Abstract
Background
Cessation of blood flow during out-of-hospital cardiac arrest (OHCA) results in microvascular thrombosis, protracted hypoperfusion after return of spontaneous circulation and damage to vital organs. We tested the hypothesis that pre-arrest antiplatelet and anticoagulant medication use would be associated with less post-arrest organ dysfunction and better outcomes.
Methods
We included OHCA patients treated from January 2005 to October 2014 at a single academic medical center. We combined our prospective OHCA registry of clinical and demographic data with a structured chart review to abstract home antiplatelet and anticoagulant medications. We fit unadjusted and adjusted regression models to test the association of antiplatelet and anticoagulant medication use with early post-arrest illness severity, survival and functionally favorable recovery.
Results
Of 1054 subjects, 295 (28%) were prescribed an antiplatelet agent and 147 (14%) were prescribed an anticoagulant prior to arrest. In adjusted models, antiplatelet agents were associated with lower post-arrest illness severity (adjusted OR 0.50 95% CI 0.33-0.77), greater odds of survival to discharge (adjusted OR 1.74 95% CI 1.08-2.80) and greater odds favorable functional outcome (adjusted OR 2.11 95% CI 1.17-3.79). By contrast, anticoagulation via any agent was not associated with illness severity, survival to discharge or favorable outcome.
Conclusion
Preventing intra-arrest and post-arrest microvascular thrombosis via antiplatelet agents could represent a novel therapeutic target to improve outcomes after OHCA.
Keywords: Thrombosis, hemostasis, platelet inhibition, anticoagulant, cardiac arrest, post-cardiac arrest syndrome
Introduction
Over 350,000 Americans suffer a cardiac arrest outside of the hospital annually and more than 125,000 achieve return of spontaneous circulation (ROSC) and are treated in the hospital[1]. Despite advances in care, mortality in this cohort is common, with only a minority of admitted patients surviving to hospital discharge and even fewer experiencing functionally favorable recovery [2]. Initial brain and extracerebral organ injury resulting from anoxic-ischemic and reperfusion injury causes significant multisystem organ dysfunction in a majority of those with ROSC [3]. Ultimately, however, most patients' survival and recovery are limited primarily by the severity of brain injury rather than other organ failure [4, 5].
Disordered thrombosis is an important and potentially modifiable mechanism of ongoing organ dysfunction after ROSC [6]. Hemostasis during cardiac arrest results in microvascular thrombosis, in turn leading to post-arrest organ hypoperfusion and areas of no-reflow that persist despite restoration of macrovascular flow [7]. The severity of both post-arrest brain and cardiopulmonary injury are strongly predictive of outcome [8, 9]. Past investigational therapies to mitigate this phenomenon have focused on anticoagulant or thrombolytic agents [10-12]. Although animal work suggested that such drugs attenuate neurological damage after cardiac arrest [12, 13], these have not translated well into human research [14, 15]. Although the contribution of platelet inhibition in acute thrombosis is recognized, and their use widespread in other cardiovascular diseases, little is known about the effects of platelet inhibition on microvascular thrombosis and blood flow after cardiac arrest [16, 17].
We sought to test whether current antiplatelet and anticoagulant medication use at the time of arrest were independently associated with outcome in patients resuscitated from out-of-hospital cardiac arrest (OHCA). We hypothesized that these medicines would reduce the severity of early post-arrest organ dysfunction, resulting in improved survival and functional outcomes at hospital discharge.
Methods
Setting and population
We included subjects admitted to a single academic medical center after resuscitation from OHCA from January 2005 to October 2014. Consistent with prior definitions, we considered arrests outside of the hospital setting or in the emergency department to be OHCA. We identified subjects from our prospective registry, and excluded those who were under 18 years of age as well as those who arrested secondary to trauma or a primary neurological catastrophe. At our hospital, an established Post-Cardiac Arrest Service (PCAS) coordinates these patients' care, as we have previously described in detail [18, 19]. Briefly, our role includes partnering with emergency and critical care providers to ensure a consistent package of initial resuscitation and diagnostic workup, intensive care, multimodal neurological prognostication, secondary prevention and post-acute rehabilitation.
Predictors
Our primary predictors of interest were anticoagulant or antiplatelet medication use immediately prior to the arrest. We considered patients to be exposed to these medications if they had an active and pharmacy-filled prescription (e.g. a 30 day medication supply filled in within 30 days of presentation, a 90 day supply filled within 90 days, etc) of at least one anticoagulant or antiplatelet medication, respectively, at the time of their arrest. We classified alteplase, argatroban, bivalirudin, apixaban, dabigatran, rivaroxaban, dalteparin, enoxaparin, fondaparinux, unfractionated heparin, and warfarin as anticoagulants. We classified anagrelide, ASA, cilostazol, clopidogrel, dipyridamole, eptifibatide, prasugrel, ticlopidine, ticagrelor, tirofiban, triflusal, and vorapaxar as antiplatelet agents. As a surrogate measure of medical and pharmacy access prior to arrest, we also determined whether each patient was prescribed any medication as a binary measure. We performed a structured chart review to derive these data from the electronic medical record. We used multiple sources within the EMR in this search including emergency department and admission physician and nursing documentation. In addition to this documentation, it is our institutional policy that an emergency department-based pharmacist reconciles all admitted patients' medication lists via direct contact with families, nursing home records, outpatient clinic documentation, and by directly contacting the outpatient pharmacy used by the patient to obtain last fill dates. We treated these exposures as binary predictors and made no adjustment according to dose or number of each agents prescribed (e.g. single vs dual antiplatelet therapy). We abstracted standard clinical and demographic data from our registry: age, sex, initial shockable rhythm, witnessed arrest, layperson cardiopulmonary resuscitation (CPR), and emergent cardiac catheterization [20]. “Cardiac etiology” of cardiac arrest was defined as arrest due to acute coronary syndrome, primary cardiac dysrhythmia, structural heart disease or either left or right ventricular failure, which we determined using a structured chart review.
Outcomes
Our primary outcome of interest was post-arrest illness severity, which we operationalized using each patients' prospectively assigned Pittsburgh Cardiac Arrest Category (PCAC). PCAC is a validated, 4-level ordinal predictor of outcome based on severity of neurological and cardiopulmonary injury after cardiac arrest [3, 9]. We assign PCAC prospectively based on the best neurological and cardiopulmonary function in the first 6 hours after ROSC. Briefly, levels of PCAC are:
–Mild or no brain injury, awake;
–Moderate brain injury without severe cardiopulmonary dysfunction;
–Moderate brain injury with cardiopulmonary dysfunction; and,
–Severe brain injury with loss of some or all brainstem reflexes.
As secondary outcomes, we examined dysfunction of separate organ systems. Because PCAC incorporates both neurological and cardiopulmonary failure, as a separate analysis we analyzed it as a 3- level outcome, grouping Category II and III patients together. We also examined measures of cardiopulmonary dysfunction, including post-arrest ejection fraction, PaO2 to FiO2 ratio, and peak troponin level.
Additional secondary outcomes were survival to hospital discharge and favorable functional status at hospital discharge. We defined a favorable functional outcome based on discharge disposition, with discharge to home or to acute rehabilitation considered good outcomes and discharge to a skilled nursing facility, long-term acute care facility, hospice or death considered unfavorable functional outcomes [21].
Statistical Analysis
We summarized baseline characteristics and report means with standard deviations (SD). In our main analysis, we tested the association between outpatient antiplatelet/anticoagulant medications and PCAC. First, we used unadjusted ordinal logistic regression to test the association between each predictor and PCAC, and standard unadjusted logistic regression to predict survival to discharge and functionally favorable outcome. Then, we built adjusted models to predict each outcome that included medication and other predictors with univariable associations significant at a level of P≤0.1. Using this method, our final model predicting PCAC included age, shockable rhythm, and witnessed status, in addition to antiplatelet and anticoagulant medication use. Our models predicting outcome at hospital discharge included age, shockable rhythm, witnessed status, and cardiac catheterization as covariates.
Because accurate information about arrest etiology was only available for the subset of the cohort presenting after February 2012, we ran separate models with (n = 1054) and without (n = 466) this predictor and compared the stability of the odds ratios between models. In a post hoc analysis, we adjusted for any current medication use as a binary predictor. Our intent was to address a potential confounding effect of access to pre-arrest medical care whereby antiplatelet medication use might simply be a marker of better access. We forced this covariate in each final adjusted model, and compared the effects for antiplatelet and anticoagulant medication usage with and without this adjustment. Finally, we used rank sum tests to compare peak troponin, left ventricular ejection fraction and PaO2:FiO2 ratio between categories of antiplatelet or anticoagulant medication use. Estimating that 30% of patients survived to discharge and 1/3 of patients were prescribed an antiplatelet or anticoagulant medication, we calculated a minimum sample size of 1000 patients to detect an odd ratio of 1.5 for the effect of interest. We used STATA 14 for all analyses (StataCorp, College Station, TX, USA).
Results
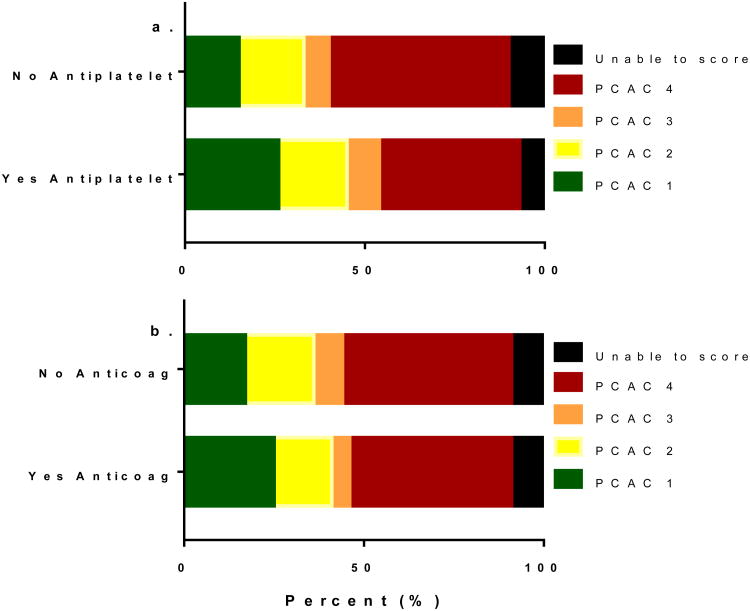
Of 1054 subjects included in analysis, 295 (28%) were prescribed an antiplatelet and 147 (14%) were prescribed an anticoagulant at the time of their arrest. Mean (SD) age was 58 (17) years and 55% were female (Table 1). As expected, baseline characteristics differed between those prescribed these medicines and those who were not (Table 1). In unadjusted ordinal logistic regression, age, shockable rhythm, witnessed arrest, cardiac etiology of arrest, antiplatelet use (Figure 1a) and anticoagulant use (Figure 1b) were all associated with PCAC at a level of P ≤ 0.1 and therefore included in adjusted models. In adjusted models, antiplatelet use was independently associated with a 50% reduction in the odds of a 1-level increase (worsening) in PCAC in adjusted models (Table 2). This effect was stable both with and without adjustment for cardiac etiology of arrest (adjusted odds ratio [OR] 0.55 [95% confidence interval {CI} 0.40 – 0.77], and adjusted OR 0.50 [95% CI 0.33–0.77], respectively). By contrast, anticoagulant use was not independently associated with PCAC in either model. These results did not change when PCAC was treated as a 3-level measure of coma severity alone (not shown). In our post hoc analysis adjusting for any pre-arrest medication use, antiplatelet medication use remained associated with PCAC (adjusted OR 0.46 [95% CI 0.31 – 0.69] without adjustment for arrest etiology; adjusted OR 0.34 [95% CI 0.20 – 0.59] with adjustment for arrest etiology). Anticoagulant use remained a non-significant predictor in this analysis.
Table 1. Baseline population characteristics and outcomes.
| Characteristic | Overall (n=1,054) | Antiplatelet use? | Anticoagulant use? | ||
|---|---|---|---|---|---|
| Yes (n=295) | No (n=759) | Yes (n=147) | No (n=907) | ||
| Age, years | 58 (17) | 65 (13) | 55 (18) | 66 (14) | 57 (17) |
| Female sex | 473 (45) | 133 (45) | 340 (45) | 36 (43) | 410 (45) |
| Shockable rhythm | 386 (37) | 118 (40) | 268 (35) | 63 (43) | 323 (36) |
| Witnessed arrest* | 364 (35) | 136 (58) | 228 (44) | 59 (56) | 305 (47) |
| Bystander CPR* | 193 (26) | 59 (25) | 134 (26) | 32 (30) | 161 (25) |
| Cardiac catheterization | 373 (36) | 130 (44) | 243 (32) | 42 (29) | 331 (37) |
| Pittsburgh Cardiac Arrest Category | |||||
| I | 191 (18) | 77 (26) | 114 (15) | 37 (25) | 154 (17) |
| II | 194 (18) | 56 (19) | 138 (18) | 23 (16) | 171 (19) |
| III | 78 (7) | 26 (9) | 52 (7) | 7 (5) | 71 (8) |
| IV | 495 (47) | 115 (39) | 380 (50) | 66 (45) | 429 (47) |
| Unable+ | 96 (9) | 21 (7) | 75 (10) | 14 (10) | 82 (9) |
| Peak troponin | 0.8 [0.2 – 8.0] | 0.8 [0.2 – 7.1] | 0.8 [0.1 – 8.5] | 0.6 [0.2 – 3.2] | 1.0 [0.2 – 8.6] |
| LV ejection fraction, % | 50 [30 – 55] | 50 [25 – 55] | 55 [35 – 60] | 50 [35 – 55] | 50 [30 – 55] |
| Survived | 417 (40) | 141 (48) | 276 (36) | 63 (43) | 354 (39) |
| Favorable outcome | 274 (26) | 85 (29) | 189 (25) | 27 (18) | 247 (27) |
Data are presented as mean (standard deviation) for normally distributed continuous data, median [interquartile range] for skewed continuous data, and raw number with corresponding percentages for categorical data.
Abbreviations: CPR – cardiopulmonary resuscitation; LV – left ventricle
Data only available after November 2009. Frequency data are presented as a percentage of the available denominator (n = 750)
Pittsburgh Cardiac Arrest Category cannot be assigned when the neurological exam is confounded by residual sedation, neuromuscular blockade, overdose or severe metabolic disarray.
Figure 1.
Distribution of Pittsburgh Cardiac Arrest Categories stratified by antiplatelet and anticoagulant medication use. Severity of illness increases from Category 1 to Category 4.
Table 2. Adjusted ordinal logistic regression models predicting post-arrest injury severity, modeled as Pittsburgh Post-Cardiac Arrest Category.
| Predictor | Adjusted OR (95%CI) | P value |
|---|---|---|
| Model 1: | ||
| Antiplatelet use | 0.55 (0.40 – 0.77) | <0.001 |
| Anticoagulant use | 0.80 (0.51 – 1.25) | 0.33 |
| Age | 1.00 (0.99 – 1.01) | 0.60 |
| Shockable rhythm | 0.33 (0.24 – 0.45) | <0.001 |
| Witnessed arrest | 0.64 (0.47 – 0.87) | 0.01 |
|
| ||
| Model 2:* | ||
| Antiplatelet use | 0.50 (0.33 – 0.77) | 0.002 |
| Anticoagulant use | 0.83 (0.46 – 1.47) | 0.51 |
| Age | 1.00 (0.99 – 1.01) | 0.74 |
| Shockable rhythm | 0.49 (0.31 – 0.76) | 0.001 |
| Witnessed arrest | 0.65 (0.04 – 0.98) | 0.04 |
| Cardiac etiology of arrest | 0.45 (0.29 – 0.71) | 0.001 |
Because accurate arrest etiology data are only available after February 2012, model 2 includes only 466 of 1,054 subjects
In unadjusted logistic regression models predicting outcome at discharge, age, shockable rhythm, witnessed arrest, and arrest etiology were associated with outcome at a level of P ≤ 0.1 and included in adjusted models. As before, we estimated separate models with (n = 1054) and without (n = 466) adjusting for arrest etiology and compared results. In both models, antiplatelet use was independently associated with increased odds of both survival to hospital discharge (adjusted OR 1.81 [95%CI 1.25 – 2.64] without adjustment for etiology; adjusted OR 1.74 [95%CI 1.08 – 2.80] with adjustment for etiology) and favorable outcome (adjusted OR 1.57 [95%CI 1.02 – 2.46] without adjustment for etiology; adjusted OR 2.11 [95%CI 1.17 – 3.76] with adjustment for etiology) (Table 3). Post hoc adjustment any medication use did not substantially change the results (adjusted OR for antiplatelet medication predicting survival 1.82 [95% CI 1.20 – 2.77] without adjustment for arrest etiology; adjusted OR 1.80 [95% CI 1.05 – 3.09] with adjustment for arrest etiology). There was no association between antiplatelet use or anticoagulant use with peak troponin, left ventricular ejection fraction, or PaO2:FiO2 ratio (data not shown).
Table 3. Adjusted logistic regression models predicting survival to hospital discharge and functionally favorable survival.
| Predictor | Predictors of survival | Predictors of favorable outcome | ||
|---|---|---|---|---|
| Adjusted OR (95%CI) | P value | Adjusted OR (95%CI) | P value | |
| Model 1: | ||||
| Age | 0.97 (0.96 – 0.98) | <0.001 | 0.95 (0.94 – 0.97) | <0.001 |
| Shockable rhythm | 1.85 (1.25 – 2.72) | 0.002 | 2.41 (1.53 – 3.77) | <0.001 |
| Witnessed arrest | 1.76 (1.25 – 2.47) | 0.001 | 1.72 (1.15 – 2.58) | 0.009 |
| Cardiac catheterization | 4.00 (2.70 – 5.94) | <0.001 | 5.03 (3.15 – 8.05) | <0.001 |
| Antiplatelet use | 1.81 (1.25 – 2.64) | 0.002 | 1.57 (1.02 – 2.46) | 0.04 |
| Anticoagulant use | 1.91 (1.18 – 3.11) | 0.009 | 0.87 (0.46 – 1.65) | 0.83 |
|
| ||||
| Model 2:* | ||||
| Age | 0.97 (0.96 – 0.98) | <0.001 | 0.94 (0.92 – 0.96) | <0.001 |
| Shockable rhythm | 1.30 (0.75 – 2.24) | 0.35 | 1.76 (0.95 – 3.24) | 0.07 |
| Witnessed arrest | 1.61 (1.05 – 2.46) | 0.03 | 1.79 (1.07 – 2.99) | 0.03 |
| Cardiac catheterization | 3.58 (2.03 – 6.29 | <0.001 | 5.30 (2.68 – 10.5) | <0.001 |
| Cardiac etiology of arrest | 1.71 (0.94 – 3.12) | 0.08 | 1.43 (0.74 – 2.77) | 0.29 |
| Antiplatelet use | 1.74 (1.08 – 2.80) | 0.02 | 2.11 (1.17 – 3.79) | 0.01 |
| Anticoagulant use | 1.65 (0.90 – 3.03) | 0.10 | 0.96 (0.43 – 2.13) | 0.62 |
Because accurate arrest etiology data are only available after February 2012, model 2 includes only 466 of 1,054 subjects
Discussion
We analyzed a large cohort of OHCA patients to test the association of pre-arrest antiplatelet or anticoagulant medications with post-arrest outcomes. A key finding of our work is that antiplatelet medications were independently associated with significantly lower early post-arrest coma severity, more frequent survival to hospital discharge and more frequent good functional outcomes. By contrast, no such association was detected for patients using an anticoagulant medication. We believe this association between antiplatelet use and outcome is biologically plausible and offers an appealing potential therapeutic target for intra-arrest or early post-arrest treatment.
Evidence from animal and human research suggest that disordered thrombosis and microcirculatory obstruction contribute substantially to organ dysfunction after cardiac arrest. In large animal models of ventricular fibrillation cardiac arrest followed by CPR, global cerebral perfusion was reduced to approximately 50% of sham control, creating local areas of low-and no-flow that were not present pre-arrest [22]. Treatment with heparin and tissue plasminogen activator significantly reduced cerebrovascular obstruction with pathological findings suggesting microthrombi as a culprit for cerebral no-reflow [12]. Unfortunately, consistent with our observation that platelet inhibition but not anticoagulant medication is associated with improved outcomes, human trials found no benefit to anticoagulation or thrombolysis [14, 15, 23]. Issues with study design or unmeasured confounders may explain this failure to translate. Alternatively, anticoagulants act predominantly in the venous system where thrombi formed due to stasis are composed largely of fibrin and trapped red cells [24]. By contrast, microvascular thrombosis following OHCA may be primarily an arteriolar phenomenon, making antiplatelets more effective protective agents.
During OHCA, cessation of blood flow abolishes laminar arterial flow and disrupts the endothelial glycolayx. The glycocalyx is a negatively charged, thin gel-like barrier surrounding the luminal vascular endothelium that modulates adhesion of inflammatory cells and platelets [25]. Combined, these mechanisms create an environment favorable to clot formation. Survivors of cardiac arrest exhibit elevated circulating components of the glycocalyx, indicating damage [26]. The consequences of this damage include inflammation [27], platelet aggregation [28], and hypercoagulability [29]. Concentrations of anti-inflammatory and anti-clotting molecules such as nitric oxide and prostacyclin are down regulated, while proinflammatory compounds such as leukocyte adhesion molecules, cytokines, endothelin, and thromboxane A2 are upregulated [30].
Inflammatory pathways result in activation of platelets, release of tissue factor, generation of thrombin, as well as low protein C and protein S [31]. Antiplatelet agents may act early on to slow the propagation of clot by tempering platelet activation and resulting inflammation. Reducing the inflammation that characterizes the “sepsis-like” state following OHCA may represent a second biologically plausible pathway for antiplatelet agents to further attenuate the severity of post-arrest organ dysfunction. These inflammatory changes are not influenced by an anticoagulation alone [31].
While beneficial effects of peri-arrest thrombolytics and heparin are not detectable in clinical studies, the peri-arrest platelet inhibition represents a mechanism not previously well characterized and independent of anticoagulation. Preexisting platelet inhibition may prevent clot initiation during the post-arrest prothrombotic state or result in a failure to develop a matrix capable of sustaining a clot in the microvasculature mitigating end organ dysfunction. Further Platelet aggregometry or platelet mapping could better characterize platelet activity during this time period. Direct assessment of the microcirculation may be possible in some patients using laser speckle, Doppler, or orthogonal polarizing spectroscopy techniques but each of these has significant limitations in the clinical environment.
As with any retrospective study, our work is not without limitations. Though our findings are consistent with a biologically plausible mechanism, we cannot assert causality. We adjusted for previously reported clinically influential covariates, however temporally associated but unmeasured confounders may have biased our results. For example, patients prescribed an antiplatelet medication may also have better access to pre-arrest care or pre-arrest functional status, improving their odds of a favorable outcome. Although we attempted to account for this effect by adjusting for any medication use, there are undoubtedly unmeasured sources of bias in pre-arrest factors that remain. Moreover, because of the observational study design we could not measure or account for all potential confounders related to post-arrest care that might affect the association between pre-arrest medication use and outcomes that were assessed at hospital discharge. Additionally, since we included a cohort of subjects that regained spontaneous circulation and survived to hospital arrival, it remains unknown how treatment affects the total population of OHCA including those who do not achieve ROSC. Future work including the entire OHCA population including those without ROSC will be important to elucidate this potential effect, but will be challenging given limited available data on those assessed by not treated by emergency medical services and those treated but not transported. The presence of antiplatelet agents may also be a surrogate for unmeasured confounders that may be associated with favorable outcomes.
In conclusion, our work suggests that antiplatelet agents may attenuate early post-arrest organ dysfunction, particularly with regards to coma severity, resulting in better survival to discharge and functional outcomes. Future work will focus on characterizing clot burden in patients suffering OHCA and identifying targets for intervention early in the post-cardiac arrest disease process.
Appendix
The Pittsburgh Post-Cardiac Arrest Service investigators are:
Clifton W Callaway
Cameron Dezfulian
Ankur A Doshi
Jonathan Elmer
Lillian Emlet
Adam Frisch
Frank X Guyette
Bradley Molyneaux
Masa Okubo
Jon C Rittenberger
Kelly Sawyer
Alex Weissman
Footnotes
Disclosures: Dr. Elmer's research time is supported by the NIH through grants 5K12HL109068 and 1K23NS097629. Dr. Kurz's research is supported by the Emergency Medicine Foundation, Society of Critical Care Medicine, American Heart Association, and NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrams HC, et al. A composite model of survival from out-of-hospital cardiac arrest using the Cardiac Arrest Registry to Enhance Survival (CARES) Resuscitation. 2013;84(8):1093–8. doi: 10.1016/j.resuscitation.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 3.Coppler PJ, et al. Validation of the Pittsburgh Cardiac Arrest Category illness severity score. Resuscitation. 2015;89:86–92. doi: 10.1016/j.resuscitation.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elmer J, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127–35. doi: 10.1016/j.resuscitation.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callaway CW, et al. Early coronary angiography and induced hypothermia are associated with survival and functional recovery after out-of-hospital cardiac arrest. Resuscitation. 2014;85(5):657–63. doi: 10.1016/j.resuscitation.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weidman JL, Shook DC, Hilberath JN. Cardiac resuscitation and coagulation. Anesthesiology. 2014;120(4):1009–14. doi: 10.1097/ALN.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 7.Elmer J, Callaway CW. The Brain after Cardiac Arrest. Semin Neurol. 2017;37(1):19–24. doi: 10.1055/s-0036-1597833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppler PJ, et al. Validation of the Pittsburgh Cardiac Arrest Category illness severity score. Resuscitation. 2015 doi: 10.1016/j.resuscitation.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rittenberger JC, et al. An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation. 2011;82(11):1399–404. doi: 10.1016/j.resuscitation.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pytte M, et al. Prearrest administration of low-molecular-weight heparin in porcine cardiac arrest: hemodynamic effects and resuscitability. Crit Care Med. 2008;36(3):881–6. doi: 10.1097/CCM.0B013E318164E781. [DOI] [PubMed] [Google Scholar]
- 11.Johansson J, et al. Antithrombin administration during experimental cardiopulmonary resuscitation. Resuscitation. 2004;62(1):71–8. doi: 10.1016/j.resuscitation.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Fischer M, et al. Thrombolysis using plasminogen activator and heparin reduces cerebral no-reflow after resuscitation from cardiac arrest: An experimental study in the cat. Intensive Care Medicine. 1996;22(11):1214–1223. doi: 10.1007/BF01709339. [DOI] [PubMed] [Google Scholar]
- 13.Gaszynski W. The use of protease inhibitor (trasylol) and heparin in cardiorespiratory resuscitation. I. Studies of the blood clotting system. Anaesth Resusc Intensive Ther. 1975;3(2):125–34. [PubMed] [Google Scholar]
- 14.Bottiger BW, et al. Thrombolysis during resuscitation for out-of-hospital cardiac arrest. N Engl J Med. 2008;359(25):2651–62. doi: 10.1056/NEJMoa070570. [DOI] [PubMed] [Google Scholar]
- 15.Pedley DK, Morrison WG. Role of thrombolytic agents in cardiac arrest. Emerg Med J. 2006;23(10):747–52. doi: 10.1136/emj.2006.038067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Connor RE, et al. Part 9: Acute Coronary Syndromes: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S483–500. doi: 10.1161/CIR.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 17.Jauch EC, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 18.Elmer J, et al. Long-term survival benefit from treatment at a specialty center after cardiac arrest. Resuscitation. 2016;108:48–53. doi: 10.1016/j.resuscitation.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rittenberger JC, et al. Outcomes of a hospital-wide plan to improve care of comatose survivors of cardiac arrest. Resuscitation. 2008;79(2):198–204. doi: 10.1016/j.resuscitation.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummins RO, et al. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: the Utstein Style. A statement for health professionals from a task force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, and the Australian Resuscitation Council. Circulation. 1991;84(2):960–75. doi: 10.1161/01.cir.84.2.960. [DOI] [PubMed] [Google Scholar]
- 21.Rittenberger JC, et al. Association between Cerebral Performance Category, Modified Rankin Scale, and discharge disposition after cardiac arrest. Resuscitation. 2011;82(8):1036–40. doi: 10.1016/j.resuscitation.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolfson SK, Jr, et al. Dynamic heterogeneity of cerebral hypoperfusion after prolonged cardiac arrest in dogs measured by the stable xenon/CT technique: a preliminary study. Resuscitation. 1992;23(1):1–20. doi: 10.1016/0300-9572(92)90158-9. [DOI] [PubMed] [Google Scholar]
- 23.Spohr F, et al. International multicentre trial protocol to assess the efficacy and safety of tenecteplase during cardiopulmonary resuscitation in patients with out-of-hospital cardiac arrest: the Thrombolysis in Cardiac Arrest (TROICA) Study. Eur J Clin Invest. 2005;35(5):315–23. doi: 10.1111/j.1365-2362.2005.01491.x. [DOI] [PubMed] [Google Scholar]
- 24.Weitz JI. Harrison's Principles of Internal Medicine: Chapter 118 Antiplatelet, Anticoagulant, and Fibrinolytic Drugs. 18. New York, NY: McGraw-Hill; 2012. [Google Scholar]
- 25.Kolarova H, et al. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediators Inflamm. 2014;2014:694312. doi: 10.1155/2014/694312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grundmann S, et al. Perturbation of the endothelial glycocalyx in post cardiac arrest syndrome. Resuscitation. 2012;83(6):715–20. doi: 10.1016/j.resuscitation.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Donati A, et al. Alteration of the sublingual microvascular glycocalyx in critically ill patients. Microvasc Res. 2013;90:86–9. doi: 10.1016/j.mvr.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Vink H, Constantinescu AA, Spaan JA. Oxidized lipoproteins degrade the endothelial surface layer: implications for platelet-endothelial cell adhesion. Circulation. 2000;101(13):1500–2. doi: 10.1161/01.cir.101.13.1500. [DOI] [PubMed] [Google Scholar]
- 29.Nieuwdorp M, et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes. 2006;55(2):480–486. doi: 10.2337/diabetes.55.02.06.db05-1103. [DOI] [PubMed] [Google Scholar]
- 30.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 31.Adrie C, et al. Coagulopathy after successful cardiopulmonary resuscitation following cardiac arrest: implication of the protein C anticoagulant pathway. J Am Coll Cardiol. 2005;46(1):21–8. doi: 10.1016/j.jacc.2005.03.046. [DOI] [PubMed] [Google Scholar]