Abstract
Background
Onychomycosis is one of the most prevalent fungal diseases in the general population. However, treatment is of limited effectiveness and must be administered for long periods of time. Systemic antifungal agents are associated with adverse effects.
Objective
We evaluated the clinical efficacy and safety of a 1,064-nm neodymium-doped yttrium aluminium garnet (Nd:YAG) laser with amorolfine nail lacquer to treat onychomycosis.
Methods
The 128 patients were randomly divided to 2 groups: 64 in the experimental group were treated with 1,064-nm Nd:YAG laser therapy and amorolfine nail lacquer; the other 64 were in a control group treated with topical amorolfine lacquer monotherapy. The laser treatment was 4 sessions at 4-week intervals and amorolfine lacquer was applied once a week for 16 weeks. Efficacy was assessed as response rate from standardized photographs with ImagePro®Plus (Media Cybernetics, Inc., USA) analysis, microscopic examination, and subjective evaluation.
Results
At 16 weeks, the experimental group showed a significantly higher cumulative cure rate than the control group (71.88% vs. 20.31%, p<0.0001). Clinical therapeutic effects were linked to patient satisfaction. The percent of “very satisfied” or “satisfied” responses was higher in the test group than the control group (81.25% vs. 23.44%). The treatment regimen was well tolerated, with transient discomfort observed in the test group.
Conclusion
The 1,064-nm Nd:YAG laser with amorolfine nail lacquer was effective and safe for treating onychomycosis. This therapy should be considered an alternative treatment, especially for patients with contraindications to systemic antifungal agents.
Keywords: Lasers, Onychomycosis, Therapeutics, Topical antifungal agent
INTRODUCTION
Onychomycosis is a common disorder with recalcitrant and recurrent fungal infection of the nails that accounts for 50%~60% of nail dystrophy1. Previous studies have estimated the prevalence of onychomycosis to be ranging from 1% to 8% in Europe and the United States2. Onychomycosis is not fatal but can cause pain, discomfort, and disfigurement and produce serious physical and occupational limitations, as well as reducing quality of life.
Treatments have advanced, including oral and topical medications. However, topical treatments are limited because they do not penetrate the nail deeply, so they generally do not cure fungal nail infections. Newer oral antifungal drugs terbinafine, fluconazole and itraconazole have replaced older therapies such as griseofulvin in the treatment of fungal nail infections. These medications are fairly safe, but they should not be taken by patients with liver disease or heart failure. Surgical approaches to fungal nail infection treatment include surgically or chemically removing the nail, but surgically removing the nail plate can cause pain, bleeding, infection and onychodystropy.
Laser systems in the near-infrared spectrum (780 nm~3,000 nm wavelength), which are commonly used in onychomycosis, exert their effect by direct heating of target tissues3. A recent systematic review found evidence about the effectiveness of laser treatment of onychomycosis is limited and of poor methodological quality4.
The purpose of this study was to evaluate the treatment of onychomycosis using a 1,064-nm neodymium-doped yttrium aluminium garnet (Nd:YAG) laser and amorolfine nail lacquer. To our best knowledge, this is the first report on the efficacy of combining a short-pulsed 1,064 nm Nd:YAG laser and amorolfine nail lacquer for the treatment of onychomycosis.
MATERIALS AND METHODS
Patients
One hundred and twenty-eight patients over age 19 with onychomycosis who visited the Department of Dermatology, Chung-Ang University Hospital, Seoul, Korea from May 2014 to March 2015 were enrolled to this prospective study. Before enrollment, patients were informed of study procedures and possible risks, benefits, and complications. Signed consent was obtained from each patient. Enrolled participants could freely terminate their participation at any time. The study protocol confirmed to the guidelines of the 1975 Declaration of Helsinki and the study was approved by the Chung-Ang University Hospital's medical ethics committee (IRB no. C2013097). Participants were excluded if they reported a history of photosensitivity, allergic reaction to amorolfine, other prediagnosed nail disorders such as nail psoriasis, pregnancy/breastfeeding, or undergoing any topical/systemic antifungal agents or laser treatments within 12 weeks before the study. Randomization was performed by block randomization method resulting in 1:1 ratio designation for case and control group, using SAS ver. 9.0 (SAS Institute, Cary, NC, USA).
Sample size
According to previously conducted clinical trial, presumed lesion-free area difference ratio and standard deviation were designated as 10% and 18, respectively. We assumed the effect size to be 0.5556, so minimum sample size for each group was calculated as 51 persons, considering the power to be 80%. Considering wastage rate as 20%, we enrolled 64 persons per group and total 128 patients were randomized.
Study treatment
The 128 patients were randomized to two groups that received either combined treatment or topical antifungal nail lacquer only. All patients were given 5% amorolfine nail lacquer once a week for 16 weeks. The test group also received four sessions of laser treatment using a 1,064 nm Nd:YAG laser (Pinpointe™ FootLaser®; Nuvolase, Inc., Chico, CA, USA). Laser treatment was applied by 4-week intervals. The laser was applied to the damaged area of the nail plate plus a 2-mm adjacent margin of unaffected area. Settings were: fluency 200 mJ/cm2, 30 ms pulse duration, 3 mm spot size and 1 Hz frequency.
There was no important change in methods after trial commencement or in clinical outcomes.
End points
A clinical photograph of the infected nail was taken at each visit using the same camera (10.0 megapixels, Canon EOS40D; Canon, Tokyo, Japan) with identical settings and lighting conditions. Response to treatment was objectively and independently assessed by two dermatologists using the clinical photographs and ImagePro®Plus software (Media Cybernetics, Inc., Rockville, MD, USA). Direct microscopic examination was performed using potassium hydroxide (KOH) and fungal culture at baseline and at the end of the study.
Primary treatment efficacy was determined by comparing the infected area at baseline and 16 weeks' changes in lesion free area (%). Second treatment efficacy was determined as cumulative cure rate at 16 weeks, mycological cure rate, changes of infected areas at 4, 8, and 12 weeks, and participant satisfaction scores. Cumulative cure rate was evaluated as the achievement of clinically normal nail or negative mycology with less than 10% involvement of nail plate. Mycological cure was assigned when the results both in KOH smear and fungal culture were negative. At the end of the study, patients documented their degree of satisfaction using a 5-point Likert scale. Patients were also asked to report treatment side effects and discomfort as 0 to 5 points at every visit.
Statistical analysis
Data were analyzed using the Wilcoxon's rank sum test, Pearson's chi-square test and Fisher's exact test. p-values of less than 0.05 were considered to be significant.
RESULTS
Study population
Demographic and clinical characteristics were similar between the two groups with no significant differences between groups in age, sex or body mass index (Table 1, 2). In both groups, treatment was more than 90% for big-toe nails and distolateral subungual onychomycosis type. All treated nails had a thickness of less than 2 mm with discoloration. Nail brittleness and spike were observed in more than 70% in both groups. All participants showed positive KOH results and positive culture rates were 96.88% in the test group and 100% in the control group.
Table 1. Demographics and baseline characteristics of the population.
| Characteristic | Case (n=64) | Control (n=64) | p-value | |
|---|---|---|---|---|
| Male (n, %) | 35 (54.7) | 39 (60.9) | 0.4741* | |
| Female (n, %) | 29 (45.3) | 25 (39.1) | ||
| Height (cm) | Mean±SD | 169.75±9.17 | 168.89±7.31 | 0.5587† |
| Median | 167.50 | 170.00 | ||
| Min, max | 153.00, 188.00 | 155.00, 186.00 | ||
| Weight (kg) | Mean±SD | 68.72±11.53 | 65.30±11.05 | 0.0890† |
| Median | 68.50 | 65.00 | ||
| Min, max | 46.00, 95.00 | 44.00, 91.00 | ||
| Body mass index (kg/m2) | Mean±SD | 23.75±2.82 | 22.81±3.05 | 0.0718† |
| Median | 23.45 | 22.79 | ||
| Min, max | 18.37, 32.49 | 17.06, 30.41 | ||
SD: standard deviation. *Pearson's chi-square test, †two sample t-test.
Table 2. Past medical history of the population.
| Past medical history | Case (n=64) | Control (n=64) | ||
|---|---|---|---|---|
| n* | Count | n* | Count | |
| Infections and infestations | 0 (0.0) | 0 | 1 (1.6) | 1 |
| Endocardidis | 0 (0.0) | 0 | 1 (1.6) | 1 |
| Renal and urinary disorders | 1 (1.6) | 1 | 0 (0.0) | 0 |
| Calculus injury | 1 (1.6) | 1 | 0 (0.0) | 0 |
| Surgical and mecial procedures | 0 (0.0) | 0 | 1 (1.6) | 1 |
| Heart valve operation | 0 (0.0) | 0 | 1 (1.6) | 1 |
| Total | 1 (1.6) | 1 | 1 (1.6) | 2 |
*Count redundancy.
Cultures revealed 42.2% Trichophyton mentagrophytes, 35.9% Trichophyton rubrum, 12.5% Candida albicans, 4.7% Epidermophyton floccosum and 1.6% Fusarium species in 64 test group samples. In the control group, T. rubrum was the most common at 65.6%, followed by 17.2% T. mentagrophytes, 15.6% C. albicans and 1.6% E. floccosum (Table 3).
Table 3. Mycological evaluation.
| Method | Case (n=64) | Control (n=64) | p-value |
|---|---|---|---|
| KOH smear | - | ||
| Positive | 64 (100.0) | 64 (100.0) | |
| Negative | 0 (0.0) | 0 (0.0) | |
| KOH culture | 0.4961* | ||
| Positive | 62 (96.9) | 64 (100.0) | |
| Negative | 2 (3.1) | 0 (0.0) | |
| Species | |||
| Dermatophyte fungi | |||
| Epidermophyton flocosum | 3 (4.7) | 1 (1.6) | |
| Trichophyton mentagrophytes | 27 (42.2) | 11 (17.2) | |
| Trichophyton rubrum | 23 (35.9) | 42 (65.6) | |
| Nondermatophyte fungi | |||
| Acremonium species | 0 (0.0) | 0 (0.0) | |
| Alternaria species | 0 (0.0) | 0 (0.0) | |
| Aspergillus species | 0 (0.0) | 0 (0.0) | |
| Botryodiplodia theobromae | 0 (0.0) | 0 (0.0) | |
| Fusarium species | 1 (1.6) | 0 (0.0) | |
| Onycochola canadensis | 0 (0.0) | 0 (0.0) | |
| Pyrenochaeta unguis-hominis | 0 (0.0) | 0 (0.0) | |
| Scopulariopsis brevicaulis | 0 (0.0) | 0 (0.0) | |
| Scytalidium dimidiatum | 0 (0.0) | 0 (0.0) | |
| Scopulariopsis species | 0 (0.0) | 0 (0.0) | |
| Scytalidium hyalimum | 0 (0.0) | 0 (0.0) | |
| Yeasts | |||
| Candida albicans | 8 (12.5) | 10 (15.6) | |
| Candida parapsilosis | 0 (0.0) | 0 (0.0) | |
| Candida tropicalis | 0 (0.0) | 0 (0.0) | |
| Others | 0 (0.0) | 0 (0.0) |
Values are presented as number (%). *Fisher's exact test.
Efficacy
Changes in lesion-free area were higher in the test group than the control group (33.63%±28.23% vs. 23.46%±21.81%). However, no significant statistical difference was detected (p=0.0705). (Table 4). The cumulative cure rate was significantly higher for the test group than the control group (71.88% vs. 20.31%, p<0.0001) (Table 5).
Table 4. Lesion-free area of toenail: baseline, 16-week.
| Case (n=64) | Control (n=64) | p-value | ||
|---|---|---|---|---|
| Baseline | Mean±SD | 40.76±27.63 | 43.13±27.33 | |
| Median | 34.66 | 46.49 | ||
| Min, max | 1.30, 86.82 | 0.66, 91.25 | ||
| 16 weeks | Mean±SD | 74.39±24.67 | 66.59±24.56 | |
| Median | 81.26 | 70.65 | ||
| Min, max | 1.85,100.00 | 1.40, 100.00 | ||
| Difference | Mean±SD | 33.63±28.23 | 23.46±21.81 | 0.0705* |
| Median | 23.43 | 17.00 | ||
| Min, max | −6.11, 98.56 | −15.21, 76.24 | ||
SD: standard deviation. *Two-sample t-test.
Table 5. Sixteen weeks cumulative cure rate.
| Cumulative cure rate | Case (n=64) | Control (n=64) | p-value |
|---|---|---|---|
| Not cured | 18 (28.1) | 51 (79.7) | <0.0001* |
| Complete remission | 46 (71.9) | 13 (20.3) |
Values are presented as number (%). *Pearson's chi-square test.
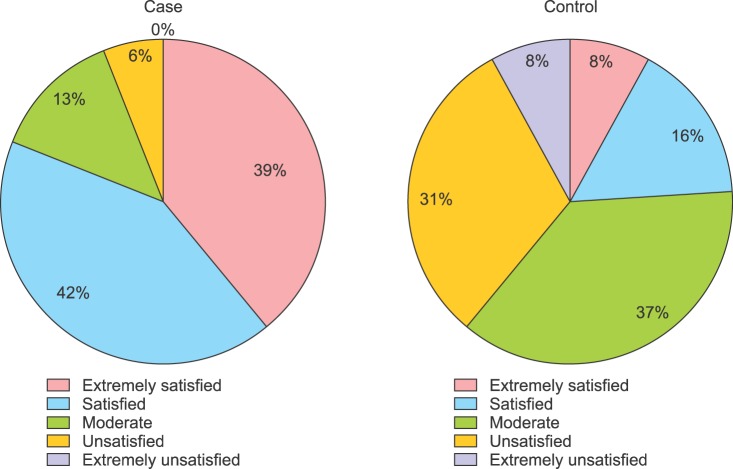
At week 16, 81.25% of test patients gave “satisfied” or “very satisfied” responses while 23.44% of control patients gave those responses. Participant satisfaction analysis revealed significance differences between the two groups (p<0.0001) (Fig. 1). Although some patients experienced mild pain and discomfort during laser treatment, treatments were well tolerated by most patients. Representative cases in combination therapy group and control group are presented respectively in Fig. 2, 3.
Fig. 1. Patient satisfaction scoring ratio.
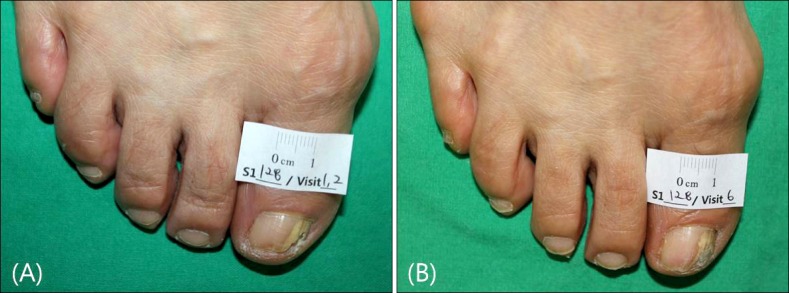
Fig. 2. Representative case of combination therapy group. (A) Onychomycotic nail of big toe, on the first visit before treatment. (B) 16 weeks after starting laser treatment. Marked improvement of nail color and texture.
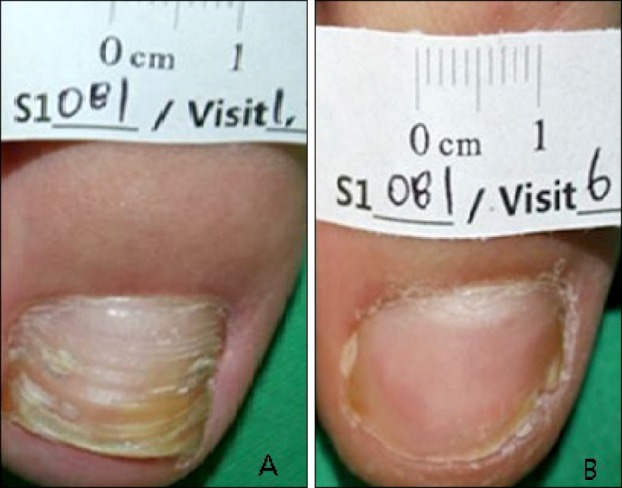
Fig. 3. Representative case of control group. (A) Onychomycotic nails of big toe, on the first visit before treatment. (B) Sixteen weeks after starting nail lacquer treatment. Subtle change in nail color and thickness.
Limitation
The biggest limitation of our study was the follow-up period of 16 weeks. Clinical outcome including recurrence rate may have been different if we had set up longer endpoint. However, our result still shows the tendency of improvement through combination treatment. Another limitation is that we did not establish an onset date for onychomycosis, which may be a relevant confounder.
DISCUSSION
Because lasers are minimally invasive and have the potential to restore clear nail growth, they have become a popular option for treatment of onychomycosis for both physicians and patients. A study evaluating the safety and efficacy of the 1,064 nm Nd:YAG laser for onychomycosis treatment showed 79% of toenails had improved clear, linear nail growth. Of 14 toenails, 11 had improved clear, linear nail growth ranging from 2.1 to 6.1 mm of nail bed clearing at 90 days after a single treatment5. A larger study with 1,064 nm Nd:YAG lasers evaluated two different treatment regimens for 154 nails from 33 patients with onychomycosis: 78 nails were treated with 8 weekly sessions and 76 were treated with 4 weekly sessions. At 24 weeks, improvement was 51% with 8 weekly treatments and 53% with 46. Some reports found no significant mycological culture or clinical nail plate clearance using 1,064 nm Nd:YAG laser7,8. Although in vitro studies of the Nd:YAG laser system yielded conflicting results9,10,11, clinical studies have shown some promising results. Laser therapy combined with other treatments might produce more rapid and effective mycological and clinical clearance12,13. In our study, lesion-free areas increased in both groups with more treatments and longer duration. Differences between the two groups was not observed at initial treatment, but lesion-free area was greater in the test group than the control group at 12 weeks after 3 laser treatments (p=0.0655 at 12 weeks; p=0.0705 at 16 weeks). The change in lesion-free area was 10% higher in the laser and nail lacquer combined-therapy group compared to the nail lacquer-only group (33.63%±28.23% vs. 23.46%±21.81%). This is similar to the 11.3% changes in lesion-free area at 24 weeks seen in previous experiments with only laser treatment (unpublished data). The 10% difference is thought to be clinically significant, considering the average lesion area at baseline in both groups was about 45%~50% in this trial.
The cure rate of 71.88% in the laser and nail lacquer combined-therapy group at 16 weeks was significantly higher than the 20.31% in the nail lacquer monotherapy group. This result confirmed the effectiveness of combination therapy. In addition, when compared with results in a meta-analysis of systemic antifungal therapies for onychomycosis, the cure rate of the laser and nail lacquer combined-therapy in this study was similar to the 60%~76% for systemic antifungal therapies such as terbinafine and itraconazole14. Clinical therapeutic effects were linked to patient satisfaction and the percent of “very satisfied” and “satisfied” responses was higher for the test group than the control group (81.25% vs. 23.44%).
Some participants experienced discomfort noted in evaluation after each visit, but this was found to be mild. Also, discomfort did not increase with increased laser times and no discomfort was reported after laser treatment was finished. This result confirmed that the discomfort was a temporary symptom and patients adapted to the laser procedure.
Amorolfine 5% nail lacquer interferes with the production of ergosterol in the fungal cell membrane by inhibiting 14-α reductase and 7, 8 isomerase, resulting in broad-spectrum fungistatic activity15,16. Topical therapy is considered a treatment alternative for mild-to-moderate disease, especially for patients unwilling or unable to receive or afford oral medications. However, topical drugs are generally not effective against onychomycosis because of their inability to penetrate the entire nail plate to eradicate the infection. Although numerous studies have shown high cure rates for onychomycosis with new oral antifungals, treatment failures and recurrences are not uncommon in clinical practice. A more careful interpretation of recent data revealed that the total cure rates for onychomycosis with terbinafine or itraconazole monotherapy are not as high as in previous studies. The failure rate 35%~50% for terbinafine and 25%~40% for itraconazole for producing a disease-free nail17.
Although the mechanism of laser treatment is still not clear, some clinical effects have been seen for onychomycosis in previous studies18,19. Some researchers have suggested that the photothermolytic effect of heating the infected nail and the fungus may result in healing of onychomycosis. Hees et al.20 has reported the stimulation of local immunologic reaction through vasodilation by nonspecific heating of tissues contribute to the improvement. Other hypothesis is that the laser induces free radical formation and activates cellular metabolic processes21.
Also, it is assumed that different species of fungi may show inconsistent response to laser treatment. Several in vitro studies to date indicate variable results regarding growth inhibition of certain fungal species in culture under laser irradiation10,11,20. For example, Paasch et al.22 have reported that laser treatment can stimulate the growth of T. rubrum while Xu et al.11 have indicated that long-pulsed Nd:YAG 1,064-nm laser can effectively inhibit the growth of T. rubrum. Therefore, it is difficult to define the fungicidal effect of independent laser treatment in onychomycosis currently. By applying amorolfine nail lacquer showing broad spectrum antifungal effect together with Nd:YAG laser, we can expect the improvement of onychomycosis23.
In our clinical trial, using changes in lesion-free area as the primary endpoint showed borderline significance. Using cure rate as a key secondary endpoint showed highly significant results in the test group compared to the control group. Although cumulative cure rate, change of infected area and patient satisfaction are not standard evaluation methods for onychomycosis therapy, we could suggest a positive trend of treatment response. Therefore, laser/nail lacquer combined therapy is thought to be an effective treatment compared with nail lacquer monotherapy. Combined therapy might be used as an active local treatment, especially in patients who cannot be treated with systemic agents. For safety, the mild warmth, pain and skin irritation were similar to previous studies. More serious or unexpected adverse reactions did not occur.
Treatment failures and relapses are not uncommon for onychomycosis, even with systemic therapy. Therefore, adjuvant measures or combination therapies should be considered to improve efficacy and reduce treatment duration and the possible serious side effects of systemic therapy.
Compared with topical amorolfine nail lacquer alone, combination with 1,064 nm Nd:YAG laser treatment achieved greater mycological cure and increased the total cure rate. Combination treatment with 1,064 nm Nd:YAG laser and amorolfine nail lacquer was safe and effective for onychomycosis, with high patient satisfaction. Based on our results, the addition of 1,064 nm Nd:YAG laser treatment to topical amorolfine nail lacquer therapy was a valuable way to treat onychomycosis.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Gupta AK, Jain HC, Lynde CW, Macdonald P, Cooper EA, Summerbell RC. Prevalence and epidemiology of onychomycosis in patients visiting physicians' offices: a multicenter canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43:244–248. doi: 10.1067/mjd.2000.104794. [DOI] [PubMed] [Google Scholar]
- 2.Sigurgeirsson B, Baran R. The prevalence of onychomycosis in the global population: a literature study. J Eur Acad Dermatol Venereol. 2014;28:1480–1491. doi: 10.1111/jdv.12323. [DOI] [PubMed] [Google Scholar]
- 3.Nenoff P, Grunewald S, Paasch U. Laser therapy of onychomycosis. J Dtsch Dermatol Ges. 2014;12:33–38. doi: 10.1111/ddg.12251. [DOI] [PubMed] [Google Scholar]
- 4.Ledon JA, Savas J, Franca K, Chacon A, Nouri K. Laser and light therapy for onychomycosis: a systematic review. Lasers Med Sci. 2014;29:823–829. doi: 10.1007/s10103-012-1232-y. [DOI] [PubMed] [Google Scholar]
- 5.Harris DM, McDowell BA, Strisower J. Laser treatment for toenail fungus. Proc SPIE Int Soc Opt Eng. 2009;7161:1–7. [Google Scholar]
- 6.Zhang RN, Wang DK, Zhuo FL, Duan XH, Zhang XY, Zhao JY. Long-pulse Nd:YAG 1064-nm laser treatment for onychomycosis. Chin Med J (Engl) 2012;125:3288–3291. [PubMed] [Google Scholar]
- 7.Hollmig ST, Rahman Z, Henderson MT, Rotatori RM, Gladstone H, Tang JY. Lack of efficacy with 1064-nm neodymium: yttrium-aluminum-garnet laser for the treatment of onychomycosis: a randomized, controlled trial. J Am Acad Dermatol. 2014;70:911–917. doi: 10.1016/j.jaad.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Carney C, Cantrell W, Warner J, Elewski B. Treatment of onychomycosis using a submillisecond 1064-nm neodymium: yttrium-aluminum-garnet laser. J Am Acad Dermatol. 2013;69:578–582. doi: 10.1016/j.jaad.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 9.Meral G, Tasar F, Kocagöz S, Sener C. Factors affecting the antibacterial effects of Nd:YAG laser in vivo. Lasers Surg Med. 2003;32:197–202. doi: 10.1002/lsm.10128. [DOI] [PubMed] [Google Scholar]
- 10.Vural E, Winfield HL, Shingleton AW, Horn TD, Shafirstein G. The effects of laser irradiation on Trichophyton rubrum growth. Lasers Med Sci. 2008;23:349–353. doi: 10.1007/s10103-007-0492-4. [DOI] [PubMed] [Google Scholar]
- 11.Xu ZL, Xu J, Zhuo FL, Wang L, Xu W, Xu Y, et al. Effects of laser irradiation on Trichophyton rubrum growth and ultrastructure. Chin Med J (Engl) 2012;125:3697–3700. [PubMed] [Google Scholar]
- 12.Xu Y, Miao X, Zhou B, Luo D. Combined oral terbinafine and long-pulsed 1,064-nm Nd: YAG laser treatment is more effective for onychomycosis than either treatment alone. Dermatol Surg. 2014;40:1201–1207. doi: 10.1097/DSS.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 13.Lim EH, Kim HR, Park YO, Lee Y, Seo YJ, Kim CD, et al. Toenail onychomycosis treated with a fractional carbondioxide laser and topical antifungal cream. J Am Acad Dermatol. 2014;70:918–923. doi: 10.1016/j.jaad.2014.01.893. [DOI] [PubMed] [Google Scholar]
- 14.Gupta AK, Ryder JE, Johnson AM. Cumulative meta-analysis of systemic antifungal agents for the treatment of onychomycosis. Br J Dermatol. 2004;150:537–544. doi: 10.1046/j.1365-2133.2003.05728.x. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz RA, Janniger CK. Onychomycosis. Cutis. 1996;57:67–74. 80–81. [PubMed] [Google Scholar]
- 16.Borgers M. Mechanism of action of antifungal drugs, with special reference to the imidazole derivatives. Rev Infect Dis. 1980;2:520–534. doi: 10.1093/clinids/2.4.520. [DOI] [PubMed] [Google Scholar]
- 17.Reinel D. Topical treatment of onychomycosis with amorolfine 5% nail lacquer: comparative efficacy and tolerability of once and twice weekly use. Dermatology. 1992;184(Suppl 1):21–24. doi: 10.1159/000247612. [DOI] [PubMed] [Google Scholar]
- 18.Helou J, Maatouk I, Hajjar MA, Moutran R. Evaluation of Nd:YAG laser device efficacy on onychomycosis: a case series of 30 patients. Mycoses. 2016;59:7–11. doi: 10.1111/myc.12425. [DOI] [PubMed] [Google Scholar]
- 19.El-Tatawy RA, Abd El-Naby NM, El-Hawary EE, Talaat RA. A comparative clinical and mycological study of Nd-YAG laser versus topical terbinafine in the treatment of onychomycosis. J Dermatolog Treat. 2015;26:461–464. doi: 10.3109/09546634.2014.998607. [DOI] [PubMed] [Google Scholar]
- 20.Hees H, Raulin C, Bäumler W. Laser treatment of onychomycosis: an in vitro pilot study. J Dtsch Dermatol Ges. 2012;10:913–918. doi: 10.1111/j.1610-0387.2012.07997.x. [DOI] [PubMed] [Google Scholar]
- 21.Manevitch Z, Lev D, Hochberg M, Palhan M, Lewis A, Enk CD. Direct antifungal effect of femtosecond laser on Trichophyton rubrum onychomycosis. Photochem Photobiol. 2010;86:476–479. doi: 10.1111/j.1751-1097.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- 22.Paasch U, Mock A, Grunewald S, Bodendorf MO, Kendler M, Seitz AT, et al. Antifungal efficacy of lasers against dermatophytes and yeasts in vitro. Int J Hyperthermia. 2013;29:544–550. doi: 10.3109/02656736.2013.823672. [DOI] [PubMed] [Google Scholar]
- 23.Li RY, Wan Z, Wang AP, Shen YN, Lu CM, Li M, et al. In vitro susceptibility testing of amorolfine in pathogenic fungi isolated from dermatomycosis patients in China. Mycoses. 2004;47:402–406. doi: 10.1111/j.1439-0507.2004.01014.x. [DOI] [PubMed] [Google Scholar]