Abstract
Background
The Multiple Endocrine Neoplasia, type 1 (MEN1) locus encodes the nuclear tumor suppressor protein menin. MEN1 mutations frequently cause neuroendocrine tumors (NETs) such as gastrinomas, remarkable for their predominant duodenal location and local metastasis at the time of diagnosis. Diffuse gastrin cell hyperplasia precedes the appearance of MEN1 gastrinomas, which develop within submucosal Brunner’s glands. We show here that loss of menin in enteric glial cells induces gastrin expression.
Aim
To determine how menin regulates gastrin gene expression and induces the generation of submucosal gastrin-expressing cell hyperplasia.
Methods
Primary enteric glial cultures were generated from the Villin-Cre:Men1FL/FL:Sst−/− mice with or without inhibition of gastric acid using omeprazole. In addition, primary enteric glial cells from wild type mice were treated with gastrin and were separated into nuclear and cytoplasmic fractions. Forskolin and H89 treatments were used to activate or inhibit protein kinase A activity. Immunoprecipitation with menin or ubiquitin was used to demonstrate posttranslational modification of menin. Primary glial cells were treated with Leptomycin b and MG132 to block nuclear export and proteasome activity, respectively.
Results
Gfap+ enteric glial cells expressed gastrin de novo through a feedforward PKA-dependent mechanism. Gastrin-induced nuclear export of menin through Cckbr-mediated PKA activation. Once exported menin was ubiquitinated and degraded by the proteasome. Gfap and other enteric glial markers co-localized with gastrin in human duodenal gastrinomas.
Conclusion
Collectively, these results suggest that MEN1-associated gastrinomas, which develop in the submucosa might arise from enteric glial cells through hormone-dependent PKA signaling that abrogates menin function leading to hypergastrinemia and associated sequelae.
Keywords: Cckbr, somatostatin, MEN1, ubiquitin, GFAP, omeprazole
Introduction
Menin is the protein product of the Multiple Endocrine Neoplasia 1 (MEN1) gene locus at 11q13 and is a known tumor suppressor that contributes to the development of different endocrine tumors 1, 2. Germline mutations in MEN1 cause a clinical syndrome characterized by pituitary adenomas, hyperparathyroidism and foregut neuroendocrine tumors (NETs). MEN1 mutations are nonsense, missense or in-frame deletions distributed across the MEN1 gene locus, invariably inactivating its function 3–5. Gastrin-secreting neuroendocrine tumors (gastrinomas) comprise one the most frequent MEN1-associated tumors and up to 60% show lymph node metastases at the time of diagnosis 6. Approximately 25–33% of MEN1 patients develop gastrinomas, which are small, multi-focal lesions present in Brunner’s glands 5, tubular structures that secrete mucus, growth factors and bicarbonate located within the submucosa of the proximal duodenum 7. Therefore, MEN1-based gastrinomas do not appear to develop from the intestinal epithelium but instead arise from glands located in the submucosa 8, 9. Less than 50% of MEN1 gastrinomas develop loss of heterozygosity (LOH) suggesting that other mechanisms contribute to loss of WT menin protein function 8, 10, 11. Although MEN1 is the locus most frequently mutated in the germline of subjects with pancreatic neuroendocrine tumors (pNETS) 12 and gastrinomas 8, deletion of Men1 in mice does not result in gastrinomas suggesting synergy with other gene loci 2, 13–16.
Given its putative tumor suppressor role in MEN1 gastrinomas, we previously established that menin suppresses the human gastrin gene (GAST) 17. Chromatin immunoprecipitation (ChiP) confirmed the presence of menin at JUND and Sp1 DNA binding sites within the proximal GAST promoter indicating direct regulation of gene expression 17, 18. We recently reported the first mouse model of a gut endocrine tumor generated by combining conditional deletion of the Men1 locus (Villin-Cre;Men1FL/FL) with deletion of somatostatin (Sst−/−) and gastric acid suppression (omeprazole) to maximally induce gastrin 19. This approach resulted in gastric carcinoids, a tumor that develops from enterochromaffin-like (ECL) cells in response to hypergastrinemia 20. Furthermore, omeprazole treatment of these Men1ΔIEC; Sst−/− (OMS) mice increased their plasma gastrin levels to nearly 16-fold above the levels observed in wild type (WT) mice 20.
To determine whether duodenal gastrin also contributed to the hypergastrinemia as observed in MEN1 gastrinomas, the proximal duodenum was analyzed and found to contain gastrin-expressing cells in the lamina propria of the OMS mice. We report here that these gastrin-expressing cells co-localized with enteric glial cell markers and expressed gastrin in response to loss of menin protein in the cell nucleus.
Materials and Methods
Human samples
De-identified surgical samples of human gastrinoma from 1996 to 2007 were obtained from the Department of Pathology at the University of Michigan, Center for Tumor Biology Barts Cancer Institute, Queen Mary University, London and the Royal Liverpool University Hospital, Liverpool, UK and are listed in Table 1. Sample access was approved by University of Michigan IRB #HUM00115310.
Table 1.
Summary of Immunohistochemistry for Human Gastrinomas
| Patient | Tissue | Gastrin | GFAP | Menin (Cytoplasmic) |
|---|---|---|---|---|
| 03-3A | Duodenal Submucosa | + | + | + |
| GA-07- 1A,B | Duodenal Submucosa | + | + | + |
| 97-B4 | Duodenal Submucosa | + | + | + |
| 03-A38 | Duodenal Submucosa | + | + | + |
| 03-A19 | Lymph Node | + | + | + |
| 08-B12 | Lymph Node | + | + | + |
| 05-1X,Y | Duodenal Submucosa | + | − | + |
| 02-1A | Pancreas | + | − | + |
| 03-1B | Pancreas | + | − | + |
| 02-1M | Pancreas | + | − | + |
| GA-09-2A | Pancreas | + | − | + |
| 06-4C | Pancreas | + | − | + |
| 96-7A | Pancreas | + | + | + |
| 04-7B | Pancreas | + | − | + |
Animals and Cell Culture
All animal experiments were approved by the University of Michigan’s Committee on the Use and Care of Animals. Men1ΔIEC; Sst−/−; and Men1ΔIEC; Sst−/− mice were generated as previously described 20. Mice were housed in a facility with access to food and water ad libitum. Experimental mice were fed omeprazole-laced chow (200 ppm, TestDiet, St. Louis, Missouri, USA) for 6 months to 1 year. Male and female mice were equivalently distributed across the control and treatment groups. STC-1 cells derived from a mouse intestinal tumor were cultured in DMEM (with glutamine, without pyruvate) with 10% FBS 21, 22.
Primary Glial Culture Isolation
Tissues from 2 mice were pooled to isolate duodenal enteric glia. The first 6–8 cm of the proximal duodenum was dissected and then flushed with DPBS (without Ca++ or Mg++). First, the longitudinal muscle/myenteric plexus (LMMP) attached to the submucosa was isolated and discarded as described previously 23. To remove the LMMP, the duodenum was threaded onto the opposing end of a cotton swab. A small incision was made vertically, and the underlying longitudinal muscle was teased away by applying gentle horizontal strokes using a cotton swab wetted with DPBS. This was continued from top to bottom until the longitudinal muscle was slowly separated from the circular muscle and then discarded. Next, the epithelium was removed by incubating the tissue twice in EDTA/HEPES/DPBS (5mM/10 mM) solution at 4°C for 10 min with vigorous shaking, until the residual solution remained almost clear. After the last incubation, remnant tissue was removed using a 40μm nylon cell strainer and then incubated in Cell Recovery Solution® (Corning) for 30 min at 4°C with rocking. The cell suspension was vortexed briefly and spun at 2000g for 5 min. The pellet was re-suspended in DMEM/F12 medium supplemented with 10% FBS, 100 IU/mL penicillin, 100 μg/mL streptomycin, 20μg/mL gentamicin, and seeded in laminin and poly-D-lysine coated 6-well plate containing DMEM/F12 medium with 1% FBS, 10 μg/mL Glial Derived Neurotrophic Factor (GDNF), 100 IU/mL penicillin, 100 μg/mL streptomycin, and 20 μg/mL gentamicin. Cells attach by 6–8 hours and remain viable for 3–4 days.
Laser Capture Micro-dissection
About 8 μM thick frozen sections were mounted onto 2μM PEN membrane slides (Leica, Inc) for LCM. Slides were thawed for 15 minutes, fixed in pre-chilled 70% ethanol for 5 minutes, and blocked with 20% serum for 10 minutes. Sections were incubated with gastrin antibody (1:50) for 30 minutes (RT), washed with 0.01% PBST, and incubated with Alexa Fluor conjugated secondary (1:200) for 15 minutes (RT). Slides were further washed with PBST, dehydrated with 70% ethanol (1 min), 100 % ethanol (1 min), and air-dried in the dark. Slides prepared as above were used for micro-dissection on the Leica LCM microscope (Leica LMD 7000, Software version V7.6) using the following parameters: power-35, aperture–32, speed–close to “Minimum”, specimen balance–<15, offset–130, head current – close to “More”, pulse frequency – Close to “less”. Micro-dissectates were collected in 0.5 mL PCR tubes (Leica) containing 50μL cold RIPA lysis buffer with protease and phosphatase inhibitors, then snap-frozen immediately. Proteins were extracted by sonicating the lysates (30 amp, two 30-second pulses separated by 10 second interval on ice) and spun at 10,000g for 15 minutes (4°C). A cutting area of ~400,000 (equivalent to ~40 intact villi) was required to extract 25–75 ng of proteins to enable detection of target proteins.
Measurement of Gastrin in plasma, tissues, and cultures
Mice were fasted for 16 h before blood collection in heparin-coated tubes, and plasma was obtained by centrifugation at 5000g for 10 min (4°C). About 50 μL of the plasma was used for measuring gastrin levels using the Human/Mouse/Rat Gastrin-I Enzyme Immunoassay Kit (RayBiotech, Georgia, USA), per manufacturer’s instructions. Tissue gastrin was extracted by boiling 10–20 mg of tissue in 300 μL of deionized water and the supernatant was used for analyses as described above. For measuring the gastrin content of primary glial cultures, cultures were scraped into PBS, pelleted, and boiled in deionized water (volume equals 10 times the size of the pellet). The extract was spun briefly and the supernatant was used for gastrin measurements. Additional details are provided in Supplementary Materials.
Results
Gastrin-expressing cells identified in duodenal lamina propria
We previously showed that the OMS mice develop gastric carcinoids (GC) in the stomach corpus 20. Examination of the duodenums of these mice revealed numerous gastrin-positive cells in the lamina propria (LP-Gastrin+; Fig. 1A, B). LP-Gastrin+ cells almost disappeared 4 months after withdrawal of OM, respectively (Fig. 1C). H&E analysis revealed misshapen villi and dilated lamina propria in the OMS mice that returned to normal 4 months after withdrawing OM (Supplemental Fig. 1A). The LP-gastrin+ cells in the OMS mice correlated with a 3-fold increase in tissue levels of gastrin peptide (Supplemental Fig. 1B, top panel), a 50% increase in gastrin mRNA (Supplemental Fig. 1B, bottom panel) and a 3-fold increase in plasma gastrin (Supplemental Fig. 1C), compared to untreated MenΔIEC; Sst−/− mice. In particular, mRNA increased 200% in isolated LP alone, compared to 50% in the epithelium (Supplemental Fig. 1D). Parallel changes occurred in the gastric antrum of OMS mice (Supplemental Fig. 1E). Gastrin mRNA was at least 30-fold higher in the duodenum compared to the jejunum, and was barely detectable in the ileum and pancreas of MenΔ IEC; Sst−/− mice (Supplemental Fig. 2A). Therefore, gastrin expression in the OMS mice increased in both the antrum and duodenum, but not in the jejunum, ileum, or pancreas. While the number of Gastrin (G) cells increased in the antral epithelium 20, the increase in gastrin-expressing cells in the duodenum occurred mainly in the submucosa. Ki67 expression in G cells did not increase suggesting that expansion of gastrin+ cell numbers in both the antral and duodenal lamina propria was not due to increased proliferation (Supplemental Fig. 2B, C).
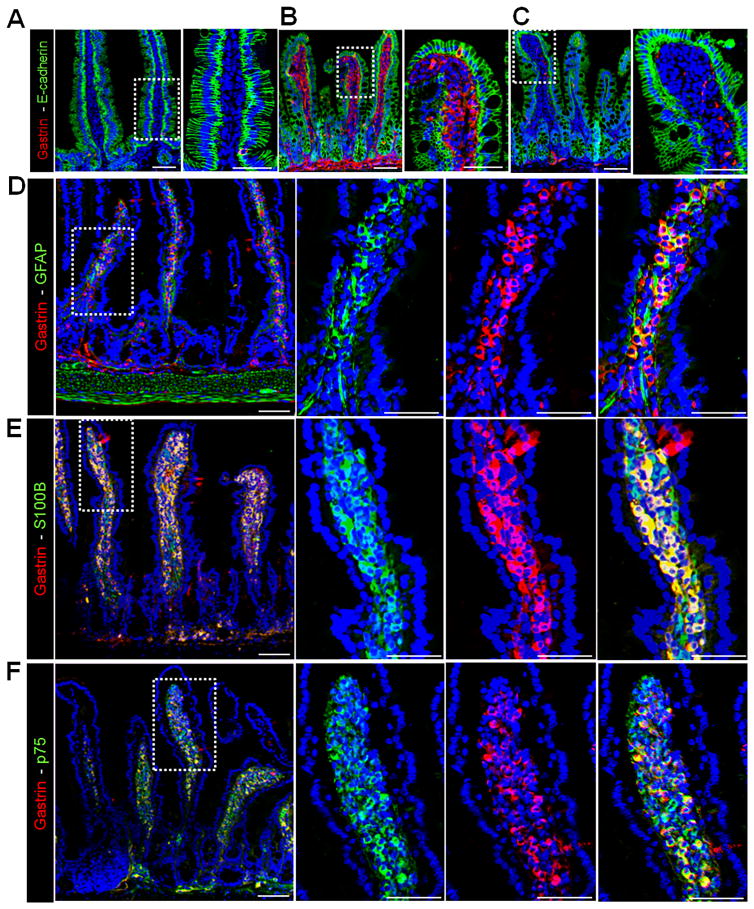
Figure 1. Gastrin-positive cells in the mucosal enteric glia of OMS mice.
(A) Immunofluorescent (IF) staining of gastrin and E-cadherin in duodenums of untreated Men1ΔIEC;Sst−/− mice, Men1ΔIEC;Sst−/− mice treated with (B) OM (OMS) for 6 months and (C) 4 months after withdrawal of OM (WD). (D–F) IF staining for gastrin and (D) Gfap, (E) S100b, and (F) p75 in duodenums of OMS mice. Scale bars: (A–C) - 50 μm, insets - 20μm, (D–F) - 50 μm, insets - 20 μm.
LP-Gastrin+ cells express enteric glial cell markers and secrete gastrin
To determine the type of cells expressing gastrin in the lamina propria, duodenal tissue from the OMS mice was stained with markers for epithelial (E-cadherin), neural (Pgp9.5), endothelial (Lyve-1) cells and myofibroblasts (Sma). The LP-gastrin+ cells were E-cadherin, Sma, Pgp9.5, and Lyve-1 negative, indicating that they did not originate from these cell types (Supplemental Fig. 2D, E). Primary glial cells isolated from the OMS mice that expressed gastrin were also negative for E-cadherin, Sma and Pgp9.5 (Supplemental Fig. 3A). By contrast, glial markers 24, 25, i.e., glial fibrillary acidic protein (Gfap), S100b, p75, Sox10, and the endocrine marker chromogranin A (CgA) co-localized with gastrin in both the duodenal lamina propria (Fig. 1D–F, Supplemental Fig. 3B), and in glial cultures isolated from the tissue (Supplemental Fig. 3C). Enteric glial cells originate from the neural crest’, consistent with their presence in the lamina propria and submucosa of the gut 26. Cultures from untreated WT mice isolated using the same protocol were also enriched in glial cells (Gfap+, S100b+, Sox10+) but were negative for gastrin (Supplemental Fig. 3D). Using flow cytometry, we determined that 93 percent of the glial cultures generated from duodenums of the OMS mice were comprised of Gfap+ glial cells with negligible contamination from Pgp9.5+ neural cells, α Sma+ myofibroblasts, and E-cadherin+ epithelial cells (Supplemental Fig. 4A–D).
Gastrin is secreted from antral G cells during a meal in response to the neuropeptides gastrin-releasing peptide Grp or bombesin (Bmb) released from enteric neurons 27. Therefore to determine whether the gastrin-positive enteric glial cells secreted gastrin peptide in response to a secretogogue, glial cells isolated from the villus cores of the OMS mice were treated with Bmb. Bmb stimulated the secretion of gastrin peptide into the media (Supplemental Fig. 5A). By contrast, Bmb did not stimulate gastrin secretion from enteric glial cells isolated from untreated MenΔIEC; Sst−/− mice. To demonstrate that glial cells in the duodenal mucosa of OMS mice express bombesin receptors, duodenal tissues were co-stained for GFAP and the Bombesin receptor 2 (BB2). We found that GFAP+ mucosal glia, and glia associated with submucosal myenteric plexi express BB2 (Supplemental Fig. 5B). Therefore, mucosal enteric glial cells from the OMS mice were capable of synthesizing and secreting the hormone gastrin.
Appearance of LP-Gastrin+ cells in the OMS mice correlates with loss of nuclear Menin
Robust nuclear expression of menin was observed in the epithelium and lamina propria of C57 WT mice but was expectedly absent in the epithelium of MenΔIEC and untreated MenΔIEC; Sst−/− duodenal villi, due to the targeted deletion of Men1 using the VillinCre transgene (Fig. 2A). However, after OM treatment, menin protein expression in the lamina propria was suppressed without changes in mRNA (Fig. 2A, Supplemental Fig. 6A), which correlated with a dramatic increase in plasma gastrin levels (~700 pg/ml). Upon OM withdrawal for 4 months, menin expression in the lamina propria cells was restored (Fig. 2A) and correlated with reduced plasma gastrin levels of 280 pg/ml (Supplemental Fig. 1C).
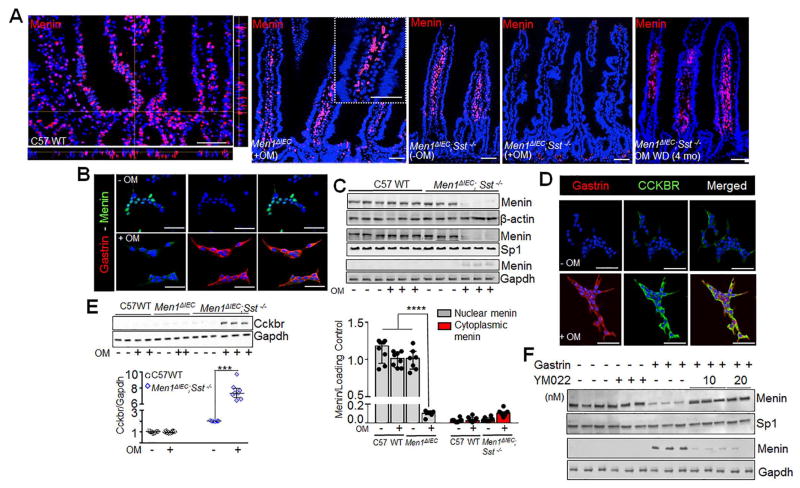
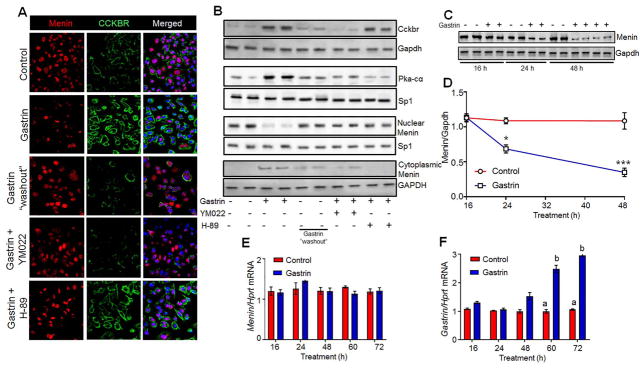
Figure 2. Gastrin induces nuclear export of menin via CCKBR activation.
(A) Left to right, IF staining of menin in duodenums of omeprazole (OM) treated C57 WT, Men1Δ IEC mice, untreated Men1ΔIEC;Sst−/−, and OMS mice. Far right panel, 4 months after withdrawal (WD) of OM from OMS mice. (B) IF staining of menin and gastrin in glial cultures isolated from duodenal lamina propria of untreated Men1ΔIEC;Sst−/− and OMS mice. (C) Representative blots showing menin expression (top) in total cell lysates (β-actin loading control), nuclear (Sp1 loading control), and cytoplasmic fractions (GAPDH loading control) of glial cultures from mice; quantitation of menin expression (bottom) in nuclear and cytoplasmic fractions of glial cultures, expressed as integrated band intensities normalized to loading controls (n=7–9 mice). (D) IF staining of gastrin and CCKBR in glial cultures from untreated Men1ΔIEC;Sst−/− (−OM, top panel) and OMS mice (+OM, bottom panel) mice. (E) Representative blot (top) and quantitation of CCKBR expression (bottom) normalized to GAPDH in glial cultures isolated from mice (n=7–9 mice). (F) Representative blot showing menin expression in nuclear (Sp1 loading control) and cytoplasmic fractions (Gapdh loading control) of glial cultures from untreated C57 WT mice treated without or with 20 nM gastrin in the presence or absence of YM022. Data shown are the Median ± Interquartile Range of median. *** p< 0.001, **** p< 0.0001. Scale bars: (A) - 50 μm, (inset in A) – 20 μm, (B, E) – 20 μm.
To directly assess changes in the villus cores, LP-Gastrin+ cells in the OMS mice were micro-dissected using laser-capture and proteins extracted were analyzed using western blot. Nearly undetectable levels of E-cadherin confirmed the absence of significant contamination from the epithelium (Supplemental Fig. 6B). The decrease in menin protein in the villus cores was observed only in the OMS mice, without changes in the levels of the glial markers, Gfap, S100b, and Sox10 consistent with changes in cell phenotype and not the number of glial cells (Supplemental Fig. 6B, C). These data confirmed that LP gastrin+ glial cells in the OMS mice have dramatically low menin expression. To determine whether menin loss in glial cells drives gastrin expression, we deleted menin in primary glial cultures generated from MenΔIEC mice using AdenoCre, and examined gastrin expression (Supplemental Fig. 7). The Cre recombinase protein was detected at an MOI as low as 1 after 24 hours of infection (Supplemental Fig. 7A). Cre-mediated recombination effectively deleted menin in >95% of primary glial cells after 48 hours of infection (Supplemental Fig. 7B). Deletion of menin led to the appearance of gastrin 24 hours later, which increased dose-dependently as a function of the viral titer (Supplemental Figure 7C, D). A 4-fold increase in gastrin mRNA was also observed in Adeno-Cre infected cells (MOI 4) compared to uninfected cells (Supplemental Figure 7E). Taken together, these data demonstrate that deletion of menin in mucosal glial cells de-repress gastrin mRNA and subsequently induce protein expression. The Adenoviral infection did not affect the viability of primary glial cells (P = 0.227, Kruskal–Wallis test) as determined by MTT assay (Supplemental Figure 7F).
To better understand why menin in decreased glial cells in vivo, we next examined menin expression in glial cultures isolated from the untreated MenΔIEC;Sst−/− or OMS mice. We observed nuclear menin in enteric glial cells cultured from the untreated MenΔIEC;Sst−/− mice and loss of nuclear menin in the OMS mice (Fig. 2B) that coincided with gastrin peptide expression. Western blot analyses of menin expression in nuclear and cytoplasmic fractions of OMS cultures showed a 90% decrease in nuclear menin along with a slight increase in cytoplasmic menin (Fig. 2C). In contrast, OM treatment had no effect on menin protein levels in C57 WT mice. Therefore, hypergastrinemia in the OMS mice correlated with a decrease in nuclear menin levels.
Gastrin induces the nuclear translocation of Menin through CCKBR-mediated PKA activation
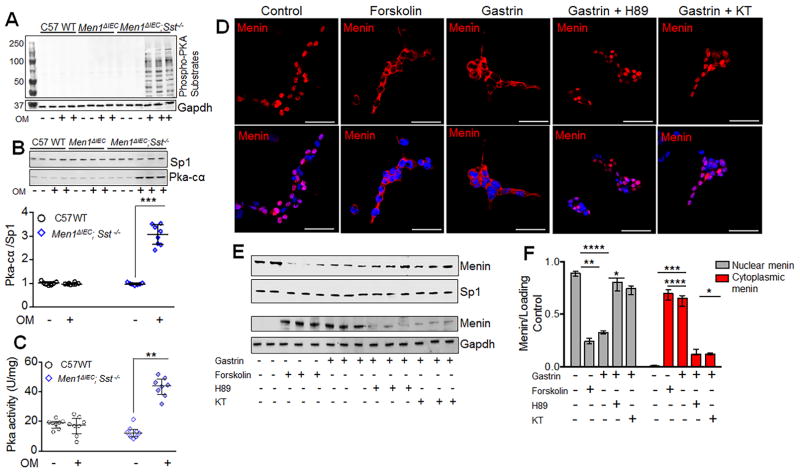
It is well established that gastrin stimulates the expression of its own receptor 20, 28, 29. Indeed, enteric glial cells isolated from the OMS mice exhibited 7-fold higher levels of Cckbr, compared to those from WT or OM treated MenΔIEC mice (Fig. 2D, E). In addition, glial cells in their native environment within the duodenal mucosa express Cckbr receptors that are potentiated in the OMS mice. Importantly, Cckbr expression was also observed on epithelial cells (Supplemental Figure 8A, B). To test whether changes in menin protein required Cckbr activation, the glial cultures were treated with the gastrin receptor antagonist YM022. Gastrin-mediated reduction of nuclear menin was blocked by YM022 treatment (Fig. 2F, Supplemental Fig. 9A, B) demonstrating a requirement for Cckbr activation. Cckbr is typically associated with Gαsq/11, intracellular Ca2+ mobilization, and increased PKC, Erk, p38 or Jun kinase activity 30. Indeed, increased intracellular Ca2+ transients [iCa2+] was observed in cultured glial cells from OMS mice (Supplemental Fig. 10). Gastrin and CCK treatment led to 225% and 400% increase in [iCa2+], respectively (Supplemental Fig. 10A–C). Gastrin induced increase in [iCa2+] was blocked by YM022 (Supplemental Fig. 10C, D), confirming that gastrin signaling in these glial cells is dependent on CCKBR. However, no change in nuclear menin levels was observed when the glial cultures were treated with PKC activator phorbol ester. Moreover, the PKC inhibitor Bis IV, or Erk inhibitor PD98059 (Supplemental Fig. 11A, B, C,) respectively did not block gastrin induced nuclear translocation. Although other GPCRs stimulate PKA signaling 31, this pathway has not been reported for Cckbr. However, since Sst stimulates menin expression by inhibiting PKA 17, we considered the possibility that Sst deletion in the OMS mice reduces the threshold for PKA activation. To determine whether Cckbr activation correlated with elevated PKA activity in the OMS mice, the presence of phosphorylated PKA substrates was assessed and found to be significantly elevated (Fig. 3A). When PKA is active, the catalytic (PKA-cα) and regulatory subunits dissociate leading to the nuclear translocation of the catalytic subunit 32, 33. Indeed, a 3-fold increase in PKA-cα was observed in the nuclear fractions of glial cultures from OMS mice (Figs. 3B). In addition, PKA enzyme activity was 2-fold higher, compared to the untreated MenΔIEC; Sst−/− or C57BL/6 WT mice (Fig. 3C). Taken together, our data demonstrated that Cckbr activation in enteric glial cells induces the nuclear export of menin via PKA activation.
Figure 3. Gastrin mediated nuclear export of menin via CCKBR requires PKA.
(A) Representative blot showing phospho-PKA substrates in glial cultures isolated from mice (GAPDH, loading control). (B) Representative blot (top) and quantitation of PKA-cα expression (bottom) in nuclear fractions of glial cultures isolated from mice (n=8–10 mice). (C) PKA activity in glial cultures, expressed as units of enzyme activity (U) normalized to protein content of cell lysates (mg) (n=8 mice). (D) IF staining of menin in glial cultures isolated from duodenal lamina propria of C57 WT mice and treated with or without gastrin (20 nM, 8 h) in the presence or absence of 10 μM H-89 and KT5720 (KT), and forskolin (10 μM, 4 h). (E) Representative blots and quantitation of menin expression (F) in nuclear and cytoplasmic fractions of glial cultures isolated from C57WT mice and treated with or without gastrin (20 nM, 8 h), in the presence or absence of 10 μM H-89, KT5720 (KT) and forskolin (10 μM, 4 h). Data shown are the Median ± Interquartile Range of median. * p< 0.05, ** p< 0.01, *** p< 0.001, **** p< 0.0001. Scale bars: (D) – 20 μm
Next, we examined whether the changes observed in the OMS mice could be recapitulated by treating normal enteric glial cells with gastrin or forskolin, a PKA agonist. We observed that within 4h, 10μM forskolin or 20nM gastrin decreased levels of nuclear menin and increased cytoplasmic levels (Fig. 3D, E). A 70% decrease in nuclear menin along with robust increase in cytoplasmic menin was observed after 4 h of gastrin stimulation (Fig. 3F). Importantly, both PKA inhibitors H89 or KT blocked gastrin-mediated export of menin (Figs. 3D–F), confirming that gastrin induces the nuclear export of menin through PKA.
To study the fate of menin protein in the cytoplasm, enteric glial cells isolated from C57 WT mice were treated with increasing concentrations of gastrin peptide for 24h. In the absence of any treatment, menin protein was detected in the nucleus of the enteric glial cells (Supplemental Fig. 12A). Gastrin-induced export of menin from the nucleus peaked between 4h and 8h (Supplemental Fig. 12B). By 24 hours, a significant loss of cytoplasmic menin was observed, suggesting that sustained gastrin stimulation is associated with loss of menin protein in these enteric glial cells.
Gastrin induces menin ubiquitination and proteasomal degradation in the cytoplasm
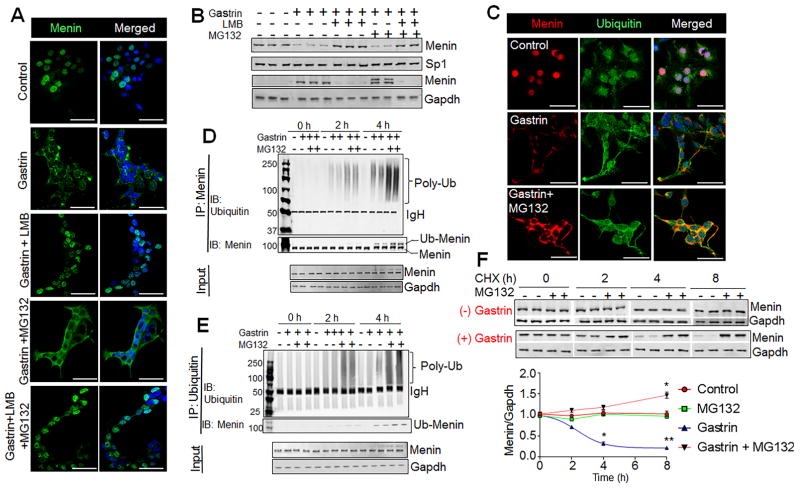
To determine if nuclear export and proteasomal degradation accounted for decrease in menin, WT enteric glial cells were treated with gastrin peptide (20 nM) for 8 hours resulting in a 90% decrease in nuclear menin protein, which was completely blocked by pre-treating the cells with the nuclear export inhibitor Leptomycin B (LMB) (Fig. 4A, B; Supplemental Fig. 12C). Menin accumulated in the cytoplasm when the enteric glial cells were treated with gastrin in the presence of the proteasome inhibitor MG132 (Fig. 4A, B). In the presence of both LMB and MG132, menin levels in the cytoplasm were not significantly higher than treating with LMB alone, demonstrating that proteasomal degradation of menin occurred in the cytoplasm and not the nucleus (Fig. 4A, B; Supplemental Fig. 12C). Unlike nuclear menin, cytoplasmic menin appeared as a doublet when the cells were treated with MG132 plus gastrin (Fig. 4B), which we subsequently determined is ubiquitinated menin protein (Ub-Menin). In glial cultures isolated from WT mice, nuclear menin did not co-localize with ubiquitin (Fig. 4C). By contrast, gastrin treatment induced the co-localization of ubiquitin with menin only in the cytoplasm, which was further enhanced by inhibiting the proteasome with MG132 (Fig. 4C). We concluded from these studies that the ubiquitin chain is added to menin in the cytoplasm after nuclear export.
Figure 4. Gastrin induced nuclear export leads to its proteasomal degradation in the cytoplasm.
(A) IF staining of menin in glial cultures isolated from duodenal lamina propria of C57 WT mice and treated without or with gastrin (20 nM, 8 h) in the presence or absence of Leptomycin b (LMB, 10 μM), or MG132 (25 μM). (B) Representative blots showing menin expression in nuclear and cytoplasmic fractions of glial cultures from C57 WT mice and treated as in (a). (C) IF staining of menin and ubiquitin in glial cultures isolated from C57 WT mice treated with or without gastrin (20 nM, 8 h) in the presence or absence of MG132 (25 μM). (D, E) Representative blots showing gastrin-induced menin ubiquitination in STC-1 cells. STC-1 cells were treated without or with gastrin (20 nM) for 0, 2, or 4 hours in the presence or absence of MG132 (25 μM). Menin was immunoprecipitated (IP) from the cytoplasmic fraction. Immunoblot (IB) for poly-Ub (top panel), and IB for menin (lower panel). Menin versus Ub-menin indicated. Blot for IgH and GAPDH in input lysates are loading controls (D). Ubiquitinated proteins were immunoprecipitated from the cytoplasmic fraction and detected with Ub antibodies (E). IB for poly-Ub (top panel), and Ub-menin (lower panel). (F) Representative blot (top) and quantitation (bottom) showing menin expression from Cycloheximide (CHX) chase experiment in STC-1 cells (n= triplicates from 4 separate experiments). STC-1 cells were treated with or without gastrin in the presence or absence of MG132 (25 uM) in the presence of CHX (25μg/mL) for 0, 2, 4, and 8 hours. Data shown are the Mean ± SEM. * p< 0.05, ** p< 0.01. Scale bars: (A, C) – 20 μm.
To test directly whether menin is ubiquitinated prior to degradation, we performed a time course of menin ubiquitination by treating the STC-1 neuroendocrine cell line with gastrin ±MG132. Cytoplasmic menin was immunoprecipitated and ubiquitin antibody was used to detect poly-ubiquitin chains (Poly-Ub) and ubiquitinated menin (Ub-Menin). As observed in the immunohistochemical analysis of primary enteric glial cultures, the addition of Ub to menin occurred within 2 h after treating the STC-1 cells with gastrin (Fig. 4D, E). Moreover, inhibiting proteasomal degradation with MG132 increased the quantity of Ub-Menin without changes in input (Fig. 4D, E). Cytoplasmic lysates showed two distinct menin immunoreactive bands in the presence of gastrin representing Ub-Menin and poly-Ub proteins (upper bands) (Fig. 4D). Blotting for menin after immunoprecipitating with poly-Ub antibody detected Ub-Menin, which was also enhanced by MG132 treatment (Fig. 4E). Ubiquitin was not detected when menin was immunoprecipitated from nuclear extracts and vice versa (data not shown). Taken together, STC-1 the data showed that gastrin induces ubiquitination of menin prior to proteasomal-dependent degradation in the cytoplasm. To determine the half-life of WT menin protein in the absence and presence of gastrin, we performed a cycloheximide (CHX) chase experiment to block de novo protein synthesis. Prior studies demonstrated that WT menin is normally stable over at least 8 h 34. Performing the chase over 8 h showed that the level of menin protein in STC-1 cells is quite stable in the absence of gastrin. However, with gastrin treatment, the half-life of WT menin was about 3h (Fig. 4F), as reported for menin protein with missense mutations identified in human subjects 34, 35. Accelerated degradation of menin eventually led to induction of gastrin gene expression, reflected by a 2.5-fold increase in its mRNA in WT cultures after 72 hours of gastrin stimulation (Supplemental Fig. 12D). Thus, the nuclear export of menin occurred over several hours; while loss of menin from the cell occurred over days eventually leading to induction of gastrin gene expression.
To test whether these mechanisms observed in the glial cultures was replicated in neuroendocrine cell lines, mouse proximal intestinal neuroendocrine cells, STC-1 were treated with gastrin. Gastrin treatment (20 nM, 24h) was associated with CCKBR activation, and the loss of nuclear menin that coincided with an increase in cytoplasmic menin (Fig. 5A, B). These effects were reversed 8 h after gastrin was removed from the media (“washout”, Fig. 5A, B), suggesting that CCKBR activation and menin export were mediated by gastrin. Gastrin treatment in the presence of YM022 and H-89 also reversed the observed effects, confirming that like glial cells, gastrin stimulation led to loss of menin in STC-1 cells, and was mediated via CCKBR and PKA activation (Fig. 5A, B). A time-dependent reduction in total menin was observed with gastrin treatment (Fig. 5C, D), that was blocked by YM022 (Supplemental Fig. 13A, B), demonstrating that CCKBR activation was essential for menin degradation. Moreover, the effects on menin protein were not associated with changes in menin mRNA. Although gastrin had no significant effect on menin mRNA (Fig. 5E, Supplemental Fig. 13C), it did induce gastrin gene expression (Fig. 5F) after 60 hours of stimulation, which was effectively blocked by YM022 (Supplemental Fig. 13D), mimicking the effects observed in glial cultures. Furthermore, nuclear export inhibitor LMB significantly blocked gastrin-mediated nuclear export of menin and proteosomal degradation in the cytoplasm (Supplemental Fig. 14A–C). MG132 treatment led to appearance of ubiquitinated menin seen as doublet. In the absence of LMB menin was detected in the cytoplasm and subsequently degraded proteosomally, leading to de-repression of gastrin mRNA (Supplemental Fig. 14D). Like primary glial cells no changes were observed in menin mRNA in response to gastrin in the presence of LMB and/or MG132 (Supplemental Fig. 14E). Thus, gastrin stimulation triggers a CCKBR-dependent feed-forward loop that eventually leads to its induction, a novel mechanism that could be applied to the pathogenesis of neuroendocrine tumors.
Figure 5. Gastrin stimulation leads to nuclear export of menin in STC-1 cells, and requires activation of CCKBR and PKA.
(A) IF staining showing menin and CCKBR expression in STC-1 cells treated with 20 nM gastrin, 8 hrs after gastrin “washout”, and gastrin in the presence of YM022, or H-89. (B) Representative blot showing CCKBR expression in total cellular lysates, PKA-c in nuclear fractions, and menin in nuclear and cytoplasmic fractions of STC-1 cells treated as above in (a). (C, D) Representative blot (C), and quantitation (D) showing menin expression in STC-1 cells treated with gastrin for 16, 24 and 48 h. Integrated band intensities of menin analyzed using LICOR Odyssey software (normalized to Gapdh loading controls) were then plotted as a function of time (n=triplicates from 3 independent experiments). (E) Menin and gastrin (F) mRNA expression in STC-1 cells treated with or without gastrin for 16–72 h, after normalization to Hprt mRNA (n = triplicates from 4 separate experiments). Data shown are the Mean ± SEM. Bars with different numbers are significantly different from each other (p<0.05).
Human MEN1 gastrinomas express enteric glial markers and no nuclear menin
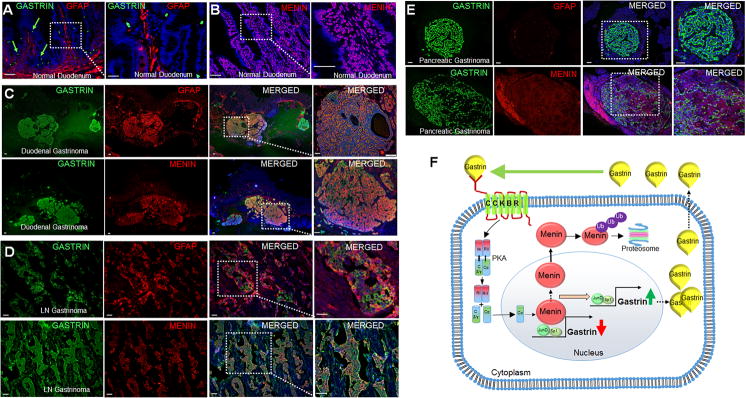
Normal human duodenum expresses GFAP in the lamina propria (Fig. 6A). Moreover, menin is expressed in the nucleus (Fig. 6B). Gastrinomas from 14 different human subjects were examined and demonstrated that most of the duodenal gastrinomas were positive for GFAP, SOX10, S100B, and p75 (Fig. 6C, Supplemental Fig. 15A, B). The lymph node gastrinomas were also positive for GFAP (Fig. 6D) but only 1 out of 8 pancreatic gastrinomas expressed GFAP+ (Fig. 6E, Table 1). Gastrin+ cell clusters showed robust CCKBR expression (Supplemental Fig. 15C), compared to normal duodenal tissues. Importantly, menin expression in the gastrinomas was cytoplasmic (Fig. 6, Table 1). Thus, duodenal gastrinomas exhibited neural crest markers, suggesting that these submucosal lesions might originate from enteric glial cells. We speculate that gastrin-induced CCKBR upregulation drives menin to the cytoplasm in these human tumors, thereby de-repressing gastrin and driving its feed-forward induction.
Figure 6. Gastrinomas from MEN1 patients express neural crest markers and negligible menin.
(A–B) IF staining of gastrin and GFAP (A) and menin (B) in normal human duodenum. (C–E) IF staining of gastrin with GFAP and menin in duodenal (C), lymph node (D), and pancreatic gastrinomas (E) from MEN1 patients. (F) Schematic of chronic hypergastrinemia and pre-gastrinoma development. Initial hypergastrinemia activates CCKBR and downstream PKA signaling pathway, which exports menin from the nucleus to the cytoplasm leading to its proteosomal degradation. Loss of menin de-represses gastrin gene expression, and triggers a feed-forward loop leading to further menin degradation and chronic hypergastrinemia. Scale bars: (A, B - 50 μm, insets - 20 μm); (C - 500 μm, insets - 100 μm); (D, E - 100 μm, insets - 50 μm).
Collectively, our data demonstrate that gastrin induced nuclear export of menin via CCKBR-PKA signaling leads to its degradation in the cytoplasm. Loss of menin protein leads to de-repression of the gastrin gene, subsequently accelerating loss of menin and further induction of gastrin (Fig. 6F).
Discussion
Menin functions as a tumor suppressor in neuroendocrine tumors, including gastrinomas 36. Accordingly, we previously showed that modulating menin levels directly regulates gastrin gene expression through JUND in cell lines 17, 18. However, deleting Men1 from the intestinal epithelium does not induce gastrinomas, suggesting that modulating additional loci restricted to the spectrum of tissues developing NETs contribute to the neoplastic transformation of gastrin-expressing neuroendocrine cells 2, 13–15. In addition, most gastrinomas do not display LOH 8, 10, 11. This long-standing observation provides additional evidence supporting the likelihood that loss of menin function occurs by mechanisms other than by gene deletion 37. Kloppel et al reported that hyperplastic G cells within the mucosa, lamina propria and Brunner’s glands of the proximal duodenum are the precursor lesions from which multi-focal MEN1 gastrinomas develop 5. Moreover chronic use of PPIs or Helicobacter –induced atrophic gastritis has been reported to increase the occurrence of non-syndromic duodenal gastrinomas, implicating a possible role for hormonal conditions or the foregut microenvironment to support neuroendocrine oncogenesis 38, 39. However, due to their small size, scattered location and lack of experimental models, extensive mechanistic analysis of these tumors has been hampered. We developed a mouse model that closely mimics the early stages of MEN1 gastrinomas, e.g., G cell hyperplasia, hypergastrinemia and gastric carcinoids, by conditionally deleting epithelial Men1 on a Sst−/− genetic background 20. When these mice were placed on the acid suppressing PPI omeprazole, enteric glial cells expressed gastrin coincidently with the nuclear export of menin.
Our results reported here show that Gfap+ enteric glial cells expressed gastrin through a ligand-receptor mediated process, demonstrating that changes in the microenvironment of these cells is sufficient to decrease nuclear menin and induce gastrin gene expression. Moreover, our findings in mice raise the novel idea that these submucosal gastrin-producing tumors in the duodenum might arise from GFAP+ enteric glial cells. In MEN1 gastrinomas, subjects presumably carry one non-functional MEN1 allele and are haploinsufficient. Reduced levels of menin protein might be sufficient for enteric glial cells expressing the gastrin receptor to respond to modest changes in circulating gastrin that builds over time to create a feedforward mechanism sustaining the nuclear export of menin and chronic de-repression of the GAST promoter.
Gastrin also stimulates the expression of its own receptor (CCKBR), which serves to further amplify CCKBR signaling in receptive cell populations 29. In the OMS mice gastrin directly stimulated PKA signaling in Gfap+ enteric glial cells, and subsequently induced the nuclear export of menin to the cytoplasm where it was ubiquitinated and degraded by the proteasome. GFAP is an intermediate filament protein expressed in CNS astrocytes and related lineages such as enteric glia, where in the small intestine they regulate barrier function 40. Moreover, GFAP binds and sequesters menin in the cytoplasm during the S→G2 phase of the cell cycle 41. Examining the expression of GFAP in human gastrinomas revealed that these submucosal duodenal tumors are positive for this enteric glial marker suggesting that these tumors could arise from enteric glial cells and not enteroendocrine cell populations residing in the epithelium. Although the intestinal Lgr5+ stem cell at the crypt base is the origin of enteroendocrine cells 42, 43, this does not preclude the possibility that under defined conditions other cells acquire the ability to express gastrointestinal peptides. Indeed, a subset of enteric glial cells exhibits stem cell properties 44. Accordingly, we suggest that the enteric glial cell population acquires the ability to express gastrin in response to signals from the microenvironment inducing PKA signaling and the nuclear export of menin. Gastrinomas overexpress somatostatin receptors e.g., SSTR2, permitting their diagnosis and treatment with somatostatin analogues 45, which presumably would suppress elevated PKA activity in the tumors. Although the secretin stimulation test used to diagnose gastrinomas is imperfect 46, the secretin receptor is coupled to heterotrimeric G proteins that stimulate adenylate cyclase, increase cAMP and activate PKA 32, 47. Thus PKA signaling appears to play a central role in the biology of gastrinomas akin to our observed regulation of gastrin in enteric glial cells.
In summary, we report a mouse model reminiscent of the early changes observed in MEN1-associated duodenal gastrinomas, suggesting their origin from enteric glial cells. Feedforward gastrin signaling in these haploinsufficient cells would abolish residual menin protein providing an alternative to genomic silencing as the mechanism for tumor development. It remains to be seen whether chronic atrophic gastritis or PPIs affects duodenal tumor growth in MEN1 patients, considering that there are anecdotal reports that low gastric acidity predispose subjects to non-syndromic gastrinomas 38.
Supplementary Material
Acknowledgments
Funding: R37 DK45729 (to JLM) and Digestive Disease Center 5P30 DK034933; R01 CA160467 (to J.G.) and R01 CA200660 (to J.G.); R01 DK66604 (to MJL); Molecular Core; UM Cancer Center P30 CA046592 Tissue and Transgenic Mouse Core
We thank Linda Samuelson for critically reviewing the manuscript, and support from R37 DK045729, Digestive Disease Center, UM Cancer Center Cores.
Abbreviations
- Men1ΔIEC
VillinCre;Men1FL/FL
- Sst−/−
somatostatin null
- OMS
Omeprazole-treated Men1ΔIEC;Sst−/−
- GFAP
glial fibrillary acidic protein
- PKA
protein kinase A
- PKC
protein kinase C
- LOH
loss of heterozygosity
- PPI
proton pump inhibitor
- GAST
gastrin
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Author Contributions
S.S. and J.L.M. designed the experiments, interpreted results and wrote the manuscript. S.S. performed experiments and analyzed data. CAM, ALP, LD, and AJK assisted with data acquisition; NS performed measurement of calcium flux; MMH maintained and genotyped the mouse colony; JG, HX, and TC assisted with the experimental design. TE, DJM, TG, FC, AMCB and NAW acquired human tissues. MJL prepared brain tissue for antibody validation. All authors discussed results and approved the manuscript.
Competing Financial Interests
Drs. Grembecka and Cierpicki receive research support from Kura Oncology. They are also receiving compensation as members of the scientific advisory board of Kura Oncology, and they have an equity ownership in the company.
References
- 1.Agarwal SK, Kester MB, Debelenko LV, et al. Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states. Hum Mol Genet. 1997;6:1169–75. doi: 10.1093/hmg/6.7.1169. [DOI] [PubMed] [Google Scholar]
- 2.Biondi CA, Gartside MG, Waring P, et al. Conditional inactivation of the MEN1 gene leads to pancreatic and pituitary tumorigenesis but does not affect normal development of these tissues. Mol Cell Biol. 2004;24:3125–31. doi: 10.1128/MCB.24.8.3125-3131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emmert-Buck MR, Lubensky IA, Dong Q, et al. Localization of the multiple endocrine neoplasia type I (MEN1) gene based on tumor loss of heterozygosity analysis. Cancer Res. 1997;57:1855–8. [PubMed] [Google Scholar]
- 4.Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2007;29:22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]
- 5.Anlauf M, Perren A, Meyer CL, et al. Precursor lesions in patients with multiple endocrine neoplasia type 1-associated duodenal gastrinomas. Gastroenterology. 2005;128:1187–98. doi: 10.1053/j.gastro.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 6.Vanoli A, La Rosa S, Klersy C, et al. Four Neuroendocrine Tumor Types and Neuroendocrine Carcinoma of the Duodenum: Analysis of 203 Cases. Neuroendocrinology. 2017;104:112–125. doi: 10.1159/000444803. [DOI] [PubMed] [Google Scholar]
- 7.Krause WJ. Brunner’s glands: a structural, histochemical and pathological profile. Prog Histochem Cytochem. 2000;35:259–367. [PubMed] [Google Scholar]
- 8.Anlauf M, Perren A, Henopp T, et al. Allelic deletion of the MEN1 gene in duodenal gastrin and somatostatin cell neoplasms and their precursor lesions. Gut. 2007;56:637–44. doi: 10.1136/gut.2006.108910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosentraeger MJ, Garbrecht N, Anlauf M, et al. Syndromic versus non-syndromic sporadic gastrin-producing neuroendocrine tumors of the duodenum: comparison of pathological features and biological behavior. Virchows Arch. 2016;468:277–87. doi: 10.1007/s00428-015-1890-9. [DOI] [PubMed] [Google Scholar]
- 10.Zhuang Z, Vortmeyer AO, Pack S, et al. Somatic mutations of the MEN1 tumor suppressor gene in sporadic gastrinomas and insulinomas. Cancer Res. 1997;57:4682–4686. [PubMed] [Google Scholar]
- 11.Debelenko LV, Zhuang Z, Emmert-Buck MR, et al. Allelic deletions on chromosome 11q13 in multiple endocrine neoplasia type 1-associated and sporadic gastrinomas and pancreatic endocrine tumors. Cancer Res. 1997;57:2238–43. [PubMed] [Google Scholar]
- 12.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scacheri PC, Crabtree JS, Kennedy AL, et al. Homozygous loss of menin is well tolerated in liver, a tissue not affected in MEN1. Mamm Genome. 2004;15:872–7. doi: 10.1007/s00335-004-2395-z. [DOI] [PubMed] [Google Scholar]
- 14.Crabtree JS, Scacheri PC, Ward JM, et al. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proc Natl Acad Sci U S A. 2001;98:1118–23. doi: 10.1073/pnas.98.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veniaminova NA, Hayes MM, Varney JM, et al. Conditional Deletion of Menin Results in Antral G Cell Hyperplasia and Hypergastrinemia. Am J Physiol Gastrointest Liver Physiol. 2012 doi: 10.1152/ajpgi.00109.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiedemann T, Pellegata NS. Animal models of multiple endocrine neoplasia. Mol Cell Endocrinol. 2016;421:49–59. doi: 10.1016/j.mce.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Mensah-Osman E, Zavros Y, Merchant JL. Somatostatin stimulates menin gene expression by inhibiting protein kinase A. Am J Physiol Gastrointest Liver Physiol. 2008;295:G843–54. doi: 10.1152/ajpgi.00607.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Gurung B, Wan B, et al. The same pocket in menin binds both MLL and JUND but has opposite effects on transcription. Nature. 2012;482:542–6. doi: 10.1038/nature10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mensah-Osman EJ, Veniaminova NA, Merchant JL. Menin and JunD regulate gastrin gene expression through proximal DNA elements. Am J Physiol Gastrointest Liver Physiol. 2011;301:G783–90. doi: 10.1152/ajpgi.00160.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundaresan S, Kang AJ, Hayes MM, et al. Deletion of Men1 and somatostatin induces hypergastrinemia and gastric carcinoids. Gut. 2016 doi: 10.1136/gutjnl-2015-310928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rindi G, Grant SG, Yiangou Y, et al. Development of neuroendocrine tumors in the gastrointestinal tract of transgenic mice. Heterogeneity of hormone expression. Am J Pathol. 1990;136:1349–1363. [PMC free article] [PubMed] [Google Scholar]
- 22.Grant SG, Seidman I, Hanahan D, et al. Early invasiveness characterizes metastatic carcinoid tumors in transgenic mice. Cancer Res. 1991;51:4917–23. [PubMed] [Google Scholar]
- 23.Smith TH, Ngwainmbi J, Grider JR, et al. An in-vitro preparation of isolated enteric neurons and glia from the myenteric plexus of the adult mouse. J Vis Exp. 2013 doi: 10.3791/50688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hol EM, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015;32:121–30. doi: 10.1016/j.ceb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Sharkey KA. Emerging roles for enteric glia in gastrointestinal disorders. J Clin Invest. 2015;125:918–25. doi: 10.1172/JCI76303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Coelho-Aguiar JM, Bon-Frauches AC, Gomes AL, et al. The enteric glia: identity and functions. Glia. 2015;63:921–35. doi: 10.1002/glia.22795. [DOI] [PubMed] [Google Scholar]
- 27.Delle Fave G, Kohn A, de Magistris L, et al. Effect of bombesin-stimulated gastrin on gastric acid secretion in man. Life Sci. 1980;27:993–9. doi: 10.1016/0024-3205(80)90110-1. [DOI] [PubMed] [Google Scholar]
- 28.Kovac S, Xiao L, Shulkes A, et al. Gastrin increases its own synthesis in gastrointestinal cancer cells via the CCK2 receptor. FEBS Lett. 2010;584:4413–8. doi: 10.1016/j.febslet.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 29.Ashurst HL, Varro A, Dimaline R. Regulation of mammalian gastrin/CCK receptor (CCK2R) expression in vitro and in vivo. Exp Physiol. 2008;93:223–36. doi: 10.1113/expphysiol.2007.040683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev. 2006;86:805–47. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- 31.Brinks HL, Eckhart AD. Regulation of GPCR signaling in hypertension. Biochim Biophys Acta. 2010;1802:1268–75. doi: 10.1016/j.bbadis.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batty NJ, Fenrich KK, Fouad K. The role of cAMP and its downstream targets in neurite growth in the adult nervous system. Neurosci Lett. 2016 doi: 10.1016/j.neulet.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 33.Calebiro D, Bathon K, Weigand I. Mechanisms of Aberrant PKA Activation by Calpha Subunit Mutations. Horm Metab Res. 2016 doi: 10.1055/s-0042-112817. [DOI] [PubMed] [Google Scholar]
- 34.Yaguchi H, Ohkura N, Takahashi M, et al. Menin missense mutants associated with multiple endocrine neoplasia type 1 are rapidly degraded via the ubiquitin-proteasome pathway. Mol Cell Biol. 2004;24:6569–80. doi: 10.1128/MCB.24.15.6569-6580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canaff L, Vanbellinghen JF, Kanazawa I, et al. Menin missense mutants encoded by the MEN1 gene that are targeted to the proteasome: restoration of expression and activity by CHIP siRNA. J Clin Endocrinol Metab. 2012;97:E282–91. doi: 10.1210/jc.2011-0241. [DOI] [PubMed] [Google Scholar]
- 36.Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional Cloning of the Gene for Multiple Endocrine Neoplasia-Type1. Science. 1997;276:404–407. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- 37.Pritchard DM. Pathogenesis of gastrinomas associated with multiple endocrine neoplasia type 1. Gut. 2007;56:606–7. doi: 10.1136/gut.2006.113985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merchant SH, VanderJagt T, Lathrop S, et al. Sporadic duodenal bulb gastrin-cell tumors: association with Helicobacter pylori gastritis and long-term use of proton pump inhibitors. Am J Surg Pathol. 2006;30:1581–7. doi: 10.1097/01.pas.0000213326.86992.98. [DOI] [PubMed] [Google Scholar]
- 39.Eyal A, Sueissa A, Braun E, et al. From hypomagnesaemia to Zollinger-Ellison syndrome: an adverse effect of a proton pump inhibitor. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-205165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savidge TC, Newman P, Pothoulakis C, et al. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344–58. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Egido J, Cunningham J, Berg M, et al. Menin’s interaction with glial fibrillary acidic protein and vimentin suggests a role for the intermediate filament network in regulating menin activity. Exp Cell Res. 2002;278:175–83. doi: 10.1006/excr.2002.5575. [DOI] [PubMed] [Google Scholar]
- 42.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 43.Andrew A, Kramer B, Rawdon BB. The origin of gut and pancreatic neuroendocrine (APUD) cells--the last word? J Pathol. 1998;186:117–8. doi: 10.1002/(SICI)1096-9896(1998100)186:2<117::AID-PATH152>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 44.Gershon MD. Behind an enteric neuron there may lie a glial cell. J Clin Invest. 2011;121:3386–9. doi: 10.1172/JCI59573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krenning EP, Bakker WH, Breeman WA, et al. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet. 1989;1:242–4. doi: 10.1016/s0140-6736(89)91258-0. [DOI] [PubMed] [Google Scholar]
- 46.Shah P, Singh MH, Yang YX, et al. Hypochlorhydria and achlorhydria are associated with false-positive secretin stimulation testing for Zollinger-Ellison syndrome. Pancreas. 2013;42:932–6. doi: 10.1097/MPA.0b013e3182847b2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ulrich CD, 2nd, Holtmann M, Miller LJ. Secretin and vasoactive intestinal peptide receptors: members of a unique family of G protein-coupled receptors. Gastroenterology. 1998;114:382–97. doi: 10.1016/s0016-5085(98)70491-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.