Abstract
Interleukin (IL)-15 is essential for natural killer (NK), NKT and memory (m) CD8+ T cell development and function, and is currently under investigation as an immunotherapeutic agent for the treatment of cancer. Recently, the creation of IL-15 superagonist by complexing IL-15 and its high affinity receptor alpha (IL-15 Rα) in solution, inspired by the natural trans-presentation of IL-15, advances the potential of IL-15-based tumor immunotherapy. IL-15 superagonist shows promising advantages over monomeric IL-15 such as sustaining high circulating concentrations due to prolonged half-life and more potently stimulating NK and CD8+ T effector lymphocytes. So far, there are three different forms of recombinant IL-15 superagonist fusion protein based on configurational modifications. Gene therapy using engineered cells co-expressing IL-15/IL-15 Rα complex for cancer treatment is also emerging. All forms have demonstrated efficacy in causing tumor regression in animal studies, which provides strong rationale for advancing IL-15 superagonist through clinical trials. To date, there are fourteen phase I/II IL-15 superagonist trials in cancer patients and one phase I trial in HIV patients. Information generated by ongoing trials regarding the toxicity and efficacy of IL-15 superagonist is awaited. Finally, we elaborate on immunotoxicity caused by IL-15 superagonist in preclinical studies and discuss important safety considerations.
Graphical abstract
Introduction
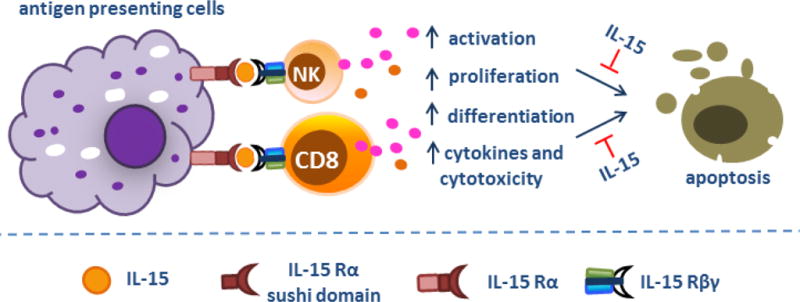
IL-15 is a 14–15 kDa four-helix bundle cytokine that is crucial for natural killer (NK), NKT and memory (m) CD8+ T cell function and homeostasis. IL-15 is minimally secreted but is effectively delivered by trans-presentation in association with its unique receptor alpha (IL-15 Rα) on the surface of IL-15 producing cells to interact with a receptor complex composed of the IL-2R β and common γ chains on target cells. The combination of IL-15 with IL-15Rα in solution results in the generation of a complex with high biological potency, which has been termed IL-15 superagonist (IL-15 SA). IL-15 SA strongly activates IL-15 responsive cells, particularly NK cells, and promotes anti-cancer and anti-viral functions. As such, IL-15 SA is being investigated as an agent to treat cancer and viral diseases.
I. Interleukin (IL)-15 and IL-15 receptor: Expression, trans-presentation and signaling
IL-15 was first identified in 1994 by Grabstein et al as a T lymphocyte growth factor that shares approximately 19% sequence homology and many of the biological properties of IL-2 (1). Like IL-2, the IL-15 three-dimensional structure consists of four-helix “up-up-down-down” bundles. Other cytokines with this structural conformation include IL-4, IL-7 and IL-9. While it is secreted in small quantities, IL-15 is unique among four-helix bundle cytokines in that it is predominantly trans-presented in conjunction with its high affinity receptor α for delivery to target cells (2). The unique high-affinity IL-15 receptor alpha, which is expressed by IL-15-producing cells, such as macrophages and dendritic cells, chaperones IL-15 through the cell and shuttles it to the cell surface for delivery to NK, NKT and memory CD8+ T cells expressing IL-15 receptor β (also known as IL-2 receptor β) and the common γ chain (shared with other cytokines including IL-2, IL-4, IL-7, IL-9 and IL-21) (3, 4) (Figure 1). Although IL-15 and the other four-helix bundle cytokines interact with a common receptor, it is likely the unique mode of presentation that confers the ability of IL-15 to mediate its distinctive functions. Mouse IL-15 shares 70% amino acid sequence identity with human IL-15 and both human and mouse IL-15 have similar properties of trans-presentation, signal transduction and biological activities (5, 6). Interestingly, a study by Neely et al indicated that Il-15 expressed on the surface of human monocytes can induce reverse signaling and cause augmentation of monocyte adhesion, activation of MAP kinase signaling and IL-8 secretion (7).
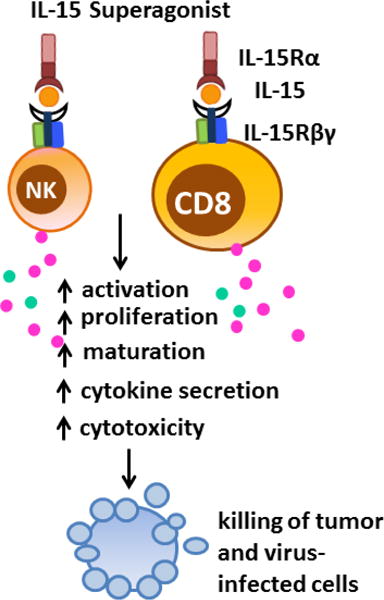
Figure 1. IL-15 transpresentation and biological functions.
Unlike most cytokines, which are secreted in soluble form, IL-15 is expressed in association with its high affinity IL-15 Rα on the surface of IL-15-producing cells and delivers signals to target cells that express IL-2 Rβ/γc receptor subunits. IL-15 stimulates proliferation and activation of NK, NKT and CD8+ T cells, especially memory phenotype CD8+ T cells, leading to increased cytotoxicity and production of IFN-γ and IFN-α. In addition, IL-15 inhibits apoptosis of immune cells by increasing expression of anti-apoptotic and decreasing production of pro-apoptotic proteins. Abbreviations: NK, natural killer cells; CD8, CD8+ T lymphocytes.
IL-15 mRNA is constitutively expressed by a wide variety of cells including dendritic cells, monocytes, macrophages, bone marrow stromal cells, and intestinal epithelial cells (8, 9). IL-15 can be further induced by stimulation with the gram negative bacterial product lipopolysaccharide (LPS), type I (IFNα/β) and type II (IFNγ) interferons (IFN), double-stranded RNA, and infection with viruses (10, 11). Trans-presentation of IL-15 is required for development and homeostasis of IL-15-dependent cell lineages and regulation of their distinct biological functions (2, 12, 13).
Trans-presented IL-15/IL-15 Rα signals through β and γ chains expressed on responding cells, leading to the recruitment and activation of Janus kinase 1 and 3 (JAK1 and JAK3) (13). Activated JAK1 and JAK3 further phosphorylate signal transducer and activator of transcription proteins 3 and 5 (STAT3 and STAT5), which prompts the transcription of IL-15-modulated genes in effector cells (Figure 2) (13). IL-15 alone can also bind to the intermediate-affinity β and γ receptor complex in the absence of the high affinity receptor α, resulting in the activation of other tyrosine kinases such as Lck, Fyn, Lyn, Syk and cross talk with the PI3K and MAPK pathways (6). Recent studies showed that the metabolic checkpoint kinase mTOR was also activated by high concentrations of IL-15 and was associated with enhanced proliferation and activation of NK cells (14, 15). Selective knockout of mTOR resulted in an NK cell maturation block in bone marrow and defective proliferation in response to high doses of IL-15. The ability of IL-15 to facilitate NK cell proliferation is likely mediated, in part, by IL-15-mediated augmentation of aerobic glycolysis since NK cell basal metabolism is low in the absence of IL-15 but is markedly enhanced by the addition of IL-15. Other investigators have confirmed the importance of IL-15-induced activation of the PI3K-Akt-mTOR pathway during NK cell maturation and early responses to viral infections (16). Interestingly, late NK cell proliferative responses to viral infection appear to be independent of the PI3K-Akt-mTOR pathway. Despite these observations, the precise mechanisms by which IL-15 activates PI3K-Akt-mTOR signaling have not been fully determined.
Figure 2. IL-15 signaling pathway.
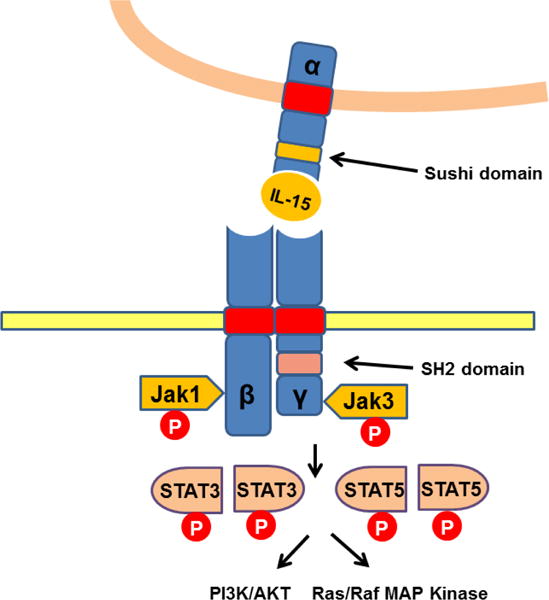
IL-15 is transpresented in association of IL-15 Rα to the IL-2 receptor β and γc subunits, leading to phosphorylation and activation of Janus kinase 1 (Jak1) and Jak3, respectively. Activated Jak1 and Jak3 result in phosphorylation and activation of signal transducer and activator of transcription 3 (STAT3) and STAT5.
II. IL-15 superagonist fusion protein
A. RLI (IL-15-linker-IL-15 Rα sushi domain)
1. Composition
In 2006, RLI was generated by a French group who coupled human IL-15 with the high-affinity IL-15 binding domain (sushi domain) of human IL-15 Rα through a linker (17) (Figure 3). IL-15 Rα, like IL-2 Ra, comprises its extracellular part at the N-terminus, which has been shown to hold most of the structural elements responsible for IL-15 binding with high affinity (Kd = 100 pM). This particular structure in IL-15 Rα, so called sushi domain, has been shown to enhance biological activities of IL-15 through cells expressing IL-15 Rβ/γc receptor subunits (17, 18). Mature IL-15 Rα contains other 4 domains, a linker region, the Pro/Thr-rich domain, the transmembrane domain and cytoplasmic tail (19, 20). As opposed to IL-15 Rα sushi domain, the cytoplasmic domain of IL-15 Rα is not necessary for IL-15 transpresentation (21).
Figure 3. Configurational structure of IL-15 superagonist fusion protein.
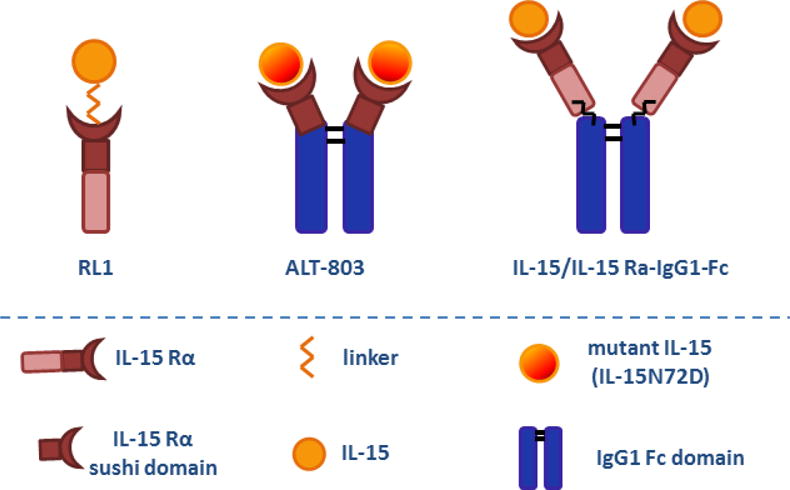
RLI is a recombinant IL-15 superagonist composed of human IL-15 with the high-affinity IL-15 binding domain (sushi domain) of human IL-15 Rα through a linker, while ALT-803 is constructed by a high-yield recombinant mammalian cell line that co-expresses a human IL-15 mutant (IL-15N72D) and the sushi domain of human IL-15 Rα fused with the Fc domain of IgG1. Additionally, IL-15 superagonist can be generated by coupling IL-15 and IL-15 Rα-IgG1-Fc chimera in solution through a high-affinity non-covalent association.
2. IL-15 Rα sushi domain, but not the entire IL-15 Rα, is required for boosting IL-15 activity in RLI
A soluble form of the complete murine IL-15 Rα complex corresponding to the entire extracellular structure (sushi domain + linker + Pro/Thr rich region) has been reported to function as an antagonist for IL-15 (22, 23). These findings are consistent with those of Mortier et al who showed that a natural form of soluble human IL-15 Rα competed with membrane-associated IL-15 Rα for binding to IL-15 and inhibited IL-15-induced cell proliferation (24). However, the IL-15 Rα sushi domain alone functions as an agonist and its binding with IL-15 potently activates IL-15 Rβ/γc complexes (17). The antagonist effect of soluble IL-15 Rα for IL-15 is caused by the exon-3 encoded 13-amino acid domain of IL-15 Rα, which exists adjacent to the sushi domain and creates a sterical constraint preventing IL-15 binding to the membrane-anchored IL-15 Rβ/γc complexes (25). However, a recent paper from Chertova et al showed a heterodimer of IL-15 and the whole extracellular region of IL-15 Rα produced from engineered human cell lines significantly increased the proliferation of NK and CD8+ T cells in vivo (26). The contained IL-15 Rα was modified at a post-translational level intracellularly, mostly by O- and N-linked glycosylation and thus boosted, but not inhibited, IL-15 bioactivity as compared to exogenous soluble IL-15 Rα (26, 27). This contention is further supported by further studies that employed plasmids co-expressing IL-15 and the extracellular domain of IL-15 Rα (28, 29).
3. Immunostimulatory functions and anti-tumor effect of RLI
RLI has been shown to greatly enhance the reconstitution of human NK and CD8+ T cells in humanized mice (30). In particular, RLI promoted proliferation and differentiation of human NK cell progenitor cells to mature peripheral NK cells with a CD56loCD16+KIR+ phenotype. In addition, expression of CD69, Bcl-2 and Bcl-xl in the reconstituted NK cells was dramatically upregulated by RLI, suggesting increased activation and longer life span (30). Therefore, all the findings indicate the therapeutic potential of RLI in NK cell- and CD8+ T cell-based cancer immunotherapeutic strategies. Indeed, RLI significantly decreased tumor burdens in mice with metastatic mouse melanoma or human colorectal carcinoma and improved long-term survival (31). A fusion protein composed of RLI and anti-GD2 antibody, which targets the tumor-associated disialoganglioside, not only retains the immunostimulatory potential of IL-15 but also improved the accuracy of tumor cell killing through anti-GD2 antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (32). In addition, RLI has been shown to activate both IL-15R α/β/γc and IL-15R β/γc and exhibit more efficacy than the noncovalent association of IL-15 and IL-15 Rα-IgG1-Fc, which only activated IL-15R βγ (17, 25, 30) (Table I).
Table I.
RLI and RLI analogs.
| Research group | IL-15 superagonsit | Effect on NK cells | Effect on CD8+ T cells | Improved survival and/or tumor clearance of | Anti-tumor effects mediated by |
|---|---|---|---|---|---|
| Vincent et al | RLI-anti-GD2 fusion protein | NA | NA | T cell lymphoma and metastatic neuroblastoma | NA |
| Huntington et al | RLI | ↑reconsitution of human NK cells in humanized mice; CD69, Bcl-2 and Bcl-xl | ↑reconsitution of human CD8+ T cells in humanized mice | NA | NA |
| Bessard et al | RLI | NA | NA | Metastatic mouse melanoma and human colorectal cancer | NK cells |
| Kermer et al | IL-15-IL-15 Rα sushi domain-4-1BBL-anti-tumor antibody | NA | ↑proliferation, IFN-γ and cytotoxicity | Metastatic melanoma | NA |
| Kermer et al | IL-15-IL-15 Rα sushi domain-anti-FAP | ↑proliferation | ↑proliferation | FAP metastatic + melanoma | NA |
| Ochoa et al | IL-15 Rα sushi domain-IL-15-Apo A-I protein | ↑number | ↑number, especially memory subset | Metastatic melanoma and colorectal carcinoma | NK and CD8+ T cells |
| Chertova et al | IL-15/IL-15 Ra | ↑proliferation | ↑number | NA | NA |
FAP: fibroblast activation protein
4. RLI analog (IL-15-IL-15 Rα sushi domain) shows a similar agonistic effect of RLI
Ochoa et al reported a triple fusion protein combining apolipoprotein A-I (Apo A-I), IL-15, and IL-15 Rα sushi domain (33). Apo A-I is the main protein component of high-density lipoprotein (HDL) and linking IL-15/IL-15 Rα sushi domain to a larger protein, like Apo A-I, significantly increased the stability of IL-15 in serum (33). Although transfer of large inocula of transfer plasmid caused NK cell-mediated immunotoxicity, transfer of lesser amounts of plasmid decreased lung metastases of melanoma and liver metastases of colorectal carcinoma in mice. The anti-tumor efficacy was mediated by expansion of NK and CD8+ T cells, especially memory CD8+ T subset. Purified recombinant IL-15 Rα sushi-IL-15-Apo A-I exhibited similar biological functions and anti-tumor effects. In an additional study, a fusion protein of IL-15-IL-15 Rα sushi domain-anti-FAP (tumor fibroblast activation protein) caused superior targeted anti-tumor killing on site, which provided rationale for future development of antibody-IL-15-IL-15 Rα sushi domain fusion proteins for cancer immunotherapy (34).
B. IL-15/IL-15 Rα-IgG1-Fc complex
1. IL-15/IL-15 Rα-IgG1-Fc complex profoundly activates NK, NKT and memory CD8+ T cells
Soon after the emergence of RLI, Rubinstein et al generated IL-15 superagonist which constitutes IL-15 and IL-15 Rα-IgG1-Fc chimera in solution through a high-affinity non-covalent association (35) (Figure 3). The IL-15/IL-15 Rα-Fc complex contains two IL-15 and two effective IL-15 binding (sushi) domains of IL-15 Rα per unit and has been shown to exhibit potent immunostimulatory effects on NK, NKT and CD8+ T cells (35, 36). In particular, the IL-15/IL-15 RαFc complex promoted proliferation of all three cell populations in spleens, livers, peripheral blood and bone marrow; preferentially expanded conventional pro-inflammatory (CD27highCD11bhigh) NK, type II (CD3+NK1.1+α-Galcer−CD1d tetramer−) NKT, and (antigen-specific) memory CD8+CD44high T cell subsets and potently activated NK cells as indicated by increased expression of activating receptors such as NKG2D, NKp46 and downregulation of inhibitory receptor expression; it also enhanced the ability of NK cells to secrete the pro-inflammatory cytokine IFN-γ and promoted perforin- and granzyme-mediated cytotoxicity. The cytotoxicity of memory CD8+ T cells was enhanced as evidenced by increased production of granzyme B (35–40) (Table II).
Table II.
IL-15/IL-15 Rα-IgG1-Fc.
| Research group | IL-15 superagonsit | Effect on NK cells | Effect on CD8+ T cells | Improved survival and/or tumor clearance of | Anti-tumor effects mediated by |
|---|---|---|---|---|---|
| Epardaud et al | IL-15/IL-15 Ra- IgG1-Fc | ↑number | ↑number, IFN granzyme B -γ and | Metastatic melanoma and pancreatic tumor | Tumor-resident, but neither circulating CD8+ nor NK or NKT cells |
| Dubois et al | IL-15/IL-15 Ra- IgG1-Fc | ↑proliferation and killing | ↑proliferation, especially memory subset | Metastatic melanoma | NK cells, but not T or B cells |
| Rubinstein et al | IL-15/IL-15 Ra- IgG1-Fc | ↑proliferation | ↑proliferation, especially antigenspecific memory subset | NA | NA |
| Guo et al | IL-15/IL-15 Ra- IgG1-Fc | ↑number, CD69, IFN-γ, granzyme B and perforin | ↑number, especially memory subset, and granzyme | NA | NA |
| Stoklasek et al | IL-15/IL-15 Ra- IgG1-Fc | ↑proliferation | ↑proliferation, especially memory subset; differentiation to effector and long-term memory subsets | Metastatic melanoma | NA |
| Huntington et al | IL-15/IL-15 Rα- IgG1-Fc | ↑reconsitution of human NK cells in humanized mice; CD69, Bcl- 2 and Bcl-xl; | ↑reconsitution of human CD8 T cells + in humanized mice | NA | NA |
| Wu et al | IL-15/IL-15 Ra- IgG1-Fc | ↑number and granzyme B | ↑number | NA | NA |
FAP: fibroblast activation protein.
2. IgG1-Fc portion may not be necessary for the agonist effect of IL-15/IL-15 Rα complex
Although most IL-15 superagonists in the literature are made up of IL-15 with IL-15 Rα-IgG1-Fc, the IL-15 Rα monomer without the Fc portion was shown to be even more effective in stimulating IL-15-mediated expansion of CD8+ T cells as compared to IL-15 Rα-IgG1-Fc complex (35). However, Dubois et al found that addition of Fc portions of immunoglobins to IL-15 Rα substantially enhanced the ability of IL-15 to stimulate NK cell proliferation as compared to IL-15 Rα alone (39). Thus, there is some controversy regarding the necessity of Ig Fc portion in IL-15 superagonist for maximal activity. Dubois et al further reported that the enhanced effect of IL-15 superagonist with IgG Fc portions may not be mediated by Fc-FcR interaction and signaling. This contention was supported by evidence that IL-15 superagonist induced similar proliferation of NK and CD8+ T cells in WT mice treated with Fc receptor neutralizing antibody as compared to vehicle. Similar results were observed after use of IL-15 superagonsit with mutated IgG1-Fc portion (39).
3. IL-15/IL-15 Rα-IgG1-Fc complex showed promising anti-tumor efficacy in preclinical studies
The IL-15/IL-15 Rα-Fc complex caused regression of solid and metastatic tumors and prolonged long-term survival in mice bearing B16 melanoma and pancreatic tumors (36, 39, 40). The augmented antitumor activity was associated with augmented proliferation and activation of NK, NKT and mCD8+ T cells. Eparduad and colleagues reported that IL-15/IL-15 Rα-Fc complex-mediated anti-tumor activity was mediated by tumor-resident T cells rather than those migrating to the tumor site (40).
C. ALT-803 (IL-15N72D/IL-15 Rα-IgG1-Fc chimera)
1. Immunological functions and efficacy of ALT-803 in experimental tumor models
ALT-803 is a novel IL-15 superagonist which is constructed by a high-yield recombinant mammalian cell line that co-expresses a human IL-15 mutant (IL-15N72D) and the sushi domain of human IL-15 Rα fused with the Fc domain of IgG1(41, 42) (Figure 3). Altor’s scientists have performed extensive studies on determining the anti-tumor efficacy of ALT-803 in different experimental models. ALT-803 has been shown to significantly stimulate CD8+ T cells, especially memory CD8+ T cells, to secrete substantial amount of IFN-γ and enhanced cytotoxicity against multiple myeloma (42). In a mouse model of glioblastoma, ALT-803 prolonged survival of tumor-bearing mice, as a single treatment as well as in combination with anti-PD-1 antibody (43). In this model, ALT-803 not only increased the percentage of CD8+ T cells infiltrating into the microenvironment, but more importantly, established a long-term immune memory against tumor rechallenge. ALT-803 also improved NK cell-mediated granulation, IFN-γ production and antibody-dependent cell-mediated cytotoxicity (ADCC) in combination with anti-CD20 monoclonal antibodies against B cell lymphoma (44, 45). In addition, ALT rescued functionality of NK cells from patients with ovarian cancer and enhanced NK cell cytotoxicity against ovarian cancer cell lines in vitro and in vivo (46). In a separate mouse model of breast carcinoma, activated NK and CD8+ T cells jointly contributed to ALT-803-mediated prevention of tumor metastasis and enhancement of long-term survival (47). Additional studies reported that combined treatment of ALT-803 with Bacillus Calmette-Guerin (BCG) enhanced anti-tumor efficacy in rat bladder cancer by reducing angiogenesis in the tumor microenvironment, decreasing proliferation and enhancing apoptosis of tumor cells (48) (Table III).
Table III.
ALT-803.
| Research group | IL-15 superagonsit | Effect on NK cells | Effect on CD8+ T cells | Improved survival and/or tumor clearance of | Anti-tumor effects mediated by |
|---|---|---|---|---|---|
| Xu et al | ALT-803 | NA | ↑number of memory subset; NKG2D and IFN -γ | Multiple myeloma | CD8+ T cells and IFN- γ, but not NK cells |
| Felices et al | ALT-803 | ↑CD107a, IFN-γ, TNF- α, and cytotoxicity of NK cells from cancer patients | NA | Ovarian cancer | NK cells |
| et al Rosario | ALT-803 + anti-CD20 mAb | ↑degranulation, IFN-γ and ADCC of human NK cells | NA | B cell lymphoma | NK cells |
| Liu et al | ALT-803 fused with anti-CD20 | ↑number, ADCC. Granzyme and perforin | ↑number and granzyme | B cell lymphoma | NA |
| Mathios et al | ALT -803 and/or anti-PD-1 | ↑number of tumor- infiltrating NK cells | ↑number of tumor- infiltrating CD8 T cells and IFN-γ + | Glioblastoma | CD8+ T, CD4+ T cells and IFN- γ, but not NK cells |
| Kim et al | ALT-803 and/or anti-CTLA4 and anti-PD-L1 | ↑preferentially expands CD27highCD11bhigh NK cell subset | ↑number of memory subset with innate property (NKG2D+PD-1−) | Metastatic breast and colon cancer | CD8+ T cells and NK cells |
| Gomes Giacoia et al | ALT-803 + BCG | ↑concentrations of NK cell-activating cytokines in serum | NA | Bladder cancer | might NK cells |
mAb: monoclonal antibodies; anti-PD-1: programmed death-1; anti-PD-L1: programmed death ligand 1; anti-CTLA-4: cytotoxic T-lymphocyte–associated antigen 4.
2. Combined treatment of ALT-803 with other immunotherapies enhanced tumor clearance
It is noteworthy to mention that combined therapy with the checkpoint inhibitors like anti-PD-1 (programmed death-1), anti-PD-L1 (programmed death ligand 1) or anti-CTLA-4 (cytotoxic T-lymphocyte–associated antigen 4), greatly enhanced the efficacy of ALT-803 in remission of primary and advanced tumors in different preclinical studies (43, 47). These findings have led to booming interest of scientists to take advantage of the combined immunotherapies in the treatment of malignant cancer. A couple of clinical trials are currently ongoing to explore the therapeutic potential of ALT-803 in patients with advanced cancer, either as single treatment or combined with standard anti-tumor agents or approaches (will be discussed Chapter VI).
3. Human IL-15 cross-reacts with murine cells, but murine IL-15 has little effect on human cells
Although ALT-803 is a human IL-15 superagonist (human IL-15N72D/human IL-15 Rα-IgG1-Fc), it has been shown to exhibit effectiveness against both human and murine tumor cells (44, 45, 47, 48). Human IL-15 shares 70% amino acid sequence identity with murine IL-15. Both human and murine IL-15 exhibit similar properties of trans-presentation, signal transduction and biological activities (5, 6). Notably, human IL-15 was more active (~ 700 fold) than murine IL-15, when signaling through the human IL-15 Rβ/γc complex, while murine IL-15 was more active (~ 200 fold) than human IL-15 on the murine IL-15 Rβ/γc complex (49). However, multiple studies showed that human IL-15, combined with either human or murine IL-15 Rα-Fc, cross-reacted on murine NK, NKT and memory CD8+ T cells with profound immunostimulatory effects, at a level comparable to murine IL-15 superagonist (35, 36, 47). However, murine IL-15, regardless of what species of IL-15 Rα it was bound with, failed to promote proliferation of human NK cells (30).
III. Engineered cells expressing IL-15/IL-15 Rα complex: Gene therapy
In 2005, a study from Tagaya’s group, who discovered IL-15 transpresentation, provided evidence that transfection of IL-15 Rα into a colon carcinoma cell line allowed these tumor cells to trans-present endogenous or exogenous IL-15 to NK cells and thus enhanced NK cell killing activity (50). These IL-15 Rα+ tumor cells failed to form tumor metastases and mice bearing IL- 15 Rα+ tumors showed better survival compared to those bearing wild type tumor cells. Other tumor cell lines transfected with IL-15/IL-15 Rα-expressing vector have also demonstrated high expression of IL-15/IL-15 Rα on the surface and improved NK and CD8+ T cell-mediated tumor lysis in vivo (51). Furthermore, Morris et al showed that vaccination of mice with engineered tumor cells expressing IL-15/IL-15 Rα significantly improved survival following subsequent challenge with the same non-transfected tumor cell line (52). Thus, the administration of tumor vaccine with engineered tumor cells may hold promise for long-term protection against tumor development in high-risk patients (Figure 4).
Figure 4. IL-15/IL-15 Rα gene therapy in tumor immunosurveillance.
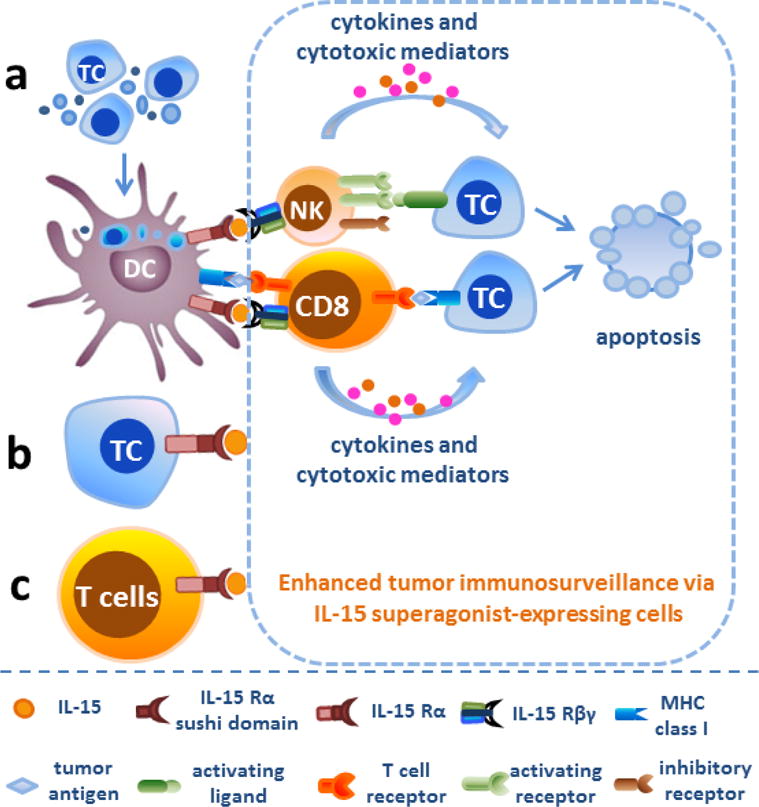
a. Transfection of IL-15 Rα and/or IL-15 into dendritic cells allowed these cells to trans-present endogenous or exogenous IL-15 to NK and CD8+ T cells and thus augmented NK and CD8+ T cell-mediated tumor lysis. b. Vaccination of mice with engineered tumor cells expressing IL-15/IL-15 Rα provides long-term protection against the same non-transfected tumor cell line. c. IL-15 cis presentation by IL-15 Rα-expressing CD8+ T cells or co-expression of IL-15/IL-15 Rα by CD8+ T cells was also able to enhance the viability and proliferation of CD8+ T lymphocytes and prompted T cell differentiation to long-lived T-memory stem cells. Abbreviations: TC, tumor cells; DC, dendritic cells; NK, natural killer cells; CD8, CD8+ T lymphocytes.
Interestingly, IL-15 cis presentation by IL-15 Rα-expressing CD8+ T cells or co-expression of IL-15/IL-15 Rα by CD8+ T cells was also able to enhance the viability and proliferation of these CD8+ T lymphocytes in vivo (53). Peripheral T cells stably coexpressing chimeric antigen receptor (anti-CD19) and IL-15/IL-15 Rα prevented leukemia engraftment and sustained resistance after tumor clearance by displaying phenotypes associated with long-lived T-memory stem cells (54). Dendritic cells are primary immune cells transpresenting IL-15 chaperoned by IL-15 Rα to NK and CD8+ T cells (55). One study from Steel et al showed a DC vaccine expressing both IL-15/IL-15 Rα plasmid and an oncoprotein enhanced host anti-tumor response against mammary carcinoma (56). A similar effect was observed when IL-15/IL-15 Rα-transduced DCs were administered to improve NK cell-mediated killing of the human Burkitt’s lymphoma cell line (57). In addition, oncolytic type-1 herpes simplex virus (oHSV) expression of IL-15 and IL-15 Rα resulted in soluble IL-15/IL-15 Rα production and greatly enhanced NK cell cytotoxic killing of tumor cells, although these tumor cell lines are not permissive to oHSV replication and killing (58). Improved anti-tumor effect was also observed in myxoma virus expressing a fusion protein of IL-15/IL-15 Rα, which increased the number of tumor-infiltrating NK and CD8+ T cells in mice challenged with melanoma (59).
In a simple way, systemic injection with plasmid co-expressing murine IL-15/murine IL-15 Rα complex resulted in production of serum IL-15/IL-15 Rα at a level sufficient to inhibit tumor growth of metastatic lung and hepatocellular cancer and significantly improved long-term survival, especially when combined with chemotherapy (29). Cheng and colleagues delivered IL-15/IL-15 Rα-expressing adenovirus in a model of hepatocellular carcinoma as an effective way to cause regression of hepatic metastases (28) (Table IV).
Table IV.
Engineered cells expressing IL-15/IL-15 Rα complex.
| Research group | IL-15/IL-15 Ra expressed by | Effect on NK cells | Effect on CD8+ T cells | Improved survival and/or tumor clearance of | Anti-tumor effects mediated by |
|---|---|---|---|---|---|
| Steel et al | Tumor antigen-expressing DCs | NA | No activation | Mammary carcinoma | Antibody response, but not NK or CD8+ T cells |
| Van den Bergh et al | Human DCs | ↑activation, IFN-γ and cytotoxicity | NA | Human B lymphoblast cell line in vitro | NK cells |
| Hurtona et al | T cells | NA | Acquire T-memory stem cell phenotype | B-cell leukemia | IL-15/IL 15 Ra - - co-expressing T cells |
| Rowley et al | CD8+ T cell | NA | ↑viability, proliferation and IFN-γ | NA | NA |
| Rowley et al | Tumor cells | ↑number of tumor- infiltrating NK cells; granzyme B | ↑number of tumor- infiltrating CD8+ T cells | Transfected glioblastoma and ovarian carcinoma cells | NK and CD8 T cells + |
| Kobayashi et al | Tumor cells | ↑cytotoxic killing | No strong activation | Transfected colon carcinioma cells | NK but not CD8+ T cells |
| Chang et al | Hepatotropic adeno-associated virus | ↑number and cytotoxic killing | ↑number | Hepatocellular carcinoma | NK but not CD8+ T cells |
| Morris et al | Tumor cells | ↑tumor- infiltrating NK cells | ↑tumor-infiltrating CD8+ T cells, killing and IFN-γ | Prostate and breast tumor | NK and CD8+ cells T |
| Tosic et al | Myxoma virus | ↑number of tumor- infiltrating NK cells | ↑the number of tumor-infiltrating CD8+ T cells | Melanoma | NK and CD8 T cells + |
| Gaston et al | Oncolytic HSV-1 | ↑proliferation and killing | NA | Neuroblastoma and glioma in vitro | NK cells |
| Cheng et al | Plasmid | ↑number | ↑number and killing | Metastatic and primary hepatocellular carcinoma | CD8+ T cells but not NK cells |
| Bergamaschi et al | Plasmid | ↑number | ↑number of effector memory CD8+ T cells | NA | NA |
| Sun, et al | Plasmid | ↑granzyme B, perforin and IFN- γ | ↑CD8 and Tbet mRNA | Metastatic lung cancer, especially with chemotherapy | NA |
IV. Comparison of IL-15 superagonist to IL-15
Multiple studies show that IL-15 SA has a prolonged half-life compared to free IL-15 in serum (~20 hours vs ~ 1 hour) and facilitates an enhanced IL-15 response by ~ 5–50 fold with regard to NK and memory CD8+ T cell proliferation, cytokine secretion and cytotoxic function (35, 36, 60). In mouse models, IL-15 superagonist caused regression of melanoma, pancreatic tumor, glioblastoma and Burkitt′s lymphoma, whereas IL-15 alone did not (31, 40, 57, 58). In addition, IL-15/IL-15Ra fusion proteins and IL-15/IL-15Ra–expressing adenovirus expression systems have also been more efficacious than IL-15 in improving survival and/or tumor clearance of primary lung cancer, mammary tumor, prostate cancer and metastatic colon carcinoma (50–52, 56) (Table V).
Table V.
Comparison of IL-15 superagonist to IL-15
| Agents | Half-life and lymphoid organ distribution | Effect on NK cells | Effect on CD8+ T cells | Anti -tumor effect |
|---|---|---|---|---|
| IL- 15 | ~40 minutes- in serum 1 hour | ↑proliferation NKp30, NKp44, NKG2D, CD69, CD56, IFN-γ and cytotoxic killing | ↑proliferation | No effect on melanoma,pancreatic tumor, glioblastoma, Burkitt′s lymphoma ; limited effect on primary lung cancer, mammary tumor, prostate cancer, metastatic colon carcinoma |
| IL-15 superagonist | ~20–25 hours in serum; a longer time in lymphoid organs | ↑↑proliferation; ↑↑NKp30, NKp44, CD69, CD56, IFN-γ and cytotoxic killing |
↑↑proliferation of total and memory CD8+ T cells; naïve cells acquire effector or memory phenotype | ↑↑survival and/or tumor regression of melanoma, pancreatic tumor, lung cancer, glioblastoma, mammary cancer, prostate cancer, multiple myeloma, metastatic colon carcinoma |
V. Anti-viral effects of IL-15 superagonist
IL-15 is known to be critical for adequate NK and CD8+ T cell expansion and functions (61). One of the main functions of NK and CD8+ T cells is to kill virus infected cells, such as those infected with human immunodeficiency virus (HIV). Virus specific cytotoxic CD8+ T lymphocytes play a critical role in controlling HIV disease progression (62). IL-15 has been shown to inhibit apoptosis of HIV-specific CD8+ T cells, and increase their activation, expansion, IFN-γ production and cytotoxic capability (63, 64). HIV infection impairs NK cell responses, causing diminished cell proliferation and IFN-γ secretion (65, 66). A pivotal study by Chehimi et al., showed that IL-15 treatment enhanced cytotoxicity of NK cells obtained from HIV patients and substantially reduced apoptosis-mediated cell death among PBMCs (67). In vitro studies have shown that NK cells have the potential to suppress HIV replication as effectively as CD8+ T cells, presumably mediated by secretion of CC-chemokines (68). These results show that IL-15 should be further investigated for its possible therapeutic efficacy to augment and restore NK cell and CD8+ T cell immune responses during HIV infection.
As discussed earlier, IL-15 superagonist has a considerably higher potency as compared to IL-15 alone. To date, only one pre-clinical study showing the beneficial effect of IL-15 SA for HIV infection in a humanized murine model of HIV infection has been published (69). In this important study, Seay et al. reported that treatment with IL-15 superagonist (ALT-803) markedly inhibited acute HIV-infection in a humanized mouse model, even when IL-15 SA treatment is delayed until 3 days after HIV-1 infection. Activated NK cells were found to be the major immune cells responsible for suppression of HIV-1 infection in this study, as NK cell depletion abrogated the protective effect. Based on this study, it can be concluded that in vivo NK cell stimulation with IL-15 superagonist might represent a new therapeutic strategy to tackle HIV infection. However, in this study, ALT-803 was effective in suppressing primary HIV infection for three weeks only when given 3 days after infection, which is the early stage of acute infection. ALT-803 failed to offer sustained protection when administered at 5 days after infection. In addition, a study by Eberly et al., has demonstrated that IL-15 treatment in macaques led to increased susceptibility of CD4+ T cells to simian immunodeficiency virus infection (70), which subsequently caused increased viral loads and accelerated disease progression (71). Thus, it is important to acquire information regarding adverse effects and therapeutic efficacy of IL-15 superagonist through clinical studies, one of which is actively underway to evaluate safety, tolerability and potential efficacy of ALT-803 in HIV patients.
VI. IL-15 superagonist clinical development
Over the past decades, immunotherapy has been a rapidly evolving field of basic and clinical research. The de-inhibition of lymphocytes by antibodies that block co-inhibitory receptors such as PD-1and CTLA-4 on T lymphocytes are effective in treating advanced cancers (72, 73). Emerging evidence shows cytokine-based treatments also hold promise for the treatment of cancer and viral diseases. IL-15 has emerged as one of the highly-promising candidates in immunotherapy and is currently undergoing clinical trials to test its efficacy for treatment of patients with advanced cancer (74).
IL-15 superagonist has demonstrated improved biological activity and augmented anti-tumor potency compared to native IL-15 in preclinical studies, thus having generated robust interest in the application of IL-15 superagonist as an anti-cancer agent (31, 40, 50–52, 56–58). As seen in Table VI, there are a total of nine actively ongoing and five prearranged phases I/II clinical trials in cancer patients with IL-15 superagonist and one phase I clinical trial on HIV patients at this time. The multi-institutional cancer clinical studies are designed to determine the safety and efficacy of IL-15 superagonist, mostly ALT-803, in patients with advanced cancer. The types of cancer under study are wide-ranging, including both solid and hematological malignancies such as melanoma, renal cell carcinoma, non-small cell lung and squamous cell head and neck cancer, as well as multiple myeloma and B cell lymphoma. ALT-803 was administrated to cancer patients with escalating doses to determine MTD (maximum tolerated dose) and MED (minimum efficacious dose) as well as DLT (dose-limiting toxicity). In some studies, ALT-803 was combined with other immunological therapies such as Nivolumab (anti-PD-1 monoclonal antibody), Rituximab (anti-CD20 monoclonal antibody) or adoptive transfer of NK cells to boost anti-tumor effect in patients with advanced tumors. Taken together, these clinical trials will advance our current knowledge of IL-15 superagonist for its safety profile, pharmacokinetics and effectiveness in treatment of human patients and may promote its fast track to the market as a novel cytokine immunotherapy.
Table VI.
IL-15 superagonist clinical development.
| Trial No. | IL-15/IL-15 Rα complex therapy | Combination | Malignancy | Phase | Status |
|---|---|---|---|---|---|
| NCT02452268 | IL15/sIL-15 Rα | NA | Metastatic solid cancer | Phase I | recruiting |
| NCT02191098 | ALT-803 | NA | HIV under antiretroviral virus | Phase I | recruiting |
| NCT01946789 | ALT-803 | NA | Advanced solid tumors | Phase I | recruiting |
| NCT01885897 | ALT-803 | NA | Relapsed hematologic malignancy after SCT | Phase I/II | recruiting |
| NCT02099539 | ALT-803 | NA | Relapsed or refractory indolent multiple myeloma | Phase I/II | recruiting |
| NCT02384954 | ALT-803 | Rituximab | Relapse/refractory B cell non-Hodgkin lymphoma | Phase I/II | recruiting |
| NCT02138734 | ALT-803 | BCG | Non-muscle invasive bladder cancer | Phase Ib/II | recruiting |
| NCT02559674 | ALT-803 | Gemcitabine and Nab-Paclitaxel | Advanced pancreatic cancer | Phase Ib/II | recruiting |
| NCT02523469 | ALT-803 | Nivolumab | Advanced non-small cell lung cancer | Phase Ib/II | recruiting |
| NCT02782546 | ALT-803 | NK cell therapy following SCT | Acute myeloid leukemia | Phase II | recruiting |
| NCT03003728 | ALT-803-treated NK cells | Elotuzumab and SCT | Multiple myeloma | Phase II | not yet recuiting |
| NCT02989844 | ALT-803 | SCT | Myelogenous leukemia and myelodysplastic syndrome | Phase II | not yet recuiting |
| NCT03022825 | ALT-803 | BCG | BCG unresponsive non-muscle invasive bladder cancer | Phase II | not yet recuiting |
| NCT02890758 | ALT-803 | NK cell therapy | Hematologic malignancy, carcinoma and sarcoma | Phase I | not yet recuiting |
| NCT03003728 | ALT-803 | NK cells, elotuzumab post SCT | Multiple myeloma | Phase II | not yet recuiting |
BCG: Bacillus Calmette-Guerin. SCT: stem cell transplant. HIV: human immunodeficiency virus
VII. IL-15 superagonist toxicity
So far there is no evidence showing IL-15 superagonist administration has adverse responses in patients, as all the human clinical trials are still in the process of collecting safety information. However, IL-15, as the primary effective component of IL-15 superagonist, has shown untoward consequences in rodents, nonhuman primates and humans. Genetic overexpression of IL-15 in mice leads to the early genesis of lymphocytic leukemia with a T-NK phenotype (75). Another study confirmed that chronic exposure of IL-15 induces transformation of large granular lymphocyte leukemia in vitro (76). A recent paper reported that IL-15 caused fetal side effects when administered in mice bearing humanized tumors (77). In nonhuman primates, IL-15 was known to cause considerable dose-dependent toxicity such as grade 3/4 transient neutropenia, weight loss and skin rash at higher doses (78). Most recently, the toxicity profile for IL-15 in cancer patients was defined. Conlon and colleagues reported grade 3 hypotension, thrombocytopenia, liver injury, fever and rigors in patients with metastatic malignancies during treatment with human recombinant IL-15 (74).
Our lab, for the first time, showed soluble IL-15/IL-15 Rα complex treatment could cause marked hypothermia, weight loss, splenomegaly and a modest increase in liver enzymes in wild type mice when given at 2 μg for 4 consecutive days but not at lower doses (37). Thus, the toxicity profile of IL-15 family immunobiologicals is similar among species. Conlon and colleagues reported expansion of NK and mCD8+T cells in blood and increased IFNγ concentrations in the plasma of cancer patients receiving IL-15 treatment. However, a cause and effect relationship between immunological alterations with IL-15-associated toxicity was not established. Our study provided a direct link between NK cell expansion and IFNγ production with the immunotoxic responses caused by IL-15 superagonist in mice.
Additional studies evaluated the toxicity of IL-15 superagonist that was expressed by engineered vectors. Chang et al showed adenovirus-delivered IL-15/IL-15 Rα did not cause apparent liver injury in wild type mice (79). Similarly, no neuropathological changes were observed in wild type mice receiving IL-15/IL-15 Rα-expressing herpes simplex viruses intracranially (58). Long-term engraftment with T cells co-expressing chimeric antigen receptors and IL-15/IL-15 Rα complex persisted, but neither proliferated aberrantly nor elicited host toxicity in the absence of tumor (54). However, lethal toxicity was observed in mice receiving a hydrodynamic gene transfer of plasmids expressing IL-15/IL-15 Rα sushi domain/apolipoprotein. Mice died from acute respiratory distress in which NK and T cells dominated a severe pro-inflammatory infiltrate in the lung (33). Thus, there was some controversy regarding the tolerability of engineered IL-15 superagonist in vivo.
It is important to define toxicity profiles of IL-15 superagonist in large-scale and long-term clinical studies. As IL-15 is heavily involved in autoimmune and chronic inflammatory diseases (80–82), IL-15 superagonist may also elicit dangerous pro-inflammatory signaling in chronic settings. Furthermore, IL-15 superagonist treatment has been shown to aggravate septic shock, in which hosts develop overwhelming pro-inflammatory responses to infections during the acute phase, through NK cell activation and IFN-γ production (83–88). Considering that people with cancer are particularly susceptible to developing sepsis compared to the general population (89, 90), IL-15 superagonist should be used with caution as it advances through drug development for cancer patients with complications of sepsis. In addition, sustained stimulation with low-dose IL-15 superagonist caused considerable impairment in activation, cytotoxicity and proliferative activity of NK cells (91). Thus, an optimal treatment regimen is needed to warrant the maximum effectiveness of IL-15 superagonist in cancer patients.
Conclusions
IL-15 is currently on top of the US National Cancer Institute’s ranking of 20 immunotherapeutic drugs with supreme potential for broad application in cancer patients (92). It significantly activates and sustains anti-tumor functions of NK and cytotoxic T cells. Although monomeric human recombinant IL-15 treatment facilitated clearance of tumor lesions in patients participating in the first-in-human clinical trial (74), this form of molecule has posed challenges on clinical trials due to its quick serum clearance and low binding affinity to receptors on target cells (6, 36). Thus, in most clinical trials, continuous infusion of IL-15 at high doses was used to reach sufficient circulating concentrations for priming host immune responses to tumor antigens. However, bolus administration with IL-15 has caused considerable toxicity including grade 3 hypotension, thrombocytopenia, and elevations of ALT and AST in cancer patients, which may prevent further approval of IL-15 as a safe immunotherapy in cancer patients (74). In this review, we showed great advantages of IL-15 superagonist over IL-15 on sustaining high concentrations in the plasma, stimulating NK and CD8+ T effector cells, and mediating effective tumor killing in preclinical studies (36, 50–52, 56). Thus, all the studies we included in this review have provided strong rantionale for advancing IL-15 superagonist through drug development as a more potent “IL-15” in cancer treatment.
As IL-15 superagonist exhibited greater bioactivity than IL-15, it also needs to be used with caution to avoid toxicity. Our group showed mice developed lethal toxicity at high doses of IL-15 superagonist, presenting with hypothermia, cachexia, acute liver injury and death (37). We also showed that hyperproliferation and hyperactivation of NK cells and production of IFN-γ, mediated IL-15 superagonist-associated immunotoxicity, whereas CD8+ T cells played a protective role. Systemic administration with IL-15 superagonist preferentially expanded the pro-inflammatory (CD11bhighCD27high) NK subset in lymphoid organs, which produces large amounts of cytokines, such as IFNγ and TNF-α, and also possesses significant cytotoxic functions (93).
Strategies to promote IL-15 superagonist therapeutic effects and limit its toxicity
For NK cell-mediated immunotoxicity, one possible explanation is that NK cells, which exhibited increased proliferation and an activated phenotype, can’t infiltrate into solid tumors for efficacious killing but would remain in the circulation mediating inflammatory pathology. Tumor cells are able to evade immune system recognition by disrupting antigen presentation, NK or cytotoxic T cell homing and activation (94). Thus, it is important to escort IL-15 superagonist to the tumor microenvironment for attracting and stimulating circulating NK cells. One way to achieve this goal is to couple IL-15 superagonist with monoclonal antibodies against tumor antigens, or to administer IL-15 superagonist topically into solid tumors.
Additionally, the simplest way to limit IL-15 superagonist-mediated immunotoxicity is to decrease the dose needed, while maintaining its anti-tumor efficacy. To achieve this goal, combined treatment with targeted therapies or other immunotherapeutic drugs could be applied. Targeted approaches aim to recognize and block biochemical pathways that are essential for tumour growth and survival, while immunotherapy acts by priming the host immune system for long-term immunity against tumor progression (95). The two approaches could have synergistic effects on tumor killing, thus lowering the dose of immunotherapeutic drug (like IL-15 superagonist) needed and limiting its toxic responses. The de-inhibition of cytotoxic lymphocytes by blocking co-inhibitory receptors such as PD-1 and CTLA-4 on T lymphocytes are effective in treating advanced cancers, which has led to fast track approval of the monoclonal antibodies pembrolizumab (anti-PD-1) and ipilimumab (anti-CTLA-4) for the treatment of metastatic melanoma (72, 73). Combined treatment with these novel immunotherapeutic drugs could also inhibit untoward responses by decreasing the doses of IL-15 superagonist in the treatment regimen.
Different cellular mechanisms IL-15 superagonist utilizes to perform tumor killing
It is also interesting to note that anti-tumor efficacy of IL-15 superagonsit may be mediated through different cellular mechanisms, mostly depending on nature of tumor. Dubois et al reported that the efficacy of IL-15 superagonist in a model of metastatic melanoma was dependent on activation of NK cells, but not T cells (39). Cheng and colleagues found that the protection of IL-15/IL-15 Rα-coexpressing plasmids against metastatic liver cancer was mediated through activation of tumor-specific CD8+ T cells (28). However, when Chang and colleagues delivered IL-15 superagonsit-expressing adenovirus in a model of hepatocellular carcinoma, the anti-tumor efficacy of the IL-15 superagonist-adenovirus construct was mediated by NK cells (79). ALT-803 protected against human B cell lymphoma by NK cells (44), multiple myeloma by CD8+ T cell (41), and metastatic breast and colon cancer by both NK and CD8+ T cells (47). Therefore, it is possible that NK and CD8+ T cells exhibit different affinity recognizing and killing a certain type of tumor cells, which provides a rationale for preferential stimulation of effector cells using IL-15 superagonist in the future drug development.
In conclusion, this review provides significant characterization of specific systemic, cellular and molecular alterations caused by IL-15 superagonist treatment as compared to native IL-15 and compares their anti-tumor effectiveness in multiple preclinical studies. To date, IL-15 superagonist, mostly ALT-803, is undergoing over 10 clinical trials to test its safety and potential efficacy in patients with advanced solid or hematological malignancies. To further improve its anti-tumor efficacy, ALT-803 has been combined with other immunotherapies such as Nivolumab (anti-PD-1 monoclonal antibody), Rituximab (anti-CD20 monoclonal antibody) or adoptive transfer of NK cells. The contents in this review provide an overview of our current knowledge of IL-15 superagonist for its history, pharmacokinetics, immunological functions, adverse responses, and effectiveness in treatment of cancer or virus-associated diseases.
Highlights.
The novel creation of IL-15 superagonist by complexing IL-15 and its high affinity receptor alpha (IL-15 Rα) in solution, inspired by the natural trans-presentation of IL-15, advances the potential of IL-15-based tumor and anti-viral immunotherapy.
The half life of IL-15 superagonist is significantly prolonged compared to IL-15 and it maximizes the biological activity of IL-15 by potently stimulating NK and CD8+ T effector lymphocytes.
Preclinical studies showed the advantage of IL-15 superagonist over native IL-15 in the treatment of multiple types of cancer including melanoma, pancreatic tumor, lung cancer, glioblastoma, mammary cancer, prostate cancer, multiple myeloma, and metastatic colon carcinoma.
The development of different forms of recombinant IL-15 superagonist fusion protein and engineered cells co-expressing IL-15/IL-15 Rα complex has provided flexible strategies for cancer treatment.
So far, there are fourteen phase I/II IL-15 superagonist trials in cancer patients and one phase I trial in HIV patients. Information generated by these clinical trials regarding the safety and efficacy of IL-15 superagonist is awaited.
Acknowledgments
This work was supported by National Institutes of Health Grant R01 GM66885. N.K.P. is supported by American Heart Association Postdoctoral Grant 16POST29920007.
Biographies
Naeem K. Patil is a Research Fellow in the laboratory of Edward Sherwood MD, PhD, in the Department of Anesthesiology at Vanderbilt University Medical Center. He received his PhD degree in Pharmacology and Toxicology in 2014 from University of Arkansas for Medical Sciences, USA and MD degree in 2009 from Byramjee Jeejeebhoy Medical College, Pune, India. During his doctoral studies, Naeem’s studies characterized the changes in renal mitochondrial function during sepsis and evaluated the therapeutic potential of mitochondria-targeted antioxidant, namely mito-TEMPO. Naeem’s current research is focused on understanding the alterations in numerous immune cells after burn injury and sepsis. Using pre-clinical models, he is also investigating the utility of novel immunotherapeutic strategies including Flt3 Ligand, interleukin-7, interleukin-15 and PD-L1/PD-1 antibodies to strengthen the host immune response against life threatening infections such as Pseudomonas aeruginosa and Staphylococcus aureus, which are commonly seen in critically ill patients such as those suffering from burn injuries.

Liming Luan is a Staff Scientist in the Department of Anesthesiology at Vanderbilt University Medical Center. After earning his Ph.D. from Northeast Agricultural University (Harbin, China) in 2006, he came to the US for his Postdoctoral training. Liming joined the Sherwood lab in 2013; and he assists with many projects in the lab but primarily studies the role of the CXCL10/CXCR3 axis in NK and NKT cell trafficking during sepsis.

Edward Sherwood is Cornelius Vanderbilt Professor and Vice Chair for Research in the Department of Anesthesiology and Professor of Pathology, Microbiology and Immunology at Vanderbilt University Medical Center. He received his PhD in Physiology from Tulane University followed by post-doctoral training at the Cancer Center of Northwestern University. He received the MD degree from the University of Chicago. He has a life-long interest in immunomodulation and immunotherapy as it applies to the treatment of infectious diseases and cancer. Specific interests include the role of natural killer cells as mediators of anti-microbial immunity and facilitators of systemic inflammation and organ injury during inflammatory syndromes such as sepsis. Further work has focused on the use of γc cytokines (IL-15, IL-7) and toll-like receptor ligands to augment the host response to infection and cancer.

Yin Guo is currently a postdoctoral research fellow in the laboratory of Dr. Edward Sherwood, MD, PhD in the Department of Pathology, Microbiology and Immunology and Department of Anesthesiology at Vanderbilt University Medical Center. She received her MD degree in 2010 from Zunyi Medical University, China and the PhD degree in Immunology in 2016 from Vanderbilt University, USA. During her graduate training, she has published 12 research papers and 1 editorial. Her thesis studies identified the immunotoxicity of interleukin (IL) -15 superagonist, an emerging novel immunotherapeutic candidate for patients with cancer and viral infections and her findings have provided mechanistic insights into the adverse responses of cancer patients treated with high doses of IL-15. Her thesis studies also showed a detrimental role of IL-15 in septic shock, a lethal disorder of systemic inflammation, through the activation of natural killer (NK) cells and interferon (IFN)-γ production, which provided the rationale for potential immunointerventions of human sepsis. She is actively taking part in several ongoing research projects exploring the ability of promising immunotherapeutic agents such as IL-15 superagonist, FLT-3 ligand, anti-PD1/PD-L1 and toll like receptor (TLR) ligands to augment host immune responses against life-threatening nosocomial infections, like Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. Her current research interest is focused on studying the effect of IL-15 superagonist on the immunometabolism of NK and CD8+ T lymphocytes in infectious diseases and cancer.

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264(5161):965–8. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 2.Castillo EF, Schluns KS. Regulating the immune system via IL-15 transpresentation. Cytokine. 2012;59(3):479–90. doi: 10.1016/j.cyto.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17(5):537–47. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 4.Hutchins NA, Unsinger J, Hotchkiss RS, Ayala A. The new normal: immunomodulatory agents against sepsis immune suppression. Trends in molecular medicine. 2014;20(4):224–33. doi: 10.1016/j.molmed.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. The Journal of experimental medicine. 1994;180(4):1395–403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jakobisiak M, Golab J, Lasek W. Interleukin 15 as a promising candidate for tumor immunotherapy. Cytokine Growth Factor Rev. 2011;22(2):99–108. doi: 10.1016/j.cytogfr.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Neely GG, Epelman S, Ma LL, Colarusso P, Howlett CJ, Amankwah EK, et al. Monocyte surface-bound IL-15 can function as an activating receptor and participate in reverse signaling. J Immunol. 2004;172(7):4225–34. doi: 10.4049/jimmunol.172.7.4225. [DOI] [PubMed] [Google Scholar]
- 8.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97(1):14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 9.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–79. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 10.Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. Journal of immunology. 2001;167(3):1179–87. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad R, Ennaciri J, Cordeiro P, El Bassam S, Menezes J. Herpes simplex virus-1 up-regulates IL-15 gene expression in monocytic cells through the activation of protein tyrosine kinase and PKC zeta/lambda signaling pathways. Journal of molecular biology. 2007;367(1):25–35. doi: 10.1016/j.jmb.2006.12.060. [DOI] [PubMed] [Google Scholar]
- 12.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26(4):503–17. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lodolce JP, Burkett PR, Koka RM, Boone DL, Ma A. Regulation of lymphoid homeostasis by interleukin-15. Cytokine & growth factor reviews. 2002;13(6):429–39. doi: 10.1016/s1359-6101(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 14.Marcais A, Cherfils-Vicini J, Viant C, Degouve S, Viel S, Fenis A, et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nature immunology. 2014;15(8):749–57. doi: 10.1038/ni.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao Y, van Hoef V, Zhang X, Wennerberg E, Lorent J, Witt K, et al. IL-15 activates mTOR and primes stress-activated gene expression leading to prolonged antitumor capacity of NK cells. Blood. 2016;128(11):1475–89. doi: 10.1182/blood-2016-02-698027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali AK, Nandagopal N, Lee SH. IL-15-PI3K-AKT-mTOR: A Critical Pathway in the Life Journey of Natural Killer Cells. Front Immunol. 2015;6:355. doi: 10.3389/fimmu.2015.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mortier E, Quemener A, Vusio P, Lorenzen I, Boublik Y, Grotzinger J, et al. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 × IL-15R alpha fusion proteins. The Journal of biological chemistry. 2006;281(3):1612–9. doi: 10.1074/jbc.M508624200. [DOI] [PubMed] [Google Scholar]
- 18.Norman DG, Barlow PN, Baron M, Day AJ, Sim RB, Campbell ID. Three-dimensional structure of a complement control protein module in solution. Journal of molecular biology. 1991;219(4):717–25. doi: 10.1016/0022-2836(91)90666-t. [DOI] [PubMed] [Google Scholar]
- 19.Anderson DM, Kumaki S, Ahdieh M, Bertles J, Tometsko M, Loomis A, et al. Functional characterization of the human interleukin-15 receptor alpha chain and close linkage of IL15RA and IL2RA genes. The Journal of biological chemistry. 1995;270(50):29862–9. doi: 10.1074/jbc.270.50.29862. [DOI] [PubMed] [Google Scholar]
- 20.Dubois S, Magrangeas F, Lehours P, Raher S, Bernard J, Boisteau O, et al. Natural splicing of exon 2 of human interleukin-15 receptor alpha-chain mRNA results in a shortened form with a distinct pattern of expression. The Journal of biological chemistry. 1999;274(38):26978–84. doi: 10.1074/jbc.274.38.26978. [DOI] [PubMed] [Google Scholar]
- 21.Wu Z, Xue HH, Bernard J, Zeng R, Issakov D, Bollenbacher-Reilley J, et al. The IL-15 receptor {alpha} chain cytoplasmic domain is critical for normal IL-15Ralpha function but is not required for trans-presentation. Blood. 2008;112(12):4411–9. doi: 10.1182/blood-2007-03-080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith XG, Bolton EM, Ruchatz H, Wei X, Liew FY, Bradley JA. Selective blockade of IL-15 by soluble IL-15 receptor alpha-chain enhances cardiac allograft survival. Journal of immunology. 2000;165(6):3444–50. doi: 10.4049/jimmunol.165.6.3444. [DOI] [PubMed] [Google Scholar]
- 23.Ruchatz H, Leung BP, Wei XQ, McInnes IB, Liew FY. Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritis: a role for IL-15 in development of antigen-induced immunopathology. Journal of immunology. 1998;160(11):5654–60. [PubMed] [Google Scholar]
- 24.Mortier E, Bernard J, Plet A, Jacques Y. Natural, proteolytic release of a soluble form of human IL-15 receptor alpha-chain that behaves as a specific, high affinity IL-15 antagonist. Journal of immunology. 2004;173(3):1681–8. doi: 10.4049/jimmunol.173.3.1681. [DOI] [PubMed] [Google Scholar]
- 25.Bouchaud G, Garrigue-Antar L, Sole V, Quemener A, Boublik Y, Mortier E, et al. The exon-3-encoded domain of IL-15ralpha contributes to IL-15 high-affinity binding and is crucial for the IL-15 antagonistic effect of soluble IL-15Ralpha. Journal of molecular biology. 2008;382(1):1–12. doi: 10.1016/j.jmb.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Chertova E, Bergamaschi C, Chertov O, Sowder R, Bear J, Roser JD, et al. Characterization and favorable in vivo properties of heterodimeric soluble IL-15.IL-15Ralpha cytokine compared to IL-15 monomer. The Journal of biological chemistry. 2013;288(25):18093–103. doi: 10.1074/jbc.M113.461756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thaysen-Andersen M, Chertova E, Bergamaschi C, Moh ES, Chertov O, Roser J, et al. Recombinant human heterodimeric IL-15 complex displays extensive and reproducible N- and O-linked glycosylation. Glycoconjugate journal. 2016;33(3):417–33. doi: 10.1007/s10719-015-9627-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng L, Du X, Wang Z, Ju J, Jia M, Huang Q, et al. Hyper-IL-15 suppresses metastatic and autochthonous liver cancers by promoting tumor-specific CD8 T cell responses. Journal of hepatology. 2014 doi: 10.1016/j.jhep.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun H, Liu D. IL-15/sIL-15Ralpha gene transfer suppresses Lewis lung cancer growth in the lungs, liver and kidneys. Cancer gene therapy. 2016;23(2–3):54–60. doi: 10.1038/cgt.2015.67. [DOI] [PubMed] [Google Scholar]
- 30.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. The Journal of experimental medicine. 2009;206(1):25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bessard A, Sole V, Bouchaud G, Quemener A, Jacques Y. High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor alpha fusion protein, in metastatic melanoma and colorectal cancer. Molecular cancer therapeutics. 2009;8(9):2736–45. doi: 10.1158/1535-7163.MCT-09-0275. [DOI] [PubMed] [Google Scholar]
- 32.Vincent M, Bessard A, Cochonneau D, Teppaz G, Sole V, Maillasson M, et al. Tumor targeting of the IL-15 superagonist RLI by an anti-GD2 antibody strongly enhances its antitumor potency. International journal of cancer. 2013;133(3):757–65. doi: 10.1002/ijc.28059. [DOI] [PubMed] [Google Scholar]
- 33.Ochoa MC, Fioravanti J, Rodriguez I, Hervas-Stubbs S, Azpilikueta A, Mazzolini G, et al. Antitumor immunotherapeutic and toxic properties of an HDL-conjugated chimeric IL-15 fusion protein. Cancer research. 2013;73(1):139–49. doi: 10.1158/0008-5472.CAN-12-2660. [DOI] [PubMed] [Google Scholar]
- 34.Kermer V, Baum V, Hornig N, Kontermann RE, Muller D. An antibody fusion protein for cancer immunotherapy mimicking IL-15 trans-presentation at the tumor site. Molecular cancer therapeutics. 2012;11(6):1279–88. doi: 10.1158/1535-7163.MCT-12-0019. [DOI] [PubMed] [Google Scholar]
- 35.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, et al. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proceedings of the National Academy of Sciences of the United States of America. 2006;103(24):9166–71. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. Journal of immunology. 2006;177(9):6072–80. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo Y, Luan L, Rabacal W, Bohannon JK, Fensterheim BA, Hernandez A, et al. IL-15 Superagonist-Mediated Immunotoxicity: Role of NK Cells and IFN-gamma. Journal of immunology. 2015;195(5):2353–64. doi: 10.4049/jimmunol.1500300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Z, Xu Y. IL-15R alpha-IgG1-Fc enhances IL-2 and IL-15 anti-tumor action through NK and CD8+ T cells proliferation and activation. Journal of molecular cell biology. 2010;2(4):217–22. doi: 10.1093/jmcb/mjq012. [DOI] [PubMed] [Google Scholar]
- 39.Dubois S, Patel HJ, Zhang M, Waldmann TA, Muller JR. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. Journal of immunology. 2008;180(4):2099–106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 40.Epardaud M, Elpek KG, Rubinstein MP, Yonekura AR, Bellemare-Pelletier A, Bronson R, et al. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer research. 2008;68(8):2972–83. doi: 10.1158/0008-5472.CAN-08-0045. [DOI] [PubMed] [Google Scholar]
- 41.Xu W, Jones M, Liu B, Zhu X, Johnson CB, Edwards AC, et al. Efficacy and mechanism-of-action of a novel superagonist interleukin-15: interleukin-15 receptor alphaSu/Fc fusion complex in syngeneic murine models of multiple myeloma. Cancer research. 2013;73(10):3075–86. doi: 10.1158/0008-5472.CAN-12-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong HC, Jeng EK, Rhode PR. The IL-15-based superagonist ALT-803 promotes the antigen-independent conversion of memory CD8+ T cells into innate-like effector cells with antitumor activity. Oncoimmunology. 2013;2(11):e26442. doi: 10.4161/onci.26442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathios D, Park CK, Marcus WD, Alter S, Rhode PR, Jeng EK, et al. Therapeutic administration of IL-15 superagonist complex ALT-803 leads to long-term survival and durable antitumor immune response in a murine glioblastoma model. International journal of cancer. 2016;138(1):187–94. doi: 10.1002/ijc.29686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosario M, Liu B, Kong L, Collins LI, Schneider SE, Chen X, et al. The IL-15-Based ALT-803 Complex Enhances FcgammaRIIIa-Triggered NK Cell Responses and In Vivo Clearance of B Cell Lymphomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(3):596–608. doi: 10.1158/1078-0432.CCR-15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu B, Kong L, Han K, Hong H, Marcus WD, Chen X, et al. A Novel Fusion of ALT-803 (Interleukin (IL)-15 Superagonist) with an Antibody Demonstrates Antigen-specific Antitumor Responses. The Journal of biological chemistry. 2016;291(46):23869–81. doi: 10.1074/jbc.M116.733600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Felices M, Chu S, Kodal B, Bendzick L, Ryan C, Lenvik AJ, et al. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecologic oncology. 2017 doi: 10.1016/j.ygyno.2017.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim PS, Kwilas AR, Xu W, Alter S, Jeng EK, Wong HC, et al. IL-15 superagonist/IL-15RalphaSushi-Fc fusion complex (IL-15SA/IL-15RalphaSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget. 2016;7(13):16130–45. doi: 10.18632/oncotarget.7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomes-Giacoia E, Miyake M, Goodison S, Sriharan A, Zhang G, You L, et al. Intravesical ALT-803 and BCG treatment reduces tumor burden in a carcinogen induced bladder cancer rat model; a role for cytokine production and NK cell expansion. PloS one. 2014;9(6):e96705. doi: 10.1371/journal.pone.0096705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eisenman J, Ahdieh M, Beers C, Brasel K, Kennedy MK, Le T, et al. Interleukin-15 interactions with interleukin-15 receptor complexes: characterization and species specificity. Cytokine. 2002;20(3):121–9. doi: 10.1006/cyto.2002.1989. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi H, Dubois S, Sato N, Sabzevari H, Sakai Y, Waldmann TA, et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 2005;105(2):721–7. doi: 10.1182/blood-2003-12-4187. [DOI] [PubMed] [Google Scholar]
- 51.Rowley J, Monie A, Hung CF, Wu TC. Inhibition of tumor growth by NK1.1+ cells and CD8+ T cells activated by IL-15 through receptor beta/common gamma signaling in trans. Journal of immunology. 2008;181(12):8237–47. doi: 10.4049/jimmunol.181.12.8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris JC, Ramlogan-Steel CA, Yu P, Black BA, Mannan P, Allison JP, et al. Vaccination with tumor cells expressing IL-15 and IL-15Ralpha inhibits murine breast and prostate cancer. Gene therapy. 2014;21(4):393–401. doi: 10.1038/gt.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rowley J, Monie A, Hung CF, Wu TC. Expression of IL-15RA or an IL-15/IL-15RA fusion on CD8+ T cells modifies adoptively transferred T-cell function in cis. European journal of immunology. 2009;39(2):491–506. doi: 10.1002/eji.200838594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurton LV, Singh H, Najjar AM, Switzer KC, Mi T, Maiti S, et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(48):E7788–E97. doi: 10.1073/pnas.1610544113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. The Journal of experimental medicine. 2008;205(5):1213–25. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steel JC, Ramlogan CA, Yu P, Sakai Y, Forni G, Waldmann TA, et al. Interleukin-15 and its receptor augment dendritic cell vaccination against the neu oncogene through the induction of antibodies partially independent of CD4 help. Cancer research. 2010;70(3):1072–81. doi: 10.1158/0008-5472.CAN-09-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van den Bergh J, Willemen Y, Lion E, Van Acker H, De Reu H, Anguille S, et al. Transpresentation of interleukin-15 by IL-15/IL-15Ralpha mRNA-engineered human dendritic cells boosts antitumoral natural killer cell activity. Oncotarget. 2015;6(42):44123–33. doi: 10.18632/oncotarget.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaston DC, Odom CI, Li L, Markert JM, Roth JC, Cassady KA, et al. Production of bioactive soluble interleukin-15 in complex with interleukin-15 receptor alpha from a conditionally-replicating oncolytic HSV-1. PloS one. 2013;8(11):e81768. doi: 10.1371/journal.pone.0081768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tosic V, Thomas DL, Kranz DM, Liu J, McFadden G, Shisler JL, et al. Myxoma virus expressing a fusion protein of interleukin-15 (IL15) and IL15 receptor alpha has enhanced antitumor activity. PloS one. 2014;9(10):e109801. doi: 10.1371/journal.pone.0109801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kokaji AI, Hockley DL, Kane KP. IL-15 transpresentation augments CD8+ T cell activation and is required for optimal recall responses by central memory CD8+ T cells. Journal of immunology. 2008;180(7):4391–401. doi: 10.4049/jimmunol.180.7.4391. [DOI] [PubMed] [Google Scholar]
- 61.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 62.McMichael AJ. Environment, sustainability and health: the learning curve steepens. Med J Aust. 2001;175(11–12):569–70. doi: 10.5694/j.1326-5377.2001.tb143728.x. [DOI] [PubMed] [Google Scholar]
- 63.Mueller YM, Bojczuk PM, Halstead ES, Kim AH, Witek J, Altman JD, et al. IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood. 2003;101(3):1024–9. doi: 10.1182/blood-2002-07-1957. [DOI] [PubMed] [Google Scholar]
- 64.Kanai T, Thomas EK, Yasutomi Y, Letvin NL. IL-15 stimulates the expansion of AIDS virus-specific CTL. J Immunol. 1996;157(8):3681–7. [PubMed] [Google Scholar]
- 65.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5(11):835–43. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 66.Mavilio D, Lombardo G, Kinter A, Fogli M, La Sala A, Ortolano S, et al. Characterization of the defective interaction between a subset of natural killer cells and dendritic cells in HIV-1 infection. J Exp Med. 2006;203(10):2339–50. doi: 10.1084/jem.20060894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chehimi J, Marshall JD, Salvucci O, Frank I, Chehimi S, Kawecki S, et al. IL-15 enhances immune functions during HIV infection. J Immunol. 1997;158(12):5978–87. [PubMed] [Google Scholar]
- 68.Kottilil S, Chun TW, Moir S, Liu S, McLaughlin M, Hallahan CW, et al. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J Infect Dis. 2003;187(7):1038–45. doi: 10.1086/368222. [DOI] [PubMed] [Google Scholar]
- 69.Seay K, Church C, Zheng JH, Deneroff K, Ochsenbauer C, Kappes JC, et al. In Vivo Activation of Human NK Cells by Treatment with an Interleukin-15 Superagonist Potently Inhibits Acute In Vivo HIV-1 Infection in Humanized Mice. J Virol. 2015;89(12):6264–74. doi: 10.1128/JVI.00563-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eberly MD, Kader M, Hassan W, Rogers KA, Zhou J, Mueller YM, et al. Increased IL-15 production is associated with higher susceptibility of memory CD4 T cells to simian immunodeficiency virus during acute infection. J Immunol. 2009;182(3):1439–48. doi: 10.4049/jimmunol.182.3.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mueller YM, Do DH, Altork SR, Artlett CM, Gracely EJ, Katsetos CD, et al. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. Journal of immunology. 2008;180(1):350–60. doi: 10.4049/jimmunol.180.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. The New England journal of medicine. 2013;369(2):134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Di Giacomo AM, Danielli R, Guidoboni M, Calabro L, Carlucci D, Miracco C, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer immunology, immunotherapy : CII. 2009;58(8):1297–306. doi: 10.1007/s00262-008-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, et al. Redistribution, Hyperproliferation, Activation of Natural Killer Cells and CD8 T Cells, and Cytokine Production During First-in-Human Clinical Trial of Recombinant Human Interleukin-15 in Patients With Cancer. J Clin Oncol. 2014 doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fehniger TA, Suzuki K, Ponnappan A, VanDeusen JB, Cooper MA, Florea SM, et al. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exp Med. 2001;193(2):219–31. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mishra A, Liu S, Sams GH, Curphey DP, Santhanam R, Rush LJ, et al. Aberrant overexpression of IL-15 initiates large granular lymphocyte leukemia through chromosomal instability and DNA hypermethylation. Cancer cell. 2012;22(5):645–55. doi: 10.1016/j.ccr.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wege AK, Weber F, Kroemer A, Ortmann O, Nimmerjahn F, Brockhoff G. IL-15 enhances the anti-tumor activity of trastuzumab against breast cancer cells but causes fatal side effects in humanized tumor mice (HTM) Oncotarget. 2017;8(2):2731–44. doi: 10.18632/oncotarget.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waldmann TA, Lugli E, Roederer M, Perera LP, Smedley JV, Macallister RP, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011;117(18):4787–95. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang CM, Lo CH, Shih YM, Chen Y, Wu PY, Tsuneyama K, et al. Treatment of hepatocellular carcinoma with adeno-associated virus encoding interleukin-15 superagonist. Human gene therapy. 2010;21(5):611–21. doi: 10.1089/hum.2009.187. [DOI] [PubMed] [Google Scholar]
- 80.Pavlakis M, Strehlau J, Lipman M, Shapiro M, Maslinski W, Strom TB. Intragraft IL-15 transcripts are increased in human renal allograft rejection. Transplantation. 1996;62(4):543–5. doi: 10.1097/00007890-199608270-00020. [DOI] [PubMed] [Google Scholar]
- 81.Liu Z, Geboes K, Colpaert S, D’Haens GR, Rutgeerts P, Ceuppens JL. IL-15 is highly expressed in inflammatory bowel disease and regulates local T cell-dependent cytokine production. Journal of immunology. 2000;164(7):3608–15. doi: 10.4049/jimmunol.164.7.3608. [DOI] [PubMed] [Google Scholar]
- 82.Rentzos M, Cambouri C, Rombos A, Nikolaou C, Anagnostouli M, Tsoutsou A, et al. IL-15 is elevated in serum and cerebrospinal fluid of patients with multiple sclerosis. Journal of the neurological sciences. 2006;241(1–2):25–9. doi: 10.1016/j.jns.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 83.Guo Y, Luan L, Patil NK, Wang J, Bohannon JK, Rabacal W, et al. IL-15 Enables Septic Shock by Maintaining NK Cell Integrity and Function. Journal of immunology. 2017;198(3):1320–33. doi: 10.4049/jimmunol.1601486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Herzig DS, Luan L, Bohannon JK, Toliver-Kinsky TE, Guo Y, Sherwood ER. The role of CXCL10 in the pathogenesis of experimental septic shock. Crit Care. 2014;18(3):R113. doi: 10.1186/cc13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patil NK, Luan L, Bohannon JK, Guo Y, Hernandez A, Fensterheim B, et al. IL-15 Superagonist Expands mCD8+ T, NK and NKT Cells after Burn Injury but Fails to Improve Outcome during Burn Wound Infection. PLoS One. 2016;11(2):e0148452. doi: 10.1371/journal.pone.0148452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Herzig D, Fang G, Toliver-Kinsky TE, Guo Y, Bohannon J, Sherwood ER. STAT1-deficient mice are resistant to cecal ligation and puncture-induced septic shock. Shock. 2012;38(4):395–402. doi: 10.1097/SHK.0b013e318265a2ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bohannon J, Guo Y, Sherwood ER. The role of natural killer cells in the pathogenesis of sepsis: the ongoing enigma. Crit Care. 2012;16(6):185. doi: 10.1186/cc11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Herzig DS, Guo Y, Fang G, Toliver-Kinsky TE, Sherwood ER. Therapeutic efficacy of CXCR3 blockade in an experimental model of severe sepsis. Crit Care. 2012;16(5):R168. doi: 10.1186/cc11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Danai PA, Moss M, Mannino DM, Martin GS. The epidemiology of sepsis in patients with malignancy. Chest. 2006;129(6):1432–40. doi: 10.1378/chest.129.6.1432. [DOI] [PubMed] [Google Scholar]
- 90.Williams MD, Braun LA, Cooper LM, Johnston J, Weiss RV, Qualy RL, et al. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Critical care. 2004;8(5):R291–8. doi: 10.1186/cc2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elpek KG, Rubinstein MP, Bellemare-Pelletier A, Goldrath AW, Turley SJ. Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Ralpha complexes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(50):21647–52. doi: 10.1073/pnas.1012128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunological reviews. 2008;222:357–68. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 93.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113(22):5488–96. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 94.Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer discovery. 2014;4(5):522–6. doi: 10.1158/2159-8290.CD-13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nature reviews Cancer. 2012;12(4):237–51. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]


