Abstract
Purpose
To determine what percentage of normal eyes follow the ISNT rule, and whether ISNT rule variants may be more generalizable to the normal population.
Design
Cross-sectional study.
Methods
Setting: Institutional setting. Study Population: 110 normal subjects. Observation Procedures: Neuroretinal rim assessments from disc photos and RNFL thickness measurements from spectral domain OCT. Main Outcome Measures: The percentages of subjects that obeyed the ISNT rule and its variants.
Results
The ISNT rule is only valid for 37.0% of disc photo rim assessments and 43.8% of RNFL measurements. Deviation of the nasal sector from the expected ISNT pattern was a major cause for the ISNT rule not being obeyed for both rim and RNFL assessments. Specifically, 10.9% of subjects had wider nasal rims than the inferior rims, 29.4% had wider nasal rims than the superior rims, 14.7% had narrower nasal rims than the temporal rims, and 42.9% had thinner nasal RNFLs compared to the temporal quadrant. Exclusion of the nasal quadrant from the ISNT rule significantly increased the validity of ISNT variant rules, with 70.9% and 76.4% of disc photos following the IST rule and the IS rule, respectively. Similarly, for RNFL thickness, 70.9% and 71.8% of patients followed the IST and IS rule, respectively.
Conclusions
The ISNT rule is only valid for about a third of disc photos and less than half of RNFL measurements in normal patients. ISNT rule variants, such as the IST and IS rule, may be considered, as they are valid in over 70% of patients.
Keywords: disc photo, ISNT rule, neuroretinal rim, optical coherence tomography, retinal nerve fiber layer
Introduction
Glaucoma is a disease of the optic nerve, which results in structural optic nerve head (ONH) damage and functional visual field (VF) loss.1, 2 It has increased as a cause of global blindness from 4.4% in 1990 to 6.3% in 2010.3 Its global prevalence is also increasing from 3.54% or 64.3 million people in 2013 to a projected 111.8 million people by 2040.4 Detection of structural ONH damage is critical for early diagnosis, because ONH changes usually precede VF loss.5 Two cornerstone elements of the ONH and peripapillary examination are stereo disc photography and optical coherence tomography (OCT) retinal nerve fiber layer (RNFL) thickness measurements.6, 7
By analyzing the neuroretinal rim in disc photos of normal subjects, Jonas et al8 found that the rim width typically exhibited a specific pattern of the inferior (I) rim being the widest, followed by the superior (S) rim, then the nasal (N) rim, and then the temporal (T) rim being the thinnest. This specific neuroretinal rim pattern was later coined by Elliot Werner as the “ISNT rule.”7, 9 Because neuroretinal rim loss is a hallmark feature of glaucoma,6, 7 patients who deviate from the ISNT rule may need to be watched more closely for glaucoma. The RNFL, on the other hand, has also been shown in histological studies in normal, non-glaucomatous eyes to exhibit a similar pattern of the inferior quadrant being the thickest, followed by the superior, nasal, then temporal quadrant.10, 11 Since RNFL thinning, particularly in the superior and inferior quadrants, is also a characteristic structural change in glaucoma,6, 7 deviation from the ISNT rule for RNFL thickness may also be an early indicator of glaucomatous structural change.
Therefore, many investigators have sought to determine whether the ISNT rule, either applied to the neuroretinal rim disc photos12–15 or to RNFL thickness measurements,16, 17 is useful in the diagnosis of glaucoma or not. However, the optic disc photo ISNT rule studies are conflicting, with some finding the ISNT rule and its variants clinically useful,13, 14 while others have not.12, 15 In contrast, RNFL ISNT rule studies based on OCT findings are in uniform agreement, stating that the ISNT rule and its variants were not helpful in the diagnosis of glaucoma.16, 17 Some have hypothesized that the ISNT rule is not easily generalizable to the individual, because the initial studies were derived from mean values.8, 10, 11 Therefore, some of the limitations of the ISNT rule may stem from the fact that it is unclear what percentage of individual normal eyes follow the ISNT rule. Other limitations may arise from the fact that perhaps other rules may be more common in the normal population.
Therefore, this study sought to determine the percentage of normal eyes that followed the ISNT rule by disc photos and RNFL thickness measurements, and secondarily, whether alternative rules may be more applicable or easily generalized. Thirdly, in the context of the ISNT rule and its variants, this study will assess how much agreement there is between disc photo neuroretinal rim assessments and RNFL thickness measurements. Although past ISNT rule studies have investigated the neuroretinal rim13, 14, 18–23 and the RNFL16 separately, none of these studies have evaluated these counterparts together in one study. The only other study17 that investigated the ISNT rule for both the neuroretinal rim and the RNFL used confocal scanning laser ophthalmoscopy (CSLO) and time domain OCT for its measurements of RNFL thickness. However, with the increased use of spectral domain OCT as the primary tool for quantitative assessment of the RNFL in clinical practice today,24 it would be important to investigate RNFL thickness using spectral domain OCT in this study.
Methods
Study participants and examinations
The study protocol adhered to the Health Insurance Portability and Accountability Act and was approved by the Institutional Review Board and the Human Studies Committee at the Massachusetts Eye and Ear Infirmary. The current study’s participants were recruited from the Glaucoma Service at the Massachusetts Eye and Ear Infirmary (MEEI) between February 2009 and December 2015; however, the current study’s patients were a small subset of a larger prospective SIG Study (Spectral Domain OCT In Glaucoma Study) of normal patients, glaucoma suspects, and glaucoma patients. Further details of the SIG study population are described elsewhere.25–38 Informed consents were obtained from all of the participants prior to collection of data.
Each patient underwent a complete ophthalmologic examination, which included: history, Snellen visual acuity (VA) testing, refraction, central corneal thickness measurements by ultrasonic pachymetry, Goldmann applanation tonometry, slit-lamp biomicroscopy, gonioscopy, dilated fundus examination, visual field (VF) testing (Swedish Interactive Threshold Algorithm Standard 24-2 test, Humphrey visual field analyzer, Carl Zeiss Meditec Inc., Dublin, CA), color optic disc photography (Visucam Pro NM, Carl Zeiss Meditec Inc., Dublin, CA), and spectral domain OCT imaging (Spectralis HRA+OCT, Heidelberg Engineering, Heidelberg, Germany).
The study included only normal subjects who satisfied the following inclusion criteria: age of greater than 18 years; a normal eye examination without ocular diseases except for cataracts; best corrected vision of > 20/40; a normal VF test result, defined by a pattern standard deviation value of > 5% compared to age-matched controls in the perimeter’s normative database and a glaucoma hemifield test within normal limits; and a spherical equivalent of between −5 and +5 diopters. Subjects were excluded if they had an unreliable VF testing with > 33% fixation losses, > 20% false positives, or > 20% false negatives. Additional exclusion criteria are as follows: any history of ocular hypertension or intraocular pressures > 21 mmHg at the time of the visit; inter-eye cup-to-disc ratio (CDR) asymmetry of > 0.2; CDR of > 0.4 in a Caucasian or Asian subject; CDR of > 0.6 in an African-American or Hispanic subject; and history of a neurologic disease or systemic medication that could produce VF defects. If both eyes were eligible after applying the inclusion and exclusion criteria, then one eye was randomly selected for analysis using a randomization table.
Peripapillary retinal nerve fiber layer thickness measurements
After pupillary dilation, all of the patients underwent OCT imaging (Spectralis HRA+OCT, Heidelberg Engineering, Heidelberg, Germany) with the peripapillary RNFL circle scan. The scan circle was 12 degrees in diameter, which is approximately 3.5 mm in diameter in an eye with a typical corneal curvature and eye length. The instrument’s built-in software automates segmentation of the internal limiting membrane and the posterior RNFL border, and then calculates the average RNFL thickness for 4 quadrants (superior, inferior, nasal, and temporal; Figure 1). Poor quality RNFL scans with an image quality score (Q) of <15 were excluded from the analysis.
Figure 1.
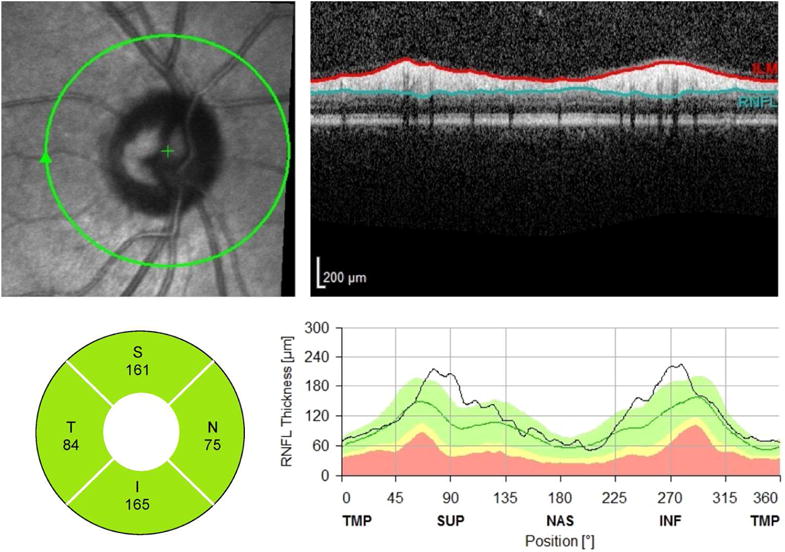
Example of peripapillary retinal nerve fiber layer (RNFL) scan (Spectralis HRA+OCT, Heidelberg Engineering, Heidelberg, Germany). In this example, the RNFL thickness (bottom, left) in the nasal quadrant is thinner than the temporal quadrant, which violates the ISNT rule.
Assessment of neuroretinal rim on optic disc photography
Digital monoscopic color disc photographs were evaluated by a committee of trained glaucoma specialists experienced in the evaluation of optic disc (LYP, DSV, AT, TCC) who were masked to the subjects’ clinical data. The graders evaluated the neuroretinal rim width at the 12, 3, 6, and 9 o’clock positions and then graded the neuroretinal rims in the order of decreasing width (Figure 2). The following definitions were used when the graders evaluated the neuroretinal rim: 1) the outer neuroretinal rim, or disc margin, was defined as the inner edge of the peripapillary scleral ring of Elschnig and 2) inner neuroretinal rim margin was determined from the position of the blood vessel bending as they exited the nerve.39 The central retinal vessel trunk was not considered a part of the neuroretinal rim. For eyes which had discrepancies between the graders on the order of the rims, a consensus meeting was convened with all 4 graders to reach unanimity on the final assessment regarding the order of the rims.
Figure 2.
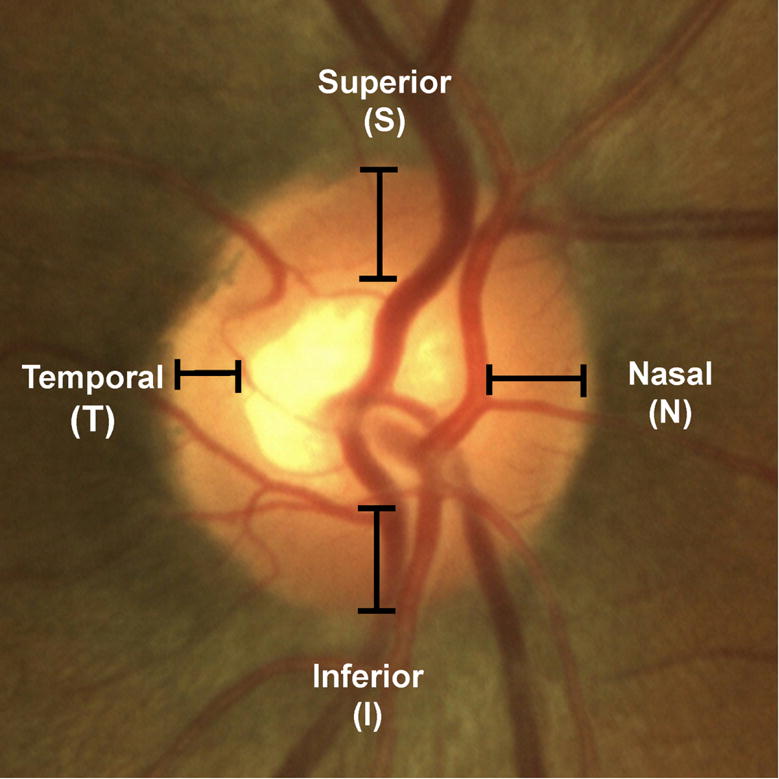
Example of a color disc photograph, which illustrates the neuroretinal rim locations at which rim width order were determined.
Definition of the ISNT rule and variants of the rule
The ISNT rule is defined as the order of the neuroretinal rim width or RNFL thickness that follows the pattern of I > S > N > T. This study also analyzed variants of the ISNT rule, such as the IST rule (I > S > T), the IS rule (I > S), and the T thinnest rule (temporal quadrant being the thinnest or the most narrow). If two quadrants were ranked to be equal in neuroretinal width order, or if two quadrants had equal RNFL thickness values, then the eye was excluded from the analysis of the given rule.
Statistical analysis
All of the statistical analyses were performed using SPSS software version 20.0 (IBM Corp., Armonk, NY, USA). The frequency of whether the ISNT rule or its variants were followed was analyzed. The McNemar test was used to compare the fulfillment rates of ISNT rule variants in RNFL thickness versus neuroretinal rim. Kappa statistics were used to evaluate how much agreement there was between when the rules were obeyed per RNFL thickness versus the neuroretinal rim. Strength of agreement was categorized based on the scale proposed by Landis and Koch40 where a kappa value of 0 – 0.20 is slight, 0.21 – 0.40 is fair, 0.41 – 0.60 is moderate, 0.61 – 0.80 is substantial, and 0.81 – 1.00 is almost perfect. Statistical significance was defined by a p value of < 0.05. Results are expressed as means ± standard deviation unless otherwise specified.
Results
A total of 110 eyes of 110 normal subjects were included in the analysis. Table 1 shows that the study subjects had a mean age of 58.8 ± 16.5 years, had a mean cup-to-disc ratio (CDR) of 0.35 ± 0.11, and were predominantly Caucasian (61.8%). For disc photo assessment of the neuroretinal rim, there was 1 eye with equal width ranking in the superior and nasal sectors, and 1 eye with equal width ranking in the nasal and temporal sectors; therefore, a total 2 eyes were excluded from analysis of the ISNT rule, and 1 eye was excluded from the analysis of the T thinnest rule. For RNFL thickness measurements, there were 5 eyes with equal thickness values in the nasal and temporal quadrants, and these eyes were excluded from the analysis of the ISNT rule and the T thinnest rule.
TABLE 1.
Patient demographics and peripapillary retinal nerve fiber layer thickness measurements
| N = 110 | |
|---|---|
| Age (years) | 58.8 ± 16.5 |
| Female, n (%) | 70 (63.6%) |
| Ethnicity, n (%) | |
| Caucasian | 68 (61.8%) |
| African-American | 17 (15.5%) |
| Hispanic | 13 (11.8%) |
| Asian | 10 (9.1%) |
| Other | 2 (1.8%) |
| Laterality, right eye, n (%) | 64 (58.2%) |
| Spherical equivalent (diopters) | −0.35 ± 1.73 |
| Cup-to-disc ratio | 0.35 ± 0.11 |
| Peripapillary RNFL thickness (μm)* | |
| Global | 96.1 ± 9.9 |
| Superior | 116.1 ± 17.9 |
| Inferior | 125.4 ± 15.9 |
| Nasal | 72.3 ± 13.2 |
| Temporal | 70.7 ± 12.8 |
Abbreviations: N = number; RNFL = retinal nerve fiber layer.
Results are expressed as means ± standard deviations unless otherwise stated.
RNFL thickness was measured using spectral domain optical coherence tomography (Spectralis HRA+OCT, Heidelberg Engineering, Heidelberg, Germany).
Table 2 summarizes the percentages of patients who fit the ISNT rule and its variants for disc photography and for RNFL thickness measurements. The ISNT rule was valid for a minority of eyes (i.e. only 37% by disc photos and 43.8% by RNFL thickness measurements, p = 0.243). In contrast, most eyes fit the IST and IS variant rules (i.e. IST rule: 70.9% for both disc photos and RNFL measurements; IS rule: 76.4% by disc photos and 71.8% by RNFL thickness measurements). The T thinnest rule was the best rule for disc photos (i.e. valid for 82.6% of eyes) but was not valid for about half of RNFL thickness assessments (i.e. 57.1%, Table 2). All 3 ISNT rule variants (i.e. IST, IS, and T thinnest rule) for disc photos were significantly better than the ISNT rule (p <0.001). Only two ISNT rule variants (i.e. IST and IS) for RNFL thickness were significantly better than the ISNT rule (p <0.001).
TABLE 2.
Percentages of patients where the ISNT rule or its variants were valid
| rule valid by disc photography neuroretinal rim assessments | rule valid by RNFL thickness measurements | p value* | |
|---|---|---|---|
| ISNT rule | 37.0% | 43.8% | 0.243 |
| IST rule | 70.9% | 70.9% | 1.000 |
| IS rule | 76.4% | 71.8% | 0.532 |
| T thinnest rule | 82.6% | 57.1% | <0.001 |
RNFL = retinal nerve fiber layer
ISNT rule = inferior > superior > nasal > temporal
IST rule = inferior > superior > temporal
IS rule = inferior > superior
T thinnest rule = temporal quadrant being the thinnest or the most narrow
p values compared percentages obtained by disc photos versus RNFL thickness measurements (McNemar test)
To explore the possible reasons for why the ISNT rule applies to only about 40% of subjects for both disc photos and for RNFL thickness, but increasing to over 70% when the nasal sector was excluded from the ISNT rule (Table 2), the relationship of the nasal quadrant to the other quadrants was assessed according to the expected trends depicted by the ISNT rule. According to Figure 3, the nasal rim width in disc photos was wider than the inferior rim in 12 out of 110 eyes (10.9%), wider than the superior rim in 32 out of 109 eyes (29.4%), and narrower than the temporal rim in 16 out of 109 eyes (14.7%). In contrast, the main reason RNFL thickness did not follow the ISNT rule was because the nasal quadrant was thicker than the temporal quadrant in 45 out of 105 eyes (42.9%; Figure 4).
Figure 3.
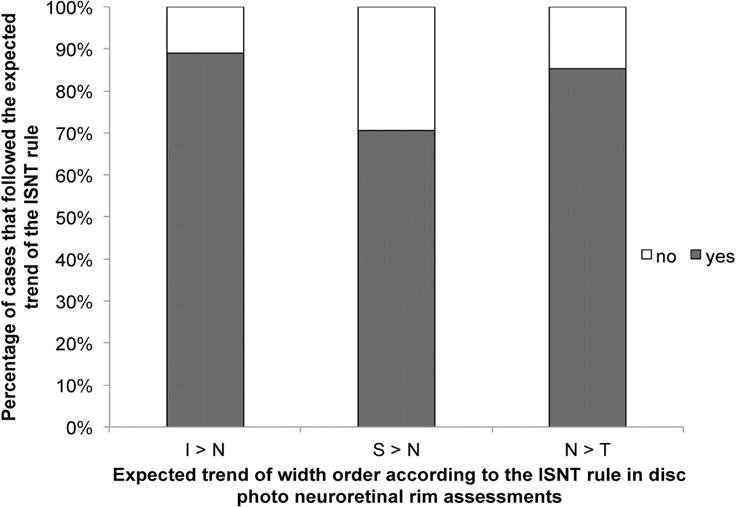
Bar graph showing the percentages of cases where the nasal quadrant did or did not follow the expected trend of the ISNT rule in disc photos. (Abbreviations: I = inferior; N = nasal; S = superior; T = temporal)
Figure 4.
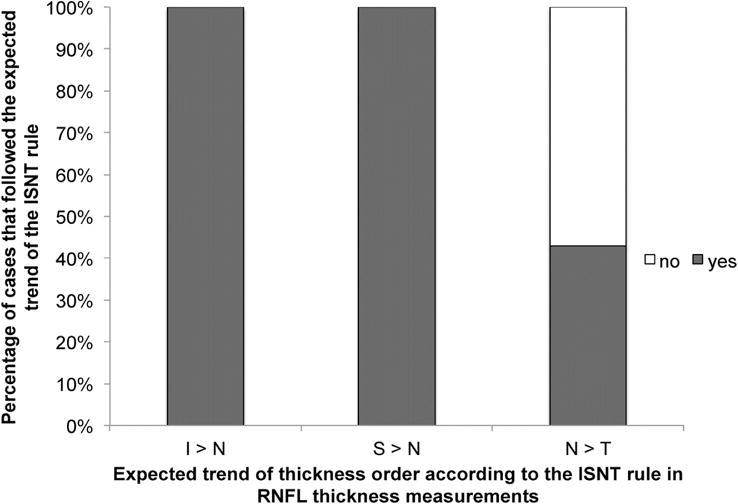
Bar graph showing the percentages of cases where the nasal quadrant did or did not follow the expected trend of the ISNT rule for retinal nerve fiber layer thickness values. (Abbreviations: I = inferior; N = nasal; S = superior; T = temporal)
The temporal sector, on the other hand, followed the expected trend of being thinner than both the inferior and superior sectors (i.e. either I>S>T or S>I>T) in102 out of 110 eyes (93%) for disc photos and in 109 out of 110 eyes (99%) for RNFL thickness measurements.
Using kappa statistics, inter-test agreement between whether the ISNT rule variants were obeyed in disc photos versus RNFL thickness measurements was further analyzed for individual cases. Although the ISNT, IST, and IS rules were fulfilled overall at similar rates by disc photos and by RNFL thickness in normal subjects (Table 2), kappa statistics showed that there was relatively low agreement between when the rules were obeyed by disc photos versus by RNFL thickness (kappa = 0.03 – 0.12; Table 3). In other words, if the disc photo neuroretinal rim width assessments obeyed the ISNT, IST, or IS rules, the RNFL thickness values would not necessarily also obey the corresponding rules, and vice versa.
TABLE 3.
Kappa statistics for inter-test agreement between whether the ISNT rule and the ISNT rule variants were obeyed in disc photos versus RNFL thickness measurements.
| Kappa statistics | |
|---|---|
| ISNT rule | 0.06 |
| IST rule | 0.12 |
| IS rule | 0.03 |
| T thinnest rule | 0.03 |
ISNT rule = inferior > superior > nasal > temporal
IST rule = inferior > superior > temporal
IS rule = inferior > superior
T thinnest rule = temporal quadrant being the thinnest or the most narrow
Discussion
The ISNT rule, first developed for the neuroretinal rim in disc photos, was based on Jonas’ previous work which found that the mean of neuroretinal rim width in a normal population of 338 subjects followed a pattern of the inferior rim being widest, followed by the superior, nasal, and lastly the temporal rim.8 While a similar pattern of I > S > N > T quadrant was found for RNFL thickness from histologic studies,10, 11 these were also reported as the mean of a population. If this rule were applied to a clinical setting, it would be important for the clinician to know how generalizable or how frequently this rule can actually be applied to an individual patient in the normal population. In the present study, we comprehensively analyzed the validity of the ISNT rule and its variants in the clinical evaluation of both the neuroretinal rim and spectral domain OCT RNFL thickness measurements in a normal population. A key finding of this paper was that the ISNT rule was valid for only a minority of eyes (i.e. only 37% by disc photos and 43.8% by RNFL thickness measurements, p = 0.243; Table 2).
In particular, for disc photo assessments, having only 37% of disc photos obey the ISNT rule is lower than previously reported rates of 52% to 79%13, 14, 19 in studies that also evaluated the neuroretinal rim width using disc photographs in the normal population. By examining the relationship between the nasal quadrant and the other quadrants with respect to the expected trend of the ISNT rule, we found that a main reason that the ISNT rule was not valid was because of considerable variation in the rim order of the nasal quadrant, in which 10.9% of subjects had a rim width that was wider nasally than inferiorly (Figure 3). In a normal population of 92 Chinese subjects, Wang et al19 found that 9 out of 92 subjects (10%) had a nasal rim that was the widest compared to all the other rims, while Harizman et al13 also reported in their study that 5 out of 66 normal subjects (7.6%) had nasal rim that was thicker than the inferior rim. Additionally, we also found that the nasal rim was wider than the superior rim in 29.4% of subjects and that the nasal rim was narrower than the temporal rim in 14.7% of subjects; and these percentages are consistent with previously reported rates for normal subjects of 28% – 36%14, 19 and 14% – 29%,14, 19 respectively. A possible reason for the high variability in nasal rim order and hence the low fulfillment rate of the ISNT rule in our study is that, although the central retinal vessel was not considered as part of the neuroretinal rim during disc assessments, there often is partial obscuration of the nasal rim by these large retinal vessels, which would make evaluation of the nasal rim width ranking more variable.13 Therefore, it is important to take into consideration the large variation that exists for the nasal rim order in the normal population when using the ISNT rule for determining whether a patient’s optic nerve has glaucoma or not.
Given that the ISNT rule is not valid most of the time due to variations in the nasal neuroretinal rim, this would support the rationale for excluding the nasal rim from the ISNT rule to make this rule more widely applicable for the normal population. When not using the “N” in the ISNT rule, alternative rules such as the IST rule or IS rule were valid over 70% of the time (Table 2). Specifically, variants of the ISNT rule were valid 70.9% of the time for the IST rule, 76.4% of the time for the IS rule, and 82.6% of the time for the T thinnest rule (Table 2). In general, previous studies have also reported similar rates, with 79.4% validity for the IST rule17 and 85% validity for the IS rule.14 A population based study conducted on Indians found that the temporal rim was thinnest in 79.8% of subjects.23 Therefore, our study and the literature seem to indicate that the ISNT rule should be at least changed to the IST rule, if not to the IST/IS/T thinnest rule; because all of these variant rules are valid over 70% of time compared to the ISNT rule which is valid only 37% of the time. However, as glaucomatous neuroretinal rim loss is more prominent at the superior and inferior poles,6, 7 the T thinnest rule may not be as useful in detecting early glaucoma, unless the superior and inferior rims are so thin as to be thinner than the temporal rim (e.g. focal notching). Other studies have also suggested that of the 3 variant rules, the IST and IS rule may be better used clinically.14, 17 For example, in a study of 189 normal subjects and 42 moderate-to-severe glaucoma subjects (VF mean deviation −11.36 ± 7.01 dB), Pradhan et al found that the IST rule had high utility in distinguishing the normal subjects (79.4% valid) from the glaucoma subjects (0% valid).17 Law et al, who evaluated the IS rule, found that this rule had an 85% specificity in differentiating normal from glaucoma subjects.14 Therefore, for direct examination or disc photo assessments of the neuroretinal rim, the IST and IS rules should be applied instead of the ISNT rule (Table 2).14, 17
Even though mean RNFL thickness values for the entire study population fit the ISNT rule (Table 1), less than half of individual patients followed the ISNT rule for RNFL thickness (43.8%, Table 2). When previous histologic studies10, 11 and OCT studies16, 27 also used mean quadrant RNFL thickness values, they also demonstrated that the ISNT rule was valid (Table 1). However, when examining individual patients, we found that only 43.8% of subjects in our normal population met the ISNT rule (Table 2), which is similar to reported rates of 42% to 55%.16, 17, 27 This highlights that when applying a rule to an aggregate population, the rule may no longer be valid for the individual due to individual variability. In contrast to our findings for disc photos, we found that the sole reason that nasal RNFL thickness deviated from the ISNT rule was that the nasal quadrant was thinner than the temporal quadrant in 42.9% of normal subjects (Figure 4), which was a surprising finding and not previously reported by other studies.16, 17, 27 Although histologic studies have demonstrated a trend of the nasal RNFL being thicker than the temporal RNFL,10, 11 both of these studies sampled the RNFL relatively close to the optic disc border. Dichtl et al11 sampled the RNFL at the disc border, while Varma et al10 sampled the RNFL at 50 and 100 μm from the disc border. However, since it has also been shown histologically10 and by OCT41 that RNFL thickness decreases as the distance away from the optic disc increases, it is possible that the RNFL thickness differences nasally and temporally are not as prominent at the location being measured by the OCT scan circle, which has about a 3.5 mm diameter or 12° distance. Therefore, since the average nasal and temporal RNFL thickness values are very similar and have overlapping normal ranges (i.e. nasal 72.3 ± 13.2 and temporal 70.7 ± 12.8, respectively; Table 1), it is easy to see how the temporal RNFL can be a few microns thicker than the nasal RNFL due to individual patient variation (i.e. T>N), and this might have accounted for a high proportion of normal patients not following the ISNT rule.
The current study supports the use of two ISNT rule variants, the IST rule and the IS rule for RNFL thickness (Table 2); and this is in contrast to some of the past literature. In the past literature, only two OCT studies have specifically investigated the use of ISNT rule variants on RNFL thickness. Dave et al measured RNFL thickness using spectral domain OCT (Spectralis OCT),16 while Pradhan et al used time-domain OCT (Stratus OCT).17 They found that the IST rule was only valid 58.7% to 60% of the time,16, 17 and the IS rule was only valid 58.7% of the time.17 Therefore, both of these studies concluded that the IST and IS rules were not clinically useful in the differentiation of normal from glaucoma subjects. This is in contrast to our study where we found that by Not including the Nasal quadrant in the ISNT rule for RNFL thickness, these two variant rules were valid the majority of the time (i.e. 70.9% for the IST rule and 71.8% for the IS rule, Table 2; versus 43.8% for the ISNT rule, Table 1). It is possible that the different results encountered in our study may be due to ethnic differences in these 2 studies (Indian subjects)16, 17 compared to our predominantly Caucasian subjects (Table 1) and to a different instrument being used for RNFL thickness measurements in the study by Pradhan et al (Stratus OCT).17 In conclusion, our findings suggest that the IST rule and IS rule, which is valid in over 70% of normal eyes, should be used for RNFL thickness assessments instead of the ISNT rule, which is only valid 43.8% of the time (Table 2).
Although our results demonstrate a trend of increasing validity of the ISNT rule when more letters are excluded (i.e. 37% for ISNT rule, 70.9% for IST rule, 76.4% for IS rule for disc photos), the validity of the IST rule variant in particular is still greater than what one would expect with random chance (i.e. Table 4, 16.7%). Notably, the percentages of patients who were valid for the IS rule compared to the IST rule for disc photos (p = 0.211) and for RNFL thickness (p = 0.851) were not statistically different; therefore, the clinical implications of the IST and IS rules in normal patients are likely very similar, if not the same. The clinical implications of the IST and IS rules are also likely the same, because our results also show that the temporal sector was almost always thinner than both the inferior and superior sectors in 93% of eyes for disc photos and in 99% of eyes for RNFL thickness values.
TABLE 4.
Probability of the ISNT rule and its variants occurring randomly
| All possible random combinations of the rule variants | Probability of random occurrence | |
|---|---|---|
| ISNT rule | ISNT | 4.2% |
| IST rule | ISNT, INST, ISTN, NIST | 16.7% |
| IS rule | ISNT, INST, INTS, ITSN, ISTN, ITNS, NIST, NTIS, NITS, TISN, TNIS, TINS | 50% |
Abbreviations: I = inferior; N = nasal; S = superior; T = temporal.
Additionally, in this study, we analyzed whether there was agreement for each individual in whether the ISNT rule or its variants were followed for disc photos versus RNFL thickness assessments, and we found that the agreement between disc photos and RNFL assessments on whether the ISNT rule and its variants were followed or not, was low (kappa = 0.032 – 0.119; p all > 0.05), despite similar aggregate rates for the ISNT, IST, and IS rules for these two structures (Table 2). One might expect that since both of these structures represent measures of the amount of nerve tissue, that there should be a higher agreement between these two structures on whether the ISNT rule variants were followed or not. However, for RNFL thickness, the measurement is taken perpendicularly to the orientation of the nerve fiber bundle (Figure 1), while for the neuroretinal rim width, the measurement is partly taken in a direction that is approximately parallel to the orientation of nerve fiber bundles (Figure 2). Furthermore, although there may be an assumption of corresponding sector-to-sector projection from the RNFL to the optic nerve head as the nerve fibers converge from the retina into the optic nerve, it has been shown that the trajectory of the RNFL bundles are complex as they converge into the optic nerve and show considerable inter-subject variability.42, 43 Additionally, to determine if an eye followed the ISNT rule, neuroretinal rim thickness on disc photos was only tested at 4 points (i.e. 3, 6, 9, 12 o’clock positions), while OCT RNFL thickness values were average values obtained over a 90 degree quadrant. Hence, not only are rim thickness and RNFL thickness different anatomic tissues, but also the regions that each are evaluating are different. Therefore, despite good concordance for the ISNT, IST, and IS rules’ validity between disc photos and RNFL values as an aggregate group, one should not assume that there is good agreement for the ISNT rule and its variants for the two structures in an individual patient.
Future alternative studies could further investigate whether better agreement exists between qualitative disc photo neuroretinal rim assessments and qualitative RNFL thickness B scan assessments compared to measured RNFL thickness values. Since OCT RNFL thickness is an average value over a 90 degree quadrant, subtle topographic changes in the RNFL may be hidden in the average value, while a qualitative assessment of the RNFL B scan may better detect such focal changes, which could possibly then result in a higher agreement between neuroretinal rim assessments and RNFL assessments. However, in this study, we chose to investigate quantitative OCT RNFL thickness measurements, because it is the most commonly used and reported parameter in OCT for glaucoma.24 Other alternative studies have also investigated the applicability of the ISNT rule using CSLO measured neuroretinal rim parameters such as the rim area18, 21, 22 and rim volume.22 In general, the validity of the ISNT rule for these rim parameters were also reported to be very low (10.7% – 18%)21, 22 but was also considerably higher for the IST and IS rule (53.3% – 77%).18, 21, 22 However, further investigation is needed to determine whether OCT derived neuroretinal rim parameters comply better with the ISNT rule compared to CSLO rim parameters.
There are several limitations to the present study. First, the method used for neuroretinal rim assessments in this study was based on monoscopic color disc photographs instead of stereoscopic disc photographs that were used by other studies.8, 13, 14, 17, 19 Although stereoscopic disc photographs have been considered the standard in the evaluation of the optic disc,44 it has specific disadvantages compared to monoscopic photographs since there needs to be perfect alignment of the stereo viewer and displayed paired photographs. Furthermore, it has been shown that when evaluated by glaucoma experts, monoscopic images demonstrated a similarly high inter-observer agreement compared to stereoscopic images for both estimated glaucoma likelihood and optic disc characteristics such as CDR (kappa = 0.72 for monoscopic images and kappa = 0.62 for stereoscopic images).45 Therefore, although we used monoscopic color disc photographs for the assessment of the neuroretinal rim, we believe that the assessments would be comparable to assessments obtained from stereoscopic disc photographs, especially since these images were assessed by 4 experienced glaucoma specialists who were unanimous in their final assessments. Secondly, all of the normal subjects in this study were recruited from a university-based Glaucoma Service, and this may pose as a potential selection bias since a patient in a university-based setting may be different from a patient in a population study-based setting. Nevertheless, all of the included subjects in this study were carefully examined by a glaucoma specialist (TCC) and were determined to be normal and without glaucoma or ocular hypertension. Also, patients with suspicious looking discs or who had physiologic cupping were excluded from this study.
In summary, the ISNT rule was valid for only a minority of normal eyes (i.e. only 37% by disc photos and 43.8% by RNFL thickness measurements, p = 0.243; Table 2). When the nasal quadrant was not used in the ISNT rule, over 70% of normal eyes followed the IST and IS rule for both disc photos and OCT RNFL thickness measurements (i.e. disc photo neuroretinal rim assessments: 70.9% obeyed the IST rule and 76.4% obeyed the IS rule; RNFL thickness assessments: 70.9% obeyed the IST rule and 71.8% obeyed the IS rule). Therefore, this study concludes that the IST and IS rule should be used instead of the ISNT for clinical disc assessments and for OCT RNFL thickness interpretations. Also, for an individual patient, validity of a specific rule for disc photos may not necessarily translate to validity of that same rule for RNFL thickness measurements.
Supplementary Material
Acknowledgments
Funding/Support: Teresa C. Chen has received funding from the American Glaucoma Society Mid-Career Award (San Francisco, California), Massachusetts Lions Eye Research Fund, Fidelity Charitable Fund (Harvard University, Boston, Massachusetts), Harvard Catalyst Grant, National Institutes of Health UL RR025758 (Bethesda, Maryland).
Financial Disclosures: No financial disclosures for all of the authors.
Other Acknowledgements: None.
Biography
Teresa C. Chen, MD is an Associate Professor of Ophthalmology at the Harvard Medical School. She works in the Glaucoma Service at the Massachusetts Eye and Ear Infirmary. Her research expertise is in imaging and the pediatric glaucomas. She edited the book Glaucoma Surgery and has published over 150 original articles, major reviews, and book chapters. Johannes F. de Boer, PhD and Teresa Chen, MD were the first to image an eye with video-rate 3-dimensional spectral domain optical coherence tomography.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238–42. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA. Glaucoma. Lancet. 2011;377(9774):1367–77. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 3.Bourne RR, Taylor HR, Flaxman SR, et al. Number of people blind or visually impaired by glaucoma worldwide and in world regions 1990 – 2010: A meta-analysis. PLoS One. 2016;11(10):e0162229. doi: 10.1371/journal.pone.0162229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–90. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Keltner JL, Johnson CA, Anderson DR, et al. The association between glaucomatous visual fields and optic nerve head features in the Ocular Hypertension Treatment Study. Ophthalmology. 2006;113(9):1603–12. doi: 10.1016/j.ophtha.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 6.Broadway DC, Nicolela MT, Drance SM. Optic disk appearances in primary open-angle glaucoma. Surv Ophthalmol. 1999;43(Suppl 1):S223–43. doi: 10.1016/s0039-6257(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 7.Jonas JB, Budde WM. Diagnosis and pathogenesis of glaucomatous optic neuropathy: morphological aspects. Prog Retin Eye Res. 2000;19(1):1–40. doi: 10.1016/s1350-9462(99)00002-6. [DOI] [PubMed] [Google Scholar]
- 8.Jonas JB, Gusek GC, Naumann GO. Optic disc, cup and neuroretinal rim size, configuration and correlations in normal eyes. Invest Ophthalmol Vis Sci. 1988;29(7):1151–8. [PubMed] [Google Scholar]
- 9.Jonas JB, Budde WM, Panda-Jonas S. Ophthalmoscopic evaluation of the optic nerve head. Surv Ophthalmol. 1999;43(4):293–320. doi: 10.1016/s0039-6257(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 10.Varma R, Skaf M, Barron E. Retinal nerve fiber layer thickness in normal human eyes. Ophthalmology. 1996;103(12):2114–9. doi: 10.1016/s0161-6420(96)30381-3. [DOI] [PubMed] [Google Scholar]
- 11.Dichtl A, Jonas JB, Naumann GO. Retinal nerve fiber layer thickness in human eyes. Graefes Arch Clin Exp Ophthalmol. 1999;237(6):474–9. doi: 10.1007/s004170050264. [DOI] [PubMed] [Google Scholar]
- 12.Jonas JB, Budde WM, Lang P. Neuroretinal rim width ratios in morphological glaucoma diagnosis. Br J Ophthalmol. 1998;82(12):1366–71. doi: 10.1136/bjo.82.12.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harizman N, Oliveira C, Chiang A, et al. The ISNT rule and differentiation of normal from glaucomatous eyes. Arch Ophthalmol. 2006;124(11):1579–83. doi: 10.1001/archopht.124.11.1579. [DOI] [PubMed] [Google Scholar]
- 14.Law SK, Kornmann HL, Nilforushan N, Moghimi S, Caprioli J. Evaluation of the “IS” rule to differentiate glaucomatous eyes from normal. J Glaucoma. 2016;25(1):27–32. doi: 10.1097/IJG.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 15.Morgan JE, Bourtsoukli I, Rajkumar KN, et al. The accuracy of the inferior>superior>nasal>temporal neuroretinal rim area rule for diagnosing glaucomatous optic disc damage. Ophthalmology. 2012;119(4):723–30. doi: 10.1016/j.ophtha.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Dave P, Shah J. Applicability of ISNT and IST rules to the retinal nerve fibre layer using spectral domain optical coherence tomography in early glaucoma. Br J Ophthalmol. 2015;99(12):1713–7. doi: 10.1136/bjophthalmol-2014-306331. [DOI] [PubMed] [Google Scholar]
- 17.Pradhan ZS, Braganza A, Abraham LM. Does the ISNT rule apply to the retinal nerve fiber layer? J Glaucoma. 2016;25(1):e1–4. doi: 10.1097/IJG.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 18.Sihota R, Srinivasan G, Dada T, et al. Is the ISNT rule violated in early primary open-angle glaucoma–a scanning laser tomography study. Eye (Lond) 2008;22(6):819–24. doi: 10.1038/sj.eye.6702798. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Xu L, Jonas JB. Shape of the neuroretinal rim and its correlations with ocular and general parameters in adult chinese: the beijing eye study. Am J Ophthalmol. 2007;144(3):462–4. doi: 10.1016/j.ajo.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 20.Hwang YH, Kim YY. Application of the ISNT rule to neuroretinal rim Thickness determined using Cirrus HD optical coherence tomography. J Glaucoma. 2015;24(7):503–7. doi: 10.1097/IJG.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 21.Nayak NV, Berezina TL, Fechtner RD, Sinai MJ, Khouri AS. Effect of age and disc size on rim order rules by Heidelberg Retina Tomograph. J Glaucoma. 2015;24(5):377–82. doi: 10.1097/IJG.0b013e31829f9c15. [DOI] [PubMed] [Google Scholar]
- 22.Iester M, Bertolotto M, Recupero SM, Perdicchi A. The “ISN’T rule” in healthy participant optic nerve head by confocal scanning laser ophthalmoscopy. J Glaucoma. 2011;20(6):350–4. doi: 10.1097/IJG.0b013e3181efb065. [DOI] [PubMed] [Google Scholar]
- 23.Arvind H, George R, Raju P, et al. Neural rim characteristics of healthy South Indians: the Chennai Glaucoma Study. Invest Ophthalmol Vis Sci. 2008;49(8):3457–64. doi: 10.1167/iovs.07-1210. [DOI] [PubMed] [Google Scholar]
- 24.Grewal DS, Tanna AP. Diagnosis of glaucoma and detection of glaucoma progression using spectral domain optical coherence tomography. Curr Opin Ophthalmol. 2013;24(2):150–61. doi: 10.1097/ICU.0b013e32835d9e27. [DOI] [PubMed] [Google Scholar]
- 25.Wu H, de Boer JF, Chen TC. Reproducibility of retinal nerve fiber layer thickness measurements using spectral domain optical coherence tomography. J Glaucoma. 2011;20(8):470–6. doi: 10.1097/IJG.0b013e3181f3eb64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu H, de Boer JF, Chen TC. Diagnostic capability of spectral-domain optical coherence tomography for glaucoma. Am J Ophthalmol. 2012;153(5):815–826 e2. doi: 10.1016/j.ajo.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alasil T, Wang K, Keane PA, et al. Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography. J Glaucoma. 2013;22(7):532–41. doi: 10.1097/IJG.0b013e318255bb4a. [DOI] [PubMed] [Google Scholar]
- 28.Alasil T, Wang K, Yu F, et al. Correlation of retinal nerve fiber layer thickness and visual fields in glaucoma: a broken stick model. Am J Ophthalmol. 2014;157(5):953–59. doi: 10.1016/j.ajo.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simavli H, Que CJ, Akduman M, et al. Diagnostic capability of peripapillary retinal thickness in glaucoma using 3D volume scans. Am J Ophthalmol. 2015;159(3):545–56 e2. doi: 10.1016/j.ajo.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H, de Boer JF, Chen L, Chen TC. Correlation of localized glaucomatous visual field defects and spectral domain optical coherence tomography retinal nerve fiber layer thinning using a modified structure-function map for OCT. Eye (Lond) 2015;29(4):525–33. doi: 10.1038/eye.2014.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Simavli H, Que CJ, et al. Patient characteristics associated with artifacts in Spectralis optical coherence tomography imaging of the retinal nerve fiber layer in glaucoma. Am J Ophthalmol. 2015;159(3):565–76 e2. doi: 10.1016/j.ajo.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Field MG, Alasil T, Baniasadi N, et al. Facilitating glaucoma diagnosis With intereye retinal nerve fiber layer asymmetry using spectral-domain optical coherence tomography. J Glaucoma. 2016;25(2):167–76. doi: 10.1097/IJG.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 33.Shieh E, Lee R, Que C, et al. Diagnostic performance of a novel three-dimensional neuroretinal rim parameter for glaucoma using high-density volume scans. Am J Ophthalmol. 2016;169:168–78. doi: 10.1016/j.ajo.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 34.Baniasadi N, Paschalis EI, Haghzadeh M, et al. Patterns of retinal nerve fiber layer loss in different subtypes of open angle glaucoma using spectral domain optical coherence tomography. J Glaucoma. 2016;25(10):865–872. doi: 10.1097/IJG.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 35.Tsikata E, Lee R, Shieh E, et al. Comprehensive three-dimensional analysis of the neuroretinal rim in glaucoma using high-density spectral-domain optical coherence tomography volume scans. Invest Ophthalmol Vis Sci. 2016;57(13):5498–5508. doi: 10.1167/iovs.16-19802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simavli H, Poon LY, Que CJ, et al. Diagnostic capability of reripapillary Retinal volume measurements in glaucoma. J Glaucoma. 2017;26(6):592–601. doi: 10.1097/IJG.0000000000000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan KC, Tsikata E, Khoueir Z, et al. Enhanced diagnostic capability for glaucoma of 3-dimensional versus 2-dimensional neuroretinal rim parameters using spectral domain optical coherence tomography. J Glaucoma. 2017;26(5):450–458. doi: 10.1097/IJG.0000000000000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khoueir Z, Jassim F, Poon LY, et al. Diagnostic capability of peripapillary three-dimensional retinal nerve fiber layer volume for glaucoma using optical coherence tomography volume scans. Am J Ophthalmol. 2017 doi: 10.1016/j.ajo.2017.08.001. Epub ahead of print. Aug 12 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Susanna R, Jr, Vessani RM. New findings in the evaluation of the optic disc in glaucoma diagnosis. Curr Opin Ophthalmol. 2007;18(2):122–8. doi: 10.1097/ICU.0b013e328040bfe0. [DOI] [PubMed] [Google Scholar]
- 40.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 41.Hirasawa H, Tomidokoro A, Araie M, et al. Peripapillary retinal nerve fiber layer thickness determined by spectral-domain optical coherence tomography in ophthalmologically normal eyes. Arch Ophthalmol. 2010;128(11):1420–6. doi: 10.1001/archophthalmol.2010.244. [DOI] [PubMed] [Google Scholar]
- 42.Jansonius NM, Schiefer J, Nevalainen J, Paetzold J, Schiefer U. A mathematical model for describing the retinal nerve fiber bundle trajectories in the human eye: average course, variability, and influence of refraction, optic disc size and optic disc position. Exp Eye Res. 2012;105:70–8. doi: 10.1016/j.exer.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Lamparter J, Russell RA, Zhu H, et al. The influence of intersubject variability in ocular anatomical variables on the mapping of retinal locations to the retinal nerve fiber layer and optic nerve head. Invest Ophthalmol Vis Sci. 2013;54(9):6074–82. doi: 10.1167/iovs.13-11902. [DOI] [PubMed] [Google Scholar]
- 44.Law SK, Tamboli DA, Ou Y, Giaconi JA, Caprioli J. Development of a resident training module for systematic optic disc evaluation in glaucoma. J Glaucoma. 2012;21(9):601–7. doi: 10.1097/IJG.0b013e31821db3c7. [DOI] [PubMed] [Google Scholar]
- 45.Chan HH, Ong DN, Kong YX, et al. Glaucomatous optic neuropathy evaluation (GONE) project: the effect of monoscopic versus stereoscopic viewing conditions on optic nerve evaluation. Am J Ophthalmol. 2014;157(5):936–44. doi: 10.1016/j.ajo.2014.01.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


