
Participant Demographics
| Gender | N | Percentage | |
|---|---|---|---|
| Male | 39 | 42 | |
| Female | 54 | 58 | |
| Variable | N | Range | Mean±SD |
| Age | 93 | 44–92 | 73.2±9.9 |
| MoCA | 93 | 20–30 | 25.5±2.5 |
| Years of Education | 93 | 12–20 | 16.3±2.8 |
3.1 Age-voxel tissue fraction relationship
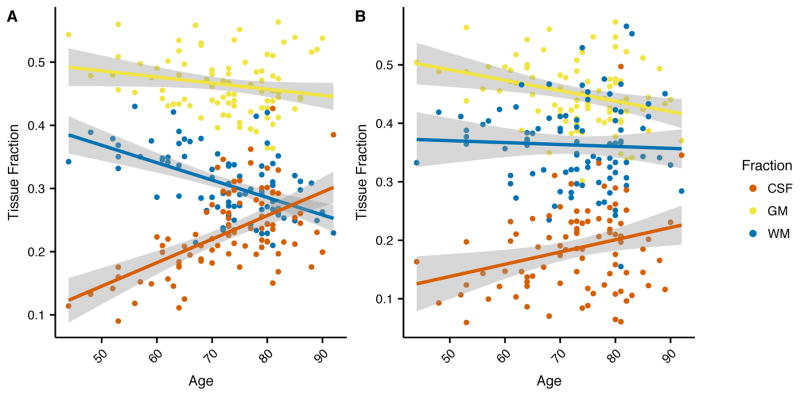
Across age, there was a significant increase in the CSF fraction in both the frontal (R2 = 0.32, p < 0.001) and posterior (R2 = 0.077, p < 0.01) voxels (Figure 2). However, the relationship between age and CSF fraction differed between the two voxels (Fischer z = 2.12, p < 0.05). The gray matter decrease across age did not differ between the two voxels (Fischer z = 0.75, p = 0.45) while the white matter decrease across the aging cohort was much greater in the frontal voxel than in the posterior voxel (Fischer z = −3.47, p < 0.001), indicating age-related atrophy occurs at different rates across the brain.
Figure 2.
Relationship between voxel tissue fractions for gray matter (yellow), white matter (blue) and CSF (red) across age for the (a) frontal and (b) posterior voxels.
3.2 Age-GABA+ relationship
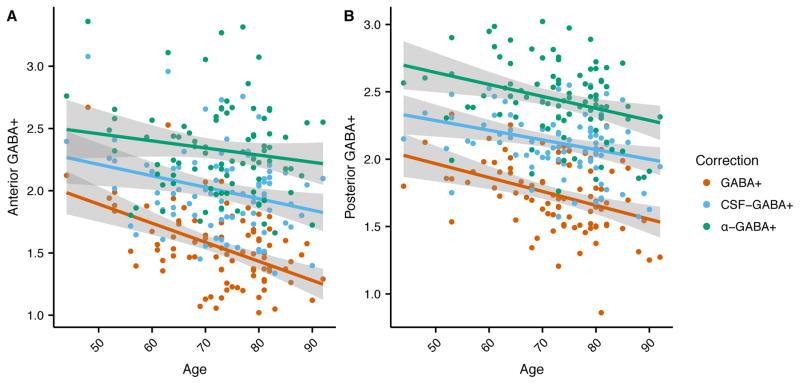
In the frontal voxel (Figure 3a), there was a significant relationship between age and uncorrected GABA+ (R2 = 0.25, F[1, 85] = 28.46, p < 0.001) and CSF-corrected GABA+ (R2 = 0.08, F[1, 85] = 7.02, p < 0.01). In contrast, the α-corrected GABA+ level was not significantly related to age (R2 = 0.02, F[1, 85] = 2.10, p = 0.15). In the posterior voxel (Figure 3b), the uncorrected GABA+ level was significantly associated with age (R2 = 0.15, F[1, 87] = 15.06, p < 0.001), as was the CSF-corrected GABA+ level (R2 = 0.09, F[1, 87] = 8.96, p < 0.01), and α-corrected GABA+ level (R2 = 0.09, F[1, 87] = 9.06, p < 0.01).
Figure 3.
Relationship between GABA+ and age for the (a) frontal and (b) posterior voxels using no tissue correction (red), CSF-correction (blue) and α-correction (green).
3.3 Cognitive performance as a function of tissue corrected GABA+
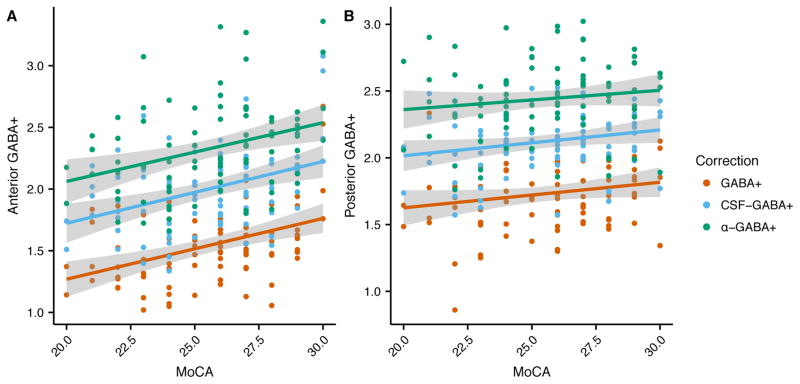
In the frontal voxel (Figure 4a), there was a significant relationship between MoCA and uncorrected GABA+ (R2 = 0.17, F[1, 85] = 17.84, p < 0.001), CSF-corrected GABA+ (R2 = 0.15, F[1, 85] = 15.11, p < 0.001), and α-corrected GABA+ (R2= 0.11, F[1, 85] = 10.72, p < 0.01).
Figure 4.
Relationship between (a) frontal and (b) posterior GABA+ and MoCA for the uncorrected (red) CSF-corrected (blue) and α-corrected (green) GABA+ levels.
The relationship between GABA+ and MoCA was then examined controlling for age and education. For uncorrected GABA+, a significant amount of the variance in cognitive performance (R2 = 0.20, F[3, 83] = 7.01, p < 0.001) was associated with GABA+ when accounting for age and education. Within this model, higher GABA+ significantly predicted better MoCA score (B = 3.01, p < 0.01), when accounting for age, and years of education, (B = −0.04, p = 0.19; B = 0.11, p = 0.24, respectively). For the CSF-corrected GABA+ and MoCA, results of the multiple regression analysis indicated that the three predictors accounted for a significant amount of the variance in cognitive performance (R2 = 0.21, F[3, 83] = 7.28, p < 0.001). Within this model, higher CSF-corrected GABA+ significantly predicted a better MoCA score (B = 2.56, p < 0.01), when accounting for the age, and years of education, (B = −0.061, p > 0.5; B = 0.10, p = 0.29, respectively). Finally, there was a significant relationship between α-corrected GABA+ and MoCA scores, again controlling for age and education (R2 = 0.19, F[3, 83] = 6.55, p < 0.001). Similarly, α-corrected GABA+ was related to MoCA (B = 2.05, p < 0.01) controlling for age and education (B = −0.07, p < 0.01; B = 0.08, p = 0.35, respectively).
In the posterior voxel (Figure 4b), uncorrected GABA+ level was not associated with MoCA score (R2 = 0.03, F[1, 87] = 3.10, p = 0.08). However, MoCA was related to CSF-corrected GABA+ level (R2 = 0.046, F[1, 87] = 4.178, p < 0.05), albeit a weak relationship, but not related to α-corrected GABA+ level (R2 = 0.02, F[1, 87] = 1.50, p = 0.22). When controlling for age and education, there was no relationship between uncorrected, CSF-corrected or α-corrected GABA+ and MoCA.
4. Discussion
This study evaluates the impact of GABA-tissue correction methods comparing no tissue correction, the most common CSF-correction approach, and a more recently proposed α-correction. The results of this study, especially considered in combination, reveal differences in the relationship between atrophy, GABA+ levels across the two voxels, and the relationship between MoCA and GABA+. The comparison between the physiological interpretation of our data implied by various tissue correction approaches supports application of the α-correction approach for tissue correction to minimize the influence of voxel composition on GABA+ measurements.
4.1 Tissue Correction Approaches: Assumptions, Limitations and Benefits
The debate as to the best approach for tissue-correction in the MRS community is ongoing. While most would agree that tissue correction is useful and improves data interpretations, a consensus on the selection of appropriate tissue correction methods is lacking.
The most common tissue correction is the CSF-correction, in which the metabolite concentration is inflated by the fraction of CSF within the voxel. It is applied because CSF does not typically contain the metabolites of interest. The CSF-correction therefore represents a voxel of pure tissue for the reported metabolite concentrations. While many MRS users realize that metabolite concentrations often vary between tissues, the CSF-correction itself does not account for these differences. It is therefore generally recognized that varying voxel fractions of gray and white matter confounds and complicates interpretation. With the α-correction, the difference in GABA+ between gray matter and white matter is included in the correction. While reported ratios of white matter to gray matter concentrations of GABA+ vary (17–21), the mean of this literature is consistent with recent work (14) that indicates there is approximately twice as much GABA+ in gray matter compared to white matter. In this manuscript, we show that accounting for this difference and applying a complete tissue correction can fundamentally change the results and their interpretation.
It should be highlighted that there are two components of the α-correction. The first correction term (1/(fWM+αfGM)) is the component that accounts for the fact that negligible GABA+ exists in the CSF and the ratio of the GABA+ concentration in white matter to gray matter, which is accomplished by setting α = 0.5. Compared to the CSF-correction or the GM-correction, in which a dependency of the corrected GABA+ on the underlying tissue fraction remains, the application of the α-ratio aims to account for these different gray matter and white matter GABA+ concentrations which limits the measurement dependencies on the voxel tissue fraction. The second component is the normalization of the voxel to the group average (the term: (μGM+αμWM)/(μGM+μWM)). Not applying the normalization but maintaining the α-factor when correcting for the tissue and CSF voxel contributions would result in a GABA-corrected measurement that assumes the voxel is 100% gray matter. Since actual voxels are not composed of 100% gray matter, we normalize to the group average voxel to more fairly represent the GABA+ level in the selected voxel. Including this factor results in a normalized voxel concentration after correction. In the current manuscript we have combined these two components and referred to the entire process as the α-correction. Statistically, because the normalization term is a constant across subjects, the result would not differ if the group normalization term were not applied.
For the sake of transparency, we wish to note we are comparing the current manuscript with our own previous work (3). This study population enables the assessment of bulk tissue changes without comorbid confounds resulting from disease pathology that impacts metabolites and function. In our previous paper we showed a significant decline in GABA+ with age for both the frontal and posterior voxel when including the fraction of CSF in the voxel as a covariate in the analysis. The current manuscript replicates this finding with the CSF-correction. Additionally, the current manuscript evaluates a newer, less accepted tissue correction, the α-correction. In the frontal voxel, the α-corrected GABA+ produces a different result from the CSF-correction and our previous work. The α-corrected GABA+ in contrast to CSF-corrected GABA+ does not show an age-related decline in GABA+. However, and importantly, the relationship between GABA+ and cognitive function, as indexed by the MoCA, persists (described further below). We suggest that the combined findings of this manuscript support the use of α-correction for GABA+ studies because we know there are differences in GABA+ between white matter and gray matter (which is accounted within the α-correction), we have shown that the CSF-correction and the α-correction can produce different results, and the functionally relevant relationship between GABA+ and MoCA is maintained with the α-correction. As a result, this study provides new information supporting the methodological utility of the α-correction as the best approach to tissue-correct data.
A complication in MRS tissue correction is the impact of the anatomical image acquisition parameters and the segmentation tool. The T1-weighted acquisition (MP-RAGE sequence) used is typical of anatomical images used for tissue segmentation, though it is recognized that changes in acquisition parameters can change image contrast, which may impact segmentation routines. Similarly, different segmentation tools can produce different segmentation results, for example the FSL-segmentation tool FAST (34) tends to show greater CSF compared to SPM segmentations (14). This underlies the importance of consistency of acquisition and processing methods within a study, but the confound remains when comparing studies that use different acquisition parameters and segmentation tools.
An alternative approach to tissue correction is to include tissue-voxel fractions in the statistical model. Depending on the factor included, the covariate approach may be equivalent to the CSF-correction (as in our previous work, (3)) or be similar to the α-correction if a ratio of gray matter to tissue fraction is used as the covariate. However, we have a defined relationship of the concentration of GABA+ in gray and white matter and a normalization approach to enable us to apply a correction. This is desirable as tissue-corrected results can be analyzed directly without having to include a covariate, thus impacting statistical power of the analysis. Theoretically, both approaches should yield the same results but when a covariate is added to an analysis, there is a loss of power. While this study has a large sample size, this manuscript serves to support this tissue-correction approach in the context of typical MRS studies with smaller sample sizes, which often suffer from power limitations.
4.2 Interpreting GABA+ Relationships with Age and Cognitive Functions
GABA+ measurements were acquired in two locations: a frontal voxel and a posterior voxel. The frontal voxel was chosen because that tissue is highly relevant to cognitive function as assessed by the MoCA, whereas the posterior voxel’s tissue is negligibly associated with the relevant cognitive processing. This healthy aging population is expected to exhibit natural tissue atrophy, which, in this analysis, is being used to explore the impact of these different tissue correction strategies in terms of age and cognitive ability. As expected, both voxels exhibit changes in tissue composition across age: the CSF fraction increases, and the gray matter and white matter fraction decreases, albeit the white matter decrease is more pronounced in the frontal voxel (Figure 2). This pattern of atrophy is typical and expected with healthy aging (10–13).
In the posterior voxel, GABA+ showed a significant relationship with age regardless of the tissue correction strategy (Figure 3). In the frontal voxel, when applying the conventional CSF-correction, there was a significant correlation between age and GABA+ levels, but this relationship was not significant when applying the α-correction. Therefore, when accounting for the fact that there is more GABA+ in gray matter than white matter, the age-related decrease in GABA+ is no longer apparent in the frontal voxel. This indicates that the previously-reported decreases in GABA+ with age, including our own work, may in fact be due primarily to tissue loss with age, i.e., the loss of tissue results in reduced measured GABA+ levels due to the fact that there is less GABA-containing tissue in the voxel. However, within the tissue that remains, the concentration of GABA+ may not be impacted by age across the sampled population. The differentiation of the source of an observed decrease in GABA+ has tremendous impact in terms of interpreting result as well as developing future interventions for pathological progressions.
The MoCA is a composite measure of cognitive function, principally dependent upon functions that are localized in frontal brain regions. Apropos our results, α-corrected GABA+ was related with MoCA performance, so GABA+ levels within tissue are related to the function of that tissue. The consistency of a significant relationship between GABA+ and MoCA using both the CSF− and the α-corrected GABA+ methods (Figure 4) confirms that the underlying GABA+ levels, not just the maintenance of gross tissue, is important for cognitive function as well as supporting the validity of the α-correction methods. It is interesting to note that tissue correction approaches alter the contribution of age to the model predicting cognitive function in frontal regions, with age becoming a significant factor in the α-corrected GABA+ model.
By contrast, the parietal cortex, as represented by the posterior voxel, is less involved in the cognitive processes assessed by the MoCA. As such, no correlation between MoCA and GABA+ levels is expected given that functional-GABA+ relationships are usually region-specific (35). Therefore, the appearance of the correlation between CSF-corrected GABA+ levels and MoCA in the posterior voxel may be a result of an inappropriate tissue correction such that the result is driven by age-related changes in tissue structure (i.e., global atrophy). This interpretation is further supported by the lack of correlation between MoCA and α-corrected GABA+ in the posterior voxel as would be expected. This further indicates that not accounting for tissue (gray and white matter) differences may introduce an erroneous relationship due to an underlying gross tissue change rather than the functional relationship of interest.
The biochemistry underlying tissue aging and atrophy is unclear; however, the distinction between a decrease in tissue and a decrease in the metabolites within the tissue impacts the interpretation and potential targeting of interventions. Previous work has suggested some metabolites decrease with aging (36–38), though the results are inconsistent with differences in regions studied, reference signals used and tissue-correction approaches. A potential limitation of the current study is the inability to assess changes in relaxation with aging, though a recent magnetic resonance spectroscopic imaging (MRSI) study (37) did not show T2 or T2′ changes in the frontal and parietal regions studied here. A second limitation of the current study is that the GABA+ measurement here is contaminated with macromolecules (MM) due to the bandwidth of the editing pulses (1). In a study of aging of a younger population (ages 21–52 y) GABA+MM was seen to increase with age while GABA measures with MM suppression appear much more stable (39). Interpretation of these results is complicated by the difficulties of MM suppression (40), but the potential influence of MM cannot be discounted, although the mechanism mediating a relationship between MM and MoCA is unclear. A limitation of applying the α-correction is that, in addition to gross tissue changes, α itself may change with age or pathology. However, any alterations in GABA in white matter, gray matter or both will be reflected in the GABA measurement. This limitation of not being able to ascribe metabolite changes to a particular location or metabolite pool within the voxel (e.g., white matter or gray matter, synaptic or metabolic origin) is a common limitation in all spectroscopy. As discussed above, decisions as to the normalization reference need to be considered for cases of pathology or aging, etc, or comparisons between different voxels. It may be appropriate to assume a standard white and gray matter composition or assuming a completely gray matter voxel may be most appropriate.
In conclusion, this paper presents several important results. The first is that, from a methodological perspective, the selection of tissue correction approach can fundamentally impact the interpretation of GABA+ results. It is therefore important to not only select an appropriate tissue correction methodology, but also consider the impact of different tissue correction approaches when evaluating the literature. The refinement of our methods and interpretations in analyzing this data strongly supports the application of the α-correction in GABA+ studies as it accounts for tissue differences. Second, it shows that in frontal regions, where atrophy was more pronounced, while the overall tissue volume decreased with age, the concentration of GABA+ in the tissue that remains was not significantly correlated with age. Finally, even after correcting for voxel tissue composition changes, frontal GABA+ is significantly correlated with cognitive performance as assessed by MoCA in this aging population. Thus, the relationship between GABA+ and MoCA exists independent of relationships between aging and bulk tissue changes, age and cognitive decline, and age and GABA+, reinforcing the importance of inhibitory signalling in cortical function.
Acknowledgments
This research was supported in part by NIH/NIA K01AG050707, K01AA025306, and R01AG054077, the Center for Cognitive Aging and Memory at the University of Florida, the McKnight Brain Research Foundation, the University of Florida Clinical and Translational Science Institute, which is supported in part by the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS) under Award No. UL1TR001427; NIH/NCATS Clinical and Translational Science Awards Grant Nos. UL1TR000064 and 1KL2TR001429; and the Claude D. Pepper Center at the University of Florida Grant No. P30 AG028740. This study applies tools developed under NIH Grant Nos. R01 EB016089 and P41 EB015909.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harris AD, Saleh MG, Edden RA. Edited 1 H magnetic resonance spectroscopy in vivo: Methods and metabolites. Magn Reson Med. 2017 doi: 10.1002/mrm.26619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao F, Edden RA, Li M, Puts NA, Wang G, Liu C, et al. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage. 2013;78:75–82. doi: 10.1016/j.neuroimage.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porges EC, Woods AJ, Edden RAE, Puts NA, Chen H, Garcia AM, et al. Frontal GABA concentrations are associated with cognitive performance in older adults. Biological Psychiatry. 2017;2(1):38–44. doi: 10.1016/j.bpsc.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He X, Koo BB, Killiany RJ. Edited Magnetic Resonance Spectroscopy Detects an Age-Related Decline in Nonhuman Primate Brain GABA Levels. Biomed Res Int. 2016;2016:6523909. doi: 10.1155/2016/6523909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang D, Liu D, Yin J, Qian T, Shrestha S, Ni H. Glutamate-glutamine and GABA in brain of normal aged and patients with cognitive impairment. Eur Radiol. 2016 doi: 10.1007/s00330-016-4669-8. Epub prior to press. [DOI] [PubMed] [Google Scholar]
- 6.Hedman AM, van Haren NE, Schnack HG, Kahn RS, Hulshoff Pol HE. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp. 2012;33(8):1987–2002. doi: 10.1002/hbm.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Royle NA, Booth T, Valdes Hernandez MC, Penke L, Murray C, Gow AJ, et al. Estimated maximal and current brain volume predict cognitive ability in old age. Neurobiol Aging. 2013;34(12):2726–33. doi: 10.1016/j.neurobiolaging.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Shea A, Cohen RA, Porges EC, Nissim NR, Woods AJ. Cognitive Aging and the Hippocampus in Older Adults. Front Aging Neurosci. 2016;8:298. doi: 10.3389/fnagi.2016.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nissim NR, O’Shea AM, Bryant V, Porges EC, Cohen R, Woods AJ. Frontal Structural Neural Correlates of Working Memory Performance in Older Adults. Front Aging Neurosci. 2016;8:328. doi: 10.3389/fnagi.2016.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dotson VM, Szymkowicz SM, Sozda CN, Kirton JW, Green ML, O’Shea A, et al. Age Differences in Prefrontal Surface Area and Thickness in Middle Aged to Older Adults. Front Aging Neurosci. 2015;7:250. doi: 10.3389/fnagi.2015.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–89. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 12.Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, et al. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 2009;19(9):2001–12. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26(9):1261–70. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 75–8. [DOI] [PubMed] [Google Scholar]
- 14.Harris AD, Puts NA, Edden RA. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J Magn Reson Imaging. 2015;42(5):1431–40. doi: 10.1002/jmri.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, et al. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 2006;55(6):1219–26. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- 16.Gussew A, Erdtel M, Hiepe P, Rzanny R, Reichenbach JR. Absolute quantitation of brain metabolites with respect to heterogeneous tissue compositions in (1)H-MR spectroscopic volumes. MAGMA. 2012;25(5):321–33. doi: 10.1007/s10334-012-0305-z. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharyya PK, Phillips MD, Stone LA, Lowe MJ. In vivo magnetic resonance spectroscopy measurement of gray-matter and white-matter gamma-aminobutyric acid concentration in sensorimotor cortex using a motion-controlled MEGA point-resolved spectroscopy sequence. Magn Reson Imaging. 2011;29(3):374–9. doi: 10.1016/j.mri.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi C, Bhardwaj PP, Kalra S, Casault CA, Yasmin US, Allen PS, et al. Measurement of GABA and contaminants in gray and white matter in human brain in vivo. Magn Reson Med. 2007;58(1):27–33. doi: 10.1002/mrm.21275. [DOI] [PubMed] [Google Scholar]
- 19.Choi IY, Lee SP, Merkle H, Shen J. In vivo detection of gray and white matter differences in GABA concentration in the human brain. Neuroimage. 2006;33(1):85–93. doi: 10.1016/j.neuroimage.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Jensen JE, de Frederick BB, Renshaw PF. Grey and white matter GABA level differences in the human brain using two-dimensional, J-resolved spectroscopic imaging. NMR Biomed. 2005;18(8):570–6. doi: 10.1002/nbm.994. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H, Edden RA, Ouwerkerk R, Barker PB. High resolution spectroscopic imaging of GABA at 3 Tesla. Magn Reson Med. 2011;65(3):603–9. doi: 10.1002/mrm.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puts NAJ, Wodka EL, Harris AD, Crocetti D, Tommerdahl M, Mostofsky SH, et al. Reduced GABA and altered somatosensory function in children with autism spectrum disorder. Autism Res. 2017;10(4):608–19. doi: 10.1002/aur.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreat Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J Am Geriatr Soc. 2005;53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 24.Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73:1738–45. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pisani V, Moschella V, Bari M, Fezza F, Galati S, Bernardi G, et al. Dynamic changes of anandamide in the cerebrospinal fluid of Parkinson’s disease patients. Mov Disord. 2010;25(7):920–4. doi: 10.1002/mds.23014. [DOI] [PubMed] [Google Scholar]
- 26.Tan JP, Li N, Gao J, Wang LN, Zhao YM, Yu BC, et al. Optimal cutoff scores for dementia and mild cognitive impairment of the Montreal Cognitive Assessment among elderly and oldest-old Chinese population. J Alzheimers Dis. 2015;43(4):1403–12. doi: 10.3233/JAD-141278. [DOI] [PubMed] [Google Scholar]
- 27.Oudman E, Postma A, Van der Stigchel S, Appelhof B, Wijnia JW, Nijboer TC. The Montreal Cogntivie Assessment (MoCA) is superior to the Mini Mental State Examination (MMSE) in detection of Korsakoff’s syndrome. Clin Neuropsychol. 2014;28(7):1123–32. doi: 10.1080/13854046.2014.960005. [DOI] [PubMed] [Google Scholar]
- 28.Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40(6):1445–52. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Wansapura JP, Holland SK, Dunn RS, Ball WS. NMR Relaxation Times in the Human Brain at 3.0 Tesla. J Magn Reson Imaging. 1999;9:531–8. doi: 10.1002/(sici)1522-2586(199904)9:4<531::aid-jmri4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 31.Lu H, Nagae-Poetscher LM, Golay X, Lin D, Pomper M, van Zijl PC. Routine clinical brain MRI sequences for use at 3.0 Tesla. J Magn Reson Imaging. 2005;22(1):13–22. doi: 10.1002/jmri.20356. [DOI] [PubMed] [Google Scholar]
- 32.Piechnik SK, Evans J, Bary LH, Wise RG, Jezzard P. Functional changes in CSF volume estimated using measurement of water T2 relaxation. Magn Reson Med. 2009;61(3):579–86. doi: 10.1002/mrm.21897. [DOI] [PubMed] [Google Scholar]
- 33.Stagg CJ, Best JG, Stephenson MC, O’Shea J, Wylezinska M, Kincses ZT, et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009;29(16):5202–6. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Brady M, Smith S. Segmentation of Brain MR Images Through a Hidden Markov Random Field Model and the Expectation-Maximization Algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 35.Boy F, Evans CJ, Edden RA, Lawrence AD, Singh KD, Husain M, et al. Dorsolateral prefrontal gamma-aminobutyric acid in men predicts individual differences in rash impulsivity. Biol Psychiatry. 2011;70(9):866–72. doi: 10.1016/j.biopsych.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haga KK, Khor YP, Farrall A, Wardlaw JM. A systematic review of brain metabolite changes, measured with 1H magnetic resonance spectroscopy, in healthy aging. Neurobiol Aging. 2009;30(3):353–63. doi: 10.1016/j.neurobiolaging.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Eylers VV, Maudsley AA, Bronzlik P, Dellani PR, Lanfermann H, Ding XQ. Detection of Normal Aging Effects on Human Brain Metabolite Concentrations and Microstructure with Whole-Brain MR Spectroscopic Imaging and Quantitative MR Imaging. AJNR Am J Neuroradiol. 2016;37(3):447–54. doi: 10.3174/ajnr.A4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu PW, Mak HK, Yau KK, Chan Q, Chang RC, Chu LW. Metabolic changes in the anterior and posterior cingulate cortices of the normal aging brain: proton magnetic resonance spectroscopy study at 3 T. Age (Dordr) 2014;36(1):251–64. doi: 10.1007/s11357-013-9545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aufhaus E, Weber-Fahr W, Sack M, Tunc-Skarka N, Oberthuer G, Hoerst M, et al. Absence of changes in GABA concentrations with age and gender in the human anterior cingulate cortex: a MEGA-PRESS study with symmetric editing pulse frequencies for macromolecule suppression. Magn Reson Med. 2013;69(2):317–20. doi: 10.1002/mrm.24257. [DOI] [PubMed] [Google Scholar]
- 40.Edden RA, Oeltzschner G, Harris AD, Puts NA, Chan KL, Boer VO, et al. Prospective frequency correction for macromolecule-suppressed GABA editing at 3T. J Magn Reson Imaging. 2016;44(6):1474–82. doi: 10.1002/jmri.25304. [DOI] [PMC free article] [PubMed] [Google Scholar]