Abstract
Background
Symptom-based screening for tuberculosis (TB) is recommended for all people living with HIV (PLHIV) resulting in unnecessary Xpert MTB/RIF testing for the vast majority of individuals living in TB endemic areas and thus, poor implementation of intensified case finding (ICF) and TB preventive therapy. Novel approaches to TB screening are therefore critical in achieving global targets for TB elimination.
Methods
In a prospective study of PLHIV with CD4+ T-cell count ≤350 cells/uL initiating antiretroviral therapy (ART) from two HIV/AIDS clinics in Uganda, we evaluated the performance of C-reactive protein (CRP) measured using a rapid and inexpensive point-of-care (POC) assay as a screening tool for active pulmonary TB.
Findings
Of 1177 HIV-infected adults (median CD4+ T-cell count 168 cells/µL) enrolled, 163 (14%) had culture-confirmed TB. POC CRP had 89% (145/163) sensitivity and 72% (731/1014) specificity for culture-confirmed TB. Compared to the WHO symptom screen, POC CRP had lower sensitivity (difference −7% [95% CI: −12 to −2], p=0.002) but substantially higher specificity (difference +58% [95% CI: +61 to +55], p<0.0001). When Xpert MTB/RIF results were used as the reference standard, sensitivity of POC CRP and the WHO symptom screen were similar (94% [79/84] vs. 99% [83/84]; difference −5% [95% CI: −12 to +2], p=0.10).
Interpretation
The performance characteristics of CRP support its use as a TB screening test for PLHIV with CD4+ T-cell count ≤350 cells/µL initiating ART. HIV/AIDS programs should consider POC CRP-based TB screening to improve the efficiency of ICF and increase uptake of TB preventive therapy.
FUNDING
National Institutes of Health; Presidential Emergency Plan for AIDS Relief; University of California, San Francisco, Nina Ireland Program for Lung Health
INTRODUCTION
Since 2000, global tuberculosis (TB) incidence has fallen by an average of 1·5% annually.1 Yet TB remains the leading infectious cause of death, responsible for 1·5 million deaths overall and 400,000 HIV-deaths (one-third of all HIV-deaths) in 2015 alone.1 To reduce the burden of TB, the World Health Organization (WHO) recommends systematic TB screening of all PLHIV, regardless of symptoms.2
The goals of screening are to: 1) detect active TB early to reduce the risk of poor disease outcomes and TB transmission and 2) identify individuals eligible for preventive therapy to reduce incident TB.2 A major barrier to implementing systematic screening of high-risk groups is the lack of an adequate TB screening test. The WHO target product profile for a TB screening test requires that sensitivity be ≥90% and specificity ≥70%.3 The high sensitivity requirement ensures that individuals who screen-negative have a low probability of active TB and can therefore initiate preventive therapy safely. The moderately high specificity requirement limits the need for confirmatory diagnostic testing to a smaller sub-group of high-risk individuals. In addition to these technical requirements, the test should be simple, low-cost, and available at the point-of-care (POC) such that TB screening could be performed by frontline healthcare workers.
For PLHIV, no current test or algorithm satisfies the minimum criteria for a TB screening test. Although simple and highly sensitive (>90%) for active TB, the WHO-recommended symptom screen has insufficient specificity.4,5 Prospective studies from sub-Saharan Africa have shown that the specificity of the symptom screen is low (range: 5–33%).6–10 If performed routinely, symptom-based TB screening would require nearly all PLHIV to undergo confirmatory testing11 before initiating life-saving TB preventive therapy. Therefore, to facilitate implementation of ICF and preventive therapy, there is an urgent need for a screening strategy that has higher specificity for active TB than the WHO symptom screen but retains high negative predictive value (NPV) and can be used at peripheral health centers in resource-limited settings.
C-reactive protein (CRP) is an acute-phase reactant whose concentrations rise in response to inflammation induced by diseases such as active TB.12–19 CRP has been consistently shown to have higher sensitivity for pulmonary TB compared to other non-specific markers of inflammation such as erythrocyte sedimentation rate, lactate dehydrogenase, and procalcitonin.12–14,20–23 Although elevations in CRP (≥10 mg/L) are not specific for active TB, two studies that evaluated CRP as a screening test among PLHIV initiating antiretroviral therapy (ART) found CRP, using stored serum specimens, to have two- to six-fold higher specificity (58% and 81%) than symptom-based TB screening.18,19 These studies suggest that CRP – which is already available as a simple, rapid, and low-cost POC test (results in 3 minutes, <$2 per test) – may be a promising approach to TB screening.
We report on the first prospective study of POC CRP-based TB screening for PLHIV presenting to prototypical HIV/AIDS clinics for ART initiation. Our objectives were to determine whether POC CRP meets the minimum sensitivity and specificity targets recommended by the WHO for TB screening, thereby assessing whether POC CRP might improve the effectiveness and efficiency of screening relative to the WHO-recommended symptom screen.
METHODS
Study population
From July 2013 to December 2015, we enrolled consecutive adults (age ≥18 years) initiating ART from two HIV/AIDS clinics within the Mulago Hospital Complex (Kampala, Uganda). Patients were included if they were ART-naïve and had a pre-ART CD4+ T-cell count ≤350 cells/µL within three months of study enrollment. Patients with a known diagnosis of active TB and/or taking medication with anti-mycobacterial activity (anti-TB or TB preventive therapy, fluoroquinolones) within three days of enrollment were excluded. All patients provided written informed consent and the study was approved by the Institutional Review Boards at the University of California, San Francisco, Makerere University, and the Uganda National Council for Science and Technology. This study conforms to the Standards for the Reporting of Diagnostic Accuracy Studies (STARD) initiative guidelines.24
Study procedures
Data collection and TB screening
Trained study personnel collected demographic and clinical data and administered the WHO symptom screen at the time of enrollment. CRP concentrations were measured at study entry using whole blood obtained from finger prick using a United States Food and Drug Administration (US FDA)-approved standard sensitivity POC assay (iCHROMA, BodiTech, South Korea).
Sputum collection and testing
Two spot sputum samples (the second induced using nebulized 3% hypertonic saline, if necessary) were collected at study entry. A minimum of one mL of sputum from the first sputum specimen was processed for Xpert MTB/RIF (Xpert) testing (Cepheid, USA). The remaining volume from the first sputum specimen and the second sputum specimen underwent decontamination with N-acetyl-L-cysteine and sodium hydroxide. Mycobacterial culture was performed on the decontaminated sediments; sediments were cultured on liquid media using the BACTEC 960 Mycobacterial Growth Indicator Tube (MGIT) system, with parallel solid (Löwenstein-Jensen) media added from June 2014 to December 2015. Laboratory technicians confirmed the identity of any growth by acid-fast bacilli smear microscopy and molecular speciation testing (Capilia TB, TAUNS, Japan or MPT64 assay, Standard Diagnostics, South Korea). All staff performing Xpert testing and culture were blinded to clinical and demographic data, including symptom screen status and POC CRP concentrations.
Definitions
Index tests
We defined a priori a POC CRP concentration of ≥10 mg/L (rounding to the nearest whole-number) as screen-positive for TB based on large-scale epidemiological studies of other conditions.25,26 In accordance with WHO guidelines, we considered patients to be symptom screen-positive if they reported any of four symptoms (current cough, fever, night sweats, weight loss) in the previous 30 days.2
Reference standard
We considered patients to have active TB if Mycobacterium tuberculosis (Mtb) was isolated from any sputum culture. We considered patients not to have active TB if: 1) Mtb was not isolated from any sputum culture, and 2) at least two sputum cultures were negative. Patients with insufficient culture data (e.g., due to contamination) were excluded from analysis. Secondarily, to evaluate CRP performance in the context of routine ICF, we also classified TB status based on Xpert results.
Statistical analysis
Comparisons between groups of categorical and continuous variables were performed using the Wilcoxon rank-sum test, t-test, or chi-square test as appropriate. For the primary analysis, we calculated the point estimates and 95% confidence intervals (CIs) for the sensitivity, specificity, NPV, and positive predictive value (PPV) of POC CRP and the WHO symptom screen in reference to culture results. We compared differences in sensitivity and specificity between the WHO symptom screen and POC CRP using McNemar’s test of paired proportions. To identify the optimal cut-point for POC CRP-based TB screening, we performed receiver operating characteristic (ROC) analysis to explore the sensitivity, specificity, and predictive values of alternate thresholds. To explore the prognostic value of POC CRP concentrations with mycobacterial load, we calculated the Pearson’s correlation coefficient between POC CRP concentrations and days-to-culture positivity. We performed all analyses using STATA 13 (STATA, USA).27
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Study population
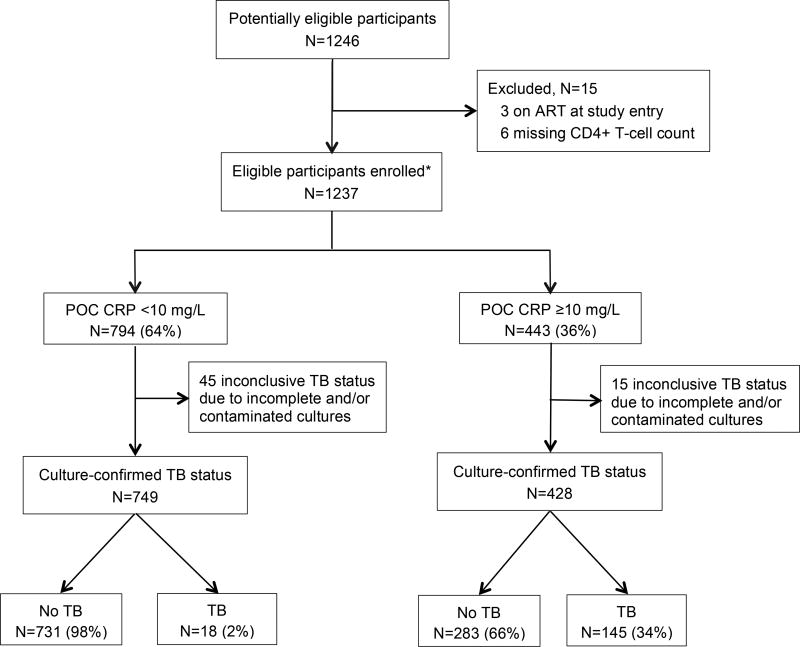
During the study period, 1246 consecutive PLHIV were screened and 1237 eligible participants were enrolled and underwent POC CRP testing. Sixty patients (5%) with incomplete and/or contaminated cultures were excluded from the analysis (Figure 1). Of the remaining 1177 patients, 626 were female (53%), median age was 33 years (interquartile range [IQR] = 27–40), and median CD4+ T-cell count was 165 cells/µL (IQR 75–271; Table 1). Overall, 163 patients (14%) had culture-confirmed TB, with TB prevalence increasing as CD4+ T-cell count decreased (p<0·001 for trend). TB prevalence was 7% (33/486), 15% (48/311), 19% (32/169), and 24% (50/2011) among patients with CD4+ T-cell counts >200, 100–200, 50–99, and <50 cells/µL, respectively. Xpert was positive in 84/163 patients (sensitivity 52%, 95% CI: 44 to 59) with culture-confirmed TB and 8/1014 patients (specificity 99%, 95% CI: 99 to 100) with negative cultures.
Figure 1.
Patient flow diagram.
Abbreviation: ART (antiretroviral therapy); POC CRP (point-of-care C-reactive protein); TB (tuberculosis).
*All enrolled participants underwent POC CRP testing and submitted two spot specimens for liquid culture. TB defined as ≥1 sputum culture positive for Mycobacterium tuberculosis. No TB defined as ≥2 sputum cultures negative for Mycobacterium tuberculosis.
Table 1.
Demographics and clinical characteristics.
| Characteristic, N (%) | Median (IQR)/Number (%) (Total N=1177) |
|---|---|
| Age (years) | 33 (27–40) |
| Female | 626 (53%) |
| New to HIV care | 742 (63%) |
| CD4+ T-cell count (cells/µL) | 165 (75–271) |
| <50 | 211 (18%) |
| 50–99 | 169 (14%) |
| 100–199 | 311 (26%) |
| ≥200 | 486 (41%) |
| BMI (kg/m2) | 21·2 (18·9–24·0) |
| Prior TB | 42 (4%) |
| TB contact | 457 (39%) |
| WHO symptom screen | 1025 (87%) |
| Current cough | 568 (48%) |
| Fever | 585 (50%) |
| Night sweats | 410 (35%) |
| Weight loss | 848 (72%) |
| Elevated POC CRP (≥10 mg/L) | 428 (36%) |
| POC CRP (mg/L) | 4·6 (2·5–24·5) |
Abbreviations: IQR (interquartile range); BMI (body mass index); TB (tuberculosis); WHO (World Health Organization); POC CRP (point-of-care C-reactive protein).
POC CRP test characteristics
Sensitivity
POC CRP concentrations were elevated (≥10 mg/L) in 428/1177 (36%) patients, including 145/163 patients with culture-confirmed TB (sensitivity 89%, 95% CI: 83 to 93; Table 2) and 79/84 patients with Xpert-positive TB (sensitivity 94%, 95% CI: 87 to 98). Among patients with CD4+ T-cell counts ≤200 cells/µL, sensitivity for culture-confirmed TB was 93% ([121/130], 95% CI: 87 to 97; Appendix, page 1) and did not vary by CD4+ strata within this group (p=0·65 for trend; Figure 2A) but was lower (73% [24/33], 95% CI: 55 to 87) for patients with CD4+ T-cell counts >200 cells/µL. NPV was high overall (98%, 95% CI: 96 to 99) and for all CD4+ strata (range: 96 to 100%).
Table 2.
Diagnostic accuracy of POC CRP vs. WHO symptom screen for active tuberculosis (in reference to culture).
| In reference to culture | POC CRP | WHO symptom screen |
% Difference (95% CI) |
p-value for difference |
|---|---|---|---|---|
| TP/Culture-positive | 145/163 | 156/163 | −7% | 0·002 |
| % Sensitivity (95% CI) | 89% (83–93) | 96% (91–98) | (−12 to −2) | |
| TN/Culture-negative | 731/1014 | 145/1014 | +58% | <0·0001 |
| % Specificity (95% CI) | 72% (69–75) | 14% (12–17) | (+55 to +61) | |
| TN/(TN+FN) | 731/749 | 145/152 | +2% | 0·13 |
| NPV (95% CI) | 98% (96–99) | 95% (91–98) | (−1 to +6) | |
| TP/(TP+FP) | 145/428 | 156/1025 | +19% | <0·0001 |
| PPV (95% CI) | 34% (29–39) | 15% (13–18) | (+14 to +24) |
Abbreviations: POC CRP (point-of-care C-reactive protein); WHO (World Health Organization); CI (confidence interval); TP (true positive); TN (true negative); FN (false negative); FP (false positive); NPV (negative predictive value); PPV (positive predictive value).
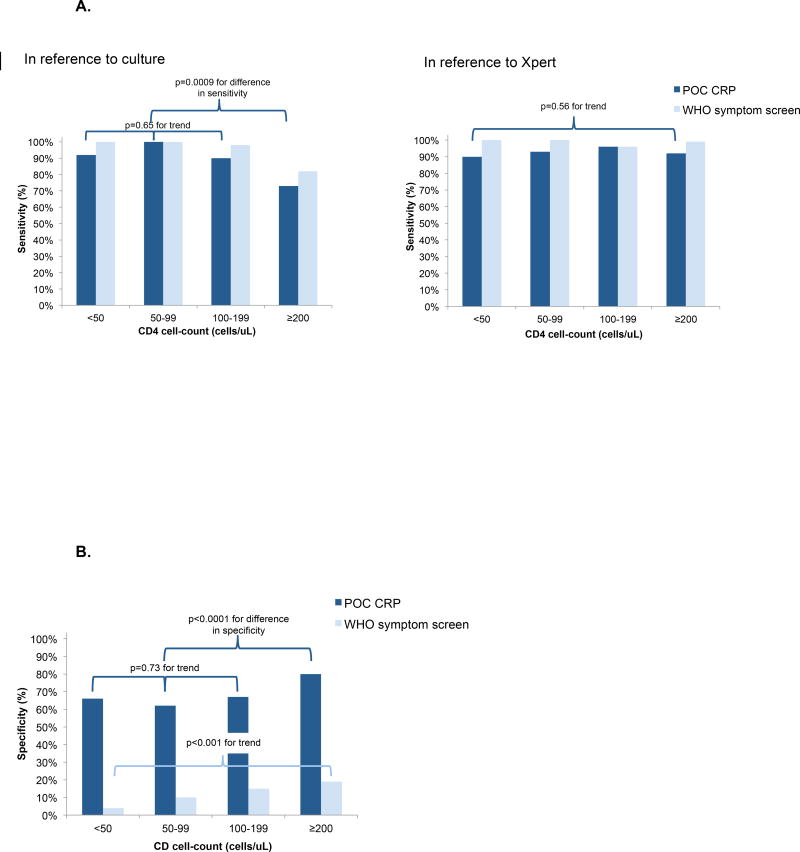
Figure 2.
A. Sensitivity of screening tests for culture-confirmed tuberculosis and Xpert-positive tuberculosis, stratified by CD4+ T-cell count.
Abbreviations: POC CRP (point-of-care C reactive protein); WHO (World Health Organization).
The dark blue bars represent the sensitivity of POC CRP while the light blue bars represent the sensitivity of the WHO symptom screen, in reference to culture (left) and Xpert (right).
In reference to culture: Among PLHIV with CD4+ T-cell counts <200 cells/uL, sensitivity of POC CRP for culture-confirmed TB did not vary significantly by CD4 strata (p = 0·65 for trend). Compared to PLHIV with CD4+ T-cell counts ≥200 cells/uL, sensitivity of POC CRP was higher among PLHIV with CD4+ T-cell counts <200 cells/uL (73% vs. 93%; difference −20% [95% CI: −36 to −5], p-value for the difference =0·0002).
In reference to Xpert: Among PLHIV with CD4+ T-cell counts ≤350 cells/uL, sensitivity of POC CRP for Xpert-positive TB did not vary significantly by CD4 strata (p=0·56 for trend).
B. Specificity of screening tests for culture-positive tuberculosis, stratified by CD4+ T-cell count.
Abbreviations: POC CRP (point-of-care C reactive protein); WHO (World Health Organization).
The dark blue bars represent the specificity of POC CRP while the light blue bars represent the specificity of the WHO symptom screen, in reference to culture. Among PLHIV with CD4+ T-cell counts ≤350 cells/uL, specificity of the WHO symptom screen decreased significantly as CD4 strata decreased (p<0·0001 for trend). Among PLHIV with CD4+ T-cell counts <200 cells/uL, specificity of POC CRP did not vary significantly by CD4 strata (p = 073 for trend). Compared to PLHIV with CD4+ T-cell counts ≥200 cells/uL, specificity of POC CRP was lower among PLHIV with CD4+ T-cell <200 cells/uL (80% vs. 66%; difference −14% [95% CI: −20 to −10], pvalue for the difference <0·0001).
Specificity
POC CRP concentrations were not elevated (<10 mg/L) in 731/1014 patients without TB (specificity 72%, 95% CI: 69 to 75; Table 2). Among patients with CD4+ T-cell counts ≤200 cells/µL, specificity was 66% ([367/561], 95% CI: 61 to 69; Appendix, page 1) and was similar (range 62–67%) for all CD4+ strata within this group (p=0·73 for trend; Figure 2B). Specificity was higher (80% [364/453], 95% CI: 76 to 84) among patients with CD4+ T-cell counts >200 cells/µL.
Correlations with measures of mycobacterial load
Median POC CRP concentrations were higher in patients with culture-confirmed TB compared to those with negative cultures (51·3 mg/L [IQR 21·9–112·8] vs. 3·4 mg/L [IQR 2·5–11·6], p<0·0001), and higher in patients with Xpert-positive TB compared to those with Xpert-negative TB (67·1 mg/L [IQR 30·7–141·2] vs. 36·9 mg/L [IQR 13·1–88·7], p=0·003). POC CRP concentrations increased as days-to-culture positivity decreased, though correlation was modest (r = −0·28, p=0·0003; Appendix, page 2).
Comparison with the WHO symptom screen
In contrast to POC CRP, the majority of patients (87%, 1025/1177) screened positive by symptoms (difference +51%, 95% CI: +48 to +54). Compared to POC CRP, the WHO symptom screen had higher sensitivity (89% [145/163] vs. 96% [156/163]; difference +7% [95% CI: +2 to +12], p=0·002; Table 2) but substantially lower specificity (72% [731/1014] vs. 14% [145/1014]; difference −58% [95% CI: −61 to −55], p<0·0001). When Xpert was used as the reference standard, sensitivity of POC CRP and the WHO were similar (94% [79/84] vs. 99% [83/84]; difference +5% [95% CI: −2 to +12], p=0·10; Appendix, page 1). For all CD4+ strata, specificity of the WHO symptom screen (range: 4–19%) was substantially lower than the specificity of POC CRP (range 62–80%; Appendix, page 1).
Diagnostic accuracy of combined TB screening strategies
Supplementary Table 3 (Appendix, page 3) shows the diagnostic accuracy of two combination TB screening strategies (any test positive and both test positive) in reference to culture. Neither combination approach to TB screening improved the diagnostic accuracy beyond that of an individual screening test.
ROC analysis
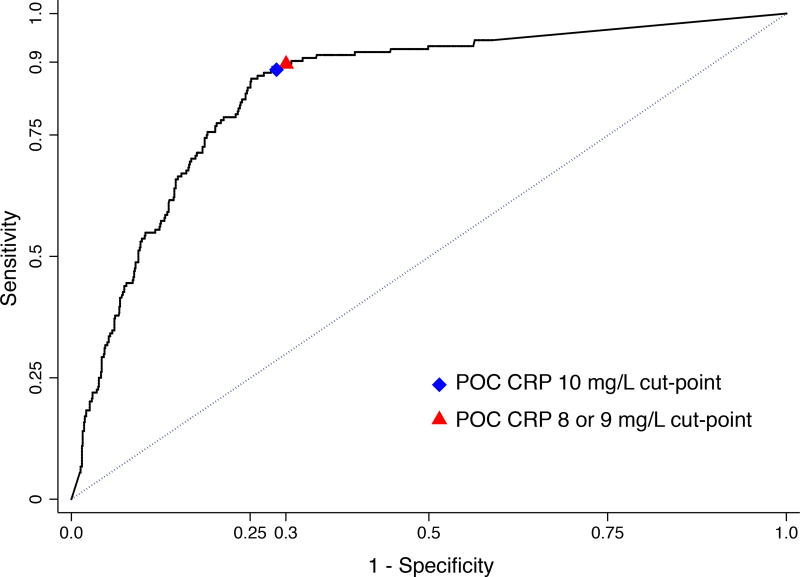
Figure 3 shows the ROC curve for POC CRP. POC CRP met the minimum TB screening test sensitivity (≥90%) and specificity (≥70%) targets when the cut-point was lowered to 8 mg/L (AUROC 0·80, 95% CI: 0·77 to 0·83) or 9 mg/L (AUROC 0·81, 95% CI: 0·78 to 0·83; Table 3).
Figure 3.
Receiver operating characteristic (ROC) curves for the detection of culture-confirmed pulmonary tuberculosis by POC CRP.
Abbreviations: POC CRP (point-of-care C-reactive protein).
Area under the receiver-operating curve for 10 mg/L (0·81, 95% CI: 0·78 to 0·83), 9 mg/L (0·81, 95% CI: 0·78 to 0·83), and 8 mg/L (0·80, 95% CI: 0·77 to 0·83) cut-points.
Table 3.
Effect of varying POC CRP threshold on diagnostic accuracy.
| POC CRP cut-point (mg/L) |
Sensitivity % (95% CI) |
Specificity % (95% CI) |
PPV % (95% CI) |
NPV % (95% CI) |
|---|---|---|---|---|
| ≥ 3 | 93·9% (89·0–97·0) | 46·5% (43·4–49·7) | 22·0% (19·0–25·3) | 97·9% (96·2–99·0) |
| ≥ 4 | 93·3% (88·2–96·6) | 53·2% (50·0–56·3) | 24·2% (20·9–27·8) | 98·0% (96·4–99·0) |
| ≥ 5 | 92·6% (87·5–96·1) | 59·7% (56·6–62·7) | 27·0% (23·3–30·8) | 98·1% (96·6–99·0) |
| ≥ 6 | 92·0% (86·7–95·7) | 63·9% (60·9–66·9) | 29·1% (25·2–33·2) | 98·0% (96·7–98·9) |
| ≥ 7 | 91·4% (86·0–95·2) | 67·6% (64·6–70·4) | 31·2% (27·0–35·5) | 98·0% (96·7–98·9) |
| ≥ 8 | 90·2% (84·5–94·3) | 69·6% (66·7–72·4) | 32·3% (28·0–36·8) | 97·8% (96·4–98·7) |
| ≥ 9 | 89·6% (83·8–93·8) | 71·5% (68·6–74·3) | 33·6% (29·1–38·2) | 97·7% (96·4–98·7) |
| ≥ 10 | 89·0% (83·1–93·3) | 72·1% (69·2–74·8) | 33·9% (29·4–38·6) | 97·6% (96·2–98·6) |
| ≥ 11 | 87·1% (81·0–91·8) | 74·6% (71·8–77·2) | 35·5% (30·8–40·4) | 97·3% (95·9–98·3) |
| ≥ 12 | 85·3% (78·9–90·3) | 75·3% (72·6–78·0) | 35·7% (31·0–40·7) | 97·0% (95·5–98·0) |
Abbreviations: POC CRP (point-of-care C-reactive protein); CI (confidence interval); PPV (positive predictive value); NPV (negative predictive value)·
DISCUSSION
In this study, we screened 1177 HIV-infected adults with CD4+ T-cell counts ≤350 cells/µL initiating ART for active TB with a rapid and inexpensive POC CRP assay using whole blood obtained from finger prick. POC CRP-based TB screening detected 89% of all culture-confirmed and 94% of all Xpert-positive TB cases. Furthermore, POC CRP correctly identified 72% of all PLHIV without active TB as immediately eligible for preventive therapy. These results identify POC CRP as the first test to meet the minimum accuracy criteria established by the WHO for a TB screening tool among PLHIV, a key high-risk population targeted for systematic screening.
Our findings are consistent with multiple prior studies that have found elevated CRP concentrations to strongly predict the presence of active TB among PLHIV.12–19 Studies evaluating CRP among patients self-reporting TB symptoms (i.e., passive case detection) have consistently found CRP to have high sensitivity (range: 89–100%) for active TB.12–17 However, because patients self-reporting symptoms have a higher prevalence of pyogenic infections (e.g., bacterial pneumonia), specificity of CRP has been generally low (range: 0–43%) in this population.13–15,17 Recently, two studies used stored serum specimens to evaluate CRP in the context of TB screening among PLHIV (i.e., active case detection).18,19 CRP had similar sensitivity but two- to six-fold higher specificity for active TB, relative to the WHO symptom screen.18,19 Our findings from the first prospective evaluation of POC CRP as a TB screening tool validate these prior analyses, and strongly support that POC CRP-based TB screening could improve the efficiency of ICF and increase the uptake of TB preventive therapy.
Despite WHO recommendations, only 7 million PLHIV were screened for TB and <1 million were provided TB preventive therapy worldwide in 2014.28 The high false-positive rate of the WHO symptom screen, the currently recommended screening test for PLHIV, has hampered efforts to scale-up both ICF and preventive therapy. Consistent with prior prospective studies,6–10 our data found that 86% of PLHIV without active TB screened false-positive by symptoms. Although the WHO symptom screen exceeded the minimum sensitivity threshold (≥90%) for a TB screening test, the poor specificity (14%) of the WHO symptom screen precludes its usefulness in this population.
For a TB screening test to be considered by policymakers and clinicians alike, the test must prioritize sensitivity over specificity. The extent to which sensitivity of a TB screening test should be prioritized over specificity is described by the WHO’s target product profile for a TB screening test, which recommends ≥90% sensitivity and ≥70% specificity.3 This trade-off between sensitivity and specificity takes into consideration the risks associated with a patient who screens false-negative (e.g., generation of isoniazid-resistant TB) and the burden patients who screen false-positive would have on the healthcare system (e.g., costs and workload of unnecessary confirmatory testing). Our data suggests that if culture is used as the confirmatory TB test, the WHO symptom screen would detect 7% more TB cases than POC CRP but would require nearly all (87%) patients to undergo culture, which is likely to be cost-prohibitive even in the few high burden countries where culture is more readily available. In contrast, POC CRP would detect 89% of all culture-confirmed TB cases but would require only 36% of all patients to undergo culture, an absolute reduction of 51% relative to the WHO symptom screen. When either an 8 or 9 mg/L cut-point was used, POC CRP met both the minimum sensitivity (≥90%) and specificity (≥70%) thresholds recommended by the WHO target product profile for a TB screening test. Furthermore, POC CRP would perform particularly well among patients with low CD4+ T-cell counts. Additional studies are needed to confirm whether POC CRP-based TB screening using a cut-point of 8 or 9 mg/L would further improve the effectiveness of TB screening and formal cost-effectiveness studies are needed to better estimate the costs and yield of POC CRP-based TB screening relative to current options.
In the vast majority of settings that use Xpert as the confirmatory test, our data show that POC CRP would detect 94% of all Xpert-positive patients but reduce by more than half (60% absolute reduction) the number of patients who would require Xpert testing relative to symptom-based screening. Therefore, POC CRP-based TB screening would identify nearly all Xpert-positive TB cases (cases that pose the greatest infectious risk to the community) but substantially lower the cost of ICF activities.
Our study has several strengths. First, our study findings are likely generalizable to a number of other HIV-endemic settings as our study participants represent a prototypical population for whom TB screening is recommended. Second, all patients, regardless of symptoms, were screened and then evaluated for TB using a robust standard for TB diagnosis: two MGIT cultures. Third, CRP concentrations were measured using a commercially available, simple and low-cost POC assay. As such, POC CRP is available for immediate scale-up for HIV/AIDS clinics wishing to implement POC CRP-based TB screening and further strengthen its evidence base. Future studies evaluating POC CRP-based TB screening should also evaluate whether the diagnostic accuracy of POC CRP-based TB screening may be further improved when used in combination with CXR and/or other promising biomarkers, particularly among patients with high CD4+ T-cell counts and/or patients treated with ART.
Our study also has limitations. First, we chose to study ART-naïve patients with advanced HIV-associated immunosuppression because TB risk is greatest in this population. Our study findings may therefore be less applicable to other HIV sub-groups. Future studies should evaluate the diagnostic accuracy of POC CRP in ART-treated PLHIV, other high-risk populations (e.g., household contacts, miners, prisoners, etc.) for whom systematic TB screening is also recommended, and other high burden settings.52 Second, we classified TB status based on culture results, the gold standard for TB diagnosis. Although TB classification based on clinical criteria may have resulted in additional TB cases identified for our analysis, empiric TB treatment was uncommon in our TB screening cohort (i.e., PLHIV undergoing active case finding rather than PLHIV seeking care for symptoms suggestive of TB). Third, we did not compare CRP concentrations measured using a POC assay in our study to a lab-based assay as prior studies including a US FDA-led investigation, have demonstrated excellent correlation of the iCHROMA POC CRP assay with the reference standard, and minimal variation with repeated testing of the same sample over a large range of CRP concentrations.29 Lastly, we did not evaluate patients for extra-pulmonary TB or for non-TB disease. Future studies should evaluate the diagnostic accuracy of POC CRP for extra-pulmonary TB and assess the causes and significance of an elevated POC CRP level among PLHIV with non-TB disease.
In conclusion, our findings have important implications for clinical care and program implementation. POC CRP-based TB screening could substantially increase the number of PLHIV initiating ART identified as eligible for TB preventive therapy and reduce the number of PLHIV requiring confirmatory TB testing. Thus, POC CRP could increase uptake of TB preventive therapy and decrease costs of implementing ICF beyond that of symptom-based screening. These results support the use of POC CRP as a part of a public health strategy to reduce the global burden of TB among PLHIV.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
C-reactive protein (CRP) is an acute-phase reactant whose levels rise in response to pyogenic infections such as active pulmonary tuberculosis (TB) and is independent of HIV status. Although elevations in CRP (≥10 mg/L) are not specific for active TB, CRP has been consistently shown to have higher sensitivity for active TB compared to other inflammatory markers. Moreover, CRP can be measured using low-cost and simple point-of-care (POC) assays from blood obtained by finger prick. To identify all studies that measured blood CRP levels from consecutive patients undergoing screening or evaluation for active pulmonary TB, we performed a systematic review of PubMed with the search terms “C-reactive protein” and “tuberculosis” for English-language studies published on or before June, 2017. We found that most studies evaluated the diagnostic accuracy of CRP for active TB in the setting of passive case finding (i.e., diagnosis-seeking patients with symptoms suggestive of TB). In these studies, CRP had high sensitivity but low specificity for active TB. We found two studies that evaluated the diagnostic accuracy of CRP for active TB in the setting of active case finding (i.e., provider-initiated screening). Using stored specimens of HIV-infected patients initiating antiretroviral therapy (ART), both studies found that CRP had comparably high sensitivity for active TB as symptom-based screening (the current standard) but substantially higher specificity. However, no study has prospectively evaluated CRP as a TB screening tool for PLHIV and it is unknown whether CRP meets the World Health Organization’s (WHO) target product profile (sensitivity ≥90% and specificity ≥70%) for a TB screening test.
Added value of this study
Our study is the first to prospectively evaluate the diagnostic accuracy of CRP in the context of TB screening. We found that CRP, using a POC assay, had 89% sensitivity and 72% specificity for culture-confirmed TB while symptom-based screening had higher sensitivity (96%) but substantially lower specificity (14%). When Xpert MTB/RIF – the confirmatory test most commonly used in resource-limited settings – was used as the reference standard, POC CRP detected 94% of all Xpert-positive TB cases. Thus, POC CRP is the first TB screening tool to meet the minimum accuracy targets established by the WHO.
Implications of all the available evidence
Previously published data and our results support the use of CRP to systematically screen PLHIV initiating ART for active TB. POC CRP-based TB screening could improve the efficiency of ICF and increase the uptake of TB preventive therapy among PLHIV. In order to support policy recommendations, studies and programs performing systematic TB screening among PLHIV should incorporate POC CRP into their TB screening protocols and evaluate costs and yield relative to current options.
Acknowledgments
This study was supported by the NIH/NIAID (K23 AI114363 to CY); NIH and University of California, San Francisco-Gladstone Institute of Virology and Immunology (UCSF-GIVI) Center for AIDS Research (CFAR; P30 AI027763 to CY); the UCSF Nina Ireland Program for Lung Health (CY); NIH/NIAID-Presidential Emergency Plan for AIDS Relief (PEPFAR) CFAR Administrative Supplement (P30 A120163 to AC); and the NIH/NHLBI (K24 HL087713 to LH). The funding organizations had no role in the design, collection, analysis and interpretation of data, or in the writing of the manuscript. We thank Amy J. Markowitz, J.D. for her editorial contribution to the manuscript and Isabel E. Allen, Ph.D. for her feedback on the analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORS’ CONTRIBUTIONS: CY and AC designed the study. FS, EA, DA, AA and MK oversaw the local collection of data. JK, SM, and LA collected the data. CY analyzed the data and wrote the first draft of the manuscript. AC and DWD critically revised the manuscript. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST: We declare that we have no conflicts of interest.
References
- 1.World Health Organization. Geneva, Switzerland: WHO; 2016. Global Tuberculosis Report. Available from: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?ua=1. [Google Scholar]
- 2.World Health Organization. WHO; [Geneva, Switzerland]. 2013. Systematic screening for active tuberculosis: principles and recommendations. Available from: http://www.who.int/tb/tbscreening/en/ [PubMed] [Google Scholar]
- 3.World Health Organization. Geneva, Switzerland: WHO; 2014. High-priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting. Available from: http://www.who.int/tb/publications/tpp_report/en/ [Google Scholar]
- 4.World Health Organization. Geneva, Switzerland: WHO; 2011. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Available from: http://apps.who.int/iris/bitstream/10665/44472/1/9789241500708_eng.pdf. [Google Scholar]
- 5.Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8(1):e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawn SD, Brooks SV, Kranzer K, et al. Screening for HIV- associated tuberculosis and rifampin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: A prospective study. PLoS Med. 2011;8(7):e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kufa T, Mngomezulu V, Charalambous S, et al. Undiagnosed tuberculosis among HIV clinic attendees: association with antiretroviral therapy and implications for intensified case finding, isoniazid preventive therapy, and infection control. J Acquir Immune Defic Syndr. 2012;60:e22–e28. doi: 10.1097/QAI.0b013e318251ae0b. [DOI] [PubMed] [Google Scholar]
- 8.Hanifa Y, Fielding KL, Charalambous S, Variava E, Luke B, Churchyard GJ, et al. Tuberculosis among adults starting antiretroviral therapy in South Africa: the need for routine case finding. Int J Tuberc Lung Dis. 2012;16(9):1252–1259. doi: 10.5588/ijtld.11.0733. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad Khan F, Verkuijl S, Parrish A, Chikwava F, Ntumy R, El-Sadr W, et al. Performance of symptom-based tuberculosis screening among people living with HIV: not as great as hoped. AIDS. 2014;28(10):1463–1472. doi: 10.1097/QAD.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swindells S, Komarow L, Tripathy S, et al. Screening for pulmonary tuberculosis in HIV-infected individuals: AIDS Clinical Trials Group Protocol A5253. Int J Tuberc Lung Dis. 2013;17(4):532–539. doi: 10.5588/ijtld.12.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Geneva, Switzerland: WHO; 2013. Policy update: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. Available from: http://apps.who.int/iris/bitstream/10665/112472/1/9789241506335_eng.pdf?ua=1. [Google Scholar]
- 12.Polzin A, Pletz M, Erbes R, et al. Procalcitonin as a diagnostic tool in lower respiratory tract infections and tuberculosis. Eur Respir J. 2003;21:939–943. doi: 10.1183/09031936.03.00055103. [DOI] [PubMed] [Google Scholar]
- 13.Schleicher GK, Herbert V, Brink A, et al. Procalcitonin and C-reactive protein levels in HIV-positive subjects with tuberculosis and pneumonia. Eur Respir J. 2005;25(4):688–692. doi: 10.1183/09031936.05.00067604. [DOI] [PubMed] [Google Scholar]
- 14.Sage EK, Noursadeghi M, Evans HE, et al. Prognostic value of C-reactive protein in HIV-infected patients with Pneumocystis jirovecii pneumonia. Int J STD AIDS. 2010;21(4):288–292. doi: 10.1258/ijsa.2010.009551. [DOI] [PubMed] [Google Scholar]
- 15.Wilson D, Nachega J, Morroni C, Chaisson R, Maartens G. Diagnosing smear-negative tuberculosis using case definitions and treatment response in HIV-infected adults. Int J Tuberc Lung Dis. 2006;10(1):31–38. [PubMed] [Google Scholar]
- 16.Wilson D, Badri M, Maartens G. Performance of serum C-reactive protein as a screening test for smear negative tuberculosis in an ambulatory high HIV prevalence population. PLoS One. 2011;6(1):e15248. doi: 10.1371/journal.pone.0015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drain P, Mayeza L, Bartman P, et al. Diagnostic accuracy and clinical role of rapid C-reactive protein testing in HIV-infected individuals with presumed tuberculosis in South Africa. Int J Tuberc Lung Dis. 2014;18(1):20–26. doi: 10.5588/ijtld.13.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon C, Davis JL, Huang L, et al. Point-of-care C-reactive protein testing to facilitate implementation of isoniazid preventive therapy for people living with HIV. J Acquir Immune Defic Syndr. 2014;65(5):551–556. doi: 10.1097/QAI.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic and prognostic value of serum C-reactive protein for screening for HIV-associated tuberculosis. Int J Tuberc Lung Dis. 2013;17:636–643. doi: 10.5588/ijtld.12.0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawn SD, Obeng J, Acheampong JW, Griffin GE. Resolution of the acute-phase response in West African patients receiving treatment for pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4(4):340–344. [PubMed] [Google Scholar]
- 21.Breen RA, Leonard O, Perrin FM, et al. How good are systemic symptoms and blood inflammatory markers at detecting individuals with tuberculosis? Int J Tuberc Lung Dis. 2008;12(1):44–49. [PubMed] [Google Scholar]
- 22.Kang YA, Kwon SY, Yoon HI, Lee JH, Lee CT. Role of C-reactive protein and procalcitonin in differentiation of tuberculosis from bacterial community acquired pneumonia. Korean J Intern Med. 2009;24(4):337–342. doi: 10.3904/kjim.2009.24.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi CM, Kang CI, Jeung WK, Kim DH, Lee CH, Yim JJ. Role of the C-reactive protein for the diagnosis of TB among military personnel in South Korea. Int J Tuberc Lung Dis. 2007;11(2):233–236. [PubMed] [Google Scholar]
- 24.Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. BMJ. 2015;351:h5527. doi: 10.1136/bmj.h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claus DR, Osmand AP, Gewurz H. Radioimmunoassay of human C-reactive protein and levels in normal sera. J Lab Clin Med. 1976;87:120–128. [PubMed] [Google Scholar]
- 26.Shine B, de Beer FC, Pepys MB. Solid phase radioimmunoassays for human C-reactive protein. Clin Chim Acta. 1981;117:13–23. doi: 10.1016/0009-8981(81)90005-x. [DOI] [PubMed] [Google Scholar]
- 27.StataCorp. Stata statistical software: Release 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 28.World Health Organization. Geneva, Switzerland: WHO; 2015. Global Tuberculosis Report. Available from: http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 29.Pfäfflin A, Schleicher E. Inflammation markers in point-of-care testing (POCT) Anal Bioanal Chem. 2009;393(5):1473–1480. doi: 10.1007/s00216-008-2561-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.