Abstract
Background
Palmar hyperhidrosis is a common disorder of excessive sweating. A number of studies have demonstrated the effectiveness of iontophoresis in the treatment of palmar hyperhidrosis. However, controlled clinical studies on iontophoresis for palmar hyperhidrosis have been limited.
Objective
To determine the efficacy and safety of iontophoresis in the treatment of palmar hyperhidrosis with a randomized, sham-controlled, single-blind, and parallel-designed study.
Methods
Twenty nine patients with significant palmar hyperhidrosis were enrolled in this study. They received active iontophoresis treatment (group A) or sham treatment (group B). Iontophoresis was performed 20 minutes each time, five times per week, for 2 weeks. Its efficacy was assessed with starch-iodine test, mean sweat secretion rate, and hyperhidrosis disease severity scale.
Results
Twenty-seven of the 29 patients completed the 2-week treatment. After completion of 10 times of treatment, results of the starch-iodine test showed clinical improvement in 92.9% of patients in group A and 38.5% of patients in group B (p=0.001). The mean sweat secretion rate was reduced by 91.8% of patients in group A and by 39.1% of patients in group B (p<0.001). Improvement in quality of life was reported by 78.6% of patients in group A and by 30.8% of patients in group B (p=0.028). In group A, one case of localized adverse event was noted, although no adverse event was encountered in group B.
Conclusion
Tap water iontophoresis could be used as an effective and safe treatment modality for palmar hyperhidrosis.
Keywords: Hyperhidrosis, Iontophoresis
INTRODUCTION
Hyperhidrosis is defined as perspiration in excess of physiologic amount necessary to maintain thermal homeostasis. Hyperhidrosis might be a primary condition or secondary condition due to medications or systemic disorders. Primary hyperhidrosis is usually focal, bilateral, and symmetric. It mainly affects the axillae, palms, soles, face, and scalp. The incidence of primary hyperhidrosis has been reported to be between 0.6%~2.8% in both genders1. Symptoms usually begin in childhood or around puberty. It interferes with daily social and occupational activities of patients and significantly impacts their quality of life2. In order to assess the degree of impairment, various types of subjective and objective measurements have been developed. Minor's starch-iodine test is a simple test that can be used to measure the approximate volume of sweat production1. Gravimetric measurement is useful for quantifying the amount of sweat3. To evaluate the effect of hyperhidrosis on patient' life, hyperhidrosis disease severity scale (HDSS) has been reported4.
Various treatments have been proposed to control primary hyperhidrosis. The treatment options for hyperhidrosis include topical therapy, anticholinergics, iontophoresis, botulinum toxin and surgery. Based on that aluminum salt is thought to block the distal eccrine sweat gland duct, 20%~25% aluminum chloride in ethanol is the most commonly used for mild to moderate hyperhidrosis of palms and axillae. Its continued use is often limited by skin irritation1. Anticholinergic agents inhibit the acetylcholine induced activation of eccrine sweat glands. Oral anticholinergic drugs, such as glycopyrrolate and oxybutynin, have been been regarded as somewhat effective for primary multifocal hyperhidrosis. However, the use of these drugs is frequently limited by systemic side effects, including dry mouth, dry eyes, difficulty in urination, constipation, blurred vision, drowsiness, dizziness and mydriasis3. Recently, topical formulation of anticholinergic agents are being investigated and may provide efficacy with fewer systemic adverse effects5,6. Botulinum toxin, administered by local intradermal injection, blocks acetylcholine release from the cholinergic nerves supplying the sweat glands. Although botulinum toxin has been widely used in the treatment of hyperhidrosis because of its well established efficacy, the use of botulinum toxin is often limited by its relative high cost and the discomfort associated with multiple needle injection7. In terms of stopping perspiration, surgical treatment including endoscopic thoracic sympathectomy (ETS) can be an effective option for primary hyperhidrosis. However, compensatory hyperhidrosis occurs in 50%~70% of patients with 1%~2% of severe cases7. ETS has been recommended to use for patients diagnosed with severe hyperhidrosis refractory to conservative therapy8.
In addition to these treatment options for hyperhidrosis, several medical devices delivering energy to destroy sweat glands are undergoing promising clinical investigation. Microwave technology, several lasers (such as 1,064-nm, 1,320-nm, and 1,440-nm neodymium:yttrium-aluminum-garnet laser, 924-nm and 975-nm diode laser, long-pulsed 800-nm diode laser), microneedle radiofrequency, and ultrasound technology could be included as example of the device8.
A number of studies have demonstrated the efficacy of iontophoresis treatment for hyperhidrosis9,10,11,12,13,14. In iontophoresis, an electromotive force is used to enhance percutaneous absorption of a drug or chemical in forms of ions on the skin. Although the exact mechanisms by which iontophoresis reduces sweating remain unclear, some hypotheses have been proposed, including sweat gland pore obstruction secondary to hyperkeratinization15, impairment of the electrochemical gradient of sweat16,17, and increased stimulus threshold of the sympathetic nervous system18. However, controlled studies are limited. Therefore, the objective of this study was to determine the effectiveness and safety of iontophoresis treatment for hyperhidrosis with a randomized, sham-controlled, single-blind, and parallel-designed study.
MATERIALS AND METHODS
Study population
Healthy volunteers with significant palmar hyperhidrosis were recruited through an advertisement in a local newspaper. Diagnosis of hyperhidrosis was made based on starch-iodine test. Patients who were pregnant or lactating, who had metal implants such as a pacemaker, who had history of ischemic heart disease or arrhythmias, who had any other diseases that could be the cause of secondary hyperhidrosis, and those who were older than 60 years old or younger than 13 years old were excluded.
Study design
This study was a single-center, randomized, sham-controlled, single-blind, and parallel-designed clinical trial. The study protocol was approved by the Institutional Review Board of Dongguk University Ilsan Hospital (IRB no. 2014-1). After obtaining informed written consent from all participants, the study was conducted in accordance with the Declaration of Helsinki principles.
Intervention
Patients were randomly assigned into either active treatment group or sham treatment group at a 1:1 ratio. Iontophoresis was performed with a single device (Hidro-X®; Medilight, Yongin, Korea) which allowed to select conventional or pulsating direct current mode. It has been reported that the pulsating direct current iontophoresis is less effective than the conventional one18,19. Therefore, the conventional direct current was used for patients in the active treatment group (group A) while low intensity pulsating direct current was used for patients in the sham treatment group (group B). Iontophoresis treatment was administered for 20 minutes each time, five times a week, for 2 weeks. To administer the treatment, both hands were immersed in pronated position in a tray filled with tap water containing electrode pads. Electrode pads were covered with a plastic grill to protect skin from burns. Patients were blinded throughout the study concerning treatment assignments.
Efficacy assessment
The severity of hyperhidrosis was assessed objectively and subjectively at baseline (week 0), after 10 times of treatment (week 2), and 1 week (week 3), 2 weeks (week 4), and 4 weeks (week 6) after completing the treatment. Objective assessment consisted of colorimetric and gravimetric measurements. The colorimetric measurement was evaluated using starch-iodine test. Briefly, after washing both hands and drying, 2% iodine solution was applied onto the palms and starch powder was sprayed. The result of starch-iodine test was graded according to the degree of sweating11. Gravimetric measurement was performed by using filter papers. They were weighted before and after having contact with the palm for 5 minutes in order to measure the volume of sweat absorbed. The weight difference was used to calculate the amount of sweat produced per minutes (mg/min)3. These assessments were conducted in a private and quiet room with temperature of 20℃~25℃ and humidity of 40%~60%. Subjective assessment was performed by asking patients to answer HDSS questionnaire in a scale of 1 to 4 (see Table 1).
Table 1. Baseline demographic and clinical characteristics of the 27 patients who completed the study.
| Characteristic | Group A (n=14) | Group B (n=13) | p-value |
|---|---|---|---|
| Sex | 0.128* | ||
| Male | 10 (71.4) | 5 (38.5) | |
| Female | 4 (28.6) | 8 (61.5) | |
| Age (yr) | 30.2±11.7 | 29.5±11.4 | 0.881 |
| Body weight (kg) | 66.4±16.3 | 61.4±10.5 | 0.362 |
| Body height (cm) | 170.1±11.2 | 166.7±7.6 | 0.371 |
| Starch-iodine test | 0.326* | ||
| Grade 3 | 10 (71.4) | 12 (92.3) | |
| Grade 4 | 4 (28.6) | 1 (7.7) | |
| Sweat secretion rates (mg/min) | 35.2±9.6 | 33.8±8.3 | 0.571 |
| HDSS | 0.159† | ||
| Grade 2 | 2 | 0 | |
| Grade 3 | 9 | 8 | |
| Grade 4 | 3 | 5 |
Values are presented as number (%) or mean±standard deviation. Starch-iodine test; grade 1: no reaction, grade 2: mild discoloration, grade 3: moderate discoloration, grade 4: severe discoloration. HDSS: hyperhidrosis disease severity scale; grade 1: my sweating is never noticeable and never interferes with my daily activities, grade 2: my sweating is tolerable but sometimes interferes with my daily activities, grade 3: my sweating is barely tolerable and frequently interferes with my daily activities, grade 4: my sweating is intolerable and always interferes with my daily activities. *Fisher's exact test, t-test, †linear by linear association.
Safety assessment
After each iontophoresis treatment prior to the next, patients were assessed for any local or systemic adverse events such as erythema, dryness, and irritation.
Statistical analysis
Data were analyzed using SAS 9.3 (SAS Inc., Cary, NC, USA). Statistical analysis was performed using the Mann-Whitney test and t-test. Statistical significance was considered when p-value was less than 0.05. To account for the multiple testing, we applied the Bonferroni correction.
RESULTS
Patient characteristics
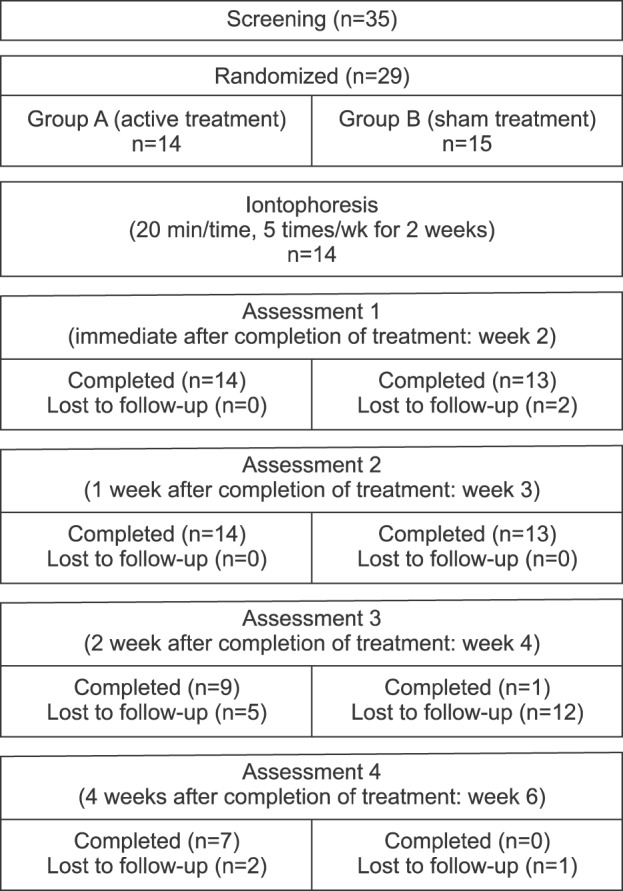
Thirty-five participants were initially screened. After excluding six patients who met the exclusion criteria, a total of 29 patients were enrolled in the study. Fourteen patients were randomly allocated to group A and 15 patients were assigned to group B. A total of 27 patients (14 in group A and 13 in group B) completed the study (Fig. 1). The demographic and clinical characteristics of these 27 patients who completed the study are summarized in Table 1. No difference in baseline demographic or clinical characteristics was observed between the two groups.
Fig. 1. Flow chart showing the number of patients used in this study.
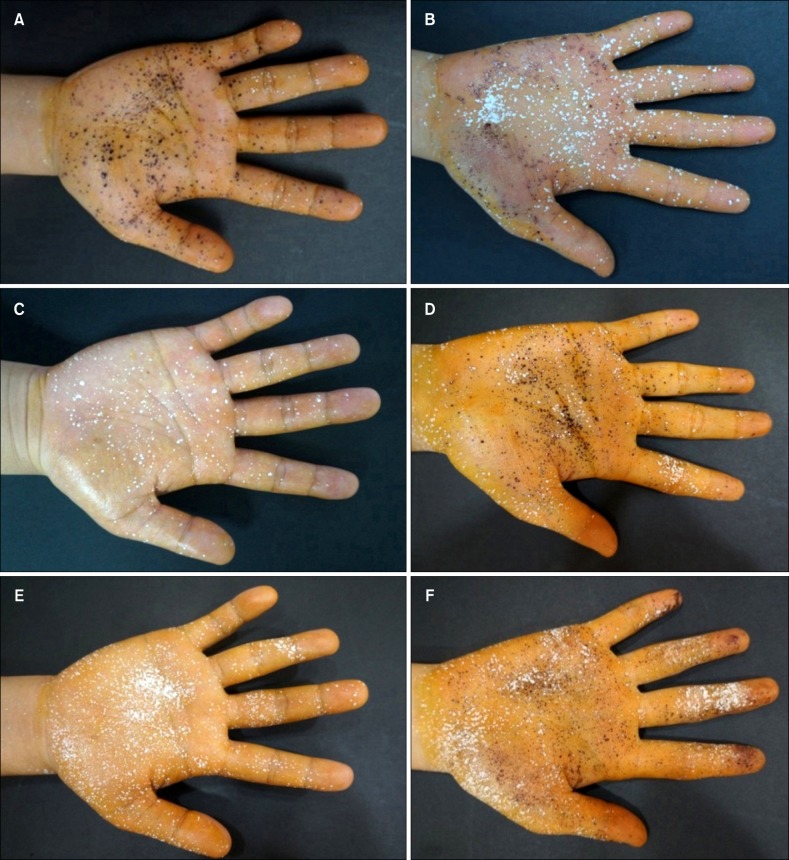
Effectiveness of treatment by colorimetric measurement
The starch-iodine test showed a clinical improvement in 92.9% (13/14), 92.9% (13/14), 88.9% (8/9), and 71.4% (5/7) of patients in group A at week 2, 3, 4, and 6, respectively. On the other hand, only 38.5% (5/13) and 23.1% (3/13) of patients in group B showed clinical improvement at week 2 and 3, respectively (Fig. 2). The clinical improvement based on starch-iodine test were significantly different between the two groups at week 2 and 3 (week 2, p=0.001; week 3, p<0.001; Table 2)11.
Fig. 2. Representative results of the starch-iodine test at baseline (A, B), immediate after completing the 10 times of treatment (C, D), and 1 week after completing the treatment (E, F) in patient group A and group B, respectively.
Table 2. Changes in starch-iodine test immediate after completing the 10 times of treatment (week 2) and 1 week (week 3), 2 weeks (week 4), and 4 weeks (week 6) after the completion of treatment11.
| Starch-iodine test | Week 2 | Week 3 | Week 4 | Week 6 | ||||
|---|---|---|---|---|---|---|---|---|
| Group A (n=14) | Group B (n=13) | Group A (n=14) | Group B (n=13) | Group A (n=9) | Group B (n=1) | Group A (n=7) | Group B (n=0) | |
| Grade 3 improvement | 4 (28.6) | 0 (0.0) | 3 (21.4) | 0 (0.0) | 3 (33.3) | 0 (0.0) | 5 (71.4) | - |
| Grade 2 improvement | 9 (64.3) | 4 (30.8) | 9 (64.3) | 2 (15.4) | 5 (55.6) | 0 (0.0) | 0 (0.0) | - |
| Grade 1 improvement | 0 (0.0) | 1 (7.7) | 1 (7.1) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 2 (28.6) | - |
| Unchanged | 1 (7.1) | 8 (61.5) | 1 (7.1) | 9 (69.2) | 1 (11.1) | 1 (100.0) | 0 (0.0) | - |
| Grade 1 worsening | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| p-value* | 0.001 | <0.001 | - | - | ||||
Values are presented as number (%). Grade 1: no reaction, grade 2: mild discoloration, grade 3: moderate discoloration, grade 4: severe discoloration. *Mann-Whitney test with Bonferroni correction.
Effectiveness of treatment by gravimetric measurement
In group A, the mean sweat secretion rate was reduced by 91.8%, 85.8%, 90.1%, and 69.0% at week 2, 3, 4, and 6, respectively. However, in group B, the mean sweat secretion rate was reduced by 39.1%, 18.0%, and 8.3% at week 2, 3, and 4, respectively. The decrease in sweat secretion rate was significantly different between the two groups at week 2 and 3 (both p<0.001, Table 3).
Table 3. Mean sweat secretion rate immediate after completing the 10 times of treatment (week 2) and 1 week (week 3), 2 weeks (week 4), and 4 weeks (week 6) after completion of treatment.
| Sweat secretion rate | Week 2 | Week 3 | Week 4 | Week 6 | ||||
|---|---|---|---|---|---|---|---|---|
| Group A (n=14) | Group B (n=13) | Group A (n=14) | Group B (n=13) | Group A (n=9) | Group B (n=1) | Group A (n=7) | Group B (n=0) | |
| Mean sweat secretion rate (mg/min) | 2.9 | 20.6 | 5.0 | 27.7 | 3.5 | 31.0 | 10.9 | - |
| Sweat decrease (%)* | 91.8 | 39.1 | 85.8 | 18.0 | 90.1 | 8.3 | 69.0 | - |
| p-value† | <0.001 | <0.001 | Not available | Not available | ||||
*Sweat decrease=[(mean sweat secretion rate at baseline)-(mean sweat secretion rate at each measurement)]/(mean sweat secretion rate at baseline)×100%, †t-test with Bonferroni correction.
Effectiveness of treatment based on HDSS
In group A, 78.6% (11/14), 78.6% (11/14), 77.8% (7/9), and 28.6% (2/7) of patients stated that they had improvement in their quality of life at week 2, 3, 4, and 6, respectively. On the other hand, in group B, 30.8% (4/13) and 23.1% (3/13) of patients stated that they had improvement in their quality of life at week 2 and 3, respectively. The improvement in patients' quality of life based on HDSS were significantly different between the two groups at week 2 and 3 (p=0.028 and p=0.014, respectively; Table 4).
Table 4. Changes in hyperhidrosis disease severity scale (HDSS) immediate after completing the 10 times of treatment (week 2) and 1 week (week 3), 2 weeks (week 4), and 4 weeks (week 6) after completion of treatment.
| HDSS | Week 2 | Week 3 | Week 4 | Week 6 | ||||
|---|---|---|---|---|---|---|---|---|
| Group A (n=14) | Group B (n=13) | Group A (n=14) | Group B (n=13) | Group A (n=9) | Group B (n=1) | Group A (n=7) | Group B (n=0) | |
| Grade 3 improvement | 1 (7.1) | 1 (7.7) | 1 (7.1) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Grade 2 improvement | 5 (35.7) | 0 (0.0) | 5 (35.7) | 0 (0.0) | 3 (33.3) | 0 (0.0) | 1 (14.3) | - |
| Grade 1 improvement | 5 (35.7) | 3 (23.1) | 5 (35.7) | 2 (15.4) | 4 (44.4) | 0 (0.0) | 1 (14.3) | - |
| Unchanged | 3 (21.4) | 9 (69.2) | 3 (21.4) | 10 (76.9) | 2 (22.2) | 1 (100.0) | 5 (71.4) | - |
| Grade 1 worsening | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| p-value* | 0.028 | 0.014 | Not available | Not available | ||||
Values are presented as number (%). Grade 1: my sweating is never noticeable and never interferes with my daily activities, grade 2: my sweating is tolerable but sometimes interferes with my daily activities, grade 3: my sweating is barely tolerable and frequently interferes with my daily activities, grade 4: my sweating is intolerable and always interferes with my daily activities. *Mann-Whitney test with Bonferroni correction.
Adverse
Regarding adverse events, one patient in group A had mild localized erythema on both hands along the water line. They subsided spontaneously after two days. No adverse event including compensatory hyperhidrosis was encountered in group B.
DISCUSSION
Iontophoresis has been especially used for the treatment of palmar and/or plantar hyperhidrosis since 195220. Although no standard protocol of iontophoresis is currently available, 10 to 30 minutes of treatment time and 3 to 5 times of treatment per week are commonly used7. Its therapeutic effect generally appears after 6~15 treatments4,21. In this study, after completion of 10 times of active treatment, 92.9% (13/14) of patients showed clinical improvement of hyperhidrosis based on colorimetric method (Table 2)8. In addition, the mean sweat secretion rate was reduced by 91.8% based on gravimetric method (Table 3). Moreover, the quality of life was improved in 78.6% (11/14) of patients based on HDSS (Table 4). The results from objective assessments generally corresponded to that from the subjective assessment (HDSS). Our results were similar to or better than those reported in previous studies using conventional direct current iontophoresis, which have resulted in improvement of hyperhidrosis in 77.8%~83.3% of patients based on colorimetric measurement, 38%~51% of sweat reduction by gravimetric measurement, and symptomatic improvement in 51%~89.5% of patients based on visual analog scale (VAS) (Table 5)9,10,11,12,13,14. Minor discrepancies of results in these studies might be related to differences in treatment protocol, iontophoretic devices, methods of assessment, and evaluation time.
Table 5. Efficacy of iontophoresis with direct current for hyperhidrosis according to the literature.
| Author (year) | Study design | No. of patients | Methods of assessment | Result | Current intensity (mA) |
|---|---|---|---|---|---|
| Stolman9 (1987) | Left-right hand comparison | 18 | Colorimetric measurement (starch-iodine test) | 83.3% of patients experienced a marked reduction in sweating | 12~20 |
| Dahl and Glent-Madsen10 (1989) | Randomized, double-blind, left-right hand comparison | 11 | Gravimetric measurement (pad grove method) | 38% reduction in sweating | 2~10 |
| Kim and Jun11 (1990) | Randomized, left-right hand comparison | 18 | Colorimetric measurement (starch-iodine test) | 77.8% of patients accomplished sufficient control | 3~14 |
| Chan et al.12 (1999) | Retrospective analysis | 9 | Gravimetric measurement & patient's rating of severity (VAS) | 51% reduction in sweating & 44.4% of patients reported significant improvement | 6.6~22.0 |
| Karakoç et al.13 (2002) | Uncontrolled | 112 | Gravimetric measurement (pad grove method) | 81.2% of patients showed significant improvement | Not specified |
| Dogruk Kacar et al.14 (2014) | Retrospective analysis | 19 | Patient's rating of severity (VAS) | 89.5% of patients stated 50% or more decrease in sweating | 6.8~24.0 |
VAS: visual analog scale.
The lasting effect of iontophoresis on sweat decrease remains controversial. In the current study, 71.4% (5/7) of patients who were followed up maintained significant improvement at 4 weeks after the last treatment. Because the treatment effect of iontophoresis usually lasts 2 to 14 weeks after the last session1, maintenance treatments are generally recommended at 1- to 4-week interval4.
Iontophoresis has a few side effects. The most common side effects are dryness, erythema, and vesicular rash in the treatment area. These lesions are completely reversible. They can be easily cured by applying emollients or topical corticosteroids. To prevent small fulgurations, patients must remove all metal objects before treatment. In addition, the electrode must be covered with insulating material. Skin defects must be covered with petrolatum in order to insulate them from current flow. Local side-effects are believed to be related to greater current22. Depending on amperage, tingling sensations may be experienced in the treated area. Compared to the current used by others9,12,13, the current applied in this study was low (4.1 mA), and only one patient had mild localized erythema on the treated area. Compensatory hyperhidrosis after iontophoresis has not been reported. For the mechanism, the lack of damage to the gangliated cord has been suggested1. On the other hand, iontophoresis has limitations as other treatment options. It is technically difficult to apply to face or axillae. In addition, the short-lived effect usually requires continuous application3.
Although the present study had some limitations, including short follow-up period and high rates of follow-up loss after treatment in the sham-treated group, this controlled study confirmed that iontophoresis could be used an effective and safe treatment modality for palmar hyperhidrosis.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Callejas MA, Grimalt R, Cladellas E. Hyperhydrosis update. Actas Dermosifiliogr. 2010;101:110–118. doi: 10.1016/j.ad.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Schlereth T, Dieterich M, Birklein F. Hyperhidrosis--causes and treatment of enhanced sweating. Dtsch Arztebl Int. 2009;106:32–37. doi: 10.3238/arztebl.2009.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vorkamp T, Foo FJ, Khan S, Schmitto JD, Wilson P. Hyperhidrosis: evolving concepts and a comprehensive review. Surgeon. 2010;8:287–292. doi: 10.1016/j.surge.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Solish N, Bertucci V, Dansereau A, Hong HC, Lynde C, Lupin M, et al. A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatol Surg. 2007;33:908–923. doi: 10.1111/j.1524-4725.2007.33192.x. [DOI] [PubMed] [Google Scholar]
- 5.Kurta AO, Glaser DA. Emerging nonsurgical treatments for hyperhidrosis. Thorac Surg Clin. 2016;26:395–402. doi: 10.1016/j.thorsurg.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Kim WO, Kil HK, Yoon KB, Yoon DM. Topical glycopyrrolate for patients with facial hyperhidrosis. Br J Dermatol. 2008;158:1094–1097. doi: 10.1111/j.1365-2133.2008.08476.x. [DOI] [PubMed] [Google Scholar]
- 7.Walling HW, Swick BL. Treatment options for hyperhidrosis. Am J Clin Dermatol. 2011;12:285–295. doi: 10.2165/11587870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto T. Pathophysiology and treatment of hyperhidrosis. Curr Probl Dermatol. 2016;51:86–93. doi: 10.1159/000446786. [DOI] [PubMed] [Google Scholar]
- 9.Stolman LP. Treatment of excess sweating of the palms by iontophoresis. Arch Dermatol. 1987;123:893–896. [PubMed] [Google Scholar]
- 10.Dahl JC, Glent-Madsen L. Treatment of hyperhidrosis manuum by tap water iontophoresis. Acta Derm Venereol. 1989;69:346–348. [PubMed] [Google Scholar]
- 11.Kim YD, Jun JB. Treatment of palmoplantar hyperhidrosis with iontophoresis. Korean J Dermatol. 1990;28:758–764. [Google Scholar]
- 12.Chan LY, Tang WY, Mok WK, Ly CY, Ip AW. Treatment of palmar hyperhidrosis using tap water iontophoresis: local experience. Hong Kong Med J. 1999;5:191–194. [PubMed] [Google Scholar]
- 13.Karakoç Y, Aydemir EH, Kalkan MT, Unal G. Safe control of palmoplantar hyperhidrosis with direct electrical current. Int J Dermatol. 2002;41:602–605. doi: 10.1046/j.1365-4362.2002.01473.x. [DOI] [PubMed] [Google Scholar]
- 14.Dogruk Kacar S, Ozuguz P, Eroglu S, Polat S, Karaca S. Treatment of primary hyperhidrosis with tap water iontophoresis in paediatric patients: a retrospective analysis. Cutan Ocul Toxicol. 2014;33:313–316. doi: 10.3109/15569527.2013.875559. [DOI] [PubMed] [Google Scholar]
- 15.Papa CM, Kligman AM. Mechanisms of eccrine anidrosis. I. High level blockade. J Invest Dermatol. 1966;47:1–9. doi: 10.1038/jid.1966.93. [DOI] [PubMed] [Google Scholar]
- 16.Sato K, Timm DE, Sato F, Templeton EA, Meletiou DS, Toyomoto T, et al. Generation and transit pathway of H+ is critical for inhibition of palmar sweating by iontophoresis in water. J Appl Physiol (1985) 1993;75:2258–2264. doi: 10.1152/jappl.1993.75.5.2258. [DOI] [PubMed] [Google Scholar]
- 17.Ohshima Y, Shimizu H, Yanagishita T, Watanabe D, Tamada Y, Sugenoya J, et al. Changes in Na+, K+ concentrations in perspiration and perspiration volume with alternating current iontophoresis in palmoplantar hyperhidrosis patients. Arch Dermatol Res. 2008;300:595–600. doi: 10.1007/s00403-008-0877-7. [DOI] [PubMed] [Google Scholar]
- 18.Anliker MD, Kreyden OP. Tap water iontophoresis. Curr Probl Dermatol. 2002;30:48–56. doi: 10.1159/000060677. [DOI] [PubMed] [Google Scholar]
- 19.Reinauer S, Neusser A, Schauf G, Hölzle E. Pulsed direct current iontophoresis as a possible new treatment for hyperhidrosis. Hautarzt. 1995;46:543–547. doi: 10.1007/s001050050296. [DOI] [PubMed] [Google Scholar]
- 20.Bouman HD, Lentzer EM. The treatment of hyperhidrosis of hands and feet with constant current. Am J Phys Med. 1952;31:158–169. [PubMed] [Google Scholar]
- 21.Hölzle E, Hund M, Lommel K, Melnik B Deutsche Dermatologische Gesellschaft. Recommendations for tap water iontophoresis. J Dtsch Dermatol Ges. 2010;8:379–383. doi: 10.1111/j.1610-0387.2009.07250.x. [DOI] [PubMed] [Google Scholar]
- 22.Reinauer S, Neusser A, Schauf G, Hölzle E. Iontophoresis with alternating current and direct current offset (AC/DC iontophoresis): a new approach for the treatment of hyperhidrosis. Br J Dermatol. 1993;129:166–169. doi: 10.1111/j.1365-2133.1993.tb03521.x. [DOI] [PubMed] [Google Scholar]