Abstract
Background & Aims
Use of monitored anesthesia care (MAC) for gastrointestinal endoscopy has increased in the Veterans Health Administration (VHA), as in fee-for-service environments, despite the absence of financial incentives. We investigated factors associated with use of MAC in an integrated healthcare delivery system with a capitated payment model.
Methods
We performed a retrospective cohort study using multi-level logistic regression, with MAC use modeled as a function of procedure year, patient- and provider-level factors, and facility effects. We collected data from 2,091,590 Veterans who underwent outpatient esophagogastroduodenoscopy and/or colonoscopy during fiscal years 2000–2013 at 133 facilities.
Results
The adjusted rate of MAC use in the VHA increased 17% per year (odds ratio for increase, 1.17; 95% CI, 1.09–1.27) from fiscal year 2000 through 2013. The most rapid increase occurred starting in 2011. VHA use of MAC was associated with patient-level factors that included obesity, obstructive sleep apnea, higher comorbidity, and use of prescription opioids and/or benzodiazepines, though the magnitude of these effects was small. Provider-level and facility factors were also associated with use of MAC, though again the magnitude of these associations was small. Unmeasured facility-level effects had the greatest effect on the trend of MAC use.
Conclusions
In retrospective study of Veterans who underwent outpatient esophagogastroduodenoscopy and/or colonoscopy from fiscal year 2000 through 2013, we found that even in a capitated system, patient factors are only weakly associated with use of MAC. Facility-level effects are the most prominent factor influencing increasing use of MAC. Future studies should focus on better defining the role of MAC and facility and organizational factors that affect choice of endoscopic sedation. It will also be important to align resources and incentives to promote appropriate allocation of MAC based on clinically-meaningful patient factors.
Keywords: gastrointestinal endoscopy, sedation, Veterans, monitored anesthesia care
INTRODUCTION
Utilization of gastrointestinal (GI) endoscopic procedures, including esophagogastroduodenoscopy (EGD) and colonoscopy and related ancillary services, is facing increased scrutiny due to the widespread use of these procedures and higher associated costs. Procedural sedation for routine GI endoscopy historically has been directed by the endoscopist (generally with short-acting opioids and benzodiazepines) and has been considered inherent to the procedure.1 In recent years, utilization of monitored anesthesia care (MAC) for GI endoscopy has been increasing. MAC requires the presence of an anesthesiologist and typically involves administration of propofol, leading to a deeper level of sedation. Potential benefits of MAC include enhanced monitoring of patients with severe cardiopulmonary comorbidities and/or potential for airway compromise, shorter recovery and discharge times, and better patient satisfaction.2 But these potential benefits must be balanced against the potential for clinical harm, including increased risk of 30-day complications3–4 and increased cost. It is estimated that utilization of MAC for EGD and colonoscopy resulted in additional national expenditures of over $1 billion dollars for Medicare and commercially-insured patients in 2009.5
Current gastroenterology society guidelines recommend consideration of MAC for patients with anticipated intolerance of standard sedatives, certain comorbidities or potential for airway compromise. These guidelines also caution that use of MAC is not cost-effective for healthy, average-risk patients undergoing routine procedures.6–7 While these guidelines broadly outline what might constitute appropriate utilization of MAC for routine GI endoscopy, they leave ample room for discretionary decision-making on the part of providers. MAC use for GI endoscopy has markedly increased in fee-for-service delivery systems, as reflected by rates of >30% in multiple studies of Medicare and commercially-insured patients.3,5,8–10 Evidence suggests that over two-thirds of MAC is used for routine endoscopy in healthy, low-risk patients, which suggests widespread guideline-discordant utilization.9 Revaluation of endoscopic sedation codes by the Centers for Medicare and Medicaid Services (CMS) may help to promote appropriate use of MAC by changing financial incentives.1 However, it is possible that this policy change might not only affect expected overuse but also could have unintended consequences, such as underuse of MAC in patients who may benefit from enhanced monitoring or deep sedation. Understanding predictors of MAC utilization in capitated healthcare systems is therefore critical to understanding the potential impact of this policy change.
The Veterans Health Administration (VHA) is the largest integrated healthcare delivery system in the United States, with over 1,700 sites of care, serving 8.76 million Veterans annually.11 Nearly 300,000 routine outpatient gastrointestinal endoscopic procedures are performed at VHA facilities each year, with the vast majority performed by salaried physicians with little if any financial incentives tied to productivity. As a capitated healthcare system, use of MAC for endoscopy does not result in additional payments to the hospital facility or endoscopists. Nonetheless, in a prior study, we found that MAC utilization in the VHA had doubled between 2000–2013 from 4.0% to 9.3%, though the overall rate of use remains significantly lower than in non-integrated health systems.12 We hypothesized that increases in obesity and prescription opioid use among Veterans, which has not been explored adequately in prior studies, might in part explain this increase in MAC use.13,14 Therefore, we sought to determine whether and to what extent these and other factors were driving this increased VHA MAC use by examining clinically-relevant patient factors such as obesity, obstructive sleep apnea and prescription opioid and/or benzodiazepine use (suggesting possible intolerance to standard sedatives), as well as provider and facility factors, such as endoscopist specialty, procedure location, and facility complexity.15
METHODS
This study was approved by the Institutional Review Board of the VA Ann Arbor Healthcare System. This was a retrospective cohort study using VHA administrative data obtained through the Corporate Data Warehouse (CDW).
Study Population
The study population consisted of Veterans who underwent outpatient EGD and/or colonoscopy in Fiscal Years (FY) 2000–2013. Cases were identified via Current Procedural Terminology (CPT) codes for colonoscopy and EGD. (Appendix A) Cases performed with MAC were identified by searching for one of the above codes occurring on the same day as one of the following anesthesia CPT codes: 00810 (anesthesia assistance for lower endoscopy); or, 00740 (anesthesia assistance for upper GI endoscopy).
Validation Study
Because no prior study has validated the use of CPT codes for MAC in the VHA, we also undertook a cross-sectional validation study using national VHA administrative data and national VHA electronic health records (the gold standard). After the study cohort was identified, a random validation sample, stratified by year of procedure (FY2000–2004, 2005–2008, 2009–2013), type of procedure (EGD, colonoscopy, or both), and presence/absence of a MAC CPT code, was identified for purposes of manual electronic medical record abstraction. Fifty records from each of the 18 strata were examined, for a total validation sample of 900 observations. Sensitivity and specificity of CPT codes for MAC were calculated, along with 95% confidence intervals (CI). Sensitivity and specificity of individual subgroups was also evaluated. Cohen’s kappa was calculated as a measure of reliability. A kappa of 0.41–0.60 was considered to reflect moderate reliability, kappa of 0.61–0.80 to reflect good reliability, and kappa >0.80 to reflect excellent reliability.16
Patient-level and Provider-level Predictors
Patient-level predictors included body mass index (BMI), Charlson-Deyo Comorbidity Index score (0, 1–2, or ≥ 3), presence of specified ICD-9 diagnosis codes in the year prior to index endoscopy (obstructive sleep apnea, drug and/or alcohol abuse or dependence), and history of prescription opioid and/or benzodiazepine use (as defined by the presence of ≥ 1 outpatient prescription filled within 6 months of endoscopy). Gender and age were also included in the model as covariates. Provider- and facility-level predictors included endoscopist specialty, endoscopy location (GI endoscopy suite vs. other location), VHA Facility Complexity Model score (which incorporates a number of factors including patient risk, clinical volume, level of teaching/research activity, and ICU level, rated on a scale from 1a (highest complexity) to 3 (lowest complexity)), and geographic region of the facility (as defined by U.S. Census Bureau region determined by zip code). The facility where the procedure was performed was included in the model as a random effect.
To retain their use in the models, variables with extensive missing information (for example, alcohol abuse or dependence) were transformed into indicators of the known presence of a variable compared against unknown or true absence. The exception to this was BMI, analyzed as a categorical variable, where patients with missing data were analyzed as a separate category. Procedures performed on patients <18 or >100 years of age, with a recorded weight <60 lbs. or >700 lbs., and with recorded height <48 inches or >84 inches were excluded given concerns regarding the reliability of the data. To allow for multilevel modeling, patients with missing procedure facility information (n=73,501) were also excluded. Facilities that never performed an endoscopic procedure with MAC (n=6), or that performed <10 total endoscopic procedures over the 14-year study period (n=5) were also excluded, resulting in 133 facilities.
Data Analysis
Multilevel random effects logistic modeling was used to analyze MAC use over time as a function of patient-, provider-, and facility-level influences as outlined above. Specifically, patients (level 1) were nested in facility (level 2). There were two random effects in the model: a random intercept (capturing overall odds of MAC use unique to facility) and a random slope (capturing the facility-specific relationship between the odds of MAC use and time). To examine time-varying effects, all predictors were crossed with time. Also, to appropriately estimate a multilevel random effects model, only the first event (first endoscopy) in the study period was included for patients with multiple procedures during the study period. Though the sample size was large, MAC use rates were relatively low. Therefore, to obtain more reasonable standard errors, a classical sandwich bias-correction estimator was used.17 Predicted probabilities were estimated at the mean year of the cohort (FY 2007). A spline analysis was also performed, to account for a potentially non-linear association over time. Analyses were run using SAS Version 7.1 of the SAS Enterprise Guide for Linux (SAS Institute, Cary, NC) and STATA 12.0 (College Station, TX). Results are reported as adjusted odds ratios with 95% confidence intervals (CI), as well as predicted probabilities.
Role of the Funding Source
The Veterans Health Administration and the National Institutes of Health had no role in the design, conduct or analysis of this study or the decision to submit the manuscript for publication.
RESULTS
A total of 2,091,590 patient/procedure encounters occurred during the study period. Of these, 3.9% (81,284 procedures) were performed with MAC. Frequencies of patient and provider characteristics and procedure type are shown in Table 1. Patients in the cohort were predominantly male and generally healthy. 31.4% of those with recorded height and weight were obese (5.2% with Class III obesity, BMI ≥40), and 19.2% had ≥1 opioid prescription filled in the 6 months prior to the procedure. The vast majority of procedures were performed by gastroenterologists in GI endoscopy units. The majority of procedures performed were colonoscopies. Mean VA facility MAC use was 5.8% in the Northeast, 4.2% in the Midwest, 3.7% in the South, and 2.9% in the West.
Table 1.
Frequency of Patient and Provider Characteristics and Procedure Type
| Characteristic | #Patients/Procedures (%) |
|---|---|
| Patient age, y | |
| <65 | 1,321,840 (63.2%) |
| ≥65 | 769,752 (36.8%) |
| BMI | |
| <25 | 289,219 (13.8%) |
| ≥ 25 to < 30 (overweight) | 471,098 (22.5%) |
| ≥ 30 to < 35 (Class I obesity) | 321,363 (15.4%) |
| ≥ 35 to < 40 (Class II obesity) | 130,845 (6.3%) |
| ≥ 40 (Class III obesity) | 66,553 (3.2%) |
| Missing | 812,514 (38.9%) |
| Gender | |
| Male | 1,973,369 (94.3%) |
| Female | 118,221 (5.7%) |
| Obstructive Sleep Apnea | |
| Yes | 121,154 (5.8%) |
| No | 1,970,438 (94.2%) |
| Drug/Alcohol Abuse or Dependence | |
| Yes | 218,543 (10.4%) |
| No | 1,873,049 (89.6%) |
| Opioid Script Filled ≤6 months | |
| Yes | 402,184 (19.2%) |
| No | 1,689,408 (80.8%) |
| Benzodiazepine Script Filled ≤6 months | |
| Yes | 97,899 (4.7%) |
| No | 1,993,693 (95.3%) |
| Charlson-Deyo Comorbidity Score | |
| 0 | 1,175,693 (56.2%) |
| 1–2 | 655,360 (31.3%) |
| ≥3 | 260,539 (12.5%) |
| Endoscopy Location | |
| Non-GI Suite/Clinic | 325,240 (15.5%) |
| GI Suite/Clinic | 1,766,352 (84.5%) |
| Endoscopist Specialty | |
| Gastroenterologist | 1,439,884 (68.8%) |
| Non-gastroenterologist | 651,708 (31.2%) |
| Type of Procedure | |
| EGD | 643,186 (27.5%) |
| Colonoscopy | 1,694,204 (72.5%) |
Validation Study
Of 900 endoscopy encounters reviewed, electronic medical records permitted a determination of the presence or absence of MAC in 89.7% (807/900) of cases. In the remaining cases, the presence or absence of MAC was unable to be discerned through available medical documentation such as procedure reports and/or sedation records. This rate of chart retrieval was similar to other validation studies performed in the VHA.18–19 The main study analyses were based on the 807 encounters for which sedation type was able to be successfully identified through electronic record review. The overall sensitivity and specificity of CPT coding for MAC in VHA databases were 81.0% (76.9%–84.7%) and 93.2% (95% CI 90.2–95.5%), respectively. Cohen’s kappa (k=0.740, 95% CI 0.694–0.787) indicated good overall reliability, as well as good reliability across individual strata. CPT codes for EGD and colonoscopy corresponded with documentation of those procedures on manual record review with good reliability (kappa = 0.723 for EGD and 0.794 for colonoscopy).
Multi-level Model Results
After adjustment, the rate of MAC use increased 17% per fiscal year (OR 1.17, 95% CI 1.09, 1.27), resulting in a nearly 7-fold net increase over the 14-year study period. (Table 2) A number of patient-level predictors were found to be statistically significant, but with small magnitude of effects. Obesity was associated with the use of MAC, with patients with Class III obesity (BMI ≥ 40) 36% more likely to receive MAC than underweight patients (ORBMI ≥ 40 = 1.35, 95% CI 1.21, 1.52, as compared with a reference of BMI <25). However, this corresponds to an increase in the predicted probability of MAC use from 0.77% in patients with BMI<25 (the reference group) to 1.04% in patients with BMI ≥ 40 kg/m2. Similarly, a diagnosis of obstructive sleep apnea increased the predicted probability of MAC use from 0.67% in patients with no known diagnosis of OSA to 1.01% in those with a diagnosis. MAC utilization was not significantly different in patients with and without a documented history of drug and/or alcohol use or dependence. On the other hand, use of a prescription opioid or benzodiazepine in the 6 months prior to the procedure slightly increased the predicted probability of MAC use (opioids: increased from 0.76% to 0.89%, benzodiazepines: increased from 0.78% to 0.87%).
Table 2.
Adjusted odds ratios, and 95% CIs from multilevel logistic regression analyses of MAC use as a function of time, person- and provider-factors.
| Predictor | adj. OR | 95% CI |
|---|---|---|
| per Fiscal Year (FY) | 1.17 | (1.09, 1.27) |
| Age | ||
| < 65 | reference | |
| ≥ 65 | 1.05 | (1.00, 1.11) |
| Gender | ||
| Male | reference | |
| Female | 1.39 | (1.23, 1.58) |
| BMI | ||
| < 25 | reference | |
| ≥ 25 to < 30 | 1.00 | (0.93, 1.07) |
| ≥ 30 to <35 | 1.02 | (0.95, 1.10) |
| ≥ 35 to < 40 | 1.11 | (1.03, 1.20) |
| ≥ 40 | 1.35 | (1.21,1.52) |
| Missing | 1.01 | (0.93, 1.09) |
| Obstructive Sleep Apnea | ||
| No evidence | reference | |
| Yes | 1.51 | (1.21, 1.88) |
| Drug/Alcohol abuse | ||
| No evidence | reference | |
| Yes | 1.11 | (.99, 1.24) |
| Opioid use | ||
| No evidence | reference | |
| Yes | 1.16 | (1.06, 1.27) |
| Benzodiazepine use | ||
| No evidence | reference | |
| Yes | 1.12 | (1.04, 1.21) |
| Charlson score category at mean FY (~ FY2007) | ||
| (1–2) vs. 0 | 1.13 | (1.07, 1.19) |
| ≥ 3 vs. 0 | 1.32 | (1.18, 1.46) |
| ≥ 3 vs. (1–2) | 1.18 | (1.10, 1.25) |
| Location of procedure | ||
| GI suite/clinic | reference | |
| Non-GI suite/clinic | 4.02 | (1.64, 9.82) |
| Provider specialty | ||
| GI | reference | |
| Non-GI | 1.43 | (1.08, 1.89) |
| FY × Charlson | ||
| at FY2000: | ||
| (1–2) vs. 0 | 1.01 | (.89, 1.14) |
| (2–3) vs. 0 | 0.92 | (.76, 1.11) |
| (2–3) vs. (1–2) | 0.91 | (.81, 1.02) |
| at FY2013: | ||
| (1–2) vs. 0 | 1.25 | (1.13, 1.37) |
| (2–3) vs. 0 | 1.84 | (1.52, 2.22) |
| (2–3) vs. (1–2) | 1.47 | (1.31, 1.66) |
| Facility Complexity | ||
| 1a | reference | |
| 1b | 0.86 | (0.42, 1.76) |
| 1c | 1.15 | (0.57, 2.31) |
| 2 | 2.09 | (0.76, 5.77) |
| 3 | 2.14 | (0.53, 8.73) |
| Region | ||
| West | Reference | |
| South | 1.2 | (0.40, 3.20) |
| Northeast | 4.9 | (1.30, 18.4) |
| Midwest | 2.0 | (0.64, 6.53) |
Higher patient comorbidity was also associated with a small increase in MAC use, and there was a significant interaction between comorbidity and time. For patients with a Charlson-Deyo score of 0, the predicted probability of MAC use ranged from 0.24% (FY 2000) to 0.58% (FY 2013), as compared to the steeper trajectory in patients with a Charlson-Deyo score ≥3, for whom the predicted probability of MAC use increased from 0.23% to 1.03%. Interactions between time and all other predictors were not statistically significant. Spline analysis stratified by comorbidity index score showed that MAC use began to increase rapidly starting in FY 2011. (Figure 1)
Figure 1. Predicted probability of MAC use over time as a function of Charlson-Deyo comorbidity index score: spline analysis.
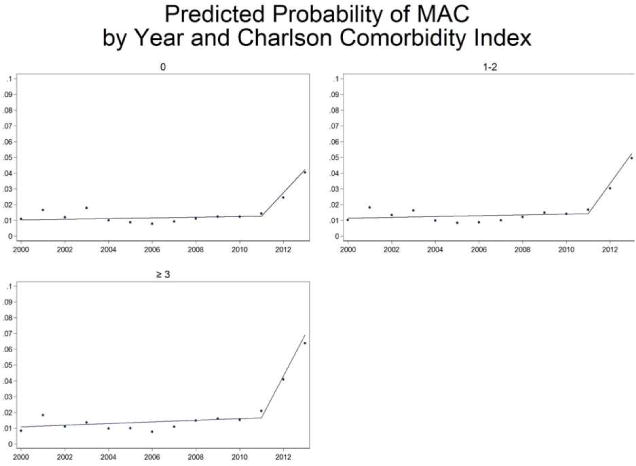
In the early years of the study period (FY 2000–2005), there was no difference in the predicted probability of MAC use between patients of different comorbidity levels. However, starting in approximately 2011, higher comorbidity patients became slightly more likely to receive MAC than lower comorbidity patients, demonstrating an interaction between comorbidity and time. In addition, spline analysis demonstrated a marked increase in MAC use starting in FY 2011.
Patients undergoing upper endoscopy were more likely to receive MAC sedation than patients undergoing colonoscopy (OREGD = 1.16, 95% CI = 1.03, 1.31, as compared to colonoscopy). Odds of MAC use at lower complexity VA facilities were not significantly different from the odds of MAC use at higher complexity VA facilities.
Both endoscopy location (non-GI suite: OR = 4.02, 95% CI = 1.64, 9.82, as compared to GI suite) and endoscopist specialty (non-GI endoscopist: OR = 1.43, 95% CI = 1.08, 1.89, as compared to GI endoscopist) were also significantly associated with MAC use. The predicted probability of MAC use was 0.68% for procedures done by GI endoscopists versus 0.99% for non-GI endoscopists, and 0.40% for procedures done in GI endoscopy suites versus 1.66% for procedures performed in other locations such as operating rooms. Examining the effect of geographic region, only a Northeast location significantly influenced the likelihood of VHA facility MAC use (OR 4.9, 95% CI = 1.3, 18.4, as compared to the West as a reference location). The predicted probability of MAC use was 0.5% for procedures done at VA facilities in the West, and 2.4% for procedures done in VA facilities in the Northeast. However, even when considered together, all of the measured patient and provider factors had a relatively small effect with the majority of the variance in MAC use trend explained by unmeasured facility factors. (Figure 2)
Figure 2. Explanation of trend in MAC use by type of analysis.
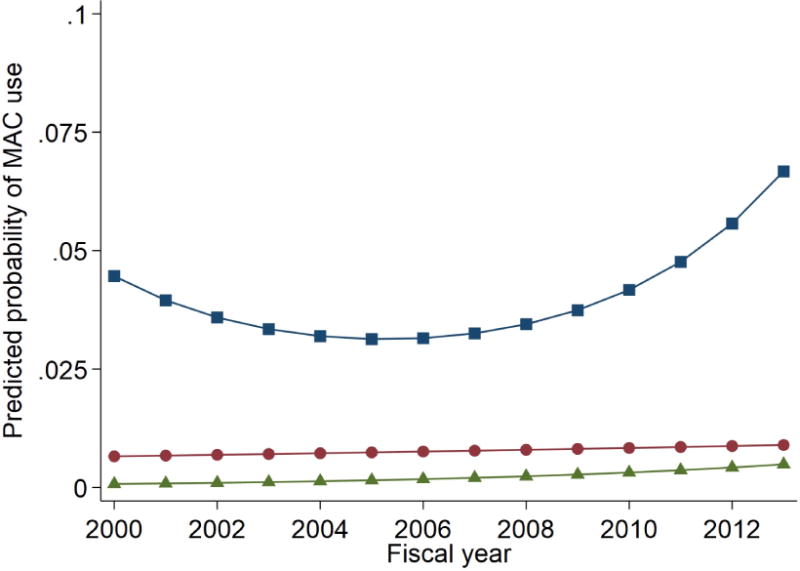
Model 1 (blue squares) includes time predictors while not accounting for person, physician, or facility-level influences and shows a nonlinear change over time. Model 2 (red circles) includes a time predictor and facility-level effects; adjusting for facility differences greatly attenuates the predicted trend in MAC use indicating that facility-specific influences explain much of the trend. Model 3 (green triangles) is fully specified and includes time, facility-level effects, and all other measured predictors (facility, provider, and patient). Most of the variability in MAC use trend can be explained by unmeasured facility-level influences.
DISCUSSION
In this study, we sought to better characterize predictors of MAC utilization in a non-fee-for-service environment in which financial incentives are largely absent. Understanding non-financial drivers of MAC use is critical to anticipating how changing payment policies may impact quality of care. Prior studies have primarily focused on potential overuse of MAC, inferring that the marked increases in MAC utilization outside the VHA are largely financially motivated.3,5,8–10 However, the results of our study suggest that, in the absence of financial incentives favoring MAC use, there still may be inappropriate use including potential underuse of MAC in the patients who may benefit the most.
Specifically, our findings show that the odds of MAC use were only slightly higher for Veterans with Class III obesity or obstructive sleep apnea, patients who may be at higher risk of airway compromise during procedural sedation, than in patients without these diagnoses. Likewise, predicted rates of MAC use in patients with the highest comorbidity scores were only slightly higher than those of healthy patients. Interestingly, the presence of an interaction between comorbidity score and time suggests that patient comorbidity has become more relevant to practitioners in aiding sedation triage decisions in recent years than previously, though the magnitude of this effect remains low. Previous studies of non-Veteran populations have yielded mixed results when examining comorbidity. Dominitz et al. found slightly higher MAC use in patients with a Charlson-Deyo comorbidity score >3 vs. 0 (10.1% vs. 8.2%, p<0.0001).15 Khiani et al. found an association between higher Elixhauser comorbidity score and MAC use in univariate analyses, but this association did not persist after adjusting for potential confounders.8 The Dominitz study also found that obstructive sleep apnea, often present in obese individuals, was more common in Medicare beneficiaries receiving MAC; however, the magnitude of this difference was small (22.9% MAC with obstructive sleep apnea vs 21.2% without; P<.003). Whether our findings are a consequence of inadequately refined or disseminated guidelines, organizational factors influencing choice of sedation (e.g. resource availability, differences in local physician culture), or other factors remains unclear.
Spline analysis showed that MAC use began to increase rapidly starting in FY 2011. (Figure 1). While to our knowledge there was no specific VA policy change at that time relating to endoscopic sedation, the American College of Gastroenterology’s petition to the FDA to change propofol labeling to allow administration by gastroenterologists was denied in 2010. The controversy surrounding this petition and ruling may have generated an enhanced awareness among the gastroenterology and anesthesiology communities regarding the importance of sedation triage, culminating in practice changes to increase access to propofol (which ultimately required the presence of an anesthesiologist following the ruling).
To our knowledge, no prior large-scale study has examined the association between MAC utilization and patient use of prescription medications that might cause tolerance to standard sedation and resultant procedural discomfort. This is important because increased rates of chronic opioid use have been documented both within and outside the VHA over this time period.20–21 Within the VHA, the overall prevalence of opioid receipt increased from 18.9% of all Veteran outpatients in FY2004 to 33.4% in FY2012 (a relative increase of ˃75%), with higher rates in females and younger Veterans.14 Prevalence varied widely by facility. Another recent VHA study found that approximately 27% of Veterans who received opioid analgesics received concurrent benzodiazepines.22 In our study, while Veterans with a prescription for an opioid or benzodiazepine in the previous 6 months had higher odds of MAC utilization, the predicted probabilities were small suggesting only a small effect related to known opioid/benzodiazepine history. Data on dosage, specific medication, and number of fills was not examined in this study. History of drug and/or alcohol abuse or dependence, which might also suggest to providers the potential for tolerance to standard sedation medications, also appeared to have no association with MAC utilization.
Provider-level predictors including endoscopist specialty and endoscopy location were relatively more influential than the patient-level predictors studied, but the absolute magnitude of these effects was modest. The reasons that MAC is more likely to be utilized by non-GI endoscopists than GI endoscopists may relate to a decreased overall comfort level on the part of the former with administering moderate sedation. Non-gastroenterologist endoscopists (including surgeons) may also be performing routine endoscopy cases in conjunction with other more traditional surgical cases that require anesthesiologist involvement, but our analysis was limited to outpatient procedures. In addition, VHA facilities with lower complexity scores were not significantly more likely to use MAC than their higher-complexity counterparts. Geographic region (specifically a location in the Northeast) was found to be influential, as in non-VA studies, suggesting the impact of local practice culture on MAC use both within and outside VA facilities. This effect may be related in part to VA providers who have a background in community practice, and transfer community practice patterns into VA facilities. However, relative to all measured variables, unmeasured facility-level factors appeared to have a comparatively large effect. This suggests that organizational structures in delivery of endoscopy at individual facilities may be leading to different allocations of MAC. Further research is needed to better define these facility-specific organizational factors influencing provider choice of endoscopic sedation.
Our study has several limitations that deserve mention, including the inherent risk of misclassification using administrative data. However, our validation study demonstrated reasonable coding accuracy, which increases confidence in the reliability of the findings. The modest sensitivity of CPT coding for MAC (81%) was likely related to under-coding resulting from a lack of financial incentive within the VA to code comprehensively. Furthermore, it is not certain whether our findings in VHA are generalizable to endoscopies performed outside the VHA. In addition, we were not able to directly examine the association between a previous failed or aborted endoscopic procedure with MAC use, given the absence of CPT modifier codes in CDW during the study period. However, this limitation is mitigated by our inclusion of only the first endoscopy during the study period.
Our study adds to the existing literature in several notable ways. First, it examined predictors of MAC use in an integrated healthcare delivery system with a capitated payment model, a delivery system that has not been well-studied previously. Previous studies have examined utilization using data primarily from Medicare and private insurers.19 While a previous Canadian study examined trends and predictors of MAC use in the single-payer Canadian healthcare system, we were able to examine more diverse patient-level variables than were analyzed in that study, and there may be other country-specific factors that make that study less applicable to the US healthcare system.23 Second, given the detailed information available through VHA databases, our study was able to investigate the association between patient-level factors such as prescription opioid and/or benzodiazepine use and history of substance abuse or dependence on MAC utilization. These factors have not been well-explored in previous studies.23,24 Third, while the majority of prior studies have examined use of MAC in Medicare patients (age >65), 63% of Veterans in our cohort were younger in age. Finally, by employing multilevel modeling techniques, rather than conventional regression for clustered data, we were able to more accurately examine individual-level versus facility-level effects. To our knowledge, only one prior study (the Canadian study referenced above) has used a multilevel modeling approach.23
CONCLUSION
While utilization of MAC has increased in the VHA, as in fee-for-service environments, this trend appears to be mostly related to unmeasured facility-level factors rather than specific patient or provider characteristics. Future studies should focus on better defining the role of MAC in endoscopic sedation and those facility and organizational factors that influence choice of endoscopic sedation. This work is critical for aligning resources and incentives to promote more appropriate allocation of MAC tailored to clinically-meaningful patient factors.
Acknowledgments
Role of the Funding Source: The Veterans Health Administration and the National Institutes of Health had no role in the design, conduct or analysis of this study or the decision to submit the manuscript for publication.
Grant Support: Dr. Adams was supported by NIH 5 T32 DK 62708-12 during this research. Dr. Krein is supported by a VA Health Services Research and Development Research Career Scientist Award (RCS 11-222). This study was also supported by the Veterans Health Administration’s Office of Informatics and Analytics.
APPENDIX A. Explanation of Current Procedural Terminology (CPT) codes for colonoscopy and EGD used in the analysis
The following colonoscopy and EGD codes were included: colonoscopy (45378-45386, G0105, G0121, 44388-44394), EGD (43200, 43202, 43204, 43215, 43220, 43226-43228, 43234-43236, 43239, 43241, 43243-43251, 43255, 43258). Colonoscopy and EGD procedure codes denoting procedures involving endoluminal stent placement (45387, 44397, 43219, 43256) were excluded because these are considered advanced interventional endoscopic procedures that may require heightened sedation monitoring.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
Megan A. Adams: study concept and design, acquisition of data, analysis and interpretation of data, statistical analysis, drafting the manuscript
Katherine M. Prenovost: acquisition of data, statistical analysis, analysis and interpretation of data, critical revision of the manuscript for important intellectual content
Jason A. Dominitz: analysis and interpretation of data, critical revision of the manuscript for important intellectual content
Robert G. Holleman: acquisition of data, analysis and interpretation of data
Eve A. Kerr: analysis and interpretation of data, critical revision of the manuscript for important intellectual content, funding
Sarah L. Krein: critical revision of the manuscript for important intellectual content
Sameer D. Saini: critical revision of the manuscript for important intellectual content
Joel H. Rubenstein: study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, study supervision
Disclosures: Megan A. Adams, MD, JD, MSc – nothing to disclose, Katherine M. Prenovost, PhD – nothing to disclose, Jason A. Dominitz, MD, MPH – nothing to disclose, Robert G. Holleman, MPH – nothing to disclose, Eve A. Kerr, MD, MPH – nothing to disclose, Sarah L. Krein PhD, RN – nothing to disclose, Sameer D. Saini, MD, MSc – nothing to disclose, Joel H. Rubenstein, MD, MSc – nothing to disclose
The opinions are those of the authors and do not represent those of the Department of Veterans Affairs.
References
- 1.Centers for Medicare & Medicaid Services; US Department of Health and Human Services. Medicare program: revisions to payment policies under the Physician Fee Schedule and other revisions to Part B for CY 2017. Fed Regist. 2016;81(136):46162–46476. [Google Scholar]
- 2.Singh H, Poluha W, Cheang M, et al. Propofol for sedation during colonoscopy. Cochrane Database of Systematic Reviews. 2008;(4) doi: 10.1002/14651858.CD006268.pub2. Art. No.: CD006268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper GS, Kou TD, Rex DK. Complications following colonoscopy with anesthesia assistance: a population-based analysis. JAMA Int Med. 2013;173:551–555. doi: 10.1001/jamainternmed.2013.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wernli KJ, Brenner AT, Rutter CM, Inadomi JM. Risks associated with anesthesia services during colonoscopy. Gastroenterology. 2016;150:888–894. doi: 10.1053/j.gastro.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khiani VS, Soulos P, Gancatco J, Gross CP. Anesthesiologist involvement in screening colonoscopy: temporal trends and cost implications in the Medicare population. Clin Gastroenterol Hepatol. 2012;10:58–64. doi: 10.1016/j.cgh.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. Sedation and anesthesia for GI endoscopy. Lichtenstein DR, Jagannath S, Baron TH. et al. Gastrointest Endosc. 2008;68(5):815–26. doi: 10.1016/j.gie.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 7.Vargo JJ, Cohen LB, Rex DK, et al. Position Statement: Nonanesthesiologist Administration of Propofol for GI Endoscopy. Am J Gastroenterol. 2009;104:2886–2892. doi: 10.1038/ajg.2009.607. [DOI] [PubMed] [Google Scholar]
- 8.Inadomi JM, Gunnarsoon CL, Rizzo JA, Fang H. Projected increased growth rate of anesthesia-professional delivered colonoscopy and EGD in the United States: 2009–2015. Gastrointest Endosc. 2010;72(3):580–86. doi: 10.1016/j.gie.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Waxman DA, Main R, Mattke S. Utilization of anesthesia services during outpatient endoscopies and colonoscopies and associated spending in 2003–2009. JAMA. 2012;307(11):1178–1184. doi: 10.1001/jama.2012.270. [DOI] [PubMed] [Google Scholar]
- 10.Predmore AB, Nie X, Main R, Mattke S, Liu H. Anesthesia service use during outpatient gastroenterology procedures continued to increase from 2010 to 2013 and potentially discretionary spending remained high. Am J Gastroenterol. 2017;112(2):297–302. doi: 10.1038/ajg.2016.266. [DOI] [PubMed] [Google Scholar]
- 11.Veterans Health Administration. http://www.va.gov/health. (Accessed January 18, 2016)
- 12.Adams MA, Prenovost KM, Dominitz JA, Kerr EA, Krein SL, Saini SD, Rubenstein JH. National trends in utilization of monitored anesthesia care for outpatient gastrointestinal endoscopy in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):436–438. doi: 10.1001/jamainternmed.2016.8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das SR, Kinsinger LS, Yancy WS, Jr, Wang A, Ciesco E, Burdick M, et al. Obesity prevalence among veterans at Veterans Affairs medical facilities. Am J Prev Med. 2005;28:291–4. doi: 10.1016/j.amepre.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Mosher HJ, Krebs EE, Carrel M, Kaboli PJ, Vander Weg MW, Lund BC. Trends in prevalent and incident opioid receipt: on observational study in Veterans Health Administration 2004–2012. J Gen Intern Med. 30(5):597–604. doi: 10.1007/s11606-014-3143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominitz JA, Baldwin L, Green P, et al. Regional variation in anesthesia assistance during outpatient colonoscopy is not associated with differences in polyp detection or complication rates. Gastroenterology. 2013;144:298–306. doi: 10.1053/j.gastro.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 17.Liang KY, Zeger SL. A comparison of two bias-corrected covariance estimators for generalized estimating equations. Biometrika. 1986;73:13–22. [Google Scholar]
- 18.Singh JA, Ayub S. Accuracy of VA databases for diagnosis of knee replacement and hip replacement. Osteoarthritis Cartilage. 2010;18:1639–1642. doi: 10.1016/j.joca.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh JA, Holmgren AR, Noorbaloochi Accuracy of Veterans Administration databases for a diagnosis of rheumatoid arthritis. Arthritis Rheum. 2004;51(6):952–7. doi: 10.1002/art.20827. [DOI] [PubMed] [Google Scholar]
- 20.Campbell CI, Weisner C, Leresche L, et al. Age and gender trends in long-term opiod analgesic use for noncancer pain. Am J Public Health. 2010;1002541(12):7. doi: 10.2105/AJPH.2009.180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morden NE, Munson JC, Colla CH, et al. Prescription opioid use among disabled Medicare beneficiaries: intensity, trends, and regional variation. Med Care. 2014;52(9):852–9. doi: 10.1097/MLR.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park TW, Saitz R, Ganoczy, Ilgen MA, Bohnert ASB. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opiod analgesics: case-cohort study. BMJ. 2015;350:h2698. doi: 10.1136/bmj.h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alharbi O, Rabeneck L, Paszat, et al. A population-based analysis of outpatient colonoscopy in adults assisted by an anesthesiologist. Anesthesiology. 2009;111(4):734–740. doi: 10.1097/ALN.0b013e3181b786d4. [DOI] [PubMed] [Google Scholar]
- 24.Adams MA, Saleh A, Rubenstein JH. A systematic review of factors associated with utilization of monitored anesthesia care for gastrointestinal endoscopy. Gastroenterol Hepatol. 2016;12(6):361–370.20. [PMC free article] [PubMed] [Google Scholar]


