Abstract
Engineered nanoparticles (NPs) have broad applications in industry and nanomedicine. When NPs enter the body, interactions with the immune system are unavoidable. The innate immune system, a non-specific first line of defense against potential threats to the host, immediately interacts with introduced NPs and generates complicated immune responses. Depending on their physicochemical properties, NPs can interact with cells and proteins to stimulate or suppress the innate immune response, and similarly activate or avoid the complement system. NPs size, shape, hydrophobicity and surface modification are the main factors that influence the interactions between NPs and the innate immune system. In this review, we will focus on recent reports about the relationship between the physicochemical properties of NPs and their innate immune response, and their applications in immunotherapy.
Keywords: immune response, engineered nanoparticle, physicochemical properties, immunomodulation, immunotherapy
1. Introduction
Due to their unique physical and chemical properties, Nanoparticles (NPs) are widely used in electronics, cosmetics, textiles and nanomedicine [1–2 3 4 5]. At present, human exposure to engineered NPs is widespread, through environmental routes (inhalation, ingestion, dermal contact and parenteral) or deliberate administration [6,7]. Interactions between nanoparticles(NPs) and the immune system have become important, and there are foundational questions about the safety of these special materials. NPs can communicate with various biological components (cells, receptors, proteins etc.) of the immune system, trigger cell signaling cascades, and consequently cause unpredictable immune responses (activation or suppression) and even harmful outcomes (autoimmune diseases or cancer) [5,7,8]. There is also evidence that NPs can alter the development of immune systems in utero in mouse models. [9]. Therefore, understanding how NPs influence or tune the immune system is critical to better knowing the potential risks in developing new nanomaterials.
The basic concept of the immune system is a biological network that reacts to foreign threats (i.e. antigens) to protect the host and maintain homeostasis [5]. The overall system is divided into two subsystems: innate immunity and adaptive immunity. Innate immunity is the first line of defense, generating a non-specific inflammatory response upon the detection of conserved biological motifs, often associated with bacteria and viruses. The adaptive immune system is a more nuanced defense mechanism that involves the development of antibodies highly-specific to detected antigens, followed by the generation of memory cells for future immunological protection [10]. Components of the innate immune system recognize pathogens mainly via pattern-recognition receptors (PRPs), while antigen presenting cells (APCs) present acquired antigens to T cells for the activation of acquired immune system. When NPs enter the body, they have a high probability of interacting with the innate immune system first, generating an immunomodulatory response based on their physicochemical properties [8,11]. Hence, understanding how NPs interact with the innate immune system is particularly important, and would provide insight into designing immune-compatible NP technologies.
Engineered NPs can be designed to either specifically interact with or avoid recognition by the immune system. Synthetic NPs have been utilized frequently to generate novel immunotherapy strategies. Immunotherapy involves intentional modulation of the immune system as a therapeutic strategy. One of the primary strengths of immunotherapy is that there can be less negative side effects than those associated with traditional therapies [12,13]. A frequent use for NPs in immunotherapy contexts has been for developing new vaccines, which has been previously discussed [14–15]. Here, we will focus on understanding the interactions between the innate immune system and engineered NPs for other immunomodulatory purposes. First, we will discuss how physicochemical properties of NPs affect the contact of NPs with the innate immune system and the resulting immune response. Then, we will demonstrate how to take advantage of NPs immunomodulatory properties for biological applications. At last, we will discuss remaining challenges that need to be considered for NP applications.
2. Innate immune system
The innate immune system is a broad, less-specific defense mechanism, which includes molecular (complement system, cytokines) and cellular (phagocytes and leukocytes) components that recognize classes of molecules particular to frequently encountered pathogens. Most components of the innate immune system are present before the onset of the infection and rapidly respond to invasion within minutes. In conjunction with this system is the highly organized complement system, which involves a set of serum proteins that circulate in an inactive state. Those proteins are converted into an active state through three pathways (classical, lection and alternative pathway) to damage and clear pathogenic organisms [19]. Activation of the complement system leads to the formation of the potent anaphylatoxins C3a and C5a. These proteins elicit physiological responses such as chemoattraction (attract phagocytes to sites of injury or inflammation) and enhanced vascular permeability [20]. The innate immune system includes several circulating and tissue-specific cell types, such as natural killer cells, granulocytes (neutrophils, basophils, eosinophils, mast cells) and antigen-presenting cells (macrophage and dendritic cells (DC)). APCs and neutrophils are responsible for recognizing pathogens via PRRs, which identify pathogen-associated molecular patterns (PAMPs). Following identification, the cells uptake and digest the pathogen, generating an inflammatory response [19,21]. APCs are also activated by damage-associated molecular pattern molecules (DAMPs) (such as ATP, uric acid, heparin sulfate) from stressed or damaged tissues or microbes [22]. These cells usually produce higher levels of reactive oxygen species (ROS), causing an accumulation of oxidative glutathione (GSSG). These changes further elicit inflammatory responses through distinct signaling pathways, such as nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and NACHT-LRR and PYD domain-containing proteins 3 (NLRP3). These changes can also cause cytokine secretion (e.g. interleukins (ILs), tumor necrosis factor (TNF-α)) [21].
Activation of PRRs is an essential part of the inflammatory immune response that direct the host cell to distinguish “self” from “non-self”. PRRs are expressed on either the cell membrane (such as Toll-like receptors (TLRs) and C-type lectin receptors (CLRs)) or in the cytosol (such as NOD-like receptors (NLRs) and RIG-I-like receptors (RLRs)) [22]. Based on their function, PRRs are divided into signaling PRRs and endocytic PRRs. Signaling PRRs (TLRs and NLRs) have a variety of functions in the regulation of inflammation and apoptotic response. For example, TLR signaling results in the activation of NF-κB, mitogen-activated protein kinase, and interferon-regulatory factors (IRFs). Those signal pathways ultimately result in gene expression and secretion of cytokines, chemokines, cell adhesion molecules, and immunoreceptors [22,23]. Endocytic PRRs can promote the attachment, engulfment and destruction of microorganisms and foreign entities by phagocytes. Each PRR family member binds a specific molecular pattern. For example, TLR4 recognizes extracellular bacterial lipopolysaccharide (LPS), and the NLR/inflammasome recognizes cytosolic bacterial DNA or peptidoglycan molecules [23,24]. By controlling the interaction with these specific receptors, the overall immunological response can be regulated.
3. Physicochemical properties of nanoparticles modulate innate immune response
NPs have been prepared with a variety of controlled structures and functionalities for delivery, therapeutic, and diagnostic purposes. Once inside the body, engineered NPs as foreign substance are immediately encounter the innate immune system and generate specific immune response based on their properties. The physicochemical properties (e.g. size, charge, shape, hydrophobicity, and stiffness) of NPs determine their interactions with soluble proteins, APCs and neutrophils, in particular effect the downstream signaling that are used to detect pathogens or other immunoregulatory events [7,11,24,25].
3.1 Size and shape
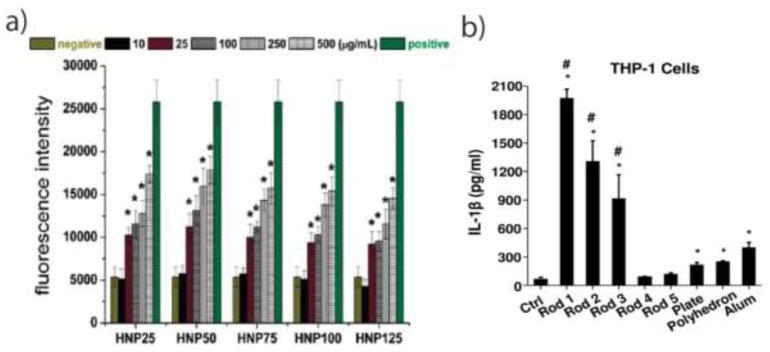
The size of NPs has a significant impact on the uptake of these materials by cells (particularly those of the innate immune system), the initiation of innate immune response, and their overall bio-distribution in vivo [7,10]. With an increase in size, the surface to volume ratio of NPs is decreased, which affects their interactions with the innate immune system. There are four endocytotic NPs uptake mechanisms: pinocytosis, macropinocytosis, phagocytosis and clathrin/caveolar mediated endocytosis. Pinocytosis and micropinocytosis, both commonly involved in NP uptake by the immune system, are non-specific processes to internalize NPs and fluids together into cells [26]. By using fluorescent PEGylated NPs, Kruth et al. reported the visualization of receptor-independent fluid-phase pinocytosis by macrophages in vitro and in a mouse atherosclerotic lesion model [27]. The uptake of NPs usually occurs with the cooperation of several endocytic uptake mechanisms. Through endocytic inhibitor analysis, Gu et al. reported that superparamagnetic iron oxide NPs (~ 10nm) were internalized into Raw 264.7 macrophage cells using clathrin-dependent endocytosis (inhibited with chlorpromazine), caveolae-dependent endocytosis (inhibited with β-cyclodextrin), and macropinocytosis (inhibited with amiloride) [28]. Rothen et al. showed that 600 nm polystyrene NPs (PS) were engulfed by phagocytosis/macropinocytosis, while 40 nm PS were internalized by both clathrin-mediated endocytosis as well as phagocytosis or macropinocytosis by macrophages (J774.1A) [29]. Čolić et al. also reported that gold NPs of 10nm and 50nm were internalized predominantly via a clathrin/dynamin-dependent mechanism by DCs, and a significantly higher number of 10 nm AuNPs per cell were uptaken than 50 nm AuNPs. It was demonstrated that smaller AuNPs inhibit the maturation of LPS induced DCs more strongly than larger ones [30]. Similarly, silica-titania hollow nanoparticles with 50 nm diameter, compared with up to 125nm, generated more ROS (Figure 1.a), and exhibited the highest induction of inflammatory cytokines (IL-1, IL-6 and TNF-α) with mouse alveolar macrophage (J774.1) in vitro [31]. However, the relationship between size of NPs and innate immune response is not linear. Jang et al. synthesized five different diameters (20 nm, 40 nm, 60 nm, 80 nm and 100 nm) of monodispersed polypyrrole (PPy) nanoparticles. Mid-sized 60 nm PPy NPs evoked the highest level of ROS and increased the expression level of costimulatory markers (CD40 and CD80) in macrophages [32]. For the bio-distribution, several studies demonstrated that smaller NPs had higher retention compared to larger NPs, and that most NPs accumulate in the liver (Kupffer cells), the amount increasing with the size of NPs [33,34]. Additionally, small NPs (20 nm to 200 nm) rapidly drained to the lymph nodes (LN), where they were taken up by resident DC. Large NPs (500 nm, 1000 nm) depended on cellular transport by DC, immigrating from the injection site (skin) to LN in vivo [35]. These data suggested that larger NPs prefer interacting with tissue-resident APCs, while smaller NPs (<200nm) could circulate through vein and lymphatic drainage, providing better antigen presentation.
Figure 1.
Size and shape effect on the innate immune system. a) silica-titania hollow NPs with 50 nm diameter generated more ROS than other size NPs, and generated the highest level of inflammation. b) AlOOH nanorods caused the secretion of more cytokine (IL-1β) than nanoplate and polyhedron in THP-1 cells.
The shape of NPs has also been demonstrated to affect interactions with the innate immune system [21,25,36]. Groll et al. found that the uptake of nanorods by macrophages was more efficient than the uptake of nanospheres. This is a result of the generation of > 1μm vesicles, allowing the nanorods to enter easily through macropinocytosis. It was also observed that neutrophil granulocytes did not fully internalize the particles but trapped them in their extracellular structures [37]. Within various aluminum oxyhydroxide nanomaterials (nanorod, nanoplate, and nanopolyhedra), nanorods are the most redox active materials, capable of NLRP3 inflammasome activation and stimulating IL-1β production in human THP-1 cells and bone marrow-derived DCs (BMDCs) (Figure 1.b) [38]. Moreover, higher aspect ratios of nanorods minimized phagocytosis and enhanced cytokine secretion (IL-6, and IFN-γ) [39]. Similarly, Moghimi et al. compared complement activation using spherical, prolate ellipsoidal (rods) and oblate ellipsoidal (disks) polystyrene NPs with equivalent surface area in pig and human blood. They found that all types of NPs induced complement activation (through measurements of C3bc, C3a, C5a, and sC5b-9) within the first 5 minutes of contact with blood, but rods and disks generate more profound complement activation than spheres at later timepoints [40]. Recently, Bigini et al. demonstrated that spherical and star-like Au NPs show the same percentage of accumulation but a different localization in liver, and only star-like Au NPs could be able to accumulate in lung [41].
The crystal phase of NPs also affects immune response. For example, Self et al. reported that titanium dioxide (TiO2) with three nanoarchitectures (anatase, rutile, and nanocube) could raise levels of proinflammatory cytokines, increase maturation, and increase expression of costimulatory molecules on DCs compared to micron-Titania [42]. Utilizing various sizes and shapes of polystyrene NPs, particles possessing the longest dimension in the range of 2–3 μm exhibited maximum tendency to attach to macrophages, mimicking the procedure of bacterium recognition by APCs, as these size particles exhibit the strongest binding to the membrane ruffles of the macrophage surface [43].
3.2 Hydrophobicity and surface modification
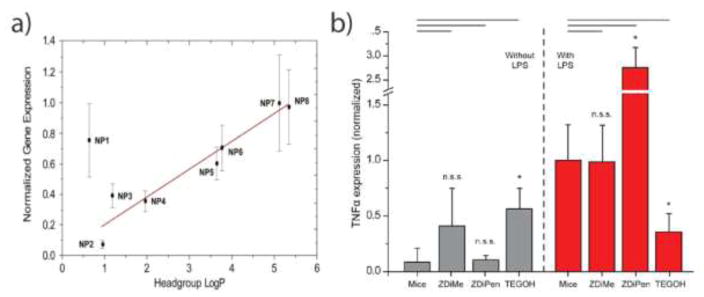
Modifying the surface properties of NPs alter their immunology response, especially in regards to recognition by hydrophobic moieties found in DAMPs and PAMPs, which are involved in danger signal pathways of the innate immune system [24,44]. Polymer NPs with different degrees of hydrophobicity were used to demonstrate that an increasing NP hydrophobicity yielded higher levels of cellular internalization and costimulatory markers (CD86) expression in DCs [45]. This pattern is consistent with other innate immune cell types; with increasingly hydrophobic positive charged NPs, proinflammatory cytokine gene expression level was upregulated in splenocytes from mice (Figure 2.a) [46]. Depending on the hydrophobicity and the arrangement of chemical motifs, cationic AuNPs bind various amounts of serum proteins (e.g. lipoproteins, immunoglobins, complements) forming a “protein corona”, which were then internalized by macrophages in a corona-dependent manner in vitro [47]. With increasing hydrophobicity, the amount of apolipoproteins and immunoglobulins adsorbed on NPs surface decreased, as well as the uptake of NPs in Raw 264.7 cells. The correlation of corona formation and NP recognition by macrophages demonstrated that adsorption of proteins from the complement system, apolipoprotein family, and coagulation factors has high positive correlation with the macrophage uptake. Similarly, a series of zwitterionic AuNPs accumulated less serum protein absorption on the NP surface (corona free), resulting in lower cellular uptake than cationic AuNPs [48]. NPs bearing a hydrophobic zwitterionic functionality boosted LPS-induced inflammatory outcomes, whereas hydrophilic zwitterionic NPs generated minimal immunology response in vivo (Figure 2.b) [49].
Figure 2.
Hydrophobicity effect on the innate immune system. a) TNF-α in vitro gene expression increased with increasing NP hydrophobicity. b) TNF-α in vivo secretion with injection of zwitterionic NPs. NPs bearing a hydrophobic zwitterionic functionality (ZDiPen) boosted LPS-induced inflammatory outcomes, whereas hydrophilic zwitterionic NPs (ZDiMe) generated minimal immunology response.
As mentioned above, proteins such as immunoglobulin, albumin, complements and apolipoproteins adsorb onto the nanoparticle surface, forming a “protein corona”, which marks them as an invader for recognition by specialized receptors, such as the TLR series, as well as the scavenger and complement receptors [50]. Particulary important is opsonization, the process in which complement proteins adsorb on the surface of NPs, priming particles for removal by immune cells. NPs incubated in serum and plasma can be rapidly opsonized with the third complement component (C3) via the alternative pathway, where C3 covalently binds to the absorbed serum proteins rather than directly to the NP shell. The protein corona accelerates the assembly of complement components on the NP surface in vitro, however C3 and other absorbed proteins were rapidly shed from the surface once the NPs were injected to mice. The exchangeable nature of the protein corona can lead to continuous shedding of the complement factors and re-opsonization in vivo [51]. Based on the composition of the protein corona, Chan et al. developed a quantitative model that uses a serum protein corona “fingerprint” to predict the cell association of 105 surface-modified AuNPs, with the larger goal of establishing predictive models for the design of NPs [52]. When “stealthy” NPs are necessary, they need to be modified to avoid or decrease potential interactions with these specialized immune receptors. For example, linear chain poly (ethylene glycol) (PEG) coating (PEGylation) provides a hydrophilic shell surrounding the NPs to block nonspecific protein adsorption by blocking potential protein-binding sites. This “stealth” coating creates a thermodynamic barrier to protein diffusion, reducing overall protein adsorption. A potential consequence of reduced protein adsorption is the subsequent slowing of cellular uptake by phagocytes that rely on the recognition of the protein corona, prolonging NP blood residence time. The stealth effect of PEGylation varies with chain length and density of PEG ligands. Above a certain density of PEG (~0.64 PEG/nm2), serum proteins adsorbed to the NPs are insignificant in the uptake process, reducing macrophage uptake of PEG-grafted NPs [53]. In addition to reducing protein adsorption, PEGylation can affect the composition of the protein corona around the surface of NPs. Specifically, PEGylation can affect the adsorption of clusterin proteins (also known as apolipoprotein J), an abundance of which are necessary to prevent non-specific uptake by innate immune system [54]. However, AuNPs with methoxy-PEG-thiol ligands can be displaced with cysteine and cystine, leading to protein absorption and cell uptake in macrophages within 24 h. By incorporating an alkyl linker between the PEG and the thiol moieties, a hydrophobic shield layer was introduced which greatly reduced protein adsorption on AuNPs as well as macrophage uptake [55].
Surface charge also has a significant effect on the interaction with the innate immune system. Positively charged NPs enhance the electrostatic attraction between NPs and negatively charged cell membrane, leading to the increased surface endocytosis (or phagocytosis) [56]. Hence, cationic NPs showed higher uptake in macrophage and DCs [57,58]. Hu et al. demonstrated that positively charged Fe2O3 NPs enhanced the cross-presentation ability of DCs, while negatively charged Fe2O3 NPs were associated with rapid autophagy [59]. Interestingly, the rate of cellular NPs internalization depends on surface charge and the phenotype of the differentiated cells. Simmet et al. reported that macrophages, differentiated from human monocytes, internalized ~4 times more PC-COOH than THP-1 cells (monocytic cell line), while THP-1 cells internalized PS-NH2 more rapidly than macrophages. It was shown that the macrophage uptake of NPs by phagocytosis depended on the specific interactions between the CD63 receptor and NPs, whereas internalization by THP-1 occurred via dynamin ||-dependent endocytosis. Meanwhile, in vivo data shown that PC-COOH NPs was accumulated in liver, where macrophages of the reticuloendothelial system reside, and PS-NH2 NPs were found in the tumor xenografts [60]. For pulmonary therapy, cationic NPs modulated the local lung environment to promote recruitment and maturation of lung DCs, but anionic NPs were found to be immunologically inert in the lung [61]. Modification of surface charge of NPs were exploited for tuning the immune response. Bao et al. demonstrated that three types of polymer coating (negative, positive, and neutral) on iron oxide NPs does not significantly change the expression of TNF-α and TLR2 in a human monocyte cell line [62]. Moreover, a silica-silane shell (neutral inorganic layer) passivated polymer NPs generated a significant decrease in the activation of innate immune cells [62].
The surface of NPs can be modified with specific ligands for receptor targeting. In the innate immune system, phagocytosis relies on a balance between prophagocytic (“eat me) and antiphagocytic (“don’t eat me”) signals on target. CD47 is a transmembrane protein that binds to signal regulatory protein α (SIRPα) expressed on the surface of macrophages. The binding of SIRPα with CD47 results in the phosphorylation of the cytoplasmic tail of SIRPα, leading to the activation of protein phosphatases that block phagocytosis [63]. Surface modification of NPs with CD47 could alter the phagocytic signaling cascade and significantly reduced phagocytosis of NPs through binding to SIRPα, which are expressed on APCs. [64]. NPs functionalized with TLR agonists, such as monophosphorly lipid A (MPLA), polyinosinic-polycytidylic acid (poly I:C) and CpG-riched oligodeoxynucleotides (CpG), enhanced cytokine production (e.g. IFN-γ, Il-6, and TNF-α), expression of activation markers (e.g. CD40, CD80 and CD86) and upregulation of immunoregulatory genes (e.g. Cxcl1, Ccl4, Il6 and Cd14) [65-]. Narasimhan et al. have shown that amphiphilic polyanhydride NPs possess pathogen-mimicking properties, and can activate DCs similarly to LPS (TLR 4 agonist) [68]. Gamazo et al. demonstrated that poly (methyl vinyl ether-co-maleic anhydride) NPs (PVMA) act as agonists of TLR2 and TLR4, and highly activated complement activation by stable binding to C3b [69]. In another approach, Thaxton et al. synthesized a suite of high-density lipoprotein-liked NPs which functions to scavenge and neutralize LPS, inhibiting TLR-4 dependent inflammatory response [70]. Moreover, Cho et al. demonstrated that NPs coated with negative regulatory complement factor H can prevent complement activation with >90% efficiency [71]. And galactose polymer modified NPs could absorbed complement H protein on their surface in vitro, providing a low level of complement activation [72].
Moreover, different inorganic NPs cores generate various levels of cellular response on the innate immune system. For example, Zubrev et al. have shown that NPs (CdTe, CuO, Au) induced distinct and distinguishable proteomics signatures on THP-1 cells. The CdTe NPs revealed a proteomics response similarly to anticancer drugs related to down-regulation of topoisomerases. CuO NPs treatment resulted in up-regulation of heat-associated proteins as well as induced ROS production. AuNPs induced up-regulation of the inflammatory mediator (NF-κB) that is mediated through dysregulation of immune homeostasis by inactivating the TIPE2 protein (negative regulator of NF-κB). [73]. At ultralow and nontoxic concentrations (10−6 ~ 10−3 μg/ml), Ag, TiO2 and ZnO NPs induce mild pro-inflammatory response by the NF-κB signaling pathway, and upregulate gene expression levels of Il6, Il1b, Nfkb1 and TNF-α in Raw 264.7 cells [74].
4. Therapeutic immune modulation by engineered nanoparticles
Engineered NPs acting as delivery vehicles or direct immunomodulation agents can manipulate the innate immune system for therapeutic purposes. The main two applications areas are vaccination and cancer immunotherapy, which both “train” the immune system to detect and eliminate foreign entities or tumors. The key to these therapeutic strategies is to induce the desired immune response (stimulation or suppression) through the recognition of engineered NPs by the innate immune system, especially APCs. [75-].
In cancer immunotherapy, the crucial process is to generate an appropriate pro-inflammatory signal in response to tumor antigens [69]. By taking advantage of the innate immune system cells’ tumor targeting ability, anti-tumor drug loaded NPs were internalized within macrophages and delivered to tumor cells via cell-cell binding, causing tumor cell death [78]. Exosomes of APCs are enriched in co-stimulatory molecules and the major histocompatibility complex (MHC). They are used as an alternative pathway to tune the immune response [79]. By mimicking their functions, MHC/peptide complexes and Fab region coated liposomes, which act like natural APCs exosomes, bound and triggered activation and expansion of T cells [80]. Similarly, when nanoporous silicon NPs were coated with cellular membranes purified from leukocytes, these “camouflaged” NPs could avoid opsonization, delay uptake by the mononuclear phagocyte system, preferentially bind inflamed endothelium, and facilitate accumulation in a tumor site [81]. In addition, CpG and MPLA coated NPs, known as “artificial bacteria”, demonstrated stronger pro-inflammatory response (higher IL-6 and IL-12 secretion) [65]. Taking advantage of the physical properties of NPs, superparamagnetic iron oxide NPs can be efficiently recognized and uptaken by DCs in vitro, while simultaneously acting as an imaging agent for magnetic resonance imaging (MRI) in vivo [82]. NPs consisting of ferumoxytol, an iron supplement approved by the FDA, inhibited cancer growth by inducing a pro-inflammatory immune response with M1 macrophage polarization [83]. In addition, NPs designed with ultrasound and photo-sensitive properties could increase the interaction with innate immune system and generate efficient antitumor effects [84-]. Ex vivo DC-based immunotherapy can also elicit a strong antigen-specific immune response. For example, near-infrared (NIR) excited up-conversion NPs (UCNPs) were efficiently engulfed by DCs and induced DC maturation (upregulation of CD80 and CD86) and cytokine release (IL-12p70 and IL-1β) in vitro. These UCNPs loaded DCs migrated to the lymph node near the injection position to initiate and trigger an immune response in vivo. The phagocytosis capability of macrophages from UCNPs treated mice was increased, demonstrating that NPs can stimulate innate immune response in vivo [87]. Recently, small interfering RNA (siRNA) modified NPs showed drastic gene silencing efficiency against the suppressor of cytokine signaling 1(SOCS1) gene in mouse DCs, leading to enhanced TNF-α and IL-6 production, and inhibited tumor growth in lymphoma bearing mice [88].
Certain diseases benefit from immune inhibition; these include allergies, atopic disorders, autoimmunity, and organ transplantation. Therapeutic NPs for these purposes need to have immunosuppressive or anti-inflammatory properties [77]. Engineered NPs as vehicles are designed to deliver anti-inflammatory compounds to phagocytes, reducing the therapeutic dose and immune based side effects. For example, rapamycin-loaded poly (lactic-co-glycolic acid) (PLGA) NPs down-regulated intercellular adhesion molecule 1 (ICAM-1) expression in DCs and maintained DCs in the immature state, exhibiting an immunosuppressive response [89]. Rapamycin and dexamethasone loaded NPs also have been shown to downregulate production of pro-inflammatory cytokines via sustained drug release, maintaining immunosuppression to transplant recipients, and prolonging viability of transplanted tissue [90,91]. In many autoimmune diseases, the expression of pro-inflammatory cytokines is overwhelming. NPs have been designed for the prevention of interactions between cytokines and their specific receptor in addition to reducing the overall cytokine gene expression. For example, NPs conjugated with antibody (e.g. Tocilizumab(anti-IL6R) and anti-IL4α) significantly decreased cytokine expression and had prolonged immunosuppressive effects [92,93]. Delivery of cytokine-targeted and signaling pathway targeted siRNA (TNF-α siRNA and COX2 siRNA) also reduced cytokine secretion and generated an anti-inflammatory response [94,95]. Moreover, excessive and prolonged activity of inflammatory monocytes is a hallmark of many autoimmune diseases. To inhibit the localization of monocytes to sites of inflammation, Nahrendorf et al. reported lipid based monocyte-targeting siRNA NPs could silence expression of the chemokine receptor CCR2 in the inflammatory monocyte subset to selectively inhibit migration. These NPs attenuated disease progression in mouse atherosclerosis, myocardial infarction, pancreatic islet transplantation and several cancer models [96]. NPs themselves can be used as anti-inflammatory materials. For example, exposure of mice to Ag-NPs resulted in significant changes in innate immune function, and generated strong anti-inflammatory effects on skin wound healing and reduced scarring [97].
5. Current challenges and perspective
Despite the potential benefits of using NPs in industry and medicine, concerns about the biosafety of these materials have not abated. Interactions between engineered NPs and different subsets of the innate immune system have become critical questions that need to be answered. These interactions can generate varied immunological responses to modulate the immune system and may cause immunotoxicity. For example, NPs create more ROS in cells in a pro-inflammatory state, which can induce protein, lipid, and membrane damage. Continuous activation of the immune system can potentially exacerbate the development of allergic and/or autoimmune diseases. On the other end of the spectrum, uncontrolled suppression of the immune system might result in increased incidence and severity of infectious diseases and cancer [98]. Therefore, understanding the immunomodulatory effect of NPs is an essential requirement to developing novel NPs for biological purposes. We have discussed how physicochemical properties of NPs determine their interactions with innate immune system, and how these interactions can be exploited for therapeutic and prophylactic applications. However, several considerations need to be addressed to better understand the situation.
To better understand the larger health implications of NPs, immunotoxicity assays of engineered NPs need to be more thoroughly evaluated in vitro and in vivo. In in vitro analysis, most studies were performed using cancer cell lines or primary cells in a relatively short time-period in two dimensional culture (2-D). Rahmani-Cherati et al. demonstrated that collagen-chitosan scaffolds could provide a suitable 3-D microenvironment for macrophage phagocytosis evaluation and could impact the expression of pro-inflammatory cytokines [99]. Therefore, one of challenges is to find better cell culture methods to mimic actual human immune systems. There are no universal agreed-upon standards for cell or mice model selection, read-out selection, end-point selection, and positive or negative control selection [7,100]. Boraschi et al. found that human primary monocyte-based in vitro assays could be used for realistically investigating the effects of engineered NPs on human innate immune responses [101]. In vitro studies on phagocytes can provide useful information on the health risks of NPs [102]. Immunotoxicity studies are usually conducted using traditional positive controls, such as LPS for cytokine induction, Triton X-100 for hemolysis, and cobra venom for complement activation. Lack of NP reference standards limits the validation of these results regarding their biocompatibility and immunotoxicity [5]. It is also difficult to translate immunotoxicity data in vivo based on in vitro data. Moreover, the mechanisms of NPs immunotoxicity are still not clear and need more effort to be determined sufficiently
Routes of NPs administration, such as intradermal, nasal, intravenous, subcutaneous, intramuscular and inhalation, can elicit different immune responses [103,104]. NPs can be recognized by different innate immune system components at first post-injection exposure. In general, NPs introduced through intravenous injection usually result in exposure to a complex environment of circulating immune cells and the complement system, and easily bind with plasma proteins on their surface (protein corona). The composition of protein corona can determine bio-distribution, therapeutic efficacy and immunotoxicity of the NPs [21]. However, NPs introduced through intradermal and intramuscular usually initiate immune response mainly dependent on the tissue residential cells, such as Langerhans’s cells, dermal DCs, and adipose tissue macrophages [25].
Although the immunomodulation of engineered NPs has been linked to the physicochemical properties of NPs, it is important to distinguish this effect from NPs or chemical or biological impurities. Contamination might be responsible for unwanted responses; contamination might be introduced during the synthesis of NPs and are difficult to remove in the purification process [5]. For example, endotoxin contaminants in NPs lead to a false positive proinflammatory result stemming from contamination and not NP behavior [105]. The best way to obtain endotoxin-free NPs is to take precaution to use endotoxin-free conditions at every part of the synthesis [106]. Currently, the FDA-approved methods to detect endotoxin are the Limulus Amebocyte Lysate (LAL) assay for in vitro assay and rabbit pyrogen test (RPT) as an in vivo test. Recently, Nelissen et al. reported that the presence of NiSO4 in culture media resulted in a decreased cellular uptake of AuNPs and sustained nickel-induced DC mutation [107]. Hence, missing this information could impair mechanistic studies of immunotoxicity.
6. Conclusion
Studies have shown that nanoparticles can interact with components of the innate immune system to various immunological endpoints. These interactions are fast, complex, and not well understood. It has been established that NPs’ physicochemical properties (size, shape, hydrophobicity and surface modification) are key to determining their interactions with plasma proteins and immune cells, especially APCs. NPs can absorb proteins on their surfaces once they enter the bloodstream, and these binding proteins guide cellular uptake, clearance route, and tissue bio-distribution. Based on their immune responses, engineered NPs are mainly used for cancer immunotherapy, vaccines, and treatment of autoimmune diseases. The cytotoxicity of NPs is useful in identifying acute host damage, but they do not detect the sub-lethal effects and dysregulation of immune system. Moreover, the mechanism and molecular pathways of how NPs affect the innate immune system are not clear. Therefore, more effort is needed to understand how NPs interact with the innate immune system, and to develop new strategies for the prevention or the treatment of human diseases.
Highlights.
Physicochemical properties of NPs affect interactions with innate immune system.
Engineered NPs are effective for immunomodulation.
Immunotoxicity assays of NPs are challenging.
Acknowledgments
V.M.R. acknowledges support from the NIH (GM077173 and EB022641).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in Medicine Therapeutic Applications and Developments. Clinical Pharmacology and Therapeutics. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 2.Kamyshny A, Magdassi S. Conductive nanomaterials for printed electronics. Small. 2014;10:3515–3535. doi: 10.1002/smll.201303000. [DOI] [PubMed] [Google Scholar]
- 3.Ssneha B. Application of nanotechnology in dentistry. Res J Pharm Technol. 2014;7:81–83. [Google Scholar]
- 4.Dastjerdi R, Montazer M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surfaces B Biointerfaces. 2010;79:5–18. doi: 10.1016/j.colsurfb.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 5.Dobrovolskaia MA, Shurin M, Shvedova AA. Current understanding of interactions between nanoparticles and the immune system. Toxicol Appl Pharmacol. 2016;299:78–89. doi: 10.1016/j.taap.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreitinger JM, Beamer CA, Shepherd DM. Environmental Immunology: Lessons Learned from Exposure to a Select Panel of Immunotoxicants. J Immunol. 2016;196:3217–3225. doi: 10.4049/jimmunol.1502149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrarca C, Clemente E, Amato V, Pedata P, Sabbioni E, Bernardini G, Iavicoli I, Cortese S, Niu Q, Otsuki T, Paganelli R, Di Gioacchino M. Engineered metal based nanoparticles and innate immunity. Clin Mol Allergy. 2015;13:13. doi: 10.1186/s12948-015-0020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kononenko V, Narat M, Drobne D. Nanoparticle interaction with the immune system. Arch Ind Hyg Toxicol. 2015;66:97–108. doi: 10.1515/aiht-2015-66-2582. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu R, Umezawa M, Okamoto S, Onoda A. Effect of maternal exposure to carbon black nanoparticle during early gestation on the splenic phenotype of neonatal mouse. J Toxicol Sci. 2014;39:571–578. doi: 10.2131/jts.39.571. [DOI] [PubMed] [Google Scholar]
- 10.Vivier E, Malissen B. Innate and adaptive immunity: specificities and signaling hierarchies revisited. Nature Immunology. 2005;6:17–22. doi: 10.1038/ni1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo YH, Chang LW, Lin P. Metal-Based Nanoparticles and the Immune System: Activation, Inflammation and Potential Applications. Biomed Res Int. 2015;2015 doi: 10.1155/2015/143720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao K, Singha S, Clemente-casares X, Tsai S, Yang Y. Nanoparticle-Based Immunotherapy. ACS Nano. 2015;9:16–30. doi: 10.1021/nn5062029. [DOI] [PubMed] [Google Scholar]
- 14.Fievez V, Garinot M, Schneider Y, Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J Control Release. 2006;116:1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev. 2008;60:915–928. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith DM, Simon JK, JRB Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13:592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irvine DJ, Hanson MC. Synthetic Nanoparticles Immunotherapy. Chem Rev. 2015;115:11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao L, Seth A, Wibowo N, Zhao C, Mitter N, Yu C, Middelberg APJ. Nanoparticle vaccines. Vaccine. 2014;32:327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 19.Szeto GL, Lavik EB. Materials design at the interface of nanoparticles and innate immunity. J Mater Chem B Mater Biol Med. 2016;4:1610–1618. doi: 10.1039/C5TB01825K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011:227–235. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Najafi-Hajivar S, Zakeri-Milani P, Mohammadi H, Niazi M, Soleymani-Goloujeh M, Baradaran B, Valizadeh H. Overview on experimental models of interactions between nanoparticles and the immune system. Biomed Pharmacother. 2016;83:1365–1378. doi: 10.1016/j.biopha.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 22.Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunological Reviews. 2012;249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landesman-Milo D, Peer D. Altering the immune response with lipid-based nanoparticles. J Control Release. 2012;161:600–608. doi: 10.1016/j.jconrel.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 24.Moyano DF, Liu Y, Peer D, Rotello VM. Modulation of Immune Response Using Engineered Nanoparticle Surfaces. Small. 2016;12:76–82. doi: 10.1002/smll.201502273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Getts DR, Shea LD, Miller SD, King NJC. Harnessing nanoparticles for immune modulation. Trends Immunol. 2015;36:419–427. doi: 10.1016/j.it.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pricop D, Andrieş M. Endocytosis and Exocytosis of Gold Nanoparticles. Int J Nano. 2014;25:1–9. [Google Scholar]
- 27.Buono C, Anzinger JJ, Amar M, Kruth HS. Fluorescent pegylated nanoparticles demonstrate fluid-phase pinocytosis by macrophages in mouse atherosclerotic lesions. Technical advance. 2009;119:1373–1381. doi: 10.1172/JCI35548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ning GU, Haiyan XU, Jimin CAO. The internalization pathway metabolic fate and biological effect of superparamagnetic iron oxide nanoparticles in the macrophage-like RAW264.7 cell. 2011;54:793–805. doi: 10.1007/s11427-011-4215-5. [DOI] [PubMed] [Google Scholar]
- 29.Kuhn DA, Vanhecke D, Michen B, Blank F, Gehr P, Petri-Fink A, Rothen-Rutishauser B. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J Nanotechnol. 2014;5:1625–1636. doi: 10.3762/bjnano.5.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomić S, Dokić J, Vasilijić S, Ogrinc N, Rudolf R, Pelicon P, Vučević D, Milosavljević P, Janković S, Anžel I, Rajković J, Rupnik MS, Friedrich B, Čolić M. Size-dependent effects of gold nanoparticles uptake on maturation and antitumor functions of human dendritic cells in vitro. PLoS One. 2014;9:1–13. doi: 10.1371/journal.pone.0096584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh WK, Kim S, Choi M, Kim C, Jeong YS, Cho BR, Hahn JS, Jang J. Cellular Uptake, Cytotoxicity and Innate Immune Response of Silica - Titania Hollow Nanoparticles Based on Size and Surface Functionality. ACS Nano. 2010;4:5301–5313. doi: 10.1021/nn100561e. [DOI] [PubMed] [Google Scholar]
- 32.Kim, Oh WK, Jeong YS, Hong JY, Cho BR, Hahn JS, Jang J. Cytotoxicity of, and innate immune response to, size-controlled polypyrrole nanoparticles in mammalian cells. Biomaterials. 2011;32:2342–2350. doi: 10.1016/j.biomaterials.2010.11.080. [DOI] [PubMed] [Google Scholar]
- 33.Hirn, Semmler-Behnke M, Schleh C, Wenk A, Lipka J, Schäffler M, Takenaka S, Möller W, Schmid G, Simon U, Kreyling WG. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur J Pharm Biopharm. 2011;77:407–416. doi: 10.1016/j.ejpb.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonavane G, Tomoda K, Makino K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surfaces B Biointerfaces. 2008;66:274–280. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 36.Mostaghaci B, Susewind J, Kickelbick G, Lehr CM, Loretz B. Transfection system of amino-functionalized calcium phosphate nanoparticles: In vitro efficacy, biodegradability and immunogenicity study. ACS Appl Mater Interfaces. 2015;7:5124–5133. doi: 10.1021/am507193a. [DOI] [PubMed] [Google Scholar]
- 37.Bartneck M, Keul HA, Singh S, Czaja K, Bockstaller M, Moeller M, Zwadlo-klarwasser G. Rapid Uptake of Gold Nanorods by Primary Human Blood Phagocytes and Chemistry. ACS Nano. 2010;4:3073–3086. doi: 10.1021/nn100262h. [DOI] [PubMed] [Google Scholar]
- 38.Sun B, Ji Z, Liao YP, Wang M, Wang X, Dong J, Chang CH, Li R, Zhang H, Nel AE, Xia T. Engineering an effective immune adjuvant by designed control of shape and crystallinity of aluminum oxyhydroxide nanoparticles. ACS Nano. 2013;7:10834–10849. doi: 10.1021/nn404211j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leong KW, Yoo HS. Multi-Functional Nanorods Serving as Nano- Bridges to Modulate T Cell-Mediated Immunity. 2013:9771–9779. doi: 10.1021/nn403275p. J. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wibroe PP, Anselmo AC, Nilsson PH, Sarode A, Gupta V, Urbanics R, Szebeni J, Hunter AC, Mitragotri S, Mollnes TE, Moghimi SM. Bypassing adverse injection reactions to nanoparticles through shape modification and attachment to erythrocytes. Nat Nanotechnol. 2017;12:589–594. doi: 10.1038/nnano.2017.47. [DOI] [PubMed] [Google Scholar]
- 41.Talamini L, Violatto MB, Cai Q, Monopoli MP, Kantner K, Krpetic Z, Perez-potti A, Cookman J, Garry D, Silveira CP, Boselli L, Pelaz B, Serchi T, Gutleb AC, Feliu N, Yan Y, Salmona M, Parak WJ, Dawson KA, Bigini P. Influence of Size and Shape on the Anatomical Distribution of Endotoxin-Free Gold Nanoparticles. ACS Nano. 2017;11:5519–5529. doi: 10.1021/acsnano.7b00497. [DOI] [PubMed] [Google Scholar]
- 42.Schanen BC, Karakoti AS, Seal S, DRD, Warren WL. Exposure to Titanium Dioxide Nanomaterials Provokes In ammation of an. ACS Nano. 2009;3:2523–2532. doi: 10.1021/nn900403h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doshi N, Mitragotri S. Macrophages recognize size and shape of their targets. PLoS One. 2010;5:1–6. doi: 10.1371/journal.pone.0010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gause KT, Wheatley AK, Cui J, Yan Y, Kent SJ, Caruso F. Immunological Principles Guiding the Rational Design of Particles for Vaccine Delivery. ACS Nano. 2017;11:54–68. doi: 10.1021/acsnano.6b07343. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Yin Y, Wang L, Zhang W, Chen X, Yang X, Xu J, Ma G. Surface hydrophobicity of microparticles modulates adjuvanticity. J Mater Chem B. 2013;1:3888. doi: 10.1039/c3tb20383b. [DOI] [PubMed] [Google Scholar]
- 46.Moyano DF, Goldsmith M, Solfiell DJ, Landesman-milo D, Miranda OR, Peer D, Rotello VM. Nanoparticle Hydrophobicity Dictates Immune Response. 2012:1–7. doi: 10.1021/ja2108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saha K, Rahimi M, Yazdani M, Kim ST, Moyano DF, Hou S, Das R, Mout R, Rezaee F, Mahmoudi M, Rotello VM. Regulation of Macrophage Recognition through the Interplay of Nanoparticle Surface Functionality and Protein Corona. ACS Nano. 2016;10:4421–4430. doi: 10.1021/acsnano.6b00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moyano DF, Saha K, Prakash G, Yan B, Kong H, Yazdani M, Rotello VM. Fabrication of Corona Free Nanoparticles with Tunable Hydrophobicity. ACS Nano. 2014;8:6748–6755. doi: 10.1021/nn5006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moyano DF, Liu Y, Ayaz F, Hou S, Puangploy P, Duncan B, Osborne BA, Rotello VM. Immunomodulatory Effects of Coated Gold Nanoparticles in LPS-Stimulated In Vitro and In Vivo Murine Model Systems. Chem. 2016;1:320–327. doi: 10.1016/j.chempr.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang W, von Roemeling CA, Chen Y, Qie Y, Liu X, Chen J, Kim BYS. Designing nanomedicine for immuno-oncology. Nat Biomed Eng. 2017;1:29. [Google Scholar]
- 51.Chen, Wang G, Griffin JI, Brenneman B, Banda NK, Holers VM, Backos DS, Wu L, Moghimi SM, Simberg D. Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat Nanotechnol. 2016;12:387–393. doi: 10.1038/nnano.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walkey CD, Olsen JB, Song F, Liu R, Guo H, Olsen DWH, Cohen Y, Emili A, Chan WCW. Protein corona fingerprinting predicts the cellular interactions of gold and silver nanoparticles. ACS Nano. 2014;8:2439–2455. doi: 10.1021/nn406018q. [DOI] [PubMed] [Google Scholar]
- 53.Walkey CD, Olsen JB, Guo H, Emili A, Chan WCW. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc. 2012;134:2139–2147. doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- 54.Schöttler, Becker G, Winzen S, Steinbach T, Mohr K, Landfester K, Mailänder V, Wurm FR. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat Nanotechnol. 2016;11:372–377. doi: 10.1038/nnano.2015.330. [DOI] [PubMed] [Google Scholar]
- 55.Larson TA, Joshi PP, Sokolov K. Preventing Protein Adsorption and Macrophage Uptake of Gold Nanoparticles via a Hydrophobic Shield. ACS Nano. 2012;6:9182–9190. doi: 10.1021/nn3035155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwon YJ, Standley SM, Goh SL, Fréchet JMJ. Enhanced antigen presentation and immunostimulation of dendritic cells using acid-degradable cationic nanoparticles. J Control Release. 2005;105:199–212. doi: 10.1016/j.jconrel.2005.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fytianos K, Chortarea S, Rodriguez-Lorenzo L, Blank F, Von Garnier C, Petri-Fink A, Rothen-Rutishauser B. Aerosol Delivery of Functionalized Gold Nanoparticles Target and Activate Dendritic Cells in a 3D Lung Cellular Model. ACS Nano. 2017;11:375–383. doi: 10.1021/acsnano.6b06061. [DOI] [PubMed] [Google Scholar]
- 58.Xu Y, Sherwood JA, Lackey KH, Qin Y, Bao Y. The responses of immune cells to iron oxide nanoparticles. J Appl Toxicol. 2016;36:543–553. doi: 10.1002/jat.3282. [DOI] [PubMed] [Google Scholar]
- 59.Mou Y, Xing Y, Ren H, Cui Z, Zhang Y, Yu G, Urba WJ, Hu Q, Hu H. The Effect of Superparamagnetic Iron Oxide Nanoparticle Surface Charge on Antigen Cross-Presentation. Nanoscale Res Lett. 2017;12:52. doi: 10.1186/s11671-017-1828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lunov O, Syrovets T, Loos C, Beil J, Delacher M, Tron K, Nienhaus GU, Musyanovych A, Mailänder V, Landfester K, Simmet T. Differential Uptake of Functionalized Polystyrene Nanoparticles by Human Macrophages and a Monocytic Cell Line. ACS Nano. 2011;5:1657–1669. doi: 10.1021/nn2000756. [DOI] [PubMed] [Google Scholar]
- 61.Fromen CA, Rahhal TB, Robbins GR, Kai MP, Shen TW, Luft JC, DeSimone JM. Nanoparticle surface charge impacts distribution, uptake, lymph node trafficking by pulmonary antigen-presenting cells. Nanomedicine Nanotechnology, Biol Med. 2016;12:677–687. doi: 10.1016/j.nano.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moser BA, Steinhardt RC, Esser-kahn AP. Surface Coating of Nanoparticles Reduces Background Inflammatory Activity while Increasing Particle Uptake and Delivery. ACS Biomater Sci Eng. 2017;3:206–213. doi: 10.1021/acsbiomaterials.6b00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, Xu H, Peng H, Fu Y, Xu MM. CD47 blockade triggers T cell – mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209–1215. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qie Y, Yuan H, von Roemeling CA, Chen Y, Liu X, Shih KD, Knight JA, Tun HW, Wharen RE, Jiang W, Kim BYS. Surface modification of nanoparticles enables selective evasion of phagocytic clearance by distinct macrophage phenotypes. Sci Rep. 2016;6:26269. doi: 10.1038/srep26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siefert AL, Caplan MJ, Fahmy TM. Artificial bacterial biomimetic nanoparticles synergize pathogen-associated molecular patterns for vaccine efficacy. Biomaterials. 2016;97:85–96. doi: 10.1016/j.biomaterials.2016.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hernández-Gil J, Cobaleda-Siles M, Zabaleta A, Salassa L, Calvo J, Mareque-Rivas JC. An Iron Oxide Nanocarrier Loaded with a Pt(IV) Prodrug and Immunostimulatory dsRNA for Combining Complementary Cancer Killing Effects. Adv Healthc Mater. 2015;4:1034–1042. doi: 10.1002/adhm.201500080. [DOI] [PubMed] [Google Scholar]
- 67.Weilhammer DR, Blanchette CD, Fischer NO, Alam S, Loots GG, Corzett M, Thomas C, Lychak C, Dunkle AD, Ruitenberg JJ, Ghanekar SA, Sant AJ, Rasley A. The use of nanolipoprotein particles to enhance the immunostimulatory properties of innate immune agonists against lethal influenza challenge. Biomaterials. 2013;34:10305–10318. doi: 10.1016/j.biomaterials.2013.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petersen LK, Ramer-Tait AE, Broderick SR, Kong CS, Ulery BD, Rajan K, Wannemuehler MJ, Narasimhan B. Activation of innate immune responses in a pathogen-mimicking manner by amphiphilic polyanhydride nanoparticle adjuvants. Biomaterials. 2011;32:6815–6822. doi: 10.1016/j.biomaterials.2011.05.063. [DOI] [PubMed] [Google Scholar]
- 69.Camacho AI, Da Costa Martins R, Tamayo I, de Souza J, Lasarte JJ, Mansilla C, Esparza I, Irache JM, Gamazo C. Poly(methyl vinyl ether-co-maleic anhydride) nanoparticles as innate immune system activators. Vaccine. 2011;29:7130–7135. doi: 10.1016/j.vaccine.2011.05.072. [DOI] [PubMed] [Google Scholar]
- 70.Foit L, Thaxton CS. Biomaterials Synthetic high-density lipoprotein-like nanoparticles potently inhibit cell signaling and production of inflammatory mediators induced by lipopolysaccharide binding Toll-like receptor 4. Biomaterials. 2016;100:67–75. doi: 10.1016/j.biomaterials.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 71.Belling JN, Jackman JA, Yorulmaz Avsar S, Park JH, Wang Y, Potroz MG, Ferhan AR, Weiss PS, Cho NJ. Stealth Immune Properties of Graphene Oxide Enabled by Surface-Bound Complement Factor H. ACS Nano. 2016;10:10161–10172. doi: 10.1021/acsnano.6b05409. [DOI] [PubMed] [Google Scholar]
- 72.Yu K, Lai BFL, Foley JH, Krisinger MJ, Conway EM, Kizhakkedathu JN. Modulation of complement activation and amplification on nanoparticle surfaces by glycopolymer conformation and chemistry. ACS Nano. 2014;8:7687–7703. doi: 10.1021/nn504186b. [DOI] [PubMed] [Google Scholar]
- 73.Tarasova NK, Gallud A, Ytterberg AJ, Chernobrovkin A, Aranzaes JR, Astruc D, Antipov A, Fedutik Y, Fadeel B, Zubarev RA. Cytotoxic and Proinflammatory Effects of Metal-Based Nanoparticles on THP-1 Monocytes Characterized by Combined Proteomics Approaches. J Proteome Res. 2017;16:689–697. doi: 10.1021/acs.jproteome.6b00747. [DOI] [PubMed] [Google Scholar]
- 74.Giovanni M, Yue J, Zhang L, Xie J, Ong CN, Leong DT. Pro-inflammatory responses of RAW264.7 macrophages when treated with ultralow concentrations of silver, titanium dioxide and zinc oxide nanoparticles. J Hazard Mater. 2015;297:146–152. doi: 10.1016/j.jhazmat.2015.04.081. [DOI] [PubMed] [Google Scholar]
- 75.Fang RH, Kroll AV, Zhang L. Nanoparticle-Based Manipulation of Antigen-Presenting Cells for Cancer Immunotherapy. Small. 2015;11:5483–5496. doi: 10.1002/smll.201501284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gomes A, Mohsen M, Bachmann M. Harnessing Nanoparticles for Immunomodulation and Vaccines. Vaccines. 2017;5:6. doi: 10.3390/vaccines5010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ilinskaya AN, Dobrovolskaia MA. Immunosuppressive and anti-inflammatory properties of engineered nanomaterials. Br J Pharmacol. 2014;171:3988–4000. doi: 10.1111/bph.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xie Z, Su Y, Kim GB, Selvi E, Ma C. Immune Cell-Mediated Biodegradable Theranostic Nanoparticles for Melanoma Targeting and Drug Delivery. Small. 2017;13:1603121. doi: 10.1002/smll.201603121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pitt JM, Charrier M, Viaud S, Andre F, Besse B, Chaput N, Zitvogel L. Dendritic Cell-Derived Exosomes as Immunotherapies in the Fight against Cancer. J Immunol. 2014;193:1006–1011. doi: 10.4049/jimmunol.1400703. [DOI] [PubMed] [Google Scholar]
- 80.De La H, Madrigal JA, Rusakiewicz S, Bencsik M, Cave GWV, Selman A, Rees RC, Travers PJ, Dodi IA. Artificial exosomes as tools for basic and clinical immunology. J Immunol Methods. 2009;344:121–132. doi: 10.1016/j.jim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 81.Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Enzo MV, Isenhart L, Ferrari M, Tasciotti E. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2012;8:61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cho N-H, Cheong T-C, Min JH, Wu JH, Lee SJ, Kim D, Yang J-S, Kim S, Kim YK, Seong S-Y. A multifunctional core–shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat Nanotechnol. 2011;6:675–682. doi: 10.1038/nnano.2011.149. [DOI] [PubMed] [Google Scholar]
- 83.Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, Coussens LM, Daldrup-Link HE. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suzuki R, Oda Y, Utoguchi N, Namai E, Taira Y, Okada N. A novel strategy utilizing ultrasound for antigen delivery in dendritic cell-based cancer immunotherapy. J Control Release. 2009;133:198–205. doi: 10.1016/j.jconrel.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 85.Unga J, Hashida M. Ultrasound induced cancer immunotherapy. Adv Drug Deliv Rev. 2014;72:144–153. doi: 10.1016/j.addr.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 86.Sano K, Nakajima T, Choyke PL, Kobayashi H. Markedly Enhanced Permeability and Retention Effects Induced by Photo-immunotherapy of Tumors. ACS Nano. 2013;7:717–724. doi: 10.1021/nn305011p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiang J, Xu L, Gong H, Zhu W, Wang C, Xu J, Feng L, Cheng L, Peng R, Liu Z. Antigen-Loaded Upconversion Nanoparticles for Dendritic Cell Stimulation, Tracking and Vaccination in Dendritic Cell-Based Immunotherapy. ACS Nano. 2015;9:6401–6411. doi: 10.1021/acsnano.5b02014. [DOI] [PubMed] [Google Scholar]
- 88.Warashina S, Nakamura T, Sato Y, Fujiwara Y, Hyodo M, Hatakeyama H, Harashima H. A lipid nanoparticle for the efficient delivery of siRNA to dendritic cells. J Control Release. 2016;225:183–191. doi: 10.1016/j.jconrel.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 89.Das S, Haddadi A, Veniamin S, Samuel J. Delivery of rapamycin-loaded nanoparticle down regulates ICAM-1 expression and maintains an immunosuppressive profile in human CD34+ progenitor-derived dendritic cells. J Biomed Mater Res - Part A. 2008;85:983–992. doi: 10.1002/jbm.a.31557. [DOI] [PubMed] [Google Scholar]
- 90.Nadig SN, Dixit SK, Levey N, Esckilsen S, Miller K, Dennis W, Atkinson C, Broome A-M. Immunosuppressive nano-therapeutic micelles downregulate endothelial cell inflammation and immunogenicity. RSC Adv. 2015;5:43552–43562. doi: 10.1039/C5RA04057D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pan Q, Xu Q, Boylan NJ, Lamb NW, Emmert DG, Yang J, Tang L, He T, Alwadani S, Eberhart CG, Stark WJ, Hanes J. Corticosteroid-loaded biodegradable nanoparticles for prevention of corneal allograft rejection in rats. J Control Release. 2015;201:32–40. doi: 10.1016/j.jconrel.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee H, Lee M-Y, Bhang SH, Kim B-S, Kim YS, Ju JH, Kim KS, Hahn SK. Hyaluronate–Gold Nanoparticle/Tocilizumab Complex for the Treatment of Rheumatoid Arthritis. ACS Nano. 2014;8:4790–4798. doi: 10.1021/nn500685h. [DOI] [PubMed] [Google Scholar]
- 93.Halwani R, Sultana Shaik A, Ratemi E, Afzal S, Kenana R, Al-Muhsen S, Al Faraj A. A novel anti-IL4Rα nanoparticle efficiently controls lung inflammation during asthma. Exp Mol Med. 2016;48:e262. doi: 10.1038/emm.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He H, Zheng N, Song Z, Kim KH, Yao C, Zhang R, Zhang C, Huang Y, Uckun FM, Cheng J, Zhang Y, Yin L. Suppression of Hepatic Inflammation via Systemic siRNA Delivery by Membrane-Disruptive and Endosomolytic Helical Polypeptide Hybrid Nanoparticles. ACS Nano. 2016;10:1859–1870. doi: 10.1021/acsnano.5b05470. [DOI] [PubMed] [Google Scholar]
- 95.Park JS, Yang HN, Jeon SY, Woo DG, Kim MS, Park KH. The use of anti-COX2 siRNA coated onto PLGA nanoparticles loading dexamethasone in the treatment of rheumatoid arthritis. Biomaterials. 2012;33:8600–8612. doi: 10.1016/j.biomaterials.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 96.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heydarnejad MS, Rahnama S, Mobini-Dehkordi M, Yarmohammadi P, Aslnai H. Sliver nanoparticles accelerate skin wound healing in mice (Mus musculus) through suppression of innate immune system. Nanomedicine Journal. 2014;1:79–87. [Google Scholar]
- 98.Pedata P, Petrarca C, Garzillo EM, Di Gioacchino M. Immunotoxicological impact of occupational and environmental nanoparticles exposure: The influence of physical, chemical and combined characteristics of the particles. Int J Immunopathol Pharmacol. 2016;29:343–353. doi: 10.1177/0394632015608933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mahmoudzadeh A, Mohsenifar A, Rahmani-cherati T. Collagen-chitosan 3-D nano-scaffolds effects on macrophage phagocytosis and pro-inflammatory cytokine release. J Immunol. 2016;13:526–534. doi: 10.3109/1547691X.2016.1139642. [DOI] [PubMed] [Google Scholar]
- 100.Kawabata TT, Evans EW. Development of Immunotoxicity Testing Strategies for Immunomodulatory Drugs. Toxicologic Pathology. 2012;40:288–293. doi: 10.1177/0192623311430238. [DOI] [PubMed] [Google Scholar]
- 101.Li Y, Italiani P, Casals E, Valkenborg D, Mertens I, Baggerman G, Nelissen I, Puntes VF, Boraschi D. Assessing the Immunosafety of Engineered Nanoparticles with a Novel in Vitro Model Based on Human Primary Monocytes. ACS Appl Mater Interfaces. 2016;8:28437–28447. doi: 10.1021/acsami.6b06278. [DOI] [PubMed] [Google Scholar]
- 102.Fröhlich E. Value of phagocyte function screening for immunotoxicity of nanoparticles in vivo. int J Nanomedicine. 2015;10:3761–3778. doi: 10.2147/IJN.S83068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheng CJ, Tietjen GT, Saucier-sawyer JK, Saltzman WM. A holistic approach to targeting disease with polymeric nanoparticles. Nat Rev Drug disc. 2015;14:239–247. doi: 10.1038/nrd4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mohanan D, Slütter B, Henriksen-lacey M, Jiskoot W, Bouwstra JA, Perrie Y, Kündig TM, Gander B, Johansen P. Administration routes affect the quality of immune responses: A cross-sectional evaluation of particulate antigen-delivery systems. J Control Release. 2010;147:342–349. doi: 10.1016/j.jconrel.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 105.Li Y, Fujita M, Boraschi D. Endotoxin Contamination in Nanomaterials Leads to the Misinterpretation of Immunosafety Results. Front Immunol. 2017;8:1–7. doi: 10.3389/fimmu.2017.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vallhov H, Qin J, Johansson SM, Ahlborg N, Muhammed MA, Scheynius A, Gabrielsson S. The Importance of an Endotoxin-Free Environment during the Production of Nanoparticles Used in Medical Applications. Nano Lett. 2006;6:1682–1686. doi: 10.1021/nl060860z. [DOI] [PubMed] [Google Scholar]
- 107.Deville S, Piella J, Tirez K, Hoet P, Monopoli M, Dawson K, Puntes VF, Nelissen I. Interaction of gold nanoparticles and nickel(II) sulfate affects dendritic cell maturation. J Nanotoxicology. 2016;10:1395–1403. doi: 10.1080/17435390.2016.1221476. [DOI] [PubMed] [Google Scholar]