Abstract
Background
Phthalates, such as dibutyl phthalate (DBP), are endocrine disruptors used in some medication coatings e.g., mesalamine to treat inflammatory bowel disease (IBD).
Objectives
Taking advantage of different mesalamine formulations with/without DBP, we assessed whether DBP from mesalamine (>1000× background) altered serum hormones.
Methods
Men (N=73) with IBD participated in a crossover-crossback prospective study and provided up to 6 serum samples (2:baseline, 2:crossover, 2:crossback). Men on non-DBP mesalamine (background) at baseline crossed-over for 4 months to DBP-mesalamine (high) and then crossed-back for 4 months to non-DBP mesalamine (B1HB2-arm) and vice versa for men on DBP-mesalamine at baseline (H1BH2-arm). We divided H1BH2-arm at the median (H1<3 yrs or H1≥3 yrs). We estimated crossover and crossback % changes in serum reproductive hormones using multivariable linear mixed effect models.
Results
When B1HB2-arm (26 men, 134 samples) crossed-over, luteinizing hormone decreased 13.9% (95% confidence interval(CI): −23.6, −3.0) and testosterone, inhibin-B, and follicle-stimulating hormone (FSH) marginally decreased; after crossback all increased 8–14%. H1BH2-arm, H1≥3 yrs (25 men, 107 samples) had no changes at crossover or crossback whereas in H1BH2-arm, H1<3 yrs (22 men, 100 samples) after crossover, inhibin-B increased 13.2% (CI: 4.2, 22.9), FSH decreased 9.9% (CI: −17.9, −1.1) and after crossback, inhibin-B further increased 11.3%, and FSH marginally increased.
Conclusions
High-DBP exposure may disrupt pituitary-gonadal hormones that largely reversed after exposure removal, but only in men with no or short previous high-exposure history. Paradoxically, men with longer duration of high-DBP exposure, exposure removal did not change hormone levels, suggesting that long-term high-DBP exposure may alter the pituitary-gonadal axis and make it insensitive to exposure changes.
Keywords: Phthalate, endocrine disruptor, crossover study, hormones, men
1. Introduction
Ortho-phthalates (hereto referred to as phthalates) are high production volume chemicals1. Because of their widespread use in consumer and personal care products, phthalate exposure is ubiquitous in the general population2.
In experimental studies, several phthalates, including dibutyl phthalate (DBP), have adverse impacts on male reproductive health3. Although banned for several uses, DBP is still used in the enteric coatings of some medications including specific formulations of mesalamine, resulting in high-DBP exposure4–10. Mesalamine, the active ingredient in Asacol®, Asacol®HD, Lialda®, Pentasa®, Apriso®, and Delzicol®, is a commonly prescribed for inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD). The enteric coating of Asacol®, and Asacol®HD contains DBP as an excipient11,12, whereas it is not used in other mesalamine formulations9. Asacol® and Asacol®HD use leads to high-DBP exposure as measured by urinary monobutyl phthalate (MBP) concentrations, the primary DBP metabolite10,13, that are approximately 1000 times higher than the median reported for men in the U.S. general population (National Health and Nutrition Examination Survey (NHANES))14.
Experimental studies have shown that DBP is anti-androgenic and a reproductive toxicant, however, most studies have focused on in utero exposure3,15,16. There is limited evidence from epidemiologic studies on the association of background DBP exposure with serum reproductive hormones17–21. However, there are no studies on effects of very high-DBP exposures from medications. Therefore, we took advantage of the difference in DBP-exposure from specific mesalamine formulations and conducted a crossover-crossback prospective cohort study to examine the associations between high-DBP exposure from mesalamine medications and serum reproductive hormones in adult men.
2. Materials and methods
2.1. Participants
As previously described5, between 2010–2016, 73 men enrolled in the Mesalamine And Reproductive health Study (MARS) from gastroenterology clinics at three Boston hospitals, Beth Israel Deaconess Medical Center (BIDMC), Brigham and Women’s Hospital (BWH) and Massachusetts General Hospital (MGH). Eligible men were 18 to 55 years old, with mild severity IBD, and on mesalamine for at least three months at enrollment. There were no exclusions based on history of infertility. Men with a history of steroid medication use in the last three months, vasectomy, diabetes mellitus, hepatic, or renal diseases were not eligible. MARS was approved by the institutional review boards of Harvard T.H. Chan School of Public Health, BIDMC, BWH and MGH. All men signed informed consents.
2.2. Study design
Men participated in up to six visits (v) (baseline: v1&v2, crossover: v3&v4 and crossback: v5&v6). Men who started on non-DBP mesalamine (background-DBP exposure) crossed-over to high-DBP mesalamine (high-DBP exposure) then crossed-back to non-DBP mesalamine (background) (B1HB2-arm; Background1-High-Background2) and vice versa in men who started on DBP mesalamine (H1BH2-arm; High1-Background-High2) (Figure 1). Crossover and crossback periods (between v2&v3 and v4&v5) were designed to be four months and durations between v1&v2, v3&v4 and v5&v6 were designed to be two weeks. We a priori chose the four month periods to be longer than the spermatogenesis cycle (around 70 days)22, as one of our aims was to study semen parameters5. At baseline, men reported demographic, lifestyle and health information on questionnaires and height and weight were measured. At each visit, men reported fever and illness in the previous three months and gave blood, semen and urine samples. 13 out of the 47 men in the H1BH2-arm participated only in a short protocol consisting of up to four visits that required only crossover without crossback as previously described5.
Figure 1. Mesalamine And Reproductive health Study (MARS) Design.
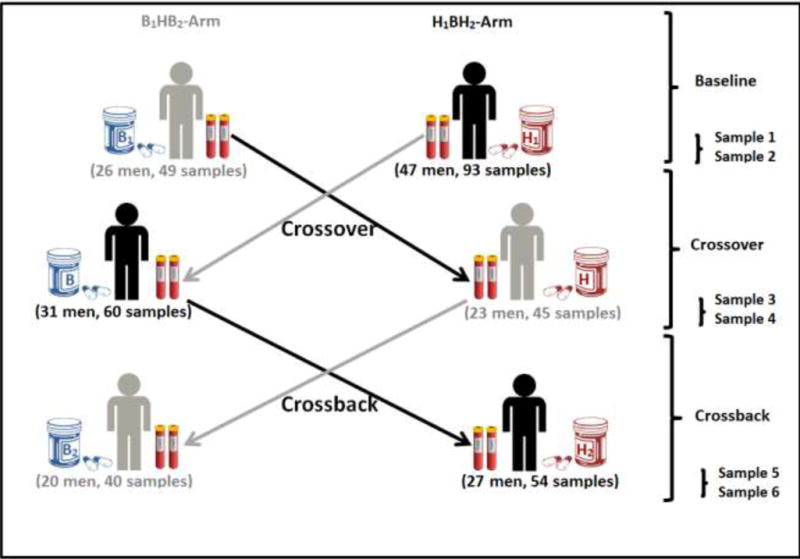
B1HB2-arm: B1 represents background-exposure at baseline, H represents high-DBP exposure after crossover and B2 represents background-exposure after crossback; included 26 men taking mesalamine that did not contain DBP at baseline. H1BH2-arm: H1 represents high-DBP exposure at baseline, B represents background-exposure after crossover and H2 represents high-DBP exposure after crossback; included 47 men taking mesalamine containing DBP at baseline (25 men for less than 3 years and 22 men with 3 years or more). Abbreviations: MARS, Mesalamine And Reproductive health Study; DBP, dibutyl phthalate.
2.3. Exposure assessment
High-DBP exposure was defined by medication type i.e., DBP-containing mesalamine versus non-DBP mesalamine. We relied on self-reported use of mesalamine medications as prescribed over the study period23.
2.4. Serum hormone analysis
Nurses collected non-fasting blood samples at each visit between 7 am and 2 pm and noted the exact time. All samples were shipped in a single batch for analysis to the laboratories at Ansh (inhibin-B and sex hormone-binding globulin (SHBG)), National Institute of Environmental Health Sciences (NIEHS) (follicle-stimulating hormone (FSH) and luteinizing hormone (LH)), and Mayo Clinic (total testosterone (TT) and albumin).
SHBG was measured by enzyme linked immunosorbent assay (ELISA) (quantitative two-step sandwich type immunoassay). Testosterone was measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Agilent Technologies, Santa Clara, CA 95051). Albumin, used to calculate the free testosterone (FT) concentration, was measured by an enzymatic colorimetric assay on the Roche Cobas c311 chemistry analyzer (Roche Diagnostics, Indianapolis, IN 46250). Inhibin-B was measured by ELISA (quantitative three-step sandwich type immunoassay). Ansh laboratory has developed highly specific antibodies for inhibin-B with no detectable cross-reactivity to inhibin-A or activin-A and AB, and negligible cross-reactivity to activin-B (0.04% at 50 ng/ml). LH and FSH concentrations were measured by chemiluminescent microparticle immunoassay (CMIA) technology (Architect i100SR Immunoassay Analyzer, Abbott Diagnostics, Abbott Park, IL).
The intra- and inter-assay coefficients of variation (CV) were for SHBG: <9.0% and <8.0%, testosterone: ≤9.0% and ≤8.9%, albumin: ≤1.4% and ≤2.1%, Inhibin-B: <4.4% and <7.4%, LH: 2.8% and 5.7%, and FSH: 1.8% and 12.7%, respectively. All laboratories were blinded to participant information. We calculated FT from the measured concentrations of TT, SHBG, and albumin using the method of Vermeulen et al.24. We also calculated TT/LH, FT/LH, and Inhibin-B/FSH ratios.
2.5. Statistical Analysis
In primary analyses, to define exposure we used a six-level indicator variable cross-classifying each observation based on the medication type (high versus non-DBP) at each period (baseline, crossover and crossback) for the two study arms (H1BH2 and B1HB2). We modeled the hormone concentrations as natural log-transformed continuous outcomes.
We performed descriptive statistics and tested for any differences between men in the different arms using Fisher’s exact test for categorical variables and Kruskal-Wallis test for continuous variables.
We selected the covariates based on directed acyclic graphs and statistical considerations (>10% change in the effect estimate). The final model included age (continuous), body mass index (BMI) (continuous), sample drawing time (continuous, time varying), season of the sample collection (warm and cold, time varying), race (Caucasian or not), current smoking (binary), and duration on high-DBP mesalamine as self-reported at baseline (continuous). In preliminary models, we considered adjustment for IBD severity, IBD diagnosis (UC/CD), duration since diagnosis, history of reproductive diseases or surgeries, education, alcohol consumption, fever and illness in the previous three months but they were not confounders and thus not retained in the final models.
We estimated the DBP crossover (crossover versus baseline), crossback (crossback versus crossover), and carryover (crossback versus baseline) percent changes in the hormone parameters within each arm, as well as the cross-sectional differences at baseline between arms. We used linear mixed effect models (LMEM) with a random intercept to account for within-man correlation among longitudinal measures of a given hormone parameter arising from man-to-man heterogeneity across the study participants.
Because men in H1BH2-arm were on their high-DBP mesalamine at baseline for a wide range of durations (4 months to 24 years), in post-hoc analyses we explored whether the duration on high-DBP mesalamine at baseline modified associations by adding an interaction term. Additionally, we subdivided men in the H1BH2-arm based on the median duration of three years on high-DBP mesalamine at baseline i.e., (H1BH2-arm, H1<3 yrs) and (H1BH2-arm, H1≥3 yrs) and conducted analyses based on the resulting three arms using the same method as the two-arm analyses above.
As a sensitivity analysis, we assessed LMEM model sensitivity to the covariance structure implied by the random intercept model, using the robust empirical standard errors25 in both two-arm and three-arm analyses. We considered two-sided alpha <0.05 as statistically significant. We conducted all statistical analyses using SAS version 9.4 (SAS Institute Inc., Cary, NC) and used the R package ggplot for generation of graphical output.
3. Results
Of the 215 eligible men who were contacted, 73 (mean age 34.6 years) agreed to participate and provided 341 blood samples (average: five samples/man) (Supplementary Fig. 1). B1HB2-arm (26 men, 134 samples) were on non-DBP mesalamine medication at baseline for a median of 1 year. H1BH2-arm (47 men, 207 serum samples) were on their high-DBP mesalamine medication at baseline (H1) for a median duration of 3 years. Men in the two arms had comparable characteristics (Table 1 and Figure 1).
Table 1.
Demographics of 73 men contributing 341 blood samples in the MARS Study by arm
| B1HB2-arm (26 men, 134 visits) |
H1BH2-arm (47 men, 207 visits) |
Total (73 men, 341 visits) |
||
|---|---|---|---|---|
|
|
||||
| N(%)/Mean (SD), [Range] | N(%)/Mean (SD), [Range] | N(%)/Mean (SD), [Range] | P-valueb | |
| Baseline characteristics (at Visit 1), # men (N)a | ||||
|
| ||||
| Duration on High-DBP mesalamine at baseline (H1, years) | 0 | 5.86 (6.16), [0.33, 24.1] | 3.77 (5.68), [0, 24.1] | – |
| Age (Years) | 33.9 (8.97), [20.2, 54.6] | 34.9 (9.66), [19.6, 55.7] | 34.6 (9.37), [19.6, 55.7] | 0.82 |
| Race | 0.27 | |||
| Caucasian | 25 (96%) | 38 (81%) | 63 (86%) | – |
| Black/African American | 1 (4%) | 2 (4%) | 3 (4%) | – |
| Asian | 0 | 4 (9%) | 4 (6%) | – |
| Other | 0 | 3 (6%) | 3 (4%) | – |
| BMI (Kg/m2) | 25.3 (3.56), [19.4, 32.7] | 26.4 (6.39), [19.5, 54.0] | 26.0 (5.54), [19.4, 54.0] | 0.88 |
| BMI-categories | 0.99 | |||
| Normal weight (18.5 ≤BMI<25) | 14 (54%) | 24 (51%) | 38 (52%) | – |
| Overweight (25≤BMI<30) | 9 (35%) | 16 (34%) | 25 (34%) | – |
| Obese (BMI≥30) | 3 (11%) | 7 (15%) | 10 (14%) | – |
| Education | 0.82 | |||
| Below college | 3 (14%) | 6 (14%) | 9 (14%) | – |
| College graduate | 10 (48%) | 16 (38%) | 26 (41%) | – |
| Graduate degree | 8 (38%) | 20 (48%) | 28 (45%) | – |
| Smoking status | 0.01 | |||
| Never smoker | 22 (85%) | 36 (77%) | 58 (80%) | – |
| Former smoker | 1 (4%) | 11 (23%) | 12 (16%) | – |
| Current smoker | 3 (11%) | 0 | 3 (4%) | – |
| Warm season at baselinec | 5 (19%) | 20 (43%) | 25 (34%) | 0.07 |
| IBD diagnosis | 0.61 | |||
| Ulcerative colitis | 16 (62%) | 32 (68%) | 48 (66%) | – |
| Crohn’s disease | 10 (38%) | 15 (32%) | 25 (34%) | – |
| IBD scored | 1.65 (1.38), [0, 4] | 1.19 (1.35), [0, 5] | 1.36 (1.37), [0, 5] | 0.13 |
| Duration since IBD diagnosis (years) | 9.00 (7.84), [0.28, 29.5] | 10.8 (9.24), [0.7, 39.0] | 10.1 (8.76), [0.28, 39.0] | 0.41 |
|
| ||||
| Time-varying characteristics, # visitsa | ||||
|
| ||||
| Warm season of blood drawc | 60 (45%) | 94 (45%) | 154 (45%) | 0.91 |
| Hour of the blood draw | 9.68 (1.62), [7.25, 13.9] | 9.72 (1.61), [7.17, 13.5] | 9.71 (1.61), [7.17, 13.9] | 0.81 |
| Hour of the blood draw-categories | 0.74 | |||
| 7 am to 10 am | 74 (55%) | 110 (53%) | 184 (54%) | – |
| After 10 am to 2 pm | 60 (45%) | 97 (47%) | 157 (46%) | – |
B1HB2-arm, B1 represents background-exposure at baseline, H represents high-DBP exposure after crossover and B2 represents background-exposure after crossback; included 26 men taking mesalamine that did not contain DBP at baseline; 16 men (62%) on Lialda®, 8 men (31%) on Pentasa®, one man (4%) on Apriso® and one man (4%) was not on mesalamine medication at the time of recruitment.
H1BH2-arm, H1 represents high-DBP exposure at baseline, B represents background-exposure after crossover and H2 represents high-DBP exposure after crossback; included 47 men taking mesalamine-containing DBP at baseline; 24 men (51%) on Asacol® and 23 men (49%) on Asacol®HD.
N (%) for categorical/binary variables and mean (SD), [Range] for continuous variables
P-values are based on Fisher exact test for categorical variables and Kruskal Wallis test for continuous variables.
Season of sample collection, Warm: April Through September
IBD score: included bowel frequency and urgency, presence of blood in the stool and general wellbeing. Mild IBD score: 5 or less on the simple clinical colitis activity index for UC and 4 or less on the Harvey-Bradshaw index for CD.
Abbreviations: MARS, Mesalamine And Reproductive health Study; BMI, body mass index; Kg, Kilogram; m, meter; SD, standard deviation; IBD, Inflammatory Bowel Disease; UC, ulcerative colitis; CD, Crohn’s disease; N, number of men; DBP, dibutyl phthalate; B1HB2, Background1-High-Background2 DBP exposure; H1BH2, High1-Background-High2 DBP exposure.
Among the 60 men who participated in the full-protocol, 51 men (85%) crossed-over and 47 (78%) crossed-back; 89% and 77%, respectively, in B1HB2-arm, 82% and 79%, respectively, in H1BH2-arm. During the follow-up, nine men were lost follow-up, three in B1HB2-arm and six in H1BH2-arm (p-value= 0.64). The median crossover duration (between v2 & v3), and crossback duration (between v4 & v5) was 121 days. The median duration between visits on the same medication (v1 & v2), (v3 & 4) and (v5 & v6) was 16 days. All serum samples had detectable concentrations for all hormones (Table 2).
Table 2.
Hormone concentrations for 73 men (341 blood samples) in the MARS study
| Hormone Parameters | Mean | SD | Geometric Mean | Minimum | 5th Perc | 25th Perc | 50th Perc | 75th Perc | 95th Perc | Maximum | QR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Testosterone (nmol/L) | 17.2 | 6.18 | 16.1 | 4.41 | 8.36 | 13.3 | 16.3 | 21.1 | 28.4 | 40.5 | 7.88 |
| Free Testosterone (pmol/L) | 345 | 105 | 329 | 44.3 | 198 | 275 | 333 | 400 | 541 | 730 | 125 |
| SHBG (nmol/L) | 34.3 | 16.6 | 30.2 | 3.89 | 11.2 | 22.8 | 31.5 | 44.5 | 64.7 | 98.7 | 21.7 |
| Inhibin-B (pg/mL) | 191 | 77.6 | 176 | 52.0 | 81.8 | 134 | 171 | 241 | 334 | 432 | 107 |
| LH (IU/L) | 3.23 | 1.37 | 2.96 | 0.90 | 1.42 | 2.22 | 3.04 | 3.94 | 5.64 | 10.8 | 1.72 |
| FSH (IU/L) | 3.20 | 1.74 | 2.77 | 0.62 | 1.13 | 1.85 | 2.97 | 4.04 | 6.73 | 9.99 | 2.19 |
| Total Testosterone/LH Ratio | 6.10 | 2.94 | 5.45 | 1.78 | 2.46 | 3.93 | 5.40 | 7.92 | 11.7 | 17.7 | 3.99 |
| Free Testosterone/LH Ratio | 123 | 56.5 | 111 | 23.8 | 51.6 | 81.6 | 111 | 155 | 223 | 360 | 73.0 |
| Inhibin-B/FSH Ratio | 90.1 | 78.4 | 63.4 | 7.59 | 15.6 | 37.5 | 61.9 | 120 | 265 | 391 | 82.7 |
Abbreviations: MARS, Mesalamine And Reproductive health Study; SD, standard deviation; Perc, percentile; QR: quartile range; SHBG, sex hormone-binding globulin; LH, luteinizing hormone; FSH, follicle-stimulating hormone.
In the adjusted two-arm analyses (Table 3 & Supplementary Fig. 3), on average, when men in B1HB2-arm (Table 3A) were newly-exposed to high-DBP mesalamine, LH decreased by 13.9% (95% confidence interval (CI): –23.6, –3.0) and TT, FT, SHBG, inhibin-B, and FSH decreased though not significantly. After crossback to their original non-DBP mesalamine, apart from SHBG which continued to decrease non-significantly, LH increased by 13.8% (CI: 0.5, 28.9), TT by 10.4% (CI: 0.4, 21.4), FT by 10.4% (CI: 0.2, 21.6), and inhibin-B and FSH marginally significantly increased. There was no carryover effect in B1HB2-arm. When men in H1BH2-arm (Table 3B) crossed-over to non-DBP mesalamine, on average, inhibin-B increased by 8.5% (CI: 2.4, 15.1) and continued to increase by an additional 7.7% (CI: 1.3, 14.5) after crossback to their original high-DBP mesalamine. LH and FSH decreased by 10.1% and 8.4%, respectively after crossover and increased by 9 to 10% after crossback (significantly for FSH). TT/LH, FT/LH and inhibin-B/FSH ratios increased significantly by 11 to 18% after crossover and decreased non-significantly after crossback despite non-significant changes in TT and FT.
Table 3A.
Adjusted means and percent changes (95% CI) of crossover, crossback and carryover on hormone concentrations among men starting on mesalamine medication not containing dibutyl phthalate (B1HB2-arm)a
Adjusted B1HB2-arm [Background (B1)- High (H)- Background (B2) dibutyl phthalate exposure]: 26 men, 134 blood samples
| Hormone serum concentrations | Fitted Geometric Means (95% CI) | Comparisons | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (B1) | Crossover (H) | Crossback (B2) | Crossover % Change (95% CI) H-B1 | P value | Crossback % Change (95% CI) B2-H | P value | Carryover % Change (95% CI) B2-B1 | P value | |
| Total Testosterone (nmol/L) | 16.3 (14.2, 18.8) | 15.6 (13.6, 18.0) | 17.2 (14.9, 19.9) | −4.28 (−12.7, 4.88) | 0.35 | 10.4 (0.37, 21.4) | 0.04 | 5.64 (−4.29, 16.6) | 0.28 |
| Free Testosterone (pmol/L) | 330 (292, 373) | 322 (284, 365) | 355 (312, 404) | −2.36 (−11.1, 7.19) | 0.61 | 10.4 (0.16, 21.6) | 0.05 | 7.77 (−2.53, 19.2) | 0.14 |
| SHBG (nmol/L) | 29.6 (24.5, 35.7) | 29.4 (24.3, 35.6) | 28.8 (23.7, 34.9) | −0.52 (−10.3, 10.3) | 0.92 | −2.14 (−12.1, 8.91) | 0.69 | −2.65 (−12.9, 8.85) | 0.64 |
| Inhibin-B (pg/mL) | 162 (137, 192) | 161 (136, 190) | 173 (146, 205) | −0.89 −7.57, 6.27) | 0.80 | 7.28 (−0.21, 15.3) | 0.06 | 6.32 (−1.44, 14.7) | 0.11 |
| LH (IU/L) | 3.29 (2.81, 3.85) | 2.83 (2.42, 3.32) | 3.22 (2.73, 3.80) | −13.9 (−23.6, −3.02) | 0.01 | 13.8 (0.53, 28.9) | 0.04 | −2.04 (−13.8, 11.4) | 0.75 |
| FSH (IU/L) | 3.23 (2.57, 4.07) | 3.05 (2.42, 3.84) | 3.30 (2.62, 4.17) | −5.79 (−12.8, 1.82) | 0.13 | 8.35 (−0.04, 17.4) | 0.05 | 2.08 (−6.19, 11.1) | 0.63 |
| Total Testosterone/L H Ratio | 4.95 (4.13, 5.92) | 5.50 (4.59, 6.6) | 5.33 (4.42, 6.42) | 11.1 (−1.11, 24.9) | 0.08 | −3.17 (−14.2, 9.33) | 0.60 | 7.62 (−5.13, 22.1) | 0.25 |
| Free Testosterone/L H Ratio | 100 (84.7, 119) | 114 (95.9, 135) | 110 (92.4, 132) | 13.4 (−0.71, 29.6) | 0.06 | −3.04 (−15.6, 11.4) | 0.66 | 9.98 (−4.68, 26.9) | 0.19 |
| Inhibin-B/FSH Ratio | 50.0 (35.0, 71.5) | 52.7 (36.8, 75.3) | 52.3 (36.5, 75.0) | 5.32 (−4.45, 16.1) | 0.30 | −0.68 (−10.2, 9.88) | 0.90 | 4.61 (−5.93, 16.3) | 0.40 |
Table 3B.
Adjusted means and percent changes (95% CI) of crossover, crossback and carryover on hormone concentrations among men starting on mesalamine medication containing dibutyl phthalate (H1BH2-arm)a
H1BH2-arm[High (H1)-Background (B) - High (H2)] dibutyl phthalate exposure]: 47 men, 207 blood samples
| Hormone serum concentrations | Fitted Geometric Means (95% CI) | Comparisons | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (Hi) | Crossover (B) | Crossback (H2) | Crossover % Change (95% CI) B-H1 | P value | Crossback % Change (95% CI) H2-B | P value | Carryover % Change (95% CI) H2-H1 | ||
| Total Testosterone (nmol/L) | 16.0 (14.5, 17.7) | 15.9 (14.3, 17.7) | 17.0 (15.3, 19.0) | −0.58 (−7.86, 7.27) | 0.88 | 7.13 (−1.18, 16.1) | 0.09 | 6.50 (−1.59, 15.3) | 0.12 |
| Free Testosterone (pmol/L) | 316 (290, 345) | 324 (294, 358) | 343 (311, 380) | 2.52 (−5.1, 10.8) | 0.53 | 5.90 (−2.49, 15.0) | 0.17 | 8.57 (0.18, 17.7) | 0.05 |
| SHBG (nmol/L) | 31.7 (27.7, 36.3) | 33.2 (28.8, 38.3) | 31.7 (27.4, 36.6) | 4.65 (−3.97, 14.1) | 0.30 | −4.50 (−12.8, 4.58) | 0.32 | −0.05 (−8.60, 9.30) | 0.99 |
| Inhibin-B (pg/mL) | 171 (152, 193) | 185 (164, 210) | 200 (176, 226) | 8.52 (2.37, 15.1) | 0.006 | 7.71 (1.31, 14.5) | 0.02 | 16.9 (10.0, 24.2) | <0.000 1 |
| LH (IU/L) | 2.96 (2.65, 3.31) | 2.66 (2.35, 3.01) | 2.90 (2.55, 3.29) | −10.1 (−18.6, −0.78) | 0.04 | 8.93 (−1.98, 21.1) | 0.11 | −2.09 (−11.7, 8.51) | 0.69 |
| FSH (IU/L) | 2.64 (2.24, 3.11) | 2.42 (2.05, 2.87) | 2.65 (2.24, 3.14) | −8.39 (−14.2, −2.23) | 0.009 | 9.63 (2.40, 17.4) | 0.00 8 | 0.43 (−6.14, 7.46) | 0.90 |
| Total Testosterone/LH Ratio | 5.41 (4.76, 6.15) | 6.01 (5.23, 6.90) | 5.90 (5.12, 6.80) | 11.0 (0.73, 22.3) | 0.04 | −1.80 (−11.4, 8.86) | 0.73 | 9.01 (−1.47, 20.6) | 0.09 |
| Free Testosterone/LH Ratio | 107 (94.9, 120) | 122 (107, 139) | 118 (103, 136) | 14.0 (2.07, 27.2) | 0.02 | −2.74 (−13.6, 9.42) | 0.64 | 10.8 (−1.16, 24.3) | 0.08 |
| Inhibin-B/FSH Ratio | 64.7 (50.1, 83.4) | 76.6 (59.1, 99.2) | 75.2 (58.0, 97.5) | 18.4 (9.12, 28.5) | <0.000 1 | −1.80 (−9.84, 6.96) | 0.68 | 16.3 (6.82, 26.6) | 0.001 |
Adjusted for race (Caucasian or not), age (continuous), BMI (continuous), hour of sample draw (continuous), season (warm or cold), current smoking, age (continuous), and duration on DBP-containing mesalamine medication at baseline (in years) in the mixed effect model.
Percentage changes are presented by exponentiating the beta coefficient. Negative sign means % decrease for the corresponding outcome compared to the measure in the previous period.
B1HB2-arm: B1 represents background low-DBP exposure at baseline, H represents high-DBP exposure after crossover and B2 represents background low-exposure after crossback.
H1BH2-arm: H1 represents high-DBP at baseline, B represents background-DBP exposure after crossover and H2 represents high-DBP after crossback.
Abbreviations: MARS, Mesalamine And Reproductive health Study; N, number of men; B1HB2, Background1-High-Background2 DBP exposure; H1BH2, High1-Background-High2 DBP exposure; 95% CI, 95% Confidence Interval; DBP, dibutyl phthalate; SHBG, sex hormone-binding globulin; LH, luteinizing hormone; FSH, follicle-stimulating hormone.
In the cross-sectional analysis of values at baseline, on average, men in H1BH2-arm had lower TT, FT, LH, and FSH than men in the B1HB2-arm. Because of the wide range in duration on high-DBP mesalamaine and to further explore duration on high-DBP mesalamine at baseline we divided the men in the H1BH2-arm at the median. At baseline, men on high-DBP mesalamine for less than 3 years (H1<3 yrs) had higher TT, FT, and LH by 15–20% and lower by 18% for FSH (although none were statistically significant) than men in H1BH2-arm on high-DBP mesalamine for 3 years or more (H1≥3 yrs). There was effect modification of the DBP exposure effect by the duration of high-DBP mesalamine at baseline for TT (p-value= 0.06), LH (p-value= 0.002) and FSH (p-value= 0.08) (data not shown).
Based on these results, we conducted a post-hoc three-arm analysis: H1BH2-arm, H1<3 yrs (25 men, 107 serum samples) had an average H1 duration of 1.2 years, H1BH2-arm, H1≥3 yrs (22 men, 100 serum samples) had an average H1 duration of 11.1 years (Supplementary Table 1& Supplementary Fig. 2) and B1HB2-arm (Figure 2 & Supplementary table 2). We found heterogeneity of the exposure responses among the three arms. Although on average, men in H1BH2-arm, H1≥3 yrs had no significant changes in hormones in crossover or crossback, men in H1BH2-arm, H1<3 yrs had significant changes similar to men in the B1HB2-arm. When men in H1BH2-arm, H1<3 years crossed-over, on average, inhibin-B increased by 13.2% (CI: 4.2, 22.9), and FSH decreased by 9.9% (CI: −17.9, −1.1), while TT/LH, FT/LH and inhibin-B/FSH ratios significantly increased by 18 to 26%. After crossback, inhibin-B had an additional increase of 11.3% (CI: 2.2, 21.3) and FSH marginally significantly increased by 9.7% (CI: −0.3, 20.8). Although not significant, there was a pattern after crossover that TT, FT, and SHBG increased and LH decreased. After crossback, SHBG decreased, TT, and FT had smaller additional increases, and LH increased. There was a significant carryover effect for FT, inhibin-B and inhibin-B/FSH ratio. Results were consistent across the adjusted and unadjusted analyses in both the primary and sensitivity analyses (Supplementary Tables 3 to 6, Figure 2 and Supplementary Figure 3).
Figure 2. Geometric means (95%CI) of reproductive hormone parameters at baseline, crossover and crossback for the 3 arms (B1HB2-arm, H1BH2-arm with duration on DBP-containing mesalamine medication at baseline <3 years and H1BH2-arm with duration ≥ 3 years) –MARS.
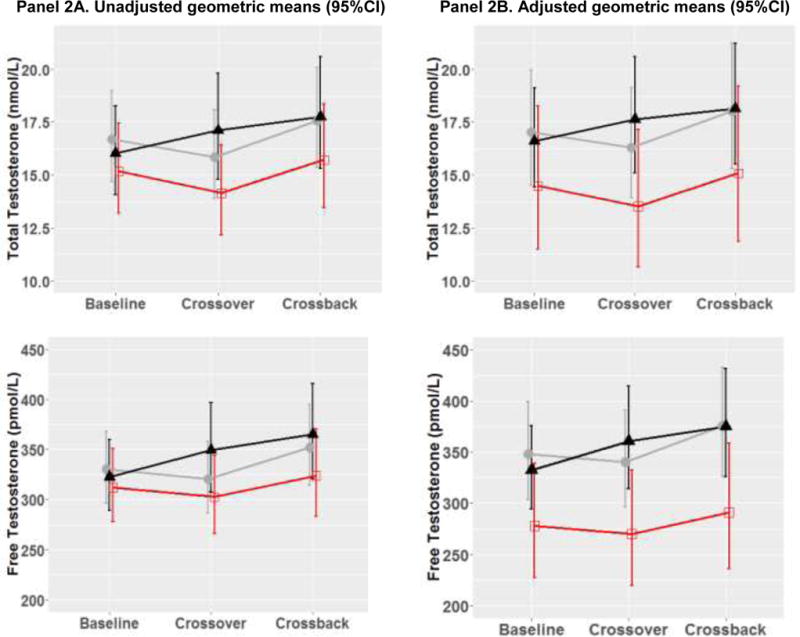
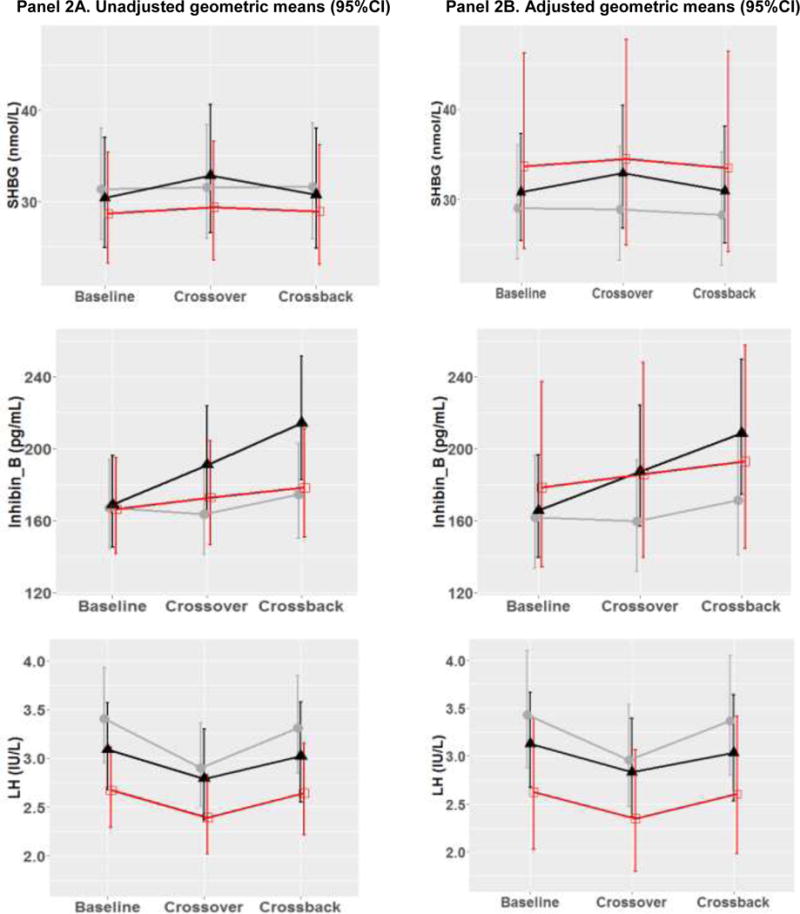
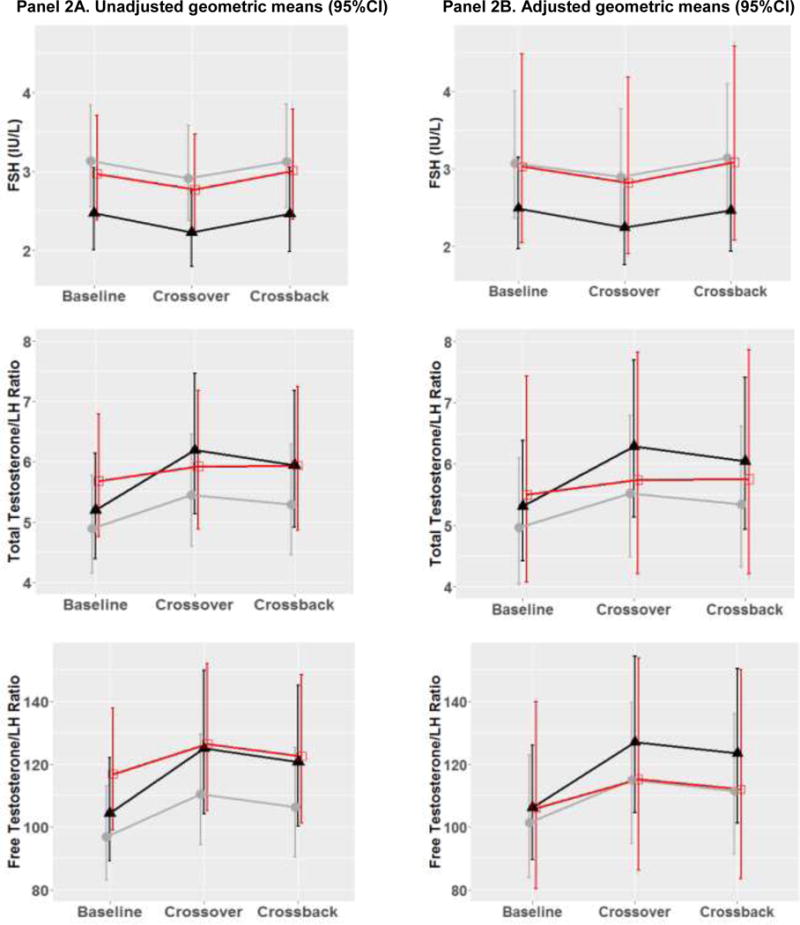
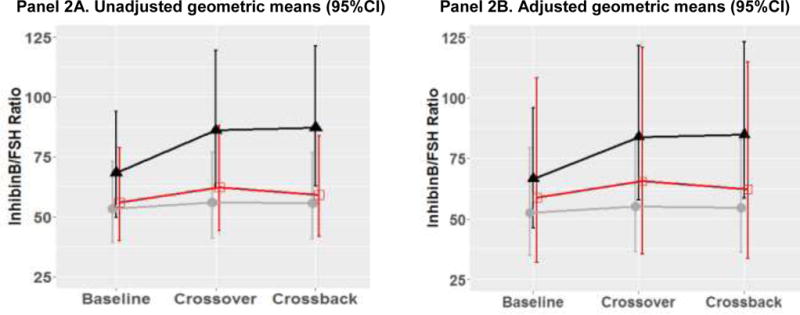
Figure 2 shows the adjusted geometric means and 95% CI [adjusted for race (Caucasian or not), age (continuous), BMI (continuous), hour of sample draw (continuous), season (warm or cold), current smoking, age (continuous), and duration on DBP-containing mesalamine medication at baseline (in years)]. B1HB2-arm, B1 represents background-exposure at baseline, H represents high-DBP exposure after crossover and B2 represents background-exposure after crossback; included 26 men taking mesalamine that did not contain DBP at baseline. H1BH2-arm, H1 represents high-DBP exposure at baseline, B represents background-exposure after crossover and H2 represents high-DBP exposure after crossback; included 47 men taking mesalamine-containing DBP at baseline. H1BH2-arm is subdivided into two arms based on the median duration on DBP-containing mesalamine medication at baseline (period) i.e., <3 years and >= 3 years
The grey lines represent B1HB2 arm, the black line represents H1BH2-arm, H1<3 yrs, and the red line represents H1BH2-arm, H1≥3 yrs.
Abbreviations: MARS, Mesalamine And Reproductive health Study; 95% CI, 95% Confidence Interval; DBP, dibutyl phthalate; SHBG, sex hormone-binding globulin; LH, luteinizing hormone; FSH, follicle-stimulating hormone.
4. Discussion
In our crossover-crossback study, on average men on non-DBP mesalamine at baseline (B1HB2-arm) and newly-exposed to high-DBP had decreased serum levels of testicular hormones (testosterone and inhibin-B) and gonadotropins (LH and FSH). After crossback to their non-DBP mesalamine, both testicular hormone and gonadotropin concentrations increased exceeding baseline concentrations. In our post-hoc three-arm analysis, in the H1BH2-arm the duration on high-DBP mesalamine at baseline modified the hormone response. The hormone changes were only found in men who were exposed to high-DBP mesalamine for < 3yrs (H1BH2-arm, H1<3 yrs) while there was no effect in those who had been receiving high-DBP mesalamine for ≥ 3 years (H1BH2-arm, ≥3 yrs). When men in the H1BH2-arm, <3 yrs crossed-over to non-DBP mesalamine, testicular hormones increased and gonadotropins decreased, suggesting a functioning pituitary negative feedback response. When they crossed-back to their original high-DBP mesalamine, their testicular hormones continued to increase but at slower rates while gonadotropins increased. TT/LH, FT/LH and inhibin-B/FSH ratios increased after crossover to non-DBP mesalamine, suggesting improved Leydig and Sertoli cell functions.
The decreases in testosterone and inhibin-B with high-DBP mesalamine new exposure in the B1HB2 arm are consistent with the decreases in semen quality parameters, primarily sperm motility seen in this same cohort5. However, after exposure removal for four months, in contrast to the observed reversal of hormonal changes, the semen quality decline was not reversed and instead further declined5. This may be explained by the long process of spermatogenesis22 which potentially may result in a lagged response to removal of high-DBP exposure. However, studies that follow sperm production over a longer follow up period will be required to confirm this hypothesis. Interestingly among men exposed to high-DBP mesalamine for a long duration before study entry (H1BH2-arm, H1≥3 yrs), the four month period during the crossover to non-DBP mesalamine was inadequate to ‘reverse’ the inhibitory effect of high-DBP exposure on LH, FSH, testosterone and inhibin-B. Similarly, semen quality did not change in H1BH2-arm after removal of high-DBP exposure5. In a cross-sectional analysis, men exposed to high-DBP for a longer duration (H1BH2-arm, H1≥3 yrs) had lower TT, FT, and LH concentrations than men exposed for a shorter duration (H1BH2-arm, H1<3 yrs) or men who were not exposed at baseline (B1HB2-arm).
In experimental studies, peri-pubertal DBP-exposure altered differential expression of proteins involved in spermatogenesis and changed number and function of Sertoli and Leydig cells26,27. Other studies in older animals showed that DBP preferentially targeted Sertoli cells28. Studies during fetal development showed that DBP was anti-androgenic resulting in decreased testosterone production by Leydig cells due to interference with steroidogenesis, down-regulating gene and/or protein expression essential for the steroidogenic pathway, and cholesterol transport and metabolism29–31. Suppression of both gonadotropin and testosterone production as well as decreased semen quality were observed in di(2-ethylhexyl)phthalate (DEHP) newly-exposed pre-pubertal and mature animals32,33, similar to our results on men newly exposed to high-DBP mesalamine. In adult DEHP-exposed animals, pituitary LH and FSH protein expression decreased significantly34,35. Decreased testosterone may indicate suppression of Leydig cell function and decreased inhibin-B may indicate suppression of Sertoli cell function, as well as germ cell production in adult testis36.
In humans, both inhibin-B and FSH decreased with higher-DEHP exposure37 suggesting that the endocrine communication between the pituitary and the testis might be disrupted27,37 hence disruption of the hypothalamo-pituitary-testis (HPT) axis. Similarly, based on our results of the suppression of both testicular hormones and gonadotropins, we speculate that high-DBP exposure from mesalamine was associated with HPT axis disruption targeting the gonads and/or the pituitary in the newly exposed men. In the H1BH2-arm, H1<3 yrs, gonadotropin negative feedback was partially regained after a relatively short removal of high-DBP exposure and partially compensated the testicular suppression after re-exposure. However, after longer durations of high-DBP exposure, as we observed in men in H1BH2-arm, H1≥3 yrs, the reproductive hormones were suppressed and the hormonal responses may be desensitized to DBP exposure removal. Therefore, our results from the three arms suggest that there may be different effects of the hormone responses modified by the duration of high-DBP mesalamine history. One caveat is that there are limited comparable animal data to explain modes of action of high-DBP exposure in adult life, especially with such unique crossover-crossback design, and no data on the potentially modified hormone responses by the duration of exposure. In addition, many of the experimental animal studies only measured testosterone and not gonadotropins.
In epidemiological studies on the association between environmental DBP-exposures and reproductive hormones in adult men, the results were inconsistent with the main focus on testosterone. Urinary MBP was negatively correlated with testosterone among occupationally exposed Chinese men38 and in infertility clinics in Slovenia39, China40, and India (only in the unadjusted analysis)41 but not in a study from Poland42. Both pooled and non-pooled analyses of infertile and fertile men in the US failed to find statistically significant associations17–19. Only a few studies included men from the general population and found that urinary MBP was inversely associated with testosterone only in men aged 40–60 years from NHANES20, but no evidence from studies in Denmark (only a significant increase in LH)21, Sweden43, or China44. All the studies described above were cross-sectional and used a single serum sample to measure the hormone concentrations at the same time when urinary MBP was measured. Janjua et al45 conducted the only human study with some similarity to our design examining the association between DBP-exposure (dermally) and short term effects on reproductive hormones. They did not find associations of topical DBP-exposure with reproductive hormones. These results must be interpreted cautiously due to much lower anticipated exposure levels with topical exposure compared to our study, likely too short washout period (one week), and a small sample size (n=26).
Several studies have characterized DBP and other phthalates in coatings of many licensed medications including over-the-counter medications in the US9,10,13, Canada4, United Kingdom6, Germany8 and China7. The urinary MBP in patients taking Asacol® was approximately 1000 times higher than the median levels reported for men in the US general population (NHANES)14. Therefore, we used a priori medication type as a proxy for high-DBP exposure5 rather than measuring urinary MBP concentrations which represent only exposure over the past several hours due to the short DBP half-life45.
Our study had several potential limitations including the lack of randomization to mesalamine formulations at baseline. However, the cross-sectional results were consistent with the longitudinal associations. In addition, physicians do not prescribe mesalamine based on DBP in the enteric coating or in relation to hormonal concentrations or semen quality, arguing against potential confounding by medication at baseline. Although our sample size was limited due to the innovative study and length of participation, the power of the study is derived from the unique design and the use of subjects as their own control. This avoids the purely cross-sectional analysis that may be confounded by inter-individual variability and lacks temporality. Post-hoc power analysis showed that the study had a sufficient number of participants to provide 80% power for detecting at least a 7% change in TT or FT, 8% in SHBG, 6% in inhibin-B and FSH and 9% in LH which were reasonable for a study of this design. Although there may be a concern of generalizing results from men with IBD, all men were classified as having mild IBD.
Our study had several important strengths including a unique innovative prospective design rarely conducted in environmental epidemiology studies. We were able to compare, within the same men, their hormone parameters during periods of high-DBP to background-DBP exposure and vice versa accounting for confounding by measured and unmeasured non-time-varying characteristics5. This is a major strength compared to cross-sectional studies. We had repeated measures up to six time points per man which increased the reliability of the measures. The different orders of exposure and non-exposure in the different arms gave us an additional opportunity to observe the reversibility of the changes under the study period. Our study was not restricted to infertile men compared to most of the previous literature. Confounding by indication was unlikely because mesalamine medications (with or without DBP coating) prescribed for treating IBD have the same active ingredient (mesalamine). We used the gold standard methods for hormone analyses especially for testosterone, which was accurately and precisely measured using LC-MS/MS methodology46. Finally, we had the opportunity to examine high-DBP exposure (1000 times background) compared to environmental background exposure.
Our results are of a concern because testosterone deficiency in adult men is a risk factor not only for decreased semen quality and infertility but also for decreased libido, energy, bone mass, and muscle strength, and is associated with erectile dysfunction, osteoporosis, metabolic syndrome, and, cardiovascular disease, and hence increased mortality47. Although manufacturers are required to disclose a listing of inactive ingredients in drug product labeling, they are not required to do so if the composition is a confidential trade secret i.e., used in patented delivery mechanisms (U.S. Pharmacopeial Convention 2009). The FDA may have information on the type and amount of phthalates used in specific approved drug products, but they do not make the information publically available because of its proprietary nature9. Recently, there was a consensus that women with IBD who are pregnant or might become pregnant may need to avoid high-DBP mesalamine4 leaving men of reproductive age with no recommendations and no guidance about any other medications that contain phthalate coating. Our results suggest the need to reconsider the use of DBP-containing mesalamine and other DBP-containing medications for men as well as improved education and access to information.
5. Conclusions
Although, some medications are a source of high-DBP exposure this source is relatively overlooked. Most studies of endocrine disrupting chemicals in men and women have focused on background environmental exposures. To our knowledge, MARS is the only and the first study that examined the association between such high-DBP exposure and health outcomes. Our results present evidence that high-DBP exposure disrupted serum levels of pituitary and gonadal hormones that largely reversed after removal of exposure, but only in men with no history of or a short duration (<3 yrs) of previous high-exposure. Paradoxically, among men with longer durations of high-DBP exposure, removal of exposure did not reverse these effects within a 4 month follow up period, suggesting that long-term high-DBP exposure may alter the pituitary-gonadal axis and make it insensitive to changes in exposures.
Supplementary Material
Highlights.
Mesalamine medications with coatings that contain dibutyl phthalate (DBP) lead to very high DBP exposure.
New exposure to high DBP from mesalamine medications disrupted pituitary-gonadal hormones in adult men.
Among men with no or short previous high DBP exposure, the hormone disruption was reversed after exposure removal.
Long term high DBP exposure may alter the pituitary-gonadal axis and make it insensitive to removal of exposure.
Acknowledgments
The authors gratefully thank Dr. Paul Foster, the Chief of the Toxicology Branch at the National Institute of Environmental Health Sciences in Research Triangle Park, North Carolina, USA, for his insightful advice. The authors thank the study participants, all members of the MARS team especially Ramace Dadd and Pat Morey and the clinical staff.
Sources of funding
The results reported herein correspond to specific aims of grant R01ES017285 to investigator Russ Hauser from the National Institute of Environmental Health Sciences (NIEHS). This work was also supported by grant P30ES000002 from the National Institute of Environmental Health Sciences (NIEHS). Also, Feiby L. Nassan received support during her doctoral studies from the Cyprus Endowment for the Environment and Public Health at the Harvard T.H. Chan School of Public Health, Leslie Silverman Industrial Hygiene Fund, and Benjamin Greely Ferris Jr. Fellowship in Environmental Epidemiology.
Abbreviations
- DBP
dibutyl phthalate
- IBD
Inflammatory Bowel Disease
- UC
ulcerative colitis
- CD
Crohn’s disease
- MBP
monobutyl phthalate
- NHANES
National Health and Nutrition Examination Survey
- MARS
Mesalamine And Reproductive health Study
- BIDMC
Beth Israel Deaconess Medical Center
- BWH
Brigham and Women’s Hospital
- MGH
Massachusetts General Hospital
- B1HB2
Background1-High-Background2 DBP exposure
- H1BH2
High1-Background-High2 DBP exposure
- H1BH2-arm
H1<3 yrs, H1BH2-arm with duration on DBP-containing mesalamine medication at baseline <3 years
- H1BH2-arm
H1≥3 yrs, H1BH2-arm with duration on DBP-containing mesalamine medication at baseline ≥ 3 years
- BMI
body mass index
- Kg
Kilogram
- m
meter
- SD
standard deviation
- LMEM
mixed effects models
- N
number of men
- 95% CI
95% Confidence Interval
- TT
total testosterone
- FT
free testosterone
- LH
luteinizing hormone
- FSH
follicle-stimulating hormone
- SHBG
sex hormone-binding globulin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The study was approved by the institutional review boards.
Disclosure Statement
J.R.K., receives research support from Abbvie, Transparency Life Sciences and Takeda, consults for Abbvie, and is a founder of a company called ColonaryConcepts. A.C.M, has no conflict currently and had previously received prior research grants from Shire, Salix and Aptalis, manufacturers of mesalamine medications. F.L.N, B.A.C, N.E.S, A.A., M.A.W, L.M, S.A.K, J.E.H, E.J.H, J.B.F, and R.H. have nothing to declare.
References
- 1.Phthalates and Their Alternatives: Health and Environmental Concerns. Lowell Center for Subtainable Production: University of Massachusetts Lowell; 2011. [Google Scholar]
- 2.CDC (Centers for Disease Control and Prevention) National Report on Human Exposure to Environmental Chemicals. 2017 www.cdc.gov/exposurereport. Accessed February 2017.
- 3.Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. International journal of andrology. 2006;29(1):140–147. doi: 10.1111/j.1365-2605.2005.00563.x. discussion 181-145. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen GC, Seow CH, Maxwell C, et al. The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy. Gastroenterology. 2016;150(3):734–757.e731. doi: 10.1053/j.gastro.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Nassan FL, Coull BA, Skakkebaek NE, et al. A crossover-crossback prospective study of dibutyl-phthalate exposure from mesalamine medications and semen quality in men with inflammatory bowel disease. Environment international. 2016;95:120–130. doi: 10.1016/j.envint.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamieson L, McCully W. Review: UK medicines likely to be affected by the proposed European Medicines Agency’s guidelines on phthalates. BMC pharmacology & toxicology. 2015;16:17. doi: 10.1186/s40360-015-0018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia LL, Lou XY, Guo Y, Leung KS, Zeng EY. Occurrence of phthalate esters in over-the-counter medicines from China and its implications for human exposure. Environment international. 2016 doi: 10.1016/j.envint.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Wittassek M, Koch HM, Angerer J, Bruning T. Assessing exposure to phthalates - the human biomonitoring approach. Molecular nutrition & food research. 2011;55(1):7–31. doi: 10.1002/mnfr.201000121. [DOI] [PubMed] [Google Scholar]
- 9.Kelley KE, Hernandez-Diaz S, Chaplin EL, Hauser R, Mitchell AA. Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environmental health perspectives. 2012;120(3):379–384. doi: 10.1289/ehp.1103998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauser R, Duty S, Godfrey-Bailey L, Calafat AM. Medications as a source of human exposure to phthalates. Environmental health perspectives. 2004;112(6):751–753. doi: 10.1289/ehp.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA. ASACOL® (mesalamine) delayed-release tablets, for oral use - HIGHLIGHTS OF PRESCRIBING INFORMATION. 2015 http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm215476.htm, http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/019651s025lbl.pdf. Accessed April, 2017.
- 12.FDA. Asacol® HD (mesalamine) delayed-release tablet for oral administration -HIGHLIGHTS OF PRESCRIBING INFORMATION. 2010 http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021830s005lbl.pdf. Accessed April, 2017.
- 13.Hait EJ, Calafat AM, Hauser R. Urinary phthalate metabolite concentrations among men with inflammatory bowel disease on mesalamine therapy. Endocr Disruptors (Austin) 2014;1(1) doi: 10.4161/endo.25066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CDC. Centers for Control Disease Prevention, Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, February 2015. 2015 http://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015.pdf. Accessed February 2016.
- 15.Kim TS, Jung KK, Kim SS, et al. Effects of in utero exposure to DI(n-Butyl) phthalate on development of male reproductive tracts in Sprague-Dawley rats. Journal of toxicology and environmental health Part A. 2010;73(21-22):1544–1559. doi: 10.1080/15287394.2010.511579. [DOI] [PubMed] [Google Scholar]
- 16.Motohashi M, Wempe MF, Mutou T, et al. Male rats exposed in utero to di(n-butyl) phthalate: Age-related changes in Leydig cell smooth endoplasmic reticulum and testicular testosterone-biosynthesis enzymes/proteins. Reproductive toxicology (Elmsford, NY) 2015;59:139–146. doi: 10.1016/j.reprotox.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Mendiola J, Meeker JD, Jorgensen N, et al. Urinary concentrations of di(2-ethylhexyl) phthalate metabolites and serum reproductive hormones: pooled analysis of fertile and infertile men. Journal of andrology. 2012;33(3):488–498. doi: 10.2164/jandrol.111.013557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendiola J, Jorgensen N, Andersson AM, et al. Associations between urinary metabolites of di(2-ethylhexyl) phthalate and reproductive hormones in fertile men. Int J Androl. 2011;34(4):369–378. doi: 10.1111/j.1365-2605.2010.01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meeker JD, Calafat AM, Hauser R. Urinary metabolites of di(2-ethylhexyl) phthalate are associated with decreased steroid hormone levels in adult men. Journal of andrology. 2009;30(3):287–297. doi: 10.2164/jandrol.108.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meeker JD, Ferguson KK. Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011-2012. The Journal of clinical endocrinology and metabolism. 2014;99(11):4346–4352. doi: 10.1210/jc.2014-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joensen UN, Frederiksen H, Jensen MB, et al. Phthalate excretion pattern and testicular function: a study of 881 healthy Danish men. Environmental health perspectives. 2012;120(10):1397–1403. doi: 10.1289/ehp.1205113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heller CG, Clermont Y. Spermatogenesis in man: an estimate of its duration. Science (New York, NY) 1963;140(3563):184–186. doi: 10.1126/science.140.3563.184. [DOI] [PubMed] [Google Scholar]
- 23.Gifford AE, Berg AH, Lahiff C, Cheifetz AS, Horowitz G, Moss AC. A random urine test can identify patients at risk of mesalamine non-adherence: a prospective study. Am J Gastroenterol. 2013;108(2):249–255. doi: 10.1038/ajg.2012.419. [DOI] [PubMed] [Google Scholar]
- 24.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. The Journal of clinical endocrinology and metabolism. 1999;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 25.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. (2nd) 2011 [Google Scholar]
- 26.Bao AM, Man XM, Guo XJ, et al. Effects of di-n-butyl phthalate on male rat reproduction following pubertal exposure. Asian journal of andrology. 2011;13(5):702–709. doi: 10.1038/aja.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moody S, Goh H, Bielanowicz A, Rippon P, Loveland KL, Itman C. Prepubertal mouse testis growth and maturation and androgen production are acutely sensitive to di-n-butyl phthalate. Endocrinology. 2013;154(9):3460–3475. doi: 10.1210/en.2012-2227. [DOI] [PubMed] [Google Scholar]
- 28.Foster PM, Mylchreest E, Gaido KW, Sar M. Effects of phthalate esters on the developing reproductive tract of male rats. Human reproduction update. 2001;7(3):231–235. doi: 10.1093/humupd/7.3.231. [DOI] [PubMed] [Google Scholar]
- 29.Barlow NJ, Phillips SL, Wallace DG, Sar M, Gaido KW, Foster PM. Quantitative changes in gene expression in fetal rat testes following exposure to di(n-butyl) phthalate. Toxicological sciences : an official journal of the Society of Toxicology. 2003;73(2):431–441. doi: 10.1093/toxsci/kfg087. [DOI] [PubMed] [Google Scholar]
- 30.Euling SY, White LD, Kim AS, et al. Use of genomic data in risk assessment case study: II. Evaluation of the dibutyl phthalate toxicogenomic data set. Toxicol Appl Pharmacol. 2013;271(3):349–362. doi: 10.1016/j.taap.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Ovacik MA, Sen B, Euling SY, Gaido KW, Ierapetritou MG, Androulakis IP. Pathway modeling of microarray data: a case study of pathway activity changes in the testis following in utero exposure to dibutyl phthalate (DBP) Toxicol Appl Pharmacol. 2013;271(3):386–394. doi: 10.1016/j.taap.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Guan HAM, Wei X, Li L, Yang P, Liu MC. Di-(2-ethylhexyl) phthalate inhibits testosterone level through disturbed hypothalamic-pituitary-testis axis and ERK-mediated 5alpha-Reductase 2. The Science of the total environment. 2016;563–564:566–575. doi: 10.1016/j.scitotenv.2016.04.145. [DOI] [PubMed] [Google Scholar]
- 33.Golshan M, Hatef A, Socha M, et al. Di-(2-ethylhexyl)-phthalate disrupts pituitary and testicular hormonal functions to reduce sperm quality in mature goldfish. Aquatic toxicology (Amsterdam, Netherlands) 2015;163:16–26. doi: 10.1016/j.aquatox.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Abdel-Maksoud FM, Leasor KR, Butzen K, Braden TD, Akingbemi BT. Prenatal Exposures of Male Rats to the Environmental Chemicals Bisphenol A and Di(2-Ethylhexyl) Phthalate Impact the Sexual Differentiation Process. Endocrinology. 2015;156(12):4672–4683. doi: 10.1210/en.2015-1077. [DOI] [PubMed] [Google Scholar]
- 35.Herreros MA, Encinas T, Torres-Rovira L, et al. Exposure to the endocrine disruptor di(2-ethylhexyl)phthalate affects female reproductive features by altering pulsatile LH secretion. Environmental toxicology and pharmacology. 2013;36(3):1141–1149. doi: 10.1016/j.etap.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Andersson AM, Muller J, Skakkebaek NE. Different roles of prepubertal and postpubertal germ cells and Sertoli cells in the regulation of serum inhibin B levels. The Journal of clinical endocrinology and metabolism. 1998;83(12):4451–4458. doi: 10.1210/jcem.83.12.5360. [DOI] [PubMed] [Google Scholar]
- 37.Joensen UN, Frederiksen H, Blomberg Jensen M, et al. Phthalate excretion pattern and testicular function: a study of 881 healthy Danish men. Environmental health perspectives. 2012;120(10):1397–1403. doi: 10.1289/ehp.1205113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan G, Hanaoka T, Yoshimura M, et al. Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a cross-sectional study in China. Environmental health perspectives. 2006;114(11):1643–1648. doi: 10.1289/ehp.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kranvogl R, Knez J, Miuc A, Voncina E, Voncina DB, Vlaisavljevic V. Simultaneous determination of phthalates, their metabolites, alkylphenols and bisphenol A using GC-MS in urine of men with fertility problems. Acta Chim Slov. 2014;61(1):110–120. [PubMed] [Google Scholar]
- 40.Li S, Dai J, Zhang L, Zhang J, Zhang Z, Chen B. An association of elevated serum prolactin with phthalate exposure in adult men. Biomedical and environmental sciences : BES. 2011;24(1):31–39. doi: 10.3967/0895-3988.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Pant N, Kumar G, Upadhyay AD, Patel DK, Gupta YK, Chaturvedi PK. Reproductive toxicity of lead, cadmium, and phthalate exposure in men. Environmental science and pollution research international. 2014;21(18):11066–11074. doi: 10.1007/s11356-014-2986-5. [DOI] [PubMed] [Google Scholar]
- 42.Jurewicz J, Radwan M, Sobala W, et al. Human urinary phthalate metabolites level and main semen parameters, sperm chromatin structure, sperm aneuploidy and reproductive hormones. Reproductive toxicology (Elmsford, NY) 2013;42:232–241. doi: 10.1016/j.reprotox.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Jonsson BA, Richthoff J, Rylander L, Giwercman A, Hagmar L. Urinary phthalate metabolites and biomarkers of reproductive function in young men. Epidemiology (Cambridge, Mass) 2005;16(4):487–493. doi: 10.1097/01.ede.0000164555.19041.01. [DOI] [PubMed] [Google Scholar]
- 44.Han X, Cui Z, Zhou N, et al. Urinary phthalate metabolites and male reproductive function parameters in Chongqing general population, China. International Journal of Hygiene and Environmental Health. 2014;217(2–3):271–278. doi: 10.1016/j.ijheh.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 45.Janjua NR, Mortensen GK, Andersson AM, Kongshoj B, Skakkebaek NE, Wulf HC. Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environmental science & technology. 2007;41(15):5564–5570. doi: 10.1021/es0628755. [DOI] [PubMed] [Google Scholar]
- 46.Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. The Journal of clinical endocrinology and metabolism. 2007;92(2):405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 47.Yeap BB, Alfonso H, Chubb SA, et al. In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. The Journal of clinical endocrinology and metabolism. 2014;99(1):E9–18. doi: 10.1210/jc.2013-3272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


