Abstract
Since the early nineteen eighties 18-hydroxycortisol and 18-oxocortisol have attracted attention when it was shown that the urinary excretion of these hybrid steroids was increased in primary aldosteronism. The development and more widespread use of specific assays has improved the understanding of their role in the (patho)physiology of adrenal disorders.
The adrenal site of synthesis is not fully understood although it is clear that for the synthesis of 18-hydroxycortisol and 18-oxocortisol the action of both aldosterone synthase (zona glomerulosa) and 17α-hydroxylase (zona fasciculata) is required with cortisol as main substrate. The major physiological regulator is ACTH and the biological activity of both steroids is very low and therefore only very high concentrations might be effective in vivo.
In healthy subjects the secretion of both steroids is low with 18-hydroxycortisol being substantially higher than that of 18-oxocortisol. The highest secretion of both steroids has been found in familial hyperaldosteronism type 1 (glucocorticoid-remediable aldosteronism) and in familial hyperaldosteronism type 3. Lower but yet substantially increased secretion is found in patients with aldosterone-producing adenomas in contrast to bilateral hyperplasia in whom the levels are similar to patients with hypertension. Several studies have attempted to show that these steroids, in particular peripheral venous plasma 18-oxocortisol, might be a useful discriminatory biomarker for subtyping PA patients. The current available limited evidence precludes the use of these steroids for subtyping.
We review the biosynthesis, regulation and function of 18-hydroxycortisol and 18-oxocortisol and their potential utility for the diagnosis and differential diagnosis of patients with primary aldosteronism.
Key terms: primary aldosteronism, adrenal cortex, steroidogenesis, hypertension
Introduction
Although the chemical synthesis of 18-oxocortisol was already reported in 1963 (1, 2), it was not until the early eighties when Chu and Ulick were searching for unidentified potential mineralocorticoids that might explain the frequently observed discrepancy between the severity of blood pressure, metabolic abnormalities and the degree of increased aldosterone secretion observed in patients with aldosterone-producing adenomas (APA) (3, 4). They isolated and identified 18-hydroxycortisol from the urine of a patient with an APA and shortly thereafter they identified 18-oxocortisol from incubations of bullfrog adrenals with cortisol and then demonstrated that these were also naturally occurring steroids in humans. Subsequent studies not only addressed the origin, biosynthesis, and biological activity of these steroids but some also suggested their increased secretion in patients with adrenocortical disease such as APAs and adrenal Cushing’s syndrome. The highest levels of these steroids were also established in patients with familial hyperaldosteronism type 1 (FH type 1) (glucocorticoid remediable aldosteronism) and in familial hyperaldosteronism type 3 (FH type 3) caused by KCNJ5 germline mutations (5, 6). Because patients FH type 1 express a hybrid or chimaeric gene responsible for the increased secretion of both 18-hydroxycortisol and 18-oxocortisol, these steroids were designated as ‘hybrid’ steroids. However the term ‘hybrid’ reflects merely their hybrid molecular structure comprising features from both the zona glomerulosa (18-hydroxylation and 18-oxidation) and the zona fasciculata (17-hydroxylation) metabolism; this is the result of a crossover recombination of the promoter region and early exons of the CYP11B1 gene and the last exons of the CYP11B2 gene resulting in an additional gene expressed in the zona fasciculata, regulated by ACTH which is responsible for the synthesis of aldosterone and both hybrid steroids (5).
Despite many in vitro and in vivo studies their function in health and disease is not fully understood. In this paper we review the biosynthesis, physiological regulation and function of 18-hydroxycortisol and 18-oxocortisol in healthy subjects and their potential utility for the diagnosis and differential diagnosis of patients with primary aldosteronism.
Biosynthesis
In 1960 two different groups chemically synthesized 17α-hydroxy-aldosterone, a steroid that later became known as 18-oxocortisol (1, 2). However, it was not known at that time whether this was a naturally occurring steroid. After the isolation of 18-hydroxycortisol from the urine of a patient with an APA, the steroid was demonstrated to be present in incubations of freshly isolated minced APA with tritium-labeled 11-deoxycortisol that was converted into 18-hydroxycortisol (3). After a report in 1982 of increased secretion of 18-hydroxycortisol in patients with two types of primary aldosteronism due to an APA and FH type 1 (7) the same group showed that using bullfrog interrenal gland slices, cortisol could be converted by 18-methyloxidase (methyl-oxidase type I and type II) now known to be exerted by a single enzyme, the aldosterone synthase (CYP11B2) (4, 8). At that time, the conversion of corticosterone to 18-hydroxycorticosterone and then to aldosterone was believed to be due to the action of two different enzymes that were called corticosterone methyloxidase I and II respectively. It was later shown that not only the two enzymatic steps, but also the 11β–hydroxylation of deoxycorticosterone to corticosterone and the further hydroxylation of corticosterone to 18-hydroxycorticosterone and aldosterone were exerted by the same enzyme, aldosterone synthase (CYP11B2) (8). Ulick demonstrated that 11-deoxycortisol served as a suboptimal substrate for the enzyme in the conversion to 18-oxocortisol compared to the conversion of deoxycorticosterone to aldosterone (4).
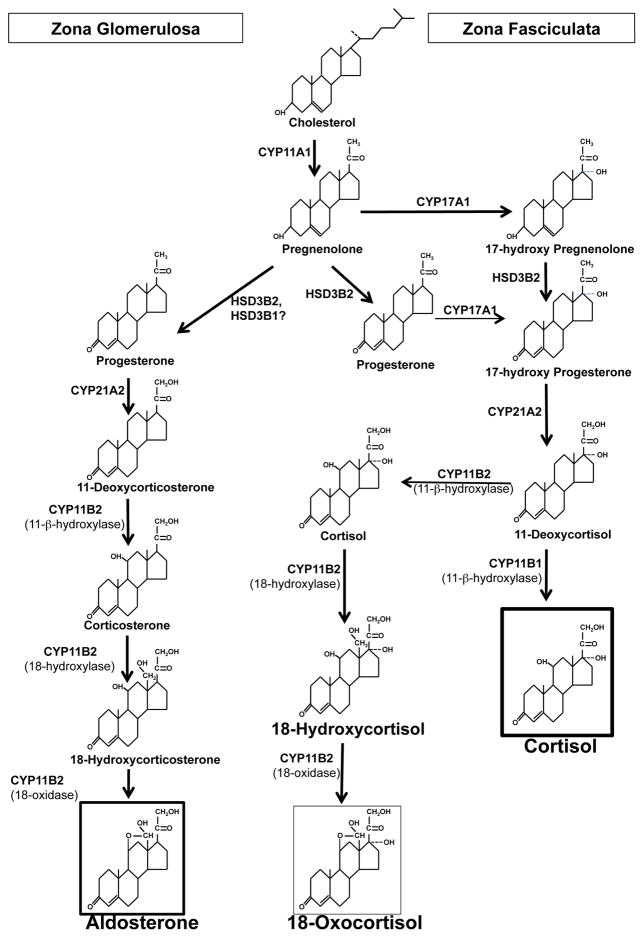
The source of precursors for the synthesis of 18-hydroxycortisol and 18-oxocortisol in the adrenal is currently not clear (Fig 1). The adrenal expresses aldosterone synthase only in the zona glomerulosa and CYP17A1 only in the zona fasciculata and the synthesis of 18-hydroxycortisol and 18-oxocortisol requires the action of both enzymes. In bovine adrenal slices, the synthesis of aldosterone and 18-hydroxycortisol and 18-oxocortisol occurs in the outer slices next to the capsule (9), and these outer slices comprise primarily zona glomerulosa that may be heavily contaminated with zona fasciculata cells.
Figure 1.
Steroidogenic pathways and enzymes leading to the synthesis of aldosterone in the zona glomerulosa, cortisol in the zona fasciculata and 18-oxocortisol in the zona glomerulosa. The potential contribution of the substrate 11-deoxycortisol for action by CYP11B2 or the alternative contribution of the CYP11B1 by synthesizing cortisol and reaching the zona glomerulosa by either from the circulation or by a paracrine mode.
Stable transfected CHO cells with the cDNAs of the CYP11B1 and the CYP11B2 and incubated with cortisol were both capable of transforming the cortisol to 18-hydroxycortisol, but the CYP11B2 was more efficient (10). Conversion of cortisol to 18-oxocortisol was only done in cells transfected with the CYP11B2 cDNA. Administration of 20 mg hydrocortisone twice daily to normal individuals who had suppressed endogenous cortisol secretion by dexamethasone showed that the urine 18-hydroxycortisol and 18-oxocortisol became detectable or increased, indicating that circulating cortisol could be converted to 18-oxocortisol by the adrenal (10). While these studies showed that circulating cortisol could be converted to 18-oxocortisol by the adrenal, it is hard to be certain that this is the main source of 18-oxocortisol in normal individuals as high doses of cortisol were used and the adrenal expresses high levels of the P-glycoprotein that promotes the excretion of polar steroids from the intracellular space to the outside of the cell (11, 12).
Biological activity
The qualitative biological activity of 18-oxocortisol is similar to aldosterone, but the potency is low with only 3–4% of that of cortisol as a glucocorticoid (13, 14) and 0.6–1.3% of that of aldosterone as a mineralocorticoid while 18-hydroxycortisol has no biological activity on either receptor (15). Administration of 18-oxocortisol to rats induced a mineralocorticoid-type hypertension with hypokalemia, cardiac and renal enlargement and cardiovascular lesions (16). Administration of 18-oxocortisol to sheep also caused a rapid increase in blood pressure (17). However, the doses required for increasing blood pressure were high.
Physiological regulation
The urinary excretion of 18-hydroxycortisol and 18-oxocortisol is increased by cosyntropin and inhibited by dexamethasone. Suppression by dexamethasone is mediated by inhibition of cortisol, the ultimate parent steroid precursor of these steroids. In addition to the pituitary-adrenal axis the renin-angiotensin system plays some role in regulating the secretion of both steroids. Dietary sodium restriction and sodium loading respectively increase and decrease the urinary excretion of both steroids (18, 19). In addition, a small study in healthy subjects showed that supine rest for two hours had no effect on the plasma levels of 18-hydroxycortisol and 18-oxocortisol but two hours in the standing position increased plasma levels by 45–50%, although substantially less than that of aldosterone (180%). However, the absence of such elevations after standing following dexamethasone suppression indicated that ACTH is a more potent physiological regulator than the renin-angiotensin system (20). Administration of Zn-ACTH twice daily for 5 days resulted in a large increase of urinary tetrahydrocortisol and tetrahydrocortisone; urinary aldosterone increased reaching a peak on the second day and decreasing to baseline levels on the fifth day (21). 18-hydroxycortisol and 18-oxocortisol remained high through the five days of ACTH gel administration further demonstrating that ACTH has a greater role in their regulation as that compared to aldosterone (21).
Assays for measurement of 18-hydroxycortisol and 18-oxocortisol
The first assay for the measurement of 18-hydroxycortisol in urine was done by gas chromatography mass spectrometry (3, 7). Shortly thereafter radioimmunoassays were developed for urine or plasma either done by a direct assay (22) or after initial purification of the urine sample by HPLC (18). In the urine the HPLC-RIA assay gave significantly lower excretion rates. Direct measurements of 18-hydroxycortisol in plasma and urine were later carried out using a solid-phase extraction and a more specific antibody (23, 24). More recently HPLC-MS/MS has been used for the measurement in plasma (25).
Assays for urinary 18-oxocortisol were initially described using an HPLC-RIA (26) and later on, after describing the metabolism of 18-oxocortisol, this was done by measuring the metabolite tetrahydro-18-oxocortisol in the urine by GC-MS (27). Plasma levels were determined by radioimmunoassay after solid phase extraction and celite purification (20). The development of a very specific monoclonal antibody to 18-oxocortisol (28) led to a more direct ELISA with values significantly lower than those measured by RIA (24). More recently LC-MS/MS was used (25) and the results are very similar to the measurement results by the ELISA assay using the monoclonal antibody (24).
LC-MS/MS measurements have been used to determine the levels of 18-hydroxycortisol and 18-oxocortisol from adrenal vein samples to help in the differentiation of aldosterone-producing adenomas and bilateral hyperplasia (BAH) (29, 30).
18-hydroxycortisol and 18-oxocortisol in healthy subjects and hypertensive patients
After the first identification of both steroids in humans (3), several studies confirmed the presence of both steroids in plasma and urine. In normotensive subjects the urinary excretion of 18-hydroxycortisol appeared to be nearly 10-fold higher than that of 18-oxocortisol (31). Using a HPLC-RIA assay, Gomez-Sanchez et al found an even higher urinary 18-hydroxycortisol/18-oxocortisol ratio (± 35) (18). In contrast, a lower ratio of these steroids was established in plasma by Yamakita et al who showed an about 3-fold higher plasma concentration of 18-hydroxycortisol than that of 18-oxocortisol (32). In a more recent study serum 18-hydroxycortisol levels appeared to be in a similar range as in the latter study (23) (Table 1).
Table 1.
Supine plasma concentrations of 18-hydroxycortisol and 18-oxocortisol in healthy subjects and in patients with hypertension and adrenal disease
| Author (ref) | Normotensive subjects | Patients with hypertension | Patients with APA | Patients with BAH | |
|---|---|---|---|---|---|
| Mosso (23) | 18-hydroxycortisol | 2.70±1.41 (n=102) | 2.81 ± 1.42 (n=101) | - | - |
| Yamakita (32) | 18-hydroxycortisol | 3.29±0.18 (n=47) | - | - | - |
| Yamakita (32) | 18-oxocortisol | 0.83±0.04 (n=47) | - | - | - |
| Eisenhofer (25) | 18-hydroxycortisol | - | 1.67 (0.45–4.33) (n=525)* | 4.49 (1.88–8.61) (n=126) | 3.54 (1.42–6.37) (n=90) |
| Eisenhofer (25) | 18-oxocortisol | - | 0.02 (<0.09) (n=525)* | 0.316 (0.032–0.133) (n=126) | 0.037 (0.027–0.332) (n=90) |
| Mulatero (24) | 18-hydroxycortisol | - | 2.24 (1.82–2.72) (n=62) ** | 4.60 (3.78–9.13) (n=20) | 2.63 (2.29–3.93) (n=61) |
| Mulatero (24) | 18-oxocortisol | - | 0.087 (0.002–0.117) (n=62) ** | 0.25 (0.17–0.28) (n=20) | 0.119 (0.084–0.181) (n=61) |
| Satoh (39) | 18-hydroxycortisol | – | - | 9.43 ± 0.91 (n=113) | 3.40 ± 0.19 (n=121) |
| Satoh (39) | 18-oxocortisol | - | - | 0.63 ± 0.09 (n=113) | 0.050 ± 0.004 (n=121) |
APA, aldosterone producing adenoma; BAH, bilateral hyperplasia
The quantitative data as provided by the different authors are as follows: Eisenhofer: median (2.5/97.5 %); Mosso: mean±SD; Mulatero: median (25/75%); Satoh: mean±SEM; Yamakita: mean±SEM
including 247 normotensives;
low-renin hypertension
For conversion of nmol/L to ng/dL multiply by 37.82 for 18-hydroxycortisol and by 37.65 for 18-oxocortisol
In patients with hypertension, the urinary excretions of both 18-hydroxycortisol and 18-oxocortisol were not different from normotensive controls and this applied to both low-renin and normal-renin hypertension (33). The same was the case for serum 18-hydroxycortisol. In a more recent study in a large group of essential hypertensives, serum 18-hydroxycortisol was not significantly different from normotensive subjects and this was the case for both low- and normal-renin hypertensives (23) (Table 1).
To contribute significantly to the pathogenesis of primary hypertension one would expect a strongly elevated secretion of one or both steroids in view of their very low biological potency. Therefore, these data do not support an abnormal secretion of both hybrids in hypertensives. As a corollary, elevations in any of these steroids should be a clue to explore an underlying cause of adrenal disease such as an APA or FH type 1 as both disorders are associated with elevations of both steroids (26, 34).
18-hydroxycortisol and 18-oxocortisol in patients with primary aldosteronism
All studies that assessed plasma and urinary levels of both hybrid steroids established clearly higher levels in patients with primary aldosteronism as compared to patients with primary hypertension. A first study reporting an increased urinary excretion of 18-hydroxycortisol using gas chromatographic mass spectrometry in patients with primary aldosteronism, noticed that this applied predominantly to patients with APA and not to patients with bilateral hyperplasia (BAH) (3). The excretion of 18-oxocortisol was found to be even more strongly increased in patients with FH type 1 (26).
Further studies confirmed later that both serum levels and urinary excretion of 18-hydroxycortisol and 18-oxocortisol were elevated in patients with primary aldosteronism but only in patients with APAs in contrast to the normal excretion in patients with BAH (31, 35). There was however overlap between the results of both patient groups which was ascribed to a few patients with angiotensin II (AII)-responsive APAs showing normal excretion of hybrid steroids (36, 37).
Similar results were described for 18-hydroxycortisol and 18-oxocortisol in serum (23, 38) and for urinary 18-oxocortisol using immunoassays (38). The study by Mosso et al reported a more than 2-fold elevation of 18-hydroxycortisol in patients with primary aldosteronism as compared to normo- and hypertensive patients. The study by Stowasser showed a nearly 5-fold elevation of 18-oxocortisol in AII-unresponsive APAs but not in most AII responsive APAs and BAH. The results of both studies were confirmed by Mulatero (24). Although that study reported also higher urinary excretion of both steroids in APAs as compared to BAH patients, levels in BAH patients were slightly but significantly higher than in low-renin hypertension and normotensives.
More importantly, the finding that no APA patient had a urinary excretion of 18-hydroxycortisol of less than 130 μg/day implicated that such patients (estimated as one third of all patients with a positive aldosterone/renin ratio) could refrain from confirmatory testing (Table 1). More recently it was demonstrated again that plasma 18-oxocortisol was about 12-fold higher in patients with APA than in those with BAH while this was only nearly 3-fold for 18-hydroxycortisol (39) (Table 1). Plasma 18-oxocortisol was positively correlated with plasma aldosterone levels in both groups of patients but plasma 18-hydroxycortisol showed this correlation only in the patients with APA.
The mechanism of the enhanced secretion of hybrid steroids and in particular of 18-oxocortisol in APA patients seems qualitatively similar as in patients with FH type 1. Both steroids display zona fasciculata characteristics as both are 17α-hydroxylated. Many APA have phenotypical characteristics of lipid laden cells as in the zona fasciculata and there is a mixture of cells that express CYP11B2, CYP11B1 and cells that co-express both the CYP11B2, CYP11B1 and cells that co-express the CYP11B1 and CYP17A1 and CYP11B2 and CYP17A1 (40, 41). Loss of functional zonation of the adrenal adenoma in patients with APAs enables cortisol to be exposed to aldosterone synthase, thus resulting in excessive production of C18-oxygenated steroids.
After the initial studies showing elevations of both steroids in patients with APA, it was surmised that these steroids and in particular 18-oxocortisol might contribute to the development and severity of hypertension in patients with primary aldosteronism. This assumption was based on the observations that in some patients the severity of hypertension was dissociated from elevations in aldosterone secretion (3, 42), thus leaving the possibility of involvement of other mineralocorticoid steroids. However, it is unlikely that the hybrid steroids play a significant role in the elevation of the blood pressure or metabolic abnormalities in primary aldosteronism due to the absent or low biological activity of both steroids (15, 17).
18-hydroxycortisol and 18-oxocortisol for the differential diagnosis of primary aldosteronism
Since it was reported that patients with APAs have an elevated secretion of both 18-hydroxycortisol and 18-oxocortisol in contrast to patients with BAH (3), several studies have investigated the diagnostic value of both steroids to distinguish APAs from BAH based on measurement of these steroids in a peripheral or adrenal plasma sample or in a 24-hours urine sample. Ulick et al established clear differences in the urinary excretion of both steroids between APA and BAH patients and they suggested cut-off levels of 15 μg/day and 60 μg/day for 24-hour urinary excretion of 18-oxocortisol and 18-hydroxycortisol respectively for separation of both subtypes (31). However these cut-off levels have not been validated in prospective studies.
A later small retrospective study on urinary excretion of 18-hydroxycortisol using 3 different assays showed that the value for the differential diagnosis was method dependent; they could establish a 100% separation between APA versus BAH and primary hypertension but this applied only to the immunoassay with fluorimetric detection (43).
This study was followed by a report on peripheral plasma levels and 24-hour urinary excretions of 18-hydroxycortisol and 18-oxocortisol before and after salt loading (24). Urinary 18-hydroxycortisol could not distinghuish APA from BAH because of gross overlap between groups. Although the sensitivity was only 30% when using a cut-off 510 μg/day, levels > 510 μg/day were diagnostic for APA, suggesting that such patients could be sent straight for adrenalectomy if they have a unilateral adrenal nodule and after genetic exclusion of FH type 1. Urinary 18-oxocortisol excretion was also lower in BAH than in APA but was still higher than in low-renin hypertension and normotensives. Nevertheless also for this compound there was considerable overlap between both subtypes of primay aldosteronism. A similar pattern was found for serum 18-hydroxycortisol and 18-oxocortisol and this was the case both before and after saline loading.
A large more recent study using measurements by LC-MS/MS showed that all patients with BAH had plasma 18-oxocortisol levels of less than 6.1 ng/dL or a plasma aldosterone of less than 32.7 ng/dL. In contrast, 84% of CT–diagnosed APAs had higher values, conferring a sensitivity of 84% (39). As no patient with APA had a plasma 18-oxocortisol of less than 1.2 ng/dL, the authors suggested the use of these cut-off levels of this compound in the diagnostic work-up of patients with primary aldosteronism. However this recommended strategy awaits further validation.
Consistent with previous studies similar results were reported by Eisenhofer et al who showed that peripheral venous plasma concentrations of 18-oxocortisol were about 8.5 times higher than in patients with BAH but again, there was considerable overlap between both groups (29). In contrast, 18-hydroxycortisol was only 1.25 times higher in patients with APA than in those with BAH. There is one additional study that demonstrated that peripheral venous plasma 18-oxocortisol concentrations were about 21-fold higher in APAs carrying KCNJ5 mutations compared with the wild-type group, while this was 16-fold higher than in APA patients with other mutations (44). A smaller difference was found for 18-hydroxycortisol which was only 2.9-fold higher among patients with APAs with KCNJ5 mutations than in the wild-type APAs (Table 1). Apparently, venous plasma concentrations of 18-oxocortisol are specific biomarkers of APAs carrying a KCNJ5 mutation.
A few studies reported on the use of the hybrid steroids measured in adrenal vein plasma samples (29, 30). In a retrospective study Nakamura et al used 18-oxocortisol instead of aldosterone in the 18-oxocortisol/cortisol ratio. They found the 18-oxocortisol levels and the 18-oxocortisol/cortisol ratios to be higher in adrenal veins emanating from APAs than in those from the contralateral non-diseased adrenal glands and those from patients with BAH (30). Although these data are promising, this study did not demonstrate that the 18-oxocortisol/cortisol ratio performed better than the conventional aldosterone/cortisol ratio to prove unilateral aldosterone production and to determine the subype of primary aldosteronism.
Finally, in a recent retrospective study in 206 patients with primary aldosteronism, the lateralization ratios for 18-oxocortisol and 18-hydroxycortisol normalized to cortisol were higher in patients with APAs compared to patients with BAH (29). In the APA group 76% had an elevated lateralization index for 18-oxocortisol/cortisol ratio while this was only 35% in the BAH group, thus underlining the limited value of adrenal venous plasma hybrid steroids for the differentiation of APA and BAH.
18-hydroxycortisol and 18-oxocortisol in patients with FH type 1
In 1980 the first two patients with dexamethasone-suppressible hyperaldosteronism were described. This new hypertensive syndrome came later known as glucorticoid remediable aldosteronism (GRA) or FH type 1 (45). The suppressibility of aldosterone with normalization of blood pressure by dexamethasone documented that aldosterone secretion was under ACTH control. A few years later Gomez-Sanchez et al demonstrated a nearly 10-fold elevated urinary excretion of 18-oxocortisol as compared to normal subjects (21). The molecular cause of this rare variant of familial aldosteronism was elucidated in the early nineties by Lifton (5) and Pascoe (46). As a result of unequal crossover during meiosis a chimaeric gene comprising the coding sequences of the CYP11B2 is expressed in the zona fasciculata where it is co-localised with CYP17A1 and cortisol which serves as a substrate for aldosterone synthase, thus explaining the suppressibility of aldosterone by ACTH. The very strong elevation (8-20 fold) of 18-oxocortisol in these patients was later confirmed by several other studies (24, 34, 38) and to a lesser degree this was also the case for 18-hydroxycortisol (24). It has to be noted however that there is overlap between the hormone levels in these patients and patients with APAs. Nevertheless it has been suggested that measurement of these hybrid steroids is a better diagnostic tool than the dexamethasone suppression test (47).
In conclusion, the diagnostic utility of measurement of these hybrid steroids is limited. In young hypertensive patients with primary aldosteronism, a strong elevation of one or both hybrid steroids may indicate the presence of familial hyperaldosteronism type I (GRA). Patients with primary aldosteronism due to an APA have higher plasma levels of both steroids as compared to patients with BAH and this pertains particularly to 18-oxocortisol. Yet there is considerable overlap between both subtypes, limiting the diagnostic utility of measurement of these hybrid steroids for this purpose. Further prospective studies are required to better determine the diagnostic place of these hybrid steroids in patients with primary aldosteronism.
Acknowledgments
Research reported in this publication was supported by National Heart, Lung and Blood Institute grant R01 HL27255 (CEGS), the National Institute of General Medical Sciences grant U54 GM115428 (CEGS). CEGS was a Visiting Fellow with the Center for Advanced Studies of the Ludwig Maximilian University of Munich. MR was supported by a grant from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No [694913]), by a grant of the Else Kröner-Fresenius Stiftung in support of the German Conn’s Registry-Else-Kröner Hyperaldosteronism Registry (2013_A182 and 2015_A171) and by grants from the Deutsche Forschungsgemeinschaft (RE 752/20-1); TAW, JL and MR were supported by the Deutsche Forschungsgemeinschaft (DFG) within the CTC/Transregio 205/1, project B15, “The Adrenal: Central Relay in Health and Disease“.
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- 1.Akhtar M, Barton DHR, Beaton JM, Hortmann A. The synthesis of substituted aldosterone. J Am Chem Soc. 1963;85:1512–1519. [Google Scholar]
- 2.Wieland P, Heusler K, Wettstein A. 18-Oxygenierte Derivate des Hydrocortisons. Über Steroide Helv Chim Acta. 1960;44:617–623. [Google Scholar]
- 3.Chu MD, Ulick S. Isolation and identification of 18-hydroxycortisol from the urine of patients with primary aldosteronism. J Biol Chem. 1982;257:2218–2224. [PubMed] [Google Scholar]
- 4.Ulick S, Chu MD, Land M. Biosynthesis of 18-oxocortisol by aldosterone-producing adrenal tissue. J Biol Chem. 1983;258:5498–5502. [PubMed] [Google Scholar]
- 5.Lifton RP, Dluhy RG, Powers M, Rich GM, Cook S, Ulick S, Lalouel JM. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355:262–265. doi: 10.1038/355262a0. [DOI] [PubMed] [Google Scholar]
- 6.Geller DS, Zhang J, Wisgerhof MV, Shackleton C, Kashgarian M, Lifton RP. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 2008;93:3117–3123. doi: 10.1210/jc.2008-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulick S, Chu MD. Hypersecretion of a new corticosteroid, 18-hydroxycortisol in two types of adrenocortical hypertension. Clin Exp Hypertens A. 1982;4:1771–1777. doi: 10.3109/10641968209061640. [DOI] [PubMed] [Google Scholar]
- 8.Kawamoto T, Mitsuuchi Y, Toda K, Yokoyama Y, Miyahara K, Miura S, Ohnishi T, Ichikawa Y, Nakao K, Imura H, et al. Role of steroid 11β-hydroxylase and steroid 18-hydroxylase in the biosynthesis of glucocorticoids and mineralocorticoids in humans. Proc Natl Acad Sci U S A. 1992;89:1458–1462. doi: 10.1073/pnas.89.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Sanchez CE, Ferris MW, Foecking MF, Gomez-Sanchez EP. Synthesis of 18-hydroxycortisol and 18-oxocortisol in bovine adrenal slices. J Steroid Biochem. 1989;33:595–598. doi: 10.1016/0022-4731(89)90046-0. [DOI] [PubMed] [Google Scholar]
- 10.Freel EM, Shakerdi LA, Friel EC, Wallace AM, Davies E, Fraser R, Connell JM. Studies on the origin of circulating 18-hydroxycortisol and 18-oxocortisol in normal human subjects. J Clin Endocrinol Metab. 2004;89:4628–4633. doi: 10.1210/jc.2004-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Kalken CK, Broxterman HJ, Pinedo HM, Feller N, Dekker H, Lankelma J, Giaccone G. Cortisol is transported by the multidrug resistance gene product P-glycoprotein. Br J Cancer. 1993;67:284–289. doi: 10.1038/bjc.1993.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bello-Reuss E, Ernest S, Holland OB, Hellmich MR. Role of multidrug resistance P-glycoprotein in the secretion of aldosterone by human adrenal NCI-H295 cells. Am J Physiol Cell Physiol. 2000;278:C1256–1265. doi: 10.1152/ajpcell.2000.278.6.C1256. [DOI] [PubMed] [Google Scholar]
- 13.Ulick S, Land M, Chu MD. 18-oxocortisol, a naturally occurring mineralocorticoid agonist. Endocrinology. 1983;113:2320–2322. doi: 10.1210/endo-113-6-2320. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Sanchez CE, Gomez-Sanchez EP, Smith JS, Ferris MW, Foecking MF. Receptor binding and biological activity of 18-oxocortisol. Endocrinology. 1985;116:6–10. doi: 10.1210/endo-116-1-6. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Sanchez EP, Gomez-Sanchez CE, Smith JS, Ferris MW, Foecking M. Receptor binding and biological activity of 18-hydroxycortisol. Endocrinology. 1984;115:462–466. doi: 10.1210/endo-115-2-462. [DOI] [PubMed] [Google Scholar]
- 16.Hall CE, Gomez-Sanchez CE. Hypertensive potency of 18-oxocortisol in the rat. Hypertension. 1986;8:317–322. doi: 10.1161/01.hyp.8.4.317. [DOI] [PubMed] [Google Scholar]
- 17.Spence CD, Coghlan JP, Denton DA, Gomez-Sanchez C, Mills EH, Whitworth JA, Scoggins BA. Blood pressure and metabolic effects of 18-oxo-cortisol in sheep. J Steroid Biochem. 1987;28:441–443. doi: 10.1016/0022-4731(87)91064-8. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Sanchez CE, Upcavage RJ, Zager PG, Foecking MF, Holland OB, Ganguly A. Urinary 18-hydroxycortisol and its relationship to the excretion of other adrenal steroids. J Clin Endocrinol Metab. 1987;65:310–314. doi: 10.1210/jcem-65-2-310. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Sanchez CE, Zager PG, Foecking MF, Holland OB, Ganguly A. 18-oxocortisol: effect of dexamethasone, ACTH and sodium restriction. J Steroid Biochem. 1989;32:409–412. doi: 10.1016/0022-4731(89)90214-8. [DOI] [PubMed] [Google Scholar]
- 20.Yamakita N, Gomez-Sanchez CE, Mune T, Yoshida H, Miyazaki S, Yasuda K, Nakai T. Regulation of 18-oxocortisol and 18-hydroxycortisol by the renin-angiotensin system and ACTH in man. J Steroid Biochem Mol Biol. 1993;46:395–399. doi: 10.1016/0960-0760(93)90230-t. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Sanchez CE, Clore JN, Estep HL, Watlington CO. Effect of chronic adrenocorticotropin stimulation on the excretion of 18-hydroxycortisol and 18-oxocortisol. J Clin Endocrinol Metab. 1988;67:322–326. doi: 10.1210/jcem-67-2-322. [DOI] [PubMed] [Google Scholar]
- 22.Corrie JE, Edwards CR, Budd PS. A radioimmunoassay for 18-hydroxycortisol in plasma and urine. Clin Chem. 1985;31:849–852. [PubMed] [Google Scholar]
- 23.Mosso L, Gomez-Sanchez CE, Foecking MF, Fardella C. Serum 18-hydroxycortisol in primary aldosteronism, hypertension, and normotensives. Hypertension. 2001;38:688–691. doi: 10.1161/01.hyp.38.3.688. [DOI] [PubMed] [Google Scholar]
- 24.Mulatero P, di Cella SM, Monticone S, Schiavone D, Manzo M, Mengozzi G, Rabbia F, Terzolo M, Gomez-Sanchez EP, Gomez-Sanchez CE, Veglio F. 18-hydroxycorticosterone, 18-hydroxycortisol, and 18-oxocortisol in the diagnosis of primary aldosteronism and its subtypes. J Clin Endocrinol Metab. 2012;97:881–889. doi: 10.1210/jc.2011-2384. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhofer G, Peitzsch M, Kaden D, Langton K, Pamporaki C, Masjkur J, Tsatsaronis G, Mangelis A, Williams TA, Reincke M, Lenders JWM, Bornstein SR. Reference intervals for plasma concentrations of adrenal steroids measured by LC-MS/MS: Impact of gender, age, oral contraceptives, body mass index and blood pressure status. Clin Chim Acta. 2017;470:115–124. doi: 10.1016/j.cca.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez-Sanchez CE, Montgomery M, Ganguly A, Holland OB, Gomez-Sanchez EP, Grim CE, Weinberger MH. Elevated urinary excretion of 18-oxocortisol in glucocorticoid-suppressible aldosteronism. J Clin Endocrinol Metab. 1984;59:1022–1024. doi: 10.1210/jcem-59-5-1022. [DOI] [PubMed] [Google Scholar]
- 27.Ulick S, Chu MD. Isolation and identification of an endogenous metabolite of 18-oxocortisol from human urine. J Steroid Biochem. 1987;28:89–94. doi: 10.1016/0022-4731(87)90129-4. [DOI] [PubMed] [Google Scholar]
- 28.Morra di Cella S, Veglio F, Mulatero P, Christensen V, Aycock K, Zhu Z, Gomez-Sanchez EP, Gomez-Sanchez CE. A time-resolved fluoroimmunoassay for 18-oxocortisol and 18-hydroxycortisol. Development of a monoclonal antibody to 18-oxocortisol. J Steroid Biochem Mol Biol. 2002;82:83–88. doi: 10.1016/s0960-0760(02)00142-5. [DOI] [PubMed] [Google Scholar]
- 29.Eisenhofer G, Dekkers T, Peitzsch M, Dietz AS, Bidlingmaier M, Treitl M, Williams TA, Bornstein SR, Haase M, Rump LC, Willenberg HS, Beuschlein F, Deinum J, Lenders JW, Reincke M. Mass Spectrometry-Based Adrenal and Peripheral Venous Steroid Profiling for Subtyping Primary Aldosteronism. Clin Chem. 2016;62:514–524. doi: 10.1373/clinchem.2015.251199. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura Y, Satoh F, Morimoto R, Kudo M, Takase K, Gomez-Sanchez CE, Honma S, Okuyama M, Yamashita K, Rainey WE, Sasano H, Ito S. 18-oxocortisol measurement in adrenal vein sampling as a biomarker for subclassifying primary aldosteronism. J Clin Endocrinol Metab. 2011;96:E1272–1278. doi: 10.1210/jc.2010-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulick S, Blumenfeld JD, Atlas SA, Wang JZ, Vaughan ED., Jr The unique steroidogenesis of the aldosteronoma in the differential diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 1993;76:873–878. doi: 10.1210/jcem.76.4.8473399. [DOI] [PubMed] [Google Scholar]
- 32.Yamakita N, Gomez-Sanchez CE, Mune T, Morita H, Yoshida H, Miyazaki S, Yasuda K. Simultaneous measurement of plasma 18-oxocortisol and 18-hydroxycortisol levels in normal man. Eur J Endocrinol. 1994;131:74–79. doi: 10.1530/eje.0.1310074. [DOI] [PubMed] [Google Scholar]
- 33.Gomez-Sanchez CE, Gomez-Sanchez EP, Holland OB. Role of 18-hydroxylated cortisols in hypertension. J Steroid Biochem. 1987;27:971–975. doi: 10.1016/0022-4731(87)90176-2. [DOI] [PubMed] [Google Scholar]
- 34.Ulick S, Chan CK, Gill JR, Jr, Gutkin M, Letcher L, Mantero F, New MI. Defective fasciculata zone function as the mechanism of glucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 1990;71:1151–1157. doi: 10.1210/jcem-71-5-1151. [DOI] [PubMed] [Google Scholar]
- 35.Yamakita N, Mune T, Morita H, Yasuda K, Miura K, Gomez-Sanchez CE. Plasma 18-oxocortisol levels in the patients with adrenocortical disorders. Clin Endocrinol (Oxf) 1994;40:583–587. doi: 10.1111/j.1365-2265.1994.tb03008.x. [DOI] [PubMed] [Google Scholar]
- 36.Gordon RD, Hamlet SM, Tunny TJ, Klemm SA. Aldosterone-producing adenomas responsive to angiotensin pose problems in diagnosis. Clin Exp Pharmacol Physiol. 1987;14:175–179. doi: 10.1111/j.1440-1681.1987.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 37.Gordon RD, Hamlet SM, Tunny TJ, Gomez-Sanchez CE, Jayasinghe LS. Distinguishing aldosterone-producing adenoma from other forms of hyperaldosteronism and lateralizing the tumour pre-operatively. Clin Exp Pharmacol Physiol. 1986;13:325–328. doi: 10.1111/j.1440-1681.1986.tb00357.x. [DOI] [PubMed] [Google Scholar]
- 38.Stowasser M, Bachmann AW, Tunny TJ, Gordon RD. Production of 18-oxo-cortisol in subtypes of primary aldosteronism. Clin Exp Pharmacol Physiol. 1996;23:591–593. doi: 10.1111/j.1440-1681.1996.tb02789.x. [DOI] [PubMed] [Google Scholar]
- 39.Satoh F, Morimoto R, Ono Y, Iwakura Y, Omata K, Kudo M, Takase K, Seiji K, Sasamoto H, Honma S, Okuyama M, Yamashita K, Gomez-Sanchez CE, Rainey WE, Arai Y, Sasano H, Nakamura Y, Ito S. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. 2015;65:1096–1102. doi: 10.1161/HYPERTENSIONAHA.114.04453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura Y, Kitada M, Satoh F, Maekawa T, Morimoto R, Yamazaki Y, Ise K, Gomez-Sanchez CE, Ito S, Arai Y, Dezawa M, Sasano H. Intratumoral heterogeneity of steroidogenesis in aldosterone-producing adenoma revealed by intensive double- and triple-immunostaining for CYP11B2/B1 and CYP17. Mol Cell Endocrinol. 2016;422:57–63. doi: 10.1016/j.mce.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura Y, Maekawa T, Felizola SJ, Satoh F, Qi X, Velarde-Miranda C, Plonczynski MW, Ise K, Kikuchi K, Rainey WE, Gomez-Sanchez EP, Gomez-Sanchez CE, Sasano H. Adrenal CYP11B1/2 expression in primary aldosteronism: immunohistochemical analysis using novel monoclonal antibodies. Mol Cell Endocrinol. 2014;392:73–79. doi: 10.1016/j.mce.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi GP, Gioco F, Fassina A, Gomez-Sanchez CE. Normoaldosteronemic aldosterone-producing adenoma: immunochemical characterization and diagnostic implications. J Hypertens. 2015;33:2546–2549. doi: 10.1097/HJH.0000000000000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reynolds RM, Shakerdi LA, Sandhu K, Wallace AM, Wood PJ, Walker BR. The utility of three different methods for measuring urinary 18-hydroxycortisol in the differential diagnosis of suspected primary hyperaldosteronism. Eur J Endocrinol. 2005;152:903–907. doi: 10.1530/eje.1.01922. [DOI] [PubMed] [Google Scholar]
- 44.Williams TA, Peitzsch M, Dietz AS, Dekkers T, Bidlingmaier M, Riester A, Treitl M, Rhayem Y, Beuschlein F, Lenders JW, Deinum J, Eisenhofer G, Reincke M. Genotype-Specific Steroid Profiles Associated With Aldosterone-Producing Adenomas. Hypertension. 2016;67:139–145. doi: 10.1161/HYPERTENSIONAHA.115.06186. [DOI] [PubMed] [Google Scholar]
- 45.New MI, Oberfield SE, Levine LS, Dupont B, Pollack M, Gill JR, Jr, Bartter FC. Autosomal dominant transmission and absence of HLA linkage in dexamethasone suppressible hyperaldosteronism. Lancet. 1980;1:550–551. doi: 10.1016/s0140-6736(80)92814-7. [DOI] [PubMed] [Google Scholar]
- 46.Pascoe L, Curnow KM, Slutsker L, Connell JM, Speiser PW, New MI, White PC. Glucocorticoid-suppressible hyperaldosteronism results from hybrid genes created by unequal crossovers between CYP11B1 and CYP11B2. Proc Natl Acad Sci U S A. 1992;89:8327–8331. doi: 10.1073/pnas.89.17.8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lifton RP, Dluhy RG, Powers M, Rich GM, Gutkin M, Fallo F, Gill JR, Jr, Feld L, Ganguly A, Laidlaw JC, et al. Hereditary hypertension caused by chimaeric gene duplications and ectopic expression of aldosterone synthase. Nat Genet. 1992;2:66–74. doi: 10.1038/ng0992-66. [DOI] [PubMed] [Google Scholar]