Abstract
Background and Purpose
It is currently unclear whether midlife systemic inflammation promotes the development of white matter (WM) abnormalities and small vessel disease in the elderly. We examined the association of midlife systemic inflammation with late-life white matter hyperintensity (WMH) volume, deep and periventricular WM microstructural integrity (fractional anisotropy [FA], mean diffusivity [MD]), cerebral infarcts and microbleeds in a biracial prospective cohort study.
Methods
Linear and logistic regression examined the relation between midlife high-sensitivity C-reactive protein (CRP), a non-specific marker of inflammation, and brain MRI markers assessed 21 years later in the Atherosclerosis Risk in Communities Study.
Results
We included 1,485 participants (baseline age 56(5), 28% African American). After adjusting for demographic factors and cardiovascular disease, each standard deviation (SD) increase in midlife CRP was associated with lower FA (−0.09 SD; 95% CI:−0.15, −0.02) and greater MD (0.08 SD; 95% CI:0.03, 0.15) in deep WM, and lower FA (−0.07 SD; 95% CI:−0.13, 0.00) in periventricular WM. We found stronger associations between CRP and periventricular WM microstructural integrity among African American participants (p-interaction=.011). While an association between higher CRP levels and greater WMH volume was found only among APOE ε4-positive participants in our primary analysis (0.14 SD; 95% CI:0.01, 0.26; p-interaction=.028), this relationship extended to the entire sample after accounting for differential attrition. Midlife CRP was not associated with the presence of cerebral infarcts or microbleeds in late-life.
Conclusions
Our findings support the hypothesis that midlife systemic inflammation may promote the development of chronic microangiopathic structural WM abnormalities in the elderly.
Keywords: inflammation, cerebrovascular disease, white matter disease, magnetic resonance imaging, diffusion tensor imaging, risk factors
Cerebral small vessel disease (SVD) and white matter abnormalities are highly prevalent among older adults and are associated with cognitive decline1 and dementia2. Although several cross-sectional studies have found evidence for an association between systemic inflammation, SVD3, and reduced white matter integrity4 among older adults, the temporal relationship between systemic inflammation, and each of these neurologic changes is still not well understood. As such, it remains unclear whether systemic inflammation contributes to the early pathogenesis of white matter changes and SVD, or if it is merely an associated feature or an epiphenomenon of these neurologic processes.
An association between midlife systemic inflammation, greater late-life white matter abnormalities, and increased risk for subsequent SVD would support an etiologic role of systemic inflammation in the development of structural brain abnormalities commonly observed in older adults. Thus, the goal of the current study was to examine the relationship between midlife high-sensitivity C-reactive protein (CRP), a widely-used marker of systemic inflammation, and late-life measures of white matter hyperintensity (WMH) volume, white matter microstructure, cerebral infarcts, and cerebral microbleeds (CMBs) in a large community sample of non-demented white and African American adults. Given evidence that race5 and APOE ε4 allele status6 can influence the effect of inflammatory stimuli on vascular function, we also examined the modifying effect of each of these factors.
Methods
Study Population
The Atherosclerosis Risk in Communities (ARIC) Study is an ongoing community-based, prospective study, which initially enrolled 15,792 adults between the ages of 45 and 657. Probability sampling was used to select participants from four communities within the U.S.: Washington County, MD; Forsyth County, NC; northwestern suburbs of Minneapolis, MN; and Jackson, MS. After the first visit (1987-89), participants were seen for three additional in-person visits taking place approximately three years apart until Visit 4 (1996-98), and for a 5th visit in 2011-13 (Visit 5).
A subset of participants completed brain a MRI at Visit 51. Participant selection was based on standard safety exclusion criteria and participation in the earlier ARIC Brain MRI Ancillary Study. Additionally, all participants who evidenced cognitive impairment at Visit 5, and an age-stratified random sample of participants without evidence of cognitive impairment were selected to undergo brain MRI. Of the 1,978 participants who completed a brain MRI at Visit 5, we excluded seven participants due to poor image quality or incomplete brain MRI data, 114 who met criteria for dementia, 94 for a diagnosis of a chronic inflammatory diseases (i.e., systemic lupus erythematosus, gout), 11 due to intracranial abnormalities (i.e., arteriovenous malformation, brain tumor, or significant space occupying lesion), 113 for missing CRP levels, 150 for missing one or more covariates, and four for race other than white or African American.
The ARIC study protocols were approved by the Institutional Review Boards at each participating center. All participants gave written informed consent at each study visit.
Inflammatory Marker Analysis
High-sensitivity CRP levels (mg/L) were measured in 2011-2013 using an immunoturbidimetric assay on the Roche Modular P chemistry analyzer (Roche Diagnostics, Indianapolis, IN) from serum samples originally collected at Visit 2 (1990-92) and stored at −70°C. We also conducted an exploratory analysis using a set of five less commonly used inflammatory biomarkers that were measured at Visit 1, i.e., fibrinogen (mg/dL), albumin (g/dL), von Willebrand factor (VWF) (% of standard), and Factor VIII activity (% of standard), and white blood cell (WBC) count using standardized protocols described previously8. A Visit 1 inflammation composite score was created by converting each of the five inflammatory markers to a standardized Z-score and calculating the mean. Albumin values were multiplied by −1 before being included in the composite Z-score.
Brain MRI
All brain MRIs were conducted at Visit 5 using a 3T MRI. Details regarding MRI acquisition have been described previously1. The following sequences were obtained from all participants: MP-RAGE, Axial T2*GRE, Axial T2 FLAIR, and Axial DTI. Cerebral infarctions and microbleeds were identified and measured by a trained imaging technician and confirmed by radiologists, as described previously1. Cortical infarctions were defined using fluid-attenuated inversion recovery (FLAIR) sequences as a hyperintense lesion ≥5 mm in size in the greatest dimension in cortical grey matter, which may extend to underlying white matter. Lacunar infarctions were defined as a hyperintense subcortical lesion with a dark center (≥3mm and ≤15mm in size) within the white matter, infratentorial, or central grey/capsular regions that is distinguishable from perivascular space. CMBs were defined using a T2* GRE MRI sequence. WMH volume was derived from proton density-weighted images using a quantitative computer-aided segmentation program which uses an algorithm to segment FLAIR images (FLAIR-histoseg) and measure the volumetric burden of leukoaraiosis9. All analyses using WMH adjusted for total intracranial volume. To assess the overall burden of SVD, we created an SVD composite score10 which accounts for the presence of lacunar infarcts, CMBs, and elevated WMH burden (see online-only Data Supplement). Diffusion tensor imaging (DTI) measures of fractional anisotropy (FA) and mean diffusivity (MD) were used to assess microstructural integrity of lobar and deep white matter regions11. Lower FA and higher MD reflect reduced coherence and microstructural integrity. The DTI protocol is described in more detail in the online-only Data Supplement. We analyzed two regions of interest: total periventricular white matter, and total deep subcortical white matter. We reported standardized DTI values for all analyses.
Covariates
Race-center (Washington County, white; Minnesota, white; Forsyth County, white; Forsyth County, African American; Jackson, African American), sex, and years of education attained (less than high school; high school/GED/vocational school; or any college) were determined by participant self-report at Visit 1. Arthritis and other inflammatory conditions were assessed by self-report of physician diagnosis (present/absent) at Visit 4. History of regular anti-inflammatory medication use (e.g., nonsteroidal anti-inflammatory drug [NSAID], arthritis medication) was assessed at Visit 5. All other covariates were assessed during Visit 2, concurrent with the measurement of CRP levels. Alcohol and cigarette use (current/former/never), and cancer diagnoses were determined by participant self-report. Body mass index (BMI) was calculated using recorded height and weight (kg/m2). Hypertension was defined as systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or use of hypertensive medication. Sitting diastolic and systolic blood pressures were calculated using a random zero sphygmomanometer. Second and third of three blood pressure measurements were averaged to define hypertension in the current analysis. Coronary heart disease was adjudicated based on participant self-report at Visit 1, and a combination of self-report and medical record evidence of previous myocardial infarction, coronary artery bypass graft or angioplasty, or myocardial infarction determined by ECG between Visits 1 and 2. Current anti-inflammatory medications were also recorded. APOE (0, 1, or 2 ε4 alleles) was genotyped using the TaqMan assay (Applied Biosystems, Foster City, CA). Dementia, used as an exclusionary criterion, was adjudicated by an expert committee based on a review of each participant's cognitive and functional status at Visit 5.
Diabetes was defined as follows: fasting glucose ≥126 mg/dl or non-fasting glucose of ≥200 mg/dl, current use of diabetes medication, or self-report of physician-diagnosed diabetes. The hexokinase method was used to measure serum glucose. The enzymatic method was used to measure total cholesterol and triglycerides12,13. High-density lipoprotein (HDL) cholesterol was measured after precipitation of non-HDL lipoprotein14.
Statistical Analysis
Participant characteristics were compared across CRP quartiles using one-way ANOVA or chi-square test. Because no evidence for nonlinear associations between CRP levels and MRI characteristics was found in exploratory analyses, we examined CRP as a linear continuous parameter. For all analyses, CRP values were log-transformed to correct for skewness. We used multivariable linear regression and logistic regression to evaluate the associations of CRP with MRI measures. All analyses were adjusted for the covariates listed in the previous section. We used multiplicative interaction terms to assess effect modification by race (white/African American) and APOE ε4 allele status.
Sampling weights were incorporated to account for the ARIC brain MRI sampling strategy. Thus, the primary results represent estimates using the entire ARIC Visit 5 population. Sensitivity analyses were conducted using inverse probability of attrition weighting (IPAW) to understand the potential effects of differential attrition due to death or dropout; IPAW included modeling for both death and dropout. These weighting procedures correct for sampling bias by applying larger weights to participants with characteristics that make their inclusion in the analytic sample less likely. In an attempt to isolate the effect of low-grade systemic inflammation, we also conducted a sensitivity analysis omitting participants with abnormally elevated CRP levels suggestive of an acute infection (>3 standard deviations above the sample mean). Lastly, we also examined the effect of excluding participants with clinically defined stroke. Because outcome variables were correlated, we did not correct for multiple comparisons using Bonferroni correction. A two-sided p value <.05 was used as the cutoff for statistical significance. All analyses were conducted using Stata Version 14 (StataCorp, College Station, Tex., USA).
Results
Study Population Characteristics
In total, 1,485 participants were included in this analysis. The mean age of the sample was 55.5 (SD 5.2) years at Visit 2 and 76.2 (SD 5.3) years at Visit 5. Sixty-two percent of the sample was female and 28% African American. The mean time between the Visit 2 CRP measurement and the Visit 5 MRI was 21 years with little variation (0.9 SD). Participants with higher midlife CRP levels were more likely to be female, African American, report a lower level of education, possess fewer copies of the APOE ε4 allele, possess higher levels of vascular risk factors, and be diagnosed with hypertension, heart failure, and arthritis (Table 1).
Table 1. Baseline (Visit 2) participant characteristics for study sample.
| Midlife high-sensitivity C-reactive protein quartiles | |||||
|---|---|---|---|---|---|
| Characteristic | Low 0.17–0.96 mg/L (n = 376) | Medium-Low 0.97–1.83 mg/L (n = 365) | Medium-High 1.84–4.03 mg/L (n = 372) | High 4.04–91.2 mg/L (n = 372) | p Value |
|
| |||||
| Demographic Variables | |||||
| Age | 55.2 (5.3) | 56.2 (5.3) | 55.4 (4.8) | 55.2 (5.3) | .027 |
| Female (%) | 53.7 | 57.0 | 60.5 | 77.7 | < .001 |
| African American (%) | 21.3 | 24.4 | 28.2 | 36.3 | < .001 |
| Education (%) | < .001 | ||||
| Less than high school | 5.6 | 16.2 | 13.4 | 16.1 | |
| High school/GED/vocational | 43.4 | 41.1 | 44.9 | 40.3 | |
| College/graduate/professional | 51.1 | 42.7 | 41.7 | 43.6 | |
| Apolipoprotein E ε4 alleles | < .001 | ||||
| 0 (%) | 64.6 | 68.0 | 76.9 | 76.1 | |
| 1 (%) | 31.7 | 30.1 | 20.4 | 21.0 | |
| 2 (%) | 3.7 | 1.9 | 2.7 | 1.9 | |
| Physiological & Lab | |||||
| Variables | |||||
| Body mass index, kg/m2 | 24.9 (3.4) | 26.6 (4.0) | 27.8 (4.5) | 30.6 (6.2) | < .001 |
| Systolic blood pressure, mm | 113.1 (14.9) | 116.2 (15.6) | 116.8 (15.2) | 119.3 (16.5) | < .001 |
| Hg | |||||
| Diastolic blood pressure, mm | 70.8 (9.8) | 72.1 (10.7) | 73.6 (10.2) | 74.4 (10.4) | < .001 |
| Hg | |||||
| Total cholesterol, mg/dl | 203.4 (33.3) | 211.0 (39.3) | 208.9 (37.6) | 206.5 (35.1) | .029 |
| HDL, mg/dl | 55.5 (17.6) | 52.4 (16.8) | 51.2 (17.8) | 52.9 (16.9) | .007 |
| LDL, mg/dl | 127.6 (31.6) | 133.7 (36.5) | 132.1 (35.8) | 127.9 (33.8) | .036 |
| Triglycerides, mg/dl | 101.7 (53.9) | 128.8 (90.9) | 132.1 (77.2) | 127.9 (63.1) | < .001 |
| Cardiovascular Disease (%) | |||||
| Hypertension | 16.2 | 19.7 | 23.4 | 36.8 | < .001 |
| Diabetes mellitus | 2.7 | 4.9 | 5.1 | 10.8 | .087 |
| Coronary heart disease | 1.9 | 2.0 | 2.2 | 3.0 | .732 |
| Myocardial infarction | 1.3 | 1.1 | 1.1 | 2.69 | .229 |
| Heart failure | 0.3 | 1.7 | 2.2 | 4.0 | .003 |
| Inflammatory Conditions (%) | |||||
| Arthritis* | 32.7 | 34.3 | 33.1 | 47.6 | < .001 |
| Cancer | 8.5 | 4.1 | 5.9 | 5.1 | .168 |
Values are displayed as means (SD) and percentages
Assessed at Visit 4 (1996-1998)
Midlife Inflammation and Late-Life White Matter Hyperintensity Volume
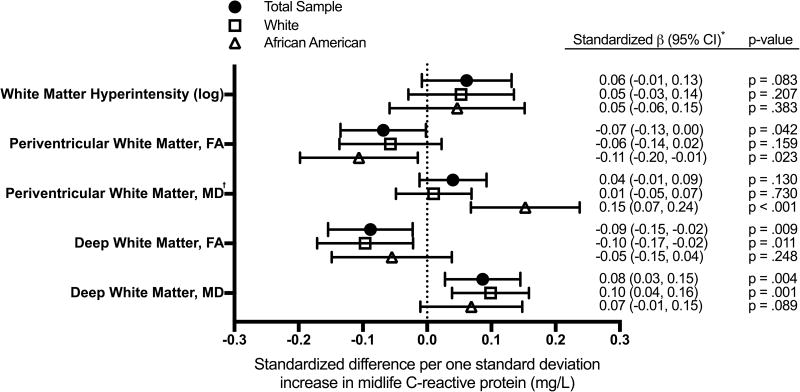
Higher CRP levels were only marginally associated with greater WMH volume in our primary analysis (Figure 1); however, a significant association was found between each SD increase in CRP level and greater WMH volume among participants with one or more copy of the APOE ε4 allele (0.14 SD; 95% CI:0.01, 0.26, p=.032), but not among APOE ε4-negative participants (0.03 SD; 95% CI:−0.06, 0.11, p=.522; p-interaction=.028; Supplemental Table I). The magnitude of the association between higher CRP levels and larger WMH volume in the full sample was considerably stronger and statistically supported, after weighting analyses to account for differential attrition (see Supplemental Table II). There was no evidence for effect modification by race. Associations were essentially unchanged in sensitivity analyses that omitted participants with clinical stroke and abnormally elevated inflammatory markers (Supplemental Tables III and IV).
Figure 1.
Covariate-adjusted associations between midlife high-sensitivity C-reactive protein and late-life MRI markers of white matter integrity.
† Race by inflammation interaction statistically significant (p-interaction=.011)
* β values represent the difference in standardized white matter hyperintensity volume (mm3), fractional anisotropy, and mean diffusivity per one standard deviation higher log CRP (mg/L).
Note. CI=confidence interval; FA=fractional anisotropy; MD=mean diffusivity
Midlife Inflammation and Late-Life White Matter Microstructural Integrity
Higher midlife CRP levels were associated with lower FA and greater MD within deep white matter (Figure 1). In our multivariable regression model, the estimated reduction in deep white matter microstructure per each SD increase in midlife CRP was similar to that for midlife hypertension and midlife diabetes. An association was also found between higher CRP levels and lower FA within periventricular white matter, but this finding did not extend to MD. There was a significant race by CRP level interaction on MD within the periventricular white matter (p-interaction=.011). The association between higher midlife CRP and reduced periventricular white matter microstructural integrity was significantly stronger among African American, compared to white, participants. In contrast, associations between CRP levels and deep white matter microstructural integrity did not appear to differ by race. No evidence for effect modification by APOE ε4 status was found (Supplemental Table I). After accounting for differential attrition, associations between higher CRP levels and reduced periventricular microstructural integrity were strengthened, particularly among white participants (Supplemental Table II). Omitting participants with clinical stroke and abnormally elevated inflammatory markers did not substantively change the findings (Supplemental Table III and IV), but effect sizes were slightly attenuated after adjusting for WMH volume (Supplemental Table V).
Midlife Inflammation, Cerebral Infarcts, Microbleeds, and Composite Small Vessel Disease Among Older Adults
Cortical and lacunar infarcts, and CMBs were present on MRI in 10% (n=148), 17% (n=257), and 25% (n=362) of participants, respectively (Supplemental Table VI). We found no association of midlife CRP levels with cortical or lacunar infarct presence, CMB presence, or SVD composite score in the total sample or in race-stratified analyses (Figure 2). Interaction terms for race and APOE ε4 status did not reach statistical significance. Results were largely unchanged in sensitivity analyses (see Supplemental Figures I-III).
Figure 2.
Covariate-adjusted odds ratios for the association between midlife high-sensitivity C-reactive protein and the presence of cerebrovascular disease in late-life.
Note. SVD = small vessel disease
Exploratory Analyses
Findings from our exploratory analyses which evaluated the association of Visit 1 inflammation composite score with brain MRI markers were null (Supplemental Figure IV; Supplemental Table VII).
Discussion
The current findings support an association between higher midlife CRP levels and reduced white matter integrity in non-demented older adults. With regard to deep white matter microstructural abnormalities, the effect of each SD increase in midlife CRP was similar to that of midlife hypertension and midlife diabetes. The associations between higher CRP and reduce white matter microstructural integrity were largely independent of clinical stroke and remained significant after adjusting for WMH volume. An association between midlife CRP and late-life WMH volume was also found, but this relationship was restricted to participants who were APOE ε4 positive in our primary analysis. However, after accounting for differential attrition, the relationship between midlife CRP and late-life WMH volume was magnified and extended to the full sample, irrespective of APOE ε4 status. Importantly, all associations were independent of age, education, cardiovascular risk factors, and anti-inflammatory medication use. With regard to cerebrovascular disease, we found no relationship between higher midlife CRP levels and risk for lacunar infarcts, cortical infarcts, or CMBs in older adults.
Although previous cross-sectional studies have demonstrated a link between systemic inflammation, reduced white matter microstructural integrity4,15 and greater WMH volume3,16 in older adults, it remains unknown whether systemic inflammation plays a pathogenic role in the development of these white matter changes. By demonstrating an association between white matter structural abnormalities among older adults and heightened systemic inflammation two decades earlier, our findings support the role of midlife systemic inflammation in the development of late-life white matter pathology. Our finding that APOE ε4 allele possession magnifies the association between midlife systemic inflammation and later WMH volume could reflect a pattern of increased sensitivity to peripheral inflammatory signaling among APOE ε4 carriers. APOE ε4 allele possession has been associated with dysregulation of the NF-κB signaling pathway and increased levels of neuroinflammation in response to inflammatory stimuli17. However, the association of midlife CRP levels with late-life WMH volume was observed in APOE ε4 carriers and non-carriers alike after adjusting for differential death and drop-out, suggesting that the modifying effect of APOE ε4 allele possession may also be explained, in part, by differential attrition. The role of APOE ε4 as a potential moderator will need to be clarified in future studies. We also found the association between higher CRP and reduced periventricular microstructural integrity to be stronger among African American, compared to white, participants. While racial differences in the response of vascular endothelial cells to peripheral inflammatory signaling5 or the differential prevalence of moderating factors (e.g., medical comorbidity) may account for some degree of the race-based differences, the reduction of these differences after adjusting for attrition suggests that this phenomenon may also result from dissimilar rates of death and drop-out over the follow-up period.
Although the current study was conducted using a non-demented sample, our results suggest white matter dysfunction may serve as a pathway through which midlife systemic inflammation increases one's risk for dementia18. The notion that cardiovascular disease during midlife influences late-life neurologic outcomes has been borne out in recent epidemiological literature19,20. Although we found associations between midlife CRP levels and late-life white matter structural abnormalities that were independent of midlife cardiovascular disease, it remains possible that these associations are mediated, at least in part, by interim cardiovascular disease occurring after midlife. The observation that our results were largely unchanged after excluding participants with abnormally elevated inflammatory markers (suggestive of an acute inflammatory response) supports current theories, which posit that low-grade systemic inflammation alone may be enough to contribute to poor neurologic outcomes21. It is possible that systemic inflammation represents a compensatory response or an epiphenomenon of another pathological process more directly linked to white matter pathology. However, evidence which suggests that systemic inflammation can promote physiological processes believed to contribute to white matter dysfunction, such as increased blood brain barrier permeability22 and microcirculatory dysfunction23, support an etiologic contribution.
Although midlife CRP level was associated with white matter integrity among older adults, it did not appear to be associated with cortical and lacunar infarcts or CMBs. Previous studies that have found an association between systemic inflammation, silent infarcts and CMBs have been cross-sectional3,16,24,25 or have had relatively short follow-up periods26. It is possible that markers of systemic inflammation are only associated with cerebral infarcts or CMBs in older adults when inflammatory biomarkers are measured during late-life or at a time more proximal to the event. Additionally, the results of our exploratory analyses suggest that perhaps not all markers of midlife inflammation are associated with late-life neurologic outcomes. However, we should note that the biomarkers used in our exploratory analyses are also regulated by non-immune processes (e.g., hemostatic, nutritional) and may therefore serve as less specific indicators of low-grade systemic inflammation compared to CRP.
Strengths of the current study include its prospective design, use of a large community sample, inclusion of a relatively large group of African Americans, and thorough assessment of potential confounding variables. However, the current findings should be interpreted within the context of several limitations. First, because we examined inflammatory markers assessed at a single time point, we were unable to examine the longitudinal properties of systemic inflammation, such as inflammation duration and trajectory, as they relate to neurologic outcomes later in life. It is currently unclear whether elevations in a single inflammatory marker during midlife can be used as a surrogate marker for chronic systemic inflammation; however, evidence suggests CRP levels are relatively stable over time in absence of disease27. Second, although CRP is a widely used marker of systemic inflammation, there are several other inflammatory proteins, such as interleukin-6 and tumor necrosis factor-alpha, which are believed to more directly affect neurologic functioning. Prospective studies which assay a wider selection of inflammatory markers will be needed. Third, we cannot rule out the possibility that the associations between CRP and measures of WM microstructural integrity were driven, at least in part, by macroscopic WM pathology (i.e., leukoaraiosis), as we did not restrict our analyses to “healthy” looking WM. Fourth, despite carefully adjusting for confounding demographic and clinical factors, it is still possible that our results are subject to residual confounding from unmeasured variables and the potential effects of treatment for medical conditions such as hypertension. Lastly, the current findings are limited by the lack of serial MRIs which precludes the assessment of change in our outcome variables over time. Because participants did not undergo an MRI at baseline, we cannot exclude the possibility that cerebrovascular disease and white matter abnormalities were already present during midlife when systemic inflammation was assessed. While some participants underwent an MRI at a previous visit, differences in imaging resolution prevents us from reliably examining change in MRI markers of interest. Despite these limitations, the current findings provide novel insight into the temporal relationship between systemic inflammation and brain health and add to the body of evidence implicating systemic inflammation in the pathogenesis of late-life white matter dysfunction.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Sources of Funding: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data is collected by U01 HL096812, HL096814, HL096899, HL096902, HL096917 with previous brain MRI examinations funded by R01-HL70825. This study was also supported by contracts T32 AG027668 (Dr. Walker) and K24 AG052573 (Dr. Gottesman) from the National Institute on Aging. The sponsors had no role in the design and conduct of the study; collection management, analysis and interpretation of the data; or preparation review, or approval of the manuscript.
Footnotes
Conflict(s)-of-Disclosure(s): Dr. Knopman serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and the DIAN study; is an investigator in clinical trials sponsored by Biogen, TauRX Pharmaceuticals, Lilly Pharmaceuticals and the Alzheimer's Disease Cooperative Study; and receives research support from NIH. Dr. Windham receives research support from NIH, is an investigator/dementia expert on a CMS Coverage with Evidence Development (CED) study, and is an investigator in a clinical trial sponsored by ACADIA Pharmaceutical. Dr. Jack Jr serves on a scientific advisory board for Eli Lilly & Company and receives research support from NIH and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation. Dr. Gottesman serves as Associate Editor for Neurology® and receives research support from NIH.
Contributor Information
Keenan A. Walker, Departments of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD.
Melinda C. Power, Department of Epidemiology and Biostatistics, George Washington University Milken Institute School of Public Health.
Ron C. Hoogeveen, Section of Cardiology, Baylor College of Medicine, Houston, TXCenter for Cardiovascular Disease Prevention, Houston Methodist DeBakey Heart and Vascular Center, Houston, TX.
Aaron R. Folsom, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, MN.
Christie M. Ballantyne, Section of Cardiology, Baylor College of Medicine, Houston, TXCenter for Cardiovascular Disease Prevention, Houston Methodist DeBakey Heart and Vascular Center, Houston, TX.
David S. Knopman, Departments of Neurology, Mayo Clinic, Rochester, MN.
B. Gwen Windham, Department of Medicine, University of Mississippi Medical Center, Jackson, MS.
Elizabeth Selvin, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
Clifford R. Jack, Jr, Departments of Radiology, Mayo Clinic, Rochester, MN.
Rebecca F. Gottesman, Departments of Neurology, Johns Hopkins University School of Medicine, Baltimore, MDDepartment of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
References
- 1.Knopman DS, Griswold ME, Lirette ST, Gottesman RF, Kantarci K, Sharrett AR, et al. Vascular Imaging abnormalities and cognition: Mediation by cortical volume in nondemented individuals: Atherosclerosis risk in communities-neurocognitive study. Stroke. 2015;46:433–440. doi: 10.1161/STROKEAHA.114.007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.JL Fu, Liu Y, Li YM, Chang C, Li W Bin. Use of Diffusion tensor imaging for evaluating changes in the microstructural integrity of white matter over 3 years in patients with amnesic-type mild cognitive impairment converting to Alzheimer's disease. J Neuroimaging. 2014;24:343–348. doi: 10.1111/jon.12061. [DOI] [PubMed] [Google Scholar]
- 3.Shoamanesh A, Preis SR, Beiser AS, Vasan RS, Benjamin EJ, Kase CS, et al. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology. 2015;84:825–832. doi: 10.1212/WNL.0000000000001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wersching H, Duning T, Lohmann H, Mohammadi S, Stehling C, Fobker M, et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74:1022–1029. doi: 10.1212/WNL.0b013e3181d7b45b. [DOI] [PubMed] [Google Scholar]
- 5.Brown MD, Feairheller DL, Thakkar S, Veerabhadrappa P, Park JY. Racial differences in tumor necrosis factor-α-induced endothelial microparticles and interleukin-6 production. Vasc Health Risk Manag. 2011;7:541–550. doi: 10.2147/VHRM.S22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero JR, Preis SR, Beiser AS, DeCarli C, Lee DY, Viswanathan A, et al. Lipoprotein phospholipase A2 and cerebral microbleeds in the Framingham Heart Study. Stroke. 2012;43:3091–4. doi: 10.1161/STROKEAHA.112.656744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill C, Gerardo D, James F, Tyroler HA, Chambless LE, Romm J, et al. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 8.Papp AC, Hatzakis H, Bracey A, Wu KK. ARIC* hemostasis study - I. Development of a blood collection and processing system suitable for multicenter hemostatic studies. Thromb Haemost. 1989;61:15–19. [PubMed] [Google Scholar]
- 9.Jack CR, O'Brien PC, Rettman DW, Shiung MM, Xu Y, Muthupillai R, et al. FLAIR histogram segmentation for measurement of leukoaraiosis volume. J Magn Reson Imaging. 2001;14:668–676. doi: 10.1002/jmri.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staals J, Makin SDJ, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology. 2014;83:1228–1234. doi: 10.1212/WNL.0000000000000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Power MC, Tingle JV, Reid RI, Huang J, Sharrett R, Coresh J, et al. Midlife and Late-Life Vascular Risk Factors and White Matter Microstructural Integrity: The Atherosclerosis Risk in Communities Neurocognitive Study. JAHA. 2017;18:e005608. doi: 10.1161/JAHA.117.005608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nägele U, Hägele EO, Sauer G, Wiedemann E, Lehmann P, Wahlefeld AW, et al. Reagent for the Enzymatic Determination of Serum Total Triglycerides with Improved Lipolytic Efficiency. Clin Chem Lab Med. 1984;22:165–174. doi: 10.1515/cclm.1984.22.2.165. [DOI] [PubMed] [Google Scholar]
- 13.Siedel J, Hagele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983;29:1075–1080. [PubMed] [Google Scholar]
- 14.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 15.Jiang J, Trollor JN, Brown DA, Crawford JD, Thalamuthu A, Smith E, et al. An inverse relationship between serum macrophage inhibitory cytokine-1 levels and brain white matter integrity in community-dwelling older individuals. Psychoneuroendocrinology. 2015;62:80–88. doi: 10.1016/j.psyneuen.2015.07.610. [DOI] [PubMed] [Google Scholar]
- 16.Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: The 3C-Dijon Study. Neurology. 2012;78:720–727. doi: 10.1212/WNL.0b013e318248e50f. [DOI] [PubMed] [Google Scholar]
- 17.Ophir G, Amariglio N, Jacob-Hirsch J, Elkon R, Rechavi G, Michaelson DM. Apolipoprotein E4 enhances brain inflammation by modulation of the NF-κB signaling cascade. Neurobiol Dis. 2005;20:709–718. doi: 10.1016/j.nbd.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: A 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52:168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 19.Gottesman RF, Schneider ALC, Zhou Y, Coresh J, Green E, Gupta N, et al. Association Between Midlife Vascular Risk Factors and Estimated Brain Amyloid Deposition. JAMA. 2017;317:1443. doi: 10.1001/jama.2017.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Power MC, Schneider ALC, Wruck L, Griswold M, Coker LH, Alonso A, et al. Life-course blood pressure in relation to brain volumes. Alzheimer's Dement. 2016;12:890–9. doi: 10.1016/j.jalz.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunningham C, Hennessy E. Co-morbidity and systemic inflammation as drivers of cognitive decline: new experimental models adopting a broader paradigm in dementia research. Alzheimers Res Ther. 2015;7:33. doi: 10.1186/s13195-015-0117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Granger DN, Kubes P. The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol. 1994;55:662–75. [PubMed] [Google Scholar]
- 24.Miwa K, Tanaka M, Okazaki S, Furukado S, Sakaguchi M, Kitagawa K. Relations of blood inflammatory marker levels with cerebral microbleeds. Stroke. 2011;42:3202–3206. doi: 10.1161/STROKEAHA.111.621193. [DOI] [PubMed] [Google Scholar]
- 25.Fornage M, Chiang YA, Omeara ES, Psaty BM, Reiner AP, Siscovick DS, et al. Biomarkers of inflammation and MRI-defined small vessel disease of the brain: The cardiovascular health study. Stroke. 2008;39:1952–1959. doi: 10.1161/STROKEAHA.107.508135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoshi T, Kitagawa K, Yamagami H, Furukado S, Hougaku H, Hori M. Relations of serum high-sensitivity C-reactive protein and interleukin-6 levels with silent brain infarction. Stroke. 2005;36:768–72. doi: 10.1161/01.STR.0000158915.28329.51. [DOI] [PubMed] [Google Scholar]
- 27.Kluft C, de Maat MP. Determination of the habitual low blood level of C-reactive protein in individuals 21. Ital Heart J. 2001;2:172–180. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.