Abstract
Adaptive learning impairments are common in cognitive and behavioral disorders, but the neurogenetic mechanisms supporting human affective learning are poorly understood. We designed a higher-order contextual learning task in which healthy participants genotyped for the Val66Met polymorphism of the brain derived neurotropic factor gene (BDNF) were required to choose the member of a picture pair most congruent with the emotion in a previously-viewed facial expression video in order to produce an advantageous monetary outcome. Functional magnetic resonance imaging (fMRI) identified frontolimbic blood oxygenation level dependent (BOLD) reactivity that was associated with BDNF Val66Met genotype during all three phases of the learning task: aversive and reward-predictive learning, contextually-challenging decision-making, and choice-related monetary loss-avoidance and gain outcomes. Relative to Val homozygotes, Met carriers showed attenuated ventromedial prefrontal response to predictive affective cues, dorsolateral prefrontal signaling that depended on decision difficulty, and enhanced ventromedial prefrontal reactivity that was specific to loss-avoidance. These findings indicate that the BDNF Val66Met polymorphism is associated with functional tuning of behaviorally-relevant frontolimbic circuitry, particularly involving the ventromedial prefrontal cortex, during higher-order learning.
INTRODUCTION
The capacity to flexibly adapt to both threatening and favorable environmental circumstances has important ramifications for survival and well-being. Rodent infralimbic cortex (Milad & Quirk 2012) and homologous human ventromedial prefrontal cortex (vMPFC) modulate such contextual processing of threats and rewards, as well as extinction recall during learning (Phelps et al. 2004; Milad & Quirk 2012). Similarly, interactions between the vMPFC and hippocampal-amygdalar circuitry are critical for inhibition of learned fear in both rodents (Likhtik et al. 2014) and humans (Milad & Quirk 2012), and the vMPFC, through its excitatory and inhibitory influences on hippocampal-amygdalar circuitry, has been shown to mediate learning in both aversive and rewarding contexts in mice (Yizhar et al. 2011; Likhtik et al. 2014) and humans Hartley & Casey 2013).
Neuromolecular mechanisms that impact this neural circuitry - critical for the ability to avoid aversive outcomes and attain rewards - have been documented in preclinical studies in mice (Kandel 2001; Lu 2003; Egan et al. 2003), rats (Hall et al. 2000; Lu 2003), and humans (Egan et al. 2003; Lu 2003). In particular, brain-derived neurotrophic factor (BDNF), a key regulator of neural development and function, is implicated in behaviorally-relevant, activity-dependent synaptic plasticity in the mammalian hippocampus (Kandel 2001; Lipsky & Marini 2007) and amygdala (Chhatwal et al. 2006; Park & Poo 2013). Hippocampus-specific deletions of the BDNF gene have been shown to cause impaired spatial and object recognition in mice (Heldt et al. 2007), while BDNF signaling in the amygdala has been implicated in fear conditioning in rats (Chatwal et al. 2006). Given BDNF’s involvement in hippocampal-amygdalar circuitry during such contextual learning, and the documented bidirectional connections between this circuitry and the vMPFC in both rodents and humans (Milad & Quirk 2012), it is likely that BDNF influences behaviors mediated by these regions. Indeed, BDNF appears to play a role in hippocampal and infralimbic mediation of fear extinction in rodents (Peters et al. 2010), as well as in modulating neural correlates of reward seeking in mice (Codiera JW et al. 2010; Koo et al. 2012) and in rats (Vargas-Perez H et al. 2009). However, the neurogenetic mechanisms mediating human behavioral adaptation to higher-order (i.e., second-order associative cues) aversive and reward learning that is representative of human adaptive behavior remain largely undefined.
Of interest to this question, variation in a human-specific single-nucleotide polymorphism (SNP) in the BDNF gene in human (Pröschel et al. 1992), which results in a valine (Val) to methionine (Met) substitution at codon 66 (Val66Met), has been linked to: 1) human neuropsychiatric disorders (Neves-Periera 2002; Angelucci et al. 2005; but see Groves 2007); 2) impairments in intracellular trafficking and with anxiety related behavior in mice (Chen et al. 2006) and 3) the regulation of memory functions in both mice and humans (Egan et al. 2003; Eisenberg et al. 2013). A BDNF Val66Met knock-in mouse model exhibited altered synaptic and neurophysiological responses in the hippocampus and amygdala, as well as impaired infralimbic vMPFC synaptic transmission and plasticity during extinction learning (Chen et al. 2006). In line with these findings, in both transgenic mice and healthy humans, impairments in fear extinction learning and altered amygdala-vMPFC function has been demonstrated in BDNF Met carriers, relative to BDNF Val homozygotes (Soliman et al. 2010). Additionally, the BDNF Val66Met polymorphism is involved in neural responses to visual observation of positive and negative emotional stimuli in humans (Montag et al. 2008; van Winkel et al. 2014). However, the role of this genotype in the neural mediation of socially relevant higher-order aversive and reward-related learning is not well defined in humans, despite the fact that such learning is proximate to the maintenance of well-being by means of the adaptive capacity to flexibly adapt to both threatening and favorable contexts in our modern, socially-complex environment.
Here, we hypothesized that the BDNF Val66Met polymorphism would functionally impact frontolimbic regions supporting socially relevant aversive and appetitive affective contextual learning. To test this hypothesis, we developed a novel affective contextual learning paradigm involving socially-relevant cues to examine the neurogenetic correlates of successful avoidance or attainment of aversive or rewarding experiences. We also designed a control replication experiment in which participants passively viewed the same social, affective stimuli, but in a context-free design without the element of prediction towards the goal of assessing the contextual specificity of the hypothesized influence of BDNF Val66Met on brain mediation of higher-order affective learning.
METHODS & PROCEDURES
Participants and Genotyping
Sixty-one healthy participants (mean age = 34.7 ± 6.7 years, range 18–54; 33 women) took part in the current study after giving written informed consent in accordance with NIH IRB-approved procedures. To account for population stratification artifacts, only Caucasians of self-identified European descent were included in the study after they successfully completed a medical history, physical examination, structural MRI, and laboratory tests to assess their health status. Individuals with current or past medical or neuropsychiatric conditions (the latter as determined by the Structured Clinical Interview for DSM-IV carried out by qualified clinicians), including alcohol or drug abuse or head trauma, were excluded, as were those with structural MRI abnormalities.
BDNF Val66Met genotype was determined using an independently validated Taqman 5-exonuclease assay as described earlier (see Egan et al. 2003). Because of the rarity of Met homozygotes, these individuals were analyzed together with heterozygotes in a Met carrier group, as in previous literature (Neves-Pereira M 2002). In the cohort of 61 participants who took part in the Affective Contextual Learning Experiment, the allelic distribution of the genotypes was as follows: 41 BDNF Val homozygotes and 20 BDNF Met carriers (three Met homozygotes and 17 heterozygotes), with a Hardy Weinberg Equilibrium value of 0.9991.
Thirty-three of the 61 subjects (mean age = 32.15 ±6.16; 14 females) also participated in the Context-Free Passive Affective Viewing Control Experiment. This passive viewing control experiment preceded the learning experiment to avoid any memory confounds that might allow participants to form contextual associations with the affective videos. The distribution of BDNF Val66Met genotypes for these 33 participants was as follows: 20 BDNF Val homozygotes and 13 BDNF Met carriers (2 Met homozygotes and 11 heterozygotes), with a Hardy Weinberg Equilibrium value of 0.7109.
Experimental Paradigms
1. Affective Contextual Learning Experiment
All sixty-one participants underwent probabilistic learning during event-related fMRI. There were four fully randomized experimental runs, each consisting of 45 trials - 15 trials of each monetary outcome condition (potential loss, potential gain, and neutral/no incentive). Thus, across all four runs there were 60 trials per monetary outcome condition, amounting to a total of 180 trials in the total experiment. Each trial consisted of (a) an initial video cue showing 3000ms of evolving (from a neutral starting point) fearful, happy, or neutral facial expressions (portraying naturally occurring eye blinks) from a sample of videos used and described in previous studies (van der Gaag et al. 2007; Jabbi et al. 2013; Jabbi et al. 2015, also see Fig. 1a); (b) a subsequent choice cue that required selecting between two non-face pictures differing in the degree of emotional concordance with the initial facial video cue (Fig. 1b); (c) a feedback cue that simply noted the picture that was chosen for each trial (Fig. 1c); and (d) an outcome cue indicating in 80% of the trials a monetary gain of $1 for a concordant happy choice, avoidance of a $1 loss for a concordant fear choice, or a blurred $1 cue indicating no gain or loss for a concordant neutral choic (Fig. 1d). As in previous literature (see, Pessiglione et al. 2006), in 20% of the trials the outcome cue was invalid in order to maximize learning efforts. Participants were informed that they could earn a maximum of $60 and will be paid in cash at the end of the fMRI sessions. However, each participant was paid $48 in cash at the end of the session regardless of the actually earned amount. Participants were debriefed amount this payment method after they received the cash amount.
Figure 1. Experimental Paradigm.
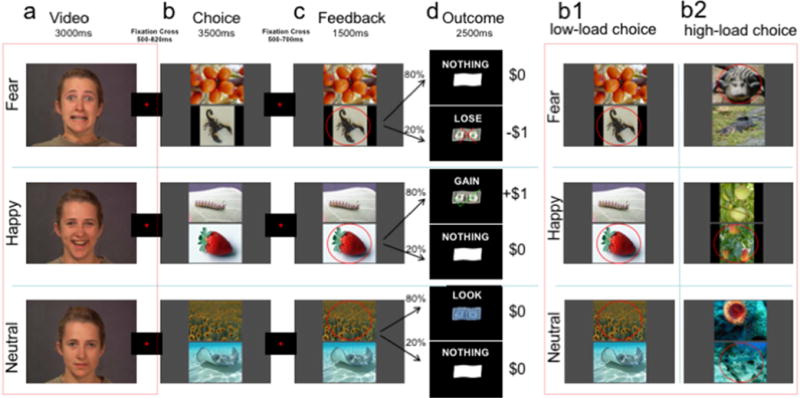
The four events within each affective contextual learning trial. Red fixation crosses (500–820ms duration) indicate inter-stimulus intervals. (a) Affective video cues: sample still frames from videos (3000ms duration) of actors expressing fearful, happy and emotionally neutral facial expressions. (b) Decision-phase: following the fixation cross, through trial and error, participants learned to choose which of two pictures (top or bottom) was most emotionally congruent with the previously observed expression in (a). (c) a feedback condition reminding participants of their choice (circled in red); (d) monetary outcome phase: an outcome window showing participants the monetary outcome resulting from their choices. The outcome was programed to an 80% probability (i.e., 20% of the trials were invalid), in accordance with the emotion congruency matching rules. (b1 & b2) example feedback for an easy/low decision-load trial (b1) and for a difficult/high decision-load trial (b2).
Prior to the fMRI session, participants were shown an example trial, but they were intentionally kept naïve of the response rules governing the actual contextual learning task. Thus, using trial and error, participants were required to learn the rules of the task that led to monetary gain or avoidance-of-loss; in sum, after seeing a fearful, happy, or neutral video cue of facial expression, they were required to press a button to choose which of the aversive, pleasant, or neutral pictures of a simultaneously presented pair was most emotionally concordant with the previously viewed video. To test for neurogenetic correlates of processing different cognitive loads, we experimentally manipulated the difficulty of choosing between the two pictures such that experimental runs 1 and 2 consisted of low decision-load (i.e., easy) trials, whereas runs 3 and 4 consisted of high decision-load (i.e., relatively difficult) trials, as described in the “Picture Stimuli” section below.
Affective Video Stimuli
For the first step of each trial, participants were shown a 3000ms video cue of an emerging facial expression (fearful, happy, or emotionally neutral with visible eye blinks). The videos portrayed 10 different actors, each of whom produced facial expressions for each of the three emotional conditions. These 30 videos were taken from a sample of validated stimuli described in previous studies (van der Gaag et al. 2007; Jabbi et al. 2013; Jabbi et al. 2015, also see Fig. 1a), and each fearful, happy, and neutral video was presented six times during the course of the entire experiment.
Picture Stimuli
To create picture pairs for the second step of each trial (Figs. 1b), a total of 936 non-face, high-resolution pictures with varying degrees of aversive or appetitive/rewarding connotations were obtained from the Internet. Contents depicted natural stimuli including flora (plants, flowers and fruits), fauna (mammals, birds, reptiles, fish, and insects), and natural phenomena (lightning, storms, and volcanoes). Of the 936 initially downloaded pictures, 360 were selected to form a final sample of 180 picture pairs using the procedure outlined below.
Picture Ratings & Pairings
To guide the formation of picture pairs for the affective contextual learning fMRI experiment, overall valence for each picture stimulus was empirically determined in a rating study performed by a separate group of 86 healthy adult volunteers (mean age = 24 years, 50 women) who did not take part in the imaging study. Each volunteer rated one of four subgroups of pictures (234 pictures in each subgroup) by considering two specific questions: “how pleasant/unpleasant is the stimulus?” and “how willing are you to be in close proximity to the content of each picture?” (as determined by imagining an encounter in real life). Ratings were performed on a Likert scale ranging from +6 for highly pleasant/approachable to −6 for highest imaginable unpleasantness/need-for-withdrawal from that particular stimulus if encountered in the natural environment. Picture pairs (180 pairs in total) were then formed such that the correct choice was the pair member with the highest or lowest average rating by the 86 behavioral raters; i.e. the more negative the mean combined rating of a picture on approachability and pleasantness, the higher the probability that that picture would be the correct choice for a fear/loss trial, and vice versa for a gain/reward trial. For neutral trials the correct choice was the picture with the average rating closest to zero.
Differential Decision-Load Picture Pairings
To test whether neurogenetic influences on higher-order contextual decision-making processes vary with decision difficulty, the ratings were additionally used to form two categories of picture pairs – low decision-load trials composed of pairs whose members had highly different average ratings, and high decision-load trials composed of pairs whose members had similar ratings. The mean difference in ratings between members of the high-load picture pairs was significantly less than the mean difference in ratings between members of the low load picture pairs (1.48+/−1.22 vs. 4.5 +/−1.23, T=14.158, p<10−5). The level of choice difficulty was further experimentally manipulated as follows: for runs 1 and 2, consisting of low decision-load trials, picture pairs were created such that the two pictures belonged to different content categories (e.g., flora vs. fauna) and differed in valence (e.g., fearful vs. happy or neutral). However, for runs 3 and 4, consisting of high decision-load trials, the choice difficulty was increased by pairing pictures from the same phylum (e.g., flora versus flora, and fauna versus fauna), and by pairing two pictures that were both emotionally concordant with the preceding video, but one more so than the other (Fig. 1b1 & b2). Thus, not only were the 90 picture pairs in the high decision-load trials (grouped in experimental runs 3 & 4) more similar in emotional valence than the 90 low decision-load pairs (grouped in experimental runs 1 & 2), they were also more similar in content, and thus more difficult to choose between. Additionally, a 3500ms choice window was maintained for both the low and high decision-load trials, adding time pressure to the already augmented difficulty of the latter.
Because the ratings were qualitatively more similar between the picture pairs across valence in the high-load condition, relative to the low-load condition with 30 trials by valence type, we assess the pleasantness rating differences within the high-load condition with 30 trials. Using a repeated measures ANOVA of pleasantness difference and found an interaction between choice (correct > wrong choices) and valence (gain > loss avoidance > neutral) at p < 0.001, F2,28 = 9.207, suggesting that pleasantness rating were still significantly different between correct and wrong choices and more so for the gain and loss avoidance choices than for the neutral choice. We additionally assessed the effect of load and choice by including both the low-load and high-load pleasantness rating scores in the model, and found no effect of load p = 0.843, F1,29 = 0.04, or choice p = 0.169, F1,29 = 1.990, or interaction between load and choice p = 0.130, F2,28 = 2.433. The lack of interaction between load and choice concerning the pleasantness rating in our behavioral cohort of 86 individuals, coupled with the significant difference in pleasantness ratings between the high-load choices suggests that load related BOLD findings should not be influenced by similarities in pleasantness between the picture pairs per se.
2. Context-Free, Passive, Affective Viewing Control Experiment
To assess whether the observed neural responses to the affective video cues during the contextual learning task were related to the predictive values of these cues, rather than to simply observing a video of facial emotion, 33 of the 61 healthy adults participated in a passive viewing control fMRI experiment. During this control task that is devoid of a learning context, participants were shown, in fully-randomized fashion (i.e. with regards to trial valence on affect and monetary outcome), the same fearful, happy, and neutral facial expression videos as in the affective contextual learning task. Here, however, participants were simply required to press a button to indicate the gender of each face after each video stimulus disappeared from the screen (see Fig. 1a). Importantly, we acquired the fMRI BOLD measures for this passive viewing control task prior to the contextual learning experiment in order to prevent participants from forming meaningful associations related to the predictive connotations of the videos with regard to the choice picture and outcome cues during the contextual learning task.
fMRI Data Acquisition and Statistical Analyses
Data were acquired with a 3T GE scanner using a 16 channel NOVA head coil at 1.95s TR, TE = 2.54. After realignment and co-registration onto ASSET MPRAGES, further preprocessing, including stereotaxic normalization and smoothing at 8mm, and subsequent analyses were carried out using SPM5 (www.fil.ion.ucl.ac.uk/spm/software/spm5/).
First-level whole-brain voxelwise analyses
First-level GLM analyses were performed to generate contrasts of interest for each participant for each of the three phases of each trial: (1) affective video viewing (Fig. 1a) in the context of both learning and passive viewing; (2) the establishment of cue-outcome association as choices between picture pairs that were made in the learning paradigm (Fig. 1b); and (3) the outcome phase of the learning experiment (Fig. 1d). In addition to modeling these time windows of interest as described below, we also modeled the onsets and durations of each feedback time window (when participants were reminded of the picture they chose prior to seeing the monetary outcome cue; Fig. 1c) as a condition of no interest.
First, we modeled the onsets and durations of the affective videos (Fig. 2a) and tagged them according to valence. For each participant, independent sample t-tests were performed to assess the differential BOLD response to fearful videos vs. baseline fixation cross (baseline), to happy videos vs. baseline fixation, and to neutral videos vs. baseline fixation. This first-level statistical analysis was carried out to test for valence-specific BOLD responses to videos both during the affective contextual learning task and during the context-free passive viewing control experiment, in which the videos were devoid of any contextual/predictive values. These first level contrasts were used for second level assessment of the effects of valence, genotype, and affective video context (predictive learning vs. passive viewing) at the second-level.
Figure 2. BOLD Response to Predictive Affective Videos.
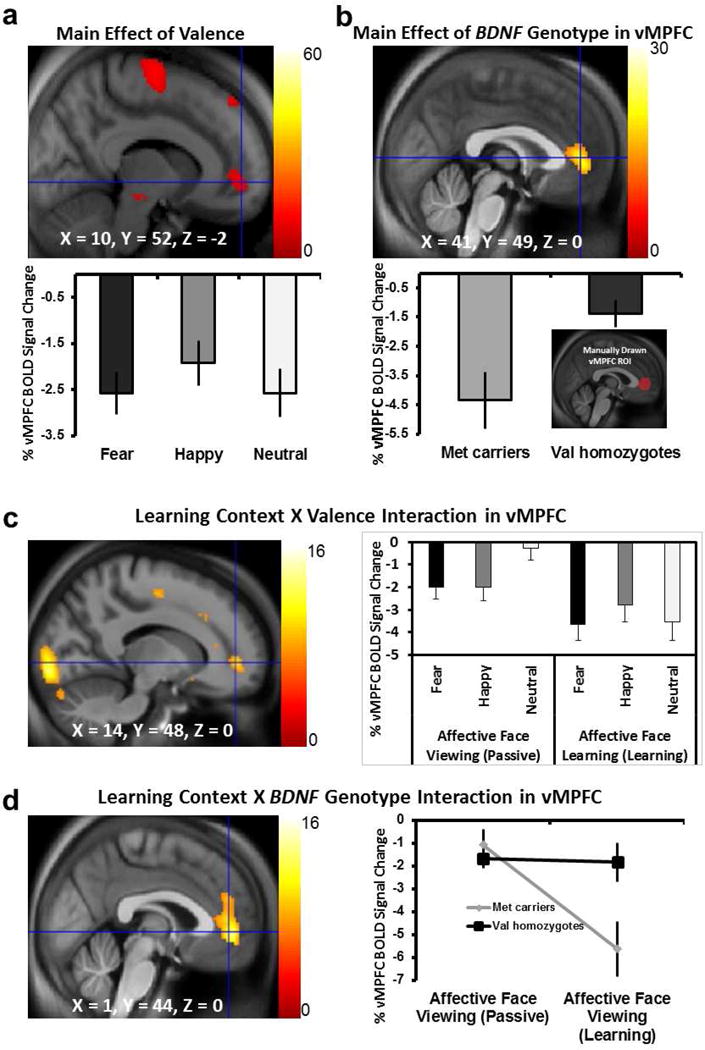
(a) Main effect of emotional valence on regional representation of the predictive facial cues in supplementary motor area, superior frontal cortex, and medial vMPFC shown voxelwise and in a graph of values extracted from the a priori vMPFC ROI (see inset in 2b; F2, 58 = 4.6, p = 0.001). (b) Main effect of BDNF Val66Met polymorphism in the vMPFC shown voxelwise and for ROI results in the graph (F1, 59 = 7.363, p = 0.01); inserted brain image in graph shows the manually defined vMPFC ROI used throughout the analysis. (c) Interaction between affective learning context (passive viewing vs. affective learning) and valence (fear happy and neutral affective videos) was shown to influence vMPFC BOLD response such that the contextual learning caused more negative/decreased regional BOLD response, and more so in the neutral valence compared to passive affective viewing of the same cues (F1,31 = 28.15, p = 0.007). (d) Interaction between affective learning context (passive viewing vs. affective learning) and BDNF Val66Met genotype in an extended vMPFC region shown voxelwise and in a graph of values extracted from the vMPFC ROI shown in the inset; when the facial cues were predictive of aversive and rewarding outcomes, BDNF Met carriers, but not Val homozygotes, showed reduced vMPFC response (F1, 31 = 5.46, p = 0.026). Color bars represent F-statistics; voxelwise images shown at p < 0.005 for display and X, Y, Z coordinates are in MNI space.
Second, we modeled the onsets and durations of the choice-related picture cues (Fig. 1b1 and 2b2) and tagged them according to potential monetary incentive. For each participant, independent sample t-tests were performed to assess BOLD response to the potential for avoidance-of-loss vs. baseline fixation, to potential monetary gain vs. baseline fixation, and to neutral choices with no monetary outcome vs. baseline fixation. Thus, while we used the fixation time window as absolute baseline, we included the neutral valence in our factorial models to be able to determine the signal directionality of the various loss avoidance and gain contexts relative to the corresponding neutral contexts. This procedure was carried out separately for high and low-load choices to enable testing of the effects of decision load/difficulty at the second-level. Because ~70 of the overall choice behavior was accurate across participants, and because the temporal sensitivity of the measured BOLD signal, including the modeled hemodynamic response function, has limited ability to differentiate the majority correct from the minority incorrect 3500ms trials, we included all choices (correct or incorrect) in the analysis of our fully randomized experiment. This way, we were able to assess overall decision-making per se, which resulted in largely successful contextual learning.
Third, we modeled the onsets and durations of the monetary outcome cues (Fig. 1d) and tagged them according to the three incentive types. For each participant, independent t-test were performed to assess BOLD response to $1 loss/loss-avoidance vs. baseline fixation, to $1 gain/failure to gain $1 vs. baseline fixation, and to no monetary outcome vs. baseline fixation. As noted earlier, these outcomes were contingent on whether participants had chosen the correct member of the previously-shown picture pair (i.e., the picture most emotionally concordant with the video that initiated that trial).
Second-level whole brain voxelwise analyses
Each of the three sets of first-level contrast images (affective video cues, picture choices, and monetary outcome cues) were included in second-level GLM models for the purpose of testing our primary hypothesis that BDNF Val66Met genotype is associated with neural response to higher-order affective learning, as well as our ancillary questions. All second-level analyses were carried out with full-factorial models in SPM5, and effects of interest were reported at p < 0.001, uncorrected. To adhered to proper inference and full reporting and transparency, we followed the recent American Statistical Association’s guidelines (Wasserstein & Lazar 2016) and avoided excluding a priori related findings that did not survive a specific correction. We additionally followed the recommendations to assess and report which of our findings survive family wise error (FWE) correction using the a priori frontolimbic search threshold of p < 0.001 and FWE cluster p-value of p < 0.05 (Chumbley & Friston 2009; Eklund et al. 2016).
First, for the affective videos, we tested the hypothesis that the BDNF Val66Met polymorphism influences neural response to affective cues predicting aversive and rewarding experience in the 61 individuals who participated in the affective contextual learning task. A whole-brain, voxelwise second-level ANOVA was performed with BDNF Val66Met genotype as a between-group factor and valence as a within-group factor (three levels: loss-predicting fearful videos, gain-predicting happy videos, and incentive-free neutral videos), and we tested for interactions between valence and genotype. To further assess whether the modulatory effects of BDNF Val66Met genotype on BOLD response to affective cues were specific to the learning-related affective contexts, rather than to simply viewing evolving facial emotions, we analyzed the 33 individuals who completed both the contextual learning task and the context-free passive viewing task. For this analysis we used the first-level contrast images representing video cues to define two within-subject levels of task (passive viewing of facial video cues without any contextual meaning, and viewing facial video cues during affective contextual learning when the video cues function as higher-order predictors for the picture-choice context and the monetary outcome), three within-subject levels of valence (fearful, happy, and neutral expressions videos), and two levels of BDNF Val66Met genotype (Met carriers and Val homozygotes) as our between-groups factor.
Second, for picture choices during contextual learning, we asked whether BDNF Val66Met genotype modulated BOLD response to instrumental choice behavior, and whether any such genetic effect varied with decision difficulty (load). The first-level contrasts constructed from the picture choice time windows were analyzed with decision load (low load vs. high load) and potential monetary incentive (choosing to avoid loss, choosing to gain, and incentive-neutral choice behavior) as within participants factors, and BDNF Val66Met polymorphism as the between groups factor.
Third, we asked whether BDNF Val66Met genotype was related to BOLD response to monetary outcome cues (fear-related loss avoidance, happiness-related gain, and neutral no incentive condition), and if such genetic influence differed between aversive and rewarding outcomes. The first-level contrasts consisting of the outcome window of interest when participants were shown visual cues indicating the monetary consequences of their previous choices were modeled with monetary outcome (i.e. $1 loss or avoidance-of-loss following fear videos, $1 gain or failure to gain following happy videos, seeing $1 or not seeing $1 following neutral videos) as a within-subject factor and genotype as a between-subjects factor.
Post-hoc region of interest analyses
Because of the role of the vMPFC in cognitive and affective processes (Montag et al. 2008; Mühlberger et al. 2014; van Winkel et al. 2014) as well as in BDNF-dependent extinction learning in preclinical and human studies (Chhatwal et al. 2006; Soliman et al. 2010; Milad & Quirk 2012; Park & Poo 2013), we performed post-hoc ROI analyses on this region in order to assess effects of interest across the various experimental questions addressed here. A bilateral vMPFC ROI based on Milad & Quirk’s 2012 vMPFC human analog of the infralimbic cortex was drawn using the anatomical ROI toolbox, ITK SNAP (see insert in Fig. 2b). Extracted averaged signals from the statistical parametric maps of the second-level analyses within this ROI were assessed in SPSS and considered significant at p < 0.05, but we reported non-significant p-values for a more comprehensive portrayal of our results.
Using the ROI data, we first tested whether vMPFC BOLD response during video viewing differed as a function of valence, genotype, and context (i.e., predictive vs. passive video viewing). Next, we tested whether vMPFC response during picture choices varied with valence, genotype, and difficulty (decision load) of choosing a picture. We also tested whether vMPFC response during outcome cues was differentially affected by incentive value (potential gain, avoidance-of-loss, or neutral) and whether this response varied by genotype. Finally, we performed correlation analyses to assess the relationship between performance (percent correct picture choices) and prefrontal BOLD signal representation of different phases of affective contextual learning.
RESULTS
No Effects of Demographics
There were no differences in demographic variables (age, sex, IQ) between Val66Met genotype groups for either the entire group who participated in the affective contextual learning task (N = 61) or the sub-group (N=33) who also performed the passive-viewing control task, see Table 1.
Table 1.
| Contextual Learning Cohort | |||||
|---|---|---|---|---|---|
|
| |||||
| BDNF Val66Met | Total | Female | Age | Performance | |
|
|
|||||
| Met Carriers | Mean | 20 | 10 | 36.30 | 67.48 |
|
|
|||||
| SEM | 1.650 | 1.911 | |||
|
|
|||||
| Val Homozygotes | Mean | 41 | 23 | 33.93 | 65.05 |
|
|
|||||
| SEM | 0.984 | 1.203 | |||
|
| |||||
| Passive Viewing Cohort | |||||
|
| |||||
| BDNF Val66Met | Total | Female | Age | ||
|
|
|||||
| Met Carriers | Mean | 13 | 4 | 32.77 | |
|
|
|||||
| SEM | 1.968 | ||||
|
|
|||||
| Val Homozygotes | Mean | 20 | 10 | 32.15 | |
|
|
|||||
| SEM | 1.071 | ||||
BDNF Val66Met Genotype Modulates BOLD Response to Predictive Affective Videos
In the contrasts representing neural response to video cues in the context of the learning experiment (N=61), we found a main effect of valence in an extended network of regions including the bilateral amygdala (including parahippocampal gyrus), bilateral DLPFC, supplementary motor area (SMA, including preSMA and mid-cingulate), left ventral and dorsal anterior insula, visual cortical areas, as well as the medial vMPFC region of particular interest to this study (Fig. 2a), with the vMPFC response surviving FWE cluster-level correction at p = 0.003. Post-hoc examination of the vMPFC response using our predefined ROI (Fig. 2b insert) showed that happy videos caused less reduction in BOLD response relative to baseline than did the fearful and neutral videos (F2, 58 = 4.6, p = 0.001; Fig. 2a graph).
In addition, in our voxelwise analyses, we found a regionally specific main effect of BDNF Val66Met genotype in a very similar vMPFC region (Fig. 2b) at FWE cluster-level corrected p = 0.049. Post-hoc examination of this vMPFC response using our predefined ROI (Fig. 2b insert) showed that BDNF Met carriers had more reduced vMPFC response to the predictive emotional facial cues than did Val homozygotes (F1, 59 = 7.363, p = 0.01; Fig. 2b graph). While there was no valence-by-genotype interaction, these results demonstrate a role for the BDNF Val66Met polymorphism in modulating vMPFC response to predictive affective cues.
BDNF Val66Met Genotype Modulation of BOLD Response to Viewing Affective Videos is Context-Specific
To assess whether the BDNF Val66Met effect on neural response to viewing facial emotion videos was dependent on their predictive significance, rather than on just observing these videos, we tested BOLD signals in the 33 individuals who participated in both the learning experiment and the passive viewing control experiment (when the videos carried no contextually relevant predictive values). There was a main effect of experimental context in which BOLD response in the amygdala, anterior insula, and DLPFC was more reduced in response to viewing the affective cues when they were contextually relevant during the learning task than when they had no predictive values during passive viewing. This effect was independent of valence. However, a context-by-valence interaction was found in the anterior cingulate cortex (ACC), pre-supplementary motor area and the vMPFC (Fig. 2c, brain map). To further assess the directionality of the context-by-valence interaction, we performed a posthoc signal analysis by extracting the vMPFC BOLD signal during viewing of facial cues. This vMPFC BOLD signal was more negative during contextual affective learning than during passive affective viewing and this reduction/negative dip in vMPFC BOLD as a function of predictive context was larger for the neutral context than for the positive and negative learning contexts (F1,31 = 28.15, p = 0.007; Fig. 2c, graph). Moreover, we found a context-by-BDNF Val66Met genotype interaction that was also localized in the same vMPFC region (Fig. 2d, brain map), and context-by-valence-by-BDNF Val66Met at FWE cluster-level corrected p = 0.03.
The vMPFC ROI analysis of these 33 individuals was consistent with the voxelwise findings. There was a main effect of context (passive vs. predictive) and a context-by-BDNF Val66Met genotype interaction (F1, 31 = 6.45, p = 0.016 and F1, 31 = 5.46, p = 0.026, respectively; Fig. 2d, graph). vMPFC BOLD signal was more reduced in response to the predictive affective cues during contextual learning relative to the passive viewing of the same affective cues, and this reduction was specific to the BDNF Met carriers. Together, these results confirmed a context-specific impact of the BDNF Val66Met polymorphism on frontolimbic BOLD response to the predictive value of socially relevant affective cues. To specifically examine the behavioral relevance of the vMPFC’s responsiveness to affective videos during the learning context, we performed a Spearman rank correlation and found no correlation between vMPFC response to the predictive videos and contextual learning performance (r = .115, p = 0.377).
BDNF Val66Met Genotype Modulates BOLD Response to Adaptive Choice Behavior
The facts that the affective videos were predictive of the best choice to be made during the decision phase of the learning task, and that the neural response to viewing these videos was context-specific and interacted with BDNF Val66Met genotype, suggested involvement of this neurogenetic mechanism in the actual instrumental choice behavior during contextual learning.
We first tested whether variation in BDNF Val66Met genotype influenced performance during choice behavior and found no differences in performance between the Met carriers and Val homozygotes (mean performance = 67.48% ±1.911 SEM versus 65.05% ±1.20 SEM, respectively; F1,59 = .96, p = .33). We then examined whether our manipulation of decision-load affected participants’ behavioral performance (percent correct choices) using repeated measures ANOVA in SPSS with decision load (low vs. high decision-load) and potential monetary outcome (loss-avoidance, gain, and incentive-free neutral choice) as our within-participant factors, and BDNF Val66Met genotype as our between-group factor. While there was no interaction between BDNF Val66Met genotype and decision-load on performance (i.e. mean performance during low-load for Met carriers was 70.93% ±2.35 SEM, and for Val homozygotes 68.87 ± 1.64 SEM; whereas performance during high-load for Met carriers was 64.03% ±1.86 SEM, and for Val homozygotes 61.23 ± 1.30 SEM; F1,59 = 0.07, p = .79), we observed an interaction between load and valence at F2, 58 = 6.14, p = 0.004 (Fig. 3a): increased decision-load led to poorer performance, with the greatest decrement in performance during potential loss trials.
Figure 3. BOLD Response during Affectively-Cued Picture Choices.
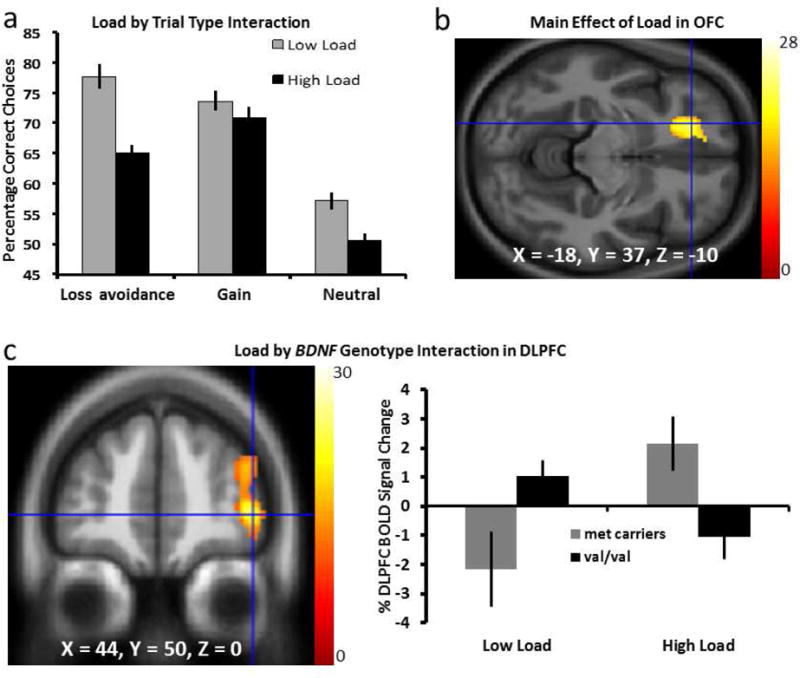
(a) Behavioral performance (percent correct pictures choices): interaction between decision load and potential outcome valence (F2, 58 = 6.14, p = 0.004). (b) Main effect of load in the left orbitofrontal cortex shown voxelwise. (c) Interaction between load and BDNF Val66Met genotype in the DLPFC region during choice behavior shown voxelwise on a coronal slice, with the graph depicting values extracted from the DLPFC peak response: BDNF Met carriers showed increased DLPFC response when decisions were more difficult, and Val homozygotes showed the opposite pattern (F1, 59=12.93, p = 0.001). Color bars represent F-statistics; voxelwise images shown at p < 0.005 for display and X, Y, Z coordinates are in MNI space.
We next assessed BOLD response during the decision phase of the learning task and found a main effect of decision load in the left orbitofrontal cortex (OFC, Fig. 3b) such that increased activation was associated with high load decisions at FWE cluster-level corrected p = 0.040. Importantly, there was an interaction between decision load and BDNF Val66Met genotype in the right DLPFC (Fig. 3c) at p < 0.001 uncorrected and FWE cluster-level corrected p = 0.696. Further extraction of the signal values from this region revealed that increased decision load was associated with increased right DLPFC BOLD response in BDNF Met carriers, but with reduced DLPFC response in BDNF Val homozygotes (F1, 59=12.93, p = 0.001, Fig. 3c). While such results may be of interest within the broader context of the higher-order affective learning task, it is necessary to interpret any activations that did not survive cluster-level FWE correction with caution.
To examine the behavioral relevance of our DLPFC findings, we perform a nonparametric Spearman’s rank correlation between overall performance across the learning task and the extracted DLPFC signal response to decision-making and found no correlation (r = −.099, p = 0.446), suggesting that on average, the degree of DLPFC response was not predictive of the level of individual performance during the affective contextual learning task. Further assessment of the signal relationship between the decision-making BOLD correlates in the DLPFC and the contextual affective video viewing correlates in the vMPFC showed a positive Spearman rank correlation (r = .352, p = 0.006, Fig. 4b).
Figure 4. BOLD Response during Affectively-Cued Monetary Outcomes.
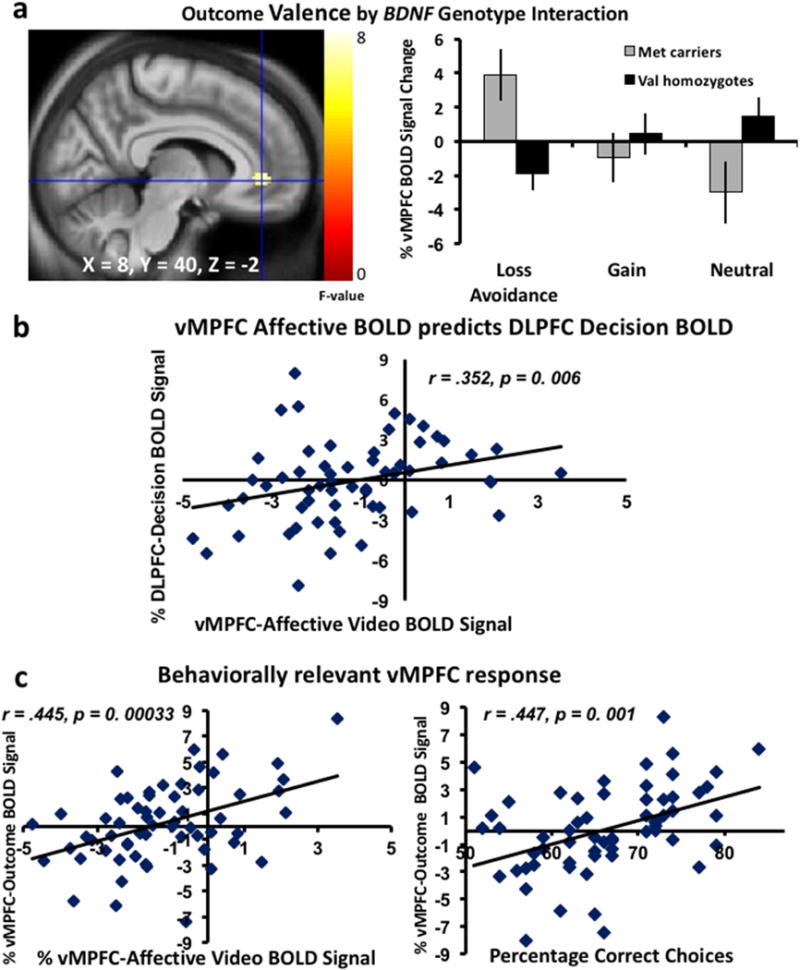
(a) Interaction between monetary outcome type and BDNF Val66Met genotype in vMPFC shown voxelwise and in a graph of values extracted from the a priori vMPFC ROI: BDNF Met carriers responded most robustly to loss/avoidance-of-loss outcomes and least to neutral outcomes, whereas the pattern was opposite in Val homozygotes (F2, 58 = 6.49, p < 0.002). (b) Scatter-plot illustrating the correlation between vMPFC BOLD responsivity to predictive affective cues and DLPFC BOLD responsivity to choice picture cues (r = .352, p = 0.006). (c) the left scatter-plot illustrates the correlation between the vMPFC BOLD response to predictive affective cues and the end-point outcome cues (r = .445, p = 0.00033). The right scatter-plot illustrates the correlation between vMPFC BOLD outcome cues and performance on picture choices (r = .417, p = 0.001). Color bars represent F-statistics; voxelwise images shown at p < 0.005 for display and X, Y, Z coordinates are in MNI space.
BDNF Val66Met Genotype Modulates BOLD Response to Outcome Experience
To better define the BDNF Val66Met polymorphism’s involvement in neural response to the monetary outcome of each trial, we modeled the BOLD response time window when individuals are presented with the monetary consequences/outcome of their choices (i.e., loss/avoidance-of-loss, gain/failure to gain, and no monetary incentive) using a factorial design with monetary outcome as a within-participants factor and BDNF Val66Met genotype as the between-groups factor at the whole brain level. We found a main effect of outcome in the bilateral amygdala and hippocampus, temporal sulcus, and the striatum (putamen, nucleus accumbens, and caudate) at p < 0.001 uncorrected, and FWE cluster-level corrected p = .723. Additionally, an interaction between outcome and BDNF Val66Met genotype was found in the right vMPFC region shown earlier to respond to predictive affective cues (Fig. 4a) at p < 0.001 uncorrected and FWE cluster-level corrected p = 0.982. These results should be taken with a cautionary note since they did not survive cluster-wise FWE correction.
BOLD signals extracted from our a priori-chosen vMPFC ROI were analyzed in a full factorial model, again using monetary outcome as a within-participants factor and BDNF Val66Met genotype as a between-groups factor. This analysis demonstrated a significant interaction that was characterized by a shift in BOLD response as a result of BDNF allelic variation such that Met carriers showed a positive vMPFC response to outcomes of loss-avoidance whereas the Val homozygotes responded with an average negative BOLD response to loss-avoidance outcomes; on the other hand, Met carriers responded with an average negative vMPFC BOLD response for gain and neutral outcomes relative to Val homozygotes and this was more pronounced for the neutral than gain outcomes (F2, 58 = 6.49, p = 0.002, Fig. 4a).
We further assessed the connectivity between this behaviorally relevant vMPFC signal response to outcome cues and the DLPFC and vMPFC responses to decision-making and predictive video viewing contexts, respectively. Using Spearman rank correlation, we found a positive correlation between vMPFC responses to predictive videos and outcome cues (r = .445, p = 0.00033, Fig 4c, left plot), but not between DLPFC response to decision-making and vMPFC response to outcome cues (r = 0.069, p = 0.599). Finally, we tested the relationship between overall performance (independent of valence) and the overall BOLD response of this extracted vMPFC ROI (independent of valence) during the predictive videos and the outcome phase. While we found no relationship between vMPFC BOLD response during observation of facial videos and performance (even though these signal values survived FWE cluster-wise multiple comparison correction at the second level), we found that vMPFC BOLD signals during outcome (a finding that did not survive FWE cluster-level correction) had a positive relationship with performance (r = .416, p = 0.001; Fig. 4c, right plot). The relationship was observed in both Val homozygotes and Met carriers (r=.331, p=0.017; and r=.531, p = 0.008, respectively). Together, these data suggest that the vMPFC response during outcome cues may be particularly relevant in coding prediction error signals at a time window when participants receive the monetary outcome/consequences of their choices in a given affective context.
DISCUSSION
While human perception and experience of emotions imbue an important adaptive value to environmental stimuli, and although impairment in adaptive emotional learning is an important aspect of neuropsychiatric disorders, experimental assessment of the neurogenetic correlates of complex human affective behavior remains a challenge in clinical neuroscience. We built upon neurogenetic insights from previous preclinical studies (Egan et al. 2003; Chen et al. 2006; Chhatwal et al. 2006; Heldt et al. 2007; Yu et al. 2009; Soliman et al. 2010; Milad & Quirk 2012) to guide our exploration of the impact of the BDNF Val66Met polymorphism ─ which has been associated with neuropsychiatric conditions ─ on brain function during affective contextual learning. We demonstrated that BDNF Val66Met genotype was associated with differential frontolimbic response, particularly involving the ventromedial prefrontal cortex (vMPFC) during adaptive, higher-order learning in healthy human adults.
We developed a novel three-step associative learning paradigm and found a valence-specific vMPFC BOLD response based on the level of contextual enrichment of the facial affective cues in that the signals became more negative when the facial cues predict decision-related outcomes. Surprisingly, this negative descent in vMPFC BOLD signaling in predictive contexts was more prominent for the neutral cues, a response pattern that is likely attributable to the ambiguous nature of the neutral cues. Moreover, we found a dissociable BDNF Val66Met polymorphism-associated BOLD response to predictive affective cues, decision-making, and monetary outcome cues that involve vMPFC and DLPFC. These convergent findings are relevant in light of BDNF’s role in mediating neural circuitry function during fear conditioning and learning (Chhatwal et al. 2006; Heldt et al. 2007; Milad & Quirk 2012; Penzo et al. 2015), in impaired extinction learning and fear generalization in both mice and humans (Chen et al. 2006; Soliman et al. 2010; Mühlberger et al. 2014), and in stress coping (Chen et al. 2006). While contextual learning in general, and the associative processes involved in such learning mechanisms, can recruit memory processes sufficiently even in non-affective contexts, our approach of including a higher-order socially relevant affective context in the current study was motivated by the idea that modern (post-hunter gatherer) human environmental adaptation is strongly rooted in socially relevant affective valuations. With that in mind, we used our novel paradigm to test the hypothesis that the BDNF Val66Met polymorphism would functionally impact frontolimbic regions supporting socially relevant aversive and appetitive affective contextual learning. In line with our predictions, the observed BDNF Val66Met genotype effect on vMPFC BOLD response to affective cues (in this case, facial expression videos) when those cues were predictive of decisions resulting in avoidance of aversive, or attainment of rewarding, outcomes was not seen when these same cues were context-free. Because this BDNF genotype effect appeared to be specific to the learning context when these cues predicted monetary outcomes, our findings cannot be attributed simply to viewing emotional faces per se, consistent with the proposed functional role of the vMPFC in mediating contextual learning (Phelps et al 2004; Milad & Quirk 2012).
We also assessed the role of the BDNF Val66Met polymorphism in influencing behavioral and neural response during decision-making as a function of decision difficulty, and we observed that variation in this genotype selectively influenced right DLPFC response when individuals were evaluating which pictures to choose in order to maximize monetary gain and minimize losses. Met carriers showed an increase in DLPFC BOLD response when the difficulty/decision load increased, whereas Val homozygotes exhibited a reduction in DLPFC BOLD response with increased choice difficulty. Of particular interest, this DLPFC region has been implicated in cognitive processes that selectively differentiate neuropsychiatric status (Eisenberg and Berman, 2010), as well as in decision-making and top-down guidance of memory, attention, executive functioning and thought (Wolf et al. 2015; Tan et al. 2012; Arnsten & Rubia 2012; Minzenberg et al. 2009; Lewis et al. 2005; Krawczyk 2002). Importantly, the degree of DLPFC response during decision-making correlated positively with the vMPFC response to the preceding affective videos and not with the same regional vMPFC response to the monetary outcome cues resulting from the decisions. Our results showing a BDNF Val66Met-modulated DLPFC BOLD coupling with the degree of decision-load, and a positive association between these signals and vMPFC response to predictive affective cues preceding the decision window, in a healthy cohort, identified a possible neurogenetic mechanism that may bias contextual adaptive behavior through a hierarchical prefrontal organization of function; this bias may enable the DLPFC to differentially exhibit a functional hegemony over downstream areas when attentional demands during difficult decision-making are high.
Our investigation of the neural response when individuals realized the monetary outcome of their decisions revealed that variation in the BDNF gene was associated with the reactivity of the same vMPFC region shown to signal higher-order predictive affective cues (facial videos). Of particular interest, this vMPFC response, at a time when individuals were encountering the monetary outcomes of their choices, correlated with both the same vMPFC area responsiveness to the predictive affective cues, and more importantly with how well they performed the affective contextual learning task. These correlations between (1) the vMPFC neural representations of viewing conspecific facial expressions that are predictive of negative and positive decision-based outcomes and same anatomical regional response during the actual experiencing of reward and loss-avoidance on the one hand, and (2) correlation of this vMPFC response during outcome experience and individual’s behavioral success in achieving those outcomes on the other, are consistent with the idea that emotions may operate, often covertly, to maximally optimize adaptive behavior (Seymour & Dolan 2008). We did not specially limit our statistical analyses to correct choices because the large majority (~70%) of the choices were correct, and because the time window within which decisions were made (correctly or erroneously) is shorter than the inherent temporal resolution of the BOLD signal, thus posing a challenge to selectively modeling the spatiotemporal BOLD response during correct choices. Together, our findings throughout this experimental paradigm suggest a critical role for the BDNF Val66Met polymorphism in prefrontal cortical circuitry mediation of higher-order affective learning in humans.
While our findings of BDNF effects were predominantly localized in the vMPFC and DLPFC, the hippocampus and amygdala are also of relevant functional interest to contextual learning and the memory coding aspects of learning in general. It is likely that our BOLD-related findings in the prefrontal cortex as mediated by the BDNF Val66Met genotype, are more proximate to higher-order end-point behaviors such as context-dependent memory recall in the vMPFC, and effortful attentional control and maintenance of learned response rules in the DLPFC during higher-order contextual learning. A putative mechanistic explanation of this localized prefrontal observation is that relative to the Val homozygotes, the prefrontal cortex of BDNF met carriers, especially in light of BDNF Met’s association with activity-dependent BDNF release, is likely to exhibit a more heightened prefrontal response cost towards achieving comparably optimal contextual learning performance. This notion is in line with our results showing BDNF Val66Met genotype effects on prefrontal tuning of contextual learning abilities and decision making in the absence of any explicit genotype difference in learning performance, as opposed to our findings of only main effects but no genetic influences on hippocampal and amygdala reactivity to stimulus valence and context.
Whether these BDNF-related prefrontal influences are human specific needs to be addressed in cross-species translational studies. Despite the lack of sensitivity of our experimental paradigm in showing BDNF Val66Met genotype effects on hippocampal and amygdala response to contextual cues, it is notable that a task-free resting state positron emission tomography study demonstrated increased regional hippocampal blood flow coupled with more robust cingulate-orbitofrontal cortical functional connectivity in BDNF Met carriers compared to Val homozygotes (Wei et al. 2012). That finding is in line with a study showing enhanced fractional anisotropy (a measure of white matter integrity) in prefrontal and visual pathways in BDNF Met carriers relative to Val homozygotes (Tost et al. 2013). Such BDNF-associated effects may also be influenced by environmental and developmental factors (Mitchell 2007). Indeed, reduced anterior cingulate grey matter volume has been reported in BDNF Met carriers with a history of adverse life experience compared with both Met carriers without and Val homozygotes with a history of adversity (Gerritsen et al. 2012). Future studies using improved spatiotemporally resolved imaging methods like magneto/electroencephalography may be necessary for more in depth millisecond-resolved delineation of genetic influences on contextually-, developmentally-, and environmentally-dependent sub-regional circuitry tuning of MPFC (Milad & Quirk 2012), hippocampus (Fanselow & Dong 2010), and amygdala (Felix-Ortiz et al. 2013) mediation of contextual decision making. Unlike the fMRI BOLD measures, such refined spatiotemporal methods like magnetoencephalography will likely be more suitable in specifying the neural response to correct choice behaviors in similar task paradigms without risking to juxtapose the neural response timing of the decision window and other task conditions.
Epistatic interactions between the BDNF Val66Met genotype and other genes should also be investigated in similar paradigms incorporating affective learning; as such, gene-gene effects have been demonstrated in other contexts. For example, the BDNF Met allele has been shown to interact with the short allelic variation (often viewed as risk allele) of the serotonin transporter gene in affecting the volume of the sub-genual cingulate (Pezawas et al. 2008, but see Notaras et al. 2015), a region encompassing our defined vMPFC region of interest in the current study. Studies of epistatic interactions between the BDNF Val66Met polymorphism and other genetic pathways, including those related to monoaminergic (Lobo et al. 2010; Graham et al. 2007; Berton et al. 2006; Martinowich & Lu 2008) and opioid function (Koo et al. 2012), will be particularly important (Krishnan & Nestler 2008).
In summary, genes code for molecular processes that directly or indirectly mediate how neurons and neural circuits adapt to influence complex behaviors (Rose & Donohue 2013; Jabbi et al. 2012), even in healthy populations, and we showed here that the BDNF Val66Met polymorphism influenced brain response repertoire in different facets of affective contextual learning in healthy adults. Our results of a differential BDNF Val66Met-modulated vMPFC and DLPFC response to predictive affective signals and cues representing decision difficulty, and the collective influence of those prediction and decision processes on brain-mediated monetary outcome, suggest a role for the BDNF Val66Met genotype in higher-order frontolimbic integration of how affective value of social signals are hierarchically coded to facilitate adaptive functioning.
Acknowledgments
We thank our colleagues at the NIH NMR center for their support; Martijn Jansma for guidance on processing16 channel head coil fMRI data; Shau-Ming Wei, Joel Bronstein, Christopher Coutlee, Gabriela Alarcon, Aarthi Padmanabhan, Molly Chalfin, James Zhang, Erik King and Anees Benferhat for their contributions to data and stimuli collection.
Funding: The Intramural Research Program of the National Institute of Mental Health, the National Institutes of Health, funded this work. ClinicalTrials.gov Identifier number: NCT00004571; Protocol ID number: 00-M-0085.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: M.J. conceived the study and wrote the manuscript. M.J. and P.K. programmed the experimental paradigm and designed the experiment, and P.K. contributed analytical tools. M.J., B.C., T.N., P.K., R.M., J.M., and J.S.K performed the experiments and analyzed the data. B.K. performed the genetic analysis. K.F.B. was involved in all aspects of this project. All authors discussed the experiments and contributed to the manuscript.
References
- Angelucci F, Brenè S, Mathé AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry. 2005;10:345–52. doi: 10.1038/sj.mp.4001637. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51:356–67. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. (Series B (Methodological)).Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- Berton O, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Chen Z, et al. Genetic variant BDNF (Val66Met) Polymorphism Alters Anxiety-Related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, et al. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbley JR, Friston KJ. False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage. 2009;44:62–70. doi: 10.1016/j.neuroimage.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Cordeira JW, et al. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J Neurosci. 2010;30:2533–41. doi: 10.1523/JNEUROSCI.5768-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, et al. The BDNF Val66Met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Eisenberg DP, Berman KF. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology. 2010;35:258–77. doi: 10.1038/npp.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DP, et al. Brain-derived neurotrophic factor (BDNF) Val(66)Met polymorphism differentially predicts hippocampal function in medication-free patients with schizophrenia. Mol Psychiatry. 2013;18:713–20. doi: 10.1038/mp.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–5. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen L, et al. BDNF Val66Met genotype modulates the effect of childhood adversity on subgenual ACC volume in healthy subjects. Mol Psychiatry. 2012;17:597–603. doi: 10.1038/mp.2011.51. [DOI] [PubMed] [Google Scholar]
- Graham DL, et al. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–37. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry. 2007;12:1079–88. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Casey BJ. Risk for anxiety and implications for treatment: developmental, environmental, and genetic factors governing fear regulation. Ann N Y Acad Sci. 2013;1304:1–13. doi: 10.1111/nyas.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, et al. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–70. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, et al. Midbrain presynaptic dopamine tone predicts sustained and transient neural response to emotional salience in humans: fMRI, MEG and FDOPA PET. Mol Psychiatry. 2013;18:4–6. doi: 10.1038/mp.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, et al. The Williams syndrome chromosome 7q11.23 hemideletion confers hypersocial, anxious personality coupled with altered insula structure and function. Proc Natl Acad Sci U S A. 2012;109:E860–6. doi: 10.1073/pnas.1114774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, et al. Convergent BOLD and Beta-Band Activity in Superior Temporal Sulcus and Frontolimbic Circuitry Underpins Human Emotion Cognition. Cerebral Cortex. 2015;25:1878–88. doi: 10.1093/cercor/bht427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, et al. Variation in the Williams syndrome GTF2I gene and anxiety proneness interactively affect prefrontal cortical response to aversive stimuli. Transl Psychiatry. 2015;18(5):e622. doi: 10.1038/tp.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–8. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Koo JW, et al. BDNF is a negative modulator of morphine action. Science. 2012;338:124–8. doi: 10.1126/science.1222265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision-making. Neurosci Biobehav Rev. 2002;26:631–64. doi: 10.1016/s0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The Molecular Neurobiology of Depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–24. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Likhtik E, et al. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 2014;17:106–13. doi: 10.1038/nn.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky RH, Marini AM. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann N Y Acad Sci. 2007;1122:130–143. doi: 10.1196/annals.1403.009. [DOI] [PubMed] [Google Scholar]
- Lobo MK, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–90. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2008;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, et al. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–22. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ. The genetics of brain wiring: from molecule to mind. PLoS Biol. 2007;4:113. doi: 10.1371/journal.pbio.0050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C, et al. The BDNF Val66Met polymorphism affects amygdala activity in response to emotional stimuli: Evidence from a genetic imaging study. NeuroImage. 2008;42:1554–1559. doi: 10.1016/j.neuroimage.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Mühlberger A, et al. The BDNF Val66Met polymorphism modulates the generalization of cued fear responses to a novel context. Neuropsychopharmacology. 2014;39:1187–95. doi: 10.1038/npp.2013.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves-Pereira M, et al. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. Am J Hum Genet. 2002;71:651–5. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaras M, Hill R, van den Buuse M. The BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: progress and controversy. Mol Psychiatry. 2015;20:916–30. doi: 10.1038/mp.2015.27. [DOI] [PubMed] [Google Scholar]
- Pattwell SS, et al. The BDNF Val66Met polymorphism impairs synaptic transmission and plasticity in the infralimbic medial prefrontal cortex. J Neurosci. 2012;32:2410–21. doi: 10.1523/JNEUROSCI.5205-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Penzo MA, et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature. 2015;519:455–9. doi: 10.1038/nature13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione M, et al. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;31:1042–5. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, et al. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–90. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, et al. Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression. Molecular Psychiatry. 2008;13:709–716. doi: 10.1038/mp.2008.32. [DOI] [PubMed] [Google Scholar]
- Phelps EA, et al. Extinction learning in humans: role of the amygdala and vMPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pröschel M, et al. Dinucleotide repeat polymorphism at the human gene for the brain-derived neurotrophic factor (BDNF) Hum Mol Genet. 1992;1:353. doi: 10.1093/hmg/1.5.353-a. [DOI] [PubMed] [Google Scholar]
- Sakata K, et al. Role of activity-dependent BDNF expression in hippocampal-prefrontal cortical regulation of behavioral perseverance. PNAS. 2013;110:15103–8. doi: 10.1073/pnas.1222872110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B, Dolan R. Emotion, decision-making, and the amygdala. Neuron. 2008;58:662–71. doi: 10.1016/j.neuron.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Soliman F, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HY, et al. Effective connectivity of AKT1-mediated dopaminergic working memory networks and pharmacogenetics of anti-dopaminergic treatment. Brain. 2012;135:1436–45. doi: 10.1093/brain/aws068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, et al. Effects of the BDNF Val66Met polymorphism on white matter microstructure in healthy adults. Neuropsychopharmacology. 2013;38:525–32. doi: 10.1038/npp.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Gaag C, Minderaa RB, Keysers C. The BOLD signal in the amygdala does not differentiate between dynamic facial expressions. Soc Cogn Affect Neurosci. 2007;2:93–103. doi: 10.1093/scan/nsm002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Winkel M, et al. Impact of variation in the BDNF gene on social stress sensitivity and the buffering impact of positive emotions: replication and extension of a gene-environment interaction. Eur Neuropsychopharmacol. 2014;24:930–8. doi: 10.1016/j.euroneuro.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Vargas-Perez H, et al. Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science. 2009;324:1732–4. doi: 10.1126/science.1168501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserstein RL, Lazar NA. The ASA’s statement on p-values: context, process, and purpose. The American Statistician. 2016;70:129–133. [Google Scholar]
- Wei SM, et al. Brain-derived neurotrophic factor Val66Met polymorphism affects resting regional cerebral blood flow and functional connectivity differentially in women versus men. J Neurosci. 2012;32:7074–81. doi: 10.1523/JNEUROSCI.5375-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DH, et al. Functional neuroimaging abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry. 2015;72:456–65. doi: 10.1001/jamapsychiatry.2014.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, et al. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J Neurosci. 2009;29:4056–64. doi: 10.1523/JNEUROSCI.5539-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


