Abstract
Purpose
To determine if different types of retinal fluid in the central macula affect the reproducibility of choroidal thickness (CT) measurements on enhanced depth imaging optical coherence tomography (EDI-OCT)
Design
retrospective, reliability analysis
Methods
EDI-OCT images were obtained and the choroidal-scleral junction was analyzed through semi-automated segmentation. CT was measured at the fovea and averaged across the central 3 mm horizontal segment. Demographic data, central macular thickness and type of fluid present were recorded. Intragrader and intergrader repeatability were assessed using the intraclass correlation coefficient (ICC) and coefficient of repeatability (CR).
Results
Of 124 eyes analyzed, 60 (48.4%) had diabetic macular edema, 32 (25.8%) had neovascular age-related macular degeneration, and 32 (25.8%) had other causes of fluid. Intergrader ICC (CR) were 0.95 (74.1 μm) and 0.96 (63.9 μm) for subfoveal and average CT, respectively. CR were similar across various causes of retinal fluid, but were worst for subretinal fluid compared to intraretinal or sub-retinal pigment epithelial fluid. CR also worsened with increasing choroidal thickness, but was not affected by retinal thickness. Intragrader repeatability was generally greater than intergrader values, and followed the same trend.
Conclusions
The presence of macular fluid reduces CT measurement reproducibility, particularly in eyes with subretinal fluid and greater choroidal thickness. A difference of 74.1 μm in subfoveal CT or 63.9 μm in average CT may be necessary to detect true clinical change in eyes with macular fluid.
Introduction
The choroid is the primary vascular supply for the outer retina and has been implicated to play a role in a variety of ocular and systemic diseases.1 Recent studies have shown that the thickness of the choroid may be affected in retinal conditions such as diabetic retinopathy, age-related macular degeneration (AMD), vascular occlusive diseases, uveitis, and pachychoroid entities.2–5 In some conditions, choroidal thickness (CT) may be used as a marker of disease activity or treatment response, although intravitreal pharmacotherapies and laser treatments may cause additional alterations to CT.6–12
Enhanced depth imaging optical coherence tomography, or EDI-OCT, improves visualization of the deeper tissue structures, allowing the choroidal-scleral interface to be more easily observed and CT to be measured.13 CT measurements may be affected by shadowing from overlying retinal pathology, especially in the presence of macular fluid. The deeper location of the choroid inherently limits its visibility, and a thicker choroid reduces measurement reliability.14,15 Finally, there is also variability in how the posterior boundary of the choroid is defined, which may include the border of the vessel lumena, the choroid stroma, or the sclera, and particularly when the suprachoroidal layer is visible. Recent studies suggest that the repeatability of CT measurements may be optimized at the stromal border.16 These factors all serve to make CT estimation imprecise, necessitating reproducibility studies to guide analysis.
Most studies to date have assessed CT measurement repeatability in healthy eyes,16–23 with a few studies examining CT in diabetic retinopathy and AMD.15,24–27 We hypothesize that the reliability of CT measurements may be less determined by the pathophysiology of particular conditions, than by the type or quantity of overlying retinal fluid in the macula. In this study, we provide a practical context to CT analysis by determining if CT measurements are affected by the presence and type of macular fluid.
Methods
Subject Selection
After obtaining approval from the Institutional Review Board, study subjects were selected from consecutive patients who were seen at the University of California, Davis Eye Center from January 1, 2015 to April 1, 2016. Patients were identified from the electronic medical record system and included if they had a diagnosis of neovascular AMD, diabetic macular edema (DME), any type of retinal vein occlusion, central serous chorioretinopathy, choroidal neovascularization, or cystoid macular edema from any cause. Eyes were selected if they underwent EDI-OCT imaging and demonstrated fovea-involving macular fluid on a central horizontal line scan. We excluded patients with high myopia (-6D), or other retinal or macular pathologies that are not among the inclusion criteria. If both eyes showed fluid, the eye with worse visual acuity was determined to be the study eye. If visual acuity was equal, the study eye was determined by birth month (right eye for even-number birth month; left eye for odd-number birth month). If EDI-OCT was obtained on multiple visits, the first visit showing retinal fluid was chosen for analysis. Patient charts were also reviewed for basic demographic data, retinal diagnosis, best corrected visual acuity, refractive error, intraocular pressure, and lens status. All steps of the study were conducted in accordance with the tenets of the Declaration of Helsinki.
EDI-OCT Imaging and Analysis
All imaging was performed by experienced ophthalmic photographers using the Spectralis OCT instrument (Heidelberg Engineering, Heidelberg, Germany), operating at 40,000 Hz. Nine mm line scans were obtained in EDI mode, with 1536 A-scans per B-scan and Automatic Real Time frame-averaging up to 100 B-scans to reduce noise. Fluid type was determined from EDI-OCT images by two experienced graders (SSW, VSV), with any discrepancies resolved by a senior grader (GY). Macular fluid was categorized as intraretinal, subretinal, or sub-retinal pigment epithelial (sub-RPE) fluid. Most eyes had predominantly one type of retinal fluid. Two eyes showing similar amounts of more than one fluid type were excluded from the analysis.
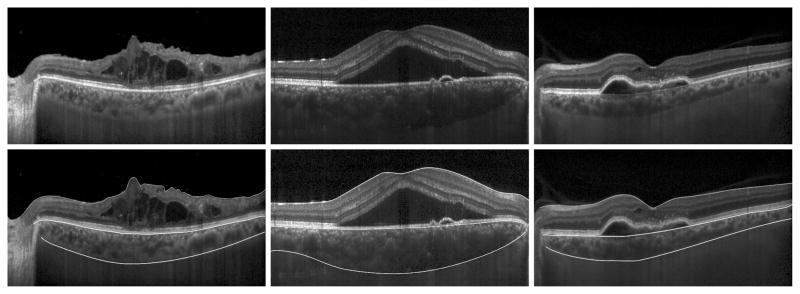
EDI-OCT images were analyzed using Duke Optical Coherence Tomography Retinal Analysis Program (DOCTRAP, v62.0), which was used for semi-automated segmentation of the internal limiting membrane and Bruch’s membrane, while the choroidal-scleral junction (CSJ) was manually traced by the two graders. The CSJ was taken to be the outermost border of the choroidal stroma (Figure 1). The fovea was manually marked based on the widest separation between the inner segment-outer segment border (or ellipsoid zone) and the cone outer-segment tips (or interdigitation zone). In cases where this anatomy was obscured by pathology, the fovea was taken to be the smallest tapering point of the inner nuclear layer. CT measured at the fovea (subfoveal CT) and at 600 other points corresponding to individual A-scans averaged across the central 3 mm of the line scan (from 1.5 mm nasal to 1.5 mm temporal to the fovea; average CT) were calculated from mean measurements of the two graders to determine intergrader repeatability. One grader (VSV) performed a second discrete set of manual segmentations at least one month later to assess intragrader repeatability. The central macular thickness (CMT), measured between the internal limiting membrane and Bruch’s membrane, was also measured by the DOCTRAP software.
FIGURE 1.
Enhanced-depth imaging optical coherence tomography (EDI-OCT) images of eyes with macular fluid using semi-automated segmentation. Scans are shown before (top) and after (bottom) segmentation, including eyes with intraretinal fluid (left), subretinal fluid (center), and sub-retinal pigment epithelial (sub-RPE) fluid (right). Segmentation lines delineate the internal limiting membrane (top line), Bruch’s membrane (middle line), and choroidal-scleral junction (bottom line).
Statistical Analysis
One-way analysis of variance was used to compare baseline CT between diagnoses and fluid type. Repeatability was evaluated using intraclass correlation coefficients (ICC) and coefficients of repeatability (CR). The ICC is a ratio of the variance between subjects over the total variance. It indicates the proportion of the total variation that is attributed to between-subject differences rather than measurement variation for an individual subject. The CR is the value under which repeated measurements of the same subject should fall with 95% probability, and represents the threshold for demonstrating true clinical change. Statistical analyses were performed using SPSS Statistics 24.0 (IBM, New York), and figures were generated with Microsoft software (Microsoft, Washington).
Results
Demographics and Ocular Characteristics
A total of 615 consecutive patient charts were reviewed, of which 491 were excluded, due to absence of macular fluid (n = 141), absence of EDI-OCT imaging (n = 269), presence of other pathologies (n = 62), or high myopia (n = 17). Two subjects were excluded for having equal amounts of more than one fluid type and two outliers were excluded due to significant pathology that precluded adequate automated segmentation of Bruch’s membrane, leaving 124 eyes of 124 subjects for final analysis. The mean age of subjects was 65.9 ± 15.2 years and 71 subjects (57.3%) were male. Of the study eyes, 67 (54%) were left eyes and 57 (46%) were right eyes. Ninety eyes (72.6%) of the sample were phakic, and the remaining 34 eyes (27.4%) were pseudophakic. No high myopes or high hyperopes were included in the final sample. All eyes had fovea-involving macular fluid based on inclusion criteria. Among these, 60 eyes (48.4%) had DME, 32 eyes (25.8%) had neovascular AMD, and 32 eyes (25.8%) had macular fluid from other causes, including vitreomacular interface disease (9 eyes), retinal vein occlusion (8 eyes), inflammatory syndromes including post-cataract cystoid macular edema and idiopathic uveitides (8 eyes), central serous chorioretinopathy (4 eyes), idiopathic choroidal neovascularization (2 eyes) and optic nerve pit (1 eye). There were 101 scans with intraretinal fluid (IRF), 14 with subretinal fluid (SRF), and 9 with sub-retinal pigment epithelial fluid (sub-RPE fluid). Scans were also divided into tertiles according to CMT, subfoveal CT and average CT.
The mean (SD) CMT was 350.4 μm (136.9 μm). Mean (SD) subfoveal CT was 267.2 μm (100.4 μm) and mean average CT was 258.5 μm (96.2 μm). CMT was lower in eyes with DME than those with fluid from other causes (p = 0.004), but was not significantly different between fluid types (p = 0.088). Subfoveal CT and average CT were not statistically different between diagnoses (p = 0.100 and p = 0.073) or fluid type (p = 0.133 and p = 0.130) (Table 1).
Table 1.
Retinal and Choroidal Thickness based on Diagnosis and Fluid Type in Eyes with Macular Fluid
| Mean CMT (SD) (μm) | Mean Subfoveal CT (SD) (μm) | Mean Average CT (SD) (μm) | ||||
|---|---|---|---|---|---|---|
| Diagnosis | ||||||
| AMD | 355.8 | (130.9) | 236.7 | (133.0) | 228.0 | (124.0) |
| DME | 314.6 | (121.7) | 268.2 | (74.9) | 261.5 | (72.3) |
| Other | 412.1 | (150.5) | 289.5 | (109.6) | 279.2 | (103.8) |
| Fluid | ||||||
| Intraretinal | 338.6 | (139.2) | 264.0 | (99.4) | 256.8 | (94.3) |
| Subretinal | 422.9 | (134.3) | 345.8 | (160.3) | 329.5 | (152.2) |
| Sub-RPE | 369.7 | (73.9) | 225.3 | (106.0) | 214.2 | (104.9) |
|
| ||||||
| Total | 350.4 | (136.9) | 267.2 | (100.4) | 258.5 | (96.2) |
CMT = central macular thickness; CT = choroidal thickness; SD = standard deviation; AMD = age-related macular degeneration; DME = diabetic macular edema
Repeatability Based on Diagnosis and Fluid Type
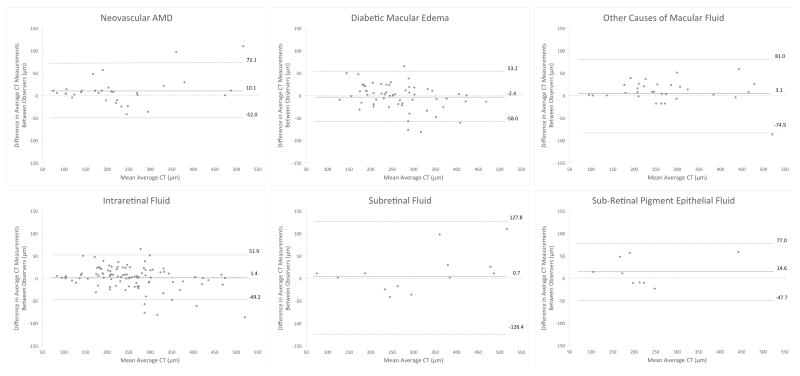
Among all eyes, intergrader ICC (CR) were 0.95 (74.1 μm) for subfoveal CT and 0.96 (63.9 μm) for average CT (Table 2). Repeatability between different diagnoses was similar, with a narrow range of CR for subfoveal CT (69.3 to 77.9 μm) and average CT (62.0 to 77.9 μm). In contrast, repeatability measures were different between fluid types, with subretinal fluid showing the highest CR (123.7 μm for subfoveal CT and 127.1 μm for average CT) when compared to eyes with intraretinal or sub-RPE fluid (Table 2). Bland-Altman analyses of the 95% limits of agreement between graders demonstrated comparable lower and upper limits between diagnoses, but a significantly wider interval for subretinal fluid compared to other fluid types (Figure 2).
Table 2.
Intergrader Repeatability of Choroidal Thickness based on Diagnosis and Fluid Type in Eyes with Macular Fluid
| Subfoveal CT | Average CT | |||
|---|---|---|---|---|
| ICC | CR (μm) | ICC | CR (μm) | |
| Diagnosis | ||||
| AMD | 0.97 | 77.9 | 0.98 | 62.0 |
| DME | 0.95 | 69.3 | 0.97 | 72.2 |
| Other | 0.97 | 76.9 | 0.97 | 77.9 |
| Fluid | ||||
| Intraretinal | 0.97 | 63.3 | 0.98 | 50.6 |
| Subretinal | 0.96 | 123.7 | 0.95 | 127.1 |
| Sub-RPE | 0.94 | 86.8 | 0.97 | 62.4 |
|
| ||||
| Total | 0.95 | 74.1 | 0.96 | 63.9 |
CT = choroidal thickness; ICC = intraclass correlation coefficient; CR = coefficient of repeatability; AMD = age-related macular degeneration; DME = diabetic macular edema
FIGURE 2.
Bland-Altman plots of intergrader agreement for measuring choroidal thickness in eyes with macular fluid. Plots are shown by diagnosis (top) and fluid type (bottom). Solid lines represent the mean difference, while dashed lines show the 95% limits of agreement.
Intragrader repeatability was better than intergrader repeatability, showing higher ICC and lower CR values. There was a similar trend toward higher CR in eyes with subretinal fluid (Supplemental Table 1; Supplemental Material at AJO.com).
Repeatability Based on Retinal and Choroidal Thickness
Reproducibility of CT measurements also appeared to be affected by choroidal thickness. We found repeatability to be worse in eyes with a thicker choroid, with the lowest ICC and highest CR values measured in eyes falling within the highest CT tertile. However, repeatability measures did not vary significantly by retinal thickness based on CMT tertiles (Table 3). Similar trends were noted in the intragrader values (Supplemental Table 2; Supplemental Material at AJO.com).
Table 3.
Intergrader Repeatability of Choroidal Thickness According to Thickness Tertiles in Eyes with Macular Fluid
| Subfoveal CT | Average CT | |||
|---|---|---|---|---|
| ICC | CR (μm) | ICC | CR (μm) | |
| CMT Range (μm) | ||||
| Lower (166–348) | 0.97 | 68.1 | 0.98 | 54.7 |
| Middle (349–531) | 0.94 | 82.5 | 0.94 | 75.3 |
| Upper (532–714) | 0.98 | 67.9 | 0.98 | 67.3 |
| Subfoveal / Average CT Range (μm) | ||||
| Lower (81–242 / 79–242) | 0.93 | 48.9 | 0.95 | 38.8 |
| Middle (243–404 / 243–407) | 0.81 | 85.3 | 0.85 | 73.1 |
| Upper (405–565 / 408–571) | 0.79 | 100.4 | 0.65 | 107.7 |
CT = choroidal thickness; CMT = central macular thickness; ICC = intraclass correlation coefficient; CR = coefficient of repeatability
Discussion
As our visualization of the choroid improves, our understanding of its impact on various retinal diseases increases. Studies have shown that CT may be increased in central serous chorioretinopathy or retinal vein occlusion,3,28 or decreased in conditions like diabetic retinopathy and DME.5,29–35 In some conditions, CT may predict clinical outcome or even be used to monitor treatment response. For instance, one study found that greater subfoveal CT may be correlated with better short-term visual and morphologic outcomes after anti-vascular endothelial growth factor (anti-VEGF) treatment for DME.36 However, therapies for treating macular fluid, such as anti-VEGF or corticosteroid injections, may decrease CT also.37–39 Hence, robust reliability studies are necessary to determine clinically significant changes in CT.
The repeatability of CT measurements has been well established in normal subjects, with reported ICC values of 0.89–0.99.17–23,40,41 Overall, ICC values appear uniformly strong across many studies, including ours, but is susceptible to being artificially increased as the between-patient differences increase. Hence, CR values may be better indicators of test-retest repeatability. Using the posterior boundary of the choroid stroma, we had determined the intergrader CR value of 50.4 μm as the threshold for determining true clinical change in subfoveal CT in normal eyes.16 Averaging CT across the central 3 mm in a horizontal line scan further improves the reliability with a CR value of 41.4 μm.16 This is within the range of previously reported CR of 30.9 μm and 78.1 μm in normal eyes.14,22
In this study, we employed a similar semi-automated method for measuring CT in diseased eyes with retinal fluid, and found that a difference of 74.1 μm or 63.9 μm is necessary to determine true change in subfoveal and average CT, respectively. These higher CR values support our hypothesis that macular fluid reduces the reliability of CT measurements in diseased eyes compared with normal eyes. Interestingly, we noted that the repeatability of CT measurements is poorest in eyes with subretinal fluid, compared with other types of macular fluid. One possible explanation is that subretinal fluid often occurs as a contiguous collection that results in greater signal attenuation or “shadowing” of the underlying choroid, in contrast to the pockets of cystic spaces seen with intraretinal fluid. In addition, eyes with subretinal fluid tend to have thicker choroids, though this relationship was not significant in our cohort. Consistent with prior studies, we also found that a thicker choroid reduces measurement repeatability,14,15,19 so we cannot exclude the possibility that CT may exert a stronger influence on measurement precision, especially since retinal thickness did not appear to have an effect. We also cannot exclude the potential effect of other OCT features such as intraretinal hyperreflective foci or subretinal hyperreflective material which may warrant future research. Additional prospective studies to address intervisit reliability of CT measurements will also enhance our understanding.
Our study suggests that the retinal diagnosis or cause of macular fluid is not as strong of a factor in determining CT repeatability as the type of retinal fluid. We hypothesize that the accuracy of CT measurements is more dependent on anatomic factors that may affect light penetration and signal strength at the posterior boundary of the choroid, than the biological process underlying the retinal exudation. Prior reports in eyes with neovascular AMD demonstrated intergrader CR values of 30–36 μm, which were comparable to other reports from normal eyes,15,22,26 but lower compared to our prior study. Those results may have been confounded by eyes with a thinner choroid, which tends to occur in advanced AMD. In eyes with diabetic retinopathy, the intergrader CR for CT measurements was reported to be 41.5 μm.24 However, none of those studies specified the presence of retinal fluid on the OCT scans analyzed, which may contribute to the lower CR values in those reports.
Our study is limited by its retrospective nature, with unequal distribution of eyes with various retinal pathologies and fluid types. We also did not assess measurement repeatability at specific locations that may correlate with the location and area of macular fluid. Thus, macular fluid outside the central foveal region, for example, may account for average CT appearing less reproducible than subfoveal CT in some subgroup of eyes in our study such as those with DME or SRF. EDI-OCT as an imaging modality also has limitations. Measurement repeatability is compromised when the choroidal-scleral junction is obscured. In comparison, swept source OCT employs a longer wavelength of light that penetrates deeper and allows greater depth of visualization. CT measurements using swept source OCT are highly repeatable, but tend to be slightly thicker compared to spectral domain OCT.23,27
In conclusion, the repeatability of CT measurements using EDI-OCT is reduced by the presence of macular fluid, particularly in eyes with greater choroidal thickness and in the presence of subretinal fluid. Clinicians and researchers should account for these characteristics when obtaining repeated CT measurements and following eyes over time. Future investigation with prospective studies is warranted to further elucidate these findings.
Supplementary Material
Acknowledgments
Funding/Support: VSV is supported by the National Center for Advancing Translational Sciences and NIH (grant UL1TR000002 and linked award TL1TR000133). SF is supported by NIH (grant P30 EY005722). AM is supported by Research to Prevent Blindness, the International Retina Research Foundation, and NIH (grant K08 EY027463). GY is supported by the E. Matilda Ziegler Foundation for the Blind, Barr Foundation for Retinal Research, Alcon Research Institute, ARVO Foundation, and NIH (grant K08 EY026101). No funding organizations had any role in the design or conduct of this research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Supplemental Material available at AJO.com
Financial Disclosures: SF has patents in OCT imaging and analysis. SSW, VSV, DC, AM, and GY have no relevant financial disclosures.
Contribution of Authors: Design of the study (SSW, GY); Conduct of the study (SSW, VSV, DC, GY); Data collection and management (SSW, VSV); Data analysis and interpretation (SSW, DC, SF, GY); Preparation of manuscript (SSW, GY); Review and approval of manuscript (SSW, VSV, DC, SF, AM, GY).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishikawa S, Taguchi M, Muraoka T, Sakurai Y, Kanda T, Takeuchi M. Changes in subfoveal choroidal thickness associated with uveitis activity in patients with Behcet’s disease. Br J Ophthalmol. 2014;98(11):1508–1513. doi: 10.1136/bjophthalmol-2014-305333. [DOI] [PubMed] [Google Scholar]
- 3.Kim KH, Lee DH, Lee JJ, Park SW, Byon IS, Lee JE. Regional Choroidal Thickness Changes in Branch Retinal Vein Occlusion with Macular Edema. Ophthalmologica. 2015;234(2):109–118. doi: 10.1159/000437276. [DOI] [PubMed] [Google Scholar]
- 4.Lu L, Xu S, He F, et al. Assessment of Choroidal Microstructure and Subfoveal Thickness Change in Eyes With Different Stages of Age-Related Macular Degeneration. Medicine (Baltimore) 2016;95(10):e2967. doi: 10.1097/MD.0000000000002967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vujosevic S, Martini F, Cavarzeran F, Pilotto E, Midena E. Macular and peripapillary choroidal thickness in diabetic patients. Retina. 2012;32(9):1781–1790. doi: 10.1097/IAE.0b013e31825db73d. [DOI] [PubMed] [Google Scholar]
- 6.Ahn SJ, Park KH, Woo SJ. Subfoveal Choroidal Thickness Changes Following Anti-Vascular Endothelial Growth Factor Therapy in Myopic Choroidal Neovascularization. Invest Ophthalmol Vis Sci. 2015;56(10):5794–5800. doi: 10.1167/iovs.14-16006. [DOI] [PubMed] [Google Scholar]
- 7.Kim YK, Park SJ, Woo SJ, Park KH. Choroidal Thickness Change after Intravitreal Anti-Vascular Endothelial Growth Factor Treatment in Retinal Angiomatous Proliferation and Its Recurrence. Retina. 2016;36(8):1516–26. doi: 10.1097/IAE.0000000000000952. [DOI] [PubMed] [Google Scholar]
- 8.Koizumi H, Kano M, Yamamoto A, et al. Subfoveal Choroidal Thickness during Aflibercept Therapy for Neovascular Age-Related Macular Degeneration: TwelveMonth Results. Ophthalmology. 2016;123(3):617–624. doi: 10.1016/j.ophtha.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 9.Oh BL, Yu HG. Choroidal Thickness after Full-Fluence and Half-Fluence Photodynamic Therapy in Chronic Central Serous Chorioretinopathy. Retina. 2015;35(8):1555–1560. doi: 10.1097/IAE.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto M, Matsuura T, Ogata N. Effects of Panretinal Photocoagulation on Choroidal Thickness and Choroidal Blood Flow in Patients with Severe Nonproliferative Diabetic Retinopathy. Retina. 2016;36(4):805–811. doi: 10.1097/IAE.0000000000000800. [DOI] [PubMed] [Google Scholar]
- 11.Unlu C, Erdogan G, Onal Gunay B, Sezgin Akcay BI, Kardes E. Subfoveal choroidal thickness changes after intravitreal bevacizumab therapy for neovascular age-related macular degeneration. Int J Ophthalmol. 2015;8(4):849–851. doi: 10.3980/j.issn.2222-3959.2015.04.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Y, Zhang T, Wang K, Xu G, Huang X. Changes in choroidal thickness after panretinal photocoagulation in patients with type 2 diabetes. Retina. 2015;35(4):695–703. doi: 10.1097/IAE.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 13.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 14.Cho AR, Choi YJ, Kim YT Medscape. Influence of choroidal thickness on subfoveal choroidal thickness measurement repeatability using enhanced depth imaging optical coherence tomography. Eye (Lond) 2014;28(10):1151–1160. doi: 10.1038/eye.2014.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JH, Kang SW, Kim JR, Kim SJ. Variability of subfoveal choroidal thickness measurements in patients with age-related macular degeneration and central serous chorioretinopathy. Eye (Lond) 2013;27(7):809–815. doi: 10.1038/eye.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vuong VS, Moisseiev E, Cunefare D, Farsiu S, Moshiri A, Yiu G. Repeatability of Choroidal Thickness Measurements on Enhanced Depth Imaging OCT using Different Posterior Boundaries. Am J Ophthalmol. 2016;169:104–12. doi: 10.1016/j.ajo.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Branchini L, Regatieri CV, Flores-Moreno I, Baumann B, Fujimoto JG, Duker JS. Reproducibility of choroidal thickness measurements across three spectral domain optical coherence tomography systems. Ophthalmology. 2012;119(1):119–123. doi: 10.1016/j.ophtha.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikuno Y, Maruko I, Yasuno Y, et al. Reproducibility of retinal and choroidal thickness measurements in enhanced depth imaging and high-penetration optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(8):5536–5540. doi: 10.1167/iovs.10-6811. [DOI] [PubMed] [Google Scholar]
- 19.Karaca EE, Ozdek S, Yalcin NG, Ekici F. Reproducibility of choroidal thickness measurements in healthy Turkish subjects. Eur J Ophthalmol. 2014;24(2):202–208. doi: 10.5301/ejo.5000351. [DOI] [PubMed] [Google Scholar]
- 20.Mansouri K, Medeiros FA, Tatham AJ, Marchase N, Weinreb RN. Evaluation of retinal and choroidal thickness by swept-source optical coherence tomography: repeatability and assessment of artifacts. Am J Ophthalmol. 2014;157(5):1022–1032. doi: 10.1016/j.ajo.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashita T, Yamashita T, Shirasawa M, Arimura N, Terasaki H, Sakamoto T. Repeatability and reproducibility of subfoveal choroidal thickness in normal eyes of Japanese using different SD-OCT devices. Invest Ophthalmol Vis Sci. 2012;53(3):1102–1107. doi: 10.1167/iovs.11-8836. [DOI] [PubMed] [Google Scholar]
- 22.Rahman W, Chen FK, Yeoh J, Patel P, Tufail A, Da Cruz L. Repeatability of manual subfoveal choroidal thickness measurements in healthy subjects using the technique of enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(5):2267–2271. doi: 10.1167/iovs.10-6024. [DOI] [PubMed] [Google Scholar]
- 23.Matsuo Y, Sakamoto T, Yamashita T, Tomita M, Shirasawa M, Terasaki H. Comparisons of choroidal thickness of normal eyes obtained by two different spectral-domain OCT instruments and one swept-source OCT instrument. Invest Ophthalmol Vis Sci. 2013;54(12):7630–7636. doi: 10.1167/iovs.13-13135. [DOI] [PubMed] [Google Scholar]
- 24.Sim DA, Keane PA, Mehta H, et al. Repeatability and reproducibility of choroidal vessel layer measurements in diabetic retinopathy using enhanced depth optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(4):2893–2901. doi: 10.1167/iovs.12-11085. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Fallah N, Forooghian F, et al. Comparative analysis of repeatability of manual and automated choroidal thickness measurements in nonneovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54(4):2864–2871. doi: 10.1167/iovs.12-11521. [DOI] [PubMed] [Google Scholar]
- 26.Hanumunthadu D, Ilginis T, Restori M, et al. Spectral-domain optical coherence tomography retinal and choroidal thickness metric repeatability in age related macular degeneration. Am J Ophthalmol. 2016;166:154–161. doi: 10.1016/j.ajo.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 27.Hanumunthadu D, Ilginis T, Restori M, et al. Repeatability of swept-source optical coherence tomography retinal and choroidal thickness measurements in neovascular age-related macular degeneration. Br J Ophthalmol. 2017;101(5):603–8. doi: 10.1136/bjophthalmol-2016-308999. [DOI] [PubMed] [Google Scholar]
- 28.Shin YU, Lee MJ, Lee BR. Choroidal Maps in Different Types of Macular Edema in Branch Retinal Vein Occlusion Using Swept-Source Optical Coherence Tomography. Am J Ophthalmol. 2015;160(2):328–334. e321. doi: 10.1016/j.ajo.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Gerendas BS, Waldstein SM, Simader C, et al. Three-dimensional automated choroidal volume assessment on standard spectral-domain optical coherence tomography and correlation with the level of diabetic macular edema. Am J Ophthalmol. 2014;158(5):1039–1048. doi: 10.1016/j.ajo.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unsal E, Eltutar K, Zirtiloglu S, Dincer N, Ozdogan Erkul S, Gungel H. Choroidal thickness in patients with diabetic retinopathy. Clin Ophthalmol. 2014;8:637–642. doi: 10.2147/OPTH.S59395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adhi M, Brewer E, Waheed NK, Duker JS. Analysis of morphological features and vascular layers of choroid in diabetic retinopathy using spectral-domain optical coherence tomography. JAMA Ophthalmol. 2013;131(10):1267–1274. doi: 10.1001/jamaophthalmol.2013.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esmaeelpour M, Brunner S, Ansari-Shahrezaei S, et al. Choroidal thinning in diabetes type 1 detected by 3-dimensional 1060 nm optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(11):6803–6809. doi: 10.1167/iovs.12-10314. [DOI] [PubMed] [Google Scholar]
- 33.Querques G, Lattanzio R, Querques L, et al. Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest Ophthalmol Vis Sci. 2012;53(10):6017–6024. doi: 10.1167/iovs.12-9692. [DOI] [PubMed] [Google Scholar]
- 34.Regatieri CV, Branchini L, Carmody J, Fujimoto JG, Duker JS. Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina. 2012;32(3):563–568. doi: 10.1097/IAE.0b013e31822f5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esmaeelpour M, Povazay B, Hermann B, et al. Mapping choroidal and retinal thickness variation in type 2 diabetes using three-dimensional 1060-nm optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(8):5311–5316. doi: 10.1167/iovs.10-6875. [DOI] [PubMed] [Google Scholar]
- 36.Rayess N, Rahimy E, Ying GS, et al. Baseline choroidal thickness as a predictor for response to anti-vascular endothelial growth factor therapy in diabetic macular edema. Am J Ophthalmol. 2015;159(1):85–91. e81–83. doi: 10.1016/j.ajo.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 37.Yiu G, Manjunath V, Chiu SJ, Farsiu S, Mahmoud TH. Effect of anti-vascular endothelial growth factor therapy on choroidal thickness in diabetic macular edema. Am J Ophthalmol. 2014;158(4):745–751. e742. doi: 10.1016/j.ajo.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lains I, Figueira J, Santos AR, et al. Choroidal thickness in diabetic retinopathy: the influence of antiangiogenic therapy. Retina. 2014;34(6):1199–1207. doi: 10.1097/IAE.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 39.Lee EK, Han JM, Hyon JY, Yu HG. Changes in choroidal thickness after intravitreal dexamethasone implant injection in retinal vein occlusion. Br J Ophthalmol. 2015;99(11):1543–1549. doi: 10.1136/bjophthalmol-2014-306417. [DOI] [PubMed] [Google Scholar]
- 40.Gupta P, Jing T, Marziliano P, et al. Distribution and determinants of choroidal thickness and volume using automated segmentation software in a population-based study. Am J Ophthalmol. 2015;159(2):293–301. doi: 10.1016/j.ajo.2014.10.034. [DOI] [PubMed] [Google Scholar]
- 41.Shao L, Xu L, Chen CX, et al. Reproducibility of subfoveal choroidal thickness measurements with enhanced depth imaging by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(1):230–233. doi: 10.1167/iovs.12-10351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.